Palmitic but Not Oleic Acid Induces Pro-Inflammatory Dysfunction of Human Endothelial Cells from Different Vascular Beds In Vitro
Abstract
1. Introduction
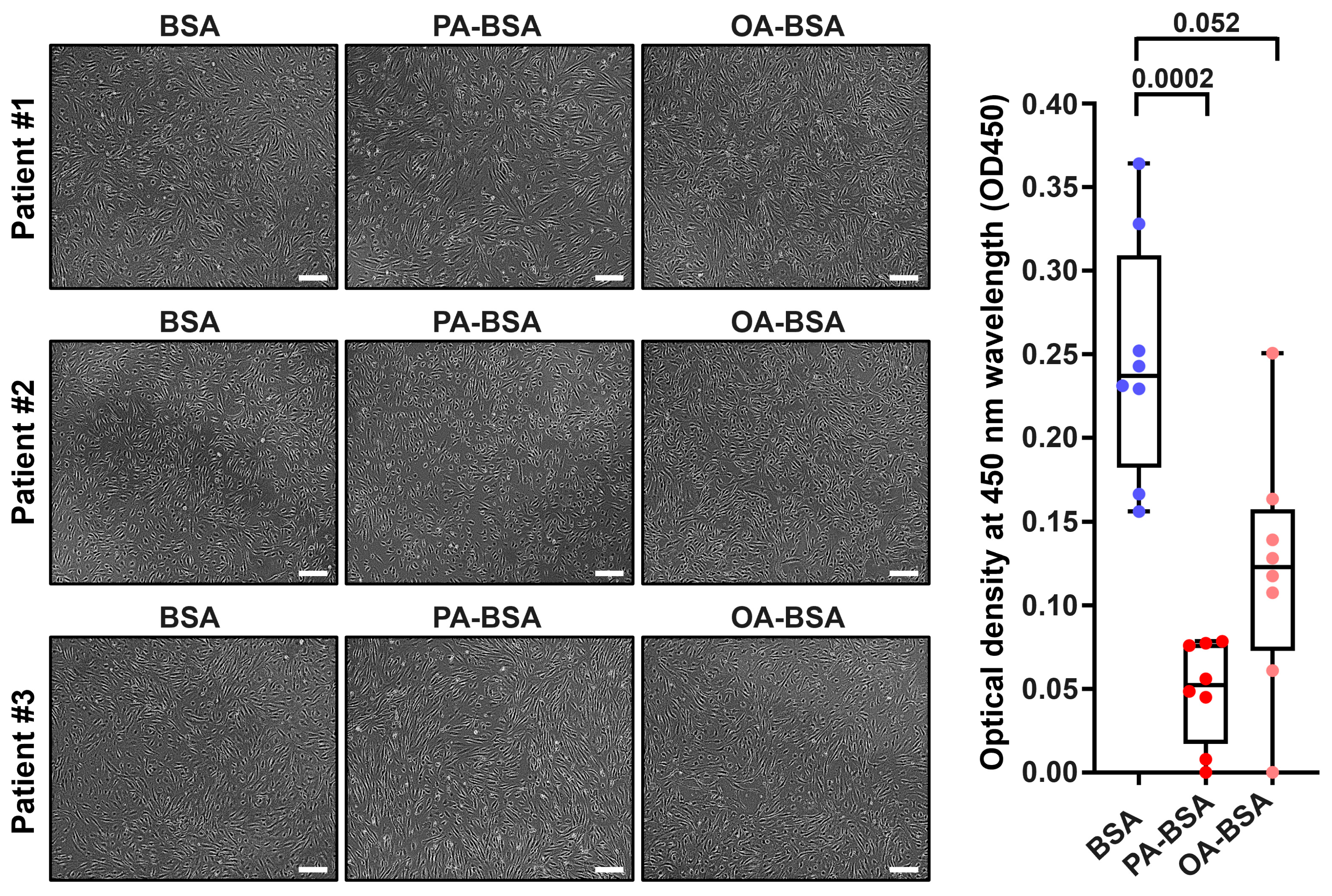
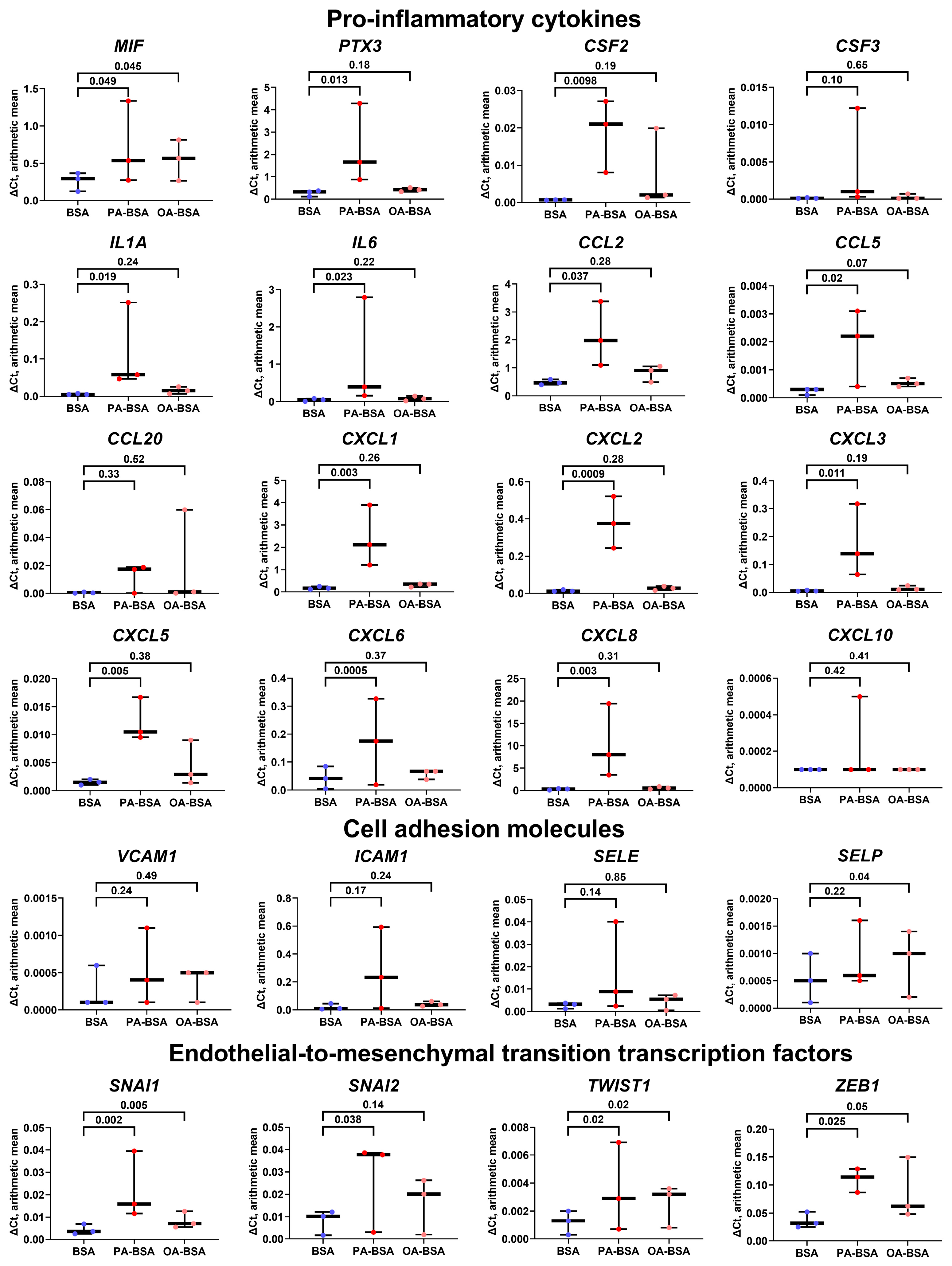
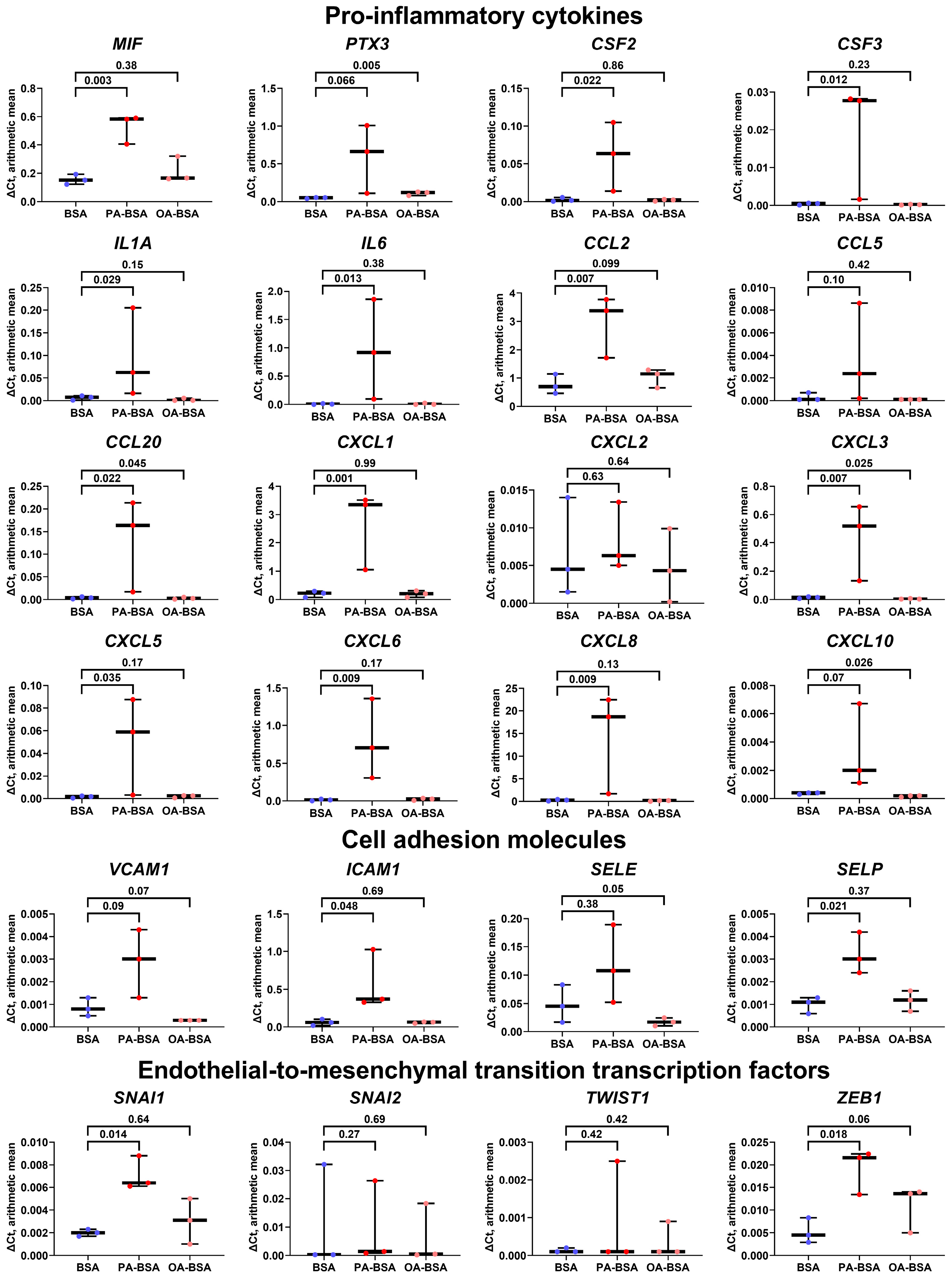
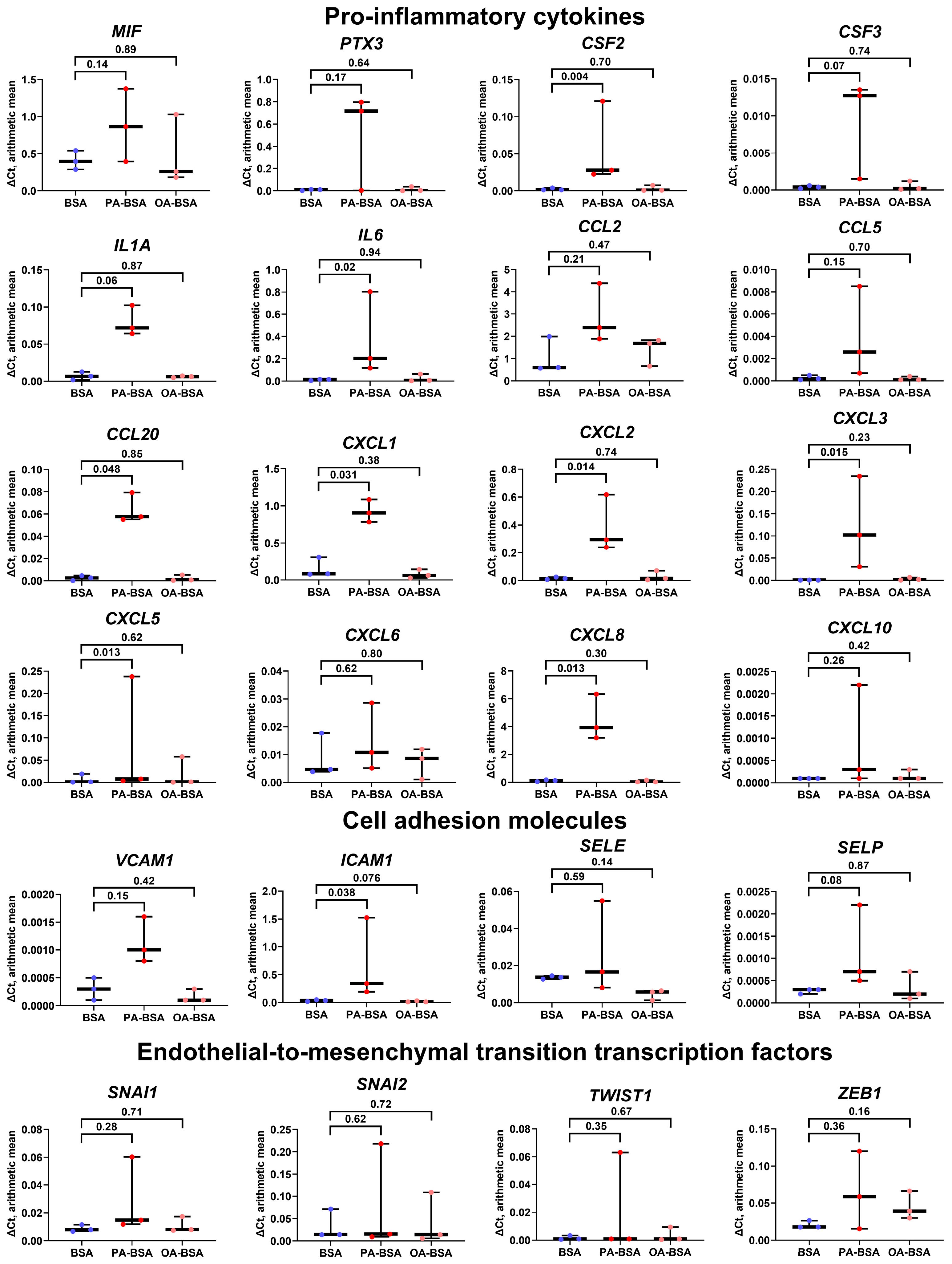
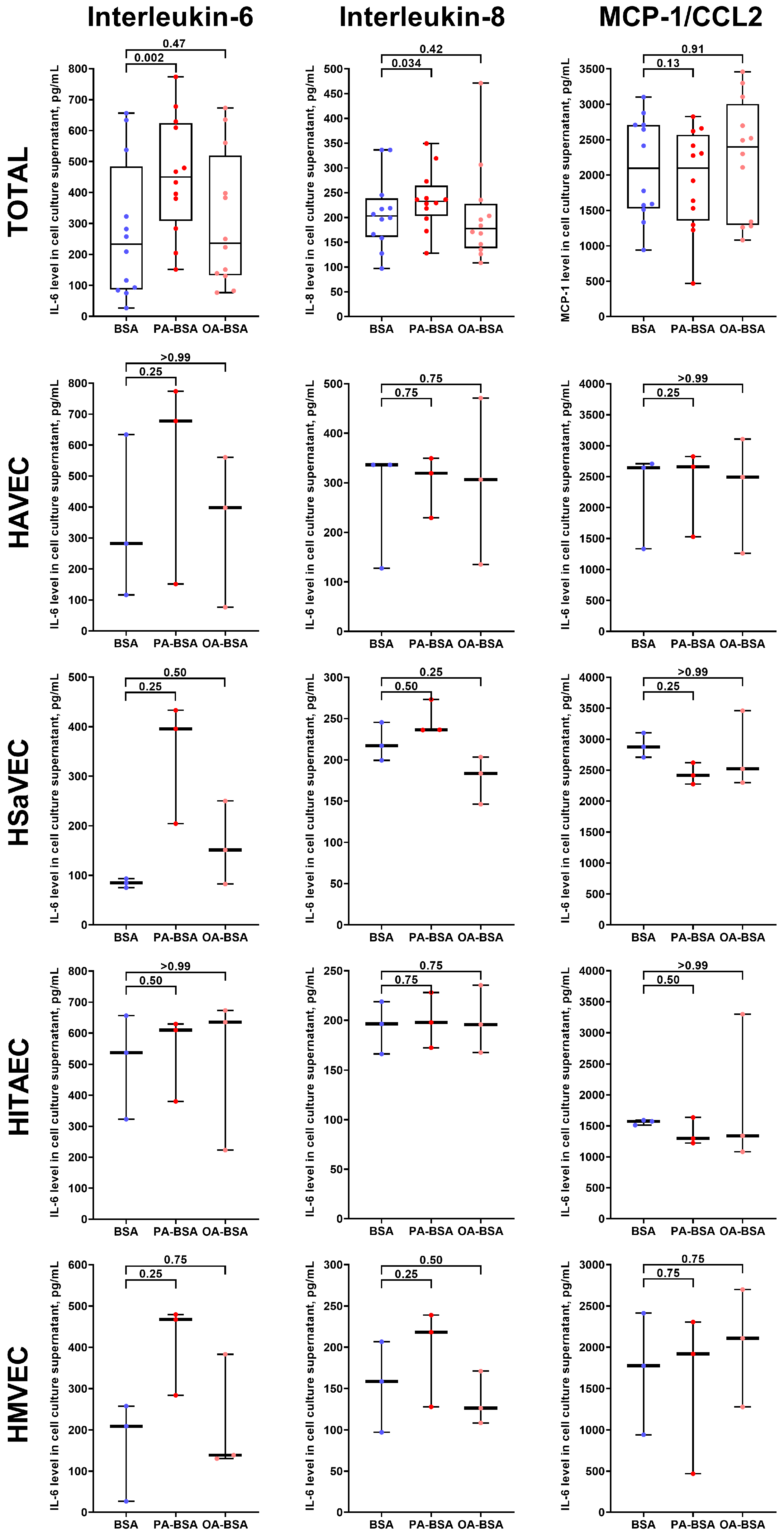
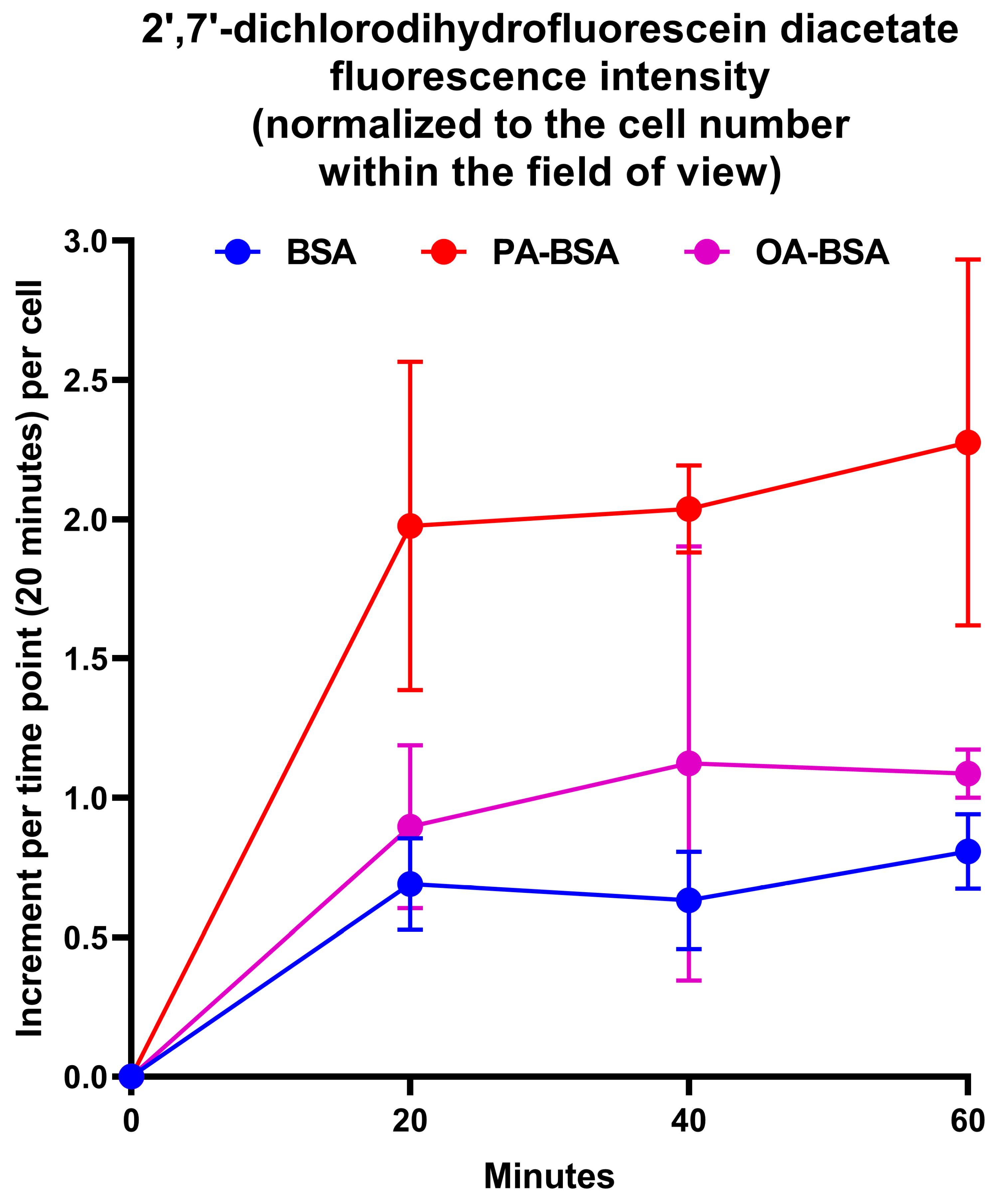
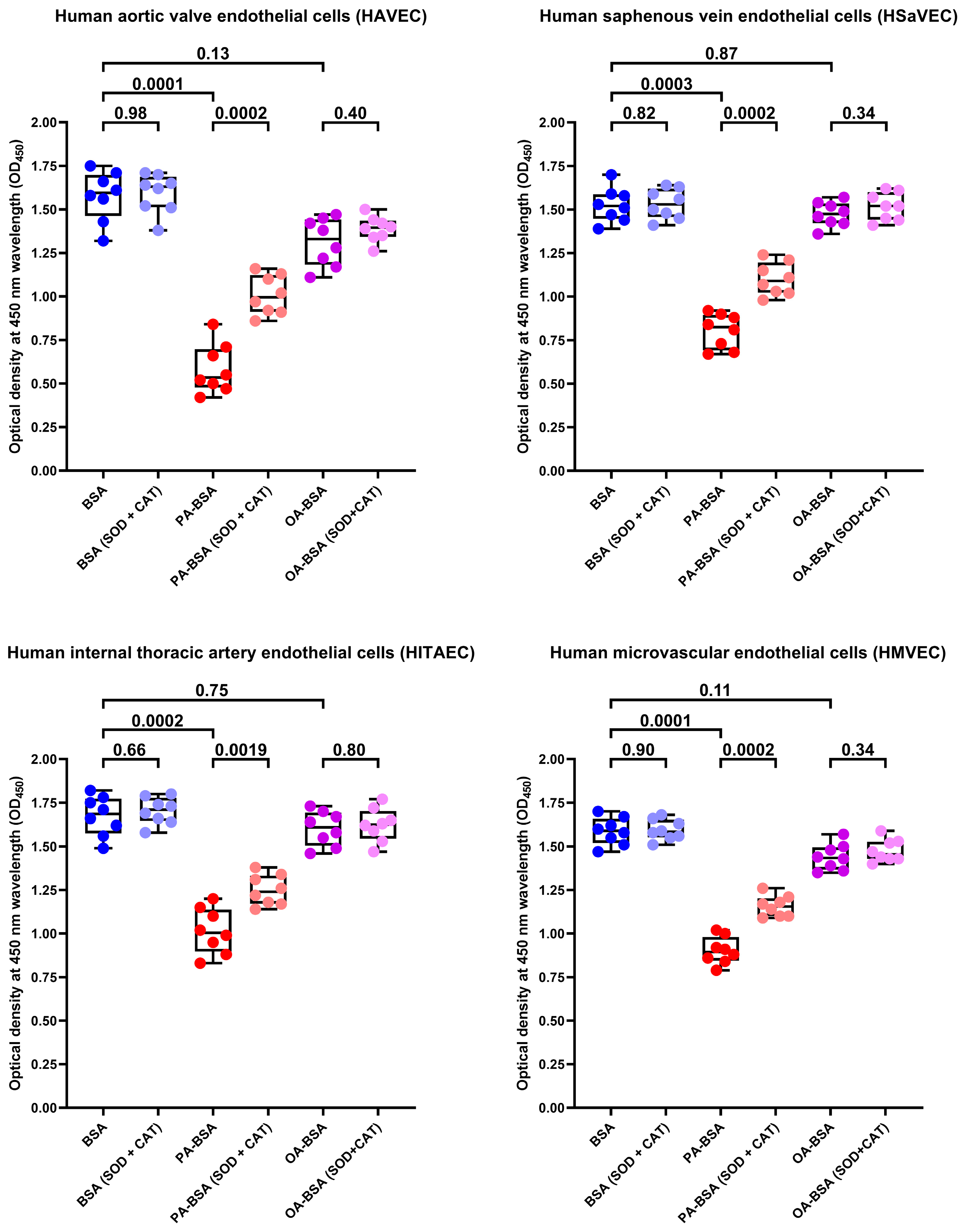
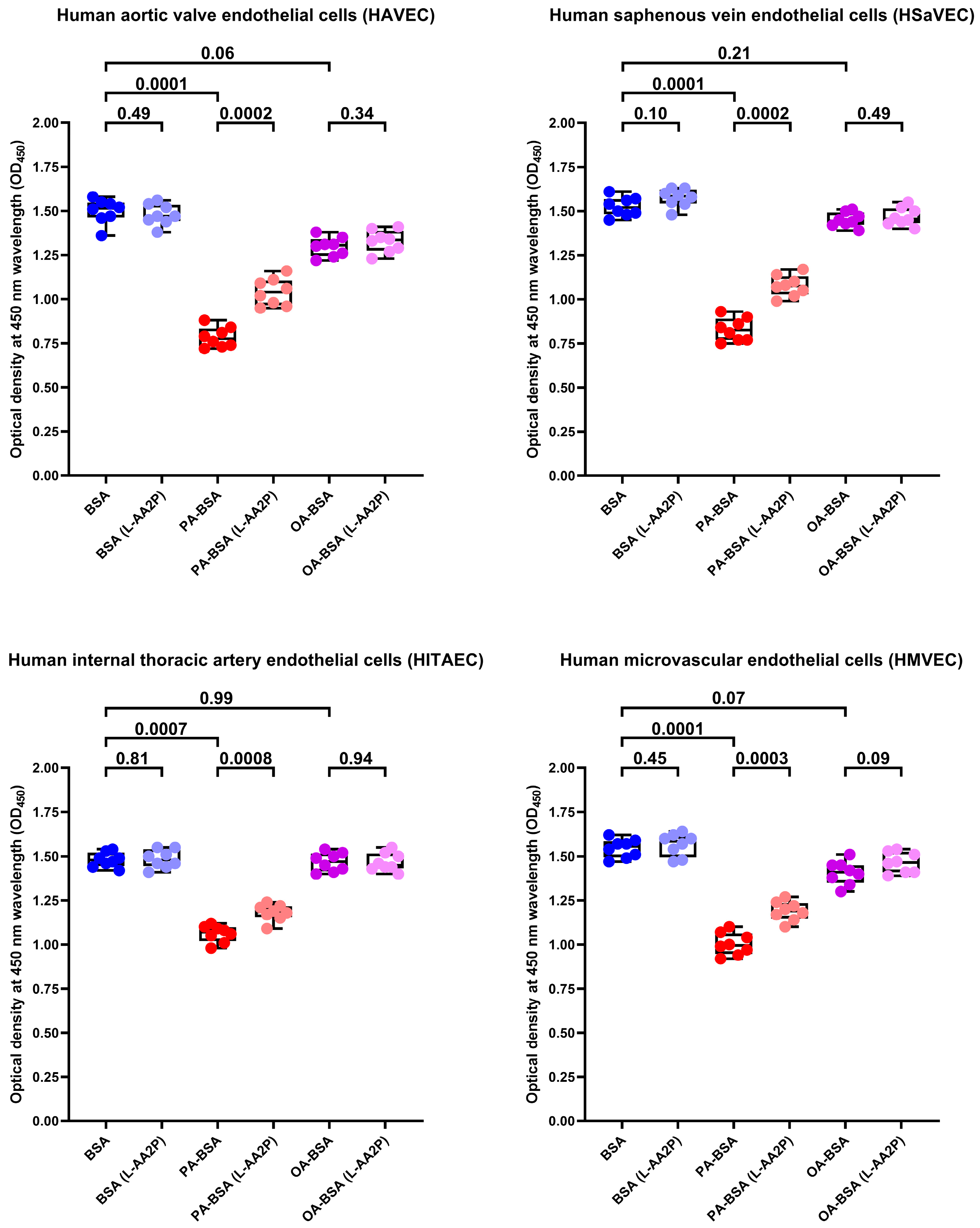
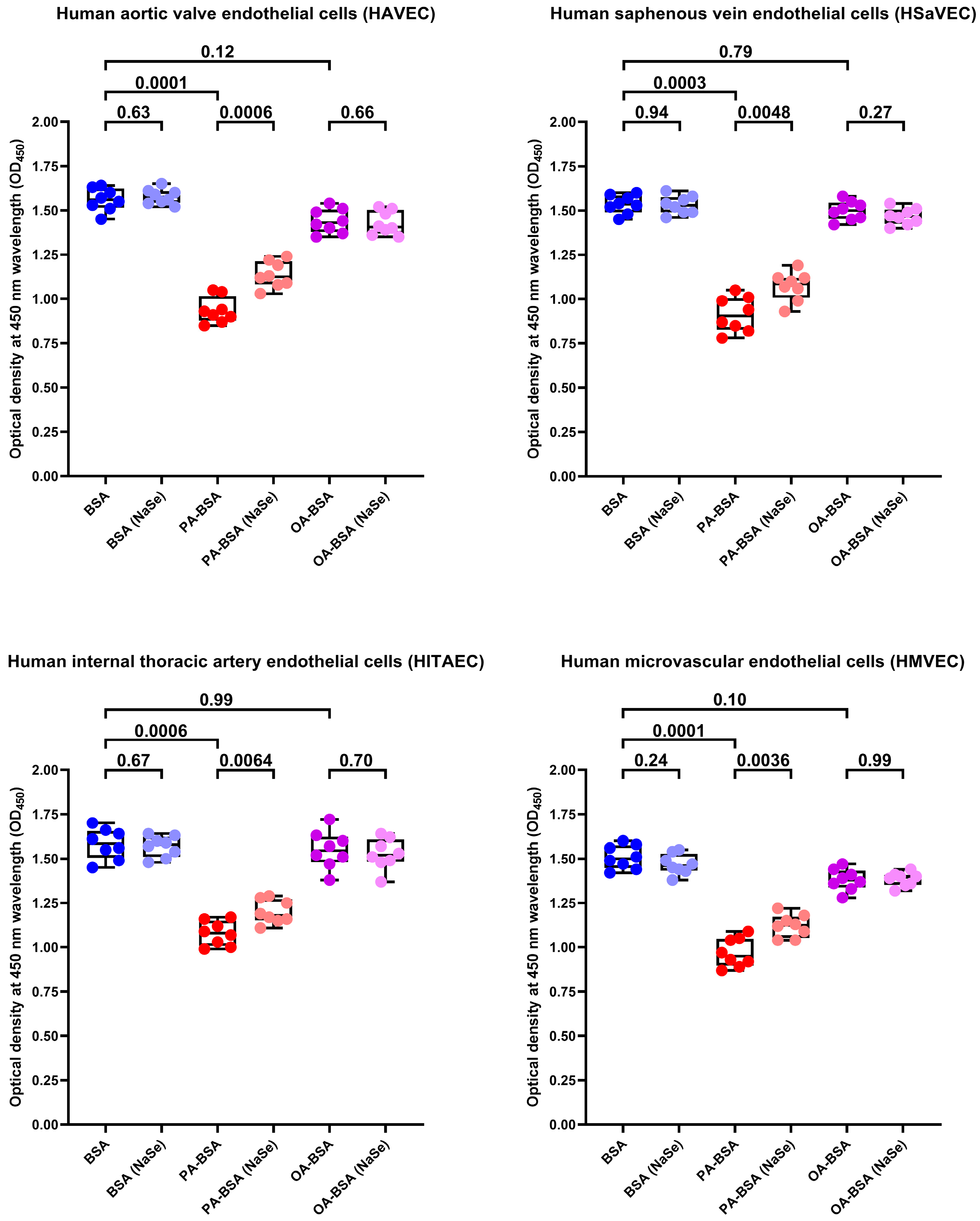
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of PA-BSA and OA-BSA Conjugates
4.2. Isolation of HAVEC, HSaVEC, HITAEC, and HMVEC
4.3. Phase Contrast Microscopy and Sample Collection
4.4. WST Assay for Cell Proliferation and Viability
4.5. Gene Expression Analysis
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Dot Blot Profiling
4.8. Measurement of Reactive Oxygen Species Generation
4.9. Rescue Experiments
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Vesga-Jiménez, D.J.; Martin, C.; Barreto, G.E.; Aristizábal-Pachón, A.F.; Pinzón, A.; González, J. Fatty Acids: An Insight into the Pathogenesis of Neurodegenerative Diseases and Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 2577. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Annevelink, C.E.; Sapp, P.A.; Petersen, K.S.; Shearer, G.C.; Kris-Etherton, P.M. Diet-derived and diet-related endogenously produced palmitic acid: Effects on metabolic regulation and cardiovascular disease risk. J. Clin. Lipidol. 2023, 17, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]
- Fattore, E.; Fanelli, R. Palm oil and palmitic acid: A review on cardiovascular effects and carcinogenicity. Int. J. Food Sci. Nutr. 2013, 64, 648–659. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty acids, epigenetic mechanisms and chronic diseases: A systematic review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Suiter, C.; Singha, S.K.; Khalili, R.; Shariat-Madar, Z. Free Fatty Acids: Circulating Contributors of Metabolic Syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2018, 16, 20–34. [Google Scholar] [CrossRef]
- Sun, L.; Zong, G.; Li, H.; Lin, X. Fatty acids and cardiometabolic health: A review of studies in Chinese populations. Eur. J. Clin. Nutr. 2021, 75, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, A.M.; Lenighan, Y.M.; O’Reilly, M.E.; McGillicuddy, F.C.; Roche, H.M. Nutritional modulation of metabolic inflammation. Biochem. Soc. Trans. 2017, 45, 979–985. [Google Scholar] [CrossRef]
- Kennedy, A.; Martinez, K.; Chuang, C.C.; LaPoint, K.; McIntosh, M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: Mechanisms of action and implications. J. Nutr. 2009, 139, 1–4. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Yang, X.; Wang, J.; Pan, C.; Chu, X.; Xiong, J.; Xie, J.; Chang, Y.; Wang, C.; Zhang, J. Obesity-induced elevated palmitic acid promotes inflammation and glucose metabolism disorders through GPRs/NF-κB/KLF7 pathway. Nutr. Diabetes 2022, 12, 23. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Gong, R.H.; Huang, C.; Chen, M.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Palmitic acid is an intracellular signaling molecule involved in disease development. Cell. Mol. Life Sci. 2019, 76, 2547–2557. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Heindel, J.J.; Lustig, R.H.; Howard, S.; Corkey, B.E. Obesogens: A unifying theory for the global rise in obesity. Int. J. Obes. 2024, 48, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Sonestedt, E.; Ericson, U.; Wallström, P.; Orho-Melander, M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br. J. Nutr. 2018, 119, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Koh, W.P.; Yuan, J.M.; Gross, M.D.; Pereira, M.A. Western-style fast food intake and cardiometabolic risk in an Eastern country. Circulation 2012, 126, 182–188. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Mendoza, K.; Smith-Warner, S.A.; Rossato, S.L.; Khandpur, N.; Manson, J.E.; Qi, L.; Rimm, E.B.; Mukamal, K.J.; Willett, W.C.; Wang, M.; et al. Ultra-processed foods and cardiovascular disease: Analysis of three large US prospective cohorts and a systematic review and meta-analysis of prospective cohort studies. Lancet Reg. Health Am. 2024, 37, 100859. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef]
- Rehman, K.; Haider, K.; Jabeen, K.; Akash, M.S.H. Current perspectives of oleic acid: Regulation of molecular pathways in mitochondrial and endothelial functioning against insulin resistance and diabetes. Rev. Endocr. Metab. Disord. 2020, 21, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Wang, Q.; Liu, R.; Chang, M.; Zhang, H.; Jin, Q.; Wang, X. Dietary oleic acid supplementation and blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 2508–2525. [Google Scholar] [CrossRef]
- Tutunchi, H.; Ostadrahimi, A.; Saghafi-Asl, M. The Effects of Diets Enriched in Monounsaturated Oleic Acid on the Management and Prevention of Obesity: A Systematic Review of Human Intervention Studies. Adv. Nutr. 2020, 11, 864–877. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Walzem, R.L.; Crouse, S.F.; Smith, D.R.; Adams, T.H.; Vaidyanathan, V.; Cao, X.; Smith, S.B. Consumption of high-oleic acid ground beef increases HDL-cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J. Nutr. 2011, 141, 1188–1194. [Google Scholar] [CrossRef]
- Nogoy, K.M.C.; Kim, H.J.; Lee, Y.; Zhang, Y.; Yu, J.; Lee, D.H.; Li, X.Z.; Smith, S.B.; Seong, H.A.; Choi, S.H. High dietary oleic acid in olive oil-supplemented diet enhanced omega-3 fatty acid in blood plasma of rats. Food Sci. Nutr. 2020, 8, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; McInerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Diabetes and the Mediterranean diet: A beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 2000, 93, 85–91. [Google Scholar] [CrossRef]
- Miklankova, D.; Markova, I.; Hüttl, M.; Stankova, B.; Malinska, H. The Different Insulin-Sensitising and Anti-Inflammatory Effects of Palmitoleic Acid and Oleic Acid in a Prediabetes Model. J. Diabetes Res. 2022, 2022, 4587907. [Google Scholar] [CrossRef] [PubMed]
- Galvão Cândido, F.; Xavier Valente, F.; da Silva, L.E.; Gonçalves Leão Coelho, O.; Gouveia Peluzio, M.D.C.; Gonçalves Alfenas, R.C. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Nutr. 2018, 57, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- Garcia, M.; Bihuniak, J.D.; Shook, J.; Kenny, A.; Kerstetter, J.; Huedo-Medina, T.B. The Effect of the Traditional Mediterranean-Style Diet on Metabolic Risk Factors: A Meta-Analysis. Nutrients 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- López-Gil, J.F.; García-Hermoso, A.; Martínez-González, M.Á.; Rodríguez-Artalejo, F. Mediterranean Diet and Cardiometabolic Biomarkers in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2421976. [Google Scholar] [CrossRef]
- Shramko, V.S.; Morozov, S.V.; Chernyak, E.I.; Shcherbakova, L.V.; Kurguzov, A.V.; Chernyavskyi, A.M.; Ragino, Y.I. Clinical characteristics of patients with coronary atherosclerosis depending on blood fatty acids. Complex Issues Cardiovasc. Dis. 2020, 9, 15–24. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Doundoulakis, I.; Farmakis, I.T.; Theodoridis, X.; Konstantelos, A.; Christoglou, M.; Kotzakioulafi, E.; Chrysoula, L.; Siargkas, A.; Karligkiotis, A.; Kyprianou, G.; et al. Effects of dietary interventions on cardiovascular outcomes: A network meta-analysis. Nutr. Rev. 2024, 82, 715–725. [Google Scholar] [CrossRef]
- Martinez-Lacoba, R.; Pardo-Garcia, I.; Amo-Saus, E.; Escribano-Sotos, F. Mediterranean diet and health outcomes: A systematic meta-review. Eur. J. Public Health 2018, 28, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Connor, W.E.; Connor, S.L. Importance of diet in the treatment of familial hypercholesterolemia. Am. J. Cardiol. 1993, 72, 42D–53D. [Google Scholar] [CrossRef]
- Padro, T.; Vilahur, G.; Sánchez-Hernández, J.; Hernández, M.; Antonijoan, R.M.; Perez, A.; Badimon, L. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J. Lipid Res. 2015, 56, 1043–1056. [Google Scholar] [CrossRef]
- Antoniazi, L.; Arroyo-Olivares, R.; Mata, P.; Santos, R.D. Association of dietary patterns and components with atherosclerosis risk biomarkers in familial hypercholesterolemia. Curr. Opin. Lipidol. 2022, 33, 89–94. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gómez, P.; Jiménez, Y.; Pérez Jiménez, F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Fischer, A. Notch Signaling in the Vasculature: Angiogenesis and Angiocrine Functions. Cold Spring Harb. Perspect. Med. 2023, 13, a041166. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R. Angiocrine endothelium: From physiology to atherosclerosis and cardiac repair. Vascul. Pharmacol. 2022, 144, 106993. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S.; Butler, J.M.; Ding, B.S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef]
- Bogdanov, L.; Shishkova, D.; Mukhamadiyarov, R.; Velikanova, E.; Tsepokina, A.; Terekhov, A.; Koshelev, V.; Kanonykina, A.; Shabaev, A.; Frolov, A.; et al. Excessive Adventitial and Perivascular Vascularisation Correlates with Vascular Inflammation and Intimal Hyperplasia. Int. J. Mol. Sci. 2022, 23, 12156. [Google Scholar] [CrossRef]
- Shishkova, D.; Markova, V.; Sinitsky, M.; Tsepokina, A.; Frolov, A.; Zagorodnikov, N.; Bogdanov, L.; Kutikhin, A. Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions. Int. J. Mol. Sci. 2020, 21, 8032. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Bringmans, T.; De Keulenaer, G.W. Endothelial dysfunction at the cellular level in three dimensions: Severity, acuteness, and distribution. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H398–H413. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Kutikhin, A.G.; Shishkova, D.K.; Velikanova, E.A.; Sinitsky, M.Y.; Sinitskaya, A.V.; Markova, V.E. Endothelial Dysfunction in the Context of Blood-Brain Barrier Modeling. J. Evol. Biochem. Physiol. 2022, 58, 781–806. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Chen, H.; Montagnani, M.; Sherman, A.; Quon, M.J. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: Insights from mechanistic modeling. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E629–E646. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial dysfunction and chronic inflammation: The cornerstones of vascular alterations in age-related diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Tardif, J.C.; Libby, P.; Camici, G.G. Inflamm-ageing: The role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Walker, K.A.; Basisty, N.; Wilson, D.M., III; Ferrucci, L. Connecting aging biology and inflammation in the omics era. J. Clin. Investig. 2022, 132, e158448. [Google Scholar] [CrossRef]
- Calila, H.; Bălășescu, E.; Nedelcu, R.I.; Ion, D.A. Endothelial Dysfunction as a Key Link between Cardiovascular Disease and Frailty: A Systematic Review. J. Clin. Med. 2024, 13, 2686. [Google Scholar] [CrossRef] [PubMed]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Does vascular endothelial dysfunction play a role in physical frailty and sarcopenia? A systematic review. Age Ageing 2021, 50, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Targosz-Korecka, M.; Suraj, J.; Proniewski, B.; Jasztal, A.; Marczyk, B.; Sternak, M.; Przybyło, M.; Kurpińska, A.; Walczak, M.; et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J. Am. Heart Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, L.; Vidal-Gómez, X.; Soleti, R.; Vergori, L.; Duluc, L.; Chwastyniak, M.; Bisserier, M.; Le Lay, S.; Villard, A.; Simard, G.; et al. Large Extracellular Vesicle-Associated Rap1 Accumulates in Atherosclerotic Plaques, Correlates with Vascular Risks and Is Involved in Atherosclerosis. Circ. Res. 2020, 127, 747–760. [Google Scholar] [CrossRef]
- Hirase, T.; Node, K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H499–H505. [Google Scholar] [CrossRef]
- Shishkova, D.; Lobov, A.; Zainullina, B.; Matveeva, V.; Markova, V.; Sinitskaya, A.; Velikanova, E.; Sinitsky, M.; Kanonykina, A.; Dyleva, Y.; et al. Calciprotein Particles Cause Physiologically Significant Pro-Inflammatory Response in Endothelial Cells and Systemic Circulation. Int. J. Mol. Sci. 2022, 23, 14941. [Google Scholar] [CrossRef]
- Shishkova, D.K.; Velikanova, E.A.; Bogdanov, L.A.; Sinitsky, M.Y.; Kostyunin, A.E.; Tsepokina, A.V.; Gruzdeva, O.V.; Mironov, A.V.; Mukhamadiyarov, R.A.; Glushkova, T.V.; et al. Calciprotein Particles Link Disturbed Mineral Homeostasis with Cardiovascular Disease by Causing Endothelial Dysfunction and Vascular Inflammation. Int. J. Mol. Sci. 2021, 22, 12458. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, A.; Shishkova, D.; Markova, V.; Markova, Y.; Frolov, A.; Lazebnaya, A.; Oshchepkova, K.; Perepletchikova, D.; Smirnova, D.; Basovich, L.; et al. Proteomic Profiling of Endothelial Cell Secretomes After Exposure to Calciprotein Particles Reveals Downregulation of Basement Membrane Assembly and Increased Release of Soluble CD59. Int. J. Mol. Sci. 2024, 25, 11382. [Google Scholar] [CrossRef]
- Shishkova, D.; Markova, V.; Sinitsky, M.; Tsepokina, A.; Velikanova, E.; Bogdanov, L.; Glushkova, T.; Kutikhin, A. Calciprotein Particles Cause Endothelial Dysfunction under Flow. Int. J. Mol. Sci. 2020, 21, 8802. [Google Scholar] [CrossRef]
- Shishkova, D.; Velikanova, E.; Sinitsky, M.; Tsepokina, A.; Gruzdeva, O.; Bogdanov, L.; Kutikhin, A. Calcium Phosphate Bions Cause Intimal Hyperplasia in Intact Aortas of Normolipidemic Rats through Endothelial Injury. Int. J. Mol. Sci. 2019, 20, 5728. [Google Scholar] [CrossRef]
- Xi, H.; Zhang, Y.; Xu, Y.; Yang, W.Y.; Jiang, X.; Sha, X.; Cheng, X.; Wang, J.; Qin, X.; Yu, J.; et al. Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells. Circ. Res. 2016, 118, 1525–1539. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Sha, X.; Xi, H.; Li, Y.-F.; Shao, Y.; Mai, J.; Lopez-Pastrana, J.; Meng, S.; Tilley, D.G.; et al. Early hyperlipidemia promotes endothelial activation via a caspase-1–sirtuin 1 pathway. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Wang, J.; Chen, J.; Zhang, Q.; Lin, X.; Zhang, R.; Bai, H.; Hua, Y.; Wang, H.; Huang, M.; et al. HCC-1 Accelerates Atherosclerosis by Inducing Endothelial Cell and Macrophage Pyroptosis and Serves as an Early Diagnostic Biomarker. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2088–2107. [Google Scholar] [CrossRef]
- Potere, N.; Garrad, E.; Kanthi, Y.; Di Nisio, M.; Kaplanski, G.; Bonaventura, A.; Connors, J.M.; De Caterina, R.; Abbate, A. NLRP3 inflammasome and interleukin-1 contributions to COVID-19-associated coagulopathy and immunothrombosis. Cardiovasc. Res. 2023, 119, 2046–2060. [Google Scholar] [CrossRef]
- Sampilvanjil, A.; Karasawa, T.; Yamada, N.; Komada, T.; Higashi, T.; Baatarjav, C.; Watanabe, S.; Kamata, R.; Ohno, N.; Takahashi, M. Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H508–H518. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, V.; Kumar, A.; Sadaf, M.I.; Lu, E.; Al’Aref, S.J.; Tarun, T.; Galiatsatos, P.; Gulati, M.; Blumenthal, R.S.; Leucker, T.M.; et al. COVID-19 in the initiation and progression of atherosclerosis: Pathophysiology during and beyond the acute phase. JACC Adv. 2024, 3, 101107. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- McMullan, R.R.; McAuley, D.F.; O’Kane, C.M.; Silversides, J.A. Vascular leak in sepsis: Physiological basis and potential therapeutic advances. Crit. Care 2024, 28, 97. [Google Scholar] [CrossRef] [PubMed]
- Maneta, E.; Aivalioti, E.; Tual-Chalot, S.; Emini Veseli, B.; Gatsiou, A.; Stamatelopoulos, K.; Stellos, K. Endothelial dysfunction and immunothrombosis in sepsis. Front. Immunol. 2023, 14, 1144229. [Google Scholar] [CrossRef]
- De Backer, D.; Ricottilli, F.; Ospina-Tascón, G.A. Septic shock: A microcirculation disease. Curr. Opin. Anaesthesiol. 2021, 34, 85–91. [Google Scholar] [CrossRef]
- Tang, F.; Zhao, X.L.; Xu, L.Y.; Zhang, J.N.; Ao, H.; Peng, C. Endothelial dysfunction: Pathophysiology and therapeutic targets for sepsis-induced multiple organ dysfunction syndrome. Biomed. Pharmacother. 2024, 178, 117180. [Google Scholar] [CrossRef]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 2021, 1, 598–615, Erratum in Nat. Aging. 2018, 1, 748. https://doi.org/10.1038/s43587-021-00102-x. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Long, Y.; Yu, F.; Ji, C.; Gui, L.; Lu, Y. Resveratrol improves gasdermin D-mediated pyroptosis of vascular endothelial cells induced by a high-fat diet and palmitic acid possibly via the SIRT1-p66Shc-NLRP3 pathway. J. Nutr. Biochem. 2025, 140, 109890. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Cai, W.; Li, Q.; Zhao, L.; Meng, Y.; Xu, H. Activation of lysosomal Ca2+ channels mitigates mitochondrial damage and oxidative stress. J. Cell Biol. 2025, 224, e202403104. [Google Scholar] [CrossRef]
- Samsonov, M.V.; Podkuychenko, N.V.; Khapchaev, A.Y.; Efremov, E.E.; Yanushevskaya, E.V.; Vlasik, T.N.; Lankin, V.Z.; Stafeev, I.S.; Skulachev, M.V.; Shestakova, M.V.; et al. AICAR protects vascular endothelial cells from oxidative injury induced by the long-term palmitate excess. Int. J. Mol. Sci. 2021, 23, 211. [Google Scholar] [CrossRef]
- Khapchaev, A.Y.; Vorotnikov, A.V.; Antonova, O.A.; Samsonov, M.V.; Shestakova, E.A.; Sklyanik, I.A.; Tomilova, A.O.; Shestakova, M.V.; Shirinsky, V.P. Shear stress and the AMP-activated protein kinase independently protect the vascular endothelium from palmitate lipotoxicity. Biomedicines 2024, 12, 339. [Google Scholar] [CrossRef]
- Belosludtseva, N.V.; Ilzorkina, A.I.; Serov, D.A.; Dubinin, M.V.; Talanov, E.Y.; Karagyaur, M.N.; Primak, A.L.; Liu, J.; Belosludtsev, K.N. ANT-mediated inhibition of the permeability transition pore alleviates palmitate-induced mitochondrial dysfunction and lipotoxicity. Biomolecules 2024, 14, 1159. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, G.; Chen, N.; Zhang, W.; Gao, Q.; Li, T.; Yuan, N.; Jin, H. CTRP13 alleviates palmitic acid-induced inflammation, oxidative stress, apoptosis and endothelial cell dysfunction in HUVECs. Tissue Cell 2024, 86, 102232. [Google Scholar] [CrossRef]
- Shamas, S.; Rahil, R.R.; Kaushal, L.; Sharma, V.K.; Wani, N.A.; Qureshi, S.H.; Ahmad, S.F.; Attia, S.M.; Zargar, M.A.; Hamid, A.; et al. Pyroptosis in endothelial cells and extracellular vesicle release in atherosclerosis via NF-κB-caspase-4/5-GSDM-D pathway. Pharmaceuticals 2024, 17, 1568. [Google Scholar] [CrossRef]
- He, Y.; Li, S.; Jiang, L.; Wu, K.; Chen, S.; Su, L.; Liu, C.; Liu, P.; Luo, W.; Zhong, S.; et al. Palmitic acid accelerates endothelial cell injury and cardiovascular dysfunction via palmitoylation of PKM2. Adv. Sci. 2025, 12, e2412895. [Google Scholar] [CrossRef]
- Lu, Y.; Qian, L.; Zhang, Q.; Chen, B.; Gui, L.; Huang, D.; Chen, G.; Chen, L. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life Sci. 2013, 92, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.; Lee, J.; Lee, E.Y.; Yoon, K.H.; Lee, S.H. Lipid Variability Induces Endothelial Dysfunction by Increasing Inflammation and Oxidative Stress. Endocrinol. Metab. 2024, 39, 511–520. [Google Scholar] [CrossRef]
- Rao, C.; Liu, B.; Huang, D.; Chen, R.; Huang, K.; Li, F.; Dong, N. Nucleophosmin contributes to vascular inflammation and endothelial dysfunction in atherosclerosis progression. J. Thorac. Cardiovasc. Surg. 2021, 161, e377–e393. [Google Scholar] [CrossRef]
- Maloney, E.; Sweet, I.R.; Hockenbery, D.M.; Pham, M.; Rizzo, N.O.; Tateya, S.; Handa, P.; Schwartz, M.W.; Kim, F. Activation of NF-κB by palmitate in endothelial cells: A key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1370–1375. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, X.L.; Zhang, D.Y.; Gao, X.J.; Zhou, L.; Qin, X.Y.; Xie, G.Y.; Liu, K.; Qin, Y.; Liu, B.L.; et al. Tectorigenin Attenuates Palmitate-Induced Endothelial Insulin Resistance via Targeting ROS-Associated Inflammation and IRS-1 Pathway. PLoS ONE 2013, 8, e66417. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, H.; Xiang, H.; Zhou, J.; Zeng, Z.; Chen, R.; Zhao, S.; Xiao, J.; Shu, Z.; Chen, S.; et al. Palmitic acid-induced autophagy increases reactive oxygen species via the Ca2+/PKCα/NOX4 pathway and impairs endothelial function in human umbilical vein endothelial cells. Exp. Ther. Med. 2019, 17, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.J.; Zhao, L.M.; Pang, Z.D.; She, G.; Song, Z.; Cheng, X.; Du, X.J.; Deng, X.L. Oxidative stress induced by palmitic acid modulates K(Ca)2.3 channels in vascular endothelium. Exp. Cell Res. 2019, 383, 111552. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, G.; Zhang, J.; Zhang, Q.; Li, X.; Qin, S.; Lv, S.; Ding, J.; Zhang, Q.; Xia, Y.; et al. Protein phosphatase 4 mediates palmitic acid-induced endothelial dysfunction by decreasing eNOS phosphorylation at serine 633 in HUVECs. Exp. Cell Res. 2024, 437, 113998. [Google Scholar] [CrossRef]
- Tan, X.H.; Gu, Y.Y.; Song, W.P.; Nan, T.G.; Song, W.D.; Fang, D.; Yuan, Y.M.; Xin, Z.C.; Li, X.S.; Guan, R.L. Transcriptome analysis highlights the role of ferroptosis in palmitic acid-induced endothelial dysfunction. Sex. Med. 2023, 11, qfac008. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, Y.P.; Zhu, J.Q.; Zhou, G.H.; Zhang, F.; An, Q.; Yang, J.; Cho, K.W.; Jin, S.N.; Wen, J.F. Physcion prevents high-fat diet-induced endothelial dysfunction by inhibiting oxidative stress and endoplasmic reticulum stress pathways. Eur. J. Pharmacol. 2023, 943, 175554. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, F.Y.; Lu, C.J.; Yi, S.W. Baicalein alleviates palmitic acid-induced endothelial cell dysfunction via inhibiting endoplasmic reticulum stress. Clin. Hemorheol. Microcirc. 2024, 88, 235–245, Erratum in Clin. Hemorheol. Microcirc. 2025, 90, 45. https://doi.org/10.1177/13860291251349301. [Google Scholar] [CrossRef]
- Ma, X.H.; Liu, G.P.; Liu, L.; Dou, Z.Y.; He, X.; Chen, X. Downregulation of HDAC9 alleviates autophagy dysfunction by inducing acetylation of ATG4B in metabolic dysfunction-associated steatotic liver disease. FASEB J. 2025, 39, e70991. [Google Scholar] [CrossRef]
- Zhu, H.X.; Ren, J.L.; Cao, W.J.; Wang, R.; Chen, L.L.; Gao, Q.; Zhou, Y.B. Intermedin(1–53) improves atherosclerosis by reducing local endothelial damage via AMPK signaling pathway in obese apoE-deficient mice. J. Inflamm. Res. 2025, 18, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ma, T.; Qu, Y.; Ye, R.; Feng, J.; Bai, G.; Hong, A.; Ma, Y. PACAP inhibits high fat-induced NLRP3 inflammasome-mediated pyroptosis in vascular endothelial cells by regulating the SIRT1/ROS pathway. Mol. Cell. Endocrinol. 2025, 608, 112633. [Google Scholar] [CrossRef]
- Hu, F.; Li, J.; Zhang, X.; Fu, Y.; Mao, Y.; Tong, S.; Xu, H. bFGF alleviates diabetic endothelial dysfunction by downregulating endoplasmic reticulum stress. Acta Diabetol. 2025, 62, 11, Erratum in Acta Diabetol. 2025, in press. https://doi.org/10.1007/s00592-025-02551-x. [Google Scholar] [CrossRef]
- Hudgins, E.C.; Johnson, E.J.; Rokka, S.; Kashyap, B.; Mahugu, A.; Nguyen, T.; Tascone, A.R.; McCarthy, E.; Halbert, C.; Fancher, I.S. Inhibition of lipolysis in visceral adipose tissue from obese mice and humans prevents impairment of endothelial Kir2.1 channels. Channels 2025, 19, 2564651. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Mohammed, S.; Mengozzi, A.; Duranti, E.; Delfine, V.; Geiger, M.A.; Hamdani, N.; Taddei, S.; Masi, S.; Virdis, A.; et al. Targeting the epigenetic remodeler GCN5 prevents vascular oxidative stress and endothelial dysfunction in obesity: Insights in patients with cardiometabolic disease. High Blood Press. Cardiovasc. Prev. 2025, 32, 577–589. [Google Scholar] [CrossRef]
- Huang, J.; Yan, J.; Wan, Z.; Ji, T.; Li, H.; Liang, W.; Huang, Y.; Yang, Z.; Xiao, Y.; Nie, H.; et al. Targeting MGLL: Terazosin regulates glycerolipid metabolism to mitigate endothelial cell senescence. J. Lipid Res. 2025, in press. [Google Scholar] [CrossRef]
- Khoi, C.S.; Lin, T.Y.; Chiang, C.K. Targeting insulin resistance, reactive oxygen species, inflammation, programmed cell death, ER Stress, and mitochondrial dysfunction for the therapeutic prevention of free fatty acid-induced vascular endothelial lipotoxicity. Antioxidants 2024, 13, 1486. [Google Scholar] [CrossRef]
- Habib, M.R.; Tokutake, Y.; Yonekura, S. Palmitic acid-induced cell death: Impact of endoplasmic reticulum and oxidative stress, mitigated by L-citrulline. Anim. Biosci. 2025, 38, 54–66. [Google Scholar] [CrossRef]
- Ceja-Galicia, Z.A.; Cespedes-Acuña, C.L.A.; El-Hafidi, M. Protection strategies against palmitic acid-induced lipotoxicity in metabolic syndrome and related diseases. Int. J. Mol. Sci. 2025, 26, 788. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Tysseling, K.A.; Rice, J.; Pham, M.; Haji, L.; Gallis, B.M.; Baas, A.S.; Paramsothy, P.; Giachelli, C.M.; Corson, M.A.; et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Azizi, P.M.; Li, Y.E.; Liu, J.; Wang, C.; Chan, K.L.; Hopperton, K.E.; Bazinet, R.P.; Heit, B.; Bilan, P.J.; et al. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E35–E44. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.D.; McMillin, S.L.; Riehle, C.; Tanner, J.; Palionyte, M.; Hillas, E.; Jones, D.; Cooksey, R.C.; Birnbaum, M.J.; McClain, D.A.; et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ. Res. 2009, 104, 1085–1094. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, Z.; Huang, B.; Luo, S.; Guo, Y. Palmitic acid promotes endothelial-to-mesenchymal transition via activation of the cytosolic DNA-sensing cGAS–STING pathway. Arch. Biochem. Biophys. 2022, 727, 109321. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, G.; Wang, Y.; Yue, Y.; Zhao, W. A key mediator, PTX3, of IKK/IκB/NF-κB exacerbates human umbilical vein endothelial cell injury and dysfunction. Int. J. Clin. Exp. Pathol. 2014, 7, 7699–7707. [Google Scholar]
- Mao, Y.; Luo, W.; Zhang, L.; Wu, W.; Yuan, L.; Xu, H.; Song, J.; Fujiwara, K.; Abe, J.I.; LeMaire, S.A.; et al. STING–IRF3 triggers endothelial inflammation in response to free fatty acid-induced mitochondrial damage in diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 920–929, Erratum in Arterioscler. Thromb. Vasc. Biol. 2018, 38, e60. https://doi.org/10.1161/ATV.0000000000000069. [Google Scholar] [CrossRef]
- Bharat, D.; Cavalcanti, R.R.M.; Petersen, C.; Begaye, N.; Cutler, B.R.; Costa, M.M.A.; Ramos, R.K.L.G.; Ferreira, M.R.; Li, Y.; Bharath, L.P.; et al. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2018, 62, 1700601. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Bhatnagar, S.; Hahn, D.J.; Kim, J.A. Long-chain acyl-CoA synthetase-1 mediates the palmitic acid-induced inflammatory response in human aortic endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E893–E903. [Google Scholar] [CrossRef]
- Chei, C.L.; Yamagishi, K.; Kitamura, A.; Kiyama, M.; Sankai, T.; Okada, T.; Imano, H.; Ohira, T.; Cui, R.; Umesawa, M.; et al. Serum fatty acid and risk of coronary artery disease—Circulatory Risk in Communities Study (CIRCS). Circ. J. 2018, 82, 3013–3020. [Google Scholar] [CrossRef]
- Satizabal, C.L.; Samieri, C.; Davis-Plourde, K.L.; Voetsch, B.; Aparicio, H.J.; Pase, M.P.; Romero, J.R.; Helmer, C.; Vasan, R.S.; Kase, C.S.; et al. APOE and the association of fatty acids with the risk of stroke, coronary heart disease, and mortality. Stroke 2018, 49, 2822–2829. [Google Scholar] [CrossRef]
- Lai, H.T.M.; de Oliveira Otto, M.C.; Lee, Y.; Wu, J.H.Y.; Song, X.; King, I.B.; Psaty, B.M.; Lemaitre, R.N.; McKnight, B.; Siscovick, D.S.; et al. Serial plasma phospholipid fatty acids in the de novo lipogenesis pathway and total mortality, cause-specific mortality, and cardiovascular diseases in the Cardiovascular Health Study. J. Am. Heart Assoc. 2019, 8, e012881. [Google Scholar] [CrossRef]
- Bockus, L.B.; Jensen, P.N.; Fretts, A.M.; Hoofnagle, A.N.; McKnight, B.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Psaty, B.M.; Sotoodehnia, N.; et al. Plasma ceramides and sphingomyelins and sudden cardiac death in the Cardiovascular Health Study. JAMA Netw. Open 2023, 6, e2343854. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, E.; Sierka, E.; Rodewald, M.; Bae, H.; Meyer, T.; Kus, E.; Chlopicki, S.; Schmitt, M.; Popp, J.; Baranska, M. Differential response of liver sinusoidal endothelial cells and hepatocytes to oleic and palmitic acid revealed by Raman and CARS imaging. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165763, Erratum in Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165804. https://doi.org/10.1016/j.bbadis.2020.165804. [Google Scholar] [CrossRef]
- Ciapaite, J.; van Bezu, J.; van Eikenhorst, G.; Bakker, S.J.; Teerlink, T.; Diamant, M.; Heine, R.J.; Krab, K.; Westerhoff, H.V.; Schalkwijk, C.G. Palmitate and oleate have distinct effects on the inflammatory phenotype of human endothelial cells. Biochim. Biophys. Acta 2007, 1771, 147–154. [Google Scholar] [CrossRef]
- de Souza, C.O.; Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Rosa Neto, J.C.; Calder, P.C. Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res. 2018, 62, e1800322. [Google Scholar] [CrossRef]
- Geng, Y.; Arroyave-Ospina, J.C.; Buist-Homan, M.; Plantinga, J.; Olinga, P.; Reijngoud, D.J.; Van Vilsteren, F.G.I.; Blokzijl, H.; Kamps, J.A.A.M.; Moshage, H. Differential effects of oleate on vascular endothelial and liver sinusoidal endothelial cells reveal its toxic features in vitro. J. Nutr. Biochem. 2023, 114, 109255. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Massaro, M.; Bonfrate, C.; Siculella, L.; Maffia, M.; Nicolardi, G.; Distante, A.; Storelli, C.; De Caterina, R. Oleic acid inhibits endothelial activation: A direct vascular antiatherogenic mechanism of a nutritional component in the Mediterranean diet. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, Ó.; Díaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75. [Google Scholar] [CrossRef]
- Scavo, M.P.; Negro, R.; Arrè, V.; Depalo, N.; Carrieri, L.; Rizzi, F.; Mastrogiacomo, R.; Serino, G.; Notarnicola, M.; De Nunzio, V.; et al. The oleic/palmitic acid imbalance in exosomes isolated from NAFLD patients induces necroptosis of liver cells via the elongase-6/RIP-1 pathway. Cell Death Dis. 2023, 14, 635. [Google Scholar] [CrossRef]
- Bass, V.L.; Soukup, J.M.; Ghio, A.J.; Madden, M.C. Oleic acid and derivatives affect human endothelial cell mitochondrial function and vasoactive mediator production. Lipids Health Dis. 2020, 19, 128. [Google Scholar] [CrossRef]
- Gremmels, H.; Bevers, L.M.; Fledderus, J.O.; Braam, B.; van Zonneveld, A.J.; Verhaar, M.C.; Joles, J.A. Oleic acid increases mitochondrial reactive oxygen species production and decreases endothelial nitric oxide synthase activity in cultured endothelial cells. Eur. J. Pharmacol. 2015, 751, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, Y.M.; Song, H.S.; Park, K.Y.; Kim, Y.M.; Kim, M.S.; Pak, Y.K.; Lee, I.K.; Lee, J.D.; Park, S.J.; et al. Oleic acid induces endothelin-1 expression through activation of protein kinase C and NF-κB. Biochem. Biophys. Res. Commun. 2003, 303, 891–895. [Google Scholar] [CrossRef]
- Pacheco, Y.M.; Bermúdez, B.; López, S.; Abia, R.; Villar, J.; Muriana, F.J. Ratio of oleic to palmitic acid is a dietary determinant of thrombogenic and fibrinolytic factors during the postprandial state in men. Am. J. Clin. Nutr. 2006, 84, 342–349. [Google Scholar] [CrossRef]
- Yajima, K.; Chiba, S.; Park, I.; Ogata, H.; Kayaba, M.; Ishihara, A.; Tanaka, Y.; Simeng, Z.; Jaehoon, S.; Katakura, M.; et al. Dietary palmitic acid to oleic acid ratio modulates energy metabolism and biological rhythms in young healthy Japanese males. Br. J. Nutr. 2024, 131, 447–460, Erratum in Br. J. Nutr. 2024, 131, 736. https://doi.org/10.1017/S0007114523002143. [Google Scholar] [CrossRef]
- Johnson, C.S.C.; Shively, C.A.; Michalson, K.T.; Lea, A.J.; DeBo, R.J.; Howard, T.D.; Hawkins, G.A.; Appt, S.E.; Liu, Y.; McCall, C.E.; et al. Contrasting effects of Western vs Mediterranean diets on monocyte inflammatory gene expression and social behavior in a primate model. eLife 2021, 10, e68293. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef]
- Svedberg, J.; Björntorp, P.; Smith, U.; Lönnroth, P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes 1990, 39, 570–574. [Google Scholar] [CrossRef]
- Römer, A.; Linn, T.; Petry, S.F. Lipotoxic impairment of mitochondrial function in beta-cells: A review. Antioxidants 2021, 10, 293. [Google Scholar] [CrossRef]
- Shishkova, D.; Yurieva, Y.; Frolov, A.; Matveeva, V.; Torgunakova, E.; Markova, V.; Lazebnaya, A.; Kutikhin, A. Isolation of Primary human saphenous vein endothelial cells, human internal thoracic artery endothelial cells, and human adipose tissue-derived microvascular endothelial cells from patients undergoing coronary artery bypass graft surgery. Int. J. Mol. Sci. 2025, 26, 9217. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Kostina, D.; Kostina, A.; Irtyuga, O.; Voronkina, I.; Smagina, L.; Ignatieva, E.; Gavriliuk, N.; Uspensky, V.; Moiseeva, O.; et al. Phenotypic and functional changes of endothelial and smooth muscle cells in thoracic aortic aneurysms. Int. J. Vasc. Med. 2016, 2016, 3107879. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Na, C.; Zhu, Y.; Bi, Y.; Yang, R.; Yang, R.; Yang, H.; Niu, M.; Huang, X.; Yang, J. Multiple Roles of Palmitic Acid in Cardiovascular Diseases. J. Inflamm. Res. 2025, 18, 14515–14533. [Google Scholar] [CrossRef]
- Huang, F.; Chen, J.; Wang, J.; Zhu, P.; Lin, W. Palmitic Acid Induces MicroRNA-221 Expression to Decrease Glucose Uptake in HepG2 Cells via the PI3K/AKT/GLUT4 Pathway. Biomed. Res. Int. 2019, 2019, 8171989. [Google Scholar] [CrossRef]
- Mthembu, S.X.H.; Mazibuko-Mbeje, S.E.; Silvestri, S.; Orlando, P.; Marcheggiani, F.; Cirilli, I.; Nkambule, B.B.; Muller, C.J.F.; Tiano, L.; Dludla, P.V. Low Levels and Partial Exposure to Palmitic Acid Improves Mitochondrial Function and the Oxidative Status of Cultured Cardiomyoblasts. Toxicol. Rep. 2024, 12, 234–243. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, J.; Ma, Y.; Qiu, C.; Liu, C.; Gong, Y.; Yuwen, Y.; Guan, G.; Zhang, Y.; et al. Palmitic Acid in Type 2 Diabetes Mellitus Promotes Atherosclerotic Plaque Vulnerability via Macrophage Dll4 Signaling. Nat. Commun. 2024, 15, 1281. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Qiu, H.; Li, P.; Hu, L.; Wang, Y.; Rao, L. Islet Protection and Amelioration of Type 2 Diabetes Mellitus by Treatment with Quercetin from the Flowers of Edgeworthia gardneri. Drug Des. Devel. Ther. 2018, 12, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Lobov, A.; Kabilov, M.; Zainullina, B.; Tupikin, A.; Shishkova, D.; Markova, V.; Sinitskaya, A.; Grigoriev, E.; Markova, Y.; et al. Multi-Omics Profiling of Human Endothelial Cells from the Coronary Artery and Internal Thoracic Artery Reveals Molecular but Not Functional Heterogeneity. Int. J. Mol. Sci. 2023, 24, 15032. [Google Scholar] [CrossRef]
- Kutikhin, A.G.; Tupikin, A.E.; Matveeva, V.G.; Shishkova, D.K.; Antonova, L.V.; Kabilov, M.R.; Velikanova, E.A. Human peripheral blood-derived endothelial colony-forming cells are highly similar to mature vascular endothelial cells yet demonstrate a transitional transcriptomic signature. Cells 2020, 9, 876. [Google Scholar] [CrossRef] [PubMed]





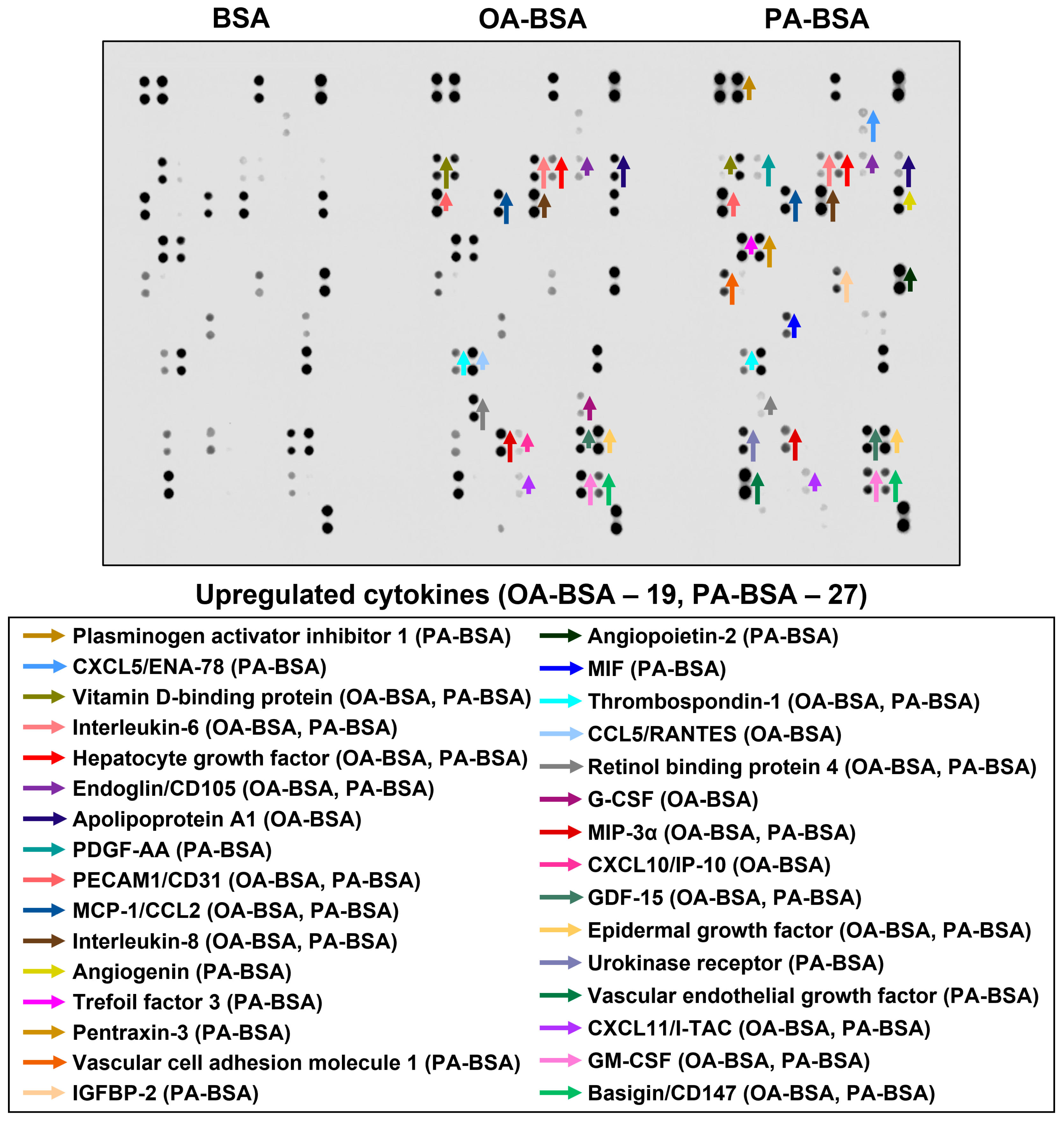
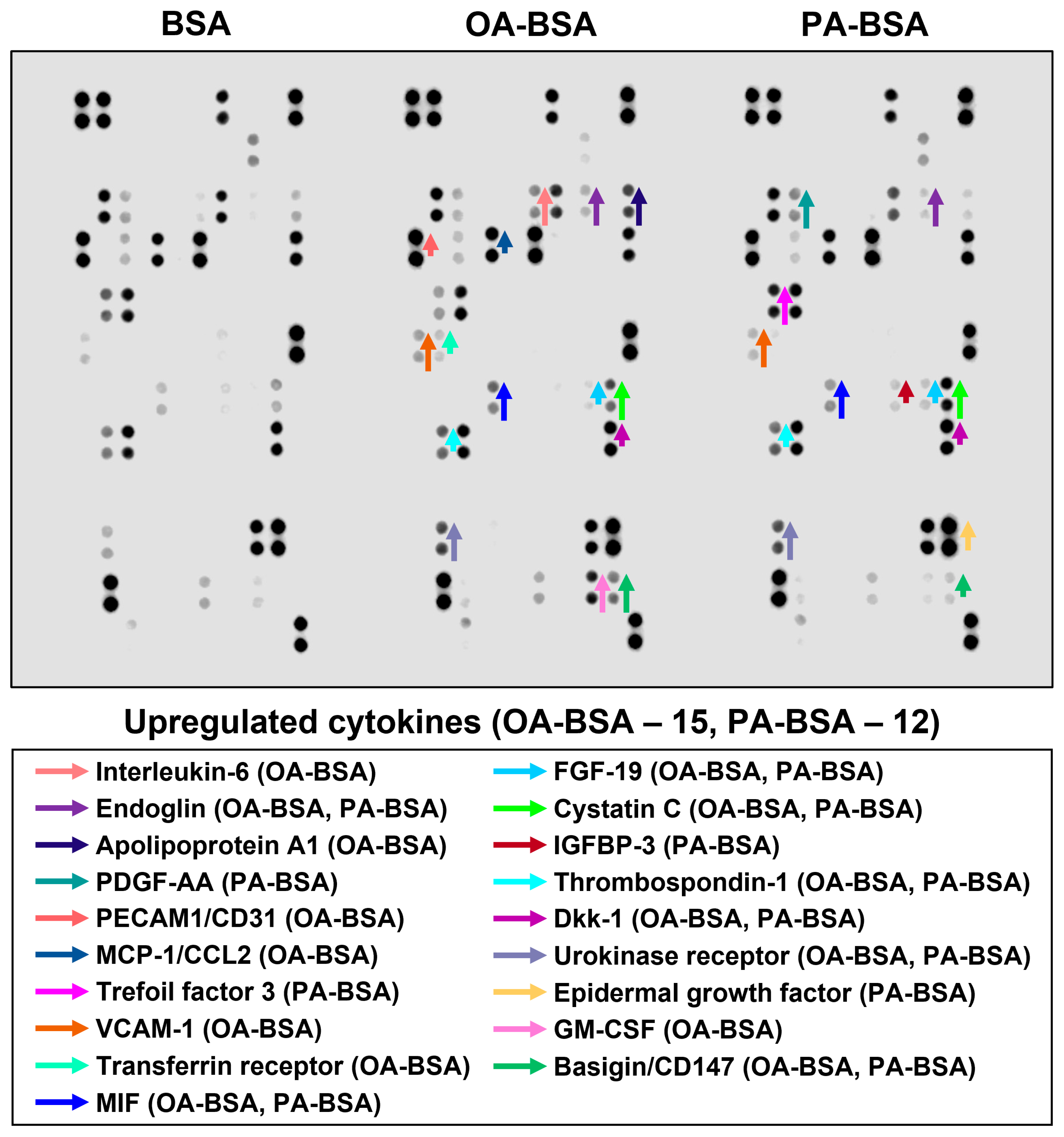

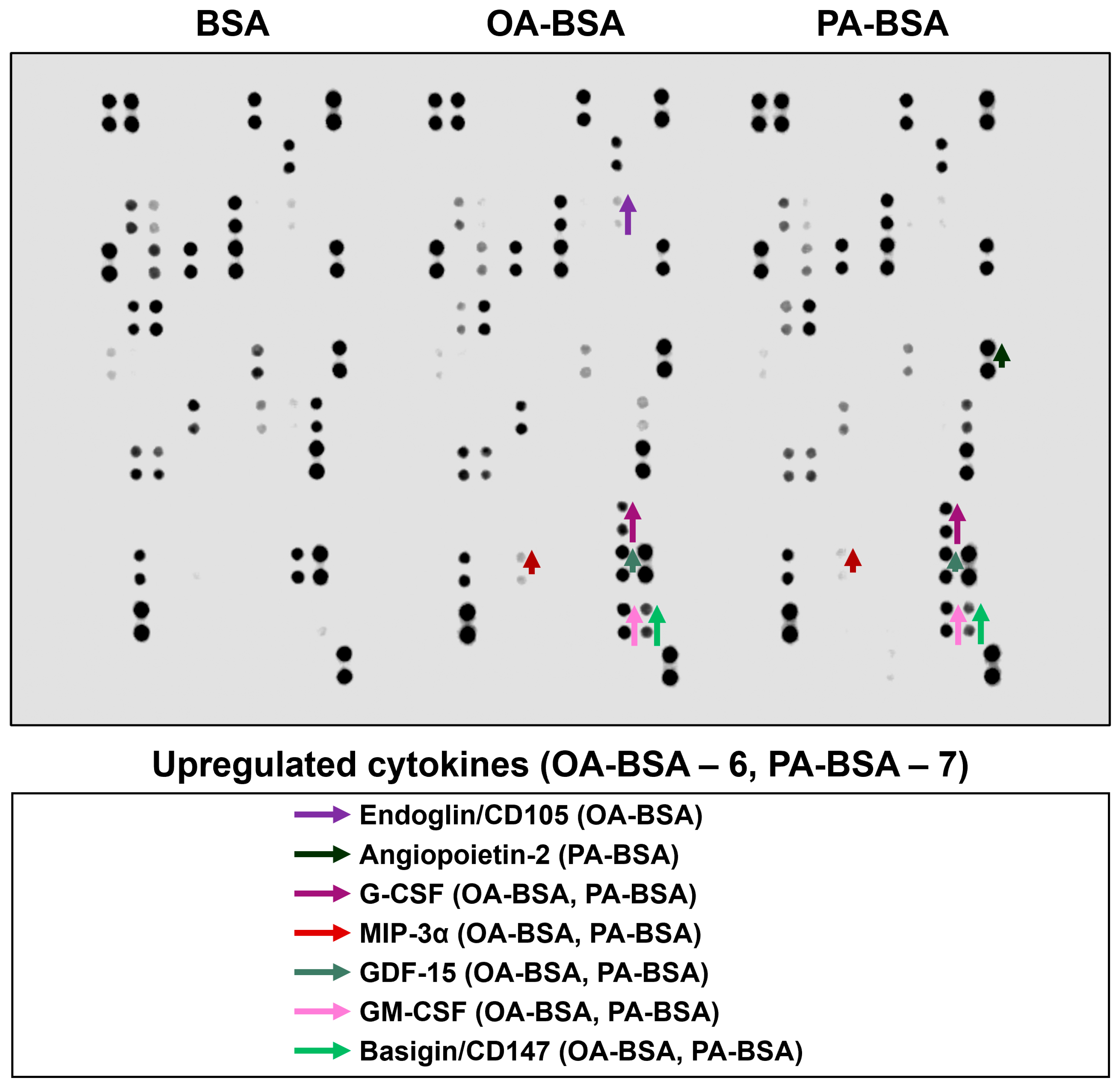
| Location on the Membrane | Analyte | BSA | OA-BSA | Fold Change | PA-BSA | Fold Change |
|---|---|---|---|---|---|---|
| Human saphenous vein endothelial cells (HSaVEC) | ||||||
| A5,6 | Apolipoprotein A1 | 708 | 22,494 | 31.78 | 6244 | 8.82 |
| A7,8 | Angiogenin | 24,976 | 25,195 | 1.01 | 31,622 | 1.27 |
| A11,12 | Angiopoietin-2 | 33,403 | 32,467 | 0.97 | 46,048 | 1.38 |
| B13,14 | Cystatin C | 8585 | 4977 | 0.58 | ||
| B15,16 | Dickkopf-related protein 1 | 33,459 | 32,276 | 0.96 | 36,140 | 1.08 |
| B19,20 | Epidermal growth factor | 27,825 | 38,830 | 1.40 | 38,199 | 1.37 |
| B21,22 | Basigin/CD147 | 4654 | 12,164 | 2.61 | 13,549 | 2.91 |
| C3,4 | Chemokine (C–X–C motif) ligand 5/Epithelial neutrophil-activating protein 78 | 15,216 | 12,803 | 0.84 | 24,412 | 1.60 |
| C5,6 | Endoglin/CD105 | 4322 | 4322 | 3155 | 3155 | |
| C17,18 | Granulocyte colony-stimulating factor | 5626 | 5626 | |||
| C19,20 | Growth/differentiation factor 15 | 17,690 | 23,369 | 1.32 | 29,984 | 1.69 |
| C21,22 | Granulocyte–macrophage colony-stimulating factor | 29,238 | 29,238 | 17,854 | 17,854 | |
| D1,2 | Chemokine (C–X–C motif) ligand 1/Growth-regulated oncogene α | 25,626 | 28,634 | 1.12 | 26,717 | 1.04 |
| D5,6 | Hepatocyte growth factor | 1114 | 9741 | 8.74 | 12,292 | 11.03 |
| D11,12 | Insulin-like growth factor-binding protein 2 | 7915 | 5710 | 0.72 | 13,468 | 1.70 |
| E5,6 | Interleukin-6 | 2059 | 24,607 | 11.95 | 6628 | 3.22 |
| E7,8 | Interleukin-8 | 24,979 | 36,636 | 1.47 | 43,670 | 1.75 |
| F19,20 | Chemokine (C–X–C motif) ligand 10/Interferon gamma-induced protein 10 | 2423 | 2423 | |||
| F21,22 | Chemokine (C–X–C motif) ligand 11/Interferon-inducible t-cell alpha chemoattractant | 2187 | 2187 | 3072 | 3072 | |
| G7,8 | Monocyte chemoattractant protein 1 | 16,406 | 25,348 | 1.55 | 27,986 | 1.71 |
| G13,14 | Macrophage migration inhibitory factor | 17,649 | 15,426 | 0.87 | 26,164 | 1.48 |
| G19,20 | Macrophage inflammatory protein-3α | 10,383 | 77,802 | 7.49 | 15,892 | 1.53 |
| H5,6 | Platelet-derived growth factor, composed of two A subunits | 820 | 3107 | 3.79 | ||
| H9,10 | Pentraxin-3 | 20,600 | 24,006 | 1.17 | 32,095 | 1.56 |
| H15,16 | Chemokine (C–C motif) ligand 5/Regulated upon activation, normal T cell expressed, and secreted | 26,970 | 35,553 | 1.32 | 29,749 | 1.10 |
| H17,18 | Retinol binding protein 4 | 33,324 | 33,324 | 2429 | 2429 | |
| I1,2 | Serpin E1/Plasminogen activator inhibitor-1 | 32,950 | 37,290 | 1.13 | 45,188 | 1.37 |
| I5,6 | Suppression of tumorigenicity 2 | 23,005 | 20,214 | 0.88 | 24,080 | 1.05 |
| I9,10 | Trefoil factor 3 | 30,586 | 30,816 | 1.01 | 37,906 | 1.24 |
| I15,16 | Thrombospondin-1 | 7327 | 10,813 | 1.48 | 9432 | 1.29 |
| I19,20 | Urokinase receptor | 8830 | 9494 | 1.08 | 23,610 | 2.67 |
| I21,22 | Vascular endothelial growth factor | 28,368 | 30,060 | 1.06 | 50,268 | 1.77 |
| J5,6 | Vitamin D-binding protein | 20,544 | 20,544 | 1183 | 1183 | |
| J7,8 | Platelet endothelial cell adhesion molecule-1/CD31 | 29,205 | 35,281 | 1.21 | 39,773 | 1.36 |
| J11,12 | Vascular cell adhesion protein 1 | 8473 | 9463 | 1.12 | 16,304 | 1.92 |
| Human microvascular endothelial cells (HMVEC) | ||||||
| A5,6 | Apolipoprotein A1 | 4941 | 19,026 | 3.85 | 2121 | 0.43 |
| A7,8 | Angiogenin | 28,435 | 23,933 | 0.84 | 30,259 | 1.06 |
| A11,12 | Angiopoietin-2 | 49,197 | 49,009 | 1.00 | 37,446 | 0.76 |
| B13,14 | Cystatin C | 8068 | 15,685 | 1.94 | 32,759 | 4.06 |
| B15,16 | Dickkopf-related protein 1 | 30,025 | 35,356 | 1.18 | 39,990 | 1.33 |
| B19,20 | Epidermal growth factor | 40,745 | 47,634 | 1.17 | 55,697 | 1.37 |
| B21,22 | Basigin/CD147 | 10,316 | 10,316 | 2549 | 2549 | |
| C3,4 | Chemokine C–X–C motif) ligand 5/Epithelial neutrophil-activating protein 78 | 30,330 | 5560 | 0.18 | 24,625 | 0.81 |
| C5,6 | Endoglin/CD105 | 460 | 4939 | 10.75 | 2239 | 4.87 |
| C13,14 | Fibroblast growth factor-19 | 1270 | 1270 | 3577 | 3577 | |
| C19,20 | Growth/differentiation factor 15 | 31,906 | 27,132 | 0.85 | 34,489 | 1.08 |
| C21,22 | Granulocyte–macrophage colony-stimulating factor | 1521 | 18,819 | 12.37 | 877 | 0.58 |
| D1,2 | Chemokine (C–X–C) ligand 1/Growth-regulated oncogene α | 24,702 | 28,720 | 1.16 | 24,389 | 0.99 |
| D5,6 | Hepatocyte growth factor | 23,493 | 23,784 | 1.01 | 18,792 | 0.80 |
| D13,14 | Insulin-like growth factor-binding protein 3 | 3150 | 3150 | |||
| E5,6 | Interleukin-6 | 1046 | 10,621 | 10.15 | ||
| E7,8 | Interleukin-8 | 38,331 | 45,555 | 1.19 | 45,083 | 1.18 |
| E21,22 | Interleukin-17a | 5145 | 5567 | 1.08 | 3329 | 0.65 |
| G7,8 | Monocyte chemoattractant protein 1 | 24,687 | 31,297 | 1.27 | 28,427 | 1.15 |
| G13,14 | Macrophage migration inhibitory factor | 5499 | 14,614 | 2.66 | 10,545 | 1.92 |
| H5,6 | Platelet-derived growth factor, composed of two A subunits | 6650 | 5718 | 0.86 | 10,787 | 1.62 |
| H7,8 | Platelet-derived growth factor, composed of A and B or two B subunits | 4318 | 3769 | 0.87 | 2617 | 0.61 |
| H9,10 | Pentraxin-3 | 30,987 | 33,459 | 1.08 | 35,450 | 1.14 |
| H15,16 | Chemokine (C–C) ligand 5/Regulated upon activation, normal T cell expressed, and secreted | 28,187 | 31,018 | 1.10 | 32,734 | 1.16 |
| I1,2 | Serpin E1 | 41,141 | 45,628 | 1.11 | 40,514 | 0.98 |
| I5,6 | Suppression of tumorigenicity 2 | 29,998 | 32,742 | 1.09 | 30,392 | 1.01 |
| I9,10 | Trefoil factor 3 | 16,638 | 9608 | 0.58 | 26,550 | 1.60 |
| I11,12 | Transferrin receptor | 2460 | 2460 | |||
| I15,16 | Thrombospondin-1 | 11,834 | 15,414 | 1.30 | 15,137 | 1.28 |
| I19,20 | Urokinase receptor | 5330 | 15,487 | 2.91 | 14,675 | 2.75 |
| I21,22 | Vascular endothelial growth factor | 42,687 | 44,673 | 1.05 | 46,020 | 1.08 |
| J7,8 | Platelet endothelial cell adhesion molecule-1/CD31 | 36,342 | 46,007 | 1.27 | 38,125 | 1.05 |
| J11,12 | Vascular cell adhesion protein 1 | 2359 | 6458 | 2.74 | 3688 | 1.56 |
| Human internal thoracic artery endothelial cells (HITAEC) | ||||||
| A3,4 | Adiponectin | 22,669 | 22,669 | |||
| A5,6 | Apolipoprotein A1 | 23,125 | 23,125 | |||
| A7,8 | Angiogenin | 33,169 | 28,838 | 0.87 | 35,768 | 1.08 |
| A11,12 | Angiopoietin-2 | 31,310 | 40,163 | 1.28 | 38,626 | 1.23 |
| B13,14 | Cystatin C | 28,793 | 6973 | 0.24 | 15,130 | 0.53 |
| B15,16 | Dickkopf-related protein 1 | 38,310 | 36,670 | 0.96 | 31,388 | 0.82 |
| B19,20 | Epidermal growth factor | 41,406 | 40,552 | 0.98 | 41,469 | 1.00 |
| B21,22 | Basigin/CD147 | 1588 | 5800 | 3.65 | 6975 | 4.39 |
| C3,4 | Chemokine (C–X–C motif) ligand 5/Epithelial neutrophil-activating protein 78 | 35,527 | 36,747 | 1.03 | 34,561 | 0.97 |
| C17,18 | Granulocyte colony-stimulating factor | 24,672 | 30,176 | 1.22 | 33,310 | 1.35 |
| C19,20 | Growth/differentiation factor 15 | 21,036 | 21,413 | 1.02 | 26,338 | 1.25 |
| C21,22 | Granulocyte–macrophage colony-stimulating factor | 29,570 | 39,667 | 1.34 | 40,004 | 1.35 |
| D1,2 | Chemokine (C–X–C motif) ligand 1/Growth-regulated oncogene α | 28,629 | 30,861 | 1.08 | 31,020 | 1.08 |
| D15,16 | Interleukin-1α | 5231 | 6784 | 1.30 | 7867 | 1.50 |
| E5,6 | Interleukin-6 | 32,060 | 28,641 | 0.89 | 31,076 | 0.97 |
| E7,8 | Interleukin-8 | 34,304 | 33,969 | 0.99 | 40,088 | 1.17 |
| F19,20 | Chemokine (C–X–C motif) ligand 10/Interferon gamma-induced protein 10 | 7751 | 16,545 | 2.13 | 15,477 | 2.00 |
| G7,8 | Monocyte chemoattractant protein 1 | 26,790 | 25,809 | 0.96 | 24,625 | 0.92 |
| G13,14 | Macrophage migration inhibitory factor | 11,970 | 24,067 | 2.01 | 10,117 | 0.85 |
| G19,20 | Macrophage inflammatory protein-3α | 33,694 | 37,162 | 1.10 | 35,738 | 1.06 |
| H9,10 | Pentraxin-3 | 29,532 | 21,859 | 0.74 | 28,106 | 0.95 |
| H15,16 | Chemokine (C–C motif) ligand 5/Regulated upon activation, normal T cell expressed, and secreted | 26,336 | 29,885 | 1.13 | 29,683 | 1.13 |
| H17,18 | Retinol binding protein 4 | 24,834 | 24,834 | |||
| I1,2 | Serpin E1/Plasminogen activator inhibitor-1 | 41,010 | 37,687 | 0.92 | 40,644 | 0.99 |
| I5,6 | Suppression of tumorigenicity 2 | 27,915 | 16,560 | 0.59 | 25,667 | 0.92 |
| I11,12 | Transferrin receptor | 15,034 | 15,034 | |||
| I15,16 | Thrombospondin-1 | 6427 | 9353 | 1.46 | 7860 | 1.22 |
| I19,20 | Urokinase receptor | 15,097 | 28,527 | 1.89 | 17,901 | 1.19 |
| I21,22 | Vascular endothelial growth factor | 41,811 | 45,097 | 1.08 | 44,888 | 1.07 |
| J5,6 | Vitamin D-binding protein | 36,030 | 36,030 | |||
| J7,8 | Platelet endothelial cell adhesion molecule-1/CD31 | 35,387 | 34,402 | 0.97 | 30,813 | 0.87 |
| J11,12 | Vascular cell adhesion protein 1 | 25,835 | 16,857 | 0.65 | 18,949 | 0.73 |
| Human aortic valve endothelial cells (HAVEC) | ||||||
| A7,8 | Angiogenin | 37,221 | 31,160 | 0.84 | 33,416 | 0.90 |
| A11,12 | Angiopoietin-2 | 36,796 | 43,772 | 1.19 | 44,475 | 1.21 |
| B13,14 | Cystatin C | 29,617 | 7449 | 0.25 | 18,692 | 0.63 |
| B15,16 | Dickkopf-related protein 1 | 42,330 | 40,481 | 0.96 | 36,586 | 0.86 |
| B19,20 | Epidermal growth factor | 42,948 | 45,228 | 1.05 | 48,909 | 1.14 |
| B21,22 | Basigin/CD147 | 15,873 | 15,873 | 12,962 | 12,962 | |
| C3,4 | Chemokine (C–X–C motif) ligand 5/Epithelial neutrophil-activating protein 78 | 29,239 | 25,628 | 0.88 | 28,887 | 0.99 |
| C5,6 | Endoglin/CD105 | 10,275 | 17,577 | 1.71 | 6085 | 0.59 |
| C17,18 | Granulocyte colony-stimulating factor | 20,111 | 20,111 | 33,590 | 33,590 | |
| C19,20 | Growth/differentiation factor 15 | 25,009 | 30,013 | 1.20 | 30,783 | 1.23 |
| C21,22 | Granulocyte–macrophage colony-stimulating factor | 28,037 | 28,037 | 25,440 | 25,440 | |
| D1,2 | Chemokine (C–X–C motif) ligand 1/Growth-regulated oncogene α | 32,560 | 34,263 | 1.05 | 31,538 | 0.97 |
| D11,12 | Insulin-like growth factor-binding protein 2 | 16,578 | 6377 | 0.38 | 6723 | 0.41 |
| D13,14 | Insulin-like growth factor-binding protein 3 | 7615 | ||||
| E5,6 | Interleukin-6 | 36,735 | 30,185 | 0.82 | 32,317 | 0.88 |
| E7,8 | Interleukin-8 | 41,077 | 36,177 | 0.88 | 36,858 | 0.90 |
| G7,8 | Monocyte chemoattractant protein 1 | 30,133 | 25,629 | 0.85 | 26,803 | 0.89 |
| G13,14 | Macrophage migration inhibitory factor | 24,785 | 25,859 | 1.04 | 10,824 | 0.44 |
| G19,20 | Macrophage inflammatory protein 3α | 3302 | 3302 | 1343 | 1343 | |
| H5,6 | Platelet-derived growth factor, composed of two A subunits | 5854 | 632 | 0.11 | 1376 | 0.24 |
| H7,8 | Platelet-derived growth factor, composed of A and B or two B subunits | 16,812 | 7818 | 0.47 | 7121 | 0.42 |
| H9,10 | Pentraxin-3 | 34,548 | 28,644 | 0.83 | 34,171 | 0.99 |
| H15,16 | Chemokine (C–C motif) ligand 5/Regulated upon activation, normal T cell expressed, and secreted | 12,561 | 11,846 | 0.94 | 12,323 | 0.98 |
| I1,2 | Serpin E1 | 44,492 | 36,145 | 0.81 | 45,135 | 1.01 |
| I5,6 | Suppression of tumorigenicity 2 | 18,996 | 8947 | 0.47 | 12,055 | 0.63 |
| I9,10 | Trefoil factor 3 | 23,557 | 6388 | 0.27 | 11,619 | 0.49 |
| I15,16 | Thrombospondin-1 | 17,631 | 19,138 | 1.09 | 13,058 | 0.74 |
| I17,18 | Tumor necrosis factor α | |||||
| I19,20 | Urokinase receptor | 19,916 | 20,838 | 1.05 | 22,339 | 1.12 |
| I21,22 | Vascular endothelial growth factor | 44,242 | 51,034 | 1.15 | 48,465 | 1.10 |
| J7,8 | Platelet endothelial cell adhesion molecule-1/CD31 | 43,180 | 39,363 | 0.91 | 38,797 | 0.90 |
| J11,12 | Vascular cell adhesion protein 1 | 2570 | 1018 | 0.40 | 956 | 0.37 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Pro-inflammatory cytokines | ||
| MIF | 5′-GGTGTCCGAGAAGTCAGGCA-3′ | 5′-GGGGCACGTTGGTGTTTACG-3′ |
| PTX3 | 5′-GAACTTTGCGTCTCTCCAGCAA-3′ | 5′-AGAGCTTGTCCCATTCCGAGT-3′ |
| CSF2 | 5′-AGCCTCACCAAGCTCAAGGG-3′ | 5′-GGGGATGACAAGCAGAAAGTCC-3′ |
| CSF3 | 5′-TCCAGGAGAAGCTGGTGAGTGA-3′ | 5′-GAGCCCCTGGTAGAGGAAAAGG-3′ |
| IL1A | 5′-TGCCCAAGATGAAGACCAACC-3′ | 5′-AGTGCCGTGAGTTTCCCAGAA-3′ |
| IL6 | 5′-GGCACTGGCAGAAAACAACC-3′ | 5′-GCAAGTCTCCTCATTGAATCC-3′ |
| CCL2 | 5′-TTCTGTGCCTGCTGCTCATAG-3′ | 5′-AGGTGACTGGGGCATTGATTG-3′ |
| CCL5 | 5′-AGTGGCAAGTGCTCCAACCC-3′ | 5′-TCAGCCGGGAGTCATACAGGA-3′ |
| CCL20 | 5′-TGAAGGCTGTGACATCAATGCT-3′ | 5′-CCATTCCAGAAAAGCCACAGTT-3′ |
| CXCL1 | 5′-GCTTGCCTCAATCCTGCATCC-3′ | 5′-ACAATCCAGGTGGCCTCTGC-3′ |
| CXCL2 | 5′-TTGTCTCAACCCCGCATCG-3′ | 5′-TGCTCAAACACATTAGGCGCAA-3′ |
| CXCL3 | 5′-TGCGCCCAAACCGAAGTCAT-3′ | 5′-TCAGCTCTGGTAAGGGCAGGGA-3′ |
| CXCL5 | 5′-ATCTGCAAGTGTTCGCCATAGG-3′ | 5′-TCCATGCGTGCTCATTTCTCTT-3′ |
| CXCL6 | 5′-CTGCGTTGCACTTGTTTACGC-3′ | 5′-GCTTCCGGGTCCAGACAAACT-3′ |
| CXCL8 | 5′-CAGAGACAGCAGAGCACAC-3′ | 5′-AGTTCTTTAGCACTCCTTGGC-3′ |
| CXCL10 | 5′-AGGAACCTCCAGTCTCAGCAC-3′ | 5′-GGACAAAATTGGCTTGCAGGA-3′ |
| Pro-inflammatory cell adhesion molecules | ||
| VCAM1 | 5′-CGTCTTGGTCAGCCCTTCCT-3′ | 5′-ACATTCATATACTCCCGCATCCTTC-3′ |
| ICAM1 | 5′-TTGGGCATAGAGACCCCGTT-3′ | 5′-GCACATTGCTCAGTTCATACACC-3′ |
| SELE | 5′-GCACAGCCTTGTCCAACC-3′ | 5′-ACCTCACCAAACCCTTCG-3′ |
| SELP | 5′-ATGGGTGGGAACCAAAAAGG-3′ | 5′-GGCTGACGGACTCTTGATGTAT-3′ |
| Endothelial-to-mesenchymal transition transcription factors | ||
| SNAI1 | 5′-CAGACCCACTCAGATGTCAAGAA-3′ | 5′-GGGCAGGTATGGAGAGGAAGA-3′ |
| SNAI2 | 5′-ACTCCGAAGCCAAATGACAA-3′ | 5′-CTCTCTCTGTGGGTGTGTGT-3′ |
| TWIST1 | 5′-GTCCGCAGTCTTACGAGGAG-3′ | 5′-GCTTGAGGGTCTGAATCTTGCT-3′ |
| ZEB1 | 5′-GATGATGAATGCGAGTCAGATGC-3′ | 5′-ACAGCAGTGTCTTGTTGTTGT-3′ |
| Housekeeping genes | ||
| PECAM1 | 5′-AAGGAACAGGAGGGAGAGTATTA-3′ | 5′-GTATTTTGCTTCTGGGGACACT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkova, D.; Markova, V.; Yurieva, Y.; Frolov, A.; Lazebnaya, A.; Sinitsky, M.; Sinitskaya, A.; Matveeva, V.; Torgunakova, E.; Stepanov, A.; et al. Palmitic but Not Oleic Acid Induces Pro-Inflammatory Dysfunction of Human Endothelial Cells from Different Vascular Beds In Vitro. Int. J. Mol. Sci. 2025, 26, 12148. https://doi.org/10.3390/ijms262412148
Shishkova D, Markova V, Yurieva Y, Frolov A, Lazebnaya A, Sinitsky M, Sinitskaya A, Matveeva V, Torgunakova E, Stepanov A, et al. Palmitic but Not Oleic Acid Induces Pro-Inflammatory Dysfunction of Human Endothelial Cells from Different Vascular Beds In Vitro. International Journal of Molecular Sciences. 2025; 26(24):12148. https://doi.org/10.3390/ijms262412148
Chicago/Turabian StyleShishkova, Daria, Victoria Markova, Yulia Yurieva, Alexey Frolov, Anastasia Lazebnaya, Maxim Sinitsky, Anna Sinitskaya, Vera Matveeva, Evgenia Torgunakova, Alexander Stepanov, and et al. 2025. "Palmitic but Not Oleic Acid Induces Pro-Inflammatory Dysfunction of Human Endothelial Cells from Different Vascular Beds In Vitro" International Journal of Molecular Sciences 26, no. 24: 12148. https://doi.org/10.3390/ijms262412148
APA StyleShishkova, D., Markova, V., Yurieva, Y., Frolov, A., Lazebnaya, A., Sinitsky, M., Sinitskaya, A., Matveeva, V., Torgunakova, E., Stepanov, A., Malashicheva, A., Khapchaev, A., Podkuychenko, N., Vorotnikov, A., Shirinsky, V., & Kutikhin, A. (2025). Palmitic but Not Oleic Acid Induces Pro-Inflammatory Dysfunction of Human Endothelial Cells from Different Vascular Beds In Vitro. International Journal of Molecular Sciences, 26(24), 12148. https://doi.org/10.3390/ijms262412148






