The Role of Natural Chalcones and Their Derivatives in Targeting Prostate Cancer: Recent Updates
Abstract
1. Introduction
2. Prostate Cancer (PCa)
3. Chalcones
4. Chalcones and PCa
4.1. Apoptosis
4.2. Cell Cycle
4.3. Cancer Cell Invasion and Migration
4.4. PI3k/Akt/mTOR Pathway
4.5. Angiogenesis Pathway
4.6. Androgen Receptor Signaling
4.7. Inflammatory Pathways
4.8. Cancer Stem Cells
5. Toxicity and Safety Profile of Chalcones
6. Insights into Structural Perspectives
7. Conclusions and Future Perspective
| No. | Chemical Structure | Origin | Research Model | Inhibitory Concentration (IC50) | Biological Effect | Target Pathway/Protein | Reference |
|---|---|---|---|---|---|---|---|
| 1 |  | Natural | Cell-line(s): CWR22Rν1, LNCaP, PC3, and DU145 Animal model: xenograft mice model | N/A |
|
| [141,208,209] |
| 2 | 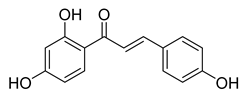 | Natural | Cell-line(s): PC-3 DU145 LNCaP 22RV1 | 19.6 µM 23.3 µM 15.7 µM 36.6 µM |
|
| [98,126,143,150,170,210,211,212] |
| Animal model: PC-3 xenograft mice model | |||||||
| 3 | 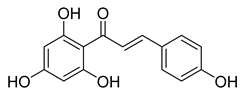 | Natural | Cell-line(s): PC3 22RV1 PNT1A | For all cell lines ≥ 1 µM |
|
| [213] |
| 4 | 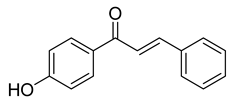 | Synthetic | Cell-line(s): PC-3 | 6.19 μM |
|
| [214] |
| 5 |  | Natural | Cell-line(s): PC3, DU145 22Rv1 PrECs PrSCs Animal models: TRAMP mice model; FVB/N mice | 22.86 μM N/A N/A >80 μM >80 μM |
|
| [132,215,216,217] |
| 6 | 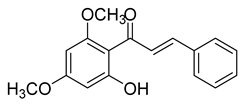 | Natural | Cell-line(s): LNCaP DU145 PC-3 LAPC4 | 48.3 µM 3.9 µM 6.2 µM 32 µM |
|
| [101,218] |
| Animal model: DU145 xenograft mice model | |||||||
| 7 |  | Natural | Cell-line(s): PC3 DU145 LNCaP RWPE-1 C4-2 PC3-PTX DU145-PTX | >50 µM in all cell lines |
|
| [186] |
| Animal model: Nude mice | |||||||
| 8 | 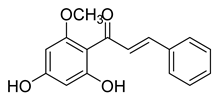 | Natural | Cell-line(s): PC3 DU145 LNCaP | 41.9 µM N/A N/A |
|
| [180,219,220] |
| 9 | 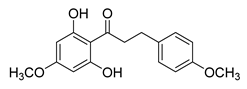 | Natural | Cell-line(s): LNCaP | N/A |
|
| [104] |
| 10 | 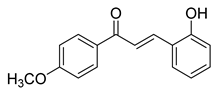 | Synthetic | Cell-line(s): PC3 | 23.14 µM |
|
| [221] |
| 11 |  | Synthetic | Cell-line(s): PC3 | 8.2 µM |
|
| [99] |
| 12 |  | Synthetic | Cell-line(s): LNCaP | 3.4 μM |
|
| [222] |
| 13 |  | Synthetic | Cell-line(s): 22Rv1 | N/A |
|
| [223] |
| Animal model: CRPC (22Rv1) xenograft mice model | |||||||
| 14 | 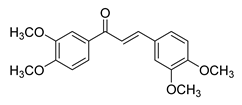 | Synthetic | Cell-line(s): PC3 LNCaP | N/A |
|
| [153] |
| 15 | 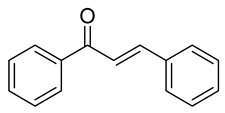 | Synthetic | Cell-line(s): PC3 RWPE-1 | 1.1 µM 34.7 µM |
|
| [224] |
| Animal model: Nude mice | |||||||
| 16 |  | Synthetic | Cell-line(s): LNCaP PC3 DU145 Animal model: PC-3 xenograft mice model | 3.74 µM 1.52 µM 4.5 µM |
|
| [130] |
| 17 | 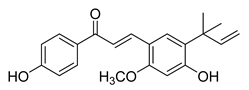 | Natural | Cell-line(s): PC3 LNCaP | N/A |
|
| [123,152] |
| 18 |  | Natural | Cell-line(s): PC3 DU145 LNCaP C4-2 MCF-10a HLMEC | 13.2 μM 12.3 μM N/A N/A |
|
| [100,102,188,195,225] |
| 19 | 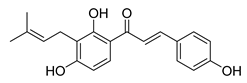 | Natural | Cell-line(s): PC3 LNCaP | 19.25 µM N/A |
|
| [105,226] |
| 20 | 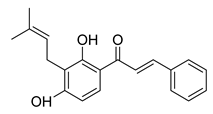 | Natural | Cell-line(s): PC3 | N/A |
|
| [227] |
| 21 | 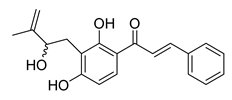 | Natural | Cell-line(s): PC3 DU145 | 11 μM 7 μM |
|
| [133] |
| 22 | 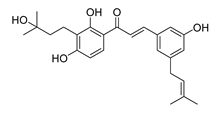 | Natural | Cell-line(s): DU145 | >10 µM |
|
| [228] |
| 23 |  | Synthetic | Cell-line(s): PC3 DU145 RWPE-1 | 4.67 µM 6.56 µM 5.00 µM |
|
| [142,166,229] |
| Animal model: PC3 xenograft mice mode | |||||||
| 24 | 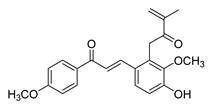 | Natural | Cell-line(s): PC3 | 1.9 μM |
|
| [230] |
| 25 | 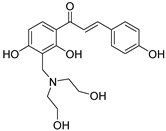 | Synthetic | Cell-line(s): PC3 | 35.14 μM |
|
| [231] |
| 26 | 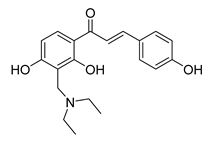 | Synthetic | Cell-line(s): PC3 | 3.9 μM |
|
| [232] |
| 27 |  | Synthetic | Cell-line(s): PC3 | 0.74 μM |
|
| [233] |
| 28 | 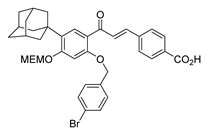 | Synthetic | Cell-line(s): PC3 | N/A |
|
| [234] |
| 29 |  | Synthetic | Cell-line(s): LNCaP PC3 DU-145 | 5.8 µM 9.2 µM 2.2 µM |
|
| [125] |
| 30 |  | Synthetic | Cell-line(s): LNCaP PC3 DU145 | 15 μM 15 μM 20 μM |
|
| [106,179] |
| 31 |  | Synthetic | Cell-line(s): PC-3 LNCaP Animal model: mouse xenografts | 29.5 μg/mL 21.4 μg/ml |
|
| [235] |
| 32 | 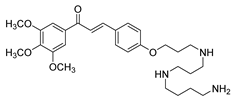 | Synthetic | Cell-line(s): PC3 DU145 | 13.73 µM 8.86 µM |
|
| [122] |
| 33 | 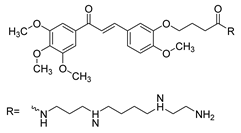 | Synthetic | Cell-line(s): PC3 DU145 | 31.8 µM 28.5 µM |
|
| [236] |
| 34 | 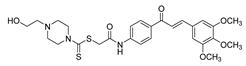 | Synthetic | Cell-line(s): PC3 | 1.05 µM |
|
| [124] |
| 35 |  | Synthetic | Cell-line(s): PC3 | 3.7 μM |
|
| [237] |
| 36 | 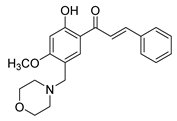 | Synthetic | Cell-line(s): PC3 | 0.78 μg/mL |
|
| [238] |
| 37 | 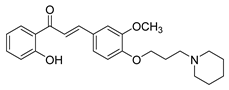 | Synthetic | Cell-line(s): PC3 DU145 | 1.95 µM 2.73 µM |
|
| [239] |
| 38 | 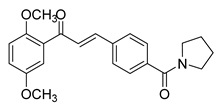 | Synthetic | Cell-line(s): PC3 | 0.53 μM |
|
| [240] |
| 39 |  | Synthetic | Cell-line(s): PC3 | 28.2 µM |
|
| [241] |
| 40 |  | Natural | Cell-line(s): LNCaP PC3 | 19.35 µM N/A |
|
| [119] |
| Animal model: SCID mice | |||||||
| 41 | 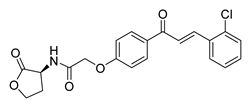 | Synthetic | Cell-line(s): PC3 DU145 Normal GES-1 | 4.61 µM 3.24 µM 13.37 µM |
|
| [107] |
| 42 | 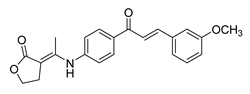 | Synthetic | Cell-line(s): PC3 FL normal cells | 69.92 µM 86.45 µM |
|
| [189] |
| 43 | 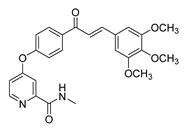 | Synthetic | Cell-line(s): PC3 | 3.15 μM |
|
| [242] |
| 44 |  | Synthetic | Cell-line(s): PC3 Animal model: Non PCa | 54 μM |
|
| [243] |
| 45 | 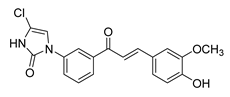 | Synthetic | Cell-line(s): PC3 | 1.95 µM |
|
| [244] |
| 46 | 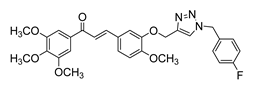 | Synthetic | Cell-line(s): DU145 | 1.3 μM |
|
| [131] |
| 47 | 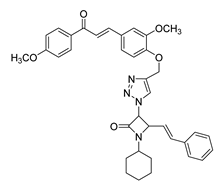 | Synthetic | Cell-line(s): PC-3 | 67.1 μM |
|
| [245] |
| 48 |  | Synthetic | Cell-line(s): PC3 DU145 | 0.45 µM 0.64 µM |
|
| [118] |
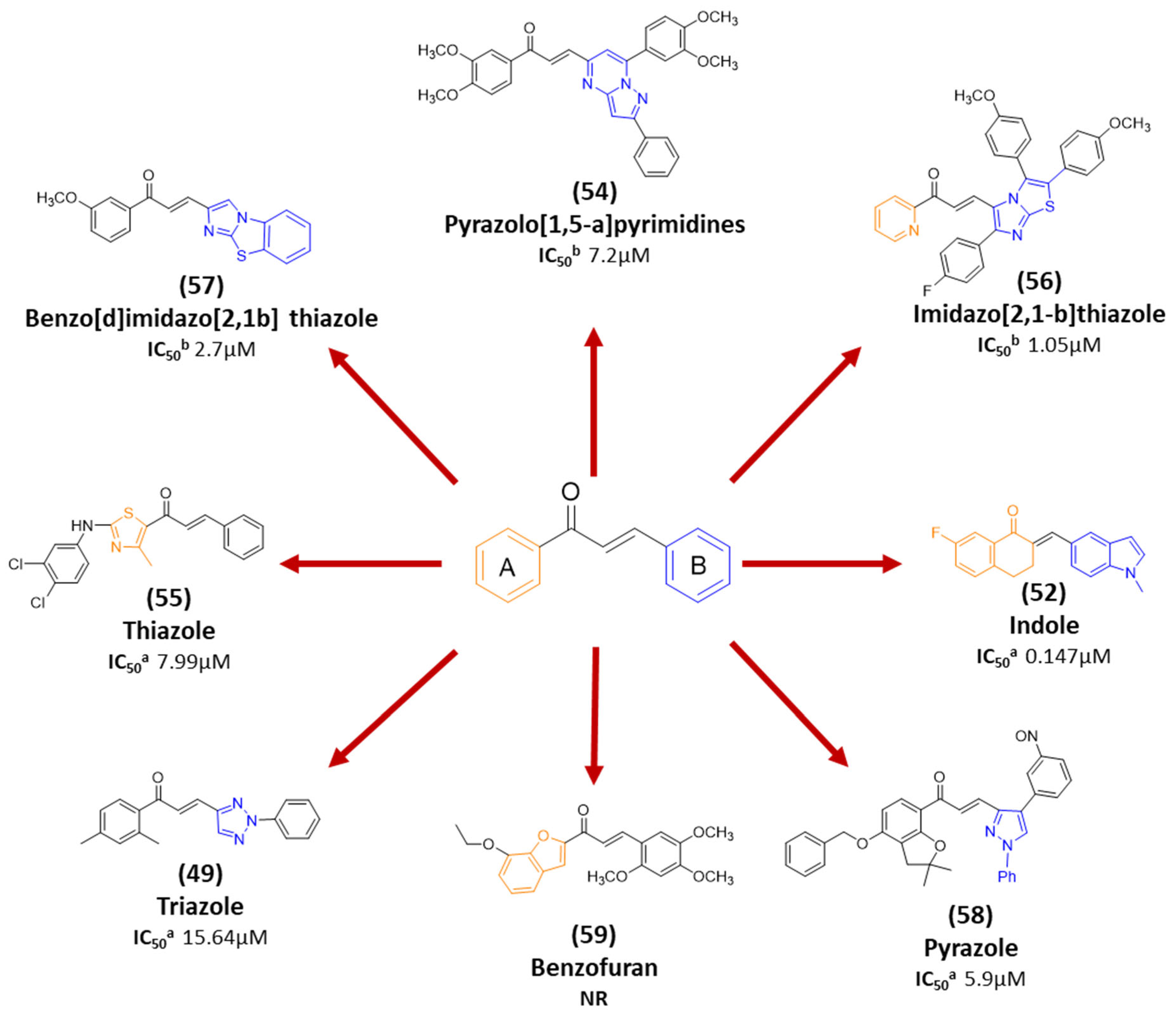
| 49 |  | Synthetic | Cell-line(s): PC3 | 15.64 µM |
|
| [246] |
| 50 |  | Synthetic | Cell-line(s): PC3 | 4.46 µM |
|
| [128] |
| 51 | 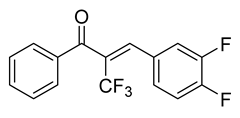 | Synthetic | Cell-line(s): PC3 DU145 LNCaP Drug resistant cell lines Animal model: SCID mice | 0.15 µM 0.19 µM N/A |
|
| [120,121] |
| 52 | 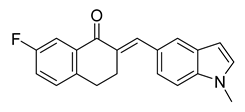 | Synthetic | Cell-line(s): PC3 | 0.147 |
|
| [247] |
| 53 |  | Synthetic | Cell-line(s): PC3 Normal lung bronchial epithelial cells | PC3 3 μM >10 µM |
|
| [248] |
| 54 | 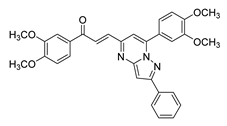 | Synthetic | Cell-line(s): DU145 | 7.2 µM |
|
| [249] |
| 55 | 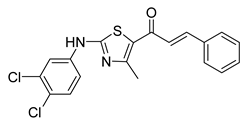 | Synthetic | Cell-line(s): PC-3 Animal model: ICR mice bearing sarcoma 180 | 7.99 μM |
|
| [250] |
| 56 | 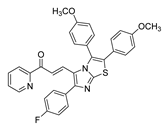 | Synthetic | Cell-line(s): DU145 | 1.05 μM |
|
| [251] |
| 57 | 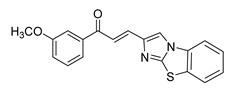 | Synthetic | Cell-line(s): DU145 | 2.7 µM |
|
| [252] |
| 58 | 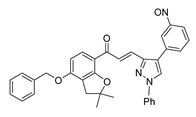 | Synthetic | Cell-line model: PC3 | 5.9 μM |
|
| [253] |
| 59 | 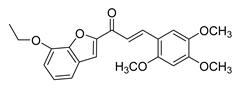 | Synthetic | Cell-line(s): PC3 | N/A |
|
| [254] |
| 60 | 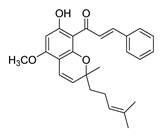 | Natural | Cell-line(s): PC3 | 27.95 µM |
|
| [255] |
| 61 | 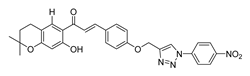 | Synthetic | Cell-line(s): DU145 | 29.9 μM |
|
| [256] |
| 62 | 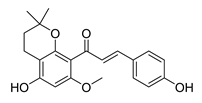 | Natural | Cell-line(s): PC3 | 10.7 µM |
|
| [257] |
| 63 | 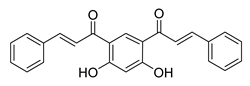 | Synthetic | Cell-line(s): DU145 | 1.70 μM |
|
| [258] |
| 64 |  | Synthetic | Cell-line(s): LNCaP PC3 | 5.04 μM 4.15 μM |
|
| [103] |
| 65 |  | Synthetic | Cell-line(s): PC3 22RV1 | 22.9 µg/mL 17.1 µg/mL |
|
| [190] |
| 66 | 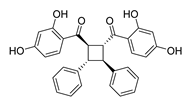 | Natural | Cell-line(s): PC3 | 3.5 µM |
|
| [259] |
| 67 | 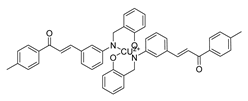 | Synthetic | Cell-line(s): PC3 | 5.95 μM |
|
| [260] |
| 68 | 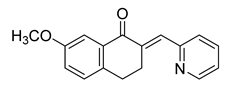 | Synthetic | Cell-line(s): PC3 DU145 | >10 µM <10 µM |
|
| [261] |
| 69 | 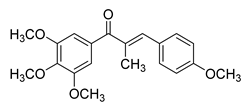 | Synthetic | Cell-line(s): LNCaP PC3 DU-145 22Rv1 C4-2 Animal model: 22Rv1 xenograft model in male Nu/Nu nude mice | 14–40 nM |
|
| [127] |
| 70 | 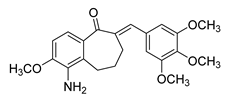 | Synthetic | Cell-line(s): DU145 HUVEC | 0.237 µM N/A |
|
| [262] |
| 71 | 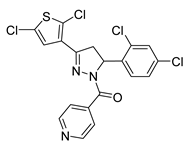 | Synthetic | Cell-line(s): DU145 | 4.95 µM |
|
| [263] |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GLOBOCAN. Global Cancer Observatory (GCO): International Agency for Research on Cancer. 2022. Available online: https://gco.iarc.fr/ (accessed on 15 August 2025).
- Dasgupta, P.; Baade, P.D.; Aitken, J.F.; Ralph, N.; Chambers, S.K.; Dunn, J. Geographical Variations in Prostate Cancer Outcomes: A Systematic Review of International Evidence. Front. Oncol. 2019, 9, 238. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Sartor, A.O. Risk Factors for Prostate Cancer UpToDate: Wolters Kluwer. 2025. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/risk-factors-for-prostate-cancer?search=prostate%20cancer%20risk%20factors&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H41 (accessed on 15 August 2025).
- SEER. Cancer Stat Facts: Prostate Cancer: National Cancer Institute. 2025. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 13 August 2025).
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans. PLoS Genet. 2011, 7, e1001387. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.; Gerke, T.; Markt, S.C.; Peisch, S.F.; Wilson, K.M.; Ahearn, T.; Giovannucci, E.; Parmigiani, G.; Mucci, L.A. Family history of breast or prostate cancer and prostate cancer risk. Clin. Cancer Res. 2018, 24, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Kalish, L.A.; McDougal, W.S.; McKinlay, J.B. Family history and the risk of prostate cancer. Urology 2000, 56, 803–806. [Google Scholar] [CrossRef]
- Attard, G.; Parker, C.; Eeles, R.A.; Schröder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Castro, E.; Mahmud, N.; Guy, M.; Edwards, S.; O’Brien, L.; Sawyer, E.; et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br. J. Cancer 2011, 105, 1230–1234. [Google Scholar] [CrossRef]
- Thompson, D.; Easton, D. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am. J. Hum. Genet. 2001, 68, 410–419. [Google Scholar] [CrossRef]
- SEER. Cancer Stat Facts: Common Cancer Sites: National Cancer Institute. 2020. Available online: https://seer.cancer.gov/statfacts/html/common.html (accessed on 15 August 2020).
- Crona, D.J.; Whang, Y.E. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.T.; Du, L.; Chowdhury, A.; Xiaoke, G.; Lu, Q.; Yin, X. Signaling pathways and proteins targeted by antidiabetic chalcones. Life Sci. 2020, 284, 118982. [Google Scholar]
- Yadav, P.; Lal, K.; Kumar, A. Antimicrobial Screening, in Silico Studies and QSAR of Chalcone-based 1,4-disubstituted 1,2,3-triazole Hybrids. Drug Res. 2020, 71, 149–156. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Butt, A.M.; Amjad, M.W.; Ahmad, W.; Shah, V.H.; Trivedi, A.R. Synthesis and evaluation of chalcone analogues based pyrimidines as angiotensin converting enzyme inhibitors. Pak. J. Biol. Sci. 2013, 16, 1368–1372. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.E.; Khalil, A. A comprehensive review on the antiviral activities of chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes. Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Feng, Q.; He, B. Androgen Receptor Signaling in the Development of Castration-Resistant Prostate Cancer. Front. Oncol. 2019, 9, 858. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Virgo, K.S.; Nam, R.; Somerfield, M.R.; Ben-Josef, E.; Mendelson, D.S.; Middleton, R.; Sharp, S.A.; Smith, T.J.; Talcott, J.; et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American society of clinical oncology practice guideline. J. Clin. Oncol. 2007, 25, 1596–1605. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Frieling, J.S.; Basanta, D.; Lynch, C.C. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control 2015, 22, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, E.Y. Castrate-resistant prostate cancer: Postdocetaxel management. Curr. Opin. Urol. 2013, 23, 201–207. [Google Scholar] [CrossRef]
- Hussain, A.; Dawson, M.A. Chemotherapy in Advanced Castration-Resistant Prostate Cancer UpToDate: Wolters Kluwer. 2025. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/chemotherapy-in-advanced-castration-resistant-prostate-cancer?sectionName=Chemotherapy-na%C3%AFve%20patients&search=castration%20resistant&topicRef=112896&anchor=H8&source=see_link#H8 (accessed on 15 August 2025).
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.A.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Crombag, M.B.S.; de Vries Schultink, A.H.M.; van Doremalen, J.G.C.; Otten, H.M.; Bergman, A.M.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Age-Associated Hematological Toxicity in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Docetaxel in Clinical Practice. Drugs Aging 2019, 36, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Elshan, N.; Rettig, M.B.; Jung, M.E. Molecules targeting the androgen receptor (AR) signaling axis beyond the AR-Ligand binding domain. Med. Res. Rev. 2019, 39, 910–960. [Google Scholar] [CrossRef]
- Dawson, N.A.; Leger, P. Overview of the Treatment of Castration-Resistant Prostate Cancer (CRPC) UpToDate: Wolters Kluwer. 2025. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/overview-of-the-treatment-of-castration-resistant-prostate-cancer-crpc?search=castration-resistant%20prostate%20cancer&source=search_result&selectedTitle=1~64&usage_type=default&display_rank=1 (accessed on 15 August 2025).
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- (FDA) USFaDA. 2010 Notifications FDA2010. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/2010-notifications (accessed on 15 August 2025).
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Prostate Cancer: NCCN. 2019. Available online: https://www2.tri-kobe.org/nccn/guideline/urological/english/prostate.pdf (accessed on 15 August 2025).
- Lexicomp. Sipuleucel-T: Drug Information: Wolters Kluwer. 2020. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/sipuleucel-t-drug-information?search=castration-resistant%20prostate%20cancer&topicRef=112896&source=see_link (accessed on 26 August 2020).
- Goldstein, N.S. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am. J. Clin. Pathol. 2002, 117, 471–477. [Google Scholar] [CrossRef]
- Lexicomp. Radium-223: Drug Information: Wolters Kluwer. 2020. Available online: https://www.google.com/search?q=wolters+kluwer&oq=wolter&aqs=chrome.0.69i59j69i57j0l3j46j69i60j69i61.1928j0j7&sourceid=chrome&ie=UTF-8 (accessed on 26 August 2020).
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III trial-FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Pozas, J.; Álvarez Rodríguez, S.; Fernández, V.A.; Burgos, J.; Santoni, M.; Manneh Kopp, R.; Molina-Cerrillo, J.; Alonso-Gordoa, T. Androgen Receptor Signaling Inhibition in Advanced Castration Resistance Prostate Cancer: What Is Expected for the Near Future? Cancers 2022, 14, 6071. [Google Scholar] [CrossRef]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef]
- Labrie, F. Mechanism of action and pure antiandrogenic properties of flutamide. Cancer 1993, 72, 3816–3827. [Google Scholar] [CrossRef]
- Longo, D.L. New Therapies for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.A.; Barrie, S.E.; Jarman, M.; Rowlands, M.G. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): Potential agents for the treatment of prostatic cancer. J. Med. Chem. 1995, 38, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Thomsen, F.B.; Røder, M.A.; Rathenborg, P.; Brasso, K.; Borre, M.; Iversen, P. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand. J. Urol. 2014, 48, 268–275. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Kita, Y.; Goto, T.; Akamatsu, S.; Yamasaki, T.; Inoue, T.; Ogawa, O.; Kobayashi, T. Castration-resistant prostate cancer refractory to second-generation androgen receptor axis-targeted agents: Opportunities and challenges. Cancers 2018, 10, 345. [Google Scholar] [CrossRef]
- (FDA) USFaDA. FDA Grants Accelerated Approval to Rucaparib for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer. 2020. Available online: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate (accessed on 15 August 2025).
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- (FDA) USFaDA. FDA Approves Olaparib for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. 2020. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer (accessed on 15 August 2025).
- (FDA) USFaDA. FDA Approves First Cancer Treatment for Any Solid Tumor with a Specific Genetic Feature. 2017. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cancer-treatment-any-solid-tumor-specific-genetic-feature (accessed on 15 August 2020).
- Nelson, P.S. Beyond the Androgen Receptor: Targeting Actionable Drivers of Prostate Cancer. JCO Precis. Oncol. 2017, 1, 1–3. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Zhou, X.C.; Wang, H.; Bassi, S.; Carducci, M.A.; Eisenberger, M.A.; Antonarakis, E.S. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur. Urol. 2014, 66, 646–652. [Google Scholar] [CrossRef]
- Cheng, H.H.; Gulati, R.; Azad, A.; Nadal, R.; Twardowski, P.; Vaishampayan, U.N.; Agarwal, N.; Heath, E.I.; Pal, S.K.; Rehman, H.T.; et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015, 18, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mezynski, J.; Pezaro, C.; Bianchini, D.; Zivi, A.; Sandhu, S.; Thompson, E.; Hunt, J.; Sheridan, E.; Baikady, B.; Sarvadikar, A.; et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: Clinical evidence for cross-resistance? Ann. Oncol. 2012, 23, 2943–2947. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.H. Chalcones in Diets. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2019; pp. 1–52. [Google Scholar]
- Aksöz, B.E.; Ertan, R. Chemical and Structural Properties of Chalcones I. FABAD J. Pharm. Sci. 2014, 36, 223–242. [Google Scholar]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Siddique, A.B.; Qusa, M.; Cyprian, F.S.; El Sayed, K.; Alali, F.; Al Moustafa, A.E.; Khalil, A. Design, synthesis, and validation of novel nitrogen-based chalcone analogs against triple negative breast cancer. Eur. J. Med. Chem. 2020, 187, 111954. [Google Scholar] [CrossRef]
- Hassan, A.F.; Hussein, O.; Al-Barazenji, T.; Allouch, A.; Kamareddine, L.; Malki, A.; Moustafa, A.E.A.; Khalil, A. The effect of novel nitrogen-based chalcone analogs on colorectal cancer cells: Insight into the molecular pathways. Heliyon 2024, 10, e27002. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef]
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar] [CrossRef]
- Das, M.; Manna, K. Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight. J. Toxicol. 2016, 2016, 7651047. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kumar, V.; Kumar, P. Heterocyclic chalcone analogues as potential anticancer agents. Anticancer Agents Med. Chem. 2013, 13, 422–432. [Google Scholar]
- Cazarolli, L.H.; Kappel, V.D.; Zanatta, A.P.; Suzuki, D.O.H.; Yunes, R.A.; Nunes, R.J.; Pizzolatti, M.G.; Silva, F.R.M.B. Chapter 2—Natural and Synthetic Chalcones: Tools for the Study of Targets of Action—Insulin Secretagogue or Insulin Mimetic? In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 47–89. [Google Scholar]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Banoth, R.; Thatikonda, A. A Review on Natural Chalcones as Update. Int. J. Pharm. Sci. Res. 2020, 11, 546–555. [Google Scholar]
- Ni, L.; Meng, C.Q.; Sikorski, J.A. Recent advances in therapeutic chalcones. Expert. Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Guex, J.J.; Avril, L.; Enrici, E.; Enriquez, E.; Lis, C.; Taïeb, C. Quality of life improvement in Latin American patients suffering from chronic venous disorder using a combination of Ruscus aculeatus and hesperidin methyl-chalcone and ascorbic acid (quality study). Int. Angiol. 2010, 29, 525–532. [Google Scholar]
- Allaert, F.A. Combination of Ruscus aculeatus extract, hesperidin methyl chalcone and ascorbic acid: A comprehensive review of their pharmacological and clinical effects and of the pathophysiology of chronic venous disease. Int. Angiol. 2016, 35, 111–116. [Google Scholar]
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Srivastava, S. Synthesis of chalcones and flavanones using Julia–Kocienski olefination. Tetrahedron 2010, 66, 9445–9449. [Google Scholar] [CrossRef]
- Kar Mahapatra, D.; Asati, V.; Bharti, S.K. An updated patent review of therapeutic applications of chalcone derivatives (2014–present). Expert. Opin. Ther. Pat. 2019, 29, 385–406. [Google Scholar] [CrossRef]
- Jandial, D.D.; Blair, C.A.; Zhang, S.; Krill, L.S.; Zhang, Y.B.; Zi, X. Molecular targeted approaches to cancer therapy and prevention using chalcones. Curr. Cancer Drug Targets 2014, 14, 181–200. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Moorthy, N.S.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activities. Recent. Pat. Anticancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, Z.; Wang, Q.; Zhou, C.; Lu, F.; Xiao, Y. Chalcone Derivatives Suppress Proliferation and Migration of Castration-resistant Prostate Cancer Cells Through FAK-mediated DNA Damage. Anticancer Res. 2023, 43, 389–403. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.G. The correlation between cancer incidence and kava consumption. Hawaii Med. J. 2000, 59, 420–422. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Dysregulation of apoptotic signaling in cancer: Molecular mechanisms and therapeutic opportunities. J. Cell. Biochem. 2008, 104, 1124–1149. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Mita, A.C.; Tolcher, A.W. Apoptosis: Mechanisms and implications for cancer therapeutics. Target. Oncol. 2006, 1, 197–214. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Kajiwara, T.; Takeuchi, T.; Ueki, T.; Moriyama, N.; Ueki, K.; Kakizoe, T.; Kawabe, K. Effect of Bcl-2 overexpression in human prostate cancer cells in vitro and in vivo. Int. J. Urol. 1999, 6, 520–525. [Google Scholar] [CrossRef]
- Cryns, V.; Yuan, J. Proteases to die for. Genes Dev. 1998, 12, 1551–1570. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Haas, N.B.; Crispen, P.L.; Kolenko, V.M. Mechanisms of apoptosis resistance and treatment strategies to overcome them in hormone-refractory prostate cancer. Cancer 2008, 112, 1660–1671. [Google Scholar] [CrossRef]
- McKenzie, S.; Kyprianou, N. Apoptosis evasion: The role of survival pathways in prostate cancer progression and therapeutic resistance. J. Cell. Biochem. 2006, 97, 18–32. [Google Scholar] [CrossRef]
- Winter, R.N.; Kramer, A.; Borkowski, A.; Kyprianou, N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001, 61, 1227–1232. [Google Scholar]
- Kagan, J.; Stein, J.; Babaian, R.J.; Joe, Y.S.; Pisters, L.L.; Glassman, A.B.; von Eschenbach, A.C.; Troncoso, P. Homozygous deletions at 8p22 and 8p21 in prostate cancer implicate these regions as the sites for candidate tumor suppressor genes. Oncogene 1995, 11, 2121–2126. [Google Scholar] [PubMed]
- Hernandez-Cueto, A.; Hernandez-Cueto, D.; Antonio-Andres, G.; Mendoza-Marin, M.; Jimenez-Gutierrez, C.; Sandoval-Mejia, A.L.; Mora-Campos, R.; Gonzalez-Bonilla, C.; Vega, M.I.; Bonavida, B.; et al. Death receptor 5 expression is inversely correlated with prostate cancer progression. Mol. Med. Rep. 2014, 10, 2279–2286. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Lim, S.S.; Choi, H.J.; Cho, H.J.; Shin, H.K.; Kim, E.J.; Chung, W.Y.; Park, K.K.; Park, J.H. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J. Nutr. Biochem. 2006, 17, 689–696. [Google Scholar] [CrossRef]
- Marquina, S.; Maldonado-Santiago, M.; Sánchez-Carranza, J.N.; Antúnez-Mojica, M.; González-Maya, L.; Razo-Hernández, R.S.; Alvarez, L. Design, synthesis and QSAR study of 2′-hydroxy-4′-alkoxy chalcone derivatives that exert cytotoxic activity by the mitochondrial apoptotic pathway. Bioorganic Med. Chem. 2019, 27, 43–54. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010, 30, 3333–3339. [Google Scholar]
- Li, X.; Pham, V.; Tippin, M.; Fu, D.; Rendon, R.; Song, L.; Uchio, E.; Hoang, B.H.; Zi, X. Flavokawain B targets protein neddylation for enhancing the anti-prostate cancer effect of Bortezomib via Skp2 degradation. Cell Commun. Signal. 2019, 17, 25. [Google Scholar] [CrossRef]
- Kłósek, M.; Mertas, A.; Król, W.; Jaworska, D.; Szymszal, J.; Szliszka, E. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in prostate cancer cells after treatment with Xanthohumol-A natural compound present in Humulus lupulus L. Int. J. Mol. Sci. 2016, 17, 837. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yun, J.; Jung, J.C.; Oh, S.; Jung, Y.S. Anti-tumor activity of benzylideneacetophenone derivatives via proteasomal inhibition in prostate cancer cells. Pharmazie 2016, 71, 274–279. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Paradysz, A.; Krol, W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 2010, 15, 5336–5353. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2009, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Fagnere, C.; Limami, Y.; Ghezali, L.; Pouget, C.; Fidanzi, C.; Ouk, C.; Gueye, R.; Beneytout, J.L.; Duroux, J.L.; et al. 2′-Hydroxy-4-methylsulfonylchalcone enhances TRAIL-induced apoptosis in prostate cancer cells. Anticancer Drugs 2015, 26, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, H.; Kong, X.; Chen, X.; Wu, C. Synthesis of new chalcone-based homoserine lactones and their antiproliferative activity evaluation. Eur. J. Med. Chem. 2019, 163, 500–511. [Google Scholar] [CrossRef]
- Thorburn, A.; Behbakht, K.; Ford, H. TRAIL receptor-targeted therapeutics: Resistance mechanisms and strategies to avoid them. Drug Resist. Updat. 2008, 11, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef]
- Carnero, A. Targeting the cell cycle for cancer therapy. Br. J. Cancer 2002, 87, 129–133. [Google Scholar] [CrossRef]
- Aaltomaa, S.; Eskelinen, M.; Lipponen, P. Expression of cyclin A and D proteins in prostate cancer and their relation to clinopathological variables and patient survival. Prostate 1999, 38, 175–182. [Google Scholar] [CrossRef]
- Comstock, C.E.S.; Revelo, M.P.; Buncher, C.R.; Knudsen, K.E. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br. J. Cancer 2007, 96, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, X.; Xu, Y.; Guo, F.; Ji, J.; Xu, H.; He, J.; Sun, Y.; Wang, F. Differential Expression and Prognostic Value of Cytoplasmic and Nuclear Cyclin D1 in Prostate Cancer. BioMed Res. Int. 2020, 2020, 1692658. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, O.J.; Haukaas, S.A.; Akslen, L.A. Combined Loss of PTEN and p27 Expression Is Associated with Tumor Cell Proliferation by Ki-67 and Increased Risk of Recurrent Disease in Localized Prostate Cancer. Clin. Cancer Res. 2003, 9, 1474–1479. [Google Scholar]
- Saito, Y.; Mizokami, A.; Maeda, S.; Takahashi, K.; Izumi, K.; Goto, M.; Nakagawa-Goto, K. Bicyclic Chalcones as Mitotic Inhibitors for Overcoming Androgen Receptor-Independent and Multidrug-Resistant Prostate Cancer. ACS Omega 2021, 6, 4842–4849. [Google Scholar] [CrossRef]
- Chen, C.; Shao, R.; Li, B.; Zhai, Y.; Wang, T.; Li, X.; Miao, L.; Huang, J.; Liu, R.; Liu, E.; et al. Neoisoliquiritin exerts tumor suppressive effects on prostate cancer by repressing androgen receptor activity. Phytomedicine 2021, 85, 153514. [Google Scholar] [CrossRef]
- Shimada, T.; Naito, R.; Toriumi, R.; Nakagawa, R.; Aoyama, S.; Kamijima, T.; Kano, H.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; et al. Novel α-Trifluoromethyl Chalcone Exerts Antitumor Effects Against Prostate Cancer Cells. Anticancer Res. 2023, 43, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Mizokami, A.; Izumi, K.; Naito, R.; Goto, M.; Nakagawa-Goto, K. α-Trifluoromethyl Chalcones as Potent Anticancer Agents for Androgen Receptor-Independent Prostate Cancer. Molecules 2021, 26, 2812. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnère, C.; Sol, V.; et al. Synthesis and biological evaluation of chalcone-polyamine conjugates as novel vectorized agents in colorectal and prostate cancer chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef]
- Fu, Y.; Hsieh, T.C.; Guo, J.; Kunicki, J.; Lee, M.Y.; Darzynkiewicz, Z.; Wu, J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [Google Scholar] [CrossRef]
- Fu, D.J.; Li, J.H.; Yang, J.J.; Li, P.; Zhang, Y.B.; Liu, S.; Li, Z.R.; Zhang, S.Y. Discovery of novel chalcone-dithiocarbamates as ROS-mediated apoptosis inducers by inhibiting catalase. Bioorganic Chem. 2019, 86, 375–385. [Google Scholar] [CrossRef]
- Sun, Y.W.; Huang, W.J.; Hsiao, C.J.; Chen, Y.C.; Lu, P.H.; Guh, J.H. Methoxychalcone induces cell-cycle arrest and apoptosis in human hormone-resistant prostate cancer cells through PI 3-kinase-independent inhibition of mTOR pathways. Prostate 2010, 70, 1295–1306. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, D.Y.; Choi, H.J.; Jung, J.I.; Chung, W.Y.; Park, J.H. Induction of cell cycle arrest in prostate cancer cells by the dietary compound isoliquiritigenin. J. Med. Food 2009, 12, 8–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Srinivasan, B.; Xing, C.; Lü, J. A new chalcone derivative (E)-3-(4-methoxyphenyl)-2-methyl-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one suppresses prostate cancer involving p53-mediated cell cycle arrests and apoptosis. Anticancer Res. 2012, 32, 3689–3698. [Google Scholar] [PubMed]
- Alam, M.J.; Alam, O.; Perwez, A.; Rizvi, M.A.; Naim, M.J.; Naidu, V.G.M.; Imran, M.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Design, Synthesis, Molecular Docking, and Biological Evaluation of Pyrazole Hybrid Chalcone Conjugates as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Pharmaceuticals 2022, 15, 280. [Google Scholar] [CrossRef]
- Peyrot, V.; Leynadier, D.; Sarrazin, M.; Briand, C.; Rodriquez, A.; Nieto, J.M.; Andreu, J.M. Interaction of tubulin and cellular microtubules with the new antitumor drug MDL 27048. A powerful and reversible microtubule inhibitor. J. Biol. Chem. 1989, 264, 21296–21301. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Mizokami, A.; Tsurimoto, H.; Izumi, K.; Goto, M.; Nakagawa-Goto, K. 5′-Chloro-2,2′-dihydroxychalcone and related flavanoids as treatments for prostate cancer. Eur. J. Med. Chem. 2018, 157, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.M.; Yedla, P.; Babu, K.S.; Shaik, T.B.; Chityal, G.K.; Kamal, A. Synthesis and Biological Evaluation of 1,2,3-triazole tethered Pyrazoline and Chalcone Derivatives. Chem. Biol. Drug Des. 2016, 88, 97–109. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Wang, Z.; Gao, M.; Wang, X.; Han, W.; Zhang, N.; Xu, X. Flavokawain A inhibits prostate cancer cells by inducing cell cycle arrest and cell apoptosis and regulating the glutamine metabolism pathway. J. Pharm. Biomed. Anal. 2020, 186, 113288. [Google Scholar] [CrossRef]
- Shaffer, C.V.; Cai, S.; Peng, J.; Robles, A.J.; Hartley, R.M.; Powell, D.R.; Du, L.; Cichewicz, R.H.; Mooberry, S.L. Texas native plants yield compounds with cytotoxic activities against prostate cancer cells. J. Nat. Prod. 2016, 79, 531–540. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-mesenchymal transition (emt) and prostate cancer. In Cell & Molecular Biology of Prostate Cancer: Updates, Insights and New Frontiers; Schatten, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 101–110. [Google Scholar]
- Montanari, M.; Rossetti, S.; Cavaliere, C.; D’Aniello, C.; Malzone, M.G.; Vanacore, D.; Di Franco, R.; La Mantia, E.; Iovane, G.; Piscitelli, R.; et al. Epithelial-mesenchymal transition in prostate cancer: An overview. Oncotarget 2017, 8, 35376–35389. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Scott, K.F.; Becker, T.M.; Lock, J.; Nimir, M.; Ma, Y.; de Souza, P. The Prospect of Identifying Resistance Mechanisms for Castrate-Resistant Prostate Cancer Using Circulating Tumor Cells: Is Epithelial-to-Mesenchymal Transition a Key Player? Prostate Cancer 2020, 2020, 7938280. [Google Scholar] [CrossRef]
- Chen, C.; Huang, S.; Chen, C.L.; Su, S.B.; Fang, D.D. Isoliquiritigenin Inhibits Ovarian Cancer Metastasis by Reversing Epithelial-to-Mesenchymal Transition. Molecules 2019, 24, 3725. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jang, H.J.; Kwak, S.; Sung, G.J.; Park, S.H.; Song, J.H.; Kim, H.; Na, Y.; Choi, K.C. Novel TGF-β1 inhibitor antagonizes TGF-β1-induced epithelial-mesenchymal transition in human A549 lung cancer cells. J. Cell Biochem. 2019, 120, 977–987. [Google Scholar] [CrossRef]
- Moon, D.O.; Choi, Y.H.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Butein suppresses the expression of nuclear factor-kappa B-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicol. Vitr. 2010, 24, 1927–1934. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, B.; Yu, J.; Huang, L.; Zeng, X.; Shen, X.; Ren, C.; Ben-David, Y.; Luo, H. Fli-1 activation through targeted promoter activity regulation using a novel 3′, 5′-diprenylated chalcone inhibits growth and metastasis of prostate cancer cells. Int. J. Mol. Sci. 2020, 21, 2216. [Google Scholar] [CrossRef]
- Kwon, G.T.; Cho, H.J.; Chung, W.Y.; Park, K.K.; Moon, A.; Park, J.H. Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased JNK/AP-1 signaling. J. Nutr. Biochem. 2009, 20, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Li, J.; Meniel, V.S.; Fennell, C.M.; Waring, P.; Montgomery, K.G.; Rebello, R.J.; Macpherson, A.A.; Koushyar, S.; Furic, L.; et al. Identification of Pik3ca Mutation as a Genetic Driver of Prostate Cancer That Cooperates with Pten Loss to Accelerate Progression and Castration-Resistant Growth. Cancer Discov. 2018, 8, 764–779. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.-M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Chung, E.; Seon, M.R.; Shin, H.K.; Kim, E.J.; Lim, S.S.; Chung, W.Y.; Park, K.K.; Park, J.H. Isoliquiritigenin (ISL) inhibits ErbB3 signaling in prostate cancer cells. Biofactors 2006, 28, 159–168. [Google Scholar] [CrossRef]
- Khor, T.O.; Yu, S.; Barve, A.; Hao, X.; Hong, J.L.; Lin, W.; Foster, B.; Huang, M.T.; Newmark, H.L.; Kong, A.N. Dietary feeding of dibenzoylmethane inhibits prostate cancer in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009, 69, 7096–7102. [Google Scholar] [CrossRef][Green Version]
- Yo, Y.T.; Shieh, G.S.; Hsu, K.F.; Wu, C.L.; Shiau, A.L. Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J. Agric. Food Chem. 2009, 57, 8266–8273. [Google Scholar] [CrossRef]
- Horta, B.; Freitas-Silva, J.; Silva, J.; Dias, F.; Teixeira, A.L.; Medeiros, R.; Cidade, H.; Pinto, M.; Cerqueira, F. Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions. Molecules 2023, 28, 2159. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.W.C.; Poon, R.T.P. Clinical implications of angiogenesis in cancers. Vasc. Health Risk Manag. 2006, 2, 97–108. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Green, M.M.L.; Hiley, C.T.; Shanks, J.H.; Bottomley, I.C.; West, C.M.L.; Cowan, R.A.; Stratford, I.J. Expression of vascular endothelial growth factor (VEGF) in locally invasive prostate cancer is prognostic for radiotherapy outcome. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.J.; Mac Gabhann, F. Dysregulation of the vascular endothelial growth factor and semaphorin ligand-receptor families in prostate cancer metastasis. BMC Syst. Biol. 2015, 9, 55. [Google Scholar] [CrossRef]
- Melegh, Z.; Oltean, S. Targeting Angiogenesis in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 2676. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Zurita, A.J.; Werner, L.; Bruce, J.Y.; Carducci, M.A.; Stein, M.N.; Heath, E.I.; Hussain, A.; Tran, H.T.; Sweeney, C.J.; et al. A randomized phase II trial of short-course androgen deprivation therapy with or without Bevacizumab for patients with recurrent prostate cancer after definitive local therapy. J. Clin. Oncol. 2016, 34, 1913–1920. [Google Scholar] [CrossRef]
- Kelly, W.K.; Halabi, S.; Carducci, M.; George, D.; Mahoney, J.F.; Stadler, W.M.; Morris, M.; Kantoff, P.; Monk, J.P.; Kaplan, E.; et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 2012, 30, 1534–1540. [Google Scholar] [CrossRef]
- Tannock, I.F.; Fizazi, K.; Ivanov, S.; Karlsson, C.T.; Fléchon, A.; Skoneczna, I.; Orlandi, F.; Gravis, G.; Matveev, V.; Bavbek, S.; et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): A phase 3, double-blind randomised trial. Lancet Oncol. 2013, 14, 760–768. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Oudard, S.; Ou, Y.C.; Sengeløv, L.; Saad, F.; Houede, N.; Ostler, P.; Stenzl, A.; Daugaard, G.; Jones, R.; et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 76–82. [Google Scholar] [CrossRef]
- Keizman, D.; Zahurak, M.; Sinibaldi, V.; Carducci, M.; Denmeade, S.; Drake, C.; Pili, R.; Antonarakis, E.S.; Hudock, S.; Eisenberger, M. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: Results of a phase I/II double-blinded, randomized study. Clin. Cancer Res. 2010, 16, 5269–5276. [Google Scholar] [CrossRef]
- Mahmoud, A.; Elkhalifa, D.; Alali, F.; Al Moustafa, A.E.; Khalil, A. Novel Polymethoxylated Chalcones as Potential Compounds Against KRAS-Mutant Colorectal Cancers. Curr. Pharm. Des. 2020, 26, 1622–1633. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Wen, Z.; Chen, X.; Yu, J.; Yuan, D.; Xu, B.; Luo, H.; Zhu, J. A novel 3′,5′-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCδ/JNK signal in prostate cancer. Aging 2020, 12, 9103–9124. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Jackson, K.M.; Frazier, M.C.; Harris, W.B. Suppression of androgen receptor expression by dibenzoylmethane as a therapeutic objective in advanced prostate cancer. Anticancer Res. 2007, 27, 1483–1488. [Google Scholar] [PubMed]
- Chen, S.; Gao, J.; Halicka, H.D.; Traganos, F.; Darzynkiewicz, Z. Down-regulation of androgen-receptor and PSA by phytochemicals. Int. J. Oncol. 2008, 32, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Geng, G.; Batist, G.; Wu, J.H. Syntheses and potential anti-prostate cancer activities of ionone-based chalcones. Bioorganic Med. Chem. Lett. 2009, 19, 1183–1186. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Reetz, L.G.; Chiesa, J.; Battisti, I.E.; Gonçalves, J.F.; Duarte, M.M.; Schetinger, M.R.; Morsch, V.M. Oxidative stress and antioxidant status in prostate cancer patients: Relation to Gleason score, treatment and bone metastasis. Biomed. Pharmacother. 2011, 65, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yamashita, H.; Yu, X.; Wang, J.; Franco, O.E.; Wang, Y.; Hayward, S.W.; Matusik, R.J. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene 2015, 34, 3700–3710. [Google Scholar] [CrossRef]
- Khurana, N.; Sikka, S.C. Targeting Crosstalk between Nrf-2, NF-κB and Androgen Receptor Signaling in Prostate Cancer. Cancers 2018, 10, 352. [Google Scholar] [CrossRef]
- Staal, J.; Beyaert, R. Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef]
- Ismail, B.; Ghezali, L.; Gueye, R.; Limami, Y.; Pouget, C.; Leger, D.Y.; Martin, F.; Beneytout, J.L.; Duroux, J.L.; Diab-Assaf, M.; et al. Novel methylsulfonyl chalcones as potential antiproliferative drugs for human prostate cancer: Involvement of the intrinsic pathway of apoptosis. Int. J. Oncol. 2013, 43, 1160–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pascoal, A.C.; Ehrenfried, C.A.; Lopez, B.G.; de Araujo, T.M.; Pascoal, V.D.; Gilioli, R.; Anhê, G.F.; Ruiz, A.L.; Carvalho, J.E.; Stefanello, M.E.; et al. Antiproliferative activity and induction of apoptosis in PC-3 cells by the chalcone cardamonin from Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study. Molecules 2014, 19, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Sarkar, F.H.; Wei, W. Targeting prostate cancer stem cells for cancer therapy. Discov. Med. 2012, 13, 135–142. [Google Scholar] [PubMed]
- Maccalli, C.; De Maria, R. Cancer stem cells: Perspectives for therapeutic targeting. Cancer Immunol. Immunother. 2015, 64, 91–97. [Google Scholar] [CrossRef]
- Hussein, O.J.; Rayan, M.; Matarid, T.R.; Elkhalifa, D.; Abunada, H.H.; Therachiyil, L.; Khalil, A.; Uddin, S.; Maccalli, C.; Korashy, H.M. The role of immune checkpoints in modulating cancer stem cells anti-tumor immune responses: Implications and perspectives in cancer therapy. J. Exp. Clin. Cancer Res. 2025, 44, 305. [Google Scholar] [CrossRef]
- Tout, I.; Bougarn, S.; Toufiq, M.; Gopinath, N.; Hussein, O.; Sathappan, A.; Chin-Smith, E.; Rehaman, F.; Mathew, R.; Mathew, L.; et al. The integrative genomic and functional immunological analyses of colorectal cancer initiating cells to modulate stemness properties and the susceptibility to immune responses. J. Transl. Med. 2025, 23, 193. [Google Scholar] [CrossRef]
- Rybak, A.P.; Bristow, R.G.; Kapoor, A. Prostate cancer stem cells: Deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget 2015, 6, 1900–1919. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Lin, F.; Singh, R.K.; Mahato, R.I. Micellar Delivery of miR-34a Modulator Rubone and Paclitaxel in Resistant Prostate Cancer. Cancer Res. 2017, 77, 3244–3254. [Google Scholar] [CrossRef]
- Lin, F.; Wen, D.; Wang, X.; Mahato, R.I. Dual responsive micelles capable of modulating miRNA-34a to combat taxane resistance in prostate cancer. Biomaterials 2019, 192, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Burcu Gürdere, M.; Aydin, A.; Yencilek, B.; Ertürk, F.; Özbek, O.; Erkan, S.; Budak, Y.; Ceylan, M. Synthesis, Antiproliferative and Cytotoxic Activities, DNA Binding Features and Molecular Docking Study of Novel Enamine Derivatives. Chem. Biodivers. 2020, 17, e2000139. [Google Scholar] [CrossRef] [PubMed]
- Ocasio-Malavé, C.; Donate, M.J.; Sánchez, M.M.; Sosa-Rivera, J.M.; Mooney, J.W.; Pereles-De León, T.A.; Carballeira, N.M.; Zayas, B.; Vélez-Gerena, C.E.; Martínez-Ferrer, M.; et al. Synthesis of novel 4-Boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorganic Med. Chem. Lett. 2020, 30, 126760. [Google Scholar] [CrossRef]
- Cancino, K.; Castro, I.; Yauri, C.; Jullian, V.; Arévalo, J.; Sauvain, M.; Adaui, V.; Castillo, D. Toxicity assessment of synthetic chalcones with antileishmanial potential in BALB/c mice. Rev. Peru. Med. Exp. Salud Publica 2021, 38, 424–433. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Shuai, W.; Sun, H.; Yao, H.; Ma, C.; Xu, S.; Yao, H.; Zhu, Z.; Yang, D.-H.; et al. Discovery of Novel Quinoline–Chalcone Derivatives as Potent Antitumor Agents with Microtubule Polymerization Inhibitory Activity. J. Med. Chem. 2019, 62, 993–1013. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; El-Shorbagy, H.M.; Hassaneen, H.M.; Abdelhamid, I.A.; Sabet, S.; Ibrahim, S.A. [1,2,4] Triazolo [3,4-a]isoquinoline chalcone derivative exhibits anticancer activity via induction of oxidative stress, DNA damage, and apoptosis in Ehrlich solid carcinoma-bearing mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1225–1238. [Google Scholar] [CrossRef]
- Zhou, P.; Gross, S.; Liu, J.H.; Yu, B.Y.; Feng, L.L.; Nolta, J.; Sharma, V.; Piwnica-Worms, D.; Qiu, S.X. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-kappaB and MAPK signaling pathways. FASEB J. 2010, 24, 4722–4732. [Google Scholar]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated Flavonoids with Selective Toxicity against Human Cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef] [PubMed]
- d’Oliveira, G.D.C.; Custodio, J.M.F.; Moura, A.F.; Napolitano, H.B.; Pérez, C.N.; Moraes, M.O.; Prókai, L.; Perjési, P. Different reactivity to glutathione but similar tumor celltoxicity of chalcones and their quinolinone analogues. Med. Chem. Res. 2019, 28, 1448–1460. [Google Scholar] [CrossRef]
- Amslinger, S. The tunable functionality of α,β-unsaturated carbonyl compounds enables their differential application in biological systems. ChemMedChem 2010, 5, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Suliman, A.; Hassan, A.F.; Saeed, S.; Al Moustafa, A.-E.; Khalil, A.; Elhissi, A. Liposomal delivery of DK14 chalcone analogue: A promising therapeutic strategy against triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2026, 115, 107601. [Google Scholar] [CrossRef]
- Souza, J.M.T.; de Negreiros Matos Silva, S.A.; de Sá, R.E.; Ribeiro, R.; de Souza Castro, A.J.; Eaton, P.; de Castro, M.R.C.; Pérez, C.N.; Machado-Neto, J.A.; da Silva, D.A.; et al. ZnO nanoparticles enhance the cytotoxic effects of a chalcone-sulfonamide hybrid by impairing the autophagic flux and upregulating HIF-1α expression. Bioorganic Chem. 2025, 166, 109127. [Google Scholar] [CrossRef]
- Nakao, I.A.; Hermenegildo, A.M.; Vaz, L.B.A.; Reis, A.C.C.; Coutinho, G.G.; Campbell, G.S.G.; Almeida, T.C.; Amparo, T.R.; de Fatima Anunciação, K.; da Silva, G.N.; et al. Development of new glycosyl-chalcones targeting cancer cells through recognition of cellular carbohydrate receptors. Future Med. Chem. 2025, 17, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Manna, T.; Pal, K.; Jana, K.; Misra, A.K. Anti-cancer potential of novel glycosylated 1,4-substituted triazolylchalcone derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 126615. [Google Scholar] [CrossRef]
- Toublet, F.-X.; Laurent, A.; Pouget, C. A Review of Natural and Synthetic Chalcones as Anticancer Agents Targeting Topoisomerase Enzymes. Molecules 2025, 30, 2498. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: A recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef]
- Laiche, M.H.; Barlow, J.W. Recent Advances in the Synthesis and Biological Applications of Prenylated Chalcones. Int. J. Mol. Sci. 2025, 26, 9845. [Google Scholar] [CrossRef]
- Buckett, L.; Sus, N.; Spindler, V.; Rychlik, M.; Schoergenhofer, C.; Frank, J. The Pharmacokinetics of Individual Conjugated Xanthohumol Metabolites Show Efficient Glucuronidation and Higher Bioavailability of Micellar than Native Xanthohumol in a Randomized, Double-Blind, Crossover Trial in Healthy Humans. Mol. Nutr. Food Res. 2023, 67, 2200684. [Google Scholar] [CrossRef]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J. Mass Spectrom. JMS 2005, 40, 289–299. [Google Scholar] [CrossRef]
- Krajnović, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef]
- Sung, B.; Cho, S.G.; Liu, M.; Aggarwal, B.B. Butein, a tetrahydroxychalcone, suppresses cancer-induced osteoclastogenesis through inhibition of receptor activator of nuclear factor-kappaB ligand signaling. Int. J. Cancer 2011, 129, 2062–2072. [Google Scholar] [CrossRef]
- Khan, N.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Butein induces apoptosis and inhibits prostate tumor growth in vitro and in vivo. Antioxid. Redox Signal. 2012, 16, 1195–1204. [Google Scholar] [CrossRef]
- Kanazawa, M.; Satomi, Y.; Mizutani, Y.; Ukimura, O.; Kawauchi, A.; Sakai, T.; Baba, M.; Okuyama, T.; Nishino, H.; Miki, T. Isoliquiritigenin inhibits the growth of prostate cancer. Eur. Urol. 2003, 43, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, Y.; Li, Y.; Shu, N.; Wang, Z.; Wang, Y.; Li, Y.; Chen, Z. Antineoplastic activity of isoliquiritigenin, a chalcone compound, in androgen-independent human prostate cancer cells linked to G2/M cell cycle arrest and cell apoptosis. Eur. J. Pharmacol. 2018, 821, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, E.D.; Wang, J.; Panzhinskiy, E.E.; Tong, C.; Li, W.; Li, J. Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 841–847. [Google Scholar] [CrossRef]
- Lackova, Z.; Buchtelova, H.; Buchtova, Z.; Klejdus, B.; Heger, Z.; Brtnicky, M.; Kynicky, J.; Zitka, O.; Adam, V. Anticarcinogenic Effect of Spices Due to Phenolic and Flavonoid Compounds-In Vitro Evaluation on Prostate Cells. Molecules 2017, 22, 1626. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Yerdelen, K.O.; Das, U.; Gul, M.; Pandit, B.; Li, P.K.; Dimmock, J.R. Synthesis and cytotoxicity of novel 3-aryl-1-(3′-dibenzylaminomethyl-4′-hydroxyphenyl)-propenones and related compounds. Chem. Pharm. Bull. 2008, 56, 1675–1681. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Ji, T.; Liu, Z.; Gu, M.; Hoang, B.H.; Zi, X. Dietary feeding of Flavokawain A, a Kava chalcone, exhibits a satisfactory safety profile and its association with enhancement of phase II enzymes in mice. Toxicol. Rep. 2014, 1, 2–11. [Google Scholar] [CrossRef]
- Li, X.; Yokoyama, N.N.; Zhang, S.; Ding, L.; Liu, H.M.; Lilly, M.B.; Mercola, D.; Zi, X. Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model. Oncotarget 2015, 6, 41809–41824. [Google Scholar] [CrossRef]
- Song, L.; Mino, M.; Yamak, J.; Nguyen, V.; Lopez, D.; Pham, V.; Fazelpour, A.; Le, V.; Fu, D.; Tippin, M.; et al. Flavokawain A Reduces Tumor-Initiating Properties and Stemness of Prostate Cancer. Front. Oncol. 2022, 12, 943846. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Liu, Z.; Simoneau, A.R.; Xie, J.; Zi, X. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int. J. Cancer 2010, 127, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N. Pre-treatment with cardamonin protects against cisplatin-induced nephrotoxicity in rats: Impact on NOX-1, inflammation and apoptosis. Toxicol. Appl. Pharmacol. 2014, 274, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Basappa; Rangappa, K.S.; et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kumar, V.; Lee, S.; Iwai, A.; Neckers, L.; Malhotra, S.V.; Trepel, J.B. Methoxychalcone inhibitors of androgen receptor translocation and function. Bioorganic Med. Chem. Lett. 2012, 22, 2105–2109. [Google Scholar] [CrossRef]
- Moses, M.A.; Kim, Y.S.; Rivera-Marquez, G.M.; Oshima, N.; Watson, M.J.; Beebe, K.E.; Wells, C.; Lee, S.; Zuehlke, A.D.; Shao, H.; et al. Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 4022–4035. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, H.Y.; Riaz, T.A.; Bhattarai, K.R.; Chaudhary, M.; Ahn, J.H.; Jeong, J.; Kim, H.R.; Chae, H.J. Chalcone suppresses tumor growth through NOX4-IRE1α sulfonation-RIDD-miR-23b axis. Redox Biol. 2021, 40, 101853. [Google Scholar] [CrossRef]
- Delmulle, L.; Bellahcène, A.; Dhooge, W.; Comhaire, F.; Roelens, F.; Huvaere, K.; Heyerick, A.; Castronovo, V.; De Keukeleire, D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine 2006, 13, 732–734. [Google Scholar] [CrossRef]
- Li, K.; Zheng, Q.; Chen, X.; Wang, Y.; Wang, D.; Wang, J. Isobavachalcone Induces ROS-Mediated Apoptosis via Targeting Thioredoxin Reductase 1 in Human Prostate Cancer PC-3 Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1915828. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Balnbury, L.; Flowers, A.; Giménez-Turba, A.; Ruiz, G.; Waterman, P.G.; Peña-Rodríguez, L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine 2007, 14, 530–533. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wen, R.; Tu, G.Z.; Chen, H.B.; Liang, H.; Zhao, Y.Y. Anti-inflammatory and antiproliferative prenylated chalcones from Hedysarum gmelinii. J. Asian Nat. Prod. Res. 2018, 20, 1009–1018. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Wang, X.; Zeng, X.; Hu, Z.; Liu, Y.; Xie, Y.; Liang, G.; Zhu, J.; Luo, H.; et al. Novel 3′,5′-diprenylated chalcones inhibited the proliferation of cancer cells in vitro by inducing cell apoptosis and arresting cell cycle phase. Eur. J. Med. Chem. 2017, 133, 227–239. [Google Scholar] [CrossRef]
- Su, S.Y.; Xue, J.J.; Yang, G.Y.; Lei, C.; Hou, A.J. New cytotoxic alkylated chalcones from fatoua villosa. Chem. Biodivers. 2017, 14, e1700076. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Y.; Wang, X.; Han, Y.; Peng, X.; Efferth, T.; Fu, Y. Synthesis and anti-tumor activity of novel aminomethylated derivatives of isoliquiritigenin. Molecules 2014, 19, 17715–17726. [Google Scholar] [CrossRef]
- Liu, G.; Ge, Z.; Zhao, M.; Zhou, Y. Design, synthesis and cytotoxic activities of novel aliphatic amino-substituted flavonoids. Molecules 2013, 18, 14070–14084. [Google Scholar] [CrossRef]
- Lorenzo, P.; Alvarez, R.; Ortiz, M.A.; Alvarez, S.; Piedrafita, F.J.; de Lera, A.R. Inhibition of IkappaB kinase-beta and anticancer activities of novel chalcone adamantyl arotinoids. J. Med. Chem. 2008, 51, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Ortiz, M.A.; Alvarez, R.; Piedrafita, F.J.; de Lera, A.R. Adamantyl arotinoids that inhibit IκB kinase α and IκB kinase β. ChemMedChem 2013, 8, 1184–1198. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Abramjuk, C.; Vásquez, L.; Gamboa, N.; Domínguez, J.; Nitzsche, B.; Höpfner, M.; Georgieva, R.; Bäumler, H.; Stephan, C.; et al. New 4-maleamic acid and 4-maleamide peptidyl chalcones as potential multitarget drugs for human prostate cancer. Pharm. Res. 2011, 28, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pouget, C.; Fidanzi-Dugas, C.; Gamond, A.; Laurent, A.; Semaan, J.; Pinon, A.; Champavier, Y.; Léger, D.Y.; Liagre, B.; et al. Design and multi-step synthesis of chalcone-polyamine conjugates as potent antiproliferative agents. Bioorganic Med. Chem. Lett. 2017, 27, 4354–4357. [Google Scholar] [CrossRef]
- Gul, H.I.; Yerdelen, K.O.; Gul, M.; Das, U.; Pandit, B.; Li, P.K.; Secen, H.; Sahin, F. Synthesis of 4′-hydroxy-3′-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch. Pharm. 2007, 340, 195–201. [Google Scholar] [CrossRef]
- Reddy, M.V.; Su, C.R.; Chiou, W.F.; Liu, Y.N.; Chen, R.Y.; Bastow, K.F.; Lee, K.H.; Wu, T.S. Design, synthesis, and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorganic Med. Chem. 2008, 16, 7358–7370. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Nekkanti, S.; Brahma, U.R.; Praveen Kumar, N.; Deshpande, N.; Prasanna, D.; Senwar, K.R.; Jaya Lakshmi, U. Synthesis of different heterocycles-linked chalcone conjugates as cytotoxic agents and tubulin polymerization inhibitors. Bioorganic Med. Chem. 2017, 25, 4805–4816. [Google Scholar] [CrossRef]
- Tu, H.Y.; Huang, A.M.; Hour, T.C.; Yang, S.C.; Pu, Y.S.; Lin, C.N. Synthesis and biological evaluation of 2′,5′-dimethoxychalcone derivatives as microtubule-targeted anticancer agents. Bioorganic Med. Chem. 2010, 18, 2089–2098. [Google Scholar] [CrossRef]
- Lei, Q.; Zhang, S.; Liu, M.; Li, J.; Zhang, X.; Long, Y. Synthesis and biological evaluation of glycosides containing triazene-chalcones. Mol. Divers. 2017, 21, 957–966. [Google Scholar] [CrossRef]
- Wang, M.; Xu, S.; Wu, C.; Liu, X.; Tao, H.; Huang, Y.; Liu, Y.; Zheng, P.; Zhu, W. Design, synthesis and activity of novel sorafenib analogues bearing chalcone unit. Bioorganic Med. Chem. Lett. 2016, 26, 5450–5454. [Google Scholar] [CrossRef] [PubMed]
- Wani, Z.A.; Guru, S.K.; Rao, A.V.; Sharma, S.; Mahajan, G.; Behl, A.; Kumar, A.; Sharma, P.R.; Kamal, A.; Bhushan, S.; et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem. Toxicol. 2016, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Ramakrishna, G.; Raju, P.; Viswanath, A.; Ramaiah, M.J.; Balakishan, G.; Pal-Bhadra, M. Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorganic Med. Chem. Lett. 2010, 20, 4865–4869. [Google Scholar] [CrossRef]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.; Kaur, T.; Saxena, A.K. 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pessôa, J.C.; Pinheiro, E.M.C.; Muri, E.M.F.; Filho, E.V.; Loureiro, L.B.; Freitas, M.C.R.; Silva Junior, C.M.D.; Fiorot, R.G.; Carneiro, J.W.M.; et al. 2H-1,2,3-Triazole-chalcones as novel cytotoxic agents against prostate cancer. Bioorganic Med. Chem. Lett. 2020, 30, 127454. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Wang, Y.; Li, M.; Jernigan, F.; Sun, L. Novel indolyl-chalcones target stathmin to induce cancer cell death. Cell Cycle 2016, 15, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hedblom, A.; Koerner, S.K.; Li, M.; Jernigan, F.E.; Wegiel, B.; Sun, L. Novel synthetic chalcones induce apoptosis in the A549 non-small cell lung cancer cells harboring a KRAS mutation. Bioorganic Med. Chem. Lett. 2016, 26, 5703–5706. [Google Scholar] [CrossRef] [PubMed]
- Bagul, C.; Rao, G.K.; Makani, V.K.K.; Tamboli, J.R.; Pal-Bhadra, M.; Kamal, A. Synthesis and biological evaluation of chalcone-linked pyrazolo [1,5-a]pyrimidines as potential anticancer agents. Medchemcomm 2017, 8, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Zhang, S.J.; Ge, Q.F.; Guo, D.W.; Cai, C.M.; Hu, W.X. Synthesis and anticancer evaluation of thiazolyl-chalcones. Bioorganic Med. Chem. Lett. 2010, 20, 6555–6559. [Google Scholar] [CrossRef]
- Kamal, A.; Balakrishna, M.; Nayak, V.L.; Shaik, T.B.; Faazil, S.; Nimbarte, V.D. Design and synthesis of imidazo[2,1-b]thiazole-chalcone conjugates: Microtubule-destabilizing agents. ChemMedChem 2014, 9, 2766–2780. [Google Scholar] [CrossRef]
- Wang, Q.; Arnst, K.E.; Wang, Y.; Kumar, G.; Ma, D.; Chen, H.; Wu, Z.; Yang, J.; White, S.W.; Miller, D.D.; et al. Structural modification of the 3,4,5-trimethoxyphenyl moiety in the tubulin inhibitor VERU-111 leads to improved antiproliferative activities. J. Med. Chem. 2018, 61, 7877–7891. [Google Scholar] [CrossRef]
- Nagaraju, M.; Gnana Deepthi, E.; Ashwini, C.; Vishnuvardhan, M.V.; Lakshma Nayak, V.; Chandra, R.; Ramakrishna, S.; Gawali, B.B. Synthesis and selective cytotoxic activity of novel hybrid chalcones against prostate cancer cells. Bioorganic Med. Chem. Lett. 2012, 22, 4314–4317. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.; Syam, S.; Narrima, P.; Cheah, S.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef]
- Chinthala, Y.; Thakur, S.; Tirunagari, S.; Chinde, S.; Domatti, A.K.; Arigari, N.K.; Srinivas, K.V.N.S.; Alam, S.; Jonnala, K.K.; Khan, F.; et al. Synthesis, docking and ADMET studies of novel chalcone triazoles for anti-cancer and anti-diabetic activity. Eur. J. Med. Chem. 2015, 93, 564–573. [Google Scholar] [CrossRef]
- Popłoński, J.; Turlej, E.; Sordon, S.; Tronina, T.; Bartmańska, A.; Wietrzyk, J.; Huszcza, E. Synthesis and Antiproliferative Activity of Minor Hops Prenylflavonoids and New Insights on Prenyl Group Cyclization. Molecules 2018, 23, 776. [Google Scholar] [CrossRef]
- Vijaya Bhaskar Reddy, M.; Shen, Y.C.; Ohkoshi, E.; Bastow, K.F.; Qian, K.; Lee, K.H.; Wu, T.S. Bis-chalcone analogues as potent NO production inhibitors and as cytotoxic agents. Eur. J. Med. Chem. 2012, 47, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Kelsang, N.; Tu, G.; Kong, D.; Lu, J.; Zhang, Y.; Liang, H.; Tu, P.; Zhang, Q. Structurally diverse cytotoxic dimeric chalcones from Oxytropis chiliophylla. J. Nat. Prod. 2018, 81, 307–315. [Google Scholar] [CrossRef]
- El Sayed Aly, M.R.; Abd El Razek Fodah, H.H.; Saleh, S.Y. Antiobesity, antioxidant and cytotoxicity activities of newly synthesized chalcone derivatives and their metal complexes. Eur. J. Med. Chem. 2014, 76, 517–530. [Google Scholar] [CrossRef]
- Gibson, M.Z.; Nguyen, M.A.; Zingales, S.K. Design, Synthesis, and Evaluation of (2-(Pyridinyl)methylene)-1-tetralone Chalcones for Anticancer and Antimicrobial Activity. Med. Chem. 2018, 14, 333–343. [Google Scholar] [CrossRef]
- Maguire, C.J.; Carlson, G.J.; Ford, J.W.; Strecker, T.E.; Hamel, E.; Trawick, M.L.; Pinney, K.G. Synthesis and biological evaluation of structurally diverse α-conformationally restricted chalcones and related analogues. Medchemcomm 2019, 10, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, B.V.S.; Prasad, Y.R.; Shaik, A.B. Synthesis, Biological Evaluation and Molecular Docking Studies of New Pyrazolines as an Antitubercular and Cytotoxic Agents. Infect. Disord. Drug Targets 2019, 19, 310–321. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, O.J.; Elkhalifa, D.; Hassan, A.F.; Alali, F.; Al Moustafa, A.-E.; Khalil, A. The Role of Natural Chalcones and Their Derivatives in Targeting Prostate Cancer: Recent Updates. Int. J. Mol. Sci. 2025, 26, 12082. https://doi.org/10.3390/ijms262412082
Hussein OJ, Elkhalifa D, Hassan AF, Alali F, Al Moustafa A-E, Khalil A. The Role of Natural Chalcones and Their Derivatives in Targeting Prostate Cancer: Recent Updates. International Journal of Molecular Sciences. 2025; 26(24):12082. https://doi.org/10.3390/ijms262412082
Chicago/Turabian StyleHussein, Ola J., Dana Elkhalifa, Arij Fouzat Hassan, Feras Alali, Ala-Eddin Al Moustafa, and Ashraf Khalil. 2025. "The Role of Natural Chalcones and Their Derivatives in Targeting Prostate Cancer: Recent Updates" International Journal of Molecular Sciences 26, no. 24: 12082. https://doi.org/10.3390/ijms262412082
APA StyleHussein, O. J., Elkhalifa, D., Hassan, A. F., Alali, F., Al Moustafa, A.-E., & Khalil, A. (2025). The Role of Natural Chalcones and Their Derivatives in Targeting Prostate Cancer: Recent Updates. International Journal of Molecular Sciences, 26(24), 12082. https://doi.org/10.3390/ijms262412082








