A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia
Abstract
:1. Introduction
2. Results
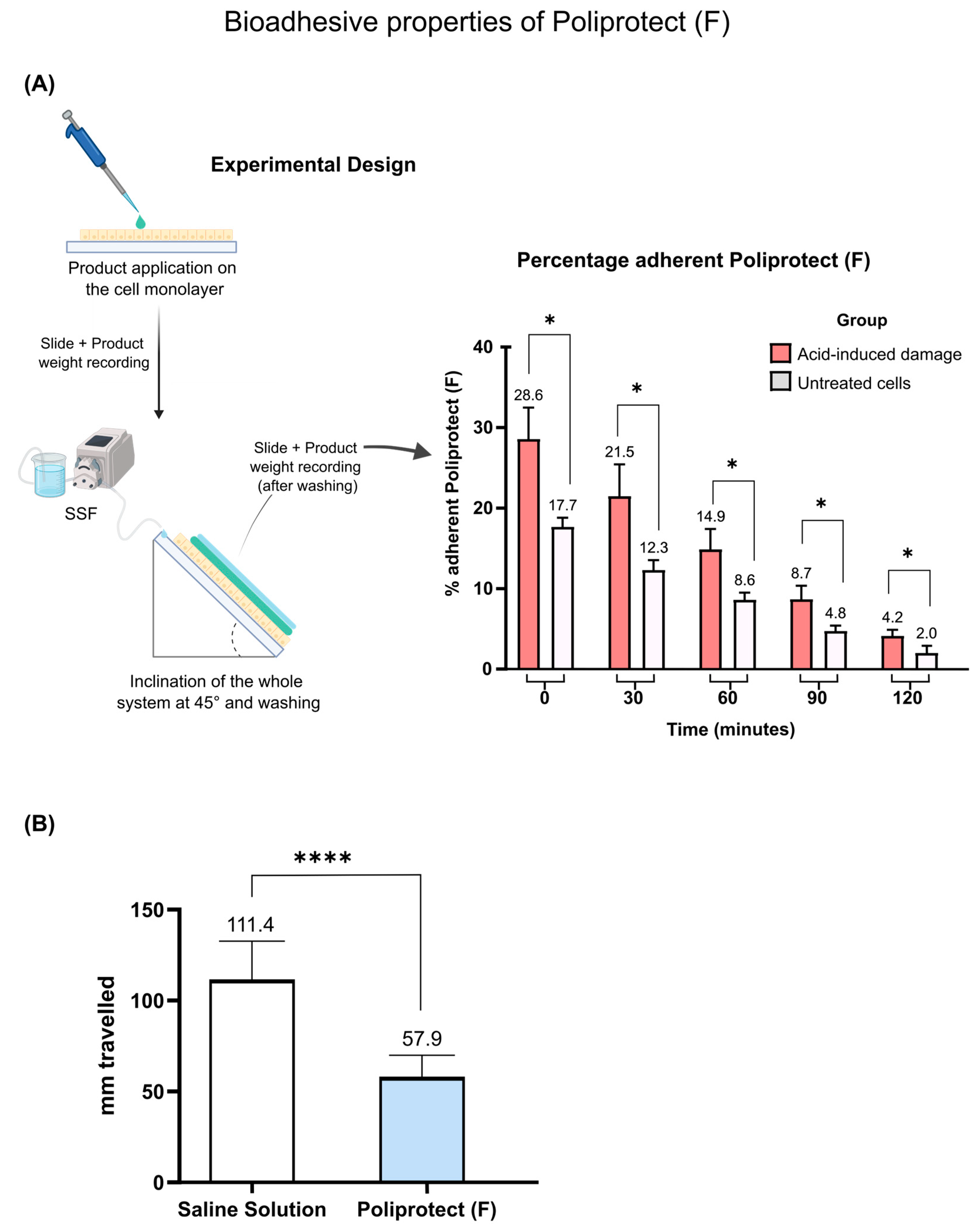
2.1. Assessment of Bioadhesive Properties of POLIPROTECT(F)
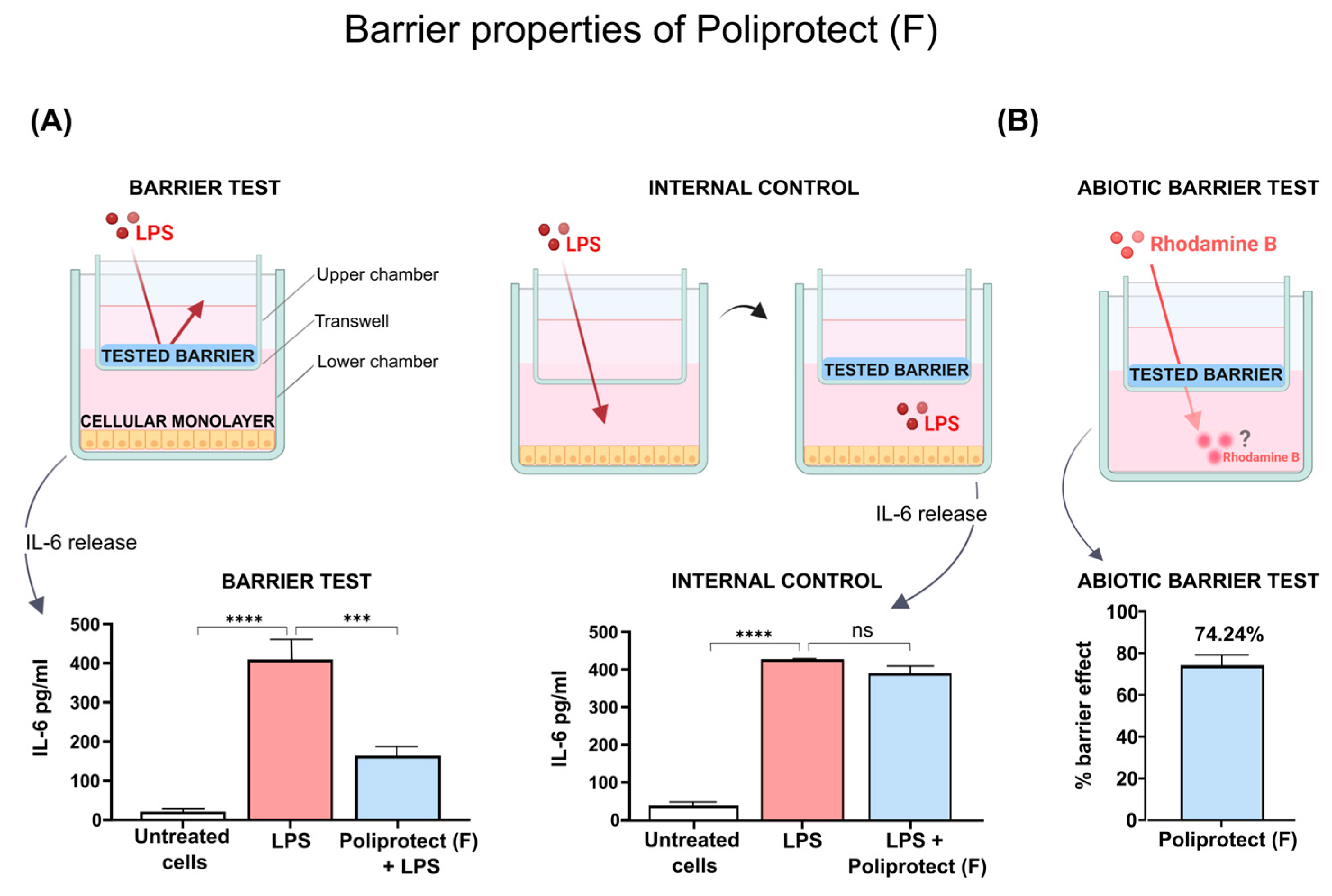
2.2. Barrier Properties of Poliprotect(F)
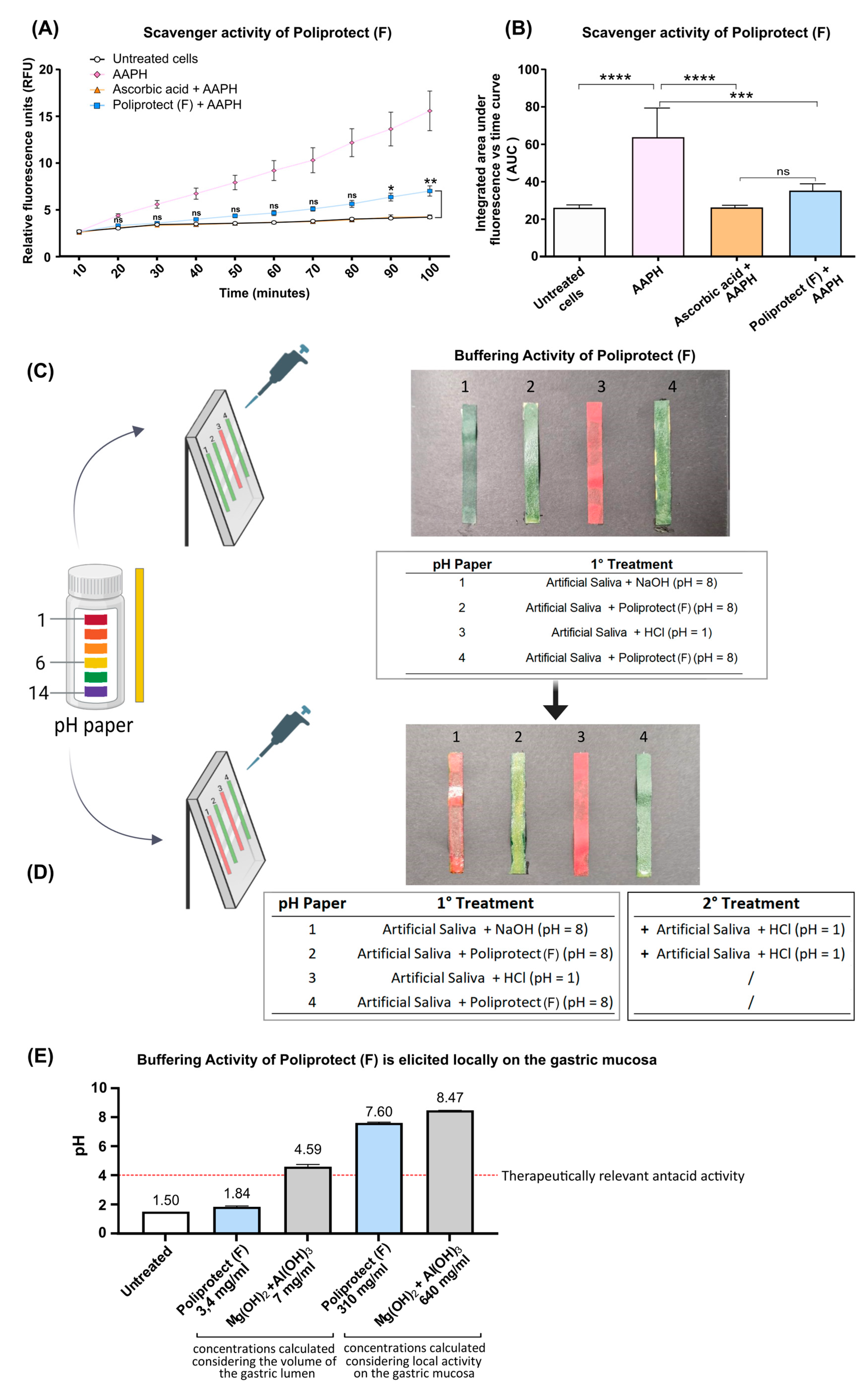
2.3. Radical Scavenging Activity
2.4. Buffering Activity and Its Localization
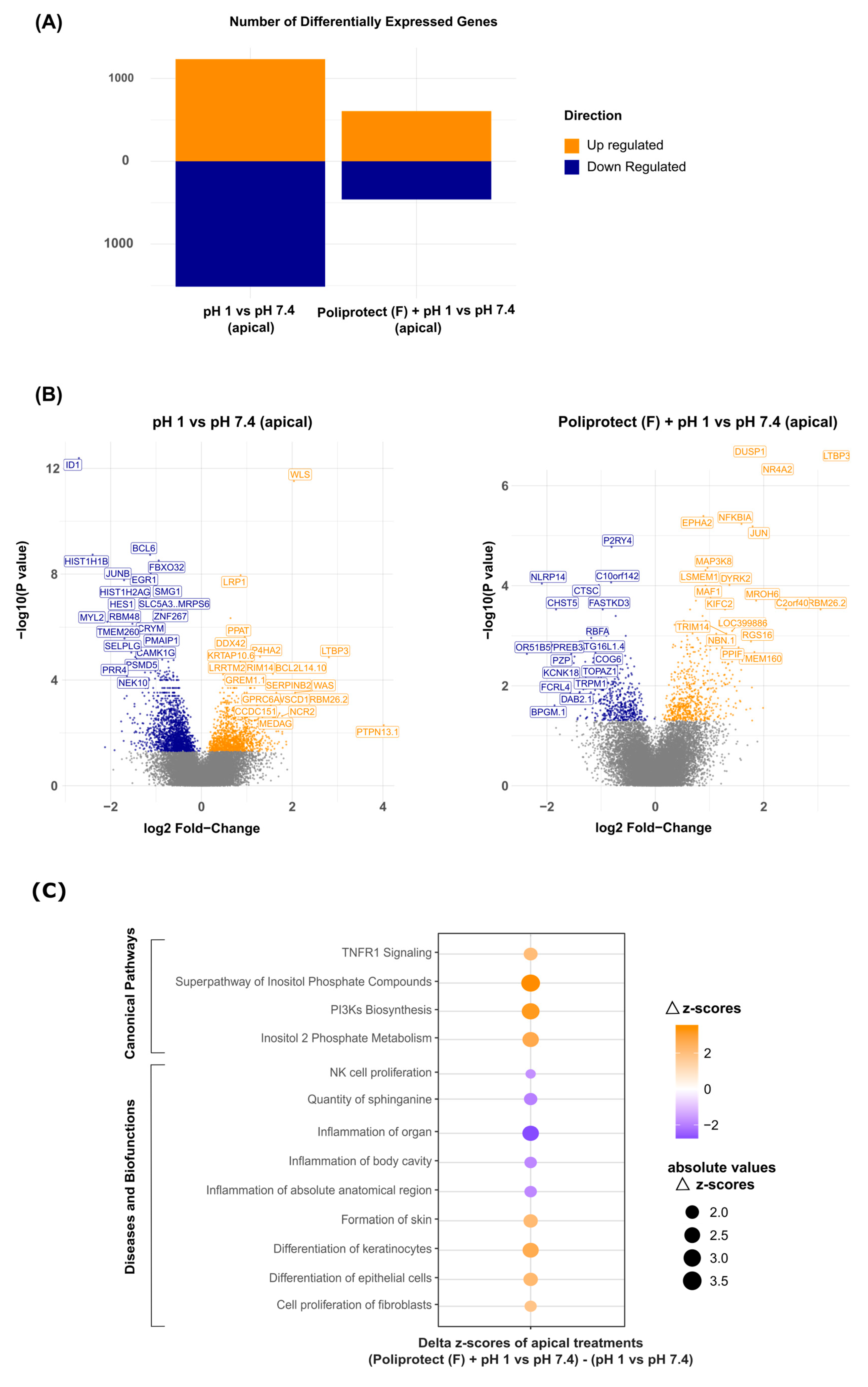
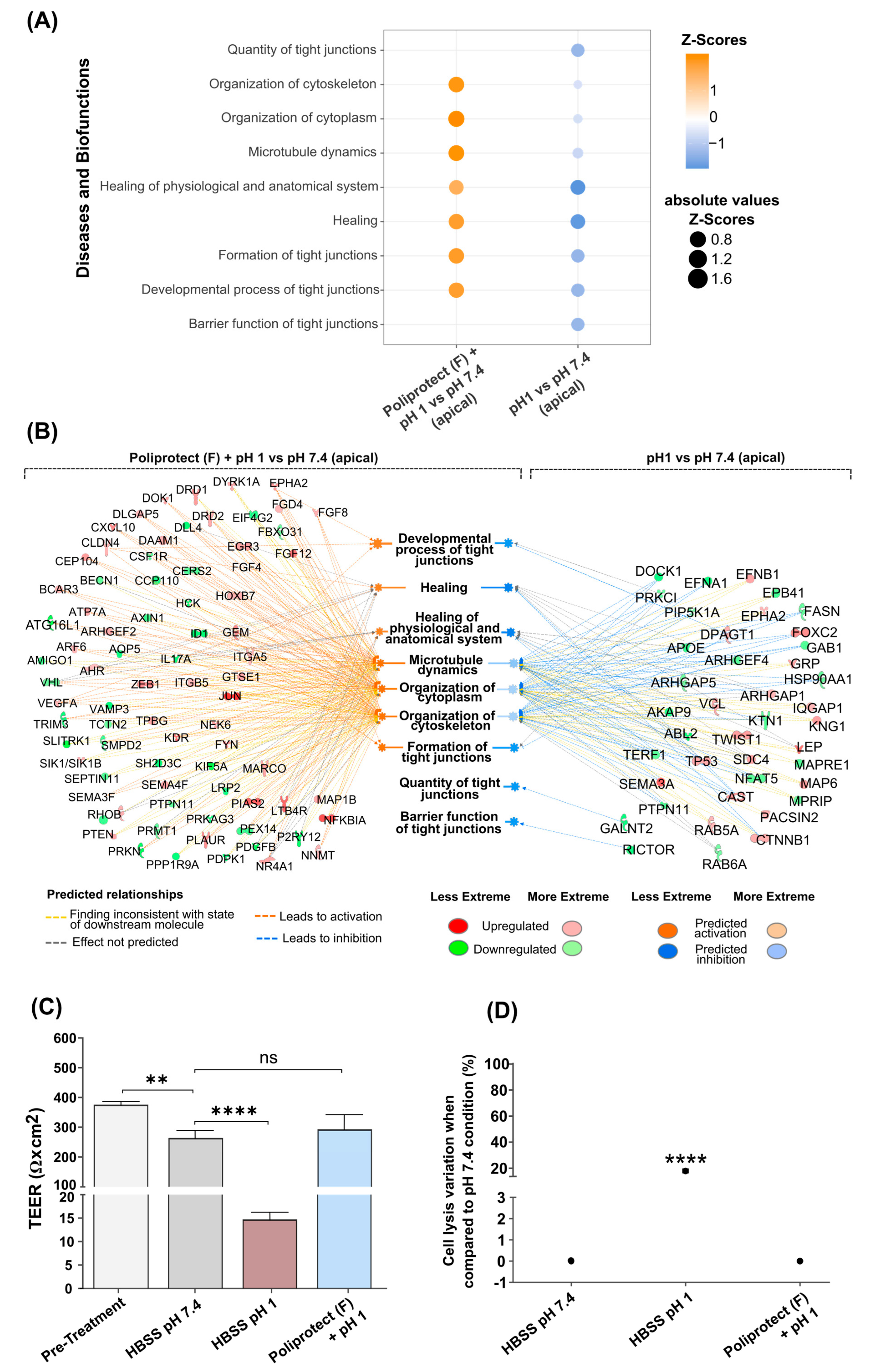
2.5. Effects of Treatment with Poliprotect(F) on Human Gastric Epithelial Cells
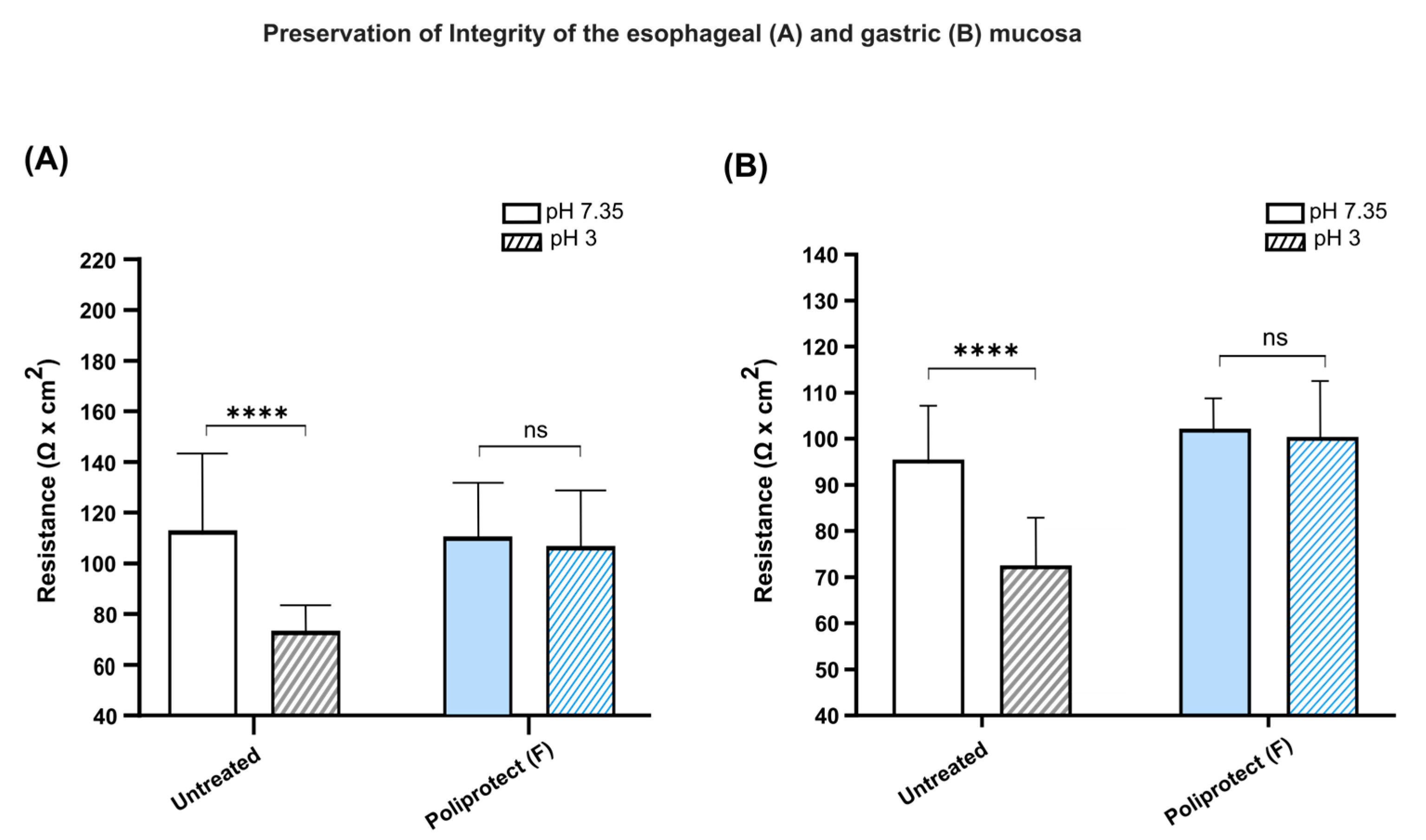
2.6. Preservation of the Permeability of Gastric and Esophageal Mucosae
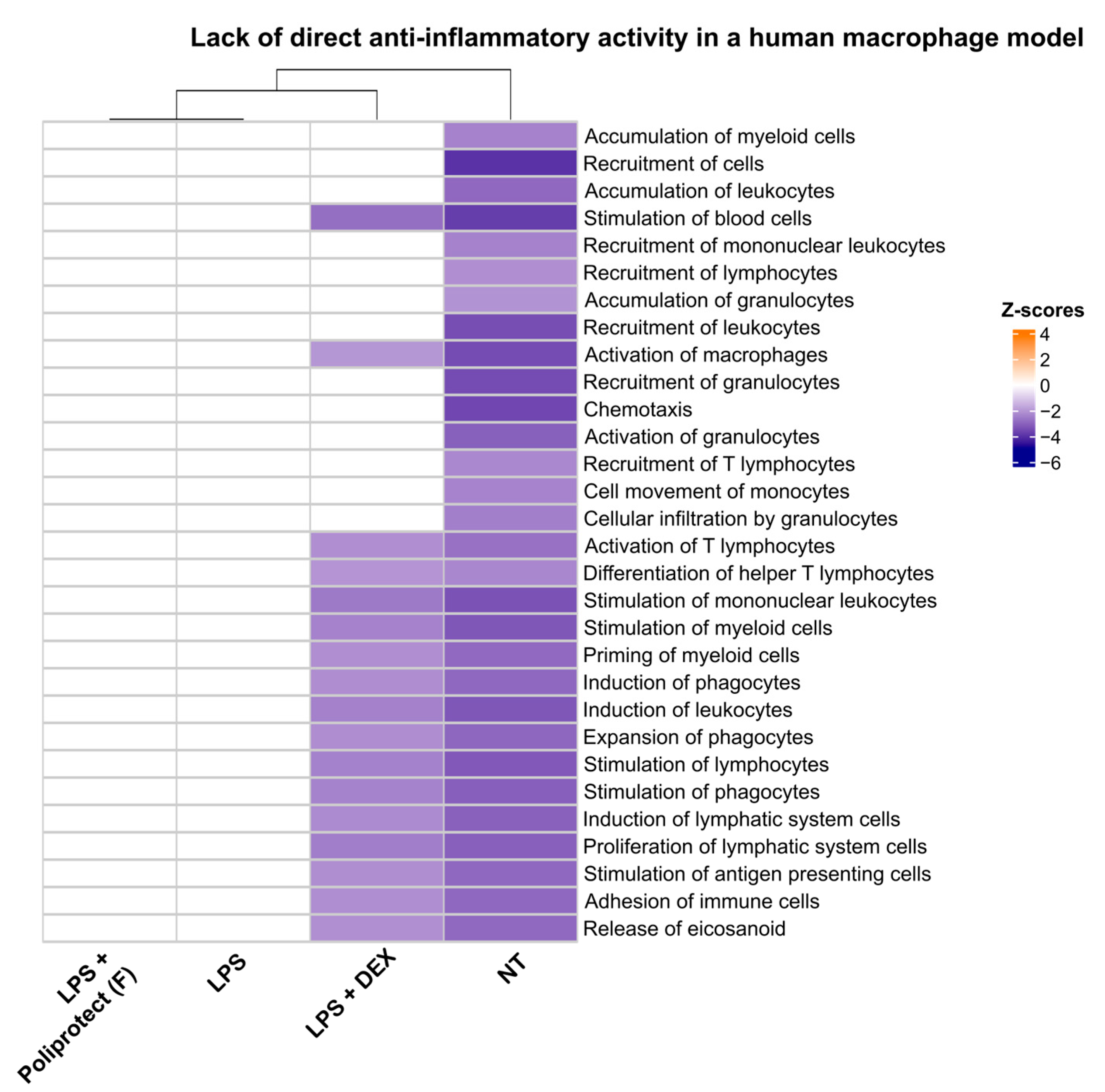
2.7. Lack of Direct Anti-Inflammatory Activity in a Human Macrophage Model
2.8. Protective Activity of Poliprotect(F) In Vivo in an Animal Model of Erosive Gastropathy
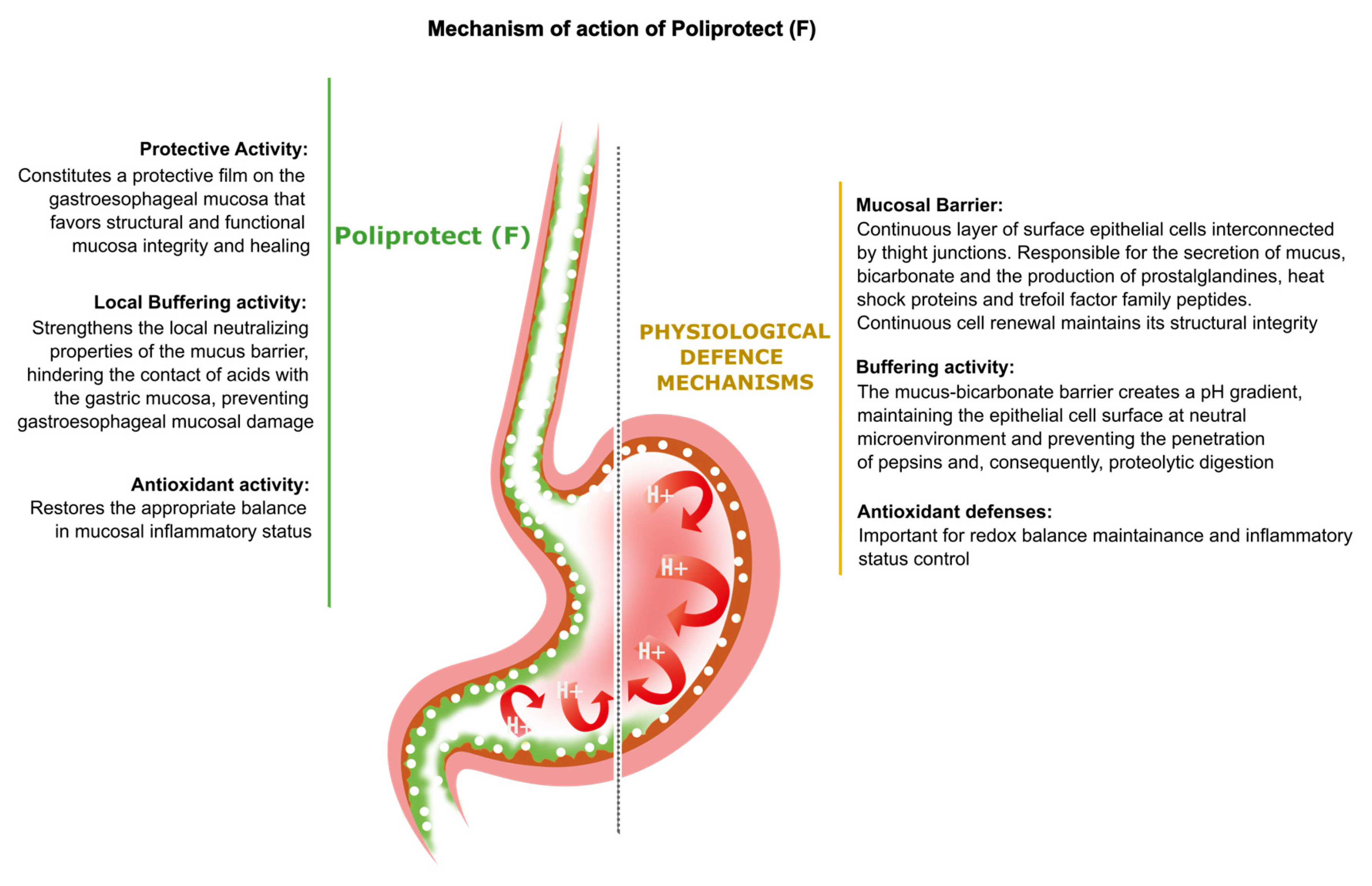
3. Discussion
4. Materials and Methods
4.1. Poliprotect Composition and Preparation
4.2. Cells and Cell Cultures
4.3. Assessment of Bioadhesive Properties of Poliprotect(F)
4.4. Barrier Properties of Poliprotect(F)
4.5. Radical Scavenging Activity
4.6. Buffering Activity and Its Localization
- (a)
- One hundred μL of a solution of HBSS acidified to pH 1 with HCl was distributed on pH paper to constitute a uniform layer, and the color change was read by comparing the color of the strip with the reference scale.
- (b)
- One hundred μL of a solution of Poliprotect(F) was distributed on pH paper to constitute a uniform layer, and the color change was read by comparing the color of the strip with the reference scale. An additional 100 µL of HBSS pH 1 was layered on top of the thus-formed barrier, and the color change was read as described above.
- (c)
- One hundred μL of a basic solution of HBSS pH 8 was distributed on pH paper to constitute a uniform layer, and the color change was read by comparing the color of the strip with the reference scale. An additional 100 µL of HBSS pH 1 was layered on top of this solution, and the color change was read as described above.
4.7. Effects of Treatment with Poliprotect(F) on Human Gastric Epithelial Cells
4.7.1. Cell Treatments and Transepithelial Electrical Resistance (TEER) Measurement
- (a)
- Acid-induced damage: The apical HBSS was replaced with 26.8 µL of HBSS acidified to pH 1 with HCl, and incubation continued with shaking for 30 min.
- (b)
- Poliprotect(F): The apical HBSS was removed, and cells were treated with 3.2 µL of the product dissolved in HBSS pH 1 at a 310 mg/mL concentration. An additional 26.8 µL of HBSS pH 1 was layered on top of the thus-formed barrier to represent the presence of the acidic stress. The incubation continued with shaking for 30 min.
- (c)
- Control: The apical HBSS was replaced with 26.8 µL of fresh HBSS pH 7.4, and incubation continued with shaking for 30 min.
- (a)
- Acid-induced damage: The apical and basolateral HBSS pH 7.4 was replaced with HBSS pH 1 (26.8 µL) and RPMI-1640 culture medium (600 µL), respectively. Incubation continued with shaking for 30 min.
- (b)
- Poliprotect(F): The apical and basolateral HBSS pH 7.4 was replaced with HBSS pH 1 (26.8 µL) and Poliprotect(F) solution (600 µL) at a concentration of 110 µg/mL in RPMI-1640, respectively. The Poliprotect(F) solution was prepared by diluting the solution used for the apical treatment in RPMI-1640 (310 mg/mL), and incubation continued with shaking for 30 min.
- (c)
- Control: The apical HBSS 7.4 was replaced with 26.8 µL of fresh HBSS pH 7.4, the basolateral HBSS 7.4 was replaced with 600 µL of RPMI-1640, and incubation continued with shaking for 30 min.
4.7.2. Gene Expression and Bioinformatics Analysis
4.8. Preservation of the Permeability of Gastric and Esophageal Mucosae
4.9. Lack of Direct Anti-Inflammatory Activity in a Human Macrophage Model (U937)
4.10. Protective Activity of Poliprotect(F) in an Animal Model of Erosive Gastropathy
Animal Models of Erosive Gastropathy
- (a)
- Rats without lesions, treated with vehicle, without other administrations (10 mL/kg per os) (n = 6, SHAM).
- (b)
- Rats treated with a vehicle and the subsequent administration of ethanol or indomethacin (10 mL/kg); (n = 12; ethanol or indomethacin).
- (c)
- Rats treated with Poliprotect(F) (650 mg/kg), Poliprotect(F) without salt (472 mg/kg), or Poliprotect(F) without plant components (587 mg/kg) (all per os), and the subsequent administration of ethanol or indomethacin (n = 12).
- (d)
- Rats treated with a reference compound (ranitidine, 50 mg/kg) and the subsequent administration of ethanol or indomethacin (n = 12).
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blevins, C.H.; Iyer, P.G.; Vela, M.F.; Katzka, A.D. The Esophageal Epithelial Barrier in Health and Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Takeuchi, K.; Tarnawski, A. Gastric Mucosal Defense and Cytoprotection: Bench to Bedside. Gastroenterology 2008, 135, 41–60. [Google Scholar] [CrossRef]
- Niv, Y.; Banić, M. Gastric Barrier Function and Toxic Damage. Dig. Dis. 2014, 32, 235–242. [Google Scholar] [CrossRef]
- Orlando, C.R. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pr. Res. Clin. Gastroenterol. 2010, 24, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.J. Prostaglandins, NSAIDs, and Gastric Mucosal Protection: Why Doesn’t the Stomach Digest Itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.-U.; Conway, B.R.; Ghori, U.M. Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Lee, K.; Kwon, C.-I.; Yeniova, A.; Koyanagi, A.; Jacob, L.; Smith, L.; Lee, S.W.; Rahmati, M.; Shin, J.-Y.; Shin, J.I.; et al. Global prevalence of functional dyspepsia according to Rome criteria, 1990–2020: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 1–11. [Google Scholar] [CrossRef]
- Nasseri-Moghaddam, S.; Mousavian, A.-H.; Kasaeian, A.; Kanno, T.; Yuan, Y.; Ford, A.C.; Moayyedi, P. What is the Prevalence of Clinically Significant Endoscopic Findings in Subjects With Dyspepsia? Updated Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 21, 1739–1749.e2. [Google Scholar] [CrossRef]
- Black, C.J.; Houghton, L.A.; Ford, C.A. Insights into the evaluation and management of dyspepsia: Recent developments and new guidelines. Ther. Adv. Gastroenterol. 2018, 11. [Google Scholar] [CrossRef]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Tolone, S.; De Bortoli, N.; Frazzoni, M.; Savarino, E. Pathophysiology, diagnosis, and pharmacological treatment of gastro-esophageal reflux disease. Expert Rev. Clin. Pharmacol. 2020, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Q.; Fass, R.; Gyawali, C.P.; Miwa, H.; Pandolfino, J.E.; Zerbib, F. Esophageal Disorders. Gastroenterology 2016, 150, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- de Bortoli, N.; Ottonello, A.; Zerbib, F.; Sifrim, D.; Gyawali, C.P.; Savarino, E. Between GERD and NERD: The relevance of weakly acidic reflux. Ann. N. Y. Acad. Sci. 2016, 1380, 218–229. [Google Scholar] [CrossRef]
- Talley, N.J.; Ford, C.A. Functional Dyspepsia. N. Engl. J. Med. 2015, 373, 1853–1863. [Google Scholar] [CrossRef]
- Woodland, P.; Ooi, J.L.S.; Grassi, F.; Nikaki, K.; Lee, C.; Evans, J.A.; Koukias, N.; Triantos, C.; McDonald, S.A.; Peiris, M.; et al. Superficial Esophageal Mucosal Afferent Nerves May Contribute to Reflux Hypersensitivity in Nonerosive Reflux Disease. Gastroenterology 2017, 153, 1230–1239. [Google Scholar] [CrossRef]
- Zerbib, F.; Duriez, A.; Roman, S.; Capdepont, M.; Mion, F. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 2007, 57, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Vanheel, H.; Carbone, F.; Valvekens, L.; Simren, M.; Tornblom, H.; Vanuytsel, T.; Van Oudenhove, L.; Tack, J. Pathophysiological Abnormalities in Functional Dyspepsia Subgroups According to the Rome III Criteria. Am. J. Gastroenterol. 2017, 112, 132–140. [Google Scholar] [CrossRef]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, J.S. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2021, 117, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.M.; Lacy, B.E.; Andrews, C.N.; Enns, R.A.; Howden, C.W.; Vakil, N. ACG and CAG Clinical Guideline: Management of Dyspepsia. Am. J. Gastroenterol. 2017, 112, 988–1013. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Dunn, J. Updated NICE Guidance on the Management of Dyspepsia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Pinto-Sanchez, M.I.; Yuan, Y.; Hassan, A.; Bercik, P.; Moayyedi, P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst. Rev. 2017, 2018, CD011194. [Google Scholar] [CrossRef]
- Sigterman, K.E.; van Pinxteren, B.; Bonis, P.A.; Lau, J.; Numans, E.M. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst. Rev. 2013, 2013, CD002095. [Google Scholar] [CrossRef]
- Khan, M.; Santana, J.; Donnellan, C.; Preston, C.; Moayyedi, P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst. Rev. 2007, 2007, CD003244. [Google Scholar] [CrossRef]
- Anjewierden, S.; Han, Z.; Foster, C.B.; Pant, C.; Deshpande, A. Risk factors for Clostridium difficile infection in pediatric inpatients: A meta-analysis and systematic review. Infect. Control. Hosp. Epidemiol. 2019, 40, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, V.; Cicala, G.; Spina, E.; Romano, C. A Narrative Review on Efficacy and Safety of Proton Pump Inhibitors in Children. Front. Pharmacol. 2022, 13, 839972. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, R.A.; Wong, C.; Paynter, S.; David, M.; Peeters, G. The Risk of Community-Acquired Enteric Infection in Proton Pump Inhibitor Therapy: Systematic Review and Meta-analysis. Ann. Pharmacother. 2018, 52, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Tsai, C.-J.; Su, C.-C.; Yen, C.-T.; Chen, J.-L.; Cheng, C.-L. Accelerated risk of renal disease progression in pre-ESRD patients with proton pump inhibitors use: A nationwide population-based study. BMC Nephrol. 2024, 25, 469. [Google Scholar] [CrossRef] [PubMed]
- Xun, X.; Yin, Q.; Fu, Y.; He, X.; Dong, Z. Proton Pump Inhibitors and the Risk of Community-Acquired Pneumonia: An Updated Meta-analysis. Ann. Pharmacother. 2021, 56, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Soo, S.; Deeks, J.; Delaney, B.; Innes, M.; Forman, D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst. Rev. 2006, 4, CD001960. [Google Scholar] [CrossRef]
- Bytzer, P.; van Zanten, S.V.; Mattsson, H.; Wernersson, B. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis—A post hoc analysis of 5796 patients. Aliment. Pharmacol. Ther. 2012, 36, 635–643. [Google Scholar] [CrossRef]
- Lacy, B.E.; Talley, N.J.; Locke, G.R.; Bouras, E.P.; DiBaise, J.K.; El-Serag, H.B.; Abraham, B.P.; Howden, C.W.; Moayyedi, P.; Prather, C. Review article: Current treatment options and management of functional dyspepsia. Aliment. Pharmacol. Ther. 2012, 36, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hillman, L.; Yadlapati, R.; Thuluvath, A.J.; Berendsen, M.A.; Pandolfino, E.J. A review of medical therapy for proton pump inhibitor nonresponsive gastroesophageal reflux disease. Dis. Esophagus 2017, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J.; Accarino, A.; Barbara, G.; Bor, S.; et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. Neurogastroenterol. Motil. 2021, 33, e14238. [Google Scholar] [CrossRef]
- Visaggi, P.; Bertin, L.; Pasta, A.; Calabrese, F.; Ghisa, M.; Marabotto, E.; Ribolsi, M.; Savarino, V.; de Bortoli, N.; Savarino, V.E. Pharmacological management of gastro-esophageal reflux disease: State of the art in 2024. Expert Opin. Pharmacother. 2024, 25, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Bor, S.; Kalkan, I.H.; Savarino, E.; Rao, S.; Tack, J.; Pasricha, J.; Cangemi, D.; Schol, J.; Karunaratne, T.; Ghisa, M.; et al. Prokinetics-safety and efficacy: The European Society of Neurogastroenterology and Motility/The American Neurogastroenterology and Motility Society expert review. Neurogastroenterol. Motil. 2024, 36, e14774. [Google Scholar] [CrossRef]
- Ko, S.; Park, J.; Kim, M.; Kim, J.; Park, J. Effects of the herbal medicine Rikkunshito, for functional dyspepsia: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020, 36, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; Giannini, E.G.; Savarino, V.E. Gastrointestinal functional disorders can benefit from the use of medical devices made of substances. Front. Drug Saf. Regul. 2023, 3, 1119353. [Google Scholar] [CrossRef]
- Savarino, V.; Pace, F.; Scarpignato, C.; The Esoxx Study Group. Randomised clinical trial: Mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease—Efficacy of Esoxx, a hyaluronic acid–chondroitin sulphate based bioadhesive formulation. Aliment. Pharmacol. Ther. 2017, 45, 631–642. [Google Scholar] [CrossRef]
- Corazziari, E.S.; Gasbarrini, A.; D’Alba, L.; D’Ovidio, V.; Riggio, O.; Passaretti, S.; Annibale, B.; Cicala, M.; Repici, A.; Bassotti, G.; et al. Poliprotect vs Omeprazole in the Relief of Heartburn, Epigastric Pain, and Burning in Patients Without Erosive Esophagitis and Gastroduodenal Lesions: A Randomized, Controlled Trial. Am. J. Gastroenterol. 2023, 118, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Haruma, K.; Ito, M.; Takahashi, N.; Takasugi, H.; Wada, Y.; Nakata, H.; Katoh, T.; Miyamoto, M.; Tanaka, S. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: A randomized clinical trial. Dis. Esophagus 2011, 25, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Reimer, C.; Lødrup, A.B.; Smith, G.; Wilkinson, J.; Bytzer, P. Randomised clinical trial: Alginate (Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment. Pharmacol. Ther. 2016, 43, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Gąsiorowska, A. The role of pH in symptomatic relief and effective treatment of gastroesophageal reflux disease. Gastroenterol. Rev. 2017, 4, 244–249. [Google Scholar] [CrossRef]
- Fujimori, S. Gastric acid level of humans must decrease in the future. World J. Gastroenterol. 2020, 26, 6706–6709. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. JALA: J. Assoc. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, M.; Bouchard, F.; Gosselin, P.; Paquin, J.; Mateescu, A.M. The NCI-N87 cell line as a gastric epithelial barrier model for drug permeability assay. Biochem. Biophys. Res. Commun. 2011, 412, 429–434. [Google Scholar] [CrossRef]
- Sharma, P.; Yadlapati, R. Pathophysiology and treatment options for gastroesophageal reflux disease: Looking beyond acid. Ann. N. Y. Acad. Sci. 2020, 1486, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ergun, P.; Samuels, T.L.; Mathison, A.J.; Plehhova, K.; Coyle, C.; Horvath, L.; Johnston, N. Global Transcriptomic Analysis of Topical Sodium Alginate Protection against Peptic Damage in an In Vitro Model of Treatment-Resistant Gastroesophageal Reflux Disease. Int. J. Mol. Sci. 2024, 25, 10714. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Garetto, S.; Montellier, E.; Liu, Y.; Chen, S.; Baldi, P.; Sassone-Corsi, P.; Lucci, J. A non-pharmacological therapeutic approach in the gut triggers distal metabolic rewiring capable of ameliorating diet-induced dysfunctions encompassed by metabolic syndrome. Sci. Rep. 2020, 10, 12915. [Google Scholar] [CrossRef]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Mannelli, L.D.C.; Antonini, G.; Panizzi, E.; Maidecchi, A.; Giovagnoni, E.; Lucci, J.; et al. Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin. Nutrients 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.; Govoni, S. The concept of non-pharmacological mechanism of action in medical devices made of substances in practice: What pharmacology can do to promote the scientific implementation of the European medical device regulation. Pharmadvances 2020, 1, 4–12. [Google Scholar] [CrossRef]
- Ustaoglu, A.; Nguyen, A.; Spechler, S.; Sifrim, D.; Souza, R.; Woodland, P. Mucosal pathogenesis in gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2020, 32, e14022. [Google Scholar] [CrossRef] [PubMed]
- Barlow, W.J.; Orlando, C.R. The pathogenesis of heartburn in nonerosive reflux disease: A unifying hypothesis. Gastroenterology 2005, 128, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Farre, R.; van Malenstein, H.; De Vos, R.; Geboes, K.; Depoortere, I.; Berghe, P.V.; Fornari, F.; Blondeau, K.; Mertens, V.; Tack, J.; et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut 2008, 57, 1366–1374. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Black, C.J.; Yuan, Y.; Veettil, S.K.; Mahadeva, S.; Kengkla, K.; Chaiyakunapruk, N.; Lee, Y.Y. Systematic review and network meta-analysis: Efficacy of drugs for functional dyspepsia. Aliment. Pharmacol. Ther. 2020, 53, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Wang, P.; Kou, G.; Zuo, X.; Wang, X.; Li, Y. Impaired gastric mucosal integrity identified by confocal endomicroscopy in Helicobacter pylori-negative functional dyspepsia. Neurogastroenterol. Motil. 2019, 32, e13719. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Perniola, V.; Lanza, E.; Mahdi, L.; Sallustio, P.; Idone, V.; Semeraro, D.; Mastrodonato, M.; Testini, M.; Desaphy, J.-F.; et al. Poliprotect®, a Medical Device Made of Substances, Potently Protects the Human Esophageal Mucosa Challenged by Multiple Agents: Evidence from In Vitro and Ex Vivo Electrophysiological Models. Int. J. Mol. Sci. 2025, 26, 791. [Google Scholar] [CrossRef] [PubMed]
- Thakre-Nighot, M.; Blikslager, T.A. Indomethacin induces increase in gastric epithelial tight junction permeability via redistribution of occludin and activation of p38 MAPK in MKN-28 Cells. Tissue Barriers 2016, 4, e1187325. [Google Scholar] [CrossRef]
- Pongpiriyadacha, Y.; Matsuda, H.; Morikawa, T.; Asao, Y.; Yoshikawa, M. Protective Effects of Polygodial on Gastric Mucosal Lesions Induced by Necrotizing Agents in Rats and the Possible Mechanisms of Action. Biol. Pharm. Bull. 2003, 26, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. 2012, 18, 2147–2160. [Google Scholar] [CrossRef]
- Cioeta, R.; Muti, P.; Rigoni, M.; Morlando, L.; Siragusa, F.; Cossu, A.; Giovagnoni, E. Effectiveness and tolerability of Poliprotect, a natural mucosal protective agent for gastroesophageal reflux disease and dyspepsia: Surveys from patients, physicians, and pharmacists. Front. Drug Saf. Regul. 2022, 2, 969831. [Google Scholar] [CrossRef]
- Mattoli, L.; Proietti, G.; Fodaroni, G.; Quintiero, C.M.; Burico, M.; Gianni, M.; Giovagnoni, E.; Mercati, V.; Santi, C. Suspect screening analysis to improve untargeted and targeted UHPLC-qToF approaches: The biodegradability of a proton pump inhibitor medicine and a natural medical device. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Aboca. Composition for Use in the Treatment of GI in Patients for Which Treatment with PPI Is. Unadvisable. Patent WO2003066022A2, 14 August 2003.
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Mackie, A.R.; Goycoolea, F.M.; Menchicchi, B.; Caramella, C.M.; Saporito, F.; Lee, S.; Stephansen, K.; Chronakis, I.S.; Hiorth, M.; Adamczak, M.; et al. Innovative Methods and Applications in Mucoadhesion Research. Macromol. Biosci. 2017, 17, 1600534. [Google Scholar] [CrossRef] [PubMed]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials 2020, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Zerrouk, N.; Caramella, C. Mucoadhesive and penetration enhancement properties of three grades of hyaluronic acid using porcine buccal and vaginal tissue, Caco-2 cell lines, and rat jejunum. J. Pharm. Pharmacol. 2004, 56, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, J.D. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, H.R. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Phillipson, M. Acid Transport through Gastric Mucus. Upsala J. Med Sci. 2004, 109, 1–24. [Google Scholar] [CrossRef]
- Konturek, P.; Konturek, S.; Hahn, E. Duodenal alkaline secretion: Its mechanisms and role in mucosal protection against gastric acid. Dig. Liver Dis. 2004, 36, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lewis, O.L.; Keener, J.P.; Fogelson, L.A. A physics-based model for maintenance of the pH gradient in the gastric mucus layer. Am. J. Physiol. Liver Physiol. 2017, 313, G599–G612. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2010, 2, 303–307. [Google Scholar]
- Cuomo, R.; Savarese, M.F.; Sarnelli, G.; Nicolai, E.; Aragri, A.; Cirillo, C.; Vozzella, L.; Zito, F.P.; Verlezza, V.; Efficie, E.; et al. The role of a pre-load beverage on gastric volume and food intake: Comparison between non-caloric carbonated and non-carbonated beverage. Nutr. J. 2011, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, K.G. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.L.; Lim, D.W.; Levesque, C.L.; Vine, D.F.; Muto, M.; Koepke, J.R.; Nation, P.N.; Wizzard, P.R.; Li, J.; Bigam, D.L.; et al. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol.—Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sheppard, D.N.; Hug, J.M. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros. 2004, 3, 123–126. [Google Scholar] [CrossRef]
- Rafiee, L.; Hajhashemi, V.; Javanmard, H.S. Fluvoxamine inhibits some inflammatory genes expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat. Iran. J. Basic Med. Sci. 2016, 19, 977–984. [Google Scholar] [PubMed]
- Rovera, G.; Santoli, D.; Damsky, C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc. Natl. Acad. Sci. USA 1979, 76, 2779–2783. [Google Scholar] [CrossRef]
- Holzer, P.; Lippe, I.T.; Heinemann, Á.; Barthó, L. Tachykinin NK1 and NK2 receptor-mediated control of peristaltic propulsion in the guinea-pig small intestine in vitro. Neuropharmacology 1998, 37, 131–138. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caterbi, S.; Buttarini, C.; Garetto, S.; Franco Moscardini, I.; Ughetto, S.; Guerrini, A.; Panizzi, E.; Rumio, C.; Mattioli, L.; Perfumi, M.; et al. A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia. Int. J. Mol. Sci. 2025, 26, 1181. https://doi.org/10.3390/ijms26031181
Caterbi S, Buttarini C, Garetto S, Franco Moscardini I, Ughetto S, Guerrini A, Panizzi E, Rumio C, Mattioli L, Perfumi M, et al. A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia. International Journal of Molecular Sciences. 2025; 26(3):1181. https://doi.org/10.3390/ijms26031181
Chicago/Turabian StyleCaterbi, Sara, Claudio Buttarini, Stefano Garetto, Isabelle Franco Moscardini, Stefano Ughetto, Angela Guerrini, Elena Panizzi, Cristiano Rumio, Laura Mattioli, Marina Perfumi, and et al. 2025. "A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia" International Journal of Molecular Sciences 26, no. 3: 1181. https://doi.org/10.3390/ijms26031181
APA StyleCaterbi, S., Buttarini, C., Garetto, S., Franco Moscardini, I., Ughetto, S., Guerrini, A., Panizzi, E., Rumio, C., Mattioli, L., Perfumi, M., Maidecchi, A., Cossu, A., Bruley des Varannes, S., Regula, J., Malfertheiner, P., Sardi, C., & Lucci, J. (2025). A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia. International Journal of Molecular Sciences, 26(3), 1181. https://doi.org/10.3390/ijms26031181






