Propolis: A Natural Substance with Multifaceted Properties and Activities
Abstract
1. Introduction
2. Chemistry of Propolis
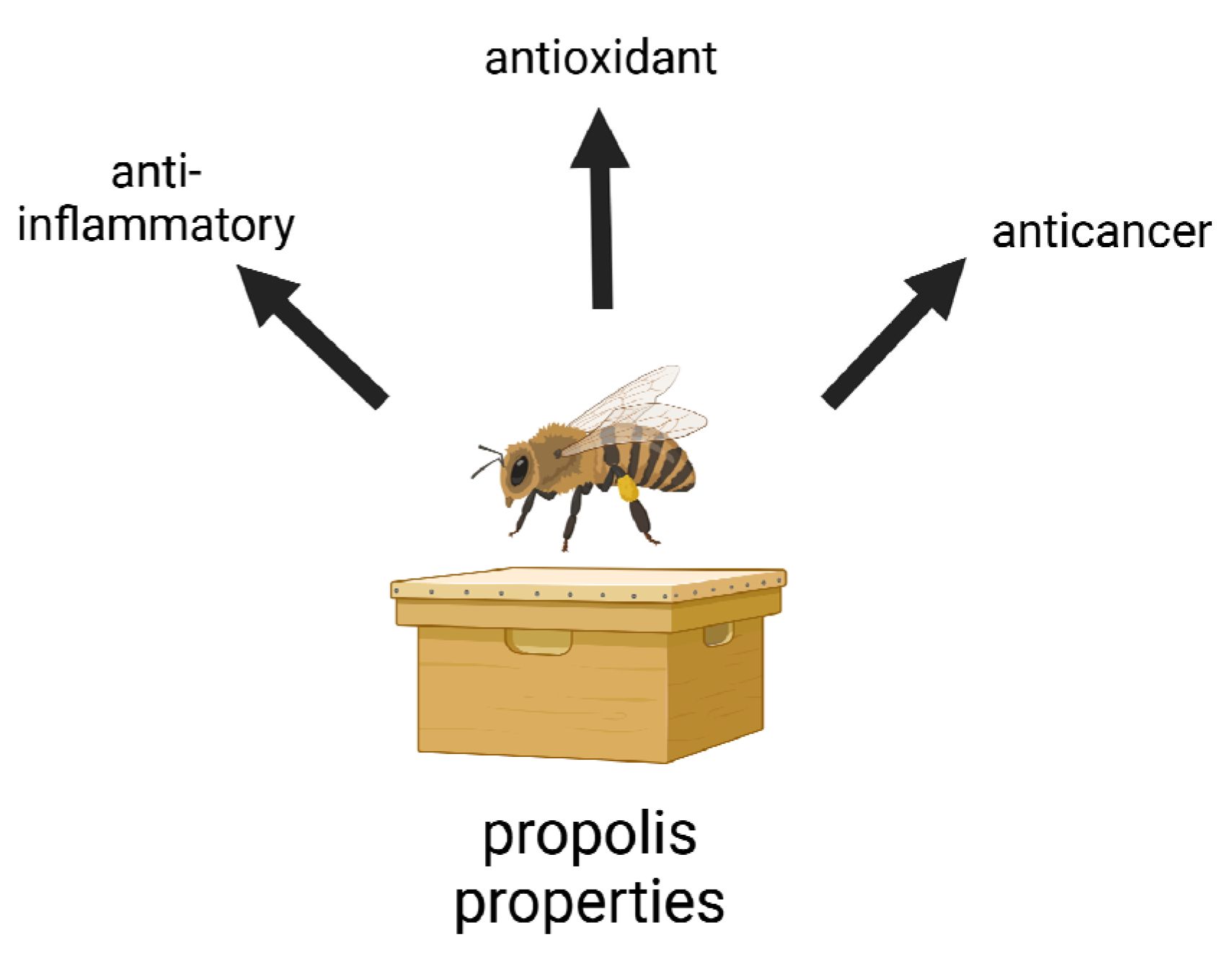
3. Antioxidant Properties
4. Anti-Inflammatory Role
4.1. Metabolic Syndrome
4.2. Type 2 Diabetes Mellitus
4.3. Rheumatoid Arthritis
4.4. Cardiovascular Disease
5. Anticancer Properties
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burns Trauma 2015, 3, 9. [Google Scholar] [CrossRef]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs human colorectal adenocarcinoma cells. Fitoterapia 2015, 106, 167–174. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222. [Google Scholar] [CrossRef]
- Martinotti, S.; Bucekova, M.; Majtan, J.; Ranzato, E. Honey: An Effective Regenerative Medicine Product in Wound Management. Curr. Med. Chem. 2019, 26, 5230–5240. [Google Scholar] [CrossRef]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Honey Bioactive Molecules: There Is a World Beyond the Sugars. BioTech 2024, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Pellavio, G.; Laforenza, U.; Ranzato, E. Propolis Induces AQP3 Expression: A Possible Way of Action in Wound Healing. Molecules 2019, 24, 1544. [Google Scholar] [CrossRef]
- Bonsignore, G.; Martinotti, S.; Ranzato, E. Propolis: A Multifaceted Approach for Wound Healing. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 689–697. [Google Scholar]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis--chemical composition and botanical origin. Evid. Based Complement. Altern. Med. 2008, 5, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Alday, E.; Valencia, D.; Garibay-Escobar, A.; Domínguez-Esquivel, Z.; Piccinelli, A.L.; Rastrelli, L.; Monribot-Villanueva, J.; Guerrero-Analco, J.A.; Robles-Zepeda, R.E.; Hernandez, J.; et al. Plant origin authentication of Sonoran Desert propolis: An antiproliferative propolis from a semi-arid region. Naturwissenschaften 2019, 106, 25. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.H.O.; Barreto, G.A.; Cerqueira, J.C.; Anjos, J.P.D.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; Machado, B.A.S. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound-assisted extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.; Cardoso, C.A.; Paredes-Gamero, E.J.; de Picoli Souza, K.; Dos Santos, E.L. Antioxidant, Cytotoxic, and Toxic Activities of Propolis from Two Native Bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, 2017, 1038153. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Chen, J.; Jiang, X.; Hu, F. Identification of Free Radical Scavengers from Brazilian Green Propolis Using Off-Line HPLC-DPPH Assay and LC-MS. J. Food Sci. 2017, 82, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Markiewicz-Żukowska, R.; Soroczyńska, J.; Teper, D.; Socha, K. Comparative Analysis of Antioxidant Properties of Honey from Poland, Italy, and Spain Based on the Declarations of Producers and Their Results of Melissopalinological Analysis. Nutrients 2022, 14, 2694. [Google Scholar] [CrossRef]
- Fabris, S.; Bertelle, M.; Astafyeva, O.; Gregoris, E.; Zangrando, R.; Gambaro, A.; Lima, G.; Stevanato, R. Antioxidant Properties and Chemical Composition Relationship of Europeans and Brazilians Propolis. Pharmacol. Pharm. 2013, 4, 45–51. [Google Scholar] [CrossRef]
- Mujica, V.; Orrego, R.; Pérez, J.; Romero, P.; Ovalle, P.; Zúñiga-Hernández, J.; Arredondo, M.; Leiva, E. The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2017, 2017, 4272940. [Google Scholar] [CrossRef]
- Jasprica, I.; Mornar, A.; Debeljak, Z.; Smolcić-Bubalo, A.; Medić-Sarić, M.; Mayer, L.; Romić, Z.; Bućan, K.; Balog, T.; Sobocanec, S.; et al. In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J. Ethnopharmacol. 2007, 110, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Xin, Z.; Gao, W.; Guo, C. Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2016, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Kawamoto, D.; Ando-Suguimoto, E.S.; Casarin, R.C.V.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P.A. Brazilian red propolis effects on peritoneal macrophage activity: Nitric oxide, cell viability, pro-inflammatory cytokines and gene expression. J. Ethnopharmacol. 2017, 207, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Saito, N.; Fujimoto, J.; Nakashima, K.I.; Fujikura, D. Brazilian propolis ethanol extract and its component kaempferol induce myeloid-derived suppressor cells from macrophages of mice in vivo and in vitro. BMC Complement. Altern. Med. 2018, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Nai, Y.S.; Chen, Y.W.; Hua, K.F. Mechanistic insight into the attenuation of gouty inflammation by Taiwanese green propolis via inhibition of the NLRP3 inflammasome. J. Cell. Physiol. 2019, 234, 4081–4094. [Google Scholar] [CrossRef]
- Mikami, N.; Tani, H.; Kawakami, R.; Sugimoto, A.; Sakaguchi, S.; Ikuta, T. Brazilian green propolis promotes TNFR2 expression on regulatory T cells. Food Sci. Nutr. 2021, 9, 3200–3208. [Google Scholar] [CrossRef]
- Kashiwakura, J.I.; Yoshihara, M.; Saitoh, K.; Kagohashi, K.; Sasaki, Y.; Kobayashi, F.; Inagaki, I.; Kitai, Y.; Muromoto, R.; Matsuda, T. Propolis suppresses cytokine production in activated basophils and basophil-mediated skin and intestinal allergic inflammation in mice. Allergol. Int. 2021, 70, 360–367. [Google Scholar] [CrossRef]
- Oliveira, L.P.G.; Conte, F.L.; de Oliveira Cardoso, E.; Conti, B.J.; Santiago, K.B.; de Assis Golim, M.; da Silva Feltran, G.; Zambuzzi, W.F.; Sforcin, J.M. A new chemotherapeutic approach using doxorubicin simultaneously with geopropolis favoring monocyte functions. Life Sci. 2019, 217, 81–90. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, E.; Santiago, K.B.; Conti, B.J.; Conte, F.L.; Tasca, K.I.; Romagnoli, G.G.; de Assis Golim, M.; Rainho, C.A.; Bastos, J.K.; Sforcin, J.M. Brazilian green propolis: A novel tool to improve the cytotoxic and immunomodulatory action of docetaxel on MCF-7 breast cancer cells and on women monocyte. Phytother. Res. 2022, 36, 448–461. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Medjeber, O.; Touri, K.; Rafa, H.; Djeraba, Z.; Belkhelfa, M.; Boutaleb, A.F.; Arroul-Lammali, A.; Belguendouz, H.; Touil-Boukoffa, C. Ex vivo immunomodulatory effect of ethanolic extract of propolis during Celiac Disease: Involvement of nitric oxide pathway. Inflammopharmacology 2018, 26, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Romeo, G.R.; Lee, J.; Shoelson, S.E. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, F.; Javadi, M.; Hashemipour, S.; Koushan, Y.; Haghighian, H.K. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement. Ther. Med. 2019, 43, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Karimian, J.; Hadi, A.; Pourmasoumi, M.; Najafgholizadeh, A.; Ghavami, A. The efficacy of propolis on markers of glycemic control in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Phytother. Res. 2019, 33, 1616–1626. [Google Scholar] [CrossRef]
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A.A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 7289. [Google Scholar] [CrossRef]
- Sakai, T.; Ohhata, M.; Fujii, M.; Oda, S.; Kusaka, Y.; Matsumoto, M.; Nakamoto, A.; Taki, T.; Nakamoto, M.; Shuto, E. Brazilian Green Propolis Promotes Weight Loss and Reduces Fat Accumulation in C57BL/6 Mice Fed A High-Fat Diet. Biol. Pharm. Bull. 2017, 40, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef]
- Cardinault, N.; Tourniaire, F.; Astier, J.; Couturier, C.; Bonnet, L.; Seipelt, E.; Karkeni, E.; Letullier, C.; Dlalah, N.; Georgé, S.; et al. Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice. Antioxidants 2021, 10, 411. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Abdalla, D.S. Emerging roles of propolis: Antioxidant, cardioprotective, and antiangiogenic actions. Evid. Based Complement. Altern. Med. 2013, 2013, 175135. [Google Scholar] [CrossRef]
- Rivera-Yañez, N.; Rodriguez-Canales, M.; Nieto-Yañez, O.; Jimenez-Estrada, M.; Ibarra-Barajas, M.; Canales-Martinez, M.M.; Rodriguez-Monroy, M.A. Hypoglycaemic and Antioxidant Effects of Propolis of Chihuahua in a Model of Experimental Diabetes. Evid. Based Complement. Altern. Med. 2018, 2018, 4360356. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Fujise, T.; Abesundara, K.J.; Doi, S.; Yamada, H.; Matsumoto, K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid. Biol. Pharm. Bull. 2004, 27, 1797–1803. [Google Scholar] [CrossRef]
- Pahlavani, N.; Malekahmadi, M.; Firouzi, S.; Rostami, D.; Sedaghat, A.; Moghaddam, A.B.; Ferns, G.A.; Navashenaq, J.G.; Reazvani, R.; Safarian, M.; et al. Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutr. Metab. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Pahlavani, N.; Ranjbar, G.; Gholizadeh Navashenaq, J.; Salehi-Sahlabadi, A.; Mahmudiono, T.; Nader Shalaby, M.; Jokar, M.; Nematy, M.; Barghchi, H.; et al. Does propolis have any effect on rheumatoid arthritis? A review study. Food Sci. Nutr. 2022, 10, 1003–1020. [Google Scholar] [CrossRef]
- Filippin, L.I.; Vercelino, R.; Marroni, N.P.; Xavier, R.M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 2008, 152, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. (Elite Ed.) 2012, 4, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, S.; Reinhold, D.; Lendeckel, U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta1 production of human immune cells. Z. Naturforsch C J. Biosci. 2003, 58, 580–589. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Takahashi, K.; Sugioka, Y.; Inui, K.; Okano, T.; Mandai, K.; Yamada, Y.; Shintani, A.; Koike, T. Double-blinded randomized controlled trial to reveal the effects of Brazilian propolis intake on rheumatoid arthritis disease activity index; BeeDAI. PLoS ONE 2021, 16, e0252357. [Google Scholar] [CrossRef]
- Ji, C.; Pan, Y.; Xu, S.; Yu, C.; Ji, J.; Chen, M.; Hu, F. Propolis ameliorates restenosis in hypercholesterolemia rabbits with carotid balloon injury by inhibiting lipid accumulation, oxidative stress, and TLR4/NF-κB pathway. J. Food Biochem. 2021, 45, e13577. [Google Scholar] [CrossRef]
- Olas, B. Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases. Nutrients 2022, 14, 2267. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Hallajzadeh, J.; Milajerdi, A.; Amirani, E.; Attari, V.E.; Maghsoudi, H.; Mirhashemi, S.M. Effects of propolis supplementation on glycemic status, lipid profiles, inflammation and oxidative stress, liver enzymes, and body weight: A systematic review and meta-analysis of randomized controlled clinical trials. J. Diabetes Metab. Disord. 2021, 20, 831–843. [Google Scholar] [CrossRef]
- Tan-No, K.; Nakajima, T.; Shoji, T.; Nakagawasai, O.; Niijima, F.; Ishikawa, M.; Endo, Y.; Sato, T.; Satoh, S.; Tadano, T. Anti-inflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol. Pharm. Bull. 2006, 29, 96–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mirzoeva, O.K.; Calder, P.C. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot. Essent. Fatty Acids 1996, 55, 441–449. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Chang, H.; Yuan, W.; Wu, H.; Yin, X.; Xuan, H. Bioactive components and mechanisms of Chinese poplar propolis alleviates oxidized low-density lipoprotein-induced endothelial cells injury. BMC Complement. Altern. Med. 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Yuan, W.; Chang, H.; Liu, M.; Hu, F. Anti-inflammatory effects of Chinese propolis in lipopolysaccharide-stimulated human umbilical vein endothelial cells by suppressing autophagy and MAPK/NF-κB signaling pathway. Inflammopharmacology 2019, 27, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Moreno, M.; García-Ruiz, J.C. The physiology of hemostasis. Blood Coagul. Fibrinolysis 2022, 33, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The Cardiovascular Therapeutic Potential of Propolis-A Comprehensive Review. Biology 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Martina, S.J.; Luthfi, M.; Govindan, P.; Wahyuni, A.S. Effectivity comparison between aspirin, propolis, and bee pollen as an antiplatelet based on bleeding time taken on mice. MATEC Web Conf. 2018, 197, 07008. [Google Scholar] [CrossRef][Green Version]
- Remirez, D.; González, R.; Rodriguez, S.; Ancheta, O.; Bracho, J.C.; Rosado, A.; Rojas, E.; Ramos, M.E. Protective effects of Propolis extract on allyl alcohol-induced liver injury in mice. Phytomedicine 1997, 4, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Li, Z.; Wang, J.; Wang, K.; Fu, C.; Yuan, J.; Hu, F. Propolis Reduces Phosphatidylcholine-Specific Phospholipase C Activity and Increases Annexin a7 Level in Oxidized-LDL-Stimulated Human Umbilical Vein Endothelial Cells. Evid. Based Complement. Altern. Med. 2014, 2014, 465383. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, G.; Patrone, M.; Grosso, F.; Martinotti, S.; Ranzato, E. Cancer Therapy Challenge: It Is Time to Look in the “St. Patrick’s Well” of the Nature. Int. J. Mol. Sci. 2021, 22, 380. [Google Scholar] [CrossRef]
- Ranzato, E.; Simona Martinotti, S.; Calabrese, C.M.; Calabrese, G. Role of nutraceuticals in cancer therapy. J. Food Res. 2014, 3, 18. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Understanding the Anticancer Properties of Honey. Int. J. Mol. Sci. 2024, 25, 1724. [Google Scholar] [CrossRef]
- Karikas, G.A. Anticancer and chemopreventing natural products: Some biochemical and therapeutic aspects. J. BUON 2010, 15, 627–638. [Google Scholar] [PubMed]
- Elumalai, P.; Muninathan, N.; Megalatha, S.T.; Suresh, A.; Kumar, K.S.; Jhansi, N.; Kalaivani, K.; Krishnamoorthy, G. An Insight into Anticancer Effect of Propolis and Its Constituents: A Review of Molecular Mechanisms. Evid. Based Complement. Altern. Med. 2022, 2022, 5901191. [Google Scholar] [CrossRef]
- Zhang, L.S.; Lum, L. Chemical Modulation of WNT Signaling in Cancer. Prog. Mol. Biol. Transl. Sci. 2018, 153, 245–269. [Google Scholar] [CrossRef]
- Wang, D.; Xiang, D.B.; He, Y.J.; Li, Z.P.; Wu, X.H.; Mou, J.H.; Xiao, H.L.; Zhang, Q.H. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of colorectal cancer cells in vitro. World J. Gastroenterol. 2005, 11, 4008–4012. [Google Scholar] [CrossRef]
- Wang, H.X.; Tang, C. Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling pathways. Oncol. Rep. 2017, 38, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Choi, S.M.; Tucker, D.F.; Gross, D.N.; Easton, R.M.; DiPilato, L.M.; Dean, A.S.; Monks, B.R.; Birnbaum, M.J. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol. Cell. Biol. 2010, 30, 5009–5020. [Google Scholar] [CrossRef]
- Woo, K.J.; Jeong, Y.J.; Park, J.W.; Kwon, T.K. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem. Biophys. Res. Commun. 2004, 325, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Yee, M.; Saba, Y.; Chino, T. Artepillin C as a targeting survivin molecule in oral squamous cell carcinoma cells in vitro: A preliminary study. J. Oral. Pathol. Med. 2018, 47, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Fan, T.P.; Watson, D.; Alenezi, S.; Saleh, K.; Sahlan, M. Preliminary studies: The potential anti-angiogenic activities of two Sulawesi Island (Indonesia) propolis and their chemical characterization. Heliyon 2019, 5, e01978. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell. Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Hayati, I. Inhibition of Mammary Gland Cancer Development by Propolis and Mangostin in Female Mice Balb/C. J. Math. Fundam. Sci. 2017, 49, 40–50. [Google Scholar] [CrossRef]
- Pai, J.T.; Lee, Y.C.; Chen, S.Y.; Leu, Y.L.; Weng, M.S. Propolin C Inhibited Migration and Invasion via Suppression of EGFR-Mediated Epithelial-to-Mesenchymal Transition in Human Lung Cancer Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 7202548. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yang, S.W.; Chang, K.P.; Feng, T.H.; Chang, K.S.; Tsui, K.H.; Shin, Y.S.; Chen, C.C.; Chao, M.; Juang, H.H. Caffeic Acid Phenethyl Ester Induces. Int. J. Mol. Sci. 2018, 19, 1397. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban Brown Propolis Interferes in the Crosstalk between Colorectal Cancer Cells and M2 Macrophages. Nutrients 2020, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- de Giffoni de Carvalho, J.T.; da Silva Baldivia, D.; Leite, D.F.; de Araújo, L.C.A.; de Toledo Espindola, P.P.; Antunes, K.A.; Rocha, P.S.; de Picoli Souza, K.; Dos Santos, E.L. Medicinal Plants from Brazilian Cerrado: Antioxidant and Anticancer Potential and Protection against Chemotherapy Toxicity. Oxid. Med. Cell. Longev. 2019, 2019, 3685264. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Abdelazim, S.A.; Darwish, H.A.; Elbaz, E.M.; Shouman, S.A. Modulation of Tamoxifen Cytotoxicity by Caffeic Acid Phenethyl Ester in MCF-7 Breast Cancer Cells. Oxid. Med. Cell. Longev. 2016, 2016, 3017108. [Google Scholar] [CrossRef] [PubMed]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and propolis on AOM/DSS induced colorectal cancer in BALB-c mice. Life Sci. 2021, 276, 119390. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.R.; Prasad, N.R. Phytochemicals reverse P-glycoprotein mediated multidrug resistance via signal transduction pathways. Biomed. Pharmacother. 2021, 139, 111632. [Google Scholar] [CrossRef]
- Kebsa, W.; Lahouel, M.; Rouibah, H.; Zihlif, M.; Ahram, M.; Abu-Irmaileh, B.; Mustafa, E.; Al-Ameer, H.J.; Al Shhab, M. Reversing Multidrug Resistance in Chemo-resistant Human Lung Adenocarcinoma (A549/DOX) Cells by Algerian Propolis Through Direct Inhibiting the P-gp Efflux-pump, G0/G1 Cell Cycle Arrest and Apoptosis Induction. Anticancer Agents Med. Chem. 2018, 18, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Wang, R.H.; Wang, H.H.; Li, C.H. Meta-analysis of randomized controlled trials of the efficacy of propolis mouthwash in cancer therapy-induced oral mucositis. Support. Care Cancer 2018, 26, 4001–4009. [Google Scholar] [CrossRef] [PubMed]
- Piredda, M.; Facchinetti, G.; Biagioli, V.; Giannarelli, D.; Armento, G.; Tonini, G.; De Marinis, M.G. Propolis in the prevention of oral mucositis in breast cancer patients receiving adjuvant chemotherapy: A pilot randomised controlled trial. Eur. J. Cancer Care 2017, 26, e12757. [Google Scholar] [CrossRef]
- AkhavanKarbassi, M.H.; Yazdi, M.F.; Ahadian, H.; SadrAbad, M.J. Randomized DoubleBlind PlaceboControlled Trial of Propolis for Oral Mucositis in Patients Receiving Chemotherapy for Head and Neck Cancer. Asian Pac. J. Cancer Prev. 2016, 17, 3611–3614. [Google Scholar] [PubMed]
- Frión-Herrera, Y.; Gabbia, D.; Díaz-García, A.; Cuesta-Rubio, O.; Carrara, M. Chemosensitizing activity of Cuban propolis and nemorosone in doxorubicin resistant human colon carcinoma cells. Fitoterapia 2019, 136, 104173. [Google Scholar] [CrossRef]
- Curti, V.; Zaccaria, V.; Tsetegho Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and In Vivo Antioxidant Activity of a Standardized Polyphenol Mixture Extracted from Brown Propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef]
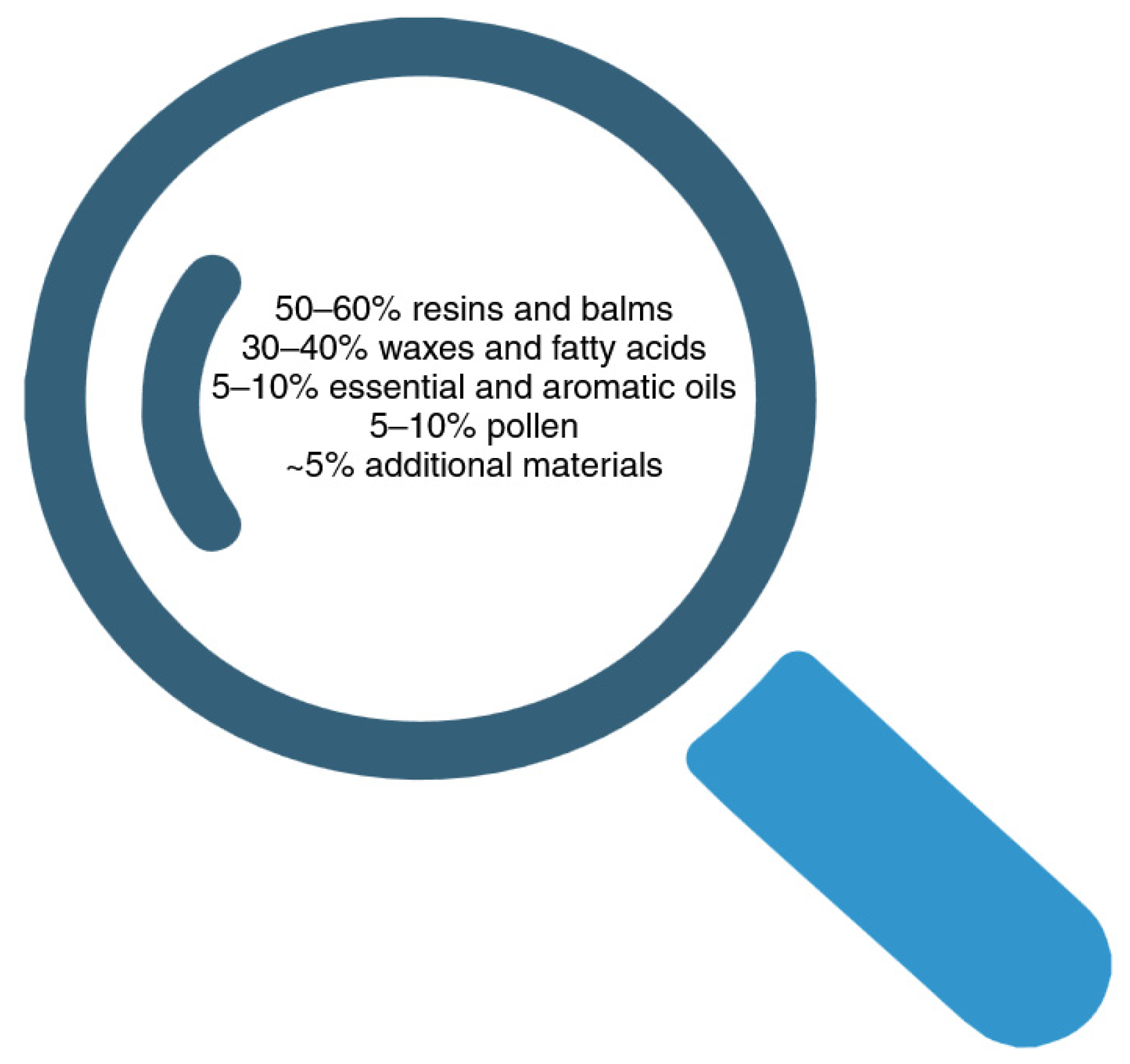
| Resin | In the hive, the bees collect plant resins and use them as polishers, sealants, disinfectants, and mummifiers for dead insects. |
| Wax | Typically, honey bees generate the yellowish, soft, and highly absorbable substance known as wax. Esters, acids, high-fat alcohols, and even free hydrocarbons are all found in waxes. Although wax is a durable and extremely moisture-proof material, it is not resistant to mechanical stresses or heat. |
| Flower pollen | The flower from which the honey bee gathers its pollen determines its precise composition. Essential amino acids, vitamins, mineral salts, and hormones are abundant in flower pollen. |
| Phenols | Flavonoids, phenolic acids, tannins, stilbenes, curcuminoids, coumarins, and quinines are all found in propolis. The proportion of these types of substances varies and depends on the place and time of collection. Strong antioxidant and antibacterial properties were due to the high total phenolic and flavone/flavonol contents in the propolis. |
| Terpenes | Primary and secondary metabolites are produced by all plants and serve a variety of purposes. Amino acids, simple sugars, nucleic acids, and lipids are all found in primary metabolites and are necessary for cellular functions. Terpenes, alkaloids, and phenolic compounds are among the substances found in secondary metabolites that are created in reaction to stress. Terpenes are also responsible for propolis’s distinctive resinous smell. |
| Hydrocarbons | Propolis from various geographical regions has been found to contain alkanes, alkenes, alkadins, monosasters, diesters, aromatic esters, fatty acids, and steroids. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinotti, S.; Bonsignore, G.; Ranzato, E. Propolis: A Natural Substance with Multifaceted Properties and Activities. Int. J. Mol. Sci. 2025, 26, 1519. https://doi.org/10.3390/ijms26041519
Martinotti S, Bonsignore G, Ranzato E. Propolis: A Natural Substance with Multifaceted Properties and Activities. International Journal of Molecular Sciences. 2025; 26(4):1519. https://doi.org/10.3390/ijms26041519
Chicago/Turabian StyleMartinotti, Simona, Gregorio Bonsignore, and Elia Ranzato. 2025. "Propolis: A Natural Substance with Multifaceted Properties and Activities" International Journal of Molecular Sciences 26, no. 4: 1519. https://doi.org/10.3390/ijms26041519
APA StyleMartinotti, S., Bonsignore, G., & Ranzato, E. (2025). Propolis: A Natural Substance with Multifaceted Properties and Activities. International Journal of Molecular Sciences, 26(4), 1519. https://doi.org/10.3390/ijms26041519







