Progress in Methylxanthine Biosynthesis: Insights into Pathways and Engineering Strategies
Abstract
1. Introduction
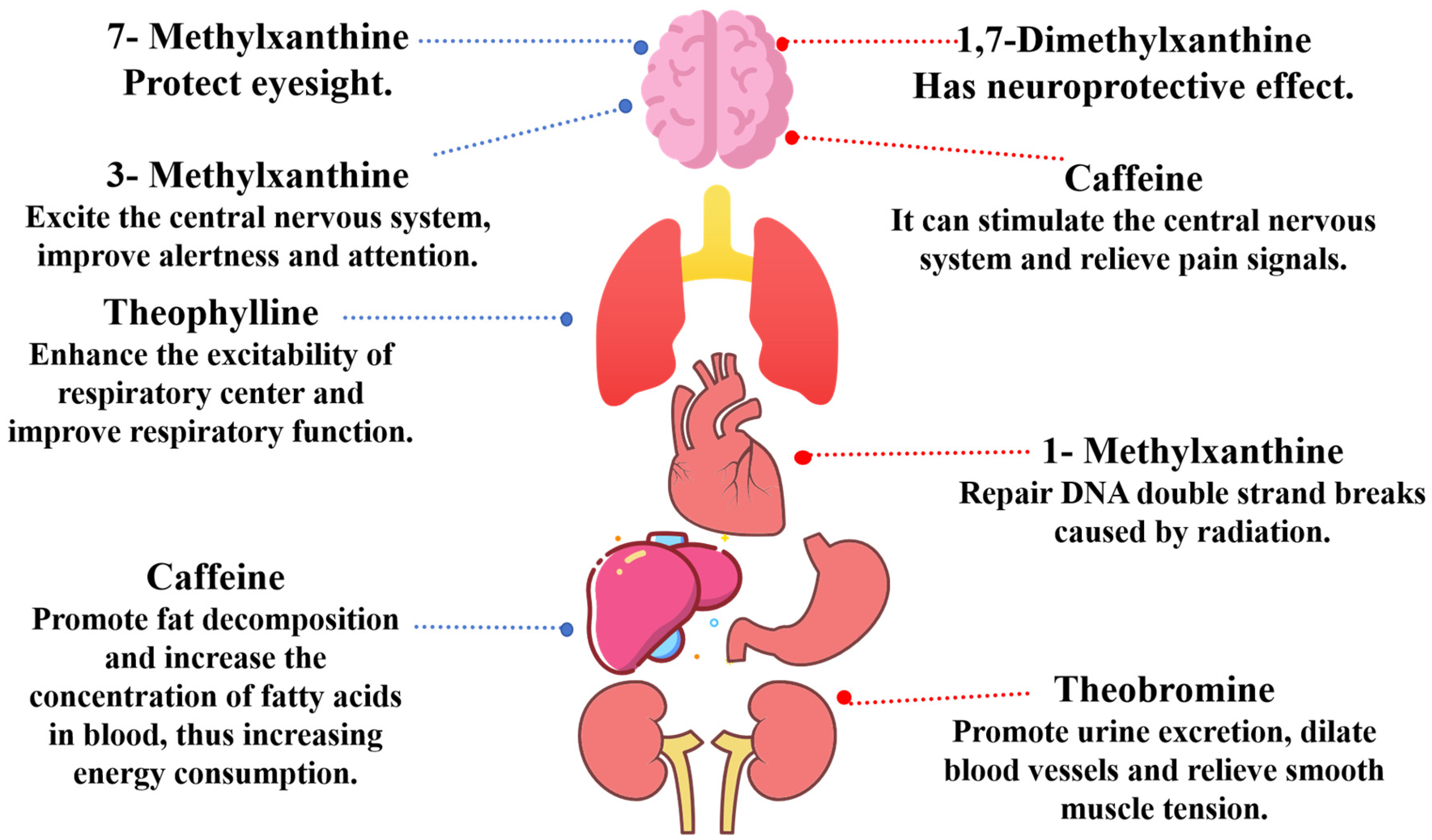
2. Biological Structures and Bioactivities of Methylxanthine Derivatives
3. Biosynthetic Pathways of Methylxanthines
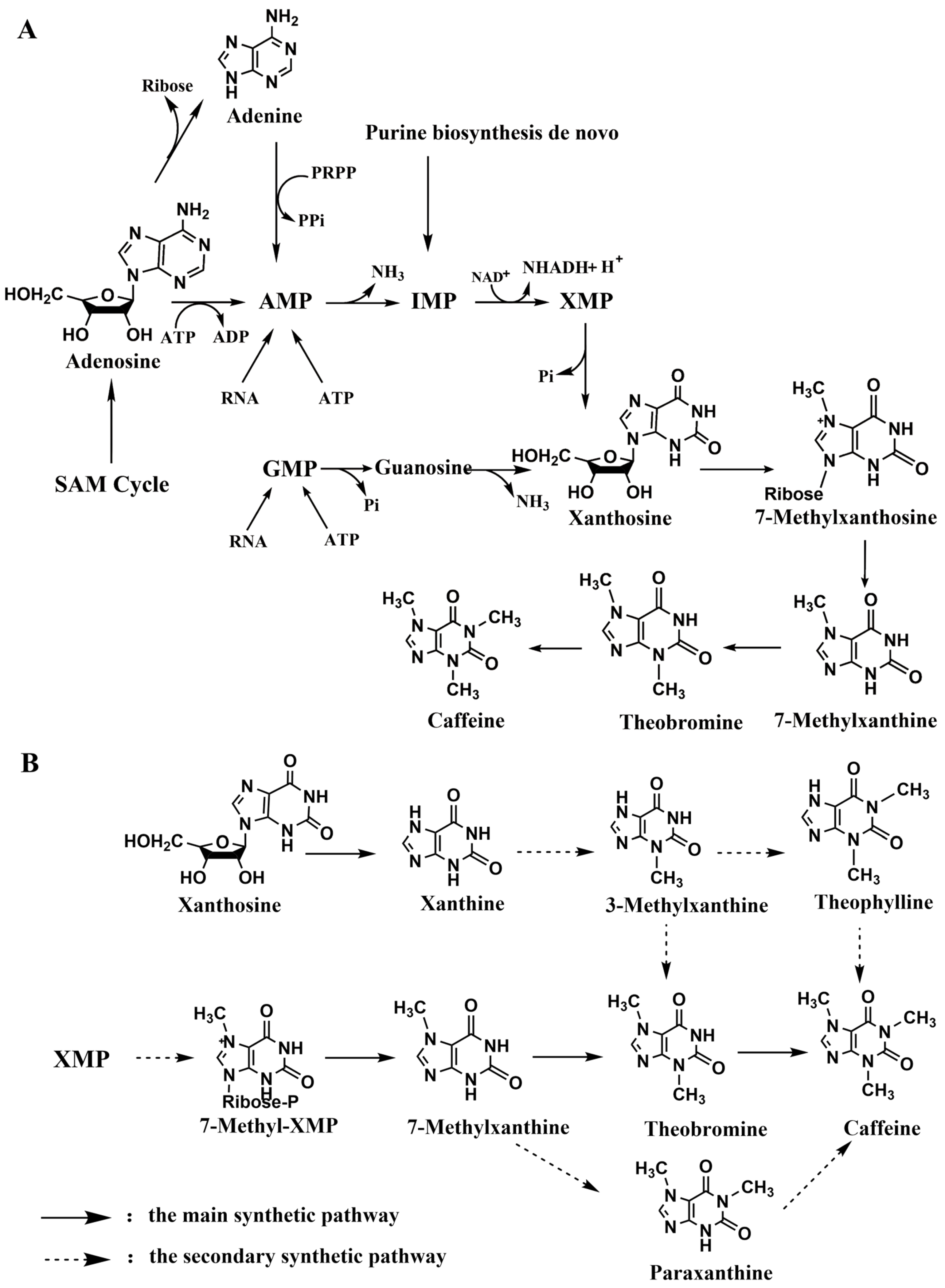
3.1. De Novo Biosynthesis of Methylxanthines
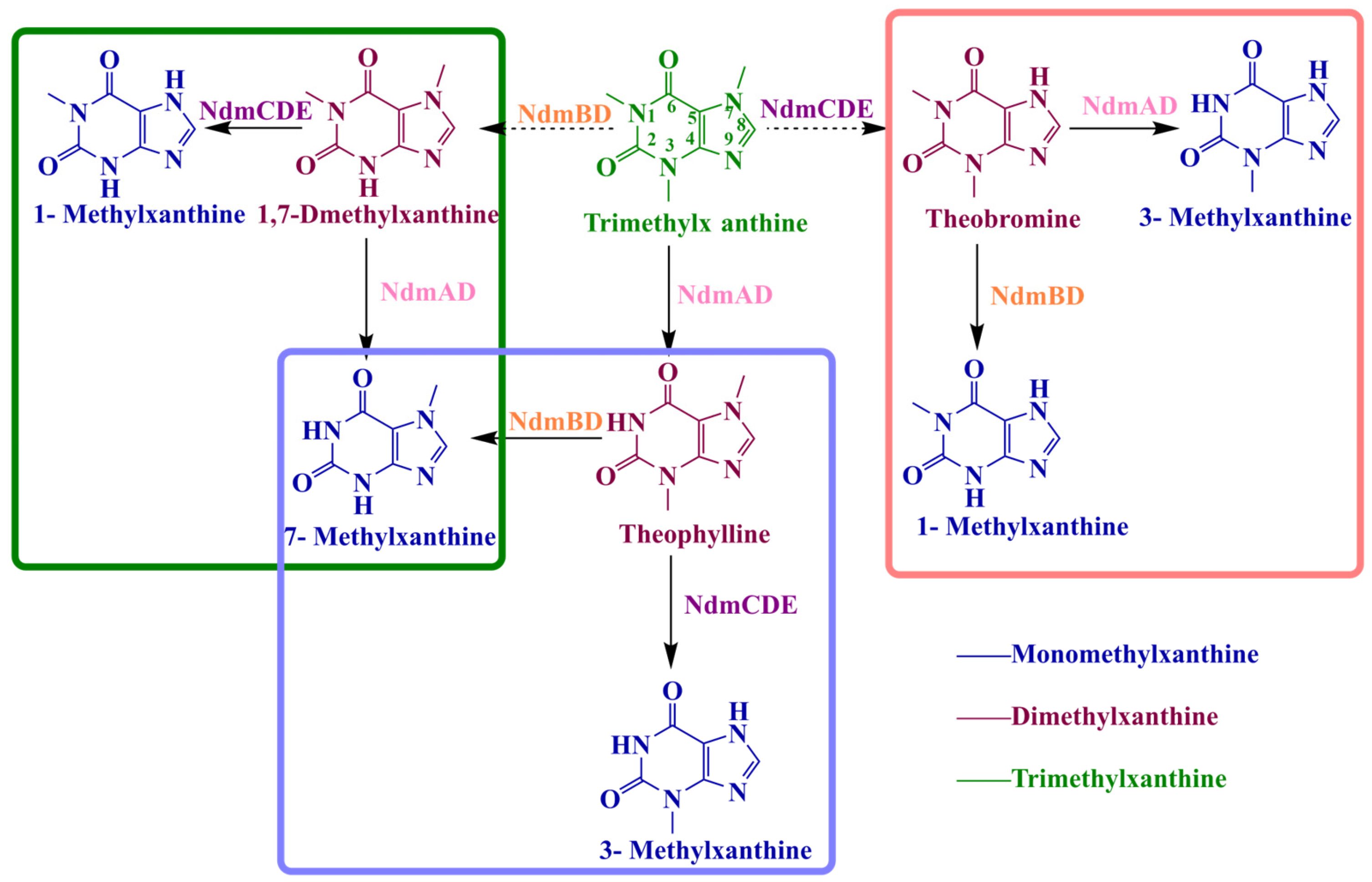
3.2. Synthesis of Methylxanthines Through Caffeine N-Demethylation
4. Advancements in Methylxanthine Biosynthesis Optimization
4.1. Advancements in the Synthesis of 1-Methylxanthine
4.2. Advances in the Synthesis of 3-Methylxanthine
4.3. Advances in the Synthesis of 7-Methylxanthine
4.4. Advances in the Synthesis of Paraxanthine
| Modification Method | Strain | Strategy | Substrate | Product | Yield (g/L) | Reference |
|---|---|---|---|---|---|---|
| Enhanced biosynthetic pathway with reutilization of byproducts to increase yield | E. coli BL21(DE3) | Targeted mutation to obtain the high-efficiency enzyme ndmA3, combined with ndmD, and introduction of frmAB for the cofactor recycling system | Theophylline | 1-MX | 1.5 | [33] |
| Enhanced biosynthetic pathway | E. coli BL21(DE3) | Introduction of the enzymes ndmB and ndmD into the cell, with the cofactor recycling system | Theophylline | 1-MX | 0.34 | [110] |
| Enhanced biosynthetic pathway | E. coli BW25113 | Use of efficient ndmA and ndmD enzymes | Theophylline | 3-MX | 3.8 | [32] |
| Enhanced biosynthetic pathway | E. coli BL21(DE3) | Use of different copy numbers of ndmA and ndmD, increasing the ndmD copy number to enhance intracellular soluble ndmD levels | Theophylline | 3-MX | 0.14 | [113] |
| Enhanced biosynthetic pathway with reutilization of byproducts to increase yield | E. coli BL21(DE3) | Targeted mutation to obtain high-efficiency enzyme ndmA4, and use of a mixed culture system with theobromine as an intermediate | Caffeine, Theobromine | 7-MX | 0.15 | [112] |
| Enhanced biosynthetic pathway with optimized microbial ratio | E. coli BL21(DE3) | Optimized initial cell density and copy numbers of the ndmB and ndmD enzymes | Theobromine | 7-MX | 0.13 | [117] |
| Enhanced biosynthetic pathway with cofactor recycling system to regenerate NADH and eliminate bottlenecks | E. coli BW25113 | Introduction of ndmA, ndmB, and modified ndmD genes, along with frmA, frmB, and FDH for cofactor regeneration system | Caffeine | 7-MX | 8.37 | [32] |
| Enhanced biosynthetic pathway with optimized enzyme copy numbers and NADPH regeneration system | E. coli BL21(DE3) | Increased the copy numbers of ndmA4 and ndmD; truncate the first 266 amino acids of ndmD to generate high-efficiency enzyme ndmDP1; establish NADPH regeneration system | Caffeine | PX | 0.12 | [34] |
| Enhanced biosynthetic pathway with cofactor recycling system and high-efficiency ndmA4 mutant | E. coli BW25113 | Introduction of the ndmB and ndmD enzymes and cofactor recycling system, and use of the ndmA4 mutant for high-efficiency enzyme production | Theophylline | PX | 0.02 | [32] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1-MX | 1-methylxanthine |
| 3-MX | 3-methylxanthine |
| 7-MX | 7-methylxanthine |
| PX | Paraxanthine |
| E. coli | Escherichia coli |
References
- Wolf, A.; Bray, G.A.; Popkin, B.M. A short history of beverages and how our body treats them. Obes. Rev. 2007, 9, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Hakami, O.; El-Shazly, M.; El-Nashar, H.A.S.; Yousif, M.N.M. Caffeine and Purine Derivatives: A comprehensive review on the chemistry, biosynthetic pathways, Synthesis-Related Reactions, biomedical prospectives and clinical applications. Chem. Biodivers. 2024, 21, e202400050. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in Foods: A Comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Kongor, J.E.; De Pascual-Teresa, S. From cocoa to chocolate: Effect of processing on flavanols and methylxanthines and their mechanisms of action. Int. J. Mol. Sci. 2022, 23, 14365. [Google Scholar] [CrossRef]
- McClure, A.P.; Spinka, C.M.; Grün, I.U. Quantitative analysis and response surface modeling of important bitter compounds in chocolate made from cocoa beans with eight roast profiles across three origins. J. Food Sci. 2021, 86, 4901–4913. [Google Scholar] [CrossRef]
- Condori, D.; Espichan, F.; Macassi, A.L.S.; Carbajal, L.; Rojas, R. Study of the post-harvest processes of the peruvian chuncho cocoa using multivariate and multi-block analysis. Food Chem. 2023, 431, 137123. [Google Scholar] [CrossRef]
- Williamson, M.; Poorun, R.; Hartley, C. Apnoea of Prematurity and Neurodevelopmental Outcomes: Current understanding and future Prospects for research. Front. Pediatr. 2021, 9, 755677. [Google Scholar] [CrossRef]
- Wang, L.; Fan, W.; Cui, L.; Yang, N.; Zhang, X.; Yu, S.; Li, Y.; Wang, B. Synthesis and biological activity evaluation of novel chalcone analogues containing a methylxanthine moiety and their N-Acyl pyrazoline derivatives. J. Agric. Food Chem. 2023, 71, 19343–19356. [Google Scholar] [CrossRef]
- Wang, L.; Fan, W.; Yang, N.; Xiong, L.; Wang, B. Novel insecticidal Butenolide-Containing methylxanthine derivatives: Synthesis, crystal structure, biological activity evaluation, DFT calculation and molecular docking. Chem. Biodivers. 2024, 21, e202400823. [Google Scholar] [CrossRef]
- Singh, H.; Singh, H.; Sahajpal, N.S.; Paul, S.; Kaur, I.; Jain, S.K. Sub-chronic and chronic toxicity evaluation of 7-methylxanthine: A new molecule for the treatment of myopia. Drug Chem. Toxicol. 2022, 45, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Marx, B.; Scuvée, É.; Scuvée-Moreau, J.; Seutin, V.; Jouret, F. Mécanismes de l’effet diurétique de la caféine. Med. Sci. 2016, 32, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a factor influencing the functioning of the human Body—Friend or foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Newmire, D.E.; Stout, J.R.; Antonio, B.; Gibbons, M.; Lowery, L.M.; Harper, J.; Willoughby, D.; Evans, C.; Anderson, D.; et al. Common questions and misconceptions about caffeine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2024, 21, 2323919. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E. Caffeine and exercise. Sports Med. 2001, 31, 785–807. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef]
- Mansfield, B.; Werner, M.; Berndt, C.; Shattuck, A.; Galt, R.; Williams, B.; Argüelles, L.; Barri, F.R.; Ishii, M.; Kunin, J.; et al. A new critical social science research agenda on pesticides. Agric. Hum. Values 2023, 41, 395–412. [Google Scholar] [CrossRef]
- Ashihara, H.; Kato, M.; Crozier, A. Distribution, Biosynthesis and Catabolism of Methylxanthines in Plants. In Methylxanthines; Handbook of Experimental Pharmacology Series; Springer: Berlin/Heidelberg, Germany, 2010; pp. 11–31. [Google Scholar] [CrossRef]
- Ashihara, H. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2007, 69, 841–856. [Google Scholar] [CrossRef]
- Deng, C.; Ku, X.; Cheng, L.; Pan, S.; Fan, L.; Deng, W.; Zhao, J.; Zhang, Z. Metabolite and Transcriptome Profiling on Xanthine Alkaloids-Fed Tea Plant (Camellia sinensis) Shoot Tips and Roots Reveal the Complex Metabolic Network for Caffeine Biosynthesis and Degradation. Front. Plant Sci. 2020, 11, 551288. [Google Scholar] [CrossRef]
- Teng, J.; Yan, C.; Zeng, W.; Zhang, Y.; Zeng, Z.; Huang, Y. Purification and characterization of theobromine synthase in a Theobromine-Enriched wild tea plant (Camellia gymnogyna Chang) from Dayao Mountain, China. Food Chem. 2020, 311, 125875. [Google Scholar] [CrossRef]
- Hebra, T.; Smrčková, H.; Elkatmis, B.; Převorovský, M.; Pluskal, T. POMBOX: A fission yeast cloning toolkit for molecular and synthetic biology. ACS Synth. Biol. 2023, 13, 558–567. [Google Scholar] [CrossRef] [PubMed]
- McKeague, M.; Wang, Y.; Cravens, A.; Win, M.N.; Smolke, C.D. Engineering a microbial platform for de novo biosynthesis of diverse methylxanthines. Metab. Eng. 2016, 38, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.M.; Louie, T.M.; Yu, C.; Gakhar, L.; Louie, K.C.; Subramanian, M. Novel, highly specific N -Demethylases enable bacteria to live on caffeine and related purine alkaloids. J. Bacteriol. 2012, 194, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Faudone, G.; Arifi, S.; Merk, D. The medicinal chemistry of caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef]
- Tkatchenko, T.V.; Tkatchenko, A.V. Pharmacogenomic Approach to Antimyopia Drug Development: Pathways lead the way. Trends Pharmacol. Sci. 2019, 40, 833–852. [Google Scholar] [CrossRef]
- Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Methylxanthines and Neurodegenerative Diseases: An update. Nutrients 2021, 13, 803. [Google Scholar] [CrossRef]
- Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Winkler, J.; Griebsch, L.V.; Pilz, S.M.; Theiss, E.L.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Methylxanthines induce a change in the AD/Neurodegeneration-Linked lipid profile in neuroblastoma cells. Int. J. Mol. Sci. 2022, 23, 2295. [Google Scholar] [CrossRef]
- Olivares-Yañez, C.; Alessandri, M.P.; Salas, L.; Larrondo, L.F. Methylxanthines modulate circadian period length independently of the action of phosphodiesterase. Microbiol. Spectr. 2023, 11, e03727-22. [Google Scholar] [CrossRef]
- Serrano-Marín, J.; Reyes-Resina, I.; Martínez-Pinilla, E.; Navarro, G.; Franco, R. Natural compounds as guides for the discovery of drugs targeting G-Protein-Coupled receptors. Molecules 2020, 25, 5060. [Google Scholar] [CrossRef]
- Baniasadi, S. Metabolism-based Drug-drug Interactions in Patients with Chronic Respiratory Diseases: A Review Focusing on Drugs Affecting the Respiratory System. Curr. Drug Metab. 2020, 21, 704–713. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Zhao, H.; Gu, X.; Gu, J.; Zhao, M.; Zuo, S.; Wang, P. Highly efficient Whole-Cell biocatalysis for the biosynthesis of 7-Methylxanthine and other xanthine derivatives. ACS Sustain. Chem. Eng. 2024, 12, 9716–9726. [Google Scholar] [CrossRef]
- Mock, M.B.; Zhang, S.; Pakulski, K.; Hutchison, C.; Kapperman, M.; Dreischarf, T.; Summers, R.M. Production of 1-methylxanthine via the biodegradation of theophylline by an optimized Escherichia coli strain. J. Biotechnol. 2024, 379, 25–32. [Google Scholar] [CrossRef]
- Mock, M.B.; Mills, S.B.; Cyrus, A.; Campo, H.; Dreischarf, T.; Strock, S.; Summers, R.M. Biocatalytic production and purification of the high-value biochemical paraxanthine. Biotechnol. Bioprocess Eng. 2022, 27, 640–651. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.H.; Brooks, S.; Kang, S.Y.; Summers, R.M.; Song, H.K. Structural and Mechanistic Insights into Caffeine Degradation by the Bacterial N-Demethylase Complex. J. Mol. Biol. 2019, 431, 3647–3661. [Google Scholar] [CrossRef] [PubMed]
- Coquis, C.; Richaud, A.; Méndez, F. Effect of methyl substituents in the reactivity of methylxanthines. J. Mol. Model. 2018, 24, 331. [Google Scholar] [CrossRef]
- Dar, M.O.; Mir, R.H.; Mohiuddin, R.; Masoodi, M.H.; Sofi, F.A. Metal complexes of xanthine and its derivatives: Synthesis and biological activity. J. Inorg. Biochem. 2023, 246, 112290. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Francescato, G.; Leitão, M.I.P.S.; Orsini, G.; Petronilho, A. Synthesis and medicinal applications of N-Heterocyclic carbene complexes based on caffeine and other xanthines. ChemMedChem 2024, 19, e202400118. [Google Scholar] [CrossRef]
- Lin, Z.; Wei, J.; Hu, Y.; Pi, D.; Jiang, M.; Lang, T. Caffeine synthesis and its mechanism and application by microbial degradation, a review. Foods 2023, 12, 2721. [Google Scholar] [CrossRef]
- Mazzafera, P. Catabolism of caffeine in plants and microorganisms. Front. Biosci. 2004, 9, 1348. [Google Scholar] [CrossRef]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health benefits of methylxanthines in cacao and chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, D.V.; Ivanov, I.D.; Baev, D.S.; Rybalova, T.V.; Mozhaitsev, E.S.; Patrushev, S.S.; Vavilin, V.A.; Tolstikova, T.G.; Shults, E.E. Design, synthesis and assay of novel Methylxanthine–Alkynylmethylamine derivatives as acetylcholinesterase inhibitors. Molecules 2022, 27, 8787. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In Methylxanthines; Handbook of Experimental Pharmacology Series; Springer: Berlin/Heidelberg, Germany, 2010; pp. 33–91. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Zheng, C.; Xia, T.; Ma, B.; Liu, X. 3-Methylxanthine production through biodegradation of theobromine by Aspergillus sydowii PT-2. BMC Microbiol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Benowitz, N. Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin. Pharmacol. Ther. 2003, 74, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Liang, L.; Huang, T.; Balasubramanian, R.; Zhao, Y.; Chandler, P.D.; Manson, J.E.; Feliciano, E.M.C.; Hayden, K.M.; Van Horn, L.; et al. Identifying metabolomic profiles of inflammatory diets in postmenopausal women. Clin. Nutr. 2019, 39, 1478–1490. [Google Scholar] [CrossRef]
- Qu, W.; Chen, Z.; Hu, X.; Zou, T.; Huang, Y.; Zhang, Y.; Hu, Y.; Tian, S.; Wan, J.; Liao, R.; et al. Profound perturbation in the metabolome of a canine obesity and metabolic disorder model. Front. Endocrinol. 2022, 13, 849060. [Google Scholar] [CrossRef]
- Muli, S.; Schnermann, M.E.; Merdas, M.; Rattner, J.; Achaintre, D.; Perrar, I.; Goerdten, J.; Alexy, U.; Scalbert, A.; Schmid, M.; et al. Metabolomics signatures of sweetened beverages and added sugar are related to anthropometric measures of adiposity in young individuals: Results from a cohort study. Am. J. Clin. Nutr. 2024, 120, 879–890. [Google Scholar] [CrossRef]
- Youn, H.; Kook, Y.H.; Oh, E.; Jeong, S.; Kim, C.; Choi, E.K.; Lim, B.U.; Park, H.J. 1-Methylxanthine enhances the radiosensitivity of tumor cells. Int. J. Radiat. Biol. 2009, 85, 167–174. [Google Scholar] [CrossRef]
- Costa-Bauza, A.; Grases, F. 7-Methylxanthine inhibits the formation of monosodium urate crystals by increasing its solubility. Biomolecules 2023, 13, 1769. [Google Scholar] [CrossRef]
- Nie, H.; Huo, L.; Yang, X.; Gao, Z.; Zeng, J.; Trier, K.; Cui, D. Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int. J. Ophthalmol. 2012, 5, 133–137. [Google Scholar] [CrossRef]
- Cui, D.; Trier, K.; Zeng, J.; Wu, K.; Yu, M.; Hu, J.; Chen, X.; Ge, J. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. 2009, 89, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.; Arumugam, B.; Ostrin, L.; Patel, N.; Trier, K.; Jong, M.; Smith, E.L., III. The adenosine receptor antagonist, 7-Methylxanthine, alters emmetropizing responses in infant macaques. Investig. Ophthalmol. Vis. Sci. 2018, 59, 472. [Google Scholar] [CrossRef] [PubMed]
- Trier, K.; Ribel-Madsen, S.M.; Cui, D.; Christensen, S.B. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: A 36-month pilot study. J. Ocul. Biol. Dis. Inform. 2008, 1, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Trier, K.; Cui, D.; Ribel-Madsen, S.; Guggenheim, J. Oral administration of caffeine metabolite 7-methylxanthine is associated with slowed myopia progression in Danish children. Br. J. Ophthalmol. 2022, 107, 1538–1544. [Google Scholar] [CrossRef]
- Singh, H.; Singh, H.; Sharma, S.; Kaur, H.; Kaur, A.; Kaur, S.; Kaur, S.; Sahajpal, N.S.; Chaubey, A.; Shahtaghi, N.R.; et al. Genotoxic and mutagenic potential of 7-methylxanthine: An investigational drug molecule for the treatment of myopia. Drug Chem. Toxicol. 2023, 47, 264–273. [Google Scholar] [CrossRef]
- Singh, H.; Sahajpal, N.S.; Singh, H.; Vanita, V.; Roy, P.; Paul, S.; Singh, S.K.; Kaur, I.; Jain, S.K. Pre-clinical and cellular toxicity evaluation of 7-methylxanthine: An investigational drug for the treatment of myopia. Drug Chem. Toxicol. 2019, 44, 575–584. [Google Scholar] [CrossRef]
- Lai, L.; Trier, K.; Cui, D. Role of 7-methylxanthine in myopia prevention and control: A mini-review. Int. J. Ophthalmol. 2023, 16, 969–976. [Google Scholar] [CrossRef]
- Rektorisova, M.; Hrbek, V.; Tomaniova, M.; Cuhra, P.; Hajslova, J. Supercritical fluid chromatography coupled to high-resolution tandem mass spectrometry: An innovative one-run method for the comprehensive assessment of chocolate quality and authenticity. Anal. Bioanal. Chem. 2022, 414, 6825–6840. [Google Scholar] [CrossRef]
- Nia, N.A.; Foroughi, M.M.; Jahani, S. Simultaneous determination of theobromine, theophylline, and caffeine using a modified electrode with petal-like MnO2 nanostructure. Talanta 2020, 222, 121563. [Google Scholar] [CrossRef]
- Chowdhury, P.; Barooah, A.K. Tea bioactive modulate innate immunity: In Perception to COVID-19 pandemic. Front. Immunol. 2020, 11, 590716. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A.; Roig, J.; Rodriguez, A. Xanthine urolithiasis: Inhibitors of xanthine crystallization. PLoS ONE 2018, 13, e0198881. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Costa-Bauza, A.; Calvó, P.; Benejam, J.; Sanchis, P.; Grases, F. Comparison of Two Dietary Supplements for Treatment of Uric Acid Renal Lithiasis: Citrate vs. Citrate + Theobromine. Nutrients 2020, 12, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Mukherjee, S.; Choi, M.J.; Kang, N.H.; Pham, H.G.; Yun, J.W. Theobromine alleviates diet-induced obesity in mice via phosphodiesterase-4 inhibition. Eur. J. Nutr. 2020, 59, 3503–3516. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, H. Koffein, Schokolade und Adenosin A2A Rezeptorantagonisten in der Behandlung des Parkinson Syndroms. Fortschritte Der Neurol. Psychiatr. 2022, 91, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Procházková, K.; Šejna, I.; Skutil, J.; Hahn, A. Ginkgo biloba extract EGb 761® versus pentoxifylline in chronic tinnitus: A randomized, double-blind clinical trial. Int. J. Clin. Pharm. 2018, 40, 1335–1341. [Google Scholar] [CrossRef]
- Mokry, J.; Urbanova, A.; Kertys, M.; Mokra, D. Inhibitors of phosphodiesterases in the treatment of cough. Respir. Physiol. Neurobiol. 2018, 257, 107–114. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Elder, H.J.; Walentiny, D.M.; Beardsley, P.M. Theophylline reverses oxycodone’s but not fentanyl’s respiratory depression in mice while caffeine is ineffective against both opioids. Pharmacol. Biochem. Behav. 2023, 229, 173601. [Google Scholar] [CrossRef]
- Park, K.; Lee, S.; Bae, S.I.; Hwang, Y.; Ok, S.; Ahn, S.H.; Sim, G.; Chung, S.; Sohn, J. Theophylline-induced endothelium-dependent vasodilation is mediated by increased nitric oxide release and phosphodiesterase inhibition in rat aorta. Gen. Physiol. Biophys. 2023, 42, 469–478. [Google Scholar] [CrossRef]
- Fredholm, B.B. Adenosine and lipolysis. Int. J. Obes. 1981, 5, 643–649. [Google Scholar]
- Barnes, P.J. Theophylline: New Perspectives for an Old Drug. Am. J. Respir. Crit. Care Med. 2002, 167, 813–818. [Google Scholar] [CrossRef]
- Talmon, M.; Massara, E.; Brunini, C.; Fresu, L.G. Comparison of anti-inflammatory mechanisms between doxofylline and theophylline in human monocytes. Pulm. Pharmacol. Ther. 2019, 59, 101851. [Google Scholar] [CrossRef] [PubMed]
- Kuremoto, T.; Kogiso, H.; Yasuda, M.; Inui, T.; Murakami, K.; Hirano, S.; Ikeuchi, Y.; Hosogi, S.; Inui, T.; Marunaka, Y.; et al. Spontaneous oscillation of the ciliary beat frequency regulated by release of Ca2+ from intracellular stores in mouse nasal epithelia. Biochem. Biophys. Res. Commun. 2018, 507, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Rong, R.; Huang, Y.; Pu, X.; Ge, N. Effects of theophylline combined with inhaled corticosteroids on patients with moderate and severe asthma and changes of T lymphocyte subsets in peripheral blood. Cent. Eur. J. Immunol. 2023, 48, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.W.; Thaw, M.M.; Ramim, J.U.; Mukherjee, R. Theophylline toxicity: A differential to consider in patients on Long-Term Theophylline presenting with nonspecific symptoms. Cureus 2023, 15, e48480. [Google Scholar] [CrossRef]
- Bin, Y.; Xiao, Y.; Huang, D.; Ma, Z.; Liang, Y.; Bai, J.; Zhang, W.; Liang, Q.; Zhang, J.; Zhong, X.; et al. Theophylline inhibits cigarette smoke-induced inflammation in skeletal muscle by upregulating HDAC2 expression and decreasing NF-κB activation. AJP Lung Cell. Mol. Physiol. 2018, 316, L197–L205. [Google Scholar] [CrossRef]
- Guerreiro, S.; Toulorge, D.; Hirsch, E.; Marien, M.; Sokoloff, P.; Michel, P.P. Paraxanthine, the Primary Metabolite of Caffeine, Provides Protection against Dopaminergic Cell Death via Stimulation of Ryanodine Receptor Channels. Mol. Pharmacol. 2008, 74, 980–989. [Google Scholar] [CrossRef]
- Ni, J.; Auston, D.A.; Freilich, D.A.; Muralidharan, S.; Sobie, E.A.; Kao, J.P.Y. Photochemical gating of intracellular CA2+ release channels. J. Am. Chem. Soc. 2007, 129, 5316–5317. [Google Scholar] [CrossRef]
- Purpura, M.; Jäger, R.; Falk, M. An assessment of mutagenicity, genotoxicity, acute-, subacute and subchronic oral toxicity of paraxanthine (1,7-dimethylxanthine). Food Chem. Toxicol. 2021, 158, 112579. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Wells, S.D.; Liao, K.; Godavarthi, A. Paraxanthine supplementation increases muscle mass, strength, and endurance in mice. Nutrients 2022, 14, 893. [Google Scholar] [CrossRef]
- Yoo, C.; Xing, D.; Gonzalez, D.E.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Lewis, M.H.; Faries, M.; et al. Paraxanthine provides greater improvement in cognitive function than caffeine after performing a 10-km run. J. Int. Soc. Sports Nutr. 2024, 21, 2352779. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, L.; Lu, M.; Zhang, J.; Han, J.; Deng, W.; Zhang, Z. Caffeine Content and Related Gene Expression: Novel Insight into Caffeine Metabolism in Camellia Plants Containing Low, Normal, and High Caffeine Concentrations. J. Agric. Food Chem. 2019, 67, 3400–3411. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, X.; Badawy, S.; Ihsan, A.; Liu, Z.; Xie, C.; Wang, X. Metabolism and mechanism of human cytochrome P450 enzyme 1A2. Curr. Drug Metab. 2021, 22, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A.; Daval, J.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- McCall, A.; Millington, W.; Wurtman, R. Blood-brain barrier transport of caffeine: Dose-related restriction of adenine transport. Life Sci. 1982, 31, 2709–2715. [Google Scholar] [CrossRef]
- Chapman, R.F.; Mickleborough, T.D. The effects of caffeine on ventilation and pulmonary function during exercise: An Often-Overlooked Response. Physician Sportsmed. 2009, 37, 97–103. [Google Scholar] [CrossRef]
- Gutierrez, A.E.; Shah, P.; Rex, A.E.; Nguyen, T.C.; Kenkare, S.P.; Barrick, J.E.; Mishler, D.M. Bioassay for detemining the concentrations of caffeine and individual methylxanthines in complex samples. Appl. Environ. Microbiol. 2019, 85, e01965-19. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, C.; Ren, X.; Xia, T.; Li, X. LC–MS/MS-based metabolomic analysis of caffeine-degrading fungus Aspergillus sydowii during tea fermentation. J. Food Sci. 2020, 85, 477–485. [Google Scholar] [CrossRef]
- Gotarkar, D.; Longkumer, T.; Yamamoto, N.; Nanda, A.K.; Iglesias, T.; Li, L.; Miro, B.; Gonzalez, E.B.; Bayon, M.M.; Olsen, K.M.; et al. A drought-responsive rice amidohydrolase is the elusive plant guanine deaminase with the potential to modulate the epigenome. Physiol. Plant. 2021, 172, 1853–1866. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, C.; Zeng, Z.; Luo, L.; Zeng, W.; Huang, Y. N-Methyltransferases of caffeine biosynthetic pathway in plants. J. Agric. Food Chem. 2020, 68, 15359–15372. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Zhou, Q.; Li, F.; Shen, Y.; Ye, Z.; Tang, D.; Chi, N.; Li, L.; Ma, S.; et al. Metabolite profiling and transcriptome analysis revealed the conserved transcriptional regulation mechanism of caffeine biosynthesis in tea and coffee plants. J. Agric. Food Chem. 2022, 70, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Summers, R.M.; Li, Y.; Mohanty, S.K.; Subramanian, M.; Pope, R.M. Rapid Identification and Quantitative Validation of a Caffeine-Degrading Pathway in Pseudomonas sp. CES. J. Proteome Res. 2014, 14, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Gummadi, S.N.; Dash, S.S.; Devarai, S. Optimization of production of caffeine demethylase by Pseudomonas sp. in a bioreactor. J. Ind. Microbiol. Biotechnol. 2009, 36, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Louie, T.M.; Summers, R.; Kale, Y.; Gopishetty, S.; Subramanian, M. Two Distinct Pathways for Metabolism of Theophylline and Caffeine Are Coexpressed in Pseudomonas putida CBB5. J. Bacteriol. 2009, 191, 4624–4632. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Wu, H.; Dong, Z.; Ye, J.; Zheng, X.; Liang, Y.; Lu, J. Different Catabolism Pathways Triggered by Various Methylxanthines in Caffeine-Tolerant Bacterium Pseudomonas putida CT25 Isolated from Tea Garden Soil. J. Microbiol. Biotechnol. 2018, 28, 1147–1155. [Google Scholar] [CrossRef]
- Blecher, R.; Lingens, F. The metabolism of caffeine by a Pseudomonas putida Strain. Hoppe-Seylers Z. Physiol. Chem. 1977, 358, 807–818. [Google Scholar] [CrossRef]
- Nanjundaiah, S.; Mutturi, S.; Bhatt, P. Modeling of caffeine degradation kinetics during cultivation of Fusarium solani using sucrose as co-substrate. Biochem. Eng. J. 2017, 125, 73–80. [Google Scholar] [CrossRef]
- Hakil, M.; Denis, S.; Viniegra-González, G.; Augur, C. Degradation and product analysis of caffeine and related dimethylxanthines by filamentous fungi. Enzym. Microb. Technol. 1998, 22, 355–359. [Google Scholar] [CrossRef]
- Brand, D.; Pandey, A.; Roussos, S.; Soccol, C.R. Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzym. Microb. Technol. 2000, 27, 127–133. [Google Scholar] [CrossRef]
- Oduro-Mensah, D.; Ocloo, A.; Lowor, S.T.; Bonney, E.Y.; Okine, L.K.; Adamafio, N.A. Isolation and characterisation of theobromine-degrading filamentous fungi. Microbiol. Res. 2017, 206, 16–24. [Google Scholar] [CrossRef]
- Dash, S.S.; Gummadi, S.N. Degradation Kinetics of Caffeine and Related Methylxanthines by Induced Cells of Pseudomonas sp. Curr. Microbiol. 2007, 55, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Harris, N.; Nordin, R.; Mazumder, A. Purification and characterization of a novel caffeine oxidase from Alcaligenes species. J. Biotechnol. 2006, 125, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.M.; Seffernick, J.L.; Quandt, E.M.; Yu, C.L.; Barrick, J.E.; Subramanian, M.V. Caffeine Junkie: An Unprecedented Glutathione S-Transferase-Dependent Oxygenase Required for Caffeine Degradation by Pseudomonas putida CBB5. J. Bacteriol. 2013, 195, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.M.; Mohanty, S.K.; Gopishetty, S.; Subramanian, M. Genetic characterization of caffeine degradation by bacteria and its potential applications. Microb. Biotechnol. 2015, 8, 369–378. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Yu, C.; Das, S.; Louie, T.M.; Gakhar, L.; Subramanian, M. Delineation of the Caffeine C-8 Oxidation Pathway in Pseudomonas sp. Strain CBB1 via Characterization of a New Trimethyluric Acid Monooxygenase and Genes Involved in Trimethyluric Acid Metabolism. J. Bacteriol. 2012, 194, 3872–3882. [Google Scholar] [CrossRef]
- Vega, F.E.; Emche, S.; Shao, J.; Simpkins, A.; Summers, R.M.; Mock, M.B.; Ebert, D.; Infante, F.; Aoki, S.; Maul, J.E. Cultivation and Genome Sequencing of Bacteria Isolated from the Coffee Berry Borer (Hypothenemus hampei), with Emphasis on the Role of Caffeine Degradation. Front. Microbiol. 2021, 12, 644768. [Google Scholar] [CrossRef]
- Mock, M.B.; Zhang, S.; Summers, R.M. Whole-cell Rieske non-heme iron biocatalysts. Methods Enzymol. 2024, 703, 243–262. [Google Scholar] [CrossRef]
- Mills, S.B.; Mock, M.B.; Summers, R.M. Rational protein engineering of bacterial n-demethylases to create biocatalysts for the production of methylxanthines. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mock, M.B.; Cyrus, A.; Summers, R.M. Biocatalytic production of 7-methylxanthine by a caffeine-degrading Escherichia coli strain. Biotechnol. Bioeng. 2022, 119, 3326–3331. [Google Scholar] [CrossRef]
- Mock, M.B.; Summers, R.M. Mixed culture biocatalytic production of the high-value biochemical 7-methylxanthine. J. Biol. Eng. 2023, 17, 2. [Google Scholar] [CrossRef]
- Algharrawi, K.H.R.; Summers, R.M.; Gopishetty, S.; Subramanian, M. Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli. Microb. Cell Factories 2015, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.M.; Louie, T.M.; Yu, C.L.; Subramanian, M. Characterization of a broad-specificity non-haem iron N-demethylase from Pseudomonas putida CBB5 capable of utilizing several purine alkaloids as sole carbon and nitrogen source. Microbiology 2010, 157, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.; Gopishetty, S.; Mohanty, S.; Subramanian, M. New genetic insights to consider coffee waste as feedstock for fuel, feed, and chemicals. Open Chem. 2014, 12, 1271–1279. [Google Scholar] [CrossRef]
- Algharrawi, K.H.; Summers, R.M.; Subramanian, M. Production of theobromine by N-demethylation of caffeine using metabolically engineered E. coli. Biocatal. Agric. Biotechnol. 2017, 11, 153–160. [Google Scholar] [CrossRef]
- Algharrawi, K.H.R.; Subramanian, M. Production of 7-methylxanthine from Theobromine by Metabolically Engineered E. coli. Iraqi J. Chem. Pet. Eng. 2020, 21, 19–27. [Google Scholar] [CrossRef]
- Retnadhas, S.; Gummadi, S.N. Identification and characterization of oxidoreductase component (NdmD) of methylxanthine oxygenase system in Pseudomonas sp. NCIM 5235. Appl. Microbiol. Biotechnol. 2018, 102, 7913–7926. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Du, C.; Li, Y.; Cao, Z. Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab. Eng. 2008, 11, 101–106. [Google Scholar] [CrossRef]
- Berríos-Rivera, S.J.; Bennett, G.N.; San, K. The Effect of Increasing NADH Availability on the Redistribution of Metabolic Fluxes in Escherichia coli Chemostat Cultures. Metab. Eng. 2002, 4, 230–237. [Google Scholar] [CrossRef]
- Denby, K.J.; Iwig, J.; Bisson, C.; Westwood, J.; Rolfe, M.D.; Sedelnikova, S.E.; Higgins, K.; Maroney, M.J.; Baker, P.J.; Chivers, P.T.; et al. The mechanism of a formaldehyde-sensing transcriptional regulator. Sci. Rep. 2016, 6, 38879. [Google Scholar] [CrossRef]
- Chen, N.H.; Djoko, K.Y.; Veyrier, F.J.; McEwan, A.G. Formaldehyde stress responses in bacterial pathogens. Front. Microbiol. 2016, 7, 257. [Google Scholar] [CrossRef]
- Gutheil, W.G.; Kasimoglu, E.; Nicholson, P.C. Induction of Glutathione-Dependent Formaldehyde Dehydrogenase Activity in Escherichia coli and Hemophilus influenza. Biochem. Biophys. Res. Commun. 1997, 238, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.D.; Blattner, F.R. Global transcriptional effects of a suppressor TRNA and the inactivation of the regulator FRMR. J. Bacteriol. 2004, 186, 6714–6720. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; De Los Santos, E.L.C.; Alkhalaf, L.M.; Challis, G.L. Rieske non-heme iron-dependent oxygenases catalyse diverse reactions in natural product biosynthesis. Nat. Prod. Rep. 2018, 35, 622–632. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Zuo, S.; Liu, C.; Xing, W.; Wang, P. Progress in Methylxanthine Biosynthesis: Insights into Pathways and Engineering Strategies. Int. J. Mol. Sci. 2025, 26, 1510. https://doi.org/10.3390/ijms26041510
Jiang T, Zuo S, Liu C, Xing W, Wang P. Progress in Methylxanthine Biosynthesis: Insights into Pathways and Engineering Strategies. International Journal of Molecular Sciences. 2025; 26(4):1510. https://doi.org/10.3390/ijms26041510
Chicago/Turabian StyleJiang, Tongtong, Shangci Zuo, Chang Liu, Wanbin Xing, and Pengchao Wang. 2025. "Progress in Methylxanthine Biosynthesis: Insights into Pathways and Engineering Strategies" International Journal of Molecular Sciences 26, no. 4: 1510. https://doi.org/10.3390/ijms26041510
APA StyleJiang, T., Zuo, S., Liu, C., Xing, W., & Wang, P. (2025). Progress in Methylxanthine Biosynthesis: Insights into Pathways and Engineering Strategies. International Journal of Molecular Sciences, 26(4), 1510. https://doi.org/10.3390/ijms26041510





