Targeting Ferroptosis/Nrf2 Pathway Ameliorates AlCl3-Induced Alzheimer’s Disease in Rats: Neuroprotective Effect of Morin Hydrate, Zeolite Clinoptilolite, and Physical Plus Mental Activities
Abstract
1. Introduction
2. Results
2.1. Treatment with MH and/or ZC Combined with PhM Improved the AlCl3-Induced Altered Behavioral, Learning, and Memory Parameters
2.1.1. Y-Maze Spontaneous Alternation Test
2.1.2. Morris Water Maze Test (MWM)
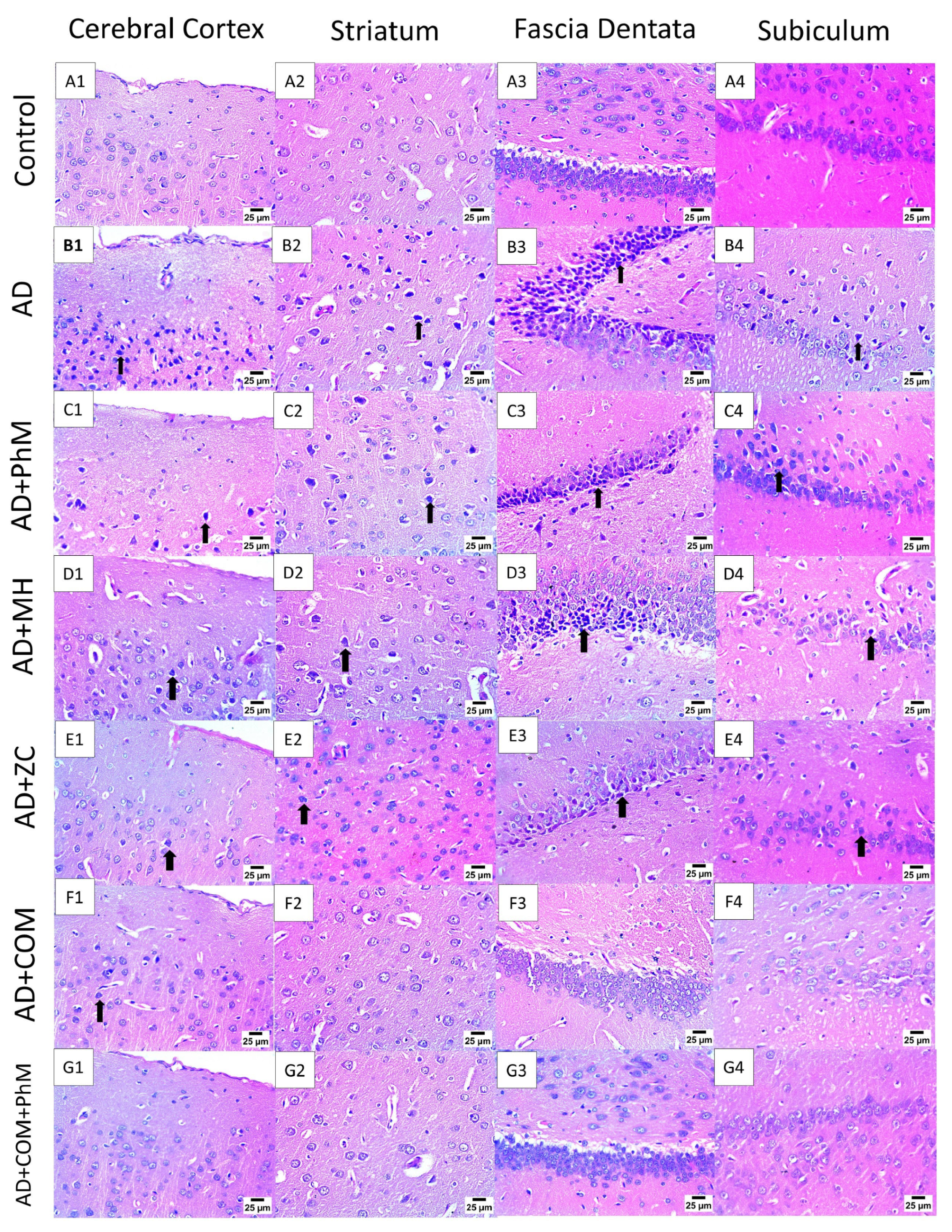
2.2. Treatment with MH and/or ZC Combined with PhM Counteracted the AlCl3-Induced Histopathological Changes in Brain Tissues
2.3. MH and/or ZC Combined with PhM Improved the AlCl3-Induced Changes in Neurotransmitter Brain Levels (DA, 5-HT, NE, and ACHE)
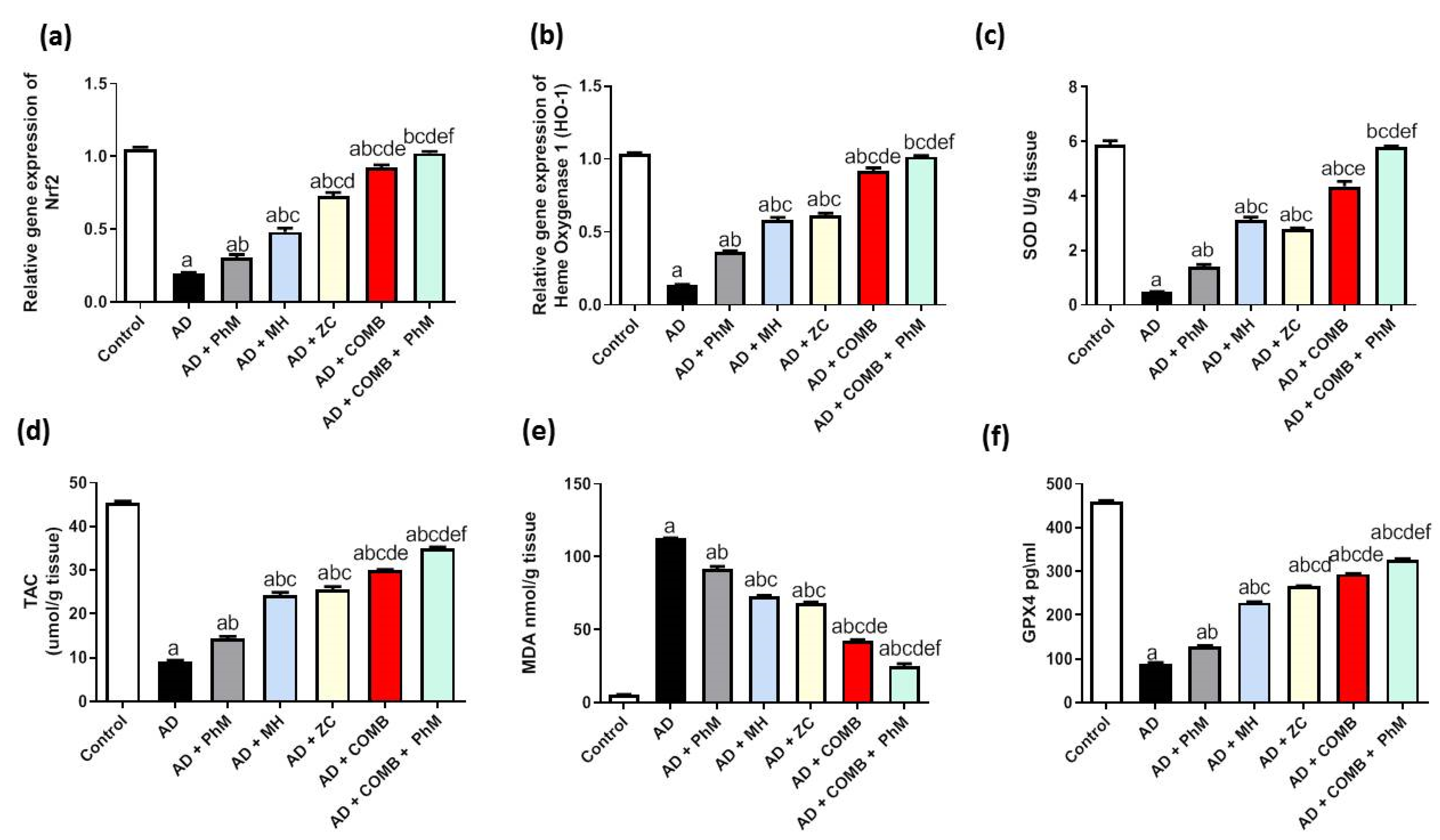
2.4. Treatment with MH and/or ZC Combined with PhM Ameliorated the AlCl3-Induced Induction of Oxidative Stress Biomarkers and Nrf2/HO-1 Signaling Pathway
2.4.1. The Impact of MH and/or ZC Therapy Combined with PhM on the AlCl3-Induced Altered Nrf2/HO-1 Signaling Pathway
2.4.2. The Impacts of MH and/or ZC Therapy Combined with PhM on the AlCl3-Induced Alterations in SOD, TAC, and MDA and GPX4 Levels
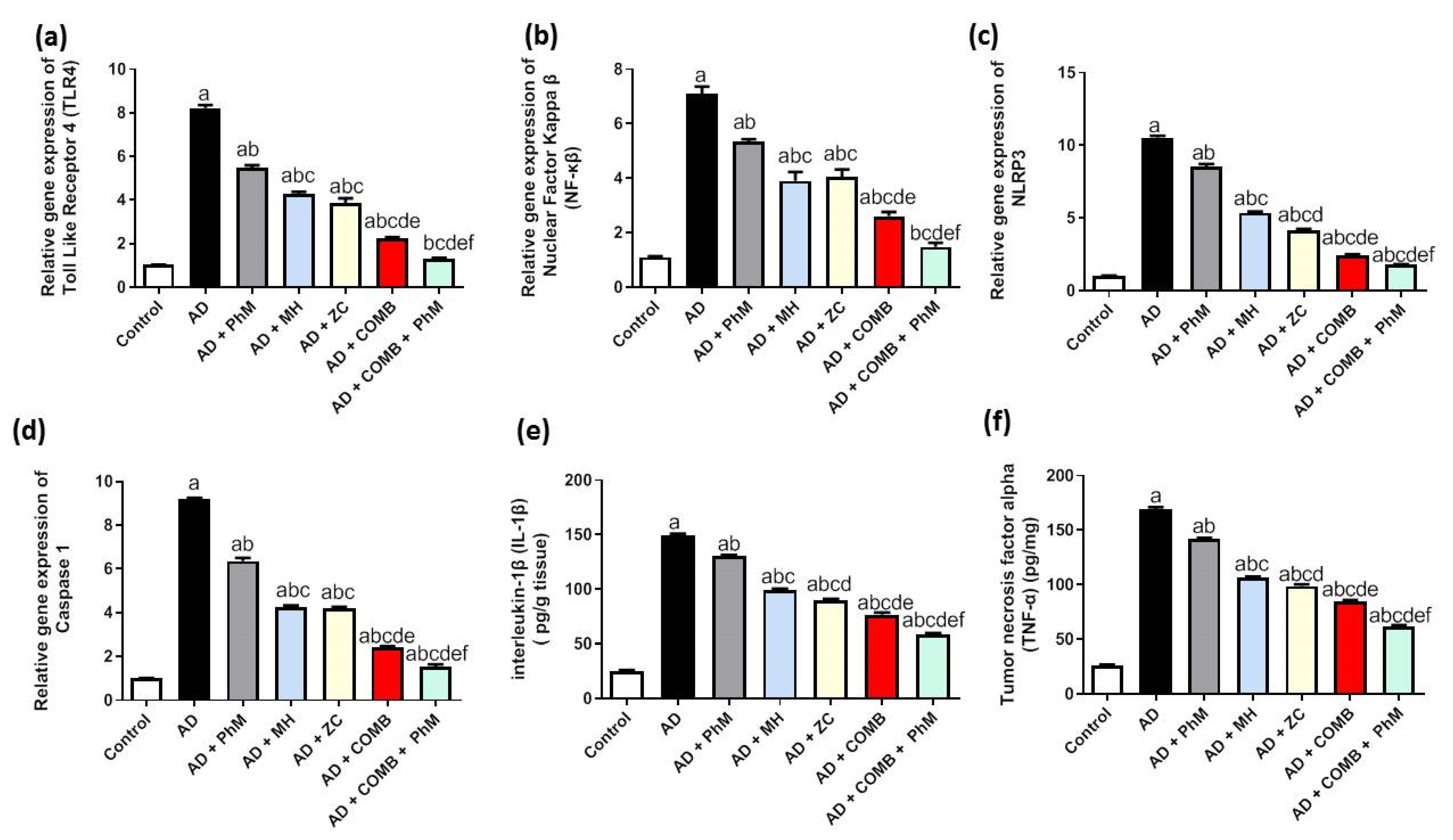
2.5. Treatment with MH and/or ZC Combined with PhM Counteracted the AlCl3-Induced Inductions in Inflammatory Pathways
2.5.1. The Impact of MH and/or ZC Therapy Combined with PhM on TLR4/NF-κB/NLRP3/caspase-1 Signaling
2.5.2. The Impact of PhM Treatment Combined with MH and ZC Therapy on Pro-Inflammatory Cytokines, IL-1β and TNF-α
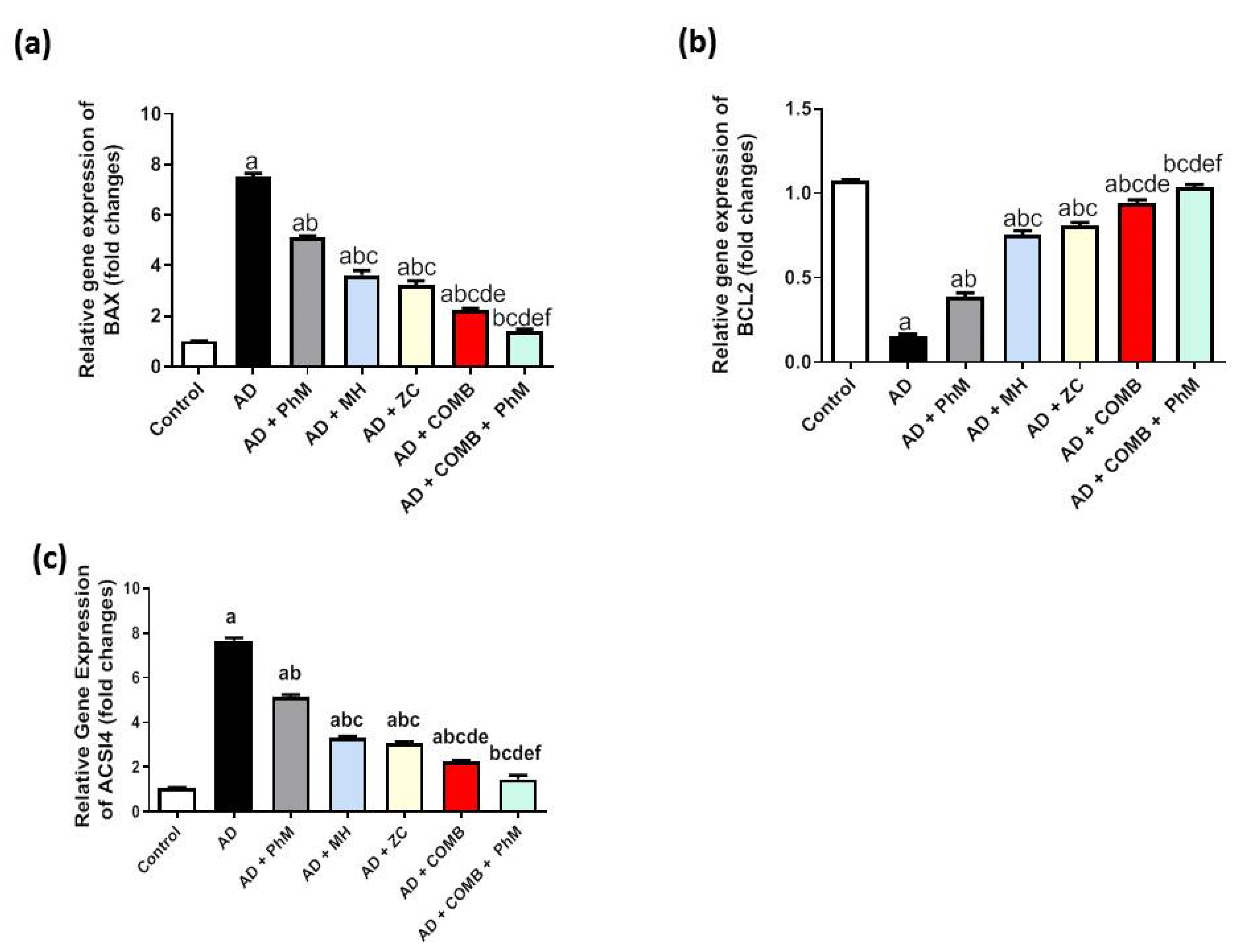
2.6. MH and/or ZC Therapy Combined with PhM Improved the AlCl3-Induced Induction of Apoptosis Biomarkers, Bax and Bcl-2
2.7. Modulatory Effect of MH and/or ZC Combined with PhM on the Ferroptosis Marker ACSL4
2.8. Treatment with MH and/or ZC Combined with PhM Improved the AlCl3-Induced Changes in BDNF and the Wnt 3/β-catenin/GSK-3β Signaling Pathway
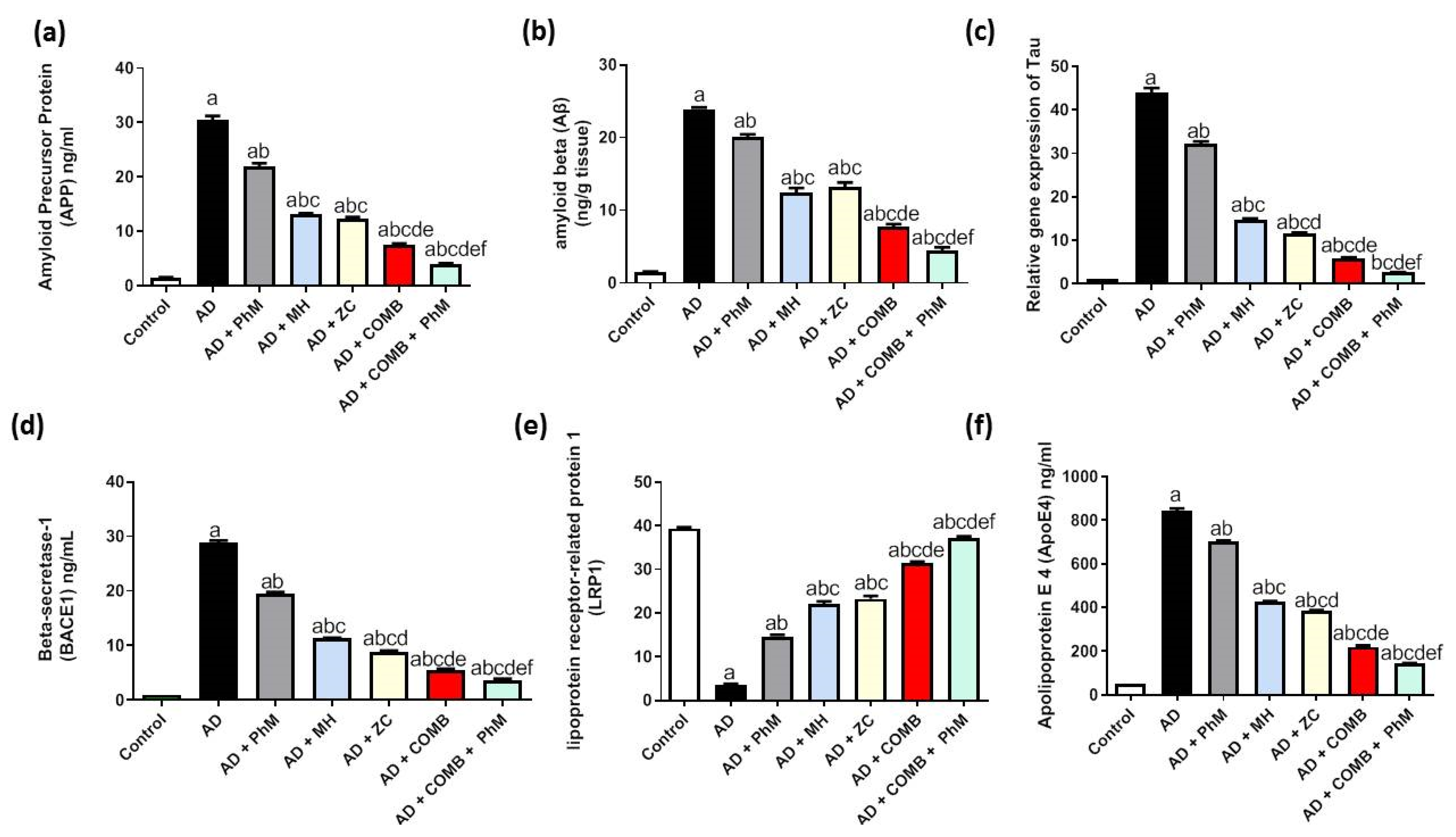
2.9. Treatment with MH and/or ZC Combined with PhM Improved the AD Progression Markers APP, Aβ, Tau, and BACE1
2.10. Modulatory Effect of MH and/or ZC Combined with PhM on the APOE4/LRP1 Signaling Pathway
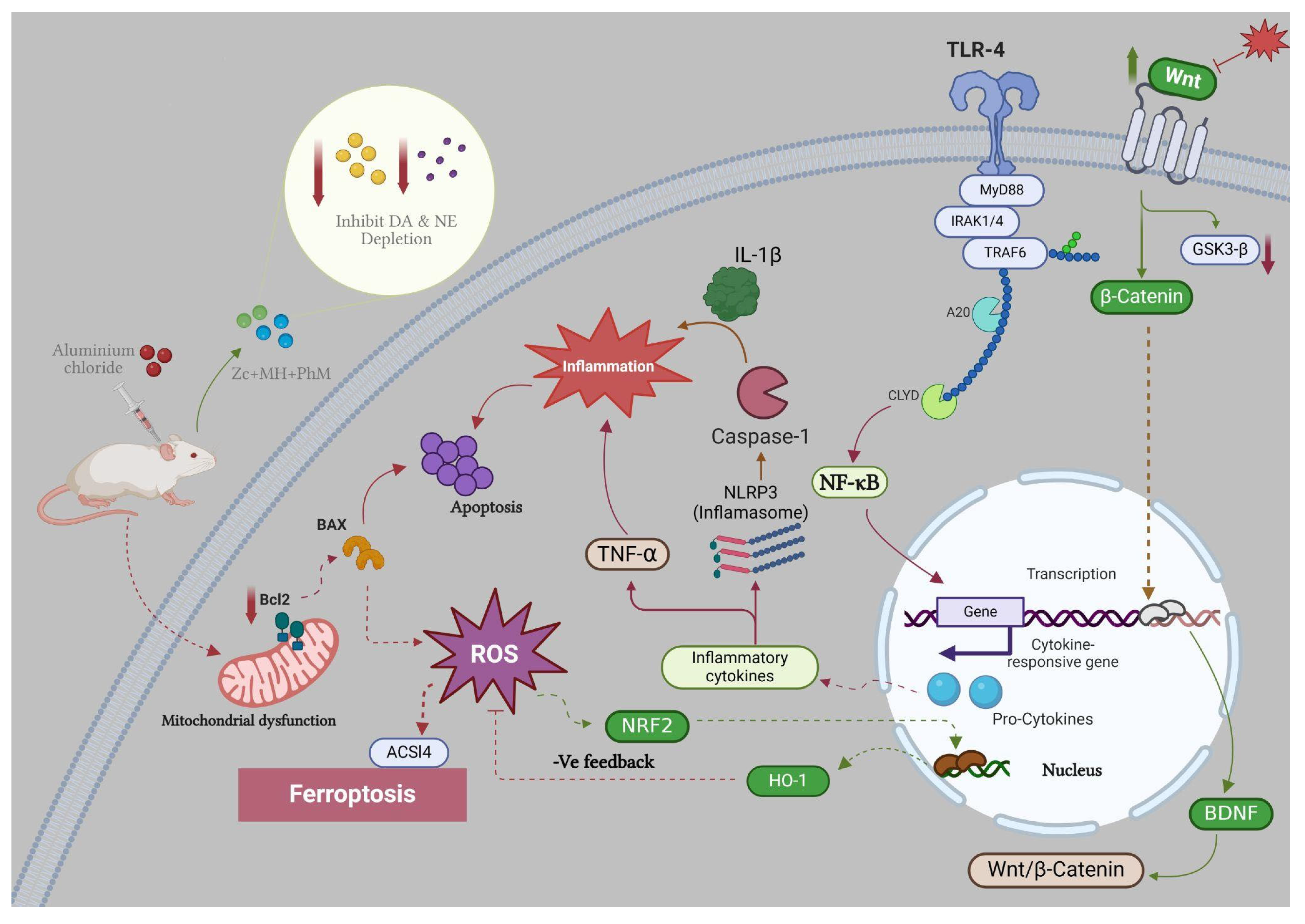
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ethical Statement
4.3. Drugs and Chemicals
4.4. Experimental Design
4.5. Physical and Mental Activities
4.6. Behavioral Tests for Evaluating the Extent of Neurodegeneration
4.7. Tissue Sampling
4.8. Histopathological Study
4.9. Immunohistochemical Study
4.10. Biochemical Analysis
4.10.1. Colorimetric Calculation of Oxidative Stress Markers
4.10.2. Fluorometric Determination of Brain Monoamines
4.10.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10.4. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD; | Alzheimer’s disease |
| AlCl3 | Aluminum chloride |
| MH | Morin hydrate |
| ZC | Zeolite clinoptilolite |
| PhM | Physical and mental activities |
| Aβ | Amyloid beta-peptide |
| BDNF | Brain derived neurotrophic factor |
| ACHE | Acetylcholinesterase |
| GSH | Reduced glutathione |
| HO-1 | Heme oxygenase-1 |
| Bax | B-cell lymphoma protein 2 (Bcl-2)-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| DA | Dopamine |
| TNF-α | Tumor necrosis factor-α |
| ELISA | Enzyme-linked immunosorbent assay |
| GSK-3β | Glycogen synthase kinase-3β |
| H&E | Haematoxylin and eosin |
| NLRP3, NOD-, LRR- | Pyrin domain-containing protein 3 |
| IL-1β | Interleukin-1β |
| NE | Norepinephrine |
| NF-κB | Nuclear factor-κappa B |
| Nrf2 | Nuclear factor erythroid 2 related factor 2 |
| Wnt3a | Wingless type MMTV integration site family, member 3a |
References
- Dregni, A.J.; Duan, P.; Xu, H.; Changolkar, L.; El Mammeri, N.; Lee, V.M.-Y.; Hong, M. Fluent molecular mixing of Tau isoforms in Alzheimer’s disease neurofibrillary tangles. Nat. Commun. 2022, 13, 2967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L. Atypical clinical variants of Alzheimer’s disease: Are they really atypical? Front. Neurosci. 2024, 18, 1352822. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef]
- Abbas, F.; Eladl, M.A.; El-Sherbiny, M.; Abozied, N.; Nabil, A.; Mahmoud, S.M.; Mokhtar, H.I.; Zaitone, S.A.; Ibrahim, D. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomed. Pharmacother. 2022, 151, 113072. [Google Scholar] [CrossRef]
- Mehrbeheshti, N.; Esmaili, Z.; Ahmadi, M.; Moosavi, M. A dose response effect of oral aluminum nanoparticle on novel object recognition memory, hippocampal caspase-3 and MAPKs signaling in mice. Behav. Brain Res. 2022, 417, 113615. [Google Scholar] [CrossRef]
- Sanajou, S.; Erkekoğlu, P.; Şahin, G.; Baydar, T. Role of aluminum exposure on Alzheimer’s disease and related glycogen synthase kinase pathway. Drug Chem. Toxicol. 2023, 46, 510–522. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum environmental pollution: The silent killer. Environ. Sci. Pollut. Res. Int. 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Klotz, K.; Weistenhöfer, W.; Neff, F.; Hartwig, A.; van Thriel, C.; Drexler, H. The Health Effects of Aluminum Exposure. Dtsch. Arztebl. Int. 2017, 114, 653–659. [Google Scholar] [CrossRef]
- ELBini-Dhouib, I.; Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease. Molecules 2021, 26, 3011. [Google Scholar] [CrossRef]
- Chen, S.X.; Xiang, J.Y.; Han, J.X.; Li, H.Z.; Chen, H.; Xu, M. Essential Oils from Spices Inhibit Cholinesterase Activity and Improve Behavioral Disorder in AlCl 3 Induced Dementia. Chem. Biodivers. 2022, 19, e202100443. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.M.E.; Alharthi, F.H.J.; Alanazi, A.H.; El-Emam, S.Z.; Zaghlool, S.S.; Metwally, K.; Albalawi, S.A.; Abdu, Y.S.; Mansour, R.E.-S.; Salem, H.A.; et al. Neuroprotective Effects of Phytochemicals against Aluminum Chloride-Induced Alzheimer’s Disease through ApoE4/LRP1, Wnt3/β-Catenin/GSK3β, and TLR4/NLRP3 Pathways with Physical and Mental Activities in a Rat Model. Pharmaceuticals 2022, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- De Plano, L.M.; Calabrese, G.; Rizzo, M.G.; Oddo, S.; Caccamo, A. The Role of the Transcription Factor Nrf2 in Alzheimer’s Disease: Therapeutic Opportunities. Biomolecules 2023, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Abu-Elfotuh, K.; Abdel-Sattar, S.A.; Abbas, A.N.; Mahran, Y.F.; Alshanwani, A.R.; Hamdan, A.M.E.; Atwa, A.M.; Reda, E.; Ahmed, Y.M.; Zaghlool, S.S.; et al. The protective effect of thymoquinone or/and thymol against monosodium glutamate-induced attention-deficit/hyperactivity disorder (ADHD)-like behavior in rats: Modulation of Nrf2/HO-1, TLR4/NF-κB/NLRP3/caspase-1 and Wnt/β-Catenin signaling pathways in rat. Biomed. Pharmacother. 2022, 155, 113799. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Li, J.; Song, Y.; Luo, C. ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries. Int. J. Mol. Sci. 2023, 24, 10021. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Lara-Reyna, S.; Caseley, E.A.; Topping, J.; Rodrigues, F.; Jimenez Macias, J.; Lawler, S.E.; McDermott, M.F. Inflammasome activation: From molecular mechanisms to autoinflammation. Clin. Transl. Immunol. 2022, 11, e1404. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Jha, D.; Bakker, E.N.T.P.; Kumar, R. Mechanistic and therapeutic role of NLRP3 inflammasome in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2023, 168, 3574–3598. [Google Scholar] [CrossRef]
- Yang, J.; Wise, L.; Fukuchi, K.-I. TLR4 Cross-Talk with NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Kostes, W.W.; Brafman, D.A. The Multifaceted Role of WNT Signaling in Alzheimer’s Disease Onset and Age-Related Progression. Cells 2023, 12, 1204. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Palomer, E.; Buechler, J.; Salinas, P.C. Wnt Signaling Deregulation in the Aging and Alzheimer’s Brain. Front. Cell. Neurosci. 2019, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Hussein, F.H.; Abbas, A.N.; Al-Rekabi, M.D.; Barghash, S.S.; Zaghlool, S.S.; El-Emam, S.Z. Melatonin and zinc supplements with physical and mental activities subside neurodegeneration and hepatorenal injury induced by aluminum chloride in rats: Inclusion of GSK-3β-Wnt/β-catenin signaling pathway. Neurotoxicology 2022, 91, 69–83. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Pires, M.; Rego, A.C. Apoe4 and Alzheimer’s Disease Pathogenesis-Mitochondrial Deregulation and Targeted Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 778. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Wang, Z.; Huang, H.-C. Roles of ApoE4 on the Pathogenesis in Alzheimer’s Disease and the Potential Therapeutic Approaches. Cell. Mol. Neurobiol. 2023, 43, 3115–3136. [Google Scholar] [CrossRef]
- Na, H.; Yang, J.B.; Zhang, Z.; Gan, Q.; Tian, H.; Rajab, I.M.; Potempa, L.A.; Tao, Q.; Qiu, W.Q. Peripheral apolipoprotein E proteins and their binding to LRP1 antagonize Alzheimer’s disease pathogenesis in the brain during peripheral chronic inflammation. Neurobiol. Aging 2023, 127, 54–69. [Google Scholar] [CrossRef]
- Koutsodendris, N.; Blumenfeld, J.; Agrawal, A.; Traglia, M.; Grone, B.; Zilberter, M.; Yip, O.; Rao, A.; Nelson, M.R.; Hao, Y.; et al. Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nat. Aging 2023, 3, 275–296. [Google Scholar] [CrossRef]
- Shinohara, M.; Tachibana, M.; Kanekiyo, T.; Bu, G. Role of LRP1 in the pathogenesis of Alzheimer’s disease: Evidence from clinical and preclinical studies. J. Lipid Res. 2017, 58, 1267–1281. [Google Scholar] [CrossRef]
- Spencer, J.P.E. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.; Lee, S.; Kim, J.; Yang, S.; Lee, M.; Ahn, J.; Lee, H.; Chang, S.-C.; Ha, N.-C.; Lee, J. Anti-Inflammatory and Neuroprotective Effects of Morin in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2022, 23, 10578. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.-Q.; Yan, H.-C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qu, J.; Zhang, W.; Bai, M.; Zhou, Q.; Zhang, Z.; Li, Z.; Miao, J. Morin reverses neuropathological and cognitive impairments in APPswe/PS1dE9 mice by targeting multiple pathogenic mechanisms. Neuropharmacology 2016, 108, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.-G.; Chung, H.Y.; Ha, N.-C.; Mattson, M.P.; Lee, J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β. Neurobiol. Dis. 2011, 44, 223–230. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Hajipour, M.J.; Barzegari, E.; Lotfabadi, A.; Ferdousi, M.; Saboury, A.A.; Ng, E.P.; Raoufi, M.; Awala, H.; Mintova, S.; et al. Zeolite Nanoparticles Inhibit Aβ-Fibrinogen Interaction and Formation of a Consequent Abnormal Structural Clot. ACS Appl. Mater. Interfaces 2016, 8, 30768–30779. [Google Scholar] [CrossRef]
- Lucas, M.J.; Keitz, B.K. Influence of Zeolites on Amyloid-β Aggregation. Langmuir 2018, 34, 9789–9797. [Google Scholar] [CrossRef]
- Panaiotov, S.; Tancheva, L.; Kalfin, R.; Petkova-Kirova, P. Zeolite and Neurodegenerative Diseases. Molecules 2024, 29, 2614. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Jiang, P.; Dang, R.-L.; Li, H.-D.; Zhang, L.-H.; Zhu, W.-Y.; Xue, Y.; Tang, M.-M. The Impacts of Swimming Exercise on Hippocampal Expression of Neurotrophic Factors in Rats Exposed to Chronic Unpredictable Mild Stress. Evid.-Based Complement. Altern. Med. 2014, 2014, 729827. [Google Scholar] [CrossRef] [PubMed]
- Serra, L.; Petrosini, L.; Mandolesi, L.; Bonarota, S.; Balsamo, F.; Bozzali, M.; Caltagirone, C.; Gelfo, F. Walking, Running, Swimming: An Analysis of the Effects of Land and Water Aerobic Exercises on Cognitive Functions and Neural Substrates. Int. J. Environ. Res. Public Health 2022, 19, 16310. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Deibel, S.H.; Karem, H.; Hong, N.S.; Hossain, S.R.; Lacoursiere, S.G.; Sutherland, R.J.; Mohajerani, M.H.; McDonald, R.J. Repeated multi-domain cognitive training prevents cognitive decline, anxiety and amyloid pathology found in a mouse model of Alzheimer disease. Commun. Biol. 2023, 6, 1145. [Google Scholar] [CrossRef] [PubMed]
- Billings, L.M.; Green, K.N.; McGaugh, J.L.; LaFerla, F.M. Learning decreases A beta*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J. Neurosci. 2007, 27, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Coria, H.; Yeung, S.T.; Ager, R.R.; Rodriguez-Ortiz, C.J.; Baglietto-Vargas, D.; LaFerla, F.M. Repeated cognitive stimulation alleviates memory impairments in an Alzheimer’s disease mouse model. Brain Res. Bull. 2015, 117, 10–15. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Y.; Gui, Y. Neuronal ApoE4 in Alzheimer’s disease and potential therapeutic targets. Front. Aging Neurosci. 2023, 15, 1199434. [Google Scholar] [CrossRef]
- Shunan, D.; Yu, M.; Guan, H.; Zhou, Y. Neuroprotective effect of Betalain against AlCl3-induced Alzheimer’s disease in Sprague Dawley Rats via putative modulation of oxidative stress and nuclear factor kappa B (NF-κB) signaling pathway. Biomed. Pharmacother. 2021, 137, 111369. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Jiang, Y.; Gluhcheva, Y.G.; Tizabi, Y.; Lobinski, R.; Tinkov, A.A. Molecular mechanisms of aluminum neurotoxicity: Update on adverse effects and therapeutic strategies. Adv. Neurotoxicology 2021, 5, 1–34. [Google Scholar] [CrossRef]
- Pan, B.; Lu, X.; Han, X.; Huan, J.; Gao, D.; Cui, S.; Ju, X.; Zhang, Y.; Xu, S.; Song, J.; et al. Mechanism by Which Aluminum Regulates the Abnormal Phosphorylation of the Tau Protein in Different Cell Lines. ACS Omega 2021, 6, 31782–31796. [Google Scholar] [CrossRef]
- Promyo, K.; Iqbal, F.; Chaidee, N.; Chetsawang, B. Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem. Toxicol. 2020, 146, 111829. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, J.; Zhao, Y.; Lu, X.; Zhang, Q.; Niu, Q. Effects of aluminium on β-amyloid (1–42) and secretases (APP-cleaving enzymes) in rat brain. Neurochem. Res. 2014, 39, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-J.; Hu, Y.-Y.; Yang, Z.-J.; Jiang, L.-P.; Shi, S.-L.; Li, Y.-R.; Guo, M.-Y.; Wu, H.-L.; Wan, Y.-Y. Amyloid β-42 induces neuronal apoptosis by targeting mitochondria. Mol. Med. Rep. 2017, 16, 4521–4528. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, H.; Nishitoh, H.; Urano, F.; Sadamitsu, C.; Matsuzawa, A.; Takeda, K.; Masutani, H.; Yodoi, J.; Urano, Y.; Nagano, T.; et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005, 12, 19–24. [Google Scholar] [CrossRef]
- Takada, E.; Okubo, K.; Yano, Y.; Iida, K.; Someda, M.; Hirasawa, A.; Yonehara, S.; Matsuzaki, K. Molecular Mechanism of Apoptosis by Amyloid β-Protein Fibrils Formed on Neuronal Cells. ACS Chem. Neurosci. 2020, 11, 796–805. [Google Scholar] [CrossRef]
- Yao, M.; Nguyen, T.-V.V.; Pike, C.J. Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J. Neurosci. 2005, 25, 1149–1158. [Google Scholar] [CrossRef]
- Song, X.; Long, D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 267. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, Z.; Zhou, L.; Wang, X.; Hui, Y.; Mao, L.; Fan, X.; Wang, B.; Zhao, X.; Sun, C. Regulating Nrf2-GPx4 axis by bicyclol can prevent ferroptosis in carbon tetrachloride-induced acute liver injury in mice. Cell Death Discov. 2022, 8, 380. [Google Scholar] [CrossRef]
- Magtanong, L.; Mueller, G.D.; Williams, K.J.; Billmann, M.; Chan, K.; Armenta, D.A.; Pope, L.E.; Moffat, J.; Boone, C.; Myers, C.L.; et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem. Biol. 2022, 29, 1409–1418.e6. [Google Scholar] [CrossRef]
- Hong, Y.; Yan, W.; Chen, S.; Sun, C.; Zhang, J. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol. Sin. 2010, 31, 1421–1430. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Mohamed, S.A.; Bakr, R.O.; Ahmed, A.H.; Atwa, A.M.; Hamdan, A.M.; Alanzai, A.G.; Alnahhas, R.K.; Gowifel, A.M.H.; et al. The potential anti-Alzheimer’s activity of Oxalis corniculata Linn. Methanolic extract in experimental rats: Role of APOE4/LRP1, TLR4/NF-κβ/NLRP3, Wnt 3/β-catenin/GSK-3β, autophagy and apoptotic cues. J. Ethnopharmacol. 2024, 324, 117731. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Wang, X.; Xu, C.; Zhang, X.; Zhang, J.; Lin, L.; Niu, Q. miR-128-3p is involved in aluminum-induced cognitive impairment by regulating the Sirt1-Keap1/Nrf2 pathway. Ecotoxicol. Environ. Saf. 2024, 271, 115966. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yan, Y.; Gao, Y.; Zhang, N.; Kumar, G.; Fang, Q.; Li, Z.; Li, J.; Zhang, Y.; Song, L.; et al. Transcriptome analysis of fasudil treatment in the APPswe/PSEN1dE9 transgenic (APP/PS1) mice model of Alzheimer’s disease. Sci. Rep. 2022, 12, 6625. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Bao, W.-D.; Pang, P.; Zhou, X.-T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.-Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Khamchai, S.; Chumboatong, W.; Hata, J.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Morin protects the blood–brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci. Rep. 2020, 10, 13379. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, X.; Xiong, N.; Wang, H.; Huang, J.; Sun, S.; Wang, T. Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo. Acta Pharmacol. Sin. 2010, 31, 900–906. [Google Scholar] [CrossRef]
- Basha, M.P.; Begum, S.; Mir, B.A. Neuroprotective Actions of Clinoptilolite and Ethylenediaminetetraacetic Acid Against Lead-induced Toxicity in Mice Mus musculus. Toxicol. Int. 2013, 20, 201–207. [Google Scholar] [CrossRef]
- Montinaro, M.; Uberti, D.; Maccarinelli, G.; Bonini, S.A.; Ferrari-Toninelli, G.; Memo, M. Dietary zeolite supplementation reduces oxidative damage and plaque generation in the brain of an Alzheimer’s disease mouse model. Life Sci. 2013, 92, 903–910. [Google Scholar] [CrossRef]
- Fisher, E.; Wood, S.J.; Elsworthy, R.J.; Upthegrove, R.; Aldred, S. Exercise as a protective mechanism against the negative effects of oxidative stress in first-episode psychosis: A biomarker-led study. Transl. Psychiatry 2020, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.; da Silva, R.A.; da Luz Scheffer, D.; Penteado, R.; Solano, A.; Barros, L.; Budde, H.; Trostchansky, A.; Latini, A. Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle. Antioxidants 2022, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Steinbauer, K.; Schuetz, B.; Greilberger, J.F.; Leber, B.; Wagner, B.; Zinser, E.; Petek, T.; Wallner-Liebmann, S.; et al. Effects of zeolite supplementation on parameters of intestinal barrier integrity, inflammation, redoxbiology and performance in aerobically trained subjects. J. Int. Soc. Sports Nutr. 2015, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Song, W. NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. Signal Transduct. Target. Ther. 2020, 5, 37. [Google Scholar] [CrossRef]
- Wu, L.; Xian, X.; Xu, G.; Tan, Z.; Dong, F.; Zhang, M.; Zhang, F. Toll-Like Receptor 4: A Promising Therapeutic Target for Alzheimer’s Disease. Mediat. Inflamm. 2022, 2022, 7924199. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Chavali, V.D.; Agarwal, M.; Vyas, V.K.; Saxena, B. Neuroprotective Effects of Ethyl Pyruvate against Aluminum Chloride-Induced Alzheimer’s Disease in Rats via Inhibiting Toll-Like Receptor 4. J. Mol. Neurosci. 2020, 70, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Thawkar, B.S.; Kaur, G. Betanin combined with virgin coconut oil inhibits neuroinflammation in aluminum chloride-induced toxicity in rats by regulating NLRP3 inflammasome. J. Tradit. Complement. Med. 2024, 14, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Q.; Huang, J.; Jin, Q.; Xu, B.; Chen, F.; Tu, C. Morin Hydrate Inhibits TREM-1/TLR4-Mediated Inflammatory Response in Macrophages and Protects Against Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Front. Pharmacol. 2019, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- López-Tenorio, I.I.; Domínguez-López, A.; Miliar-García, Á.; Escalona-Cardoso, G.N.; Real-Sandoval, S.A.; Gómez-Alcalá, A.; Jaramillo-Flores, M.E. Modulation of the mRNA of the Nlrp3 inflammasome by Morin and PUFAs in an obesity model induced by a high-fat diet. Food Res. Int. 2020, 137, 109706. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Z.; Shen, B.; Zhang, Q.; Feng, H. Protective effects of morin on lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting TLR4/NF-κB and activating Nrf2/HO-1 signaling pathways. Int. Immunopharmacol. 2017, 45, 148–155. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Li, Y.; Zhao, G.; Guo, Z.; Luo, Y.; et al. Exercise suppresses NLRP3 inflammasome activation in mice with diet-induced NASH: A plausible role of adropin. Lab. Investig. 2021, 101, 369–380. [Google Scholar] [CrossRef]
- Yapislar, H.; Taskin, E.; Ozdas, S.; Akin, D.; Sonmez, E. Counteraction of Apoptotic and Inflammatory Effects of Adriamycin in the Liver Cell Culture by Clinopitolite. Biol. Trace Elem. Res. 2016, 170, 373–381. [Google Scholar] [CrossRef]
- Krajišnik, D.; Stepanović-Petrović, R.; Tomić, M.; Micov, A.; Ibrić, S.; Milić, J. Application of artificial neural networks in prediction of diclofenac sodium release from drug-modified zeolites physical mixtures and antiedematous activity assessment. J. Pharm. Sci. 2014, 103, 1085–1094. [Google Scholar] [CrossRef]
- Yan, N.; Xu, Z.; Qu, C.; Zhang, J. Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int. Immunopharmacol. 2021, 98, 107844. [Google Scholar] [CrossRef]
- Gupta, U.; Ghosh, S.; Wallace, C.T.; Shang, P.; Xin, Y.; Nair, A.P.; Yazdankhah, M.; Strizhakova, A.; Ross, M.A.; Liu, H.; et al. Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD. Autophagy 2023, 19, 92–111. [Google Scholar] [CrossRef]
- Oliva, C.A.; Vargas, J.Y.; Inestrosa, N.C. Wnt signaling: Role in LTP, neural networks and memory. Ageing Res. Rev. 2013, 12, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. Roles of Wnt proteins in neural development and maintenance. Curr. Opin. Neurobiol. 2000, 10, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Rosso, S.B.; Inestrosa, N.C. WNT signaling in neuronal maturation and synaptogenesis. Front. Cell. Neurosci. 2013, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Mota, A.S.; Norwitz, S.G.; Clarke, K. Multi-Loop Model of Alzheimer Disease: An Integrated Perspective on the Wnt/GSK3β, α-Synuclein, and Type 3 Diabetes Hypotheses. Front. Aging Neurosci. 2019, 11, 184. [Google Scholar] [CrossRef]
- Lee, S.; Hong, D.G.; Yang, S.; Kim, J.; Baek, M.; Kim, S.; Thirumalai, D.; Chung, H.Y.; Chang, S.-C.; Lee, J. Anti-Inflammatory Effect of IKK-Activated GSK-3β Inhibitory Peptide Prevented Nigrostriatal Neurodegeneration in the Rodent Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 998. [Google Scholar] [CrossRef]
- Götz, J.; Xia, D.; Leinenga, G.; Chew, Y.L.; Nicholas, H. What Renders TAU Toxic. Front. Neurol. 2013, 4, 72. [Google Scholar] [CrossRef]
- Nilson, A.N.; English, K.C.; Gerson, J.E.; Barton Whittle, T.; Nicolas Crain, C.; Xue, J.; Sengupta, U.; Castillo-Carranza, D.L.; Zhang, W.; Gupta, P.; et al. Tau Oligomers Associate with Inflammation in the Brain and Retina of Tauopathy Mice and in Neurodegenerative Diseases. J. Alzheimers. Dis. 2017, 55, 1083–1099. [Google Scholar] [CrossRef]
- Shafiei, S.S.; Guerrero-Muñoz, M.J.; Castillo-Carranza, D.L. Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front. Aging Neurosci. 2017, 9, 83. [Google Scholar] [CrossRef]
- Omran, A.; Mohamed, E.; Abd Elraouf, O.; Georgy, G.; Balah, A. Modulatory effect of zeolite in an experimental rat model of Alzheimer’s disease. Azhar Int. J. Pharm. Med. Sci. 2022, 2, 105–115. [Google Scholar] [CrossRef]
- Cooper, J.M.; Lathuiliere, A.; Migliorini, M.; Arai, A.L.; Wani, M.M.; Dujardin, S.; Muratoglu, S.C.; Hyman, B.T.; Strickland, D.K. Regulation of tau internalization, degradation, and seeding by LRP1 reveals multiple pathways for tau catabolism. J. Biol. Chem. 2021, 296, 100715. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Niu, Q. Effect of Aluminum-Maltolate on the Content of Aβ Protein and the Expression of ApoER2, VLDLRs, and LRP1 in PC12-ApoE4 Cells. Neurotox. Res. 2019, 35, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Ragab, G.M.; Salahuddin, A.; Jamil, L.; Abd Al Haleem, E.N. Attenuative Effects of Fluoxetine and Triticum aestivum against Aluminum-Induced Alzheimer’s Disease in Rats: The Possible Consequences on Hepatotoxicity and Nephrotoxicity. Molecules 2021, 26, 6752. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Abo El-Ella, D.M.; El-Emam, S.Z.; Shahat, A.S.; El-Sayed, R.M. Physical & mental activities enhance the neuroprotective effect of vinpocetine & coenzyme Q10 combination against Alzheimer & bone remodeling in rats. Life Sci. 2019, 229, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Khalil, M.G.; Elariny, H.A.; Abu Elfotuh, K. The Role of Mental and Physical Activities against Development of Alzheimer ’s Disease in Socialized and Isolated Rats. Brain Disord. Ther. 2017, 6, 2. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Cunha, J.M.; Masur, J. Evaluation of Psychotropic Drugs with a Modified Open Field Test. Pharmacology 1978, 16, 259–267. [Google Scholar] [CrossRef]
- Hritcu, L.; Cioanca, O.; Hancianu, M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 2012, 19, 529–534. [Google Scholar] [CrossRef]
- Ali, A.A.; Kamal, M.M.; Khalil, M.G.; Ali, S.A.; Elariny, H.A.; Bekhit, A.; Wahid, A. Behavioral, Biochemical and Histopathological effects of Standardised Pomegranate extract with Vinpocetine, Propolis or Cocoa in a rat model of Parkinson’s disease. Exp. Aging Res. 2022, 48, 191–210. [Google Scholar] [CrossRef]
- Sanderson, T.; Wild, G.; Cull, A.M.; Marston, J.; Zardin, G. Immunohistochemical and immunofluoresent techniques. In Bancroft’s Theory and Practice of Histological Techniques; Suvarna, K.S., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–396. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Parameter | Control | AD | AD + PhM | AD + MH | AD + ZC | AD + COM | AD + COM + PhM |
|---|---|---|---|---|---|---|---|
| DA | 71.22 ± 1.8 | 13.63 ± 1.8 a | 23.7 ± 2.4 ab | 40 ± 0.1 abc | 35.85 ± 2 abc | 49.87 ± 0.1 abcde | 58.12 ± 2 abcdef |
| 5-HT | 11.82 ± 0.9 | 1.93 ± 0.1 a | 4.21 ± 0.4 ab | 5.95 ± 0.4 abc | 7.26 ± 0.5 abcd | 9.71 ± 0.5 abcde | 11.23 ± 0.8 bcdef |
| NE | 737.2 ± 4 | 176.6 ± 8 a | 241.3 ± 14 ab | 390.7 ± 14 abc | 441.4 ± 13 abcd | 533.5 ± 26 abcde | 635.4 ± 34 abcdef |
| ACHE | 11.75 ± 1.08 | 70.47 ± 0.8 a | 62.45 ± 1.8 ab | 44.83 ± 0.7 abcde | 35.87 ± 1.2 abcde | 22.42 ± 1.8 abce | 13.76 ± 1 bcd |
| Gene | Forward Primer | Reverse Primer | Accession No. |
|---|---|---|---|
| NF-kβ | 5′-GGACAGCACCACCTACGATG-3′ | 5′-CTGGATCACTTCAATGGCCTC-3′ | NM_001276711 |
| Bax | 5′-CACGTCTGCGGGGAGTCA-3′ | 5′-TAGGAAAGGAGGCCATCCCA-3′ | NM_017059 |
| Bcl-2 | 5′-CATCTCATGCCAAGGGGGAA-3′ | 5′-TATCCCACTCGTAGCCCCTC- 3′ | NM_016993 |
| Caspase-1 | 5′-GAACAAAGAAGGTGGCGCAT-3′ | 5′-GAGGTCAACATCAGCTCCGA-3′ | NM_012762 |
| NLRP3 | 5′-TGCATGCCGTATCTGGTTGT-3′ | 5′-ACCTCTTGCGAGGGTCTTTG-3′ | NM_001191642 |
| GSK3β | 5′-AGCCTATATCCATTCCTTGG-3′ | 5′-CCTCGGACCAGCTGCTTT-3′ | NM_032080 |
| HO-1 | 5′-CACCAGCCACACAGCACTAC-3′ | 5′-CACCCACCCCTCAAAAGACA-3′ | NM_012580 |
| Nrf-2 | 5′-CTCTCTGGAGACGGCCATGACT-3′ | 5′-CTGGGCTGGGGACAGTGGTAGT-3′ | NM_031789 |
| TLR4 | 5′-TCAGCTTTGGTCAGTTGGCT-3′ | 5′-GTCCTTGACCCACTGCAAGA-3′ | NM_019178 |
| Tau | 5′-TAGCTGACGAGGTGTCTGCC-3′ | 5′-ATTTGAAGGACTTGGGGAGG-3′ | NM_017212 |
| ACSL4 | 5′-TCCCTGGACTAGGACCGAAG-3′ | 5′-GGGGCGTCATAGCCTTTCTT-3′ | NM_001431649 |
| β-actin | 5′-CCGTAAAGACCTCTATGCCA- 3′ | 5′-AAGAAAGGGTGTAAAACGCA- 3′ | NM_031144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Elfotuh, K.; Mahran, Y.; Bayoumie El Gazzar, W.; Youssef, H.S.; Hamdan, A.M.E.; Albalawi, T.M.; Alsunbul, M.; ALQahtani, R.; Mohammed, A.A. Targeting Ferroptosis/Nrf2 Pathway Ameliorates AlCl3-Induced Alzheimer’s Disease in Rats: Neuroprotective Effect of Morin Hydrate, Zeolite Clinoptilolite, and Physical Plus Mental Activities. Int. J. Mol. Sci. 2025, 26, 1260. https://doi.org/10.3390/ijms26031260
Abu-Elfotuh K, Mahran Y, Bayoumie El Gazzar W, Youssef HS, Hamdan AME, Albalawi TM, Alsunbul M, ALQahtani R, Mohammed AA. Targeting Ferroptosis/Nrf2 Pathway Ameliorates AlCl3-Induced Alzheimer’s Disease in Rats: Neuroprotective Effect of Morin Hydrate, Zeolite Clinoptilolite, and Physical Plus Mental Activities. International Journal of Molecular Sciences. 2025; 26(3):1260. https://doi.org/10.3390/ijms26031260
Chicago/Turabian StyleAbu-Elfotuh, Karema, Yasmin Mahran, Walaa Bayoumie El Gazzar, Heba S. Youssef, Ahmed M. E. Hamdan, Tariq Mohammed Albalawi, Maha Alsunbul, Reem ALQahtani, and Asmaa A. Mohammed. 2025. "Targeting Ferroptosis/Nrf2 Pathway Ameliorates AlCl3-Induced Alzheimer’s Disease in Rats: Neuroprotective Effect of Morin Hydrate, Zeolite Clinoptilolite, and Physical Plus Mental Activities" International Journal of Molecular Sciences 26, no. 3: 1260. https://doi.org/10.3390/ijms26031260
APA StyleAbu-Elfotuh, K., Mahran, Y., Bayoumie El Gazzar, W., Youssef, H. S., Hamdan, A. M. E., Albalawi, T. M., Alsunbul, M., ALQahtani, R., & Mohammed, A. A. (2025). Targeting Ferroptosis/Nrf2 Pathway Ameliorates AlCl3-Induced Alzheimer’s Disease in Rats: Neuroprotective Effect of Morin Hydrate, Zeolite Clinoptilolite, and Physical Plus Mental Activities. International Journal of Molecular Sciences, 26(3), 1260. https://doi.org/10.3390/ijms26031260






