Mint3 as a Molecular Target Activated in the Early Stage of Hepatocarcinogenesis
Abstract
1. Introduction
2. Results
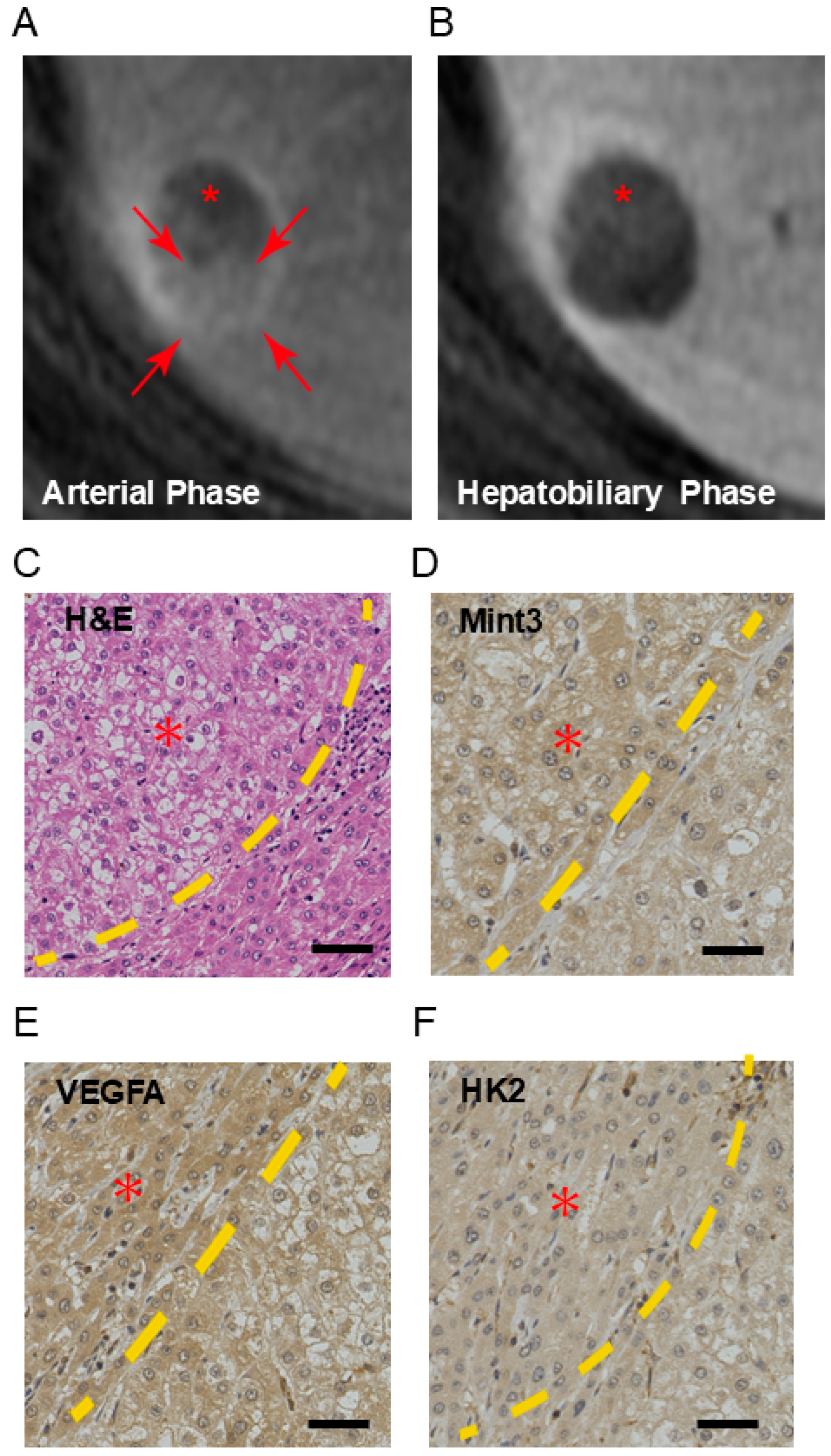
2.1. Mint3 Is Overexpressed in Well-Differentiated HCC
2.2. Downregulation of Mint3 Inhibits HCC Growth In Vitro and In Vivo
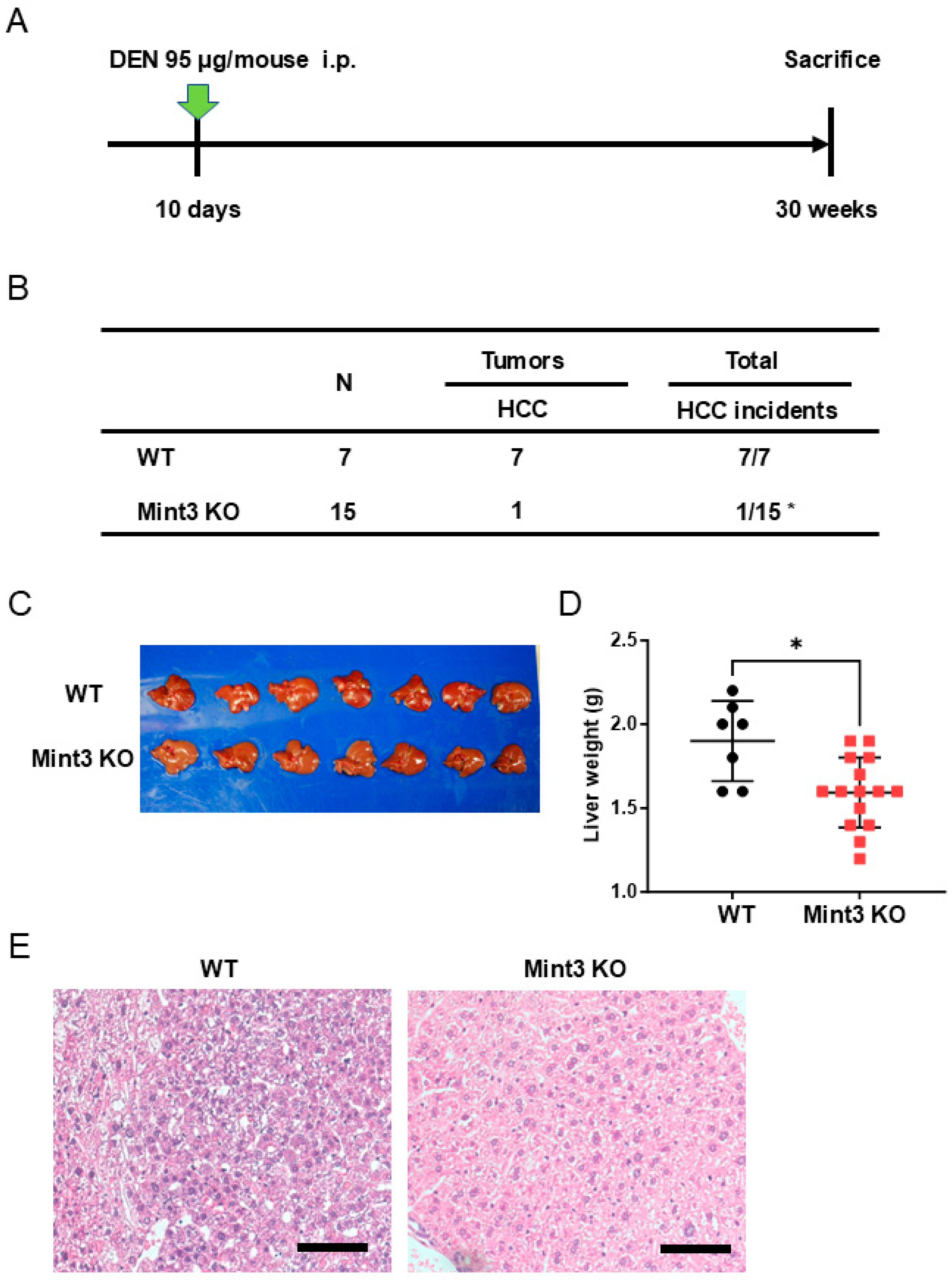
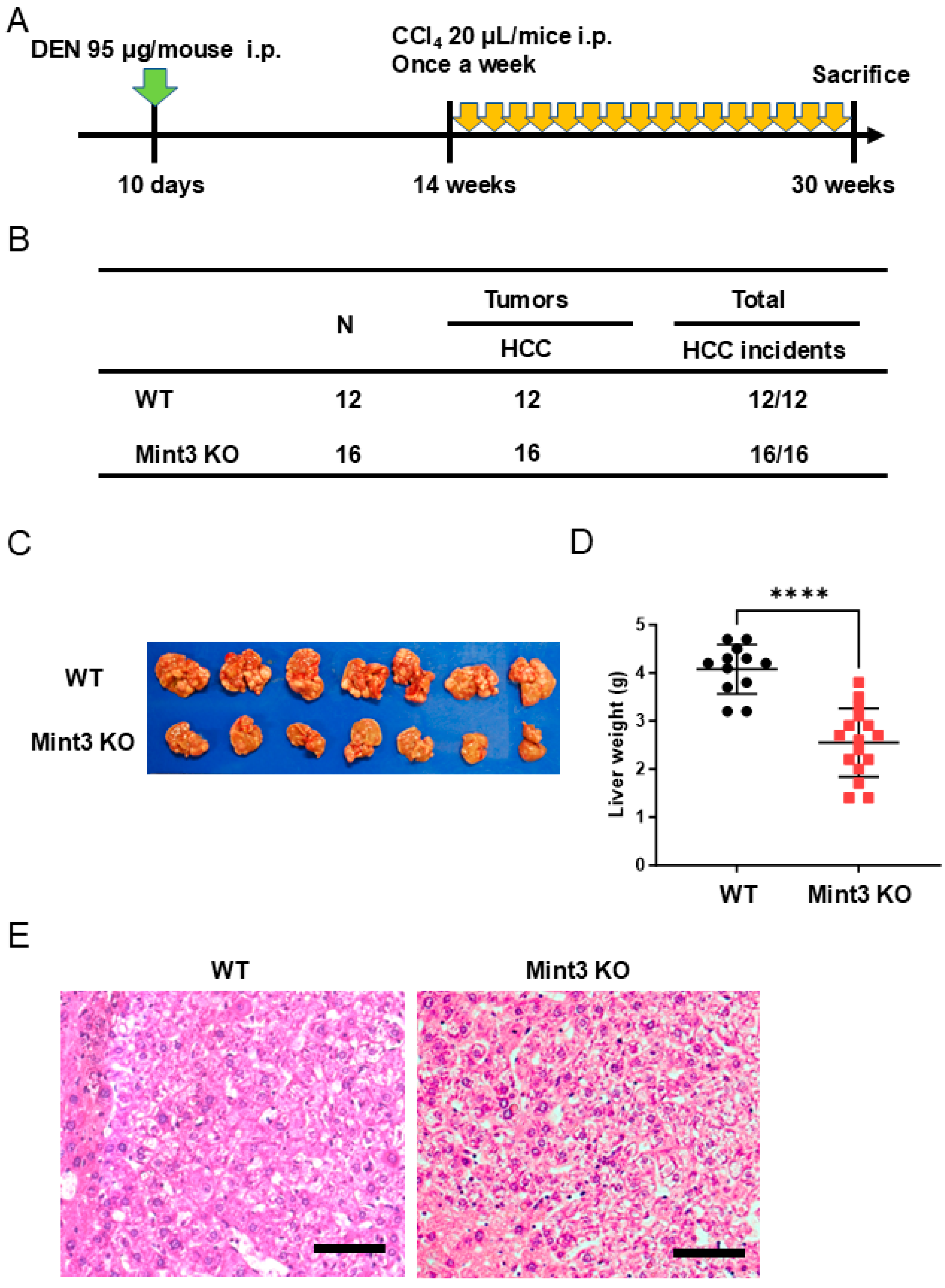
2.3. Chemical HCC Development Initiated and Promoted by DEN/CCl4 Is Impaired in Mint3 KO Mice
3. Discussion
4. Materials and Methods
4.1. Human HCC Samples
4.2. Cell Lines and Reagents
4.3. Spheroid Formation Assay
4.4. Immunohistochemistry (IHC)
4.5. Western Blotting Analysis
4.6. RNA Isolation, Reverse, Transcription (RT), and Quantitative Polymerase Chain Reaction (PCR)
4.7. Animal Models
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Willoughby, C.E.; Singal, A.G.; Greten, T.F.; Heikenwälder, M.; El-Serag, H.B.; Finn, R.S.; Friedman, S.L. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 487–503. [Google Scholar] [CrossRef]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Takai, A.; Eso, Y.; Takahashi, K.; Marusawa, H.; Seno, H. Genetic Landscape of Multistep Hepatocarcinogenesis. Cancers 2022, 14, 568. [Google Scholar] [CrossRef]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, Q.; Yang, F.; Liu, R.; Gao, Q.; Cheng, B.; Lin, X.; Huang, L.; Chen, C.; Xiang, J.; et al. Signaling metabolite succinylacetone activates HIF-1alpha and promotes angiogenesis in GSTZ1-deficient hepatocellular carcinoma. JCI Insight 2023, 8, e164968. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, Z.; Yang, L.; Gao, Y.; Zhu, Q.; Hu, L.; Huang, D.; Xu, Q. Hypoxia-inducible factors in hepatocellular carcinoma (Review). Oncol. Rep. 2020, 43, 3–15. [Google Scholar] [CrossRef]
- Walko, C.M.; Grande, C. Management of common adverse events in patients treated with sorafenib: Nurse and pharmacist perspective. Semin. Oncol. 2014, 41 (Suppl. S2), S17–S28. [Google Scholar] [CrossRef]
- Berretta, M.; Rinaldi, L.; Di Benedetto, F.; Lleshi, A.; De Re, V.; Facchini, G.; De Paoli, P.; Di Francia, R. Angiogenesis Inhibitors for the Treatment of Hepatocellular Carcinoma. Front. Pharmacol. 2016, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Solinas, A.; Calvisi, D.F. Cabozantinib for HCC Treatment, From Clinical Back to Experimental Models. Front. Oncol. 2021, 11, 756672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.-H.; He, A.R.; Ryoo, B.-Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Kang, Y.-K.; Yen, C.-J.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Motomura, K.; Ohno, I.; et al. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br. J. Cancer 2021, 124, 1388–1397. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef]
- Alberghina, L. The Warburg Effect Explained: Integration of Enhanced Glycolysis with Heterogeneous Mitochondria to Promote Cancer Cell Proliferation. Int. J. Mol. Sci. 2023, 24, 15787. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.N.; Rao, S.; Roth, B.; Bryan, T.; Fernando, D.M.; Dayyani, F.; Imagawa, D.; Abi-Jaoudeh, N. Targeting Hypoxia-Inducible Factor-1alpha for the Management of Hepatocellular Carcinoma. Cancers 2023, 15, 2738. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell. Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, H.; Tabira, T. X11L2, a new member of the X11 protein family, interacts with Alzheimer’s beta-amyloid precursor protein. Biochem. Biophys. Res. Commun. 1999, 255, 663–667. [Google Scholar] [CrossRef]
- Sakamoto, T.; Seiki, M. Mint3 enhances the activity of hypoxia-inducible factor-1 (HIF-1) in macrophages by suppressing the activity of factor inhibiting HIF-1. J. Biol. Chem. 2009, 284, 30350–303509. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Seiki, M. Integrated functions of membrane-type 1 matrix metalloproteinase in regulating cancer malignancy: Beyond a proteinase. Cancer Sci. 2017, 108, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Sakamoto, T. Mint3 as a Potential Target for Cooling Down HIF-1alpha-Mediated Inflammation and Cancer Aggressiveness. Biomedicines 2023, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, J.; Ohe, C.; Tanaka, N.; Yoshida, T.; Saito, R.; Atsumi, N.; Kobayashi, T.; Kinoshita, H.; Tsuta, K.; Sakamoto, T. Hypoxia inducible factor-1 activator munc-18-interacting protein 3 promotes tumour progression in urothelial carcinoma. Clin. Transl. Discov. 2023, 3, e158. [Google Scholar] [CrossRef]
- Kanamori, A.; Matsubara, D.; Saitoh, Y.; Fukui, Y.; Gotoh, N.; Kaneko, S.; Seiki, M.; Murakami, Y.; Inoue, J.-I.; Sakamoto, T. Mint3 depletion restricts tumor malignancy of pancreatic cancer cells by decreasing SKP2 expression via HIF-1. Oncogene 2020, 39, 6218–6230. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.J.; Hara, T.; Yoshino, S.; Kanamori, A.; Matsui, Y.; Shimamura, T.; Sato, H.; Murakami, Y.; Seiki, M.; Sakamoto, T. NECAB3 Promotes Activation of Hypoxia-inducible factor-1 during Normoxia and Enhances Tumourigenicity of Cancer Cells. Sci. Rep. 2016, 6, 22784. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Niiya, D.; Seiki, M. Targeting the Warburg effect that arises in tumor cells expressing membrane type-1 matrix metalloproteinase. J. Biol. Chem. 2011, 286, 14691–14704. [Google Scholar] [CrossRef]
- Sakamoto, T.; Weng, J.S.; Hara, T.; Yoshino, S.; Kozuka-Hata, H.; Oyama, M.; Seiki, M. Hypoxia-inducible factor 1 regulation through cross talk between mTOR and MT1-MMP. Mol. Cell. Biol. 2014, 34, 30–42. [Google Scholar] [CrossRef]
- Tanaka, N.; Okada, H.; Yamaguchi, K.; Seki, M.; Matsubara, D.; Gotoh, N.; Suzuki, Y.; Furukawa, Y.; Yamashita, T.; Inoue, J.-I.; et al. Mint3-depletion-induced energy stress sensitizes triple-negative breast cancer to chemotherapy via HSF1 inactivation. Cell Death. Dis. 2023, 14, 815. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tang, C.; Luo, R.; Gu, X.; Zhou, J.; Gao, J. Current Concepts of Precancerous Lesions of Hepatocellular Carcinoma: Recent Progress in Diagnosis. Diagnostics 2023, 13, 1211. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.F.; Harri, P.; Little, B.; Moreno, C.C.; Mittal, P.K. Magnetic Resonance Imaging of Primary Hepatic Malignancies in Patients With and Without Chronic Liver Disease: A Pictorial Review. Cureus 2017, 9, e1539. [Google Scholar] [CrossRef]
- Moawad, A.W.; Szklaruk, J.; Lall, C.; Blair, K.J.; Kaseb, A.O.; Kamath, A.; Rohren, S.A.; Elsayes, K.M. Angiogenesis in Hepatocellular Carcinoma; Pathophysiology, Targeted Therapy, and Role of Imaging. J. Hepatocell. Carcinoma 2020, 7, 77–89. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kozaka, K.; Gabata, T.; Matsui, O.; Minami, T. Intraarterial and intravenous contrast enhanced CT and MR imaging of multi-step hepatocarcinogenesis defining the early stage of hepatocellular carcinoma development. Hepatoma Res. 2020, 2020, 36. [Google Scholar] [CrossRef]
- Levine, A.J.; Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Sakamoto, T.; Fukui, Y.; Kondoh, Y.; Honda, K.; Shimizu, T.; Hara, T.; Hayashi, T.; Saitoh, Y.; Murakami, Y.; Inoue, J.-I.; et al. Pharmacological inhibition of Mint3 attenuates tumour growth, metastasis, and endotoxic shock. Commun. Biol. 2021, 4, 1165. [Google Scholar] [CrossRef]
- Hara, T.; Nakaoka, H.J.; Hayashi, T.; Mimura, K.; Hoshino, D.; Inoue, M.; Nagamura, F.; Murakami, Y.; Seiki, M.; Sakamoto, T. Control of metastatic niche formation by targeting APBA3/Mint3 in inflammatory monocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E4416–E4424. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Morishita, W.; Atasoy, D.; Liu, X.; Tabuchi, K.; Hammer, R.E.; Malenka, R.C.; Südhof, T.C. Genetic analysis of Mint/X11 proteins: Essential presynaptic functions of aneuronal adaptor protein family. J. Neurosci. 2006, 26, 13089–13101. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Honda, M.; Nakamoto, Y.; Baba, M.; Nio, K.; Hara, Y.; Zeng, S.S.; Hayashi, T.; Kondo, M.; Takatori, H.; et al. Discrete nature of EpCAM+ and CD90+cancer stem cells in human hepatocellular carcinoma. Hepatology 2013, 57, 1484–1497. [Google Scholar] [CrossRef]
- Yamashita, T.; Kitao, A.; Matsui, O.; Hayashi, T.; Nio, K.; Kondo, M.; Ohno, N.; Miyati, T.; Okada, H.; Yamashita, T.; et al. Gd-EOB-DTPA-Enhanced Magnetic Resonance Imaging and Alpha-Fetoprotein Predict Prognosis of Early-Stage Hepatocellular Carcinoma. Hepatology 2014, 60, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, T.; Yamashita, T.; Okada, H.; Nio, K.; Hayashi, T.; Asahina, Y.; Hayashi, T.; Terashima, T.; Iida, N.; Takatori, H. Inactivation of Transcriptional Repressor Capicua Confers Sorafenib Resistance in Human Hepatocellular Carcinoma. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 269–285. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Yoshino, S.; Matsui, Y.; Fukui, Y.; Seki, M.; Yamaguchi, K.; Kanamori, A.; Saitoh, Y.; Shimamura, T.; Suzuki, Y.; Furukawa, Y.; et al. EXOSC9 depletion attenuates P-body formation, stress resistance, and tumorigenicity of cancer cells. Sci. Rep. 2020, 10, 9275. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Murakami, Y.; Seiki, M.; Sakamoto, T. Mint3 in bone marrow-derived cells promotes lung metastasis in breast cancer model mice. Biochem. Biophys. Res. Commun. 2017, 490, 688–692. [Google Scholar] [CrossRef]


| Parameters | Mint3 Negative | Mint3 Positive | p Value |
|---|---|---|---|
| (n = 56) | (n = 44) | ||
| Age (year, mean ± SE) | 64.7 ± 1.3 | 65.3 ± 1.6 | 0.775 |
| Sex (male/female) | 40/16 | 32/12 | 1 |
| Etiology (HBV/HCV/B + C/other) | 15/19/20/2 | 8/18/18/0 | 0.519 |
| AFP (ng/mL, mean ± SE) | 3830 ± 1366 | 364.6 ± 217.5 | 0.0285 |
| PIVKAII (mAU/mL, mean ± SE) | 4201 ± 1530 | 2751 ± 1633 | 0.521 |
| AST (IU/L, mean ± SE) | 42.9 ± 4.09 | 41.3 ± 2.87 | 0.761 |
| ALT (IU/L, mean ± SE) | 37.1 ± 3.54 | 38.9 ± 2.60 | 0.691 |
| Plt (×103, mean ± SE) | 139 ± 7.26 | 152 ± 12.1 | 0.316 |
| LC/non-LC | 27/29 | 25/19 | 0.426 |
| Histological grade of HCC | |||
| Well-differentiated | 7 | 14 | |
| Moderately differentiated | 38 | 28 | |
| Poorly differentiated | 11 | 2 | 0.0128 |
| Tumor size (<5 cm/>5 cm) | 36/20 | 33/11 | 0.282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishitani, M.; Okada, H.; Nio, K.; Hayashi, T.; Terashima, T.; Iida, N.; Shimakami, T.; Takatori, H.; Honda, M.; Kaneko, S.; et al. Mint3 as a Molecular Target Activated in the Early Stage of Hepatocarcinogenesis. Int. J. Mol. Sci. 2025, 26, 1430. https://doi.org/10.3390/ijms26041430
Nishitani M, Okada H, Nio K, Hayashi T, Terashima T, Iida N, Shimakami T, Takatori H, Honda M, Kaneko S, et al. Mint3 as a Molecular Target Activated in the Early Stage of Hepatocarcinogenesis. International Journal of Molecular Sciences. 2025; 26(4):1430. https://doi.org/10.3390/ijms26041430
Chicago/Turabian StyleNishitani, Masaki, Hikari Okada, Kouki Nio, Tomoyuki Hayashi, Takeshi Terashima, Noriho Iida, Tetsuro Shimakami, Hajime Takatori, Masao Honda, Shuichi Kaneko, and et al. 2025. "Mint3 as a Molecular Target Activated in the Early Stage of Hepatocarcinogenesis" International Journal of Molecular Sciences 26, no. 4: 1430. https://doi.org/10.3390/ijms26041430
APA StyleNishitani, M., Okada, H., Nio, K., Hayashi, T., Terashima, T., Iida, N., Shimakami, T., Takatori, H., Honda, M., Kaneko, S., Sakamoto, T., & Yamashita, T. (2025). Mint3 as a Molecular Target Activated in the Early Stage of Hepatocarcinogenesis. International Journal of Molecular Sciences, 26(4), 1430. https://doi.org/10.3390/ijms26041430






