Infrared Laser-Assisted Extraction of Bioactive Compounds from Rosa canina L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition Profile of Formulated Extracts
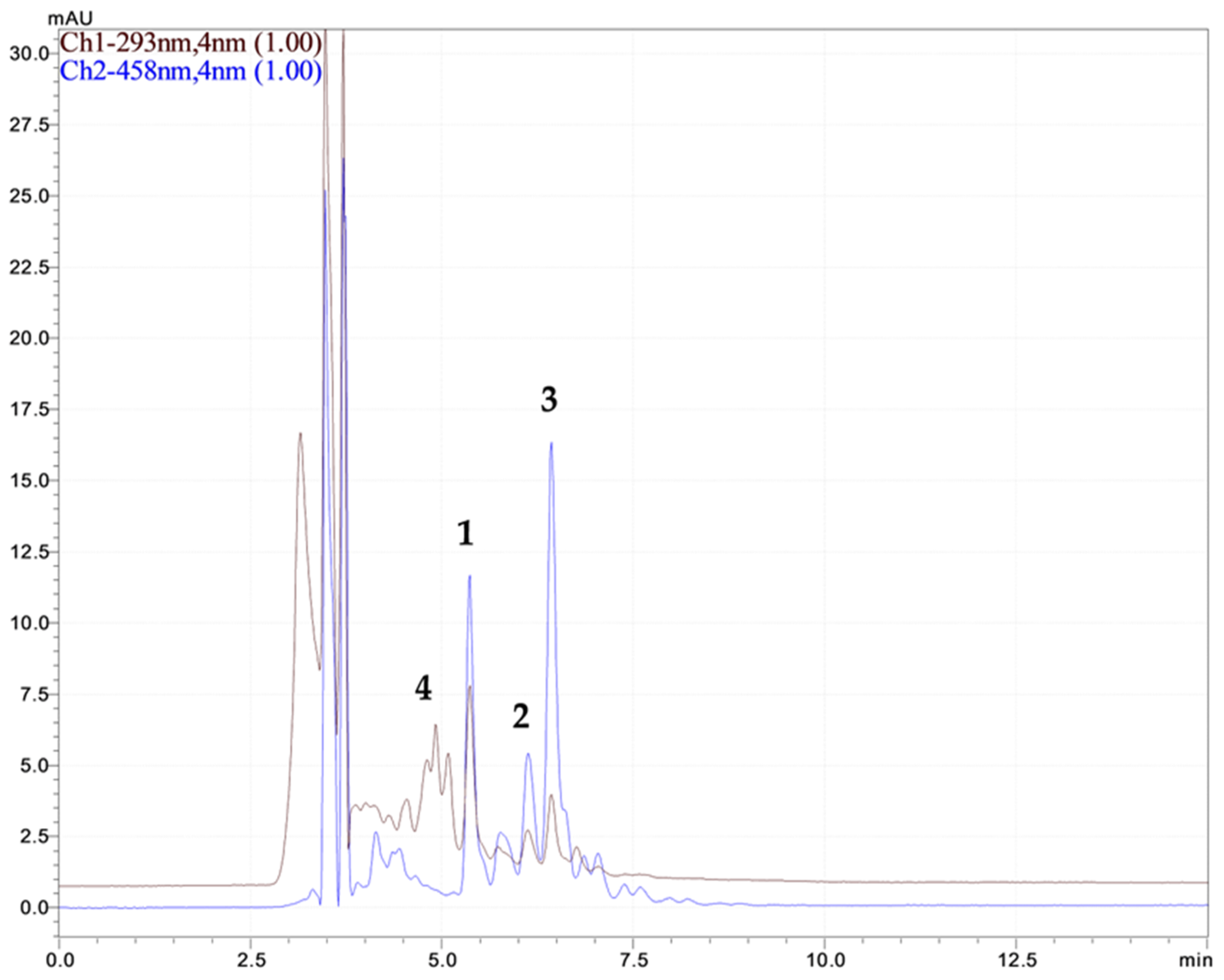
2.1.1. Polyphenol Compound Analysis of Rosa canina L. Extracts
2.1.2. Lipophilic Compound Analysis of Rosa canina L. Extracts
2.2. Assessment of Formulated Extract Efficacy as Antioxidants
2.2.1. The Antioxidant Capacity of the Rosa canina L. Polyphenolic Extracts
2.2.2. The Antioxidant Capacity of the Rosa canina L. Lipophilic Extracts
3. Materials and Methods
3.1. Reagents
3.2. Preparation of Rosa canina L. Extracts
3.2.1. Rosa canina L. Extracts Obtained by IRLIR
3.2.2. Rosa canina L. Extracts Obtained by ASE
3.3. Methods
3.3.1. HPLC-MS Analysis of Rosa canina L. Polyphenolic Extracts
3.3.2. HPLC-DAD Analysis of Rosa canina L. Lipophilic Extracts
3.3.3. Antioxidant Capacity
3.3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Pirvu, L.C.; Nita, S.; Rusu, N.; Bazdoaca, C.; Neagu, G.; Bubueanu, C.; Udrea, M.; Udrea, R.; Enache, A. Effects of Laser Irradiation at 488, 514, 532, 552, 660, and 785 nm on the Aqueous Extracts of Plantago lanceolata L.: A Comparison on Chemical Content, Antioxidant Activity and Caco-2 Viability. Appl. Sci. 2022, 12, 5517. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Alecu, A.; Albu, C.; Seciu-Grama, A.-M.; Radu, G.L. Evaluating the Antioxidant and Antidiabetic Properties of Medicago sativa and Solidago virgaurea Polyphenolic-Rich Extracts. Molecules 2024, 29, 326. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Hanafiah, M.M.; Taha, Z.A.; AlHilfy, I.H.H.; Said, M.N.M. Laser Irradiation Effects at Different Wavelengths on Phenology and Yield Components of Pretreated Maize Seed. Appl. Sci. 2020, 10, 1189. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Tashish, W.; Al-Osaif, S.S.; Atif, M. Effects of He–Ne laser and argon laser irradiation on growth, germination, and physico-biochemical characteristics of wheat seeds (Triticumaestivum L.). Laser Phys. 2019, 29, 015602. [Google Scholar] [CrossRef]
- Perveen, R.; Wang, X.; Jamil, Y.; Ali, Q.; Ali, S.; Zakaria, M.Q.; Afzaal, M.; Kasana, R.A.; Saleem, M.H.; Fiaz, S. Quantitative Determination of the Effects of He–Ne Laser Irradiation on Seed Thermodynamics, Germination Attributes and Metabolites of Safflower (Carthamus tinctorius L.) in Relation with the Activities of Germination Enzymes. Agronomy 2021, 11, 1411. [Google Scholar] [CrossRef]
- Garner, A.L.; Neculaes, B.; Dylov, D.V. Infrared Laser-Based Single Cell Permeabilization by Plasma Membrane Temperature Gradients. Membranes 2022, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, D.; Zabic, M.; Terakawa, M.; Boch, J. Laser-based molecular delivery and its applications in plant science. Plant Methods 2022, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Gazor, M.; Talesh, S.S.A.; Kavianpour, A.; Khatami, M.; Javidanbardan, A.; Hosseini, S.N. A Novel Cell Disruption Approach: Effectiveness of Laser-induced Cell Lysis of Pichia pastoris in the Continuous System. Biotechnol. Bioprocess Eng. 2018, 23, 49–54. [Google Scholar] [CrossRef]
- Cios, A.; Ciepielak, M.; Szyma´nski, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef]
- Tinne, N.; Kaune, B.; Ripken, K.A. Interaction Mechanisms of Cavitation Bubbles Induced by Spatially and Temporally Separated fs-Laser Pulses. PLoS ONE 2014, 9, e114437. [Google Scholar] [CrossRef] [PubMed]
- Korneev, N.; Rodŕıguez-Montero, P.; Duŕan-Śanchez, M.; Ibarra-Escamilla, B.; Kuzin, E. Thermoactivated cavitation induced in water by low power, continuous wave thulium-doped fiber laser. Rev. Mex. De Física 2019, 65, 185–189. [Google Scholar] [CrossRef]
- Kaub, L.; Schmitz, C. Comparison of the Penetration Depth of 905 nm and 1064 nm Laser Light in Surface Layers of Biological Tissue Ex Vivo. Biomedicines 2023, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Ding, L.; Zhou, W.; Xu, G.; Wang, Y.; Zhang, Y.; Ni, Q. Accelerated Solvent Extraction of Antioxidant Compounds from Gardeniae Fructus and Its Acetylcholinesterase Inhibitory and PC12 Cell Protective Activities. Foods 2021, 10, 2805. [Google Scholar] [CrossRef]
- Suna, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A. 2012, 1237, 1–23. [Google Scholar] [CrossRef]
- Wibisono, R.; Zhang, J.; Saleh, Z.; Stevenson, D.E.; Joyce, N.I. Optimisation of accelerated solvent extraction for screening of the health benefits of plant food materials. Health 2009, 1, 220–230. [Google Scholar] [CrossRef]
- Mottaleb, M.A.; Sarker, S.D. Accelerated Solvent Extraction for Natural Products Isolation. In Natural Products Isolation, 3rd ed.; Sarker, S.D., Nahar, L., Eds.; Humana Press: Clifton, NJ, USA, 2012; Volume 864, pp. 75–87. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. Compr. Pharmacol. 2022, 2, 408–422. [Google Scholar] [CrossRef]
- Ghiorghita, G.; Antohe, N.; Rati, I.V.; Maftei, D.E. The Study of Some Parameters of Rosa canina L. Genotypes from Different Native Populations and from The Same Population. An. Stiint. Univ. Al. I. Cuza Iasi 2012, 58, 19–27. [Google Scholar]
- Rajakaruna, A.; Manful, C.F.; Abu-Reidah, I.M.; Critch, A.L.; Vidal, N.P.; Pham, T.P.; Cheema, M.; Thomas, R. Application of solvent pH under pressurized conditions using accelerated solvent extraction and green solvents to extract phytonutrients from wild berries. Food Biosci. 2022, 47, 101471. [Google Scholar] [CrossRef]
- Bozhuyuk, M.R.; Ercisli, S.; Karatas, N.; Ekiert, H.; Elansary, H.O.; Szopa, A. Morphological and Biochemical Diversity in Fruits of Unsprayed Rosa canina and Rosa dumalis Ecotypes Found in Different Agroecological Conditions. Sustainability 2021, 13, 8060. [Google Scholar] [CrossRef]
- Winther, K.; Vinther Hansen, A.S.; Campbell-Tofte, J. Bioactive ingredients of rose hips (Rosa canina L.) with special reference to antioxidative and anti-inflammatory properties: In vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Igual, M.; García-Herrera, P.; Cámara, R.M.; Martínez-Monzó, J.; García-Segovia, P.; Cámara, M. Bioactive Compounds in Rosehip (Rosa canina) Powder with Encapsulating Agents. Molecules 2022, 27, 4737. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.; Valencia, S.; Tereucán, G.; Nahuelcura, J.; Jiménez-Aspee, F.; Cornejo, P.; Ruiz, A. Bioactive Compounds and Antioxidant Activity in the Fruit of Rosehip (Rosa canina L. and Rosa rubiginosa L.). Molecules 2023, 28, 3544. [Google Scholar] [CrossRef]
- Hendrysiak, A.; Brzezowska, J.; Nicolet, N.; Bocquel, D.; Andlauer, W.; Michalska-Ciechanowska, A. Juice Powders from Rosehip (Rosa canina L.): Physical, Chemical, and Antiglycation Properties. Molecules 2023, 28, 1674. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A.; Zapater, J.M.M.; Fernandez, S.D.P.T. Composition and biological activity of the Algerian plant Rosa canina L. by HPLC-UV-MS. Arab. J. Chem. 2020, 13, 1105–1119. [Google Scholar] [CrossRef]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.L.; Kačániová, M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, iPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef] [PubMed]
- Christina Lustrup, D.; Winther, K. Rose Hip as a Nutraceutical. In Medicinal Plants; Kumar, S., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80356-033-5. [Google Scholar] [CrossRef]
- Fan, C.; Pacier, C.; Martirosyan, D.M. Rose hip (Rosa canina L.): A functional food perspective. FFHD 2014, 4, 493–509. [Google Scholar] [CrossRef]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Rosa canina-derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2022, 41 (Suppl. S1), 44S–60S. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, V.; Chiocchio, I.; Mandrone, M.; Cirrincione, M.; Protti, M.; Farruggia, G.; Abruzzo, A.; Luppi, B.; Bigucci, F.; Mercolini, L. Extraction, Encapsulation into Lipid Vesicular Systems, and Biological Activity of Rosa canina L. Bioactive Compounds for Dermocosmetic Use. Molecules 2022, 27, 3025. [Google Scholar] [CrossRef] [PubMed]
- Angelov, G.; Boyadzhieva, S.S.; Georgieva, S.S. Rosehip extraction: Process optimization and antioxidant capacity of extracts. Cent. Eur. J. Chem. 2014, 12, 502–508. [Google Scholar] [CrossRef]
- Radu, G.L.; Litescu, S.C.; Albu, C.; Teodor, E.; Truica, G. Beta-carotene and lycopene determination in new enriched bakery productsby HPLC-DAD method. Rom. Biotechnol. Lett. 2012, 17, 7005–70012. [Google Scholar]
- Badea, G.I.; Litescu-Filipescu, S.C.; Radu, G.L.; Diaconu, I. Evaluation of the Efficacy of Various Green Extraction Methods for High Valorisation of Vegetal Antioxidant Sources. Rev. Chim. 2018, 69, 2708–2711. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Development of an Efficient System for Recovering Hydro- and Liposoluble Antioxidant Active Principles from Plant by-Products and Yeast Types. Using Customized Laser Treatments/SC Apel Laser SRL. Available online: https://proiecte.incdsb.ro/FITOCOMP (accessed on 20 December 2024).
- Jiménez, S.; Gascón, S.; Luquin, A.; Laguna, M.; Ancin-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Rosa canina Extracts Have Antiproliferative and Antioxidant Effects on Caco-2 Human Colon Cancer. PLoS ONE 2016, 11, e0159136. [Google Scholar] [CrossRef]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic Composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea Extracts and Assessment of Their Antioxidant Activity in Human Endothelial Cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef]
- Liu, S.C.; Lin, J.T.; Yang, D.J. Determination of cis- and trans- [alpha]- and [beta]-carotenoids in Taiwanese sweet potatoes (Ipomoea batatas (L.) Lam.) harvested at various times. Food Chem. 2009, 116, 605–610. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, B.H. Determination of carotenoids in tomato juice by liquid chromatography. J. Chromatogr. A 2003, 1012, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Kayahan, S.; Ozdemir, Y.; Gulbag, F. Functional Compounds and Antioxidant Activity of Rosa Species Grown In Turkey. Appl. Fruit Sci. Erwerbs-Obstbau 2023, 65, 1079–1086. [Google Scholar] [CrossRef]
- Zilic, S.; Mogol, B.A.; Akıllıoglu, G.; Serpen, A.; Babic, M.; Gokmen, V. Effects of infrared heating on phenolic compounds and Maillard reaction products in maize flour. J. Cereal Sci. 2013, 58, 1–7. [Google Scholar] [CrossRef]
- Badhani, B.; Sharmaand, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.Y.; Kim, Y.J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.-L.; Alecu, A.; Brinduse, E. The Influence of Melatonin Treatment in the Vinification of Feteasca Neagra and Cabernet Sauvignon Wines on the Profile of Polyphenolic Compounds and Antioxidant Activity. Antioxidants 2023, 12, 1214. [Google Scholar] [CrossRef]
- Litescu, S.C.; Eremia, S.A.V.; Tache, A.; Vasilescu, I.; Radu, G.L. The Use of Oxygen Radical Absorbance Capacity (ORAC) and Trolox Equivalent Antioxidant Capacity (TEAC) Assays in the Assessment of Beverages’ Antioxidant Properties. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; King’s College London: London, UK, 2014; pp. 245–251. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.C.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]


| Compound | [M − H]− | tR, min. | Linear Regression Equations | R | Linearity Range of Response, µg mL−1 | LoD, µg mL−1 | LoQ, µg mL−1 |
|---|---|---|---|---|---|---|---|
| Gallic acid | 169 | 5.82 | A = 48,686.49 × C − 4680.433 | 0.9993 | 1–100 | 0.16 | 0.30 |
| Chlorogenic acid | 353 | 15.18 | A = 15,009.58 × C − 434.9512 | 0.9999 | 1–100 | 0.33 | 0.45 |
| Quercetin | 301 | 35.52 | A = 72,301.05 × C – 10,684.39 | 0.9997 | 1–100 | 0.19 | 0.29 |
| Quercitrin | 447 | 27.78 | A = 56,594.18 × C − 3157.114 | 0.9998 | 1–100 | 0.11 | 0.23 |
| Quercetin 3-β-D-glucoside | 463 | 25.48 | A = 47,325.40 × C − 8096.301 | 0.9992 | 1–100 | 0.23 | 0.38 |
| Rutin | 609 | 24.81 | A = 35,613.21 × C − 747.2902 | 0.9995 | 1–100 | 0.11 | 0.30 |
| (+)-Catechin | 289 | 15.65 | A = 19,405.45 × C − 6469.358 | 0.9999 | 1–100 | 0.35 | 0.38 |
| Ellagic acid | 301 | 25.52 | A = 58,850.16 × C – 16,277.28 | 0.9993 | 1–100 | 0.33 | 0.45 |
| IRLIR, 20% Ethanol | Polyphenolic Compounds, µg g−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Laser Wavelength, nm | Laser Power, mW | Extraction Time, min. | (+)-Catechin | Rutin | Quercitrin | Quercetin | Quercetin 3-β-D-Glucoside | Gallic Acid | Ellagic Acid | Chlorogenic Acid |
| 1064 | 150 | 5 | 126.13 ± 1.23 | 6.30 ± 0.07 | 25.38 ± 0.28 | 8.52 ± 0.08 | 57.51 ± 0.45 | 107.61 ± 0.95 | 24.83 ± 0.18 | 19.17 ± 0.15 |
| 15 | 173.37 ± 1.68 | 6.87 ± 0.06 | 35.02 ± 0.31 | 8.98 ± 0.09 | 76.69 ± 0.62 | 131.66 ± 1.19 | 35.36 ± 0.24 | 23.07 ± 0.19 | ||
| 300 | 5 | 150.7 ± 1.48 | 6.09 ± 0.05 | 31.14 ± 0.40 | 9.19 ± 0.09 | 62.37 ± 0.61 | 104.54 ± 0.87 | 28.85 ± 0.23 | 24.4 ± 0.21 | |
| 15 | 104.85 ± 0.82 | 6.05 ± 0.04 | 25.75 ± 0.32 | 10.64 ± 0.11 | 50.83 ± 0.48 | 98.59 ± 0.69 | 30.02 ± 0.29 | 18.21 ± 0.15 | ||
| 1270 | 150 | 5 | 135.78 ± 1.62 | 6.48 ± 0.06 | 27.36 ± 0.22 | 9.83 ± 0.09 | 63.49 ± 0.54 | 123.13 ± 1.09 | 26.67 ± 0.21 | 20.98 ± 0.18 |
| 15 | 138.79 ± 1.37 | 6.03 ± 0.04 | 30.93 ± 0.26 | 9.61 ± 0.08 | 68.47 ± 0.61 | 123.65 ± 1.12 | 34.22 ± 0.30 | 19.95 ± 0.17 | ||
| 300 | 5 | 138.37 ± 1.05 | 5.54 ± 0.04 | 27.68 ± 0.24 | 9.96 ± 0.09 | 55.28 ± 0.52 | 101.71 ± 0.85 | 29.25 ± 0.27 | 18.87 ± 0.16 | |
| 15 | 110.58 ± 0.85 | 6.22 ± 0.08 | 27.67 ± 0.23 | 10.97 ± 0.12 | 53.29 ± 0.48 | 113.30 ± 0.96 | 27.16 ± 0.26 | 19.64 ± 0.19 | ||
| 1550 | 150 | 5 | 142.34 ± 1.22 | 6.49 ± 0.04 | 27.88 ± 0.29 | 10.23 ± 0.10 | 61.04 ± 0.56 | 120.16 ± 1.14 | 28.95 ± 0.29 | 20.53 ± 0.20 |
| 15 | 139.11 ± 1.57 | 6.07 ± 0.09 | 28.66 ± 0.27 | 8.68 ± 0.08 | 61.23 ± 0.57 | 112.88 ± 1.07 | 32.95 ± 0.29 | 18.28 ± 0.21 | ||
| 300 | 5 | 115.07 ± 0.87 | 6.06 ± 0.04 | 29.77 ± 0.30 | 10.47 ± 0.11 | 58.33 ± 0.55 | 101.82 ± 1.00 | 34.28 ± 0.31 | 18.76 ± 0.18 | |
| 15 | 137.96 ± 1.33 | 5.81 ± 0.04 | 27.28 ± 0.25 | 12.83 ± 0.13 | 58.50 ± 0.52 | 112.97 ± 0.93 | 34.17 ± 0.32 | 17.29 ± 0.16 | ||
| IRLIR, 80% Ethanol | Polyphenolic Compounds, µg g−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Laser Wavelength, nm | Laser Power, mW | Extraction Time, min. | (+)-Catechin | Rutin | Quercitrin | Quercetin | Quercetin 3-β-D-Glucoside | Gallic Acid | Ellagic Acid | Chlorogenic Acid |
| 1064 | 150 | 5 | 44.00 ± 0.42 | 2.71 ± 0.03 | 47.33 ± 0.43 | 15.38 ± 0.15 | 89.06 ± 0.82 | <LoD | 16.72 ± 1.12 | 11.64 ± 0.10 |
| 15 | 47.13 ± 0.49 | 2.65 ± 0.03 | 40.37 ± 0.39 | 14.59 ± 0.13 | 77.68 ± 0.79 | <LoD | 104.42 ± 1.03 | 9.00 ± 0.08 | ||
| 300 | 5 | 8.02 ± 0.08 | 2.62 ± 0.02 | 38.41 ± 0.35 | 15.36 ± 0.15 | 54.89 ± 0.52 | 8.17 ± 0.07 | 107.66 ± 1.02 | 16.93 ± 0.14 | |
| 15 | 7.65 ± 0.07 | 2.68 ± 0.04 | 41.42 ± 0.40 | 16.44 ± 0.17 | 59.29 ± 0.57 | 8.91 ± 0.08 | 118.92 ± 1.12 | 18.38 ± 0.17 | ||
| 1270 | 150 | 5 | 42.72 ± 0.41 | 2.55 ± 0.02 | 36.42 ± 0.34 | 13.94 ± 0.12 | 71.35 ± 0.69 | <LoD | 99.40 ± 0.95 | 7.87 ± 0.06 |
| 15 | 37.88 ± 0.39 | 2.54 ± 0.02 | 39.02 ± 0.38 | 14.20 ± 0.14 | 71.54 ± 0.71 | <LoD | 102.65 ± 0.99 | 8.07 ± 0.07 | ||
| 300 | 5 | 7.57 ± 0.07 | 2.65 ± 0.02 | 41.43 ± 0.41 | 16.06 ± 0.15 | 57.88 ± 0.57 | 8.68 ± 0.07 | 113.49 ± 1.05 | 18.05 ± 0.18 | |
| 15 | 6.69 ± 0.06 | 3.14 ± 0.03 | 40.31 ± 0.41 | 15.98 ± 0.16 | 55.74 ± 0.52 | 8.57 ± 0.07 | 112.56 ± 1.04 | 17.92 ± 0.16 | ||
| 1550 | 150 | 5 | 37.72 ± 0.35 | 2.39 ± 0.02 | 35.88 ± 0.36 | 13.75 ± 0.11 | 64.49 ± 0.68 | <LoD | 95.88 ± 0.93 | 7.62 ± 0.06 |
| 15 | 38.38 ± 0.41 | 2.62 ± 0.03 | 37.03 ± 0.38 | 15.40 ± 0.13 | 63.83 ± 0.62 | 4.12 ± 0.04 | 104.42 ± 1.01 | 11.03 ± 0.13 | ||
| 300 | 5 | 13.04 ± 0.12 | 2.62 ± 0.02 | 34.36 ± 0.32 | 15.69 ± 0.15 | 42.98 ± 0.40 | 10.63 ± 0.09 | 92.46 ± 0.89 | 16.37 ± 0.15 | |
| 15 | 17.63 ± 0.15 | 4.28 ± 0.04 | 43.21 ± 0.42 | 16.12 ± 0.16 | 60.55 ± 0.58 | 14.43 ± 0.13 | 97.72 ± 0.92 | 15.66 ± 0.14 | ||
| Extraction Solvent | Polyphenolic Compounds, mg g−1 | |||||||
|---|---|---|---|---|---|---|---|---|
| (+)-Catechin | Rutin | Quercitrin | Quercetin | Quercetin 3-β-D-Glucoside | Gallic Acid | Ellagic Acid | Chlorogenic Acid | |
| 20% ethanol | 99.50 ± 5.59 | 23.00 ± 1.78 | 110.00 ± 2.07 | 31.16 ± 2.01 | 205.68 ± 7.09 | 9.52 ± 0.39 | 266.87 ± 14.81 | 25.11 ± 0.18 |
| 80% ethanol | 60.44 ± 6.58 | 11.14 ± 0.42 | 63.75 ± 4.14 | 27.56 ± 0.63 | 70.20 ± 5.05 | <LoD | 160.54 ± 10.08 | 15.25 ± 0.75 |
| Compound | tR, min. | Linear Regression Equations | R | Linearity Range of Response, µg mL−1 | LoD, µg mL−1 | LoQ, µg mL−1 |
|---|---|---|---|---|---|---|
| α-tocoferol | 4.91 | A =13,185 × C − 7529 | 0.9976 | 5–115 | 2.20 | 3.25 |
| β-caroten | 6.44 | A = 5313 × C − 27752 | 0.9983 | 45–275 | 13.10 | 28.20 |
| IRLIR | Lipophilic Compounds, µg g−1 | |||||
|---|---|---|---|---|---|---|
| Laser Wavelength, nm | Laser Power, mW | Extraction Time, min. | α-Tocopherol | β-Carotene | Lycopene | Lutein |
| 1064 | 150 | 5 | 251.04 | 1595.79 | 1627.08 | 414.56 |
| 15 | 283.23 | 1804.23 | 1892.33 | 450.09 | ||
| 300 | 5 | 258.36 | 1708.86 | 1852.18 | 429.94 | |
| 15 | 255.70 | 1716.57 | 1808.09 | 436.92 | ||
| Extract Solvent | Laser Wavelength, nm | Laser Power, mW | Extraction Time, min. | TEAC, µmol g−1 |
|---|---|---|---|---|
| 20% ethanol | 1064 | 150 | 5 | 23,456 ± 55 |
| 15 | 22,672 ± 48 | |||
| 300 | 5 | 17,492 ± 384 | ||
| 15 | 21,825 ± 154 | |||
| 1270 | 150 | 5 | 23,488 ± 48 | |
| 15 | 22,289 ± 315 | |||
| 300 | 5 | 20,114 ± 138 | ||
| 15 | 21,441 ± 154 | |||
| 1550 | 150 | 5 | 22,401 ± 182 | |
| 15 | 23,619 ± 305 | |||
| 300 | 5 | 20,786 ± 499 | ||
| 15 | 22,193 ± 584 | |||
| 80% ethanol | 1064 | 150 | 5 | 5238 ± 44 |
| 15 | 3330 ± 194 | |||
| 300 | 5 | 3791 ± 75 | ||
| 15 | 4331 ± 82 | |||
| 1270 | 150 | 5 | 4650 ± 168 | |
| 15 | 3370 ± 52 | |||
| 300 | 5 | 3863 ± 52 | ||
| 15 | 4225 ± 50 | |||
| 1550 | 150 | 5 | 4656 ± 102 | |
| 15 | 3462 ± 99 | |||
| 300 | 5 | 4100 ± 142 | ||
| 15 | 3988 ± 97 |
| Laser Wavelength, nm | Laser Power, mW | Extraction Time, min. | TEAC, µmol g−1 |
|---|---|---|---|
| 1064 | 150 | 5 | 31,701 ± 63 |
| 15 | 117,260 ± 235 | ||
| 300 | 5 | 68,872 ± 138 | |
| 15 | 33,398 ± 67 | ||
| 1270 | 150 | 5 | 9855 ± 20 |
| 15 | 100,944 ± 202 | ||
| 300 | 5 | 55,465 ± 111 | |
| 15 | 11,828 ± 24 | ||
| 1550 | 150 | 5 | 22,117 ± 44 |
| 15 | 53,392 ± 107 | ||
| 300 | 5 | 33,722 ± 67 | |
| 15 | 32,619 ± 65 |
| Extract Solvent | TEAC (ABTS), µmol g−1 | TEAC (ORAC), µmol g−1 |
|---|---|---|
| 20% | 14,699 ± 282 | 19,707 ± 158 |
| 80% | 1299.3 ± 5.7 | 10,058 ± 57 |
| Laser Wavelength, nm | Laser Power, mW | Extraction Time, min. | AC, µmol g−1 |
|---|---|---|---|
| 1064 | 150 | 5 | 6881 ± 33 |
| 15 | 8475 ± 35 | ||
| 300 | 5 | 6202 ± 28 | |
| 15 min | 6353 ± 18 |
| Parameters | Characteristics | ||
|---|---|---|---|
| Wavelength, nm | 1064 ± 1 | 1270 ± 10 | 1550 ± 20 |
| Operating mode | Continuous | Continuous | Continuous |
| Output power before fiber, W | 1.637 | 1.275 | 1.246 |
| Output power after fiber, W | 1.511 | 1.100 | 1.100 |
| Power control, % | 0–100 | 0–100 | 0–100 |
| Power stability, % | <3 | <3 | <3 |
| Warm-up time, min. | <10 | <5 | <5 |
| Fiber connecter | SMA905 | SMA905 | SMA905 |
| Fiber, um@1 m | 400 | 400 | 400 |
| Beam dimension, mm | ~5 × 8 | ~5 × 8 | ~5 × 8 |
| Beam divergence, mrad | <1.5 | <3.0 | <3.0 |
| Beam diameter at 1/e2, mm | ~1.5 | ~1.5 | ~1.5 |
| Spot diameter after adding end collimator @10 cm, mm | ~35 | ~35 | ~35 |
| Beam high from base plate, mm | 24.8 | 29 | 24.8 |
| Operating temperature, °С | 10~35 | 10~35 | 10~35 |
| Wavelength, nm | Current, A/Power, mW | Energy Consumption, kW |
|---|---|---|
| 1064 | 0.71/150 | 0.020–0.030 |
| 0.97/300 | ||
| 1270 | 0.9/150 | 0.029–0.030 |
| 1.22/300 | ||
| 1550 | 0.80/150 | 0.037–0.038 |
| 1.22/300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alecu, A.; Albu, C.; Badea, G.-I.; Alionte, A.; Enache, A.-A.; Radu, G.-L.; Litescu, S.-C. Infrared Laser-Assisted Extraction of Bioactive Compounds from Rosa canina L. Int. J. Mol. Sci. 2025, 26, 992. https://doi.org/10.3390/ijms26030992
Alecu A, Albu C, Badea G-I, Alionte A, Enache A-A, Radu G-L, Litescu S-C. Infrared Laser-Assisted Extraction of Bioactive Compounds from Rosa canina L. International Journal of Molecular Sciences. 2025; 26(3):992. https://doi.org/10.3390/ijms26030992
Chicago/Turabian StyleAlecu, Andreia, Camelia Albu, Georgiana-Ileana Badea, Aurelia Alionte, Alin-Alexandru Enache, Gabriel-Lucian Radu, and Simona-Carmen Litescu. 2025. "Infrared Laser-Assisted Extraction of Bioactive Compounds from Rosa canina L." International Journal of Molecular Sciences 26, no. 3: 992. https://doi.org/10.3390/ijms26030992
APA StyleAlecu, A., Albu, C., Badea, G.-I., Alionte, A., Enache, A.-A., Radu, G.-L., & Litescu, S.-C. (2025). Infrared Laser-Assisted Extraction of Bioactive Compounds from Rosa canina L. International Journal of Molecular Sciences, 26(3), 992. https://doi.org/10.3390/ijms26030992







