Relevance of Lipoprotein Composition in Endothelial Dysfunction and the Development of Hypertension
Abstract
1. Introduction
2. Relationship Between Lipoprotein Components and Endothelial Function
2.1. Lipoproteins
Lipoproteins Function
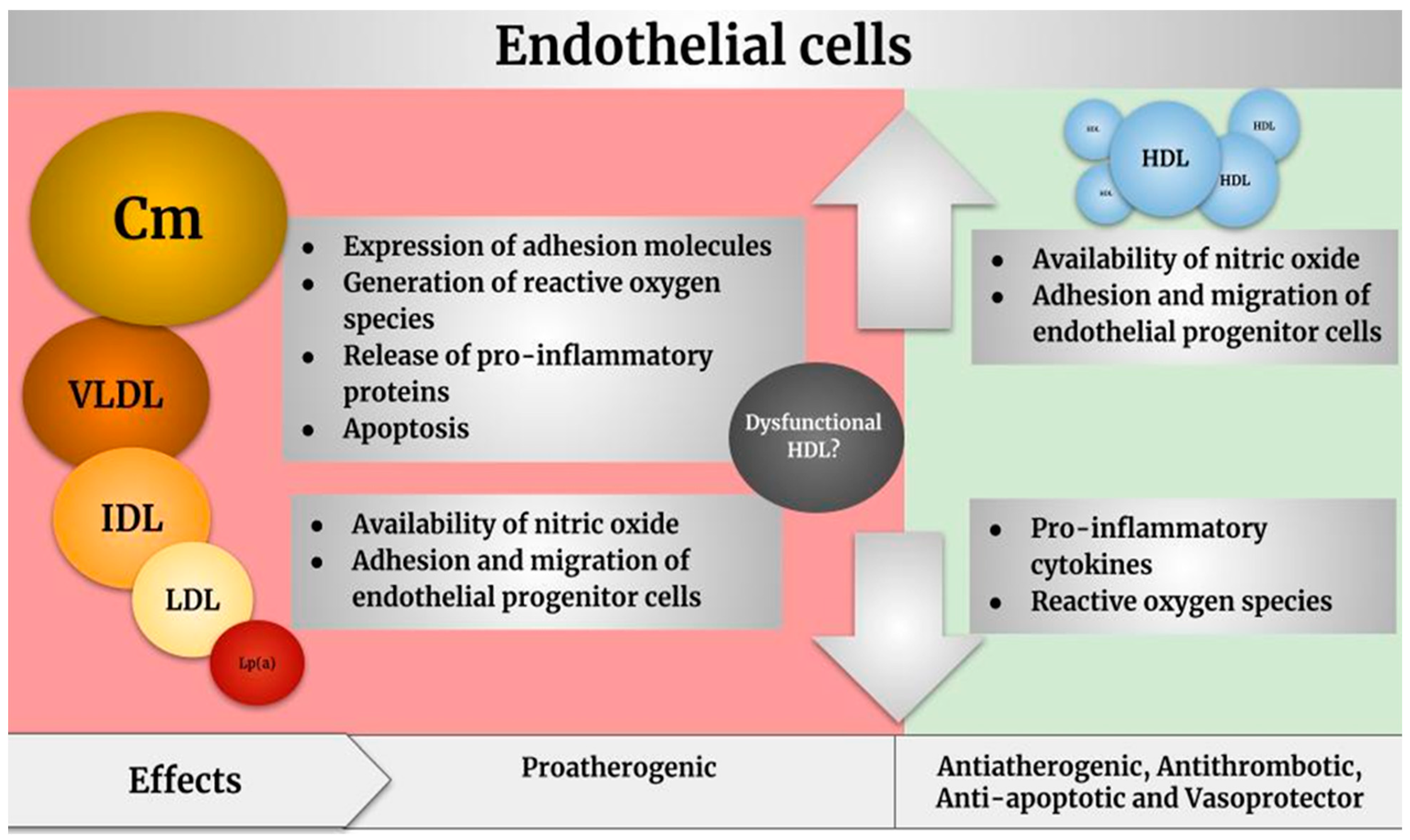
2.2. Endothelium
Endothelial Dysfunction Induced by Lipoproteins
| Apolipoproteins | Functions | Refs. |
|---|---|---|
| Apo A-I | Involved in the transport of cholesterol and other lipids. | [66,67,68] |
| Involved the formation of new lipoproteins. | ||
| Antioxidant and anti-inflammatory properties. | ||
| Apo A-II | The second most abundant protein in HDLs. | |
| Apo A-IV | Increased in the intestine during fat absorption. | |
| Apo A-V | Activator of lipolysis mediated by LPL; metabolism of lipoproteins rich in triglycerides. | |
| Apo B | Two related proteins, B-48 and B-100. Biomarker of cardiovascular risk and development of atherosclerosis. B-100 is large in size, has moderate hydrophobicity, and is unable to be transferred to other lipoproteins. | [69,70] |
| Apo C | Three different proteins; C-I, C-II, and C-III. Involved in the clearance of triglyceride-rich lipoproteins. C-III reduces the clearance of lipoproteins containing apo B and triglyceride-rich lipoproteins. Inhibits the binding of apo E and apo B to LDLr. Associated with HDL, inhibiting the apoptosis of endothelial cells and their capacity to inhibit monocyte adhesion to endothelial cells. A deficit of C-II increases Tgs, VLDL, and Cm while decreasing LDL, IDL, HDL, apo B, and apo A-I. Activates LPL. | [71,72,73] |
| Apo D | Also named lipocalin. Associated with lipid metabolism, inflammation, and antioxidative response. Involved in the transport of arachidonic acid. Modulates eicosanoid acid production. Provides neuroprotection. | [74,75,76] |
| Apo E2,3,4 | Mediates the elimination of Cms and VLDLs. Helps to enrich the nuclei of HDLs with CEs. Reduced levels are related to hypercholesterolemia and atherosclerosis. | [77,78] |
| Apo J | Called “clusterin”. Protects cells against damage from oxidation, inflammation, and apoptosis. Associated with atherosclerosis, obesity, and diabetes. | [79,80] |
| Apo M | Involved in HDL metabolism and pre-β-HDL formation. Promotes the flow of cholesterol associated with HDLs. Anti-atherogenic, anti-inflammatory, and antioxidant effects. Primarily carries S1P. | [81,82,83,84] |
| Apo (a) | Binds to LDL-particles containing modified apo B-100.Pro-atherogenic, pro-inflammatory, and pro-thrombotic effects.Carrier of oxidized phospholipids. | [85,86,87,88,89] |
3. Hypertension and Lipoproteins
4. Pharmacological vs. No Pharmacological Therapy for Endothelial Dysfunction
Bioactive Compounds and Their Effect on Lipoproteins
| Bioactive Compound | Food Matrix | Dose/Concentration | Experimental Model | Effects and Mechanisms of Action | Refs. |
|---|---|---|---|---|---|
| Phytosterols | Plant stanols | 2 g/day | Healthy volunteers | Reduced cholesterol absorption and LDL-C, apo B, and oxLDL levels. | [136] |
| Plant sterols | 1.6–2.5 g/day | Dyslipidemic subjects | Decreased triglyceride levels. | [144] | |
| Phytosterol esters (β-sitosterol) (Fitocor®) | Phytosterols (2.6 g/day) were prescribed and supplied in 650 mg gelatin capsules for 12 weeks | Patients >18 years, with LDL-c ≥130 mg/dL and <190 mg/dL and triglycerides <400 mg/dL. | Decreased total cholesterol, increased HDL-C, and reduced IDL. | [145] | |
| Plant sterols | 400 mL of soy milk enriched with 1.6 g of phytosterols/4 weeks | Thirty-eight moderately hypercholesterolemic volunteers | Reduced endothelin-1 plasma concentration by 11%.Reduced total plasma cholesterol concentration, triglycerides, and apo B. | [146] | |
| Terpenes | Reagent | Vitamin E: 10 mmol/L α-13′-OH and 5 mmol/L α-13′-COOH | THP-1 monocytes and human monocyte-derived macrophages | Decreased CD36 expression and oxLDL absorption. | [134] |
| Iraqi Cicer areitinum | Terpenes (500 mg/kg)/56 days | Hyperlipidemic mice | Decreased levels of total cholesterol, triglycerides, LDL-C, and VLDL-C. Reduced ALT, AST, and ALP enzymatic activities also in total serum bilirubin levels. Increased levels of HDL-C. | [147] | |
| Callistemon citrinus | High-fat-sucrose diet + 1,8-cineole (0.88 mg/kg body weight), limonene (0.43 mg/kg body weight), α-terpineol (0.32 mg/kg body weight), and a mixture of the three terpenes/15 weeks | Obese rats | Reduced triglycerides levels, advanced oxidation protein products and hydroxyalkenals, a lipid peroxidation product. Restored levels of reduced glutathione. | [148] | |
| Polyunsaturated fatty acids | Fish oil | Mice diet containing 19% fish oil alone, mice diet containing 19% fish oil + aspirin (via drinking water 30 mg/L) | COX-1 neo mice (a neomycin (neo)-resistant cassette inserted in COX-1 intron 10 to ensure hypomorphic expression of COX-1 gene) | Hypolipidemic and antihypertensive effects. Increased levels of omega-3 PUFAs, including EPA, DPA, and DHA. Anti-inflammatory effect. Decreased expression of adhesion molecules. | [149] |
| Fish oil | Capsule omega-3 PUFA equivalent to 640 mg (520 mg DHA and 120 mg EPA) | Healthy subjects and subjects with a history of CVD | Decreased P-selectin expression and the percentage of platelet–monocyte aggregates. | [150] | |
| Purified omega-3 | 460 mg EPA and 380 mg DHA twice a day for 5 weeks | Hypertriglyceridemic patients | Decreased triglycerides and HDL-triglyceride plasma concentrations. Anti-inflammatory properties. Increase in endothelial function. | [12] | |
| Fish oil | 12 g/day of EPA- or DHA-rich fish oil supplement (~4.8 g/d total EPA + DHA) | Healthy normolipidemic adults | Decreased plasma TG levels, as well as overall particle numbers of VLDLs and TG-rich lipoprotein subfractions. Increase in plasma levels of apo M. | [151] | |
| Fish oil | 1 g fish oil capsules consumed for 8 weeks—370 mg of EPA and 230 mg of DHA, 3 times per day; total EPA + DHA = 1800 mg | 30–74-year-old subjects with at least one cardiovascular risk factor (dyslipidemia, high blood pressure, diabetes, or smoking) | Increased large HDLs and reduced small HDLs and the non-esterified fatty acids in HDL (NEFAs-HDL) levels. Reduced CETP activity. Decreased apo CIII and increased apo CII and PON1. | [152] |
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1/G1 | ATP-binding cassette class A type 1/class G type 1 |
| ACAT | Acyl-coenzyme A: cholesterol acyltransferase |
| Apos | Apolipoproteins |
| AT1R | Angiotensin II type 1 receptor |
| BP | Blood pressure |
| CE | Cholesterol ester |
| CETP | Cholesteryl ester transfer protein |
| Cm | Chylomicron |
| COX-1 | Cyclooxygenase-1 |
| CVD | Cardiovascular disease |
| ED | Endothelial dysfunction |
| eGlx | Endothelial glycocalyx |
| EndoMT | Endothelial–mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| ERK | Extracellular-signal-regulated kinase |
| FC | Free cholesterol |
| FFA | Free fatty acid |
| GM-CSF | Granulocyte–monocyte colony stimulating factor |
| HDL | High-density lipoprotein |
| HDL-C | HDL cholesterol |
| HL | Hepatic lipase |
| HUVECs | Human umbilical vein endothelial cells |
| ICAM-1 | Intracellular adhesion molecule-1 |
| IDL | Intermediate-density lipoprotein |
| iNOS | Inducible nitric oxide synthase |
| LCAT | Lecithin-cholesterol acyltransferase |
| LDL | Low-density lipoprotein |
| LDL-C | LDL cholesterol |
| LDLr | LDL receptor |
| LOX-1 | Lectin-like ox-LDL scavenger receptor-1 |
| Lp (a) | Lipoprotein (a) |
| LPL | Lipoprotein lipase |
| LPS | Lipopolysaccharide |
| LSR | Lipolysis-stimulated lipoprotein receptor |
| MCP-1 | Monocyte chemotactic protein-1 |
| MDA | Malondialdehyde |
| MPO | Oxidative myeloperoxidase |
| NF-kB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NPC1L1 | Niemann-Pick C1-like 1 protein |
| oxLDL | Oxidized low-density lipoprotein |
| oxPL | Oxidized phospholipid |
| Php | Phospholipid |
| PLTP | Phospholipid transfer protein |
| PON1 | Paraoxonase-1 |
| RCT | Reverse cholesterol transport |
| ROS | Reactive oxygen species |
| S1P | Sphingosine-1-phosphate |
| SAA | Serum amyloid protein A |
| SCFA | Short-chain fatty acid |
| SRA/B1 | Scavenger receptor A/class B type 1 |
| Tg | Triglyceride |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| VLDL | Very low-density lipoprotein |
| VLDLr | Very low-density lipoprotein receptor |
| VSMCs | Vascular smooth muscle cells |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 13 May 2024).
- Ray, K.K.; Ference, B.A.; Séverin, T.; Blom, D.; Nicholls, S.J.; Shiba, M.H.; Almahmeed, W.; Alonso, R.; Daccord, M.; Ezhov, M.; et al. World Heart Federation Cholesterol Roadmap 2022. Glob. Heart 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Charbonneau, F.; Schiffrin, E.L. Correlation of endothelial function in large and small arteries in human essential hypertension. J. Hypertens. 2001, 19, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Fang, J.; Liu, Z.; Shi, Y.; Agostini, M.; Bernassola, F.; Bove, P.; Candi, F.; Rovella, V.; Soca, G.; et al. Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 2023, 14, 691. [Google Scholar] [CrossRef]
- Casas, R.; Estruch, R.; Sacanella, E. Influence of bioactive nutrients on the atherosclerotic process: A review. Nutrients 2018, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.K.; Sufian, H.B.; Choudhury, M.; Yamasaki, M.; Al-Harrasi, A.; Moustaid-Moussa, N.; Rahman, S.M. Role of macrophage autophagy in atherosclerosis: Modulation by bioactive compounds. Biochem. J. 2021, 478, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, D.E. Health Promoting Effects of Bioactive Compounds in Plants. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2013. [Google Scholar]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Anti-atherosclerotic effects of fruit bioactive compounds: A review of current scientific evidence. Can. J. Plant Sci. 2012, 92, 407–419. [Google Scholar] [CrossRef]
- Scolaro, B.; Soo Jin Kim, H.; de Castro, I.A. Bioactive compounds as an alternative for drug co-therapy: Overcoming challenges in cardiovascular disease prevention. Crit. Rev. Food Sci. Nutr. 2018, 58, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Morales, A.; Estrada-Luna, D.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Luna-Luna, M.; Flores-Castillo, C.; Vargas-Alarcón, G.; Fragoso, J.M.; Pérez-Méndez, O.; Carreón-Torres, E. Microencapsulated Pomegranate Modifies the Composition and Function of High-Density Lipoproteins (HDL) in New Zealand Rabbits. Molecules 2020, 25, 3297. [Google Scholar] [CrossRef]
- Peña-De-La-Sancha, P.; Muñoz-García, A.; Espínola-Zavaleta, N.; Bautista-Pérez, R.; Mejía, A.M.; Luna-Luna, M.; López-Olmos, V.; Rodríguez-Pérez, J.M.; Fragoso, J.M.; Carreón-Torres, E.; et al. Eicosapentaenoic and docosahexaenoic acid supplementation increases HDL content in n-3 fatty acids and improves endothelial function in hypertriglyceridemic patients. Int. J. Mol. Sci. 2023, 24, 5390. [Google Scholar] [CrossRef]
- Dieckmann, M.; Dietrich, M.F.; Herz, J. Lipoprotein receptors--an evolutionarily ancient multifunctional receptor family. Biol. Chem. 2010, 391, 1341–1363. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Chang, M.Y.; Wang, S.; Wight, T.N.; McMillen, T.S.; Oram, J.F.; Vaisar, T.; Heinecke, J.W.; De Beer, F.C.; De Beer, M.C.; et al. Serum amyloid A facilitates the binding of high-density lipoprotein from mice injected with lipopolysaccharide to vascular proteoglycans. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1326–1332. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Yates, A.G.; Rizvi, L.; Anthony, D.C.; Probert, F. HDL and LDL have distinct, opposing effects on LPS-induced brain inflammation. Lipids Health Dis. 2023, 22, 54. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Kim, M.S.; Memon, R.A.; Shigenaga, A.; Moser, A.; Feingold, K.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Keuter, M.; Wagener, F.; Stalenhoef, A.F.H.; van der Meer, J.W.M.; Kullberg, B.J. Circulating lipoproteins are a crucial component of host defense against invasive Salmonella typhimurium infection. PLoS ONE 2009, 4, e4237. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Luna, D.; Ortiz-Rodriguez, M.A.; Medina-Briseño, L.; Carreón-Torres, E.; Izquierdo-Vega, J.A.; Sharma, A.; Cancino-Díaz, J.C.; Pérez-Méndez, O.; Belefant-Miller, H.; Betanzos-Cabrera, G. Current therapies focused on high-density lipoproteins associated with cardiovascular disease. Molecules 2018, 23, 2730. [Google Scholar] [CrossRef]
- Kuklenyik, Z.; Jones, J.I.; Gardner, M.S.; Schieltz, D.M.; Parks, B.A.; Toth, C.A.; Rees, J.C.; Andrews, M.L.; Carter, K.; Lehtikoski, A.K.; et al. Core lipid, surface lipid and apolipoprotein composition analysis of lipoprotein particles as a function of particle size in one workflow integrating asymmetric flow field-flow fractionation and liquid chromatography-tandem mass spectrometry. PLoS ONE 2018, 13, e0194797. [Google Scholar] [CrossRef]
- Stapleton, P.A.; Goodwill, A.G.; James, M.E.; Brock, R.W.; Frisbee, J.C. Hypercholesterolemia and microvascular dysfunction: Interventional strategies. J. Inflamm. 2010, 7, 54. [Google Scholar] [CrossRef]
- Vinagre, C.G.; Freitas, F.R.; de Mesquita, C.H.; Vinagre, J.C.; Mariani, A.C.; Kalil-Filho, R.; Maranhão, R.C. Removal of Chylomicron Remnants from the Bloodstream is Delayed in Aged Subjects. Aging Dis. 2018, 9, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Real, J.T.; Ascaso, J.F. Lipid metabolism and classification of hyperlipaemias. Metabolismo lipídico y clasificación de las hiperlipemias. Clin. Investig. Arterioscler. 2021, 33, 3–9. [Google Scholar] [PubMed]
- Errico, T.L.; Chen, X.; Martin Campos, J.M.; Julve, J.; Escolà-Gil, J.C.; Blanco-Vaca, F. Mecanismos básicos: Estructura, función y metabolismo de las lipoproteínas plasm. [Basic mechanisms: Structure, function and metabolism of plasma lipoproteins]. Clin. Investig. Arterioscler. 2013, 25, 98–103. [Google Scholar]
- Yen, F.T.; Roitel, O.; Bonnard, L.; Notet, V.; Pratte, D.; Stenger, C.; Magueur, E.; Bihain, B.E. Lipolysis stimulated lipoprotein receptor: A novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem. 2008, 283, 25650–25659. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Stern, J.; Bouzid, D.; Robert, T.; Dehoux, M.; Snauwaert, A.; Zappella, N.; Cournot, M.; Lortat-Jacob, B.; Augustin, P.; et al. Relationship between lipoprotein concentrations and short-term and 1-year mortality in intensive care unit septic patients: Results from the HIGHSEPS study. Ann. Intensive Care 2021, 11, 11. [Google Scholar] [CrossRef]
- Xu, Q.; Li, C.; Jing, P.; Li, H.; Tian, X.; Xia, X.; Zhang, Y.; Zhang, X.; Wang, Y.; Wang, A.; et al. Low-Density Lipoprotein Cholesterol to Triglyceride Ratio and Clinical Outcomes after Acute Ischaemic Stroke or Transient Ischaemic Attack. J. Atheroscler. Thromb. 2024, 31, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.; Leeuwenburgh, C.; Brusko, T.; Moldawer, L.; Reddy, S.T.; Guirgis, F.W. Lipid and lipoprotein dysregulation in sepsis: Clinical and mechanistic insights into chronic critical illness. J. Clin. Med. 2021, 10, 1693. [Google Scholar] [CrossRef]
- Muñoz-Vega, M.; Massó, F.; Páez, A.; Vargas-Alarcón, G.; Coral-Vázquez, R.; Mas-Olivia, J. Carreón-Torres, E.; Pérez-Méndez, O. HDL-Mediated Lipid Influx to Endothelial Cells Contributes to Regulating Intercellular Adhesion Molecule (ICAM)-1 Expression and eNOS Phosphorylation. Int. J. Mol. Sci. 2018, 19, 3394. [Google Scholar] [CrossRef]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.I.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Ginsberg, H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011, 22, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hesse, D.; Jaschke, A.; Chung, B.; Schürmann, A. Trans-Golgi proteins participate in the control of lipid droplet and chylomicron formation. Biosci. Rep. 2013, 33, e00001. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Dahlbäck, B. Blood coagulation. Lancet 2000, 355, 1627–1632. [Google Scholar] [CrossRef]
- Gimbrone Jr, M.A.; García-Cardeña, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar]
- Tousoulis, D.; Antoniades, C.; Stefanadis, C. Evaluating endothelial function in humans: A guide to invasive and non-invasive techniques. Heart 2005, 91, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L.A. Endothelial glycocalyx. Compr. Physiol. 2022, 12, 3781. [Google Scholar]
- Enseleit, F.; Hürlimann, D.; Lüscher, T.F. Vascular protective effects of angiotensin converting enzyme inhibitors and their relation to clinical events. J. Cardiovasc. Pharmacol. 2001, 37, S21–S30. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; De Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. Low body mass index is a risk factor for impaired endothelium-dependent vasodilation in humans: Role of nitric oxide and oxidative stress. J. Am. Coll. Cardiol. 2003, 42, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Khaddaj Mallat, R.; Mathew John, C.; Kendrick, D.J.; Braun, A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017, 54, 458–470. [Google Scholar] [CrossRef]
- Sandoo, A.; Van Zanten, J.J.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302. [Google Scholar] [CrossRef] [PubMed]
- Weseler, A.R.; Bast, A. Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010, 12, 154–161. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Lawrence-Mills, S.J.; Hughes, D.; Hezzell, M.J.; Butler, M.; Neal, C.; Foster, R.R.; Welsh, G.I.; Finch, N. The microvascular endothelial glycocalyx: An additional piece of the puzzle in veterinary medicine. Vet. J. 2022, 285, 105843. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Donato, L.; Alibrandi, S.; Scimone, C.; D’Angelo, R.; Sidoti, A. Oxidative stress and the neurovascular unit. Life 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Cominacini, L.; Pasini, A.F.; Garbin, U.; Davoli, A.; Tosetti, M.L.; Campagnola, M.; Rigoni, A.; Pastorino, A.M.; Lo Cascio, V.; Sawamura, T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-κB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 2000, 275, 12633–12638. [Google Scholar] [CrossRef]
- Mitra, R.; O’Neil, G.L.; Harding, I.C.; Cheng, M.J.; Mensah, S.A.; Ebong, E.E. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr. Atheroscler. Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Brosolo, G.; Da Porto, A.; Marcante, S.; Picci, A.; Capilupi, F.; Capilupi, P.; Bulfone, L.; Vacca, A.; Bertin, N.; Vivarelli, C.; et al. Lipoprotein (a): Just an innocent bystander in arterial hypertension? Int. J. Mol. Sci. 2023, 24, 13363. [Google Scholar] [CrossRef]
- Barlage, S.; Gnewuch, C.; Liebisch, G.; Wolf, Z.; Audebert, F.X.; Glück, T.; Fröhlich, D.; Krämer, B.K.; Rothe, G.; Schmitz, G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009, 35, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Munno, M.; Mallia, A.; Greco, A.; Modafferi, G.; Banfi, C.; Eligini, S. Radical Oxygen Species, Oxidized Low-Density Lipoproteins, and Lectin-like Oxidized Low-Density Lipoprotein Receptor 1: A Vicious Circle in Atherosclerotic Process. Antioxidants 2024, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Zhao, W.; Tang, S.Y.; Levi, M.G.; Ibrahim, A.; Yang, Y.; Roberts, E.; Lai, L.; Li, J.; Assoian, R.K.; et al. Endothelial lipid droplets suppress eNOS to link high fat consumption to blood pressure elevation. J. Clin. Investig. 2023, 133, e173160. [Google Scholar] [CrossRef]
- Alipour, A.; Elte, J.W.; van Zaanen, H.C.; Rietveld, A.P.; Cabezas, M.C. Postprandial inflammation and endothelial dysfuction. Biochem. Soc. Trans. 2007, 35, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Alaupovic, P. The concept of apolipoprotein-defined lipoprotein families and its clinical significance. Curr. Atheroscler. Rep. 2003, 5, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.-L.; Wu, C.-L.; Zhao, S.-P. Plasma apolipoprotein O level increased in the patients with acute coronary syndrome. J. Lipid Res. 2012, 53, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
- Segrest, J.P.; Jones, M.K.; De Loof, H.; Dashti, N. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 2001, 42, 1346–1367. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, E.P. Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Springer International Publishing: Cham, Switzerland, 2020; pp. 399–420. [Google Scholar]
- Kanter, J.E.; Shao, B.; Kramer, F.; Barnhart, S.; Shimizu-Albergine, M.; Vaisar, T.; Graham, M.J.; Crooke, R.M.; Manuel, C.R.; Haeusler, R.A.; et al. Increased apolipoprotein C3 drives cardiovascular risk in type 1 diabetes. J. Clin. Investig. 2019, 129, 4165–4179. [Google Scholar] [CrossRef]
- Chan, D.C.; Watts, G.F. Apolipoproteins as markers and managers of coronary risk. QJM Int. J. Med. 2006, 99, 277–287. [Google Scholar] [CrossRef]
- Kastelein, J.J.; Van Der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yi, J.; Li, W.; Zheng, X.; Liu, J.; Wang, J.; Du, G. Apolipoproteins and cancer. Cancer Med. 2019, 8, 7032–7043. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, A.N.; Heinecke, J.W. Lipoproteomics: Using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins: Thematic Review Series: Proteomics. J. Lipid Res. 2009, 50, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef]
- Hubacek, J.A. Apolipoprotein A5 fifteen years anniversary: Lessons from genetic epidemiology. Gene 2016, 592, 193–199. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Lo, C.M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: A protein intimately involved in metabolism. J. Lipid Res. 2015, 56, 1403–1418. [Google Scholar] [CrossRef]
- Olofsson, S.O.; Borèn, J. Apolipoprotein B: A clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 2005, 258, 395–410. [Google Scholar] [CrossRef]
- Whitfield, A.J.; Barrett, P.H.R.; van Bockxmeer, F.M.; Burnett, J.R. Lipid disorders and mutations in the APOB gene. Clin. Chem. 2004, 50, 1725–1732. [Google Scholar] [CrossRef]
- Zheng, C.; Khoo, C.; Furtado, J.; Sacks, F.M. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 2010, 121, 1722–1734. [Google Scholar] [CrossRef]
- Morton, A.M.; Koch, M.; Mendivil, C.O.; Furtado, J.D.; Tjønneland, A.; Overvad, K.; Wang, L.; Jensen, M.K.; Sacks, F.M. Apolipoproteins E and CIII interact to regulate HDL metabolism and coronary heart disease risk. JCI Insight 2018, 3, e98045. [Google Scholar] [CrossRef]
- Kawakami, A.; Aikawa, M.; Libby, P.; Alcaide, P.; Luscinskas, F.W.; Sacks, F.M. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 2006, 113, 691–700. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Rubio-Sardón, N.; Peláez, R.; García-Álvarez, E.; Del Valle, E.; Tolivia, J.; Larráyoz, I.M.; Navarro, A. Neuroprotective effect of apolipoprotein D in cuprizone-induced cell line models: A potential therapeutic approach for multiple sclerosis and demyelinating diseases. Int. J. Mol. Sci. 2021, 22, 1260. [Google Scholar] [CrossRef]
- Pérez, C.; Navarro, A.; Martínez, E.; Ordóñez, C.; Del Valle, E.; Tolivia, J. Age-related changes of apolipoprotein D expression in female rat central nervous system with chronic estradiol treatment. Age 2012, 34, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Forest, J.C.; Giguère, Y.; Masse, A.; Lafond, J.; Rassart, E. Modulation of Apolipoprotein D levels in human pregnancy and association with gestational weight gain. Reprod. Biol. Endocrinol. 2009, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J. Lipid Res. 2009, 50, S183–S188. [Google Scholar] [CrossRef]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Büttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlötzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef]
- Herz, J.; Bock, H.H. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2022, 71, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Turner, J.; McCarthy, S.; Scaltriti, M.; Bettuzzi, S.; Yeatman, T.J. Clusterin-mediated apoptosis is regulated by adenomatous polyposis coli and is p21 dependent but p53 independent. Cancer Res. 2004, 64, 7412–7419. [Google Scholar] [CrossRef]
- Yi-zhou, Y.; Bing, C.; Ming-qiu, L.; Wei, W.; Ru-xing, W.; Jun, R.; Liu-yan, W.; Zhao-hui, J.; Yong, J.; Guo qing, J.; et al. Dihydrotestosterone regulating apolipoprotein M expression mediates via protein kinase C in HepG2 cells. Lipids Health Dis. 2012, 11, 68–168. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, C.; Jauhiainen, M.; Moser, M.; Porse, B.; Ehnholm, C.; Boesl, M.; Boesl, M.; Dahlbacck, B.; Nielsen, L.B. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J. Biol. Chem. 2008, 283, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Gao, J.-L.; Pu, C.; Zhang, P.-H.; Wang, L.-Z.; Feng, G.; Zhang, Y. Apolipoprotein M: Research progress, regulation and metabolic functions. Mol. Med. Rep. 2015, 12, 1617–1624. [Google Scholar]
- Mattisson, I.Y.; Christoffersen, C. Apolipoprotein M and its impact on endothelial dysfunction and inflammation in the cardiovascular system. Atherosclerosis 2021, 334, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; van Kimmenade, R.R.J.; Liu, Y.; Hu, X.; Browne, A.; Plutzky, J.; Tsimikas, S.; Blankstein, R.; Natarajan, R. Lipoprotein(a), Oxidized Phospholipids, and Progression to Symptomatic Heart Failure: The CASABLANCA Study. J. Am. Heart Assoc. 2024, 13, e034774. [Google Scholar] [CrossRef]
- Tsimikas, S.; Witztum, J.L. Oxidized phospholipids in cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 170–191. [Google Scholar] [CrossRef]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein (a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef]
- Simantiris, S.; Antonopoulos, A.S.; Papastamos, C.; Benetos, G.; Koumallos, N.; Tsioufis, K.; Tousoulis, D. Lipoprotein (a) and inflammation-pathophysiological links and clinical implications for cardiovascular disease. J. Clin. Lipidol. 2023, 17, 55–63. [Google Scholar] [CrossRef]
- Gilliland, T.C.; Liu, Y.; Mohebi, R.; Miksenas, H.; Haidermota, S.; Wong, M.; Hu, X.; Cristino, J.R.; Browne, A.; Plutzky, J.; et al. Lipoprotein(a), Oxidized Phospholipids, and Coronary Artery Disease Severity and Outcomes. J. Am. Coll. Cardiol. 2023, 81, 1780–1792. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The Road Ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Park, -S.; Yoon, S.-J.; Tae, H.-J.; Shim, C.Y. RAGE and cardiovascular disease. Front. Biosci. 2011, 16, 486–497. [Google Scholar] [CrossRef]
- Dauphinee, S.M.; Karsan, A. Lipopolysaccharide signaling in endothelial cells. Lab. Investig. 2006, 86, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Lidgard, B.; Hoofnagle, A.N.; Zelnick, L.R.; de Boer, I.H.; Fretts, A.M.; Kestenbaum, B.R.; Lemaitre, R.N.; Robinson-Cohen, C.; Bansal, N. High-Density Lipoprotein Lipidomics and Mortality in CKD. Kidney Med. 2023, 5, 100708. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, X.; Xing, S.; Bian, F.; Yao, W.; Bai, X.; Zheng, T.; Wu, G.; Jin, S. Endogenous ceramide contributes to the transcytosis of oxLDL across endothelial cells and promotes its subendothelial retention in vascular wall. Oxidative Med. Cell. Longev. 2014, 2014, 823071. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Kuebler, W.M.; Simmons, S. Sphingolipids in Atherosclerosis: Chimeras in Structure and Function. Int. J. Mol. Sci. 2022, 23, 11948. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Freeman, M.W. Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1702–1711. [Google Scholar] [CrossRef]
- Younis, N.; Sharma, R.; Soran, H.; Charlton-Menys, V.; Elseweidy, M.; Durrington, P.N. Glycation as an atherogenic modification of LDL. Curr. Opin. Lipidol. 2008, 19, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Tonti, L.; Roma, P.; Catapano, A.L. Apoptosis and proliferation of endothelial cells in early atherosclerotic lesions: Possible role of oxidised LDL. Nutr. Metab. Cardiovasc. Dis. 2002, 12, 297–305. [Google Scholar]
- Bhale, A.S.; Meilhac, O.; d’Hellencourt, C.L.; Vijayalakshmi, M.A.; Venkataraman, K. Cholesterol transport and beyond: Illuminating the versatile functions of HDL apolipoproteins through structural insights and functional implications. BioFactors 2024, 50, 922–956. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, X.A. Dysfunctional high-density lipoprotein. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ambale, V.B.; Volpe, G.J.; Donekal, S.; Mewton, N.; Liu, C.Y.; Shea, S.; Liu, K.; Burke, G.; Wu, C.; Bluemke, D.A.; et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: The Multi-Ethnic Study of Atherosclerosis study. Hypertension 2014, 64, 508–515. [Google Scholar] [CrossRef]
- Virk, R.; Cook, K.; Cavazos, A.; Wassall, S.R.; Gowdy, K.M.; Shaikh, S.R. How membrane phospholipids containing long chain polyunsaturated fatty acids and their oxidation products orchestrate lipid raft dynamics to control inflammation. J. Nutr. 2024, 154, 2862–2870. [Google Scholar] [CrossRef]
- Vladimirova-Kitova, L.; Deneva, T.; Angelova, E.; Nikolov, F.; Marinov, B.; Mateva, N. Relationship of asymmetric dimethylarginine with flow-mediated dilatation in subjects with newly detected severe hypercholesterolemia. Clin. Physiol. Funct. Imaging 2008, 28, 417–425. [Google Scholar] [CrossRef]
- Stancu, C.S.; Toma, L.; Sima, A.V. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012, 349, 433–446. [Google Scholar] [CrossRef]
- Ku, I.A.; Imboden, J.B.; Hsue, P.Y.; Ganz, P. Rheumatoid arthritis a model of systemic inflammation driving atherosclerosis. Circ. J. 2009, 73, 977–985. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefe Chroni, A.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708. [Google Scholar] [CrossRef] [PubMed]
- Speer, T.; Rohrer, L.; Blyszczuk, P.; Shroff, R.; Kuschnerus, K.; Kränkel, N.; Kania, G.; Zewinger, S.; Akhmedov, A.; Shi, Y.; et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013, 38, 754–768. [Google Scholar] [CrossRef]
- Mehta, J.L.; Chen, J.; Hermonat, P.L.; Romeo, F.; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006, 69, 36–45. [Google Scholar] [CrossRef]
- Aldonza, M.B.D.; Son, Y.S.; Sung, H.J.; Ahn, J.M.; Choi, Y.; Kim, Y.; Cho, S.; Cho, J. Paraoxonase-1 (PON1) induces metastatic potential and apoptosis escape via its antioxidative function in lung cancer cells. Oncotarget 2017, 8, 42817–42835. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Z.; Riwanto, M.; Gao, S.; Levison, B.S.; Gu, X.; Fu, X.; Wagner, M.A.; Besler, C.; Gerstenecker, G.; et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013, 123, 3815–3828. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, G.; May-Zhang, L.S.; Yermalitsky, V.; Dikalov, S.; Voynov, M.A.; Amarnath, V.; Kon, V.; Linton, M.F.; Vickers, K.C.; Davies, S.S. Myeloperoxidase-induced modification of HDL by isolevuglandins inhibits paraoxonase-1 activity. J. Biol. Chem. 2021, 297, 101019. [Google Scholar] [CrossRef]
- Sirca, T.B.; Mureșan, M.E.; Pallag, A.; Marian, E.; Jurca, T.; Vicaș, L.G.; Tunduc, I.P.; Manole, F.; Stefan, L. The Role of Polyphenols in Modulating PON1 Activity Regarding Endothelial Dysfunction and Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 2962. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated Pomegranate Reverts High-Density Lipoprotein (HDL)-Induced Endothelial Dysfunction and Reduces Postprandial Triglyceridemia in Women with Acute Coronary Syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef]
- Li, C.; Tu, Y.; Liu, T.R.; Guo, Z.G.; Xie, D.; Zhong, J.K.; Fan, Y.Z.; Lai, W.Y.; Lai, W.Y. Rosiglitazone attenuates atherosclerosis and increases high-density lipoprotein function in atherosclerotic rabbits. Int. J. Mol. Med. 2015, 35, 715–723. [Google Scholar] [CrossRef][Green Version]
- Engin, A. Endothelial Dysfunction in Obesity and Therapeutic Targets. Adv. Exp. Med. Biol. 2024, 1460, 489–538. [Google Scholar] [PubMed]
- Kirkpatrick, C.F.; Sikand, G.; Petersen, K.S.; Anderson, C.A.M.; Aspry, K.E.; Bolick, J.P.; Kris-Etherton, P.M.; Maki, K.C. Nutrition interventions for adults with dyslipidemia: A Clinical Perspective from the National Lipid Association. J. Clin. Lipidol. 2023, 17, 428–451. [Google Scholar] [CrossRef] [PubMed]
- Charchar, F.J.; Prestes, P.R.; Mills, C.; Mooi, C.S.; Dinesh, N.; Francine, M.; James, S.; Liffert, V.; Louise, B.; Lyudmilla, K.; et al. Lifestyle management of hypertension: International Society of Hypertension position paper endorsed by the World Hypertension League and European Society of Hypertension. J. Hypertens. 2024, 42, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Khan, A.A.; Has, P.; Jacobson, A.; Bogin, S.; Khalid, M.; Khan, A.; Kim, S.; Erqou, S.; Choudhary, G.; et al. Proprotein convertase subtilisn/kexin type 9 inhibitors and small interfering RNA therapy for cardiovascular risk reduction: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0295359. [Google Scholar] [CrossRef]
- Giglio, R.V.; Pantea Stoian, A.; Al-Rasadi, K.; Banach, M.; Patti, A.M.; Ciaccio, M.; Rizvi, A.A.; Rizzo, M. Novel Therapeutical Approaches to Managing Atherosclerotic Risk. Int. J. Mol. Sci. 2021, 22, 4633. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Sakuma, I.; Sohn, I.S.; Hayashi, T.; Shimada, K.; Koh, K.K. Best Treatment Strategies With Statins to Maximize the Cardiometabolic Benefits. Circ. J. 2018, 82, 937–943. [Google Scholar] [CrossRef]
- Jiang, J.; Gan, Z.; Li, Y.; Zhao, W.; Li, H.; Zheng, J.-P.; Ke, Y. REM sleep deprivation induces endothelial dysfunction and hypertension in middle-aged rats: Roles of the eNOS/NO/cGMP pathway and supplementation with L-arginine. PLoS ONE 2017, 12, e0182746. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Lerman, A. Endothelial dysfunction and coronary artery disease: Assessment, prognosis and treatment. Coron. Artery Dis. 2014, 25, 713. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Q.; Zhang, H.; Wang, C.; Xiu, R. Effects of nebivolol versus other antihypertensive drugs on the endothelial dysfunction in patients with essential hypertension. Biosci. Rep. 2020, 40, BSR20200436. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Abel, L.; Langford, O.; Monaghan, G.; Aronson, J.K.; Stevens, R.J.; Lay-Flurrie, S.; Koshiaris, C.; McManus, R.J.; Hobbs, F.D.R.; et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: Systematic review with pairwise, network, and dose-response meta-analyses. Bmj 2021, 374, n1537. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gutierrez, R.; Garcia-Leal, M.; Raygoza-Cortez, K.; Flores-Rodríguez, A.; Moreno-Alvarado, M.; Heredia-Martínez, E.M.; Vazquez-Baquerizo, B.; Guerra-Espiricueta, R.; Muñoz-Silva, V.; Gonzalez-Gonzalez, J.G. Benefits and harms of fibrate therapy in patients with type 2 diabetes: A systematic review and meta-analysis. Endocrine 2023, 81, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Tome, J.; Sehgal, K.; Kamboj, A.K.; Harmsen, W.S.; Khanna, S.; Pardi, D.S. Bile acid sequestrants in microscopic colitis: Clinical outcomes and utility of bile acid testing. Clin. Gastroenterol. Hepatol. 2023, 21, 3125–3131. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Meily, S.; Petersen, K.S.; Kris-Etherton, P.M. Effects of Cranberry Juice Supplementation on Cardiovascular Disease Risk Factors in Adults with Elevated Blood Pressure: A Randomized Controlled Trial. Nutrients 2021, 13, 2618. [Google Scholar] [CrossRef]
- Cho, K.H.; Yadav, D.; Kim, S.J.; Kim, J.R. Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules 2018, 23, 1080. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarallah, A.; Babiker, F. High Density Lipoprotein Reduces Blood Pressure and Protects Spontaneously Hypertensive Rats Against Myocardial Ischemia-Reperfusion Injury in an SR-BI Dependent Manner. Front. Cardiovasc. Med. 2022, 9, 825310. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Fermented soybean foods and diabetes. J. Diabetes Investig. 2023, 14, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Long, X.; Zou, Y.; Liu, F.; Li, Q. Mulberry leaf phenolics and fiber exert anti-obesity through the gut microbiota-host metabolism pathway. J. Food Sci. 2021, 86, 1432–1447. [Google Scholar] [CrossRef]
- Irondi, E.A.; Agboola, S.O.; Oboh, G.; Boligon, A.A.; Athayde, M.L.; Shode, F.O. Guava leaves polyphenolics-rich extract inhibits vital enzymes implicated in gout and hypertension in vitro. J. Intercult. Ethnopharmacol. 2016, 5, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Puhl, H.; Waeg, G.; Krebs, A.; Dieber-Rotheneder, M. The role of vitamin E in lipoprotein oxidation. In Vitamin E in Health and Disease; CRC Press: Boca Raton, FL, USA, 2023; pp. 649–672. [Google Scholar]
- Esser, D.; Mars, M.; Oosterink, E.; Stalmach, A.; Müller, M.; Afinan, L.A. Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men. FASEB J. 2014, 28, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.M.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: A pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 2013, 52, 153–160. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, L.; Zhang, J.; Mo, J.; Zhuang, P.; Zheng, L. Oxyresveratrol from mulberry branch extract protects HUVECs against oxidized Low-density Lipoprotein-induced oxidative injury via activation of the Nrf-2/HO-1 pathway. J. Funct. Foods 2022, 100, 105371. [Google Scholar] [CrossRef]
- Basu, A. Role of Berry Bioactive Compounds on Lipids and Lipoproteins in Diabetes and Metabolic Syndrome. Nutrients 2019, 11, 1983. [Google Scholar] [CrossRef]
- Tsiantas, K.; Konteles, S.J.; Kritsi, E.; Sinanoglou, V.J.; Tsiaka, T.; Zoumpoulakis, P. Effects of Non-Polar Dietary and Endogenous Lipids on Gut Microbiota Alterations: The Role of Lipidomics. Int. J. Mol. Sci. 2022, 23, 4070. [Google Scholar] [CrossRef]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.D.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Andersen, C.J.; Blesso, C.N.; Lee, J.; Barona, J.; Shah, D.; Thomas, M.J.; Fernandez, M.L. Egg consumption modulates HDL lipid composition and increases the cholesterol-accepting capacity of serum in metabolic syndrome. Lipids 2013, 48, 57–567. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative stress in human atherothrombosis: Sources, markers and therapeutic targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef]
- Bard, J.-M.; Paillard, F.; Lecerf, J.-M. Effect of phytosterols/stanols on LDL concentration and other surrogate markers of cardiovascular risk. Diabetes Metab. 2015, 41, 69–75. [Google Scholar] [CrossRef]
- Machado, V.A.; Santisteban, A.R.N.; Martins, C.M.; Damasceno, N.R.T.; Fonseca, F.A.; Neto, A.M.F.; Izar, M.C. Effects of phytosterol supplementation on lipoprotein subfractions and LDL particle quality. Sci. Rep. 2024, 14, 11108. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Godoy Ilha, A.; Sutti Nunes, V.; Silva Afonso, M.; Regina Nakandakare, E.; da Silva Ferreira, G.; de Paula Assis Bombo, R.; Rodrigues Giorgi, R.; Marcondes Machado, R.; Rocha Quintão, E.C.; Lottenberg, A.M. Phytosterols supplementation reduces endothelin-1 plasma concentration in moderately hypercholesterolemic individuals independently of their cholesterol-lowering properties. Nutrients 2020, 12, 1507. [Google Scholar] [CrossRef] [PubMed]
- Hwerif, N.; Raghif, A.; Kadhim, E. Effect of terpenes fraction of Iraqi cicer arietinum in experimentally induced hyperlipidemic mice. Int. J. Health Sci. 2022, 6, 10514–10530. [Google Scholar] [CrossRef]
- Ayala-Ruiz, L.A.; Ortega-Pérez, L.G.; Piñón-Simental, J.S.; Magana-Rodriguez, O.R.; Meléndez-Herrera, E.; Rios-Chavez, P. Role of the major terpenes of Callistemon citrinus against the oxidative stress during a hypercaloric diet in rats. Biomed. Pharmacother. 2022, 153, 113505. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, M.; Piao, L.; Li, X.; Yang, F.; Zhang, J.; Xiao, B.; Zhang, Q.; Song, W.L.; Yin, H.; et al. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br. J. Pharmacol. 2015, 172, 5647–5660. [Google Scholar] [CrossRef]
- McEwen, B.J.; Morel-Kopp, M.-C.; Chen, W.; Tofler, G.H.; Ward, C.M. Effects of omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease. Semin. Thromb. Hemost. 2013, 39, 025–032. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Amar, M.; Sampson, M.; Courville, A.B.; Sorokin, A.V.; Gordon, S.M.; Aponte, A.M.; Stagliano, M.; Playford, M.P.; Fu, Y.; et al. Comparison of omega-3 eicosapentaenoic acid versus docosahexaenoic acid-rich fish oil supplementation on plasma lipids and lipoproteins in normolipidemic adults. Nutrients 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Cartolano, F.D.C.; Dias, G.D.; Miyamoto, S.; Damasceno, N.R.T. Omega-3 fatty acids improve functionality of high-density lipoprotein in individuals with high cardiovascular risk: A randomized, parallel, controlled and double-blind clinical trial. Front. Nutr. 2022, 8, 767535. [Google Scholar] [CrossRef] [PubMed]
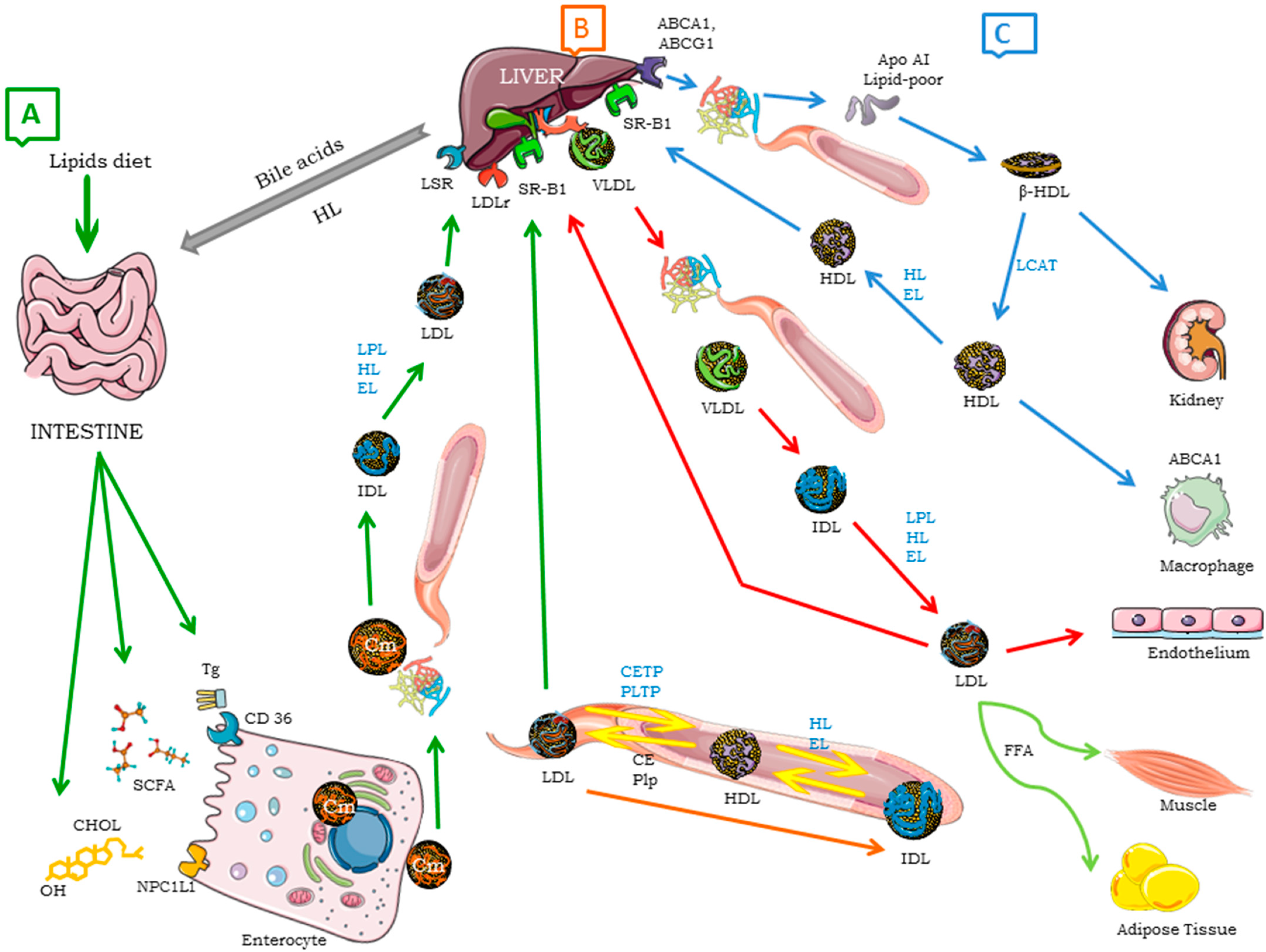
| Lipoproteins | Electrophoretic Mobility | Source of Synthesis | Flotation Density (g/mL) | Size (nm) | Main Chemical Composition | Main Apolipoproteins |
|---|---|---|---|---|---|---|
| Cm | Origin | Intestine | <0.940 | >70 | Triglycerides 90–95%, phospholipids 3–6%, cholesterol 1–3%, protein 1–2% * | Apo B-48, apo C-I, II, III, apo E, apo A-I, II, IV, V |
| VLDL | pre- β | Liver | 1.006 | 30–70 | Triglycerides 45–65%, phospholipids 15–20%, cholesterol 4–8%, protein 6–10% * | Apo B-100, apo C-I, II, III, apo E |
| IDL | β | Blood vessel | 1.019 | 20–30 | Triglycerides 20–30%, phospholipids 20–30%, cholesterol 35–40%, protein 15–20% * | Apo B-100, apo C-I, II, III, apo E |
| LDL | β | Blood vessel | 1.063 | 19–23 | Triglycerides 4–8%, phospholipids 18–24%, cholesterol 50–60%, protein 18–22% * | Apo B-100 |
| Lp (a) | pre-β | Liver | 1.085 | 21–26 | Triglycerides 3–5%, phospholipids 19–21%, cholesterol 40–45%, protein 27–29% * | Apo (a) |
| HDL (Subclasses 2a, 2b, 3a, 3b, 3c) | α | Liver, intestine | 1.210 | 7.5–12.5 | Triglycerides 2–7%, phospholipids 26–32%, cholesterol 20–25%, protein 45–55% * | Apo A-I, II, IV, apo C-I, II, III Apo D, apo E, apo M, apo J |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Melo, L.M.; Estrada-Luna, D.; Rubio-Ruiz, M.E.; Castañeda-Ovando, A.; Fernández-Martínez, E.; Jiménez-Osorio, A.S.; Pérez-Méndez, Ó.; Carreón-Torres, E. Relevance of Lipoprotein Composition in Endothelial Dysfunction and the Development of Hypertension. Int. J. Mol. Sci. 2025, 26, 1125. https://doi.org/10.3390/ijms26031125
Ramírez-Melo LM, Estrada-Luna D, Rubio-Ruiz ME, Castañeda-Ovando A, Fernández-Martínez E, Jiménez-Osorio AS, Pérez-Méndez Ó, Carreón-Torres E. Relevance of Lipoprotein Composition in Endothelial Dysfunction and the Development of Hypertension. International Journal of Molecular Sciences. 2025; 26(3):1125. https://doi.org/10.3390/ijms26031125
Chicago/Turabian StyleRamírez-Melo, Lisette Monsibaez, Diego Estrada-Luna, María Esther Rubio-Ruiz, Araceli Castañeda-Ovando, Eduardo Fernández-Martínez, Angélica Saraí Jiménez-Osorio, Óscar Pérez-Méndez, and Elizabeth Carreón-Torres. 2025. "Relevance of Lipoprotein Composition in Endothelial Dysfunction and the Development of Hypertension" International Journal of Molecular Sciences 26, no. 3: 1125. https://doi.org/10.3390/ijms26031125
APA StyleRamírez-Melo, L. M., Estrada-Luna, D., Rubio-Ruiz, M. E., Castañeda-Ovando, A., Fernández-Martínez, E., Jiménez-Osorio, A. S., Pérez-Méndez, Ó., & Carreón-Torres, E. (2025). Relevance of Lipoprotein Composition in Endothelial Dysfunction and the Development of Hypertension. International Journal of Molecular Sciences, 26(3), 1125. https://doi.org/10.3390/ijms26031125







