Endothelial Dysfunction in Huntington’s Disease: Pathophysiology and Therapeutic Implications
Abstract
1. Introduction
2. Endothelial Dysfunction in HD
2.1. Impaired Angiogenesis
- Reduced blood flow velocity and volume, leading to decreased oxygen and nutrient delivery
- Increased vascular resistance, potentially compromising the brain’s ability to regulate blood flow in response to metabolic demands
- Impaired waste clearance, which may contribute to the accumulation of toxic metabolites
2.2. Altered Cerebral Blood Flow (CBF)
2.3. Impaired Cerebrovascular Reactivity (CVR)
2.4. Impaired Neurovascular Coupling (NVC)
- Mitochondrial-targeted antioxidants: compounds designed to accumulate in mitochondria and reduce oxidative stress, potentially preserving both mitochondrial and endothelial function.
- NO pathway modulators: therapeutics targeting the NO signaling cascade to restore proper vascular tone regulation and improve neurovascular coupling.
2.5. Increased Permeability of BBB
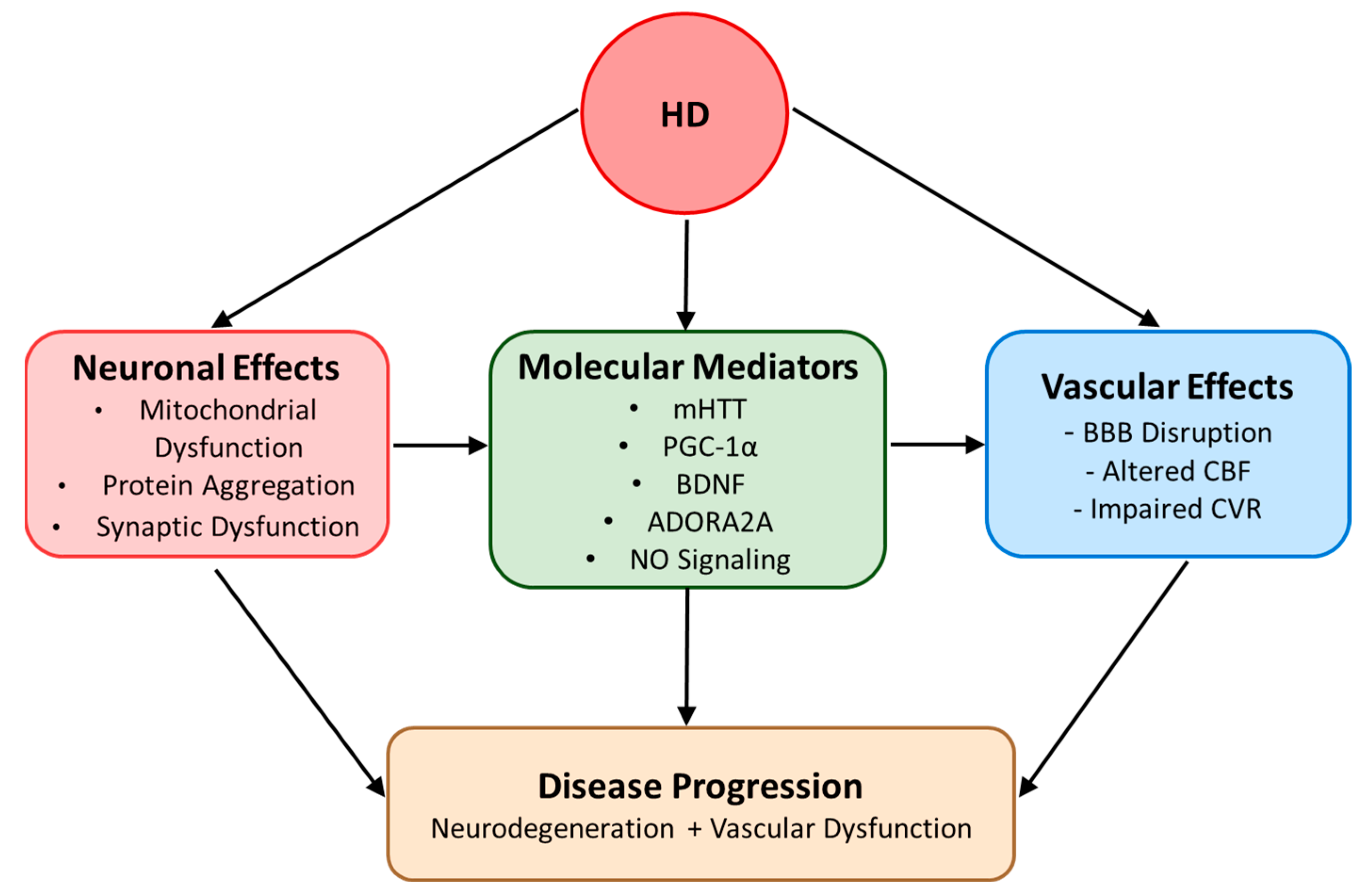
3. Genetic Factors Influencing Endothelial Functions in HD
3.1. mHTT
3.2. PGC-1α
3.3. BDNF
3.4. ADORA2A
| Vascular Effect | Genetic Factor | Therapeutic Opportunities | References |
|---|---|---|---|
| Altered angiogenesis | mHTT | ASO, small molecule aggregation inhibitors | [17,39] |
| PGC-1α | Mitochondrial enhancers, antioxidants | [48] | |
| ADORA2A | Selective agonists/antagonists | [54] | |
| BBB disruption | mHTT | ASO, small molecule aggregation inhibitors | [17,39] |
| PGC-1α | Mitochondrial enhancers, antioxidants | [47] | |
| ADORA2A | Selective agonists/antagonists | [53] | |
| NO production | PGC-1α | Mitochondrial enhancers, antioxidants | [46] |
| BDNF | TrkB agonists, BDNF mimetics | [49] |
3.5. Integrated Genetic Factor Network in HD Vascular Pathology
4. Therapeutic Approaches Targeting Endothelial Functions in HD
4.1. Antioxidants
4.2. Enhancing NO Bioavailability
4.3. Pharmacological Agents
4.4. Glycemic Control: Focus on Metformin
5. Possible Mechanism of Endothelial Dysfunctions in HD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef]
- Wilton, D.K.; Stevens, B. The contribution of glial cells to Huntington’s disease pathogenesis. Neurobiol. Dis. 2020, 143, 104963. [Google Scholar] [CrossRef]
- Ferguson, M.W.; Kennedy, C.J.; Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Current and Possible Future Therapeutic Options for Huntington’s Disease. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221092517. [Google Scholar] [CrossRef]
- Kremer, R.; Williams, A.; Wardlaw, J. Endothelial cells as key players in cerebral small vessel disease. Nat. Rev. Neurosci. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; Sawiak, S.J.; Cisbani, G.; Lagace, M.; Kuan, W.L.; Saint-Pierre, M.; Dury, R.J.; Alata, W.; St-Amour, I.; Mason, S.L.; et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann. Neurol. 2015, 78, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, S.; Ryu, J.K.; Shim, Y.; Hill, A.; Connolly, C.; Hayden, M.R.; McLarnon, J.G.; Leavitt, B.R. Age-dependent neurovascular abnormalities and altered microglial morphology in the YAC128 mouse model of Huntington disease. Neurobiol. Dis. 2012, 45, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsu, Y.H.; Lin, M.H.; Yang, T.H.; Chen, H.M.; Chen, Y.C.; Hsiao, H.Y.; Chen, C.C.; Chern, Y.; Chang, C. Neurovascular abnormalities in humans and mice with Huntington’s disease. Exp. Neurol. 2013, 250, 20–30. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Arribas, V.; Onetti, Y.; Ramiro-Pareta, M.; Villacampa, P.; Beck, H.; Alberola, M.; Esteve-Codina, A.; Merkel, A.; Sperandio, M.; Martinez-Estrada, O.M.; et al. Endothelial TDP-43 controls sprouting angiogenesis and vascular barrier integrity, and its deletion triggers neuroinflammation. JCI Insight 2024, 9, e177819. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Ross, C.A.; Aylward, E.H.; Wild, E.J.; Langbehn, D.R.; Long, J.D.; Warner, J.H.; Scahill, R.I.; Leavitt, B.R.; Stout, J.C.; Paulsen, J.S.; et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014, 10, 204–216. [Google Scholar] [CrossRef]
- Van de Roovaart, H.J.; Nguyen, N.; Veenstra, T.D. Huntington’s Disease Drug Development: A Phase 3 Pipeline Analysis. Pharmaceuticals 2023, 16, 1513. [Google Scholar] [CrossRef] [PubMed]
- Vis, J.C.; Nicholson, L.F.; Faull, R.L.; Evans, W.H.; Severs, N.J.; Green, C.R. Connexin expression in Huntington’s diseased human brain. Cell Biol. Int. 1998, 22, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Chen, Y.C.; Huang, C.H.; Chen, C.C.; Hsu, Y.H.; Chen, H.M.; Chiu, F.L.; Kuo, H.C.; Chang, C.; Chern, Y. Aberrant astrocytes impair vascular reactivity in Huntington disease. Ann. Neurol. 2015, 78, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Ciammola, A.; Sassone, J.; Cannella, M.; Calza, S.; Poletti, B.; Frati, L.; Squitieri, F.; Silani, V. Low brain-derived neurotrophic factor (BDNF) levels in serum of Huntington’s disease patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.G.; Quan, C.; Reyes-Ortiz, A.M.; Lutz, S.E.; Kedaigle, A.J.; Gipson, T.A.; Wu, J.; Vatine, G.D.; Stocksdale, J.; Casale, M.S.; et al. Huntington’s Disease iPSC-Derived Brain Microvascular Endothelial Cells Reveal WNT-Mediated Angiogenic and Blood-Brain Barrier Deficits. Cell Rep. 2017, 19, 1365–1377. [Google Scholar] [CrossRef]
- Harris, G.J.; Aylward, E.H.; Peyser, C.E.; Pearlson, G.D.; Brandt, J.; Roberts-Twillie, J.V.; Barta, P.E.; Folstein, S.E. Single photon emission computed tomographic blood flow and magnetic resonance volume imaging of basal ganglia in Huntington’s disease. Arch. Neurol. 1996, 53, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.J.; Codori, A.M.; Lewis, R.F.; Schmidt, E.; Bedi, A.; Brandt, J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington’s disease. Brain 1999, 122 Pt 9, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, S.G.; Oberg, G.; Sorensen, S.A.; Andersen, A.R.; Waldemar, G.; Schmidt, J.F.; Fenger, K.; Paulson, O.B. Reduced regional cerebral blood flow in Huntington’s disease studied by SPECT. J. Neurol. Neurosurg. Psychiatry 1992, 55, 1018–1023. [Google Scholar] [CrossRef]
- Sax, D.S.; Powsner, R.; Kim, A.; Tilak, S.; Bhatia, R.; Cupples, L.A.; Myers, R.H. Evidence of cortical metabolic dysfunction in early Huntington’s disease by single-photon-emission computed tomography. Mov. Disord. 1996, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, N.; Meyer, J.S.; Ishikawa, Y.; Kandula, P.; Mortel, K.F.; Rogers, R.L.; Gandhi, S.; Walker, M. Cerebral blood flow and cognitive testing correlate in Huntington’s disease. Arch. Neurol. 1985, 42, 1169–1175. [Google Scholar] [CrossRef]
- Deckel, A.W.; Weiner, R.; Szigeti, D.; Clark, V.; Vento, J. Altered patterns of regional cerebral blood flow in patients with Huntington’s disease: A SPECT study during rest and cognitive or motor activation. J. Nucl. Med. 2000, 41, 773–780. [Google Scholar]
- Weinberger, D.R.; Berman, K.F.; Iadarola, M.; Driesen, N.; Zec, R.F. Prefrontal cortical blood flow and cognitive function in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 94–104. [Google Scholar] [CrossRef]
- Wolf, R.C.; Gron, G.; Sambataro, F.; Vasic, N.; Wolf, N.D.; Thomann, P.A.; Saft, C.; Landwehrmeyer, G.B.; Orth, M. Magnetic resonance perfusion imaging of resting-state cerebral blood flow in preclinical Huntington’s disease. J. Cereb. Blood Flow. Metab. 2011, 31, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Salat, D.H.; Rosas, H.D. Complex relationships between cerebral blood flow and brain atrophy in early Huntington’s disease. Neuroimage 2012, 59, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; Charron, O.; Colpo, G.D.; Latham, L.B.; Patino, J.E.; Stimming, E.F.; Freeman, L.; Teixeira, A.L. Cerebral blood flow is associated with markers of neurodegeneration in Huntington’s disease. Parkinsonism Relat. Disord. 2022, 102, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sleight, E.; Stringer, M.S.; Marshall, I.; Wardlaw, J.M.; Thrippleton, M.J. Cerebrovascular Reactivity Measurement Using Magnetic Resonance Imaging: A Systematic Review. Front. Physiol. 2021, 12, 643468. [Google Scholar] [CrossRef]
- Chan, S.T.; Mercaldo, N.D.; Kwong, K.K.; Hersch, S.M.; Rosas, H.D. Impaired Cerebrovascular Reactivity in Huntington’s Disease. Front. Physiol. 2021, 12, 663898. [Google Scholar] [CrossRef]
- Rahman, A.; Ekman, M.; Shakirova, Y.; Andersson, K.E.; Morgelin, M.; Erjefalt, J.S.; Brundin, P.; Li, J.Y.; Sward, K. Late onset vascular dysfunction in the R6/1 model of Huntington’s disease. Eur. J. Pharmacol. 2013, 698, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.D.; Niu, Y.; Herrera, E.A.; Morton, A.J.; Giussani, D.A. Impaired Nitric Oxide Mediated Vasodilation In The Peripheral Circulation In The R6/2 Mouse Model Of Huntington’s Disease. Sci. Rep. 2016, 6, 25979. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Cepeda-Prado, E.; Popp, S.; Khan, U.; Stefanov, D.; Rodriguez, J.; Menalled, L.B.; Dow-Edwards, D.; Small, S.A.; Moreno, H. R6/2 Huntington’s disease mice develop early and progressive abnormal brain metabolism and seizures. J. Neurosci. 2012, 32, 6456–6467. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Amico, E.; Scalabri, F.; Pepe, G.; Castaldo, S.; Elifani, F.; Capocci, L.; De Sanctis, C.; Comerci, L.; Pompeo, F.; et al. Impairment of blood-brain barrier is an early event in R6/2 mouse model of Huntington Disease. Sci. Rep. 2017, 7, 41316. [Google Scholar] [CrossRef]
- Kim, G.W.; Gasche, Y.; Grzeschik, S.; Copin, J.C.; Maier, C.M.; Chan, P.H. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: Role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J. Neurosci. 2003, 23, 8733–8742. [Google Scholar] [CrossRef]
- Nishino, H.; Kumazaki, M.; Fukuda, A.; Fujimoto, I.; Shimano, Y.; Hida, H.; Sakurai, T.; Deshpande, S.B.; Shimizu, H.; Morikawa, S.; et al. Acute 3-nitropropionic acid intoxication induces striatal astrocytic cell death and dysfunction of the blood-brain barrier: Involvement of dopamine toxicity. Neurosci. Res. 1997, 27, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Shimano, Y.; Kumazaki, M.; Sakurai, T.; Hida, H.; Fujimoto, I.; Fukuda, A. Hypothalamic neurons are resistant to the intoxication with 3-nitropropionic acid that induces lesions in the striatum and hippocampus via the damage in the blood-brain barrier. Neurobiology 1995, 3, 257–267. [Google Scholar] [PubMed]
- Linville, R.M.; Nerenberg, R.F.; Grifno, G.; Arevalo, D.; Guo, Z.; Searson, P.C. Brain microvascular endothelial cell dysfunction in an isogenic juvenile iPSC model of Huntington’s disease. Fluids Barriers CNS 2022, 19, 54. [Google Scholar] [CrossRef]
- Vignone, D.; Gonzalez Paz, O.; Fini, I.; Cellucci, A.; Auciello, G.; Battista, M.R.; Gloaguen, I.; Fortuni, S.; Cariulo, C.; Khetarpal, V.; et al. Modelling the Human Blood-Brain Barrier in Huntington Disease. Int. J. Mol. Sci. 2022, 23, 7813. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh-Fard, E.; Saft, C.; Andrich, J.; Wieczorek, S.; Arning, L. PGC-1alpha as modifier of onset age in Huntington disease. Mol. Neurodegener. 2009, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Alberch, J.; Lopez, M.; Badenas, C.; Carrasco, J.L.; Mila, M.; Munoz, E.; Canals, J.M. Association between BDNF Val66Met polymorphism and age at onset in Huntington disease. Neurology 2005, 65, 964–965. [Google Scholar] [CrossRef] [PubMed]
- Dhaenens, C.M.; Burnouf, S.; Simonin, C.; Van Brussel, E.; Duhamel, A.; Defebvre, L.; Duru, C.; Vuillaume, I.; Cazeneuve, C.; Charles, P.; et al. A genetic variation in the ADORA2A gene modifies age at onset in Huntington’s disease. Neurobiol. Dis. 2009, 35, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Rius-Perez, S.; Torres-Cuevas, I.; Millan, I.; Ortega, A.L.; Perez, S. PGC-1alpha, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Craige, S.M.; Kroller-Schon, S.; Li, C.; Kant, S.; Cai, S.; Chen, K.; Contractor, M.M.; Pei, Y.; Schulz, E.; Keaney, J.F., Jr. PGC-1alpha dictates endothelial function through regulation of eNOS expression. Sci. Rep. 2016, 6, 38210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Geng, X.Y.; Cong, X.L. PGC-1alpha ameliorates AngiotensinII-induced eNOS dysfunction in human aortic endothelial cells. Vascul Pharmacol. 2016, 83, 90–97. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Zhou, Q.; Zhu, F.; He, S. Protective Effects of PGC-1alpha on the Blood Brain Barrier After Acute Kidney Injury. Neurochem. Res. 2020, 45, 1086–1096. [Google Scholar] [CrossRef]
- Fujiwara, T.; Takeda, N.; Hara, H.; Ishii, S.; Numata, G.; Tokiwa, H.; Katoh, M.; Maemura, S.; Suzuki, T.; Takiguchi, H.; et al. PGC-1alpha-mediated angiogenesis prevents pulmonary hypertension in mice. JCI Insight 2023, 8, e162632. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef]
- Santhanam, A.V.; Smith, L.A.; Katusic, Z.S. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke 2010, 41, 350–356. [Google Scholar] [CrossRef] [PubMed]
- del Toro, D.; Canals, J.M.; Gines, S.; Kojima, M.; Egea, G.; Alberch, J. Mutant huntingtin impairs the post-Golgi trafficking of brain-derived neurotrophic factor but not its Val66Met polymorphism. J. Neurosci. 2006, 26, 12748–12757. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jiang, W.; Liu, H.; Wang, J.; Zheng, K.; Cui, P.; Feng, Y.; Dang, C.; Bu, Y.; Wang, Q.M.; et al. Upregulation of neuronal PGC-1alpha ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Theranostics 2020, 10, 2832–2848. [Google Scholar] [CrossRef]
- Yamamoto, M.; Guo, D.H.; Hernandez, C.M.; Stranahan, A.M. Endothelial Adora2a Activation Promotes Blood-Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance. J. Neurosci. 2019, 39, 4179–4192. [Google Scholar] [CrossRef]
- Liu, Y.; Alahiri, M.; Ulloa, B.; Xie, B.; Sadiq, S.A. Adenosine A2A receptor agonist ameliorates EAE and correlates with Th1 cytokine-induced blood brain barrier dysfunction via suppression of MLCK signaling pathway. Immun. Inflamm. Dis. 2018, 6, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; Dedeoglu, A.; Ferrante, R.J.; Jenkins, B.G.; Ferrante, K.L.; Thomas, M.; Friedlich, A.; Browne, S.E.; Schilling, G.; Borchelt, D.R.; et al. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol. Dis. 2001, 8, 479–491. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Andreassen, O.A.; Dedeoglu, A.; Ferrante, K.L.; Jenkins, B.G.; Hersch, S.M.; Beal, M.F. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J. Neurosci. 2002, 22, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Andreassen, O.A.; Jenkins, B.G.; Dedeoglu, A.; Kuemmerle, S.; Kubilus, J.K.; Kaddurah-Daouk, R.; Hersch, S.M.; Beal, M.F. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000, 20, 4389–4397. [Google Scholar] [CrossRef] [PubMed]
- Kalonia, H.; Kumar, P.; Kumar, A. Targeting oxidative stress attenuates malonic acid induced Huntington like behavioral and mitochondrial alterations in rats. Eur. J. Pharmacol. 2010, 634, 46–52. [Google Scholar] [CrossRef]
- Miyamoto, M.; Coyle, J.T. Idebenone attenuates neuronal degeneration induced by intrastriatal injection of excitotoxins. Exp. Neurol. 1990, 108, 38–45. [Google Scholar] [CrossRef]
- Wright, D.J.; Renoir, T.; Smith, Z.M.; Frazier, A.E.; Francis, P.S.; Thorburn, D.R.; McGee, S.L.; Hannan, A.J.; Gray, L.J. N-Acetylcysteine improves mitochondrial function and ameliorates behavioral deficits in the R6/1 mouse model of Huntington’s disease. Transl. Psychiatry 2015, 5, e492. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Schifitto, G.; Oakes, D.; Bredlau, A.L.; Meyers, C.M.; Nahin, R.; Rosas, H.D.; For the Huntington Study Group CREST-E Investigators and Coordinators. The CREST-E study of creatine for Huntington disease: A randomized controlled trial. Neurology 2017, 89, 594–601. [Google Scholar] [CrossRef] [PubMed]
- McGarry, A.; McDermott, M.; Kieburtz, K.; de Blieck, E.A.; Beal, F.; Marder, K.; Ross, C.; Shoulson, I.; Gilbert, P.; Mallonee, W.M.; et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology 2017, 88, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Deckel, A.W.; Volmer, P.; Weiner, R.; Gary, K.A.; Covault, J.; Sasso, D.; Schmerler, N.; Watts, D.; Yan, Z.; Abeles, I. Dietary arginine alters time of symptom onset in Huntington’s disease transgenic mice. Brain Res. 2000, 875, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Puerta, E.; Hervias, I.; Barros-Minones, L.; Jordan, J.; Ricobaraza, A.; Cuadrado-Tejedor, M.; Garcia-Osta, A.; Aguirre, N. Sildenafil protects against 3-nitropropionic acid neurotoxicity through the modulation of calpain, CREB, and BDNF. Neurobiol. Dis. 2010, 38, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, A.; Shetty, S.; Shirole, T.; Jagtap, A.G. Potential of protease inhibitor in 3-nitropropionic acid induced Huntington’s disease like symptoms: Mitochondrial dysfunction and neurodegeneration. Neurotoxicology 2014, 45, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, J.; Bryer, A. Huntington’s disease: Deterioration in clinical state during treatment with angiotensin converting enzyme inhibitor. Br. Med. J. (Clin. Res. Ed.) 1987, 294, 1659–1660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siddiqi, F.H.; Menzies, F.M.; Lopez, A.; Stamatakou, E.; Karabiyik, C.; Ureshino, R.; Ricketts, T.; Jimenez-Sanchez, M.; Esteban, M.A.; Lai, L.; et al. Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing. Nat. Commun. 2019, 10, 1817. [Google Scholar] [CrossRef] [PubMed]
- Joviano-Santos, J.V.; Valadao, P.A.C.; Magalhaes-Gomes, M.P.S.; Fernandes, L.F.; Diniz, D.M.; Machado, T.C.G.; Soares, K.B.; Ladeira, M.S.; Massensini, A.R.; Gomez, M.V.; et al. Neuroprotective effect of CTK 01512-2 recombinant toxin at the spinal cord in a model of Huntington’s disease. Exp Physiol 2022, 107, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Joviano-Santos, J.V.; Valadao, P.A.C.; Magalhaes-Gomes, M.P.S.; Fernandes, L.F.; Diniz, D.M.; Machado, T.C.G.; Soares, K.B.; Ladeira, M.S.; Miranda, A.S.; Massensini, A.R.; et al. Protective effect of a spider recombinant toxin in a murine model of Huntington’s disease. Neuropeptides 2021, 85, 102111. [Google Scholar] [CrossRef]
- Schultz, J.L.; Nopoulos, P.C.; Killoran, A.; Kamholz, J.A. Statin use and delayed onset of Huntington’s disease. Mov. Disord. 2019, 34, 281–285. [Google Scholar] [CrossRef]
- Ma, T.C.; Buescher, J.L.; Oatis, B.; Funk, J.A.; Nash, A.J.; Carrier, R.L.; Hoyt, K.R. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci. Lett. 2007, 411, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, I.; Willam, M.; Griesche, N.; Krummeich, J.; Watari, H.; Offermann, N.; Weber, S.; Narayan Dey, P.; Chen, C.; Monteiro, O.; et al. Metformin reverses early cortical network dysfunction and behavior changes in Huntington’s disease. eLife 2018, 7, e38744. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, A.; Garcia-Gimeno, M.A.; Canada-Martinez, A.J.; Sequedo, M.D.; Millan, J.M.; Sanz, P.; Vazquez-Manrique, R.P. Metformin treatment reduces motor and neuropsychiatric phenotypes in the zQ175 mouse model of Huntington disease. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vallee, A.; Lecarpentier, Y.; Guillevin, R.; Vallee, J.N. Aerobic glycolysis in amyotrophic lateral sclerosis and Huntington’s disease. Rev. Neurosci. 2018, 29, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Fang, P.; Li, S.; Xia, D.; Zhang, J.; Wu, X.; Pan, J.; Cai, H.; Fu, L.; Sun, G.; et al. Lactylation of Histone H3k18 and Egr1 Promotes Endothelial Glycocalyx Degradation in Sepsis-Induced Acute Lung Injury. Adv. Sci. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Cunha-Oliveira, T.; Nascimento, M.V.; Ribeiro, M.; Proenca, M.T.; Januario, C.; Oliveira, C.R.; Rego, A.C. Bioenergetic dysfunction in Huntington’s disease human cybrids. Exp. Neurol. 2011, 231, 127–134. [Google Scholar] [CrossRef] [PubMed]

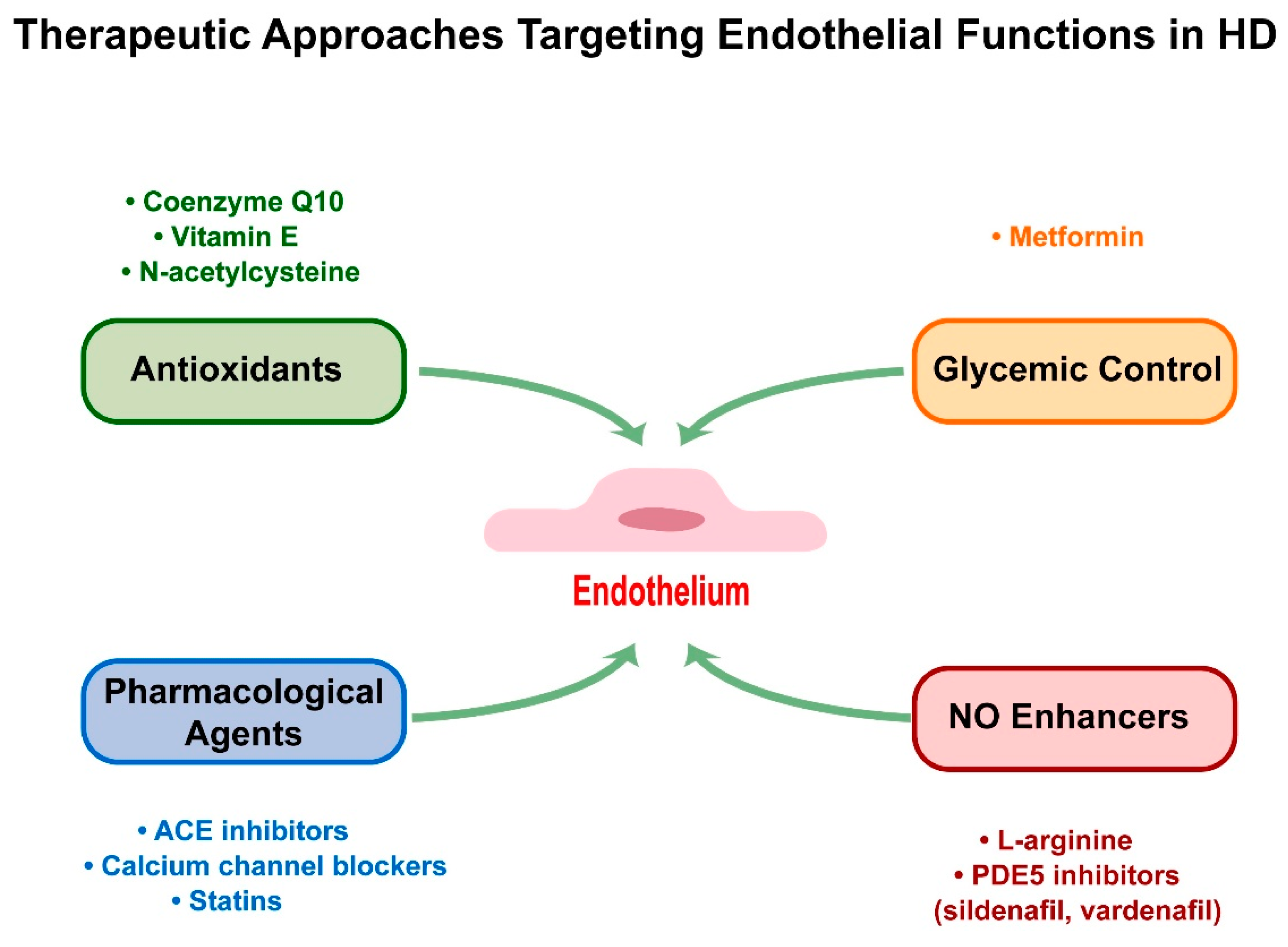
| Approach | Key Advantages | Limitations | Optimization Strategies |
|---|---|---|---|
| Antioxidants | Multiple targets, well-tolerated | Poor BBB penetration, timing issues | Novel delivery systems, early intervention |
| NO Enhancers | Direct vascular effects | Systemic side effects | CNS-targeted delivery, biomarker monitoring |
| ACE Inhibitors | Proven safety profile | Variable neurological effects | Patient-specific titration |
| CCBs | Strong preclinical evidence | Cardiovascular effects | Novel formulations, targeted delivery |
| Statins | Multiple mechanisms | Muscle-related side effects | Combination therapy, dose optimization |
| Metformin | Multi-target effects | Variable response | Personalized dosing, early intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, N.; Chen, Z.; Zhao, X.; Peng, X.; Wu, Y.; Yang, K.; Sun, T. Endothelial Dysfunction in Huntington’s Disease: Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 1432. https://doi.org/10.3390/ijms26041432
Hu N, Chen Z, Zhao X, Peng X, Wu Y, Yang K, Sun T. Endothelial Dysfunction in Huntington’s Disease: Pathophysiology and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(4):1432. https://doi.org/10.3390/ijms26041432
Chicago/Turabian StyleHu, Ning, Zihao Chen, Xinyue Zhao, Xin Peng, Yimeng Wu, Kai Yang, and Taolei Sun. 2025. "Endothelial Dysfunction in Huntington’s Disease: Pathophysiology and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 4: 1432. https://doi.org/10.3390/ijms26041432
APA StyleHu, N., Chen, Z., Zhao, X., Peng, X., Wu, Y., Yang, K., & Sun, T. (2025). Endothelial Dysfunction in Huntington’s Disease: Pathophysiology and Therapeutic Implications. International Journal of Molecular Sciences, 26(4), 1432. https://doi.org/10.3390/ijms26041432






