Exploring the Molecular Mechanism of Hepatic Dysfunction Among Workers Exposed to Nickel and Chromium in Electroplating
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Clinical Manifestation Prevalence Among Electroplaters
3.2. Impacts of Occupational Exposure on Serum Cr, Ni, and Liver Function Parameters
3.3. Oxidative Markers Among Electroplaters
3.4. Gene Expression and Inflammatory Markers
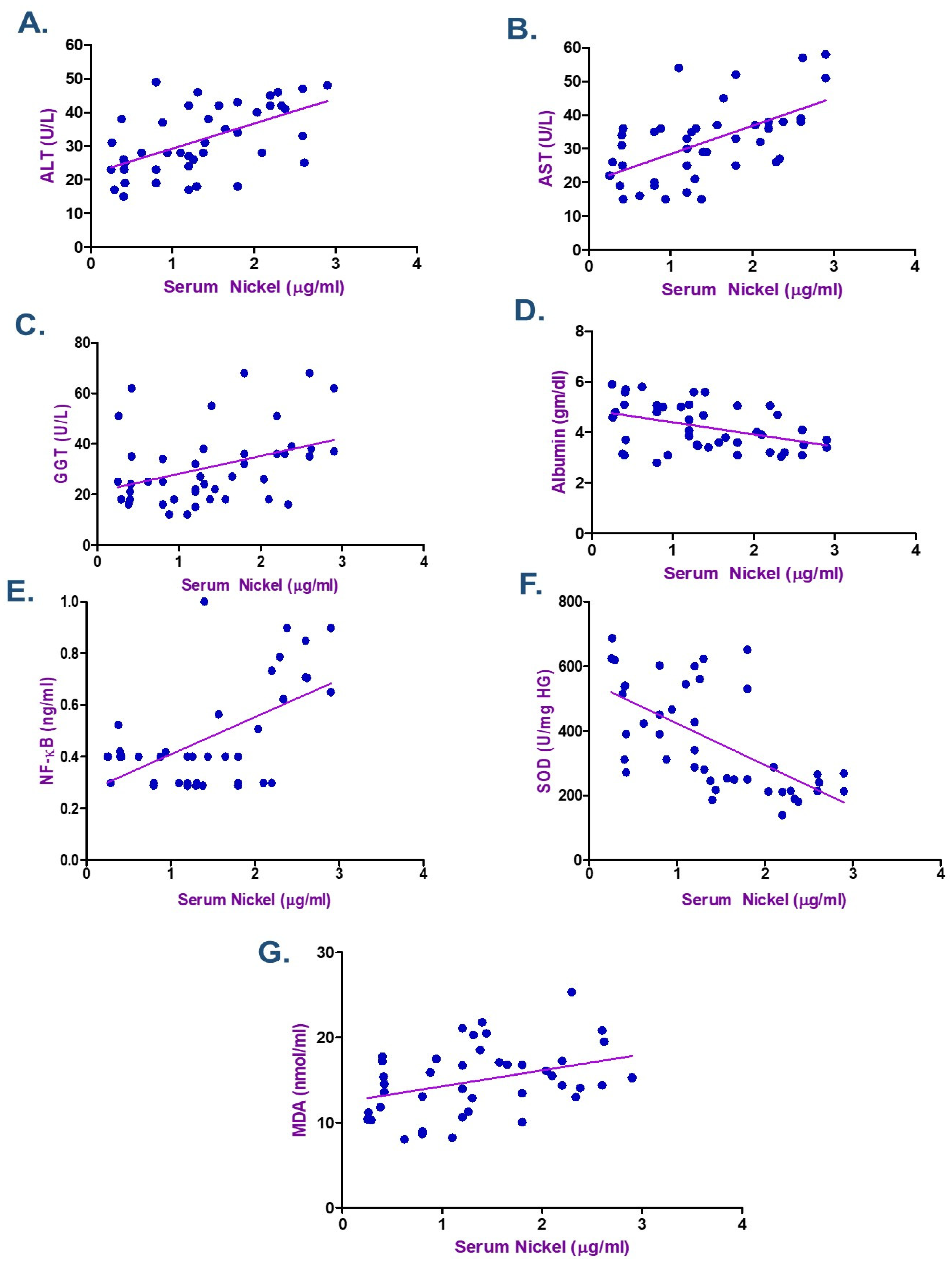
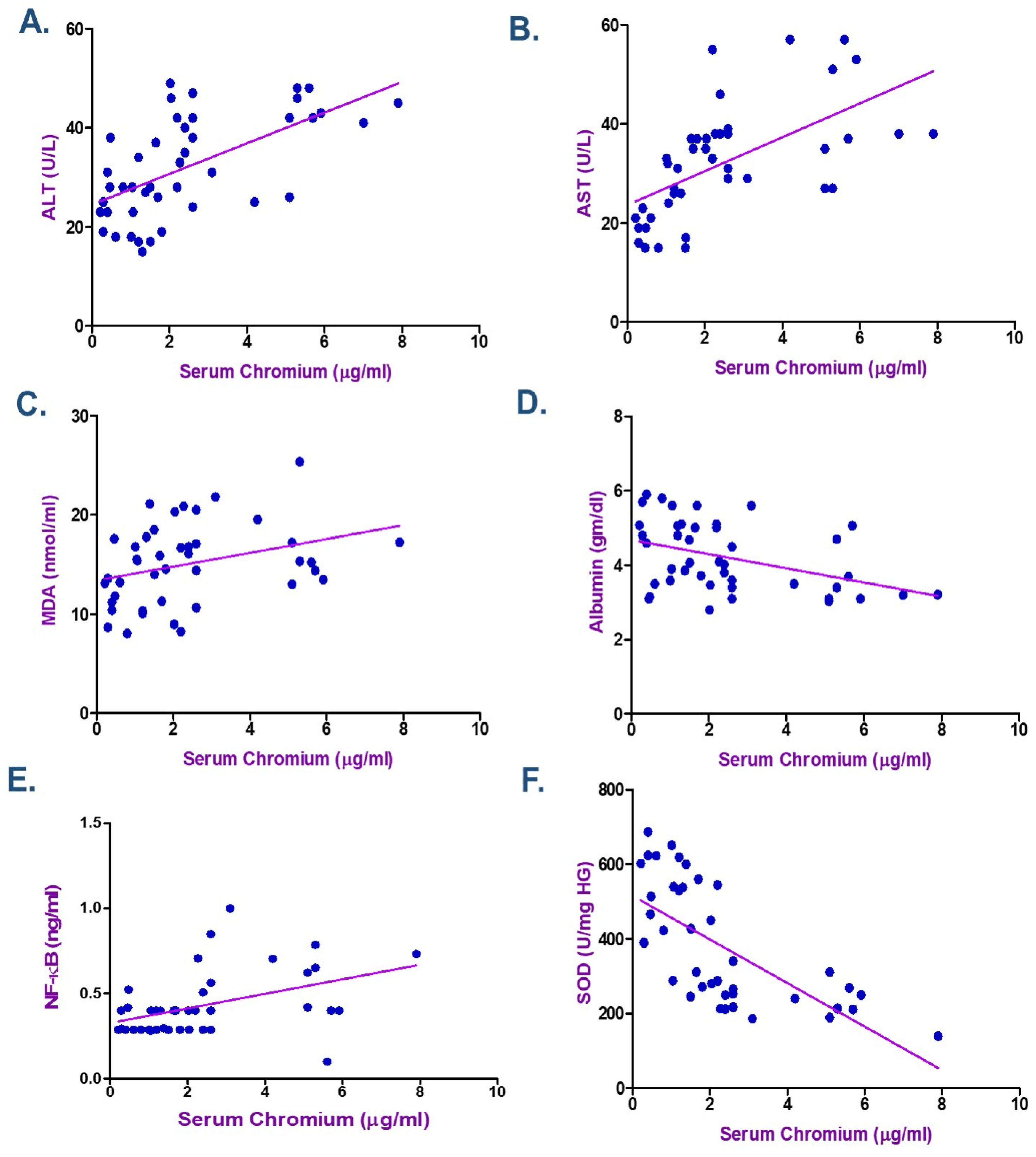
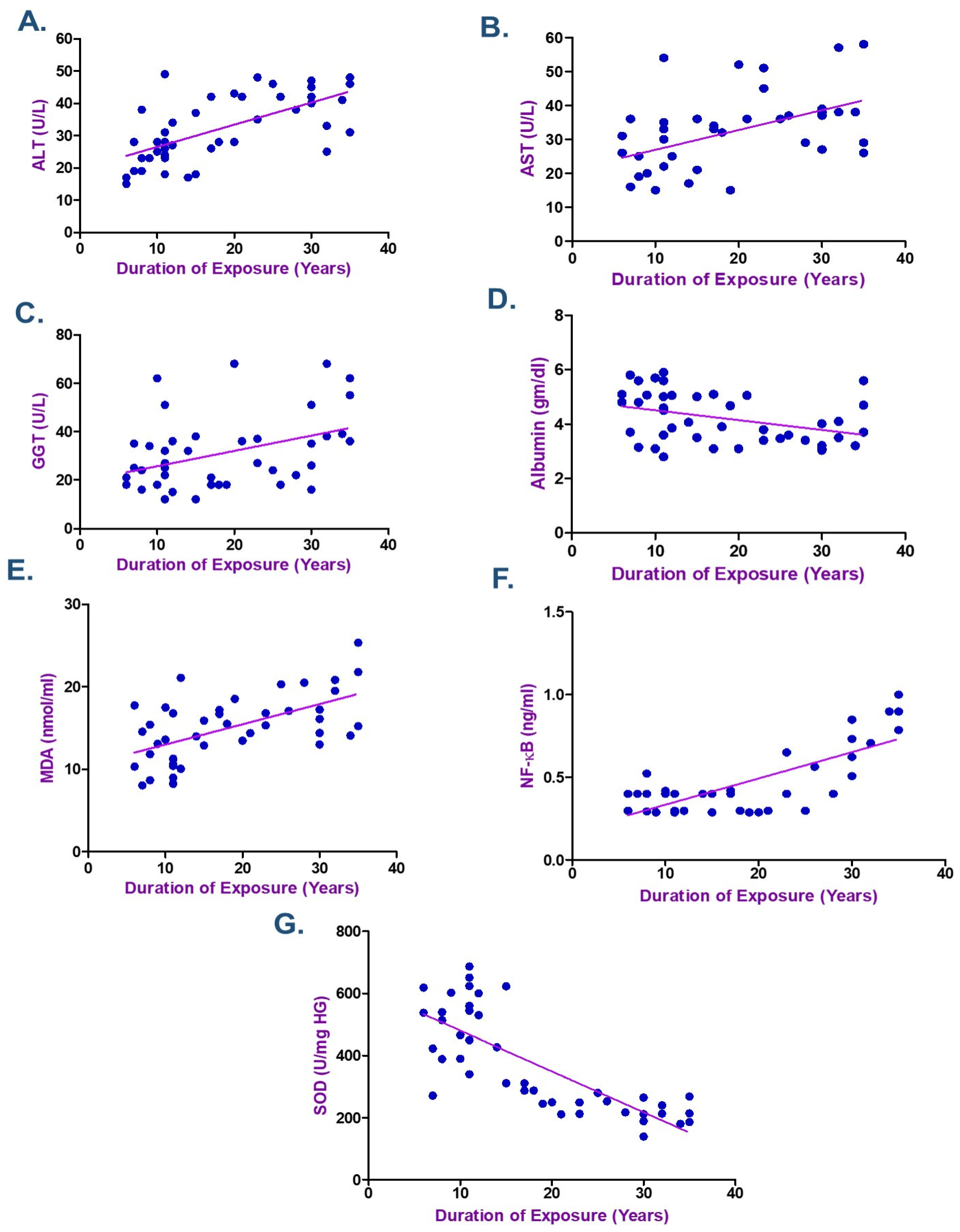
3.5. Correlation Between Metals Exposure and Biochemical Indicators
3.6. Occupational Exposure Determinants
3.7. Strengths and Limitations
4. Materials and Methods
4.1. Population of Study and Disease Condition
4.1.1. Exclusion Criteria
4.1.2. Sample Size
4.2. Questionnaire and Clinical Examination
4.3. Sample Collection and Analysis
4.3.1. Liver Function Tests
4.3.2. Evaluation of Gene Interaction with Chromium
4.3.3. Measurement of miR-223 and Keap-1, Nrf2, and Ho-1 mRNAs: Fold Changes Isolation of RNA
4.4. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oginawati, K.; Susetyo, S.H.; Pratiwi, I. Biomarker Of Kidney Function In Electroplating Workers Exposed To Chromium. IJSTR 2020, 9, 247–253. [Google Scholar]
- Qayyum, S.; Ara, A.; Usmani, J.A. An epidemiological study of electroplaters occupationally exposed to Nickel and Chromium. Biomed Res-India 2012, 23, 609–613. [Google Scholar]
- Xiao, F.; Feng, X.; Zeng, M.; Guan, L.; Hu, Q.; Zhong, C. Hexavalent chromium induces energy metabolism disturbance and p53-dependent cell cycle arrest via reactive oxygen species in L-02 hepatocytes. Mol. Cell Biochem. 2012, 371, 65–76. [Google Scholar] [CrossRef]
- Jin, L.; Kom, M.C.; Fu, G.; Xie, Y.; Gao, Y.; Shen, J.; Huang, H.; Hu, B.; Yan, J. Hexavalent chromium induces hepatocyte apoptosis via regulation of apoptosis signal-regulating kinase 1/c-Jun amino-terminal kinase signaling. Environ. Toxicol. 2022, 37, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Renu, K.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Mirza, A.K.; Vellingiri, B.; Iyer, M.; Dey, A.; Gopalakrishnan, A.V. Mechanism of chromium-induced toxicity in lungs, liver, and kidney and their ameliorative agents. Biomed. Pharmacother. 2022, 151, 113119, Correction in Biomed. Pharmacother. 2024, 181, 117629. https://doi.org/10.1016/j.biopha.2024.117629. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Gopalakrishnan, A.V. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ge, X.; Xu, J.; Li, A.; Mei, Y.; Yin, G.; Wu, J.; Liu, X.; Wei, L.; Xu, Q. Association between urine metals and liver function biomarkers in Northeast China: A cross-sectional study. Ecotoxicol. Environ. Saf. 2022, 231, 113163. [Google Scholar] [CrossRef]
- Zheng, G.H.; Liu, C.M.; Sun, J.M.; Feng, Z.J.; Cheng, C. Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat Toxicol. 2014, 147, 105–111. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Lucano-Landeros, S.; Monroy-Ramirez, H.C.; Silva-Gomez, J.; Gutierrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Roles of Nrf2 in Liver Diseases: Molecular, Pharmacological, and Epigenetic Aspects. Antioxidants 2020, 9, 980. [Google Scholar] [CrossRef]
- Han, B.; Li, S.; Lv, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Lv, Z.; Zhang, Z. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019, 10, 5555–5565. [Google Scholar] [CrossRef]
- Hu, G.; Long, C.; Hu, L.; Zhang, Y.; Hong, S.; Zhang, Q.; Zheng, P.; Su, Z.; Xu, J.; Wang, L.; et al. Blood chromium exposure, immune inflammation and genetic damage: Exploring associations and mediation effects in chromate exposed population. J. Hazard. Mater. 2022, 425, 127769. [Google Scholar] [CrossRef]
- Yang, Q.; Han, B.; Li, S.; Wang, X.; Wu, P.; Liu, Y.; Li, J.; Han, B.; Deng, N.; Zhang, Z. The link between deacetylation and hepatotoxicity induced by exposure to hexavalent chromium. J. Adv. Res. 2021, 35, 129–140. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Reece, E.A.; Wang, L.; Harman, C.R.; Yang, P. microRNA expression profiling and functional annotation analysis of their targets modulated by oxidative stress during embryonic heart development in diabetic mice. Reprod. Toxicol. 2016, 65, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The Role of miRNAs in the Pathophysiology of Liver Diseases and Toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.; Masters, S.L. miR-223: Infection, inflammation and cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Wu, Y.; Zuo, Y.; Li, J.; Lv, H.; Ma, L.; Ren, B.; Zhao, L.; Li, W.; et al. Ellagic acid ameliorates oxidative stress and insulin resistance in high glucose-treated HepG2 cells via miR-223/keap1-Nrf2 pathway. Biomed. Pharmacother. 2019, 110, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.Z.; Jiang, B.H. Dysregulation of microRNAs in metal-induced angiogenesis and carcinogenesis. Semin. Cancer Biol. 2021, 76, 279–286. [Google Scholar] [CrossRef]
- El Safty, A.M.K.; Samir, A.M.; Mekkawy, M.K.; Fouad, M.M. Genotoxic Effects Due to Exposure to Chromium and Nickel Among Electroplating Workers. Int. J. Toxicol. 2018, 37, 234–240. [Google Scholar] [CrossRef]
- Shaker, D.A.; Mohamed, R.S. Reproductive Hormones among Electroplaters Exposed to Chromium and Nickel at a Factory for Metallic Industries in Egypt. Egypt. J. Occup. Med. 2019, 43, 345–359. [Google Scholar] [CrossRef]
- Jeyamala, S.; Kumaraguru, A.K.; Nagarani, N. Occupational health effects due to nickel and chromium exposure in electroplating workers. Toxicol. Environ. Chem. 2012, 94, 1583–1590. [Google Scholar] [CrossRef]
- Were, F.H.; Charles Moturi, M.; Kamau, G.N.; Wafula, G.A. Respiratory Diseases Due to Occupational Exposure to Nickel and Chromium among Factory Workers in Kenya. J. Community Med. Health Educ. 2013, 3, 252. [Google Scholar] [CrossRef]
- Tang, S.; Luo, S.; Wu, Z.; Su, J. Association between blood heavy metal exposure levels and risk of metabolic dysfunction associated fatty liver disease in adults: 2015–2020 NHANES large cross-sectional study. Front. Public Health 2024, 12, 1280163. [Google Scholar] [CrossRef]
- Ling, S.; Diao, H.; Lu, G.; Shi, L. Associations between serum levels of liver function biomarkers and all-cause and cause-specific mortality: A prospective cohort study. BMC Public Health 2024, 24, 3302. [Google Scholar] [CrossRef]
- El-Shafei, H.M. Assessment of liver function among nickel-plating workers in Egypt. East. Mediterr. Health J. 2011, 17, 490–494. [Google Scholar] [CrossRef]
- Muhammad, A.; Fawad, A. Lipid Peroxidation and Biochemical Abnormalities in Tannery Workers exposed to Hexavalent Chromium. Res. J. Biotech. 2016, 11, 75–82. [Google Scholar]
- David, M.; Ain, Q.U.; Afzal, M.; Shoaib, M.; Aman, F.; Cloete, K.J.; Turi, N.; Jahan, S. Study of occupational exposure to brick kiln emissions on heavy metal burden, biochemical profile, cortisol level and reproductive health risks among female workers at Rawat, Pakistan. Environ. Sci. Pollut. Res. Int. 2020, 27, 44073–44088. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Houseiny, W.; El-Murr, A.E.; Ebraheim, L.L.M.; Ahmed, A.I.; El-Hakim, Y.M.A. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Sayed, R.A.; Abdel-Daim, M.M. Hepatoprotective potential of Rosmarinus officinalis essential oil against hexavalent chromium-induced hematotoxicity, biochemical, histological, and immunohistochemical changes in male rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 17445–17456. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, P.; Savari, A.; Movahedinia, A.; Safahieh, A.; Azhdari, D. An assessment of hematological and biochemical responses in the tropical fish Epinephelus stoliczkae of Chabahar Bay and Gulf of Oman under chromium exposure: Ecological and experimental tests. Environ. Sci. Pollut. Res. Int. 2014, 21, 6076–6088. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ye, S.; Ma, Y.; Liang, Y.; Liang, N.; Xiao, F. Clusterin alleviates Cr(VI)-induced mitochondrial apoptosis in L02 hepatocytes via inhibition of Ca-ROS-Drp1-mitochondrial fission axis. Ecotoxicol. Environ. Saf. 2020, 205, 111326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, W.; Liu, X.; Song, X.; Chai, L. Probing the effects of hexavalent chromium exposure on histology and fatty acid metabolism in liver of Bufo gargarizans tadpoles. Chemosphere 2020, 243, 125437. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Z.; Cao, X.; Qi, Q.; Wang, L.; Liu, P.; Chen, Y.; Hu, G.; Guo, X.; Gao, X. Mechanism of Mitochondrial Kinetic Imbalance and Nrf2 Signaling Pathway-Mediated Oxidative Stress in Nickel and/or Chromium-Induced Kidney Injury in Mice. Antioxidants 2024, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Seo, Y.R. Molecular and genomic approach for understanding the gene-environment interaction between Nrf2 deficiency and carcinogenic nickel-induced DNA damage. Oncol. Rep. 2012, 28, 1959–1967. [Google Scholar] [CrossRef]
- Tripathi, S.; Kharkwal, G.; Mishra, R.; Singh, G. Nuclear factor erythroid 2-related factor 2 (Nrf2) signaling in heavy metals-induced oxidative stress. Heliyon 2024, 10, e37545. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, S.; Zeng, Y.; Shi, Y.; Du, J.; Huang, C.; Shen, Y.; Liu, P.; Guo, X.; Gao, X. Effects of chronic Cr and Ni co-exposure on liver inflammation and autophagy in mice by regulating the TLR4/mTOR pathway. Sci. Total Environ. 2024, 926, 171921. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, H.; Li, S.; Han, B.; Liu, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Zhang, Z. Sulforaphane prevents chromium-induced lung injury in rats via activation of the Akt/GSK-3β/Fyn pathway. Environ. Pollut. 2020, 259, 113812. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Shen, J.; Kom, M.C.; Huang, H.; Fu, G.; Xie, Y.; Gao, Y.; Tang, Y.; Yan, J.; Jin, L. Role of NF-κB signaling pathway in hexavalent chromium-induced hepatotoxicity. Environ. Toxicol. 2023, 38, 1361–1371. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, M.; Liu, Y.; Lundh, T.; Pineda, D.; Schenk, L.; Saber, A.T.; Vogel, U.; Ljunggren, S.; Ricklund, N. Integrative analyses of circulating microRNA expression profile in hexavalent chromium exposed workers—A cross-sectional study within the SafeChrom project. J. Hazard. Mater. 2025, 488, 137367. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, T.; Lou, G.; Liu, Y. Role of miR-223 in the pathophysiology of liver diseases. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Chen, Y.; Li, N.; Hou, X. MiR-223 as a Regulator and Therapeutic Target in Liver Diseases. Front. Immunol. 2022, 13, 860661. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, C.; Hu, G.; Hong, S.; Su, Z.; Zhang, Q.; Zheng, P.; Wang, T.; Yu, S.; Jia, G. Two-week repair alleviates hexavalent chromium-induced hepatotoxicity, hepatic metabolic and gut microbial changes: A dynamic inhalation exposure model in male mice. Sci Total. Environ. 2023, 857 Pt 1, 159429. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Hong, S.; Zhang, Q.; Xu, J.; Hu, G.; Zhu, X.; Yuan, F.; Yu, S.; Wang, T.; et al. Relationships between blood chromium exposure and liver injury: Exploring the mediating role of systemic inflammation in a chromate-exposed population. J. Environ. Sci. 2024, 143, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Sazakli, E.; Villanueva, C.M.; Kogevinas, M.; Maltezis, K.; Mouzaki, A.; Leotsinidis, M. Chromium in drinking water: Association with biomarkers of exposure and effect. Int. J. Environ. Res. Public Health 2014, 11, 10125–10145. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Egyptian Environmental Affairs Agency (EEAA). Egyptian Environmental Law 4 for Year 1994. Promulgating the Environment Law and Its Executive Regulation for the Year 2005. Available online: https://www.eeaa.gov.eg/ (accessed on 3 March 2025).
- Kalahasthi, R.B.; Rajmohan, H.R.; Rajan, B.K. Assessment of functional integrity of liver among workers exposed to soluble nickel compounds during nickel plating. Indian J. Occup. Environ. Med. 2006, 1, 78–81. [Google Scholar] [CrossRef]
- Zakim, D.; Boyer, T.D.; Stolz, A.; Kaplowitz, N. Biochemical tests for liver disease. In Hepatology, 2nd ed.; Zakim, D., Boyer, T.D., Eds.; WB Saunders: Philadelphia, PA, USA, 1990; pp. 637–657. [Google Scholar]
- Ostrow, J.D.; Blanckaertm, N.; Heirwegh, K.P. Analysis and preparation of bilirubins and biliverdins. In Bile Pigments and Jaundice; Ostrow, J.D., Ed.; Marcel Dekker: New York, NY, USA, 1986; pp. 31–79. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R.; Bruns, D.E. (Eds.) Tietz Textbook of Clinical Chemistry. In Philadelphia: WB Saunders Company, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Christensen, J.M.; Kristiansen, J.; Nielsen, N.H.; Menné, T.; Byrialsen, K. Nickel concentrations in serum and urine of patients with nickel eczema. Toxicol Lett. 1999, 108, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics102: Quantitative Data—Parametric & Non-parametric Tests. Singap. Med. J. 2003, 44, 391–396. [Google Scholar]
- Chan, Y.H. Biostatistics 103: Qualitative Data–Tests of Independence. Singap. Med. J. 2003, 44, 498–503. [Google Scholar]
- Chan, Y.H. Biostatistics 104: Correlational Analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
| Electroplating Workers | Referent Group | p-Value | ||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | |||
| Chronic fatigue | Yes | 14 | 32.6% | 6 | 14.0% | 0.041 * |
| No | 29 | 67.4% | 37 | 86.0% | ||
| Cough | Yes | 13 | 30.2% | 4 | 9.3% | 0.015 * |
| No | 30 | 69.8% | 39 | 90.7% | ||
| Expectoration | Yes | 11 | 25.6% | 2 | 4.7% | 0.007 * |
| No | 32 | 74.4% | 41 | 95.3% | ||
| Dyspnea | Yes | 6 | 14% | 2 | 4.7% | 0.14 |
| No | 37 | 86% | 41 | 95.3% | ||
| Allergic rhinitis | Yes | 8 | 18.6% | 0 | 0.0% | 0.005 * |
| No | 35 | 81.4% | 43 | 100.0% | ||
| Sinusitis | Yes | 7 | 16.3% | 2 | 4.7% | 0.156 |
| No | 36 | 83.7% | 41 | 95.3% | ||
| Conjunctivitis | Yes | 5 | 11.6% | 1 | 2.3% | 0.202 |
| No | 38 | 88.4% | 42 | 97.7% | ||
| Contact dermatitis | Yes | 8 | 18.6% | 0 | 0.0% | 0.005 * |
| No | 35 | 81.4% | 43 | 100.0% | ||
| Electroplating Workers | Referent Group | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Min–Max | Mean ± SD | Median | Min–Max | ||
| Age | 40.74 ± 9.86 | 39 | 28–59 | 39.07 ± 7.64 | 38 | 29–54 | 0.381 |
| Duration of exposure (years) | 18.26 ± 9.58 | 15 | 6–35 | 16.56 ± 7.36 | 15 | 7–33 | 0.571 |
| BMI (kg/m2) | 25.25 ± 2.42 | 25.7 | 18.8–29.1 | 25.73 ± 2.61 | 26.4 | 18.8–29.1 | 0.380 |
| Systolic blood pressure | 124.88 ± 11.1 | 125 | 110–140 | 123.26 ± 11.01 | 120 | 110–145 | 0.497 |
| Diastolic blood pressure | 77.44 ± 8.12 | 80 | 60–90 | 75.58 ± 7.25 | 75 | 60–90 | 0.266 |
| Serum Ni (µg/L) | 1.39 ± 0.79 | 1.30 | 0.25–2.9 | 0.51 ± 0.33 | 0.44 | 0.02–1.8 | <0.001 * |
| Serum Cr (µg/L) | 2.47 ± 2.00 | 2.02 | 0.21–7.90 | 0.45 ± 0.26 | 0.40 | 0.10–1.2 | <0.001 * |
| ALT (u/L) | 32.16 ± 10.25 | 31 | 15–49 | 26.47 ± 7.11 | 26.00 | 15–44 | 0.013 * |
| AST (u/L) | 31.72 ± 11.36 | 32 | 15–58 | 26.47 ± 8.56 | 22.00 | 13–42 | 0.048 * |
| GGT (u/L) | 30.91 ± 15.15 | 26 | 12–68 | 25 ± 5.07 | 24.00 | 16–38 | 0.278 |
| Total bilirubin (gm/dL) | 0.91 ± 0.45 | 0.94 | 0.25–1.9 | 0.67 ± 0.18 | 0.68 | 0.36–0.98 | 0.015 * |
| Albumin (gm/dL) | 4.21 ± 0.93 | 4.02 | 2.8–5.9 | 4.65 ± 0.67 | 4.70 | 3.26–5.8 | 0.013 * |
| MDA (nmol/mL) | 15.02 ± 3.99 | 15.24 | 8.04–25.35 | 5.55 ± 2.38 | 4.80 | 2.07–10.2 | <0.001 * |
| SOD (U/mg HG) | 372.31 ± 162.44 | 311 | 140–687 | 1241.07 ± 221.41 | 1297 | 717–1587 | <0.001 * |
| GPx (mU/mL) | 34.73 ± 13.45 | 34.53 | 13.98–77.81 | 109.85 ± 24.85 | 115.58 | 58.36–143.89 | <0.001 * |
| NF-κB (ng/mL) | 0.46 ± 0.20 | 0.38 | 0.29–0.95 | 0.26 ± 0.04 | 0.26 | 0.2–0.36 | <0.001 * |
| miR-223 expression | 0.38 ± 0.17 | 0.36 | 0.13–0.73 | 1 ± 0 | 1.00 | 1–1 | <0.001 * |
| Keap-1 expression | 1.47 ± 0.23 | 1.5 | 1.07–1.86 | 1 ± 0 | 1.00 | 1–1 | <0.001 * |
| Nrf2 expression | 0.34 ± 0.19 | 0.29 | 0.10–0.73 | 1 ± 0 | 1.00 | 1–1 | <0.001 * |
| Ho-1 expression | 0.35 ± 0.21 | 0.32 | 0.10–0.83 | 1 ± 0 | 1.00 | 1–1 | <0.001 * |
| Use of PPE | |||
|---|---|---|---|
| Yes (n = 17) | No (n = 26) | p-Value | |
| Blood nickel (µg/L) | 0.72 ± 0.44 | 1.83 ± 0.65 | <0.001 * |
| Blood chromium (µg/L) | 1.07 ± 0.75 | 3.38 ± 2.04 | <0.001 * |
| ALT (u/L) | 26.76 ± 9.45 | 35.69 ± 99.31 | 0.005 * |
| AST (u/L) | 23.88 ± 7.01 | 36.84 ± 10.79 | <0.001 * |
| GGT (u/L) | 28.29 ± 12.53 | 32.61 ± 16.66 | 0.384 |
| Total bilirubin (gm/dL) | 0.93 ± 0.44 | 0.89 ± 0.46 | 0.813 |
| Albumin (gm/dL) | 4.63 ± 0.98 | 3.93 ± 0.79 | 0.021 * |
| MDA (nmol/mL) | 12.52 ± 3.1 | 16.66 ± 3.69 | 0.001 * |
| GPx (mU/mL) | 3.68 ± 12.56 | 34.11 ± 14.21 | 0.486 |
| NF-kB (ng/mL) | 0.35 ± 0.06 | 0.53 ± 0.23 | 0.054 |
| miR-223 expression | 0.39 ± 0.18 | 0.37 ± 0.17 | 0.921 |
| Keap-1 expression | 1.44 ± 0.21 | 1.49 ± 0.24 | 0.455 |
| Nrf2 expression | 0.45 ± 0.17 | 0.26 ± 0.17 | 0.002 * |
| Ho-1 expression | 0.47 ± 0.2 | 0.27 ± 0.19 | 0.002 * |
| Serum Ni (µg/L) | Serum Cr (µg/L) | Duration of Exposure (Years) | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| Duration of exposure (years) | 0.830 | <0.001 * | 0.765 | <0.001 * | ||
| ALT (u/L) | 0.558 | <0.001 * | 0.626 | <0.001 * | 0.647 | <0.001 * |
| AST (u/L) | 0.590 | <0.001 * | 0.741 | <0.001 * | 0.549 | <0.001 * |
| GGT (u/L) | 0.411 | 0.006 * | 0.294 | 0.056 | 0.390 | 0.010 * |
| Total bilirubin (gm/dL) | 0.044 | 0.780 | 0.191 | 0.220 | 0.123 | 0.434 |
| Albumin (gm/dL) | −0.365 | 0.016 * | −0.406 | 0.007 * | −0.352 | 0.021 * |
| MDA (nmol/mL) | 0.371 | 0.014 * | 0.358 | 0.018 * | 0.535 | <0.001 * |
| GPx (mU/mL) | −0.078 | 0.621 | 0.162 | 0.299 | −0.141 | 0.369 |
| NF-kB (ng/mL) | 0.449 | 0.003 * | 0.562 | <0.001 * | 0.560 | <0.001 * |
| SOD (U/mg HG) | −0.698 | <0.001 * | −0.794 | <0.001 * | −0.782 | <0.001 * |
| ALT (u/L) | AST (u/L) | |||
|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| Duration of exposure (years) | 0.647 | <0.001 * | 0.549 | <0.001 * |
| MDA (nmol/mL) | 0.178 | 0.253 | 0.207 | 0.183 |
| GPx (mU/mL) | 0.064 | 0.683 | 0.017 | 0.915 |
| NF-κB (ng/mL) | 0.528 | <0.001 * | 0.421 | 0.005 * |
| SOD (U/mg HG) | −0.612 | <0.001 * | −0.489 | 0.001 * |
| miR-223 expression | 0.005 | 0.974 | −0.068- | 0.666 |
| Keap-1 expression | −0.053 | 0.735 | −0.019 | 0.906 |
| Nrf2 expression | −0.390 | 0.010 * | −0.299 | 0.051 |
| Ho-1 expression | −0.293 | 0.057 | −0.258 | 0.095 |
| miR-223 Expression | Keap-1 Expression | Nrf2 Expression | Ho-1 Expression | |||||
|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| Duration of exposure (years) | −0.094 | 0.547 | 0.107 | 0.495 | −0.665 | <0.001 * | −0.544 | <0.001 * |
| Serum Ni (µg/L) | −0.001 | 0.993 | 0.043 | 0.785 | −0.443 | 0.003 * | −0.395 | 0.009 * |
| Serum Cr (µg/L) | −0.149 | 0.340 | 0.003 | 0.983 | −0.433 | 0.004 * | −0.291 | 0.058 |
| ALT (u/L) | 0.005 | 0.974 | −0.053 | 0.735 | −0.390 | 0.010 * | −0.293 | 0.057 |
| AST (u/L) | −0.068 | 0.666 | −0.019 | 0.906 | −0.299 | 0.051 | −0.258 | 0.095 |
| GGT (u/L) | −0.126 | 0.420 | 0.059 | 0.709 | −0.388 | 0.010 * | −0.121 | 0.441 |
| Total bilirubin (gm/dL) | −0.211 | 0.175 | 0.175 | 0.261 | −0.229 | 0.140 | 0.002 | 0.991 |
| Albumin (gm/dL) | 0.225 | 0.147 | 0.236 | 0.128 | 0.159 | 0.310 | 0.209 | 0.179 |
| MDA (nmol/mL) | −0.167 | 0.283 | 0.116 | 0.459 | −0.308 | 0.044 * | −0.328 | 0.032 * |
| GPx (mU/mL) | −0.004 | 0.982 | −0.064 | 0.684 | 0.141 | 0.366 | 0.219 | 0.158 |
| NF-κB (ng/mL) | −0.164 | 0.292 | −0.040 | 0.798 | −0.260 | 0.092 | −0.129 | 0.410 |
| SOD (U/mg HG) | 0.164 | 0.294 | 0.072 | 0.645 | 0.589 | <0.001 * | 0.480 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, M.A.; Abdelgwad, M.; Atawia, R.T.; Badr, A.M.; Khalifa, E.M.; Alkharashi, L.A.; Mohammed, R.S. Exploring the Molecular Mechanism of Hepatic Dysfunction Among Workers Exposed to Nickel and Chromium in Electroplating. Int. J. Mol. Sci. 2025, 26, 11954. https://doi.org/10.3390/ijms262411954
Ramadan MA, Abdelgwad M, Atawia RT, Badr AM, Khalifa EM, Alkharashi LA, Mohammed RS. Exploring the Molecular Mechanism of Hepatic Dysfunction Among Workers Exposed to Nickel and Chromium in Electroplating. International Journal of Molecular Sciences. 2025; 26(24):11954. https://doi.org/10.3390/ijms262411954
Chicago/Turabian StyleRamadan, Mona Abdallah, Marwa Abdelgwad, Reem T. Atawia, Amira M. Badr, Eman Mahmoud Khalifa, Layla A. Alkharashi, and Rateba Said Mohammed. 2025. "Exploring the Molecular Mechanism of Hepatic Dysfunction Among Workers Exposed to Nickel and Chromium in Electroplating" International Journal of Molecular Sciences 26, no. 24: 11954. https://doi.org/10.3390/ijms262411954
APA StyleRamadan, M. A., Abdelgwad, M., Atawia, R. T., Badr, A. M., Khalifa, E. M., Alkharashi, L. A., & Mohammed, R. S. (2025). Exploring the Molecular Mechanism of Hepatic Dysfunction Among Workers Exposed to Nickel and Chromium in Electroplating. International Journal of Molecular Sciences, 26(24), 11954. https://doi.org/10.3390/ijms262411954





