Therapeutic Potential of the β3-Adrenergic Receptor and Its Ligands in Cardiovascular Diseases
Abstract
1. Introduction
2. β3-Adrenergic Receptor Pharmacology
2.1. General Information
| Agonist | Efficacy | Potency | Selectivity | Isoproterenol Concentration [M] | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β1-AR Maximal Agonist Effect ± SEM [%] | β2-AR Maximal Agonist Effect ± SEM [%] | β3-AR Maximal Agonist Effect ± SEM [%] | β1-AR EC50 [nM] | β2-AR EC50 [nM] | β3-AR EC50 [nM] | EC50 Ratio β1-AR/ β3-AR | EC50 Ratio β2-AR/ β3-AR | |||
| Mirabegron | 10 | 10 | 80 | >10,000 | >10,000 | 22.4 | >446 | >446 | 10−4 | [22] |
| Solabegron | 4.2 ± 1.7 | 8.8 ± 2.4 | 89.1 ± 4.2 | <10,000 * | <10,000 * | 6.92 * | <1445 * | <1445 * | 10−5 | [23] |
| Vibegron | 5 | 7 | 84 | >10,000 | >10,000 | 1 | >10,000 * | >10,000 * | 10−6 | [24] |
| BRL37344 | 50 | 70 | 60 | 12,900 | 360 | 457 | 28.2 | 0.79 | 10−4 | [22] |
| 55.7 ± 3.7 | 50.7 ± 6.5 | 46.7 ± 6 | 986 ± 108 | 153 ± 21 | 728 ± 203 | 70.1 | 10.1 | 10−5 | [25] | |
| 100.7 ± 8.6 | 80.1 ± 4.1 | 79.7 ± 3.6 | 288.4 * | 131.8 * | 33.9 * | 8.51 * | 3.89 * | 10−5 | [21] | |
| CL316243 | ND | ND | 54.9 ± 2.1 | >10,000 | >10,000 | 11,200 ± 4000 | >46.2 | >43 | 10−5 | [25] |
| 0 | 10 | 50 | >10,000 | >10,000 | 4430 | >2.26 * | >2.26 * | 10−4 | [22] | |
| Zinterol | 113.5 ± 3.8 | 105.3 ± 4.6 | 101.2 ± 3.4 | 58.9 * | 0.3 * | 8.1 * | 7.27 * | 0.04 * | 10−5 | [21] |
| L755507 | 101.6 ± 3 | 3 ± 0.2 | 101.1 ± 3.4 | 23.4 * | 89.1 * | 0.1 * | 234 * | 891 * | 10−5 | [21] |
| Nebivolol | 2.8 ± 0.3 a | ND | ND | 1.1 *a | ND | ND | ND | ND | 10−5 | [21] |
| ICI215001 | ND | ND | 60.3 ± 2.9 | ND | ND | 87.1 * | ND | ND | 10−5 | [26] |
| CGP12177 | ND a | ND a | 61.4 ± 1.5 | ND a | ND a | 269.2 * | ND | ND | 10−5 | [26] |
| ZD7114 | 72.0 ± 2.9 | 2.2 ± 0.4 | 58.2 ± 2 | 8.9 * | 22.4 * | 28.2 * | 0.32 * | 0.79 * | 10−5 | [21] |
| Carazolol | 38.1 ± 4.9 | 1.9 ± 0.3 | 75.7 ± 1.1 | 40.7 * | 0.2 * | 109.6 * | 0.37 * | 0 * | 10−5 | [21] |
| Fenoterol | 106 ± 1.7 | 100.9 ± 2.7 | 100.5 ± 4 | 29.5 * | 1.3 * | 23.4 * | 1.26 * | 0.06 * | 10−5 | [21] |
2.2. Gene Polymorphisms of β3-AR and Their Influence on Its Pharmacology
2.3. Ligand-Directed Agonism
2.4. β3-AR Agonists
2.4.1. Structure–Activity Relationship of β3-AR Agonists
2.4.2. Mirabegron and Vibegron—Agonists for Overactive Bladder Treatment
Mirabegron
Vibegron
2.4.3. Nebivolol, Celiprolol, and Carvedilol—Third-Class β-Blockers and Their Influence on β3-AR
Nebivolol
Carvedilol
Celiprolol
3. Role of β3-AR in Blood Vessels
3.1. Endothelial Dysfunction
3.2. Atherosclerosis
3.3. β3 Adrenergic Receptor and Blood Pressure
3.3.1. Pulmonary Hypertension
3.3.2. Arterial Hypertension
3.3.3. Preeclampsia
3.3.4. Portal Hypertension
4. Role of β3-AR in the Heart
4.1. Stimulation Effects and Ion Channels
4.1.1. Hyperpolarization-Activated Current (If)
4.1.2. Inward Rectifier Potassium Current (IK1)
4.1.3. Transient Outward Potassium Current (Ito)
4.1.4. Slow Delayed Rectifier Potassium Current (IKs)
4.1.5. L-Type Calcium Current (ICaL)
4.1.6. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
4.1.7. β3-AR in Atria
4.2. Cardiac Arrhythmias
4.2.1. Ventricular Tachycardia
4.2.2. Atrial Fibrillation
Electrophysiological Alterations
Structural Alterations
Biochemical Alterations
4.3. Myocardial Ischemia
4.4. Heart Failure
4.4.1. Role of β3-AR in Cardiac Remodelling and Haemodynamic Changes
4.4.2. Other β3-AR Effects in HF
Na+/K+-ATPase Stimulation in HF
Sepsis-Related HF
Autoantibodies Against β3-AR in Heart Failure
Role of β3-AR Agonism in Chemotherapy-Induced HF
β3-AR Upregulation in Diabetic Hearts
Role in Improving Exercise Ability of HF-Mediated Through β3-AR
Cardiorenal Syndrome
4.4.3. Clinical Trials Involving β3-Adrenergic Receptor Stimulation in Heart Failure Patients
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β3-AR | Beta-3 Adrenergic Receptor | IKs | Slow delayed rectifier potassium current |
| ABCA1 | ATP-binding cassette A1 | If | Hyperpolarization-activated current |
| ABCG1 | ATP-binding cassette G1 | Ito | Transient outward potassium current |
| ADMA | Asymmetric dimethylarginine | JNK | c-Jun N-terminal kinase (isoforms 1 and 2) |
| AERP | Atrial effective refractory period | Ki67 | Cellular Proliferation Marker |
| AF | Atrial fibrillation | Kir | Inwardly rectifying potassium channels |
| AKT | Protein kinase B | Kir2.x | Vascular smooth muscle and endothelial cell Kir family (Kir2.1–2.3) |
| AMPK | AMP-activated protein kinase | Kv | Voltage-gated potassium channels |
| AP1 | Activator protein 1 | L-NMA | L-Nω-Methylarginine—Non-selective nitric oxide synthase inhibitor |
| APD | Action potential duration | LDL | Low-density lipoprotein |
| ApoAI | Apolipoprotein AI | LPS | Lipopolysaccharide |
| ApoAII | Apolipoprotein AII | LV | Left ventricle |
| ApoE | Apolipoprotein E | LVEDP | Left ventricular end-diastolic pressure |
| AR | Adrenergic receptor | LVEF | Left ventricular ejection fraction |
| ATP | Adenosine triphosphate | MAPK/p38 MAPK | Mitogen-activated protein kinase/p38 subtype |
| BEAT-HF | Beta-3 Agonist Treatment in Heart Failure | MHRA | Medicines and Healthcare Products Regulatory Agency |
| BEAT-HF-II | Beta-3 Agonist Treatment in Heart Failure II | miR-21 | MicroRNA-21 |
| β3-LVH | Beta-3 Adrenergic Receptor in Left Ventricular Hypertrophy Trial | miR-26b | MicroRNA-26b |
| CAD | Coronary artery disease | miR-320 | MicroRNA-320 |
| cAMP | Cyclic adenosine monophosphate | miRNA | MicroRNA |
| CFTR | Cystic fibrosis transmembrane conductance regulator | mRNA | Messenger ribonucleic acid |
| cGMP | Cyclic guanosine monophosphate | NADPH | Nicotinamide adenine dinucleotide phosphate |
| CHO | Chinese Hamster Ovary | NFAT | Nuclear factor of activated T-cells |
| CHO K1 | Chinese Hamster Ovary K1 | NFκB | Nuclear factor κ-light-chain-enhancer of activated B cells |
| COMET | Carvedilol Or Metoprolol European Trial | nNOS | Neuronal nitric monoxide synthase |
| COX2 | Cyclooxygenase-2 | NO | Nitric monoxide |
| CTGF | Connective tissue growth factor | NOS | Nitric monoxide synthase |
| DOCA | Deoxycorticosterone Acetate | NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| EAT | Epicardial adipose tissue | NOX2 | NADPH oxidase 2 |
| EC50 | Half-maximal effective concentration | NT-proBNP | N-terminal pro b-type natriuretic peptide |
| EMA | European Medicines Agency | NYHA Class | New York Heart Association Classification |
| eNOS | Endothelial nitric monoxide synthase | ox-LDL | Oxidized low-density lipoprotein |
| ERK1 | Extracellular signal-regulated kinases 1 | p27 | Cyclin-Dependent Kinase Inhibitor 1B |
| ERK2 | Extracellular signal-regulated kinases 2 | PAH | Pulmonary arterial hypertension |
| ESC | European Society of Cardiology | PH | Pulmonary hypertension |
| FOXO-3A | Forkhead box protein O3A (proapoptotic transcription factor) | PI3K | Phosphatidylinositol 3-kinase |
| G(i) | G(i)—Inhibitory G-protein | PKA | Protein kinase A |
| GRK | G-protein coupled receptor kinase | PKC | Protein kinase C |
| HCN | Cyclic nucleotide-gated | PPARα | Peroxisome proliferator-activated receptor α |
| HDL | High-density lipoprotein | PPARγ | Peroxisome proliferator-activated receptor γ |
| HEK293 | Human embryonic kidney 293 | PTEN | Phosphatase and Tensin Homolog |
| HF | Heart failure | PVR | Pulmonary vascular resistance |
| HFpEF | Heart Failure with Preserved Ejection Fraction | QTc | Corrected QT interval (heart rate–corrected measure of ventricular repolarization) |
| HFrEF | Heart Failure with Reduced Ejection Fraction | Rho-kinase | Rho-associated Protein Kinase |
| HNF-3/4 | Hepatocyte nuclear factors 3 and 4 | RhoA | Ras Homolog Family Member A |
| HSP90 | Heat-shock protein 90 | ROS | Reactive oxygen species |
| HUVEC | Human umbilical vein endothelial cells | RV | Right ventricle |
| IGF1R | Insulin-like Growth Factor 1 Receptor | SENIORS | Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure |
| IL-1β | Interleukin-1β | STAR-PAD | Stimulating β3-AR for peripheral artery disease |
| iNOS | Inducible nitric monoxide synthase | TGFβ | Transforming growth factor beta |
| IRI | Heart ischemia-reperfusion injury | TGFβ1 | Transforming growth factor beta 1 |
| ICaL | L-type calcium current | UCP1, UCP2 | Uncoupling protein 1 and 2 |
| IK1 | Inward rectifier potassium current | VT | Ventricular tachycardia |
References
- Insel, P.A. Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors--evolving concepts and clinical implications. N. Engl. J. Med. 1996, 334, 580–585. [Google Scholar] [CrossRef]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Kannabiran, S.A.; Gosejacob, D.; Niemann, B.; Nikolaev, V.O.; Pfeifer, A. Real-time monitoring of cAMP in brown adipocytes reveals differential compartmentation of beta1 and beta3-adrenoceptor signalling. Mol. Metab. 2020, 37, 100986. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.Y.M.; Farah, C.; Balligand, J.L. The Beta3 Adrenergic Receptor in Healthy and Pathological Cardiovascular Tissues. Cells 2020, 9, 2584. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hutchinson, D.S.; Bengtsson, T.; Floren, A.; Langel, U.; Horinouchi, T.; Evans, B.A.; Summers, R.J. Functional domains of the mouse beta3-adrenoceptor associated with differential G protein coupling. J. Pharmacol. Exp. Ther. 2005, 315, 1354–1361. [Google Scholar] [CrossRef]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular characterization of the human beta 3-adrenergic receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef]
- Dessy, C.; Balligand, J.L. Beta3-adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Adv. Pharmacol. 2010, 59, 135–163. [Google Scholar] [CrossRef]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional beta3-adrenoceptor in the human heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about beta3-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, L.; Li, X.; Xue, Y.; Wang, B.; Lv, Z.; Chen, J.; Sun, D.; Zheng, Q. beta3-Adrenoreceptor stimulation protects against myocardial infarction injury via eNOS and nNOS activation. PLoS ONE 2014, 9, e98713. [Google Scholar] [CrossRef]
- García-Álvarez, A.; Pereda, D.; Garcia-Lunar, I.; Sanz-Rosa, D.; Fernandez-Jimenez, R.; Garcia-Prieto, J.; Nuno-Ayala, M.; Sierra, F.; Santiago, E.; Sandoval, E.; et al. Beta-3 adrenergic agonists reduce pulmonary vascular resistance and improve right ventricular performance in a porcine model of chronic pulmonary hypertension. Basic Res. Cardiol. 2016, 111, 49. [Google Scholar] [CrossRef] [PubMed]
- Arioglu-Inan, E.; Kayki-Mutlu, G.; Michel, M.C. Cardiac beta3-adrenoceptors—A role in human pathophysiology? Br. J. Pharmacol. 2019, 176, 2482–2495. [Google Scholar] [CrossRef] [PubMed]
- Grazia Perrone, M.; Scilimati, A. beta(3)-Adrenoceptor agonists and (antagonists as) inverse agonists history, perspective, constitutive activity, and stereospecific binding. Methods Enzymol. 2010, 484, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Bagchi, D.; Bagchi, M. Physiological and metabolic functions of the beta3-adrenergic receptor and an approach to therapeutic achievements. J. Physiol. Biochem. 2024, 80, 757–774. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Balligand, J.L. New and Emerging Therapies and Targets: Beta-3 Agonists. In Heart Failure; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2017; Volume 243, pp. 205–223. [Google Scholar] [CrossRef]
- Kennelly, M.J.; Rhodes, T.; Girman, C.J.; Thomas, E.; Shortino, D.; Mudd, P.N., Jr. Efficacy of Vibegron and Mirabegron for Overactive Bladder: A Systematic Literature Review and Indirect Treatment Comparison. Adv. Ther. 2021, 38, 5452–5464. [Google Scholar] [CrossRef]
- Okeke, K.; Michel-Reher, M.B.; Gravas, S.; Michel, M.C. Desensitization of cAMP Accumulation via Human beta3-Adrenoceptors Expressed in Human Embryonic Kidney Cells by Full, Partial, and Biased Agonists. Front. Pharmacol. 2019, 10, 596. [Google Scholar] [CrossRef]
- Milano, S.; Gerbino, A.; Schena, G.; Carmosino, M.; Svelto, M.; Procino, G. Human beta3-Adrenoreceptor is Resistant to Agonist-Induced Desensitization in Renal Epithelial Cells. Cell. Physiol. Biochem. 2018, 48, 847–862. [Google Scholar] [CrossRef]
- Okeke, K.; Angers, S.; Bouvier, M.; Michel, M.C. Agonist-induced desensitisation of beta3 -adrenoceptors: Where, when, and how? Br. J. Pharmacol. 2019, 176, 2539–2558. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef]
- Baker, J.G. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br. J. Pharmacol. 2010, 160, 1048–1061. [Google Scholar] [CrossRef]
- Takasu, T.; Ukai, M.; Sato, S.; Matsui, T.; Nagase, I.; Maruyama, T.; Sasamata, M.; Miyata, K.; Uchida, H.; Yamaguchi, O. Effect of (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J. Pharmacol. Exp. Ther. 2007, 321, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.; McCafferty, G.P.; Riedel, E.; Aiyar, N.; Pullen, M.; Evans, C.; Luce, T.D.; Coatney, R.W.; Rivera, G.C.; Westfall, T.D.; et al. GW427353 (solabegron), a novel, selective beta3-adrenergic receptor agonist, evokes bladder relaxation and increases micturition reflex threshold in the dog. J. Pharmacol. Exp. Ther. 2007, 323, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, J.; Nagabukuro, H.; Wickham, L.A.; Abbadie, C.; DeMartino, J.A.; Fitzmaurice, A.; Gichuru, L.; Kulick, A.; Donnelly, M.J.; Jochnowitz, N.; et al. Pharmacological Characterization of a Novel Beta 3 Adrenergic Agonist, Vibegron: Evaluation of Antimuscarinic Receptor Selectivity for Combination Therapy for Overactive Bladder. J. Pharmacol. Exp. Ther. 2017, 360, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Kanie, S.; Otsuka, A.; Yoshikawa, S.; Morimoto, T.; Hareyama, N.; Okazaki, S.; Kobayashi, R.; Hasebe, K.; Nakao, K.; Hayashi, R.; et al. Pharmacological effect of TRK-380, a novel selective human β3-adrenoceptor agonist, on mammalian detrusor strips. Urology 2012, 79, 744.e1–744.e7. [Google Scholar] [CrossRef]
- Baker, J.G. Evidence for a secondary state of the human beta3-adrenoceptor. Mol. Pharmacol. 2005, 68, 1645–1655. [Google Scholar] [CrossRef]
- Cohen, M.L.; Bloomquist, W.; Kriauciunas, A.; Shuker, A.; Calligaro, D. Aryl propanolamines: Comparison of activity at human beta3 receptors, rat beta3 receptors and rat atrial receptors mediating tachycardia. Br. J. Pharmacol. 1999, 126, 1018–1024. [Google Scholar] [CrossRef][Green Version]
- Pott, C.; Brixius, K.; Bundkirchen, A.; Bolck, B.; Bloch, W.; Steinritz, D.; Mehlhorn, U.; Schwinger, R.H. The preferential beta3-adrenoceptor agonist BRL 37344 increases force via beta1-/beta2-adrenoceptors and induces endothelial nitric oxide synthase via beta3-adrenoceptors in human atrial myocardium. Br. J. Pharmacol. 2003, 138, 521–529. [Google Scholar] [CrossRef]
- Brown, L.; Deighton, N.M.; Bals, S.; Sohlmann, W.; Zerkowski, H.R.; Michel, M.C.; Brodde, O.E. Spare receptors for beta-adrenoceptor-mediated positive inotropic effects of catecholamines in the human heart. J. Cardiovasc. Pharmacol. 1992, 19, 222–232. [Google Scholar] [CrossRef]
- Haji, E.; Al Mahri, S.; Aloraij, Y.; Malik, S.S.; Mohammad, S. Functional Characterization of the Obesity-Linked Variant of the beta3-Adrenergic Receptor. Int. J. Mol. Sci. 2021, 22, 5721. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, Q.; Li, X.M.; Liu, F.; Zhao, Q.; Men, L.; Chen, Q.J.; Zhai, H.; Yang, Y.N. Association of an ADRB3 Variant with Coronary Artery Disease Within the Chinese Han Population: Construction of a Predictive Nomogram Model. Genet. Test. Mol. Biomark. 2023, 27, 81–89. [Google Scholar] [CrossRef]
- Michel, M.C. Are beta3 -adrenoceptor gene polymorphisms relevant for urology? Neurourol. Urodyn. 2023, 42, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Matayoshi, T.; Kamide, K.; Takiuchi, S.; Yoshii, M.; Miwa, Y.; Takami, Y.; Tanaka, C.; Banno, M.; Horio, T.; Nakamura, S.; et al. The thiazide-sensitive Na(+)-Cl(−) cotransporter gene, C1784T, and adrenergic receptor-beta3 gene, T727C, may be gene polymorphisms susceptible to the antihypertensive effect of thiazide diuretics. Hypertens. Res. 2004, 27, 821–833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vrydag, W.; Alewijnse, A.E.; Michel, M.C. Do gene polymorphisms alone or in combination affect the function of human beta3-adrenoceptors? Br. J. Pharmacol. 2009, 156, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Pelat, M.; Verwaerde, P.; Galitzky, J.; Lafontan, M.; Berlan, M.; Senard, J.M.; Montastruc, J.L. High isoproterenol doses are required to activate beta3-adrenoceptor- mediated functions in dogs. J. Pharmacol. Exp. Ther. 2003, 304, 246–253. [Google Scholar] [CrossRef]
- Galitzky, J.; Reverte, M.; Portillo, M.; Carpene, C.; Lafontan, M.; Berlan, M. Coexistence of beta 1-, beta 2-, and beta 3-adrenoceptors in dog fat cells and their differential activation by catecholamines. Am. J. Physiol. 1993, 264 Pt 1, E403–E412. [Google Scholar] [CrossRef]
- Gauthier, C.; Seze-Goismier, C.; Rozec, B. Beta 3-adrenoceptors in the cardiovascular system. Clin. Hemorheol. Microcirc. 2007, 37, 193–204. [Google Scholar]
- Sato, M.; Horinouchi, T.; Hutchinson, D.S.; Evans, B.A.; Summers, R.J. Ligand-directed signaling at the beta3-adrenoceptor produced by 3-(2- Ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate (SR59230A) relative to receptor agonists. Mol. Pharmacol. 2007, 72, 1359–1368. [Google Scholar] [CrossRef]
- Kenakin, T. Biased agonism. F1000 Biol. Rep. 2009, 1, 87. [Google Scholar] [CrossRef]
- Michel, M.C.; Korstanje, C. beta3-Adrenoceptor agonists for overactive bladder syndrome: Role of translational pharmacology in a repositioning clinical drug development project. Pharmacol. Ther. 2016, 159, 66–82. [Google Scholar] [CrossRef]
- Evans, B.A.; Sato, M.; Sarwar, M.; Hutchinson, D.S.; Summers, R.J. Ligand-directed signalling at beta-adrenoceptors. Br. J. Pharmacol. 2010, 159, 1022–1038. [Google Scholar] [CrossRef]
- Hutchinson, D.S.; Sato, M.; Evans, B.A.; Christopoulos, A.; Summers, R.J. Evidence for pleiotropic signaling at the mouse beta3-adrenoceptor revealed by SR59230A [3-(2-Ethylphenoxy)-1-[(1,S)-1,2,3,4- tetrahydronapth-1-ylamino]-2S-2-propanol oxalate]. J. Pharmacol. Exp. Ther. 2005, 312, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Pocognoli, A.; D’Anzeo, M.; Siquini, W.; Minardi, D.; Muzzonigro, G.; Dessi-Fulgheri, P.; Sarzani, R. Nebivolol induces, via beta3 adrenergic receptor, lipolysis, uncoupling protein 1, and reduction of lipid droplet size in human adipocytes. J. Hypertens. 2014, 32, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hutchinson, D.S.; Evans, B.A.; Summers, R.J. The beta3-adrenoceptor agonist 4-[[(Hexylamino)carbonyl]amino]-N-[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxyphenoxy)propyl]amino]ethyl]-phenyl]-benzenesulfonamide (L755507) and antagonist (S)-N-[4-[2-[[3-[3-(acetamidomethyl)phenoxy]-2-hydroxypropyl]amino]-ethyl]phenyl]benzenesulfonamide (L748337) activate different signaling pathways in Chinese hamster ovary-K1 cells stably expressing the human beta3-adrenoceptor. Mol. Pharmacol. 2008, 74, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Laurence, L.; Brunton, B.C.K. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 14th ed.; McGraw-Hill Education: New York, NY, USA, 2023. [Google Scholar]
- Moghadam Farid, S.; Noori, M.; Nazari Montazer, M.; Khalili Ghomi, M.; Mollazadeh, M.; Dastyafteh, N.; Irajie, C.; Zomorodian, K.; Mirfazli, S.S.; Mojtabavi, S.; et al. Synthesis and structure-activity relationship studies of benzimidazole-thioquinoline derivatives as α-glucosidase inhibitors. Sci. Rep. 2023, 13, 4392. [Google Scholar] [CrossRef]
- Wei, H.; McCammon, J.A. Structure and dynamics in drug discovery. Npj Drug Discov. 2024, 1, 1. [Google Scholar] [CrossRef]
- Ujiantari, N.S.O.; Ham, S.; Nagiri, C.; Shihoya, W.; Nureki, O.; Hutchinson, D.S.; Schuster, D. Pharmacophore-guided Virtual Screening to Identify New β(3)-adrenergic Receptor Agonists. Mol. Inform. 2022, 41, e2100223. [Google Scholar] [CrossRef]
- Sacco, E.; Bientinesi, R. Mirabegron: A review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 2012, 4, 315–324. [Google Scholar] [CrossRef]
- Lightner, D.J.; Gomelsky, A.; Souter, L.; Vasavada, S.P. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 2019, 202, 558–563. [Google Scholar] [CrossRef]
- European Medicines Agency. Betmiga (Mirabegron). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/betmiga (accessed on 5 January 2025).
- Maki, T.; Kajioka, S.; Itsumi, M.; Kareman, E.; Lee, K.; Shiota, M.; Eto, M. Mirabegron induces relaxant effects via cAMP signaling-dependent and -independent pathways in detrusor smooth muscle. Low Urin. Tract Symptoms 2019, 11, O209–O217. [Google Scholar] [CrossRef]
- Nureki, I.; Kobayashi, K.; Tanaka, T.; Demura, K.; Inoue, A.; Shihoya, W.; Nureki, O. Cryo-EM structures of the beta3 adrenergic receptor bound to solabegron and isoproterenol. Biochem. Biophys. Res. Commun. 2022, 611, 158–164. [Google Scholar] [CrossRef]
- Li, W.; Ma, Z.; Du, L.; Li, M. Development and Characterization of a Highly Selective Turn-On Fluorescent Ligand for beta3-Adrenergic Receptor. Anal. Chem. 2023, 95, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Yu, Q.; Tamalunas, A.; Stief, C.G.; Hennenberg, M. Ligand-Receptor Interactions and Structure-Function Relationships in Off-Target Binding of the β(3)-Adrenergic Agonist Mirabegron to α(1A)-Adrenergic Receptors. Int. J. Mol. Sci. 2024, 25, 7468. [Google Scholar] [CrossRef] [PubMed]
- Krauwinkel, W.; van Dijk, J.; Schaddelee, M.; Eltink, C.; Meijer, J.; Strabach, G.; van Marle, S.; Kerbusch, V.; van Gelderen, M. Pharmacokinetic properties of mirabegron, a beta3-adrenoceptor agonist: Results from two phase I, randomized, multiple-dose studies in healthy young and elderly men and women. Clin. Ther. 2012, 34, 2144–2160. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.O.; Jose, J.; Paul, F.; Sreedharan, S.; Uthaman, N. Therapeutic effectiveness and adverse drug reactions of mirabegron versus solifenacin in the treatment of overactive bladder syndrome. Perspect. Clin. Res. 2024, 15, 147–151. [Google Scholar] [CrossRef]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis is Driven by beta2-AR Stimulation. Cell Metab. 2020, 32, 287–300.e7. [Google Scholar] [CrossRef]
- Korstanje, C.; Suzuki, M.; Yuno, K.; Sato, S.; Ukai, M.; Schneidkraut, M.J.; Yan, G.X. Translational science approach for assessment of cardiovascular effects and proarrhythmogenic potential of the beta-3 adrenergic agonist mirabegron. J. Pharmacol. Toxicol. Methods 2017, 87, 74–81. [Google Scholar] [CrossRef]
- van Gelderen, M.; Stolzel, M.; Meijer, J.; Kerbusch, V.; Collins, C.; Korstanje, C. An Exploratory Study in Healthy Male Subjects of the Mechanism of Mirabegron-Induced Cardiovascular Effects. J. Clin. Pharmacol. 2017, 57, 1534–1544. [Google Scholar] [CrossRef]
- Nitti, V.W.; Auerbach, S.; Martin, N.; Calhoun, A.; Lee, M.; Herschorn, S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J. Urol. 2013, 189, 1388–1395. [Google Scholar] [CrossRef]
- Medicines and Healthcare products Regulatory Agency (MHRA). Mirabegron (Betmiga▼): Risk of Severe Hypertension and Associated Cerebrovascular and Cardiac Events. Available online: https://www.gov.uk/drug-safety-update/mirabegron-betmiga-risk-of-severe-hypertension-and-associated-cerebrovascular-and-cardiac-events#:~:text=An%20EU-wide%20review%20of%20the%20latest%20safety%20data (accessed on 5 January 2025).
- Mo, W.; Michel, M.C.; Lee, X.W.; Kaumann, A.J.; Molenaar, P. The beta3-adrenoceptor agonist mirabegron increases human atrial force through beta1 -adrenoceptors: An indirect mechanism? Br. J. Pharmacol. 2017, 174, 2706–2715. [Google Scholar] [CrossRef]
- Igawa, Y.; Aizawa, N.; Michel, M.C. beta3-Adrenoceptors in the normal and diseased urinary bladder-What are the open questions? Br. J. Pharmacol. 2019, 176, 2525–2538. [Google Scholar] [CrossRef]
- Malik, M.; van Gelderen, E.M.; Lee, J.H.; Kowalski, D.L.; Yen, M.; Goldwater, R.; Mujais, S.K.; Schaddelee, M.P.; de Koning, P.; Kaibara, A.; et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: A randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin. Pharmacol. Ther. 2012, 92, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.C.; Kiguti, L.R.; Calmasini, F.B.; Silva, F.H.; da Silva, K.P.; Ferreira, R.; Ribeiro, C.A.; Monica, F.Z.; Pupo, A.S.; Antunes, E. Mirabegron relaxes urethral smooth muscle by a dual mechanism involving beta3 -adrenoceptor activation and alpha1 -adrenoceptor blockade. Br. J. Pharmacol. 2016, 173, 415–428. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Schinzari, F.; Di Daniele, N.; Sica, G.; Gentileschi, P.; Vizioli, G.; Cardillo, C.; Tesauro, M. Mirabegron relaxes arteries from human visceral adipose tissue through antagonism of alpha1-adrenergic receptors. Vasc. Pharmacol. 2022, 146, 107094. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tocino, M.; Pun-Garcia, A.; Gomez, M.; Clemente-Moragon, A.; Oliver, E.; Villena-Gutierrez, R.; Trigo-Anca, S.; Diaz-Guerra, A.; Sanz-Rosa, D.; Prados, B.; et al. beta3-Adrenergic receptor overexpression in cardiomyocytes preconditions mitochondria to withstand ischemia-reperfusion injury. Basic Res. Cardiol. 2024, 119, 773–794. [Google Scholar] [CrossRef]
- Andersson, K.E. Pharmacology: On the mode of action of mirabegron. Nat. Rev. Urol. 2016, 13, 131–132. [Google Scholar] [CrossRef]
- European Medicines Agency. Obgemsa (Vibegron). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/obgemsa (accessed on 5 January 2025).
- Gleicher, S.; Sebesta, E.M.; Reynolds, W.S.; Dmochowski, R. Vibegron for the treatment of overactive bladder: A comprehensive update. Expert. Opin. Pharmacother. 2022, 23, 1479–1484. [Google Scholar] [CrossRef]
- Brucker, B.M.; King, J.; Mudd, P.N., Jr.; McHale, K. Selectivity and Maximum Response of Vibegron and Mirabegron for beta3-Adrenergic Receptors. Curr. Ther. Res. Clin. Exp. 2022, 96, 100674. [Google Scholar] [CrossRef]
- Edmondson, S.D.; Zhu, C.; Kar, N.F.; Di Salvo, J.; Nagabukuro, H.; Sacre-Salem, B.; Dingley, K.; Berger, R.; Goble, S.D.; Morriello, G.; et al. Discovery of Vibegron: A Potent and Selective beta3 Adrenergic Receptor Agonist for the Treatment of Overactive Bladder. J. Med. Chem. 2016, 59, 609–623. [Google Scholar] [CrossRef]
- Staskin, D.; Frankel, J.; Varano, S.; Shortino, D.; Jankowich, R.; Mudd, P.N., Jr. International Phase III, Randomized, Double-Blind, Placebo and Active Controlled Study to Evaluate the Safety and Efficacy of Vibegron in Patients with Symptoms of Overactive Bladder: EMPOWUR. J. Urol. 2020, 204, 316–324. [Google Scholar] [CrossRef]
- Staskin, D.; Frankel, J.; Varano, S.; Shortino, D.; Jankowich, R.; Mudd, P.N., Jr. Once-Daily Vibegron 75 mg for Overactive Bladder: Long-Term Safety and Efficacy from a Double-Blind Extension Study of the International Phase 3 Trial (EMPOWUR). J. Urol. 2021, 205, 1421–1429. [Google Scholar] [CrossRef]
- Staskin, D.; Owens-Grillo, J.; Thomas, E.; Rovner, E.; Cline, K.; Mujais, S. Efficacy and Safety of Vibegron for Persistent Symptoms of Overactive Bladder in Men Being Pharmacologically Treated for Benign Prostatic Hyperplasia: Results From the Phase 3 Randomized Controlled COURAGE Trial. J. Urol. 2024, 212, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Haag-Molkenteller, C.; King, J.; Walker, A.; Mudd, P.N., Jr.; White, W.B. Effects of vibegron on ambulatory blood pressure in patients with overactive bladder: Results from a double-blind, placebo-controlled trial. Blood Press. Monit. 2022, 27, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Karmakar, S.; Mainkar, P.S.; Madhavachary, R.; Chandrasekhar, S. A Chiron Approach to the Practical and Scalable Synthesis of the β3-Adrenergic Receptor Agonist Vibegron. Eur. J. Org. Chem. 2025, 28, e202500469. [Google Scholar] [CrossRef]
- Kalinowski, L.; Dobrucki, L.W.; Szczepanska-Konkel, M.; Jankowski, M.; Martyniec, L.; Angielski, S.; Malinski, T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: A novel mechanism for antihypertensive action. Circulation 2003, 107, 2747–2752. [Google Scholar] [CrossRef]
- Broeders, M.A.; Doevendans, P.A.; Bekkers, B.C.; Bronsaer, R.; van Gorsel, E.; Heemskerk, J.W.; Egbrink, M.G.; van Breda, E.; Reneman, R.S.; van Der Zee, R. Nebivolol: A third-generation beta-blocker that augments vascular nitric oxide release: Endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation 2000, 102, 677–684. [Google Scholar] [CrossRef]
- de Groot, A.A.; Mathy, M.J.; van Zwieten, P.A.; Peters, S.L. Involvement of the beta3 adrenoceptor in nebivolol-induced vasorelaxation in the rat aorta. J. Cardiovasc. Pharmacol. 2003, 42, 232–236. [Google Scholar] [CrossRef]
- Dessy, C.; Saliez, J.; Ghisdal, P.; Daneau, G.; Lobysheva, I.I.; Frerart, F.; Belge, C.; Jnaoui, K.; Noirhomme, P.; Feron, O.; et al. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation 2005, 112, 1198–1205. [Google Scholar] [CrossRef]
- Abdelkrim, M.A.; Martignat, L.; Gogny, M.; Desfontis, J.C.; Noireaud, J.; Mallem, M.Y. Celiprolol induces beta(3)-adrenoceptors-dependent relaxation in isolated porcine coronary arteries. Can. J. Physiol. Pharmacol. 2013, 91, 791–796. [Google Scholar] [CrossRef]
- Ferri, C. The role of nebivolol in the management of hypertensive patients: From pharmacological profile to treatment guidelines. Future Cardiol. 2021, 17, 1421–1433. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for Nebivolol, CID 71301. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nebivolol (accessed on 10 November 2025).
- Pauwels, P.J.; Van Gompel, P.; Leysen, J.E. Human beta 1- and beta 2-adrenergic receptor binding and mediated accumulation of cAMP in transfected Chinese hamster ovary cells. Profile of nebivolol and known beta-adrenergic blockers. Biochem. Pharmacol. 1991, 42, 1683–1689. [Google Scholar] [CrossRef]
- Quang, T.T.; Rozec, B.; Audigane, L.; Gauthier, C. Investigation of the different adrenoceptor targets of nebivolol enantiomers in rat thoracic aorta. Br. J. Pharmacol. 2009, 156, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Garban, H.J.; Buga, G.M.; Ignarro, L.J. Estrogen receptor-mediated vascular responsiveness to nebivolol: A novel endothelium-related mechanism of therapeutic vasorelaxation. J. Cardiovasc. Pharmacol. 2004, 43, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Ladage, D.; Brixius, K.; Hoyer, H.; Steingen, C.; Wesseling, A.; Malan, D.; Bloch, W.; Schwinger, R.H. Mechanisms underlying nebivolol-induced endothelial nitric oxide synthase activation in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 2006, 33, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Vecchione, C.; Aretini, A.; Poulet, R.; Bettarini, U.; Gentile, M.T.; Cifelli, G.; Lembo, G. Characterization of nitric oxide release by nebivolol and its metabolites. Am. J. Hypertens. 2006, 19, 579–586. [Google Scholar] [CrossRef][Green Version]
- Rozec, B.; Erfanian, M.; Laurent, K.; Trochu, J.N.; Gauthier, C. Nebivolol, a vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the nonfailing transplanted human heart. J. Am. Coll. Cardiol. 2009, 53, 1532–1538. [Google Scholar] [CrossRef]
- Oelze, M.; Daiber, A.; Brandes, R.P.; Hortmann, M.; Wenzel, P.; Hink, U.; Schulz, E.; Mollnau, H.; von Sandersleben, A.; Kleschyov, A.L.; et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 2006, 48, 677–684. [Google Scholar] [CrossRef]
- Cominacini, L.; Fratta Pasini, A.; Garbin, U.; Nava, C.; Davoli, A.; Criscuoli, M.; Crea, A.; Sawamura, T.; Lo Cascio, V. Nebivolol and its 4-keto derivative increase nitric oxide in endothelial cells by reducing its oxidative inactivation. J. Am. Coll. Cardiol. 2003, 42, 1838–1844. [Google Scholar] [CrossRef]
- Frazier, E.P.; Michel-Reher, M.B.; van Loenen, P.; Sand, C.; Schneider, T.; Peters, S.L.; Michel, M.C. Lack of evidence that nebivolol is a beta(3)-adrenoceptor agonist. Eur. J. Pharmacol. 2011, 654, 86–91. [Google Scholar] [CrossRef]
- Gosgnach, W.; Boixel, C.; Nevo, N.; Poiraud, T.; Michel, J.B. Nebivolol induces calcium-independent signaling in endothelial cells by a possible beta-adrenergic pathway. J. Cardiovasc. Pharmacol. 2001, 38, 191–199. [Google Scholar] [CrossRef]
- Food and Drug Administration. Coronis (Carvedilol)—Summary of Product Characteristics. Available online: https://www.efda.gov.et/wp-content/uploads/2023/09/Coronis_Carvedilol_Bilim-ilac-sanayi-ve-ticaret-AS.pdf#:~:text=Carvedilol%20is%20indicated%20for%20the%20treatment%20of%20mild,disease%20in%20the%20treatment%20of%20chronic%20heart%20failure (accessed on 5 January 2025).
- Schnabel, P.; Maack, C.; Mies, F.; Tyroller, S.; Scheer, A.; Bohm, M. Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors. J. Cardiovasc. Pharmacol. 2000, 36, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, L.X.; Fuenmayor, N.; Varin, F.; Villagra, V.G.; Colindres, R.E.; Powell, J.R. Mechanism of the vasodilatory effect of carvedilol in normal volunteers: A comparison with labetalol. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. S11), S81–S84. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, B.; Prichard, B.N.; Graham, B.R.; Walden, R.J. Clinical pharmacology of carvedilol. Clin. Investig. 1992, 70 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, R.R., Jr.; Gellai, M.; Hieble, J.P.; Willette, R.N.; Nichols, A.J. The pharmacology of carvedilol. Eur. J. Clin. Pharmacol. 1990, 38 (Suppl. S2), S82–S88. [Google Scholar] [CrossRef]
- Bank, A.J.; Kelly, A.S.; Thelen, A.M.; Kaiser, D.R.; Gonzalez-Campoy, J.M. Effects of carvedilol versus metoprolol on endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am. J. Hypertens. 2007, 20, 777–783. [Google Scholar] [CrossRef]
- Toyoda, S.; Haruyama, A.; Inami, S.; Arikawa, T.; Saito, F.; Watanabe, R.; Sakuma, M.; Abe, S.; Nakajima, T.; Tanaka, A.; et al. Effects of carvedilol vs bisoprolol on inflammation and oxidative stress in patients with chronic heart failure. J. Cardiol. 2020, 75, 140–147. [Google Scholar] [CrossRef]
- Feuerstein, G.Z.; Ruffolo, R.R., Jr. Carvedilol, a novel multiple action antihypertensive agent with antioxidant activity and the potential for myocardial and vascular protection. Eur. Heart J. 1995, 16 (Suppl. F), 38–42. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for Carvedilol, CID 2585. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2585 (accessed on 10 November 2025).
- Nawarskas, J.J.; Cheng-Lai, A.; Frishman, W.H. Celiprolol: A Unique Selective Adrenoceptor Modulator. Cardiol. Rev. 2017, 25, 247–253. [Google Scholar] [CrossRef]
- Mehta, J.L.; Lopez, L.M.; Chen, L.; Cox, O.E. Alterations in nitric oxide synthase activity, superoxide anion generation, and platelet aggregation in systemic hypertension, and effects of celiprolol. Am. J. Cardiol. 1994, 74, 901–905. [Google Scholar] [CrossRef]
- Dhein, S.; Titzer, S.; Wallstein, M.; Muller, A.; Gerwin, R.; Panzner, B.; Klaus, W. Celiprolol exerts microvascular dilatation by activation of beta 2-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1992, 346, 27–31. [Google Scholar] [CrossRef]
- Kakoki, M.; Hirata, Y.; Hayakawa, H.; Nishimatsu, H.; Suzuki, Y.; Nagata, D.; Suzuki, E.; Kikuchi, K.; Nagano, T.; Omata, M. Effects of vasodilatory beta-adrenoceptor antagonists on endothelium- derived nitric oxide release in rat kidney. Hypertension 1999, 33, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.J.; Buckley, M.M. Celiprolol. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in cardiovascular disease. Drugs 1991, 41, 941–969. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for Celiprolol, CID 2663. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/celiprolol (accessed on 10 November 2025).
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bubb, K.J.; Ravindran, D.; Cartland, S.P.; Finemore, M.; Clayton, Z.E.; Tsang, M.; Tang, O.; Kavurma, M.M.; Patel, S.; Figtree, G.A. beta 3 Adrenergic Receptor Stimulation Promotes Reperfusion in Ischemic Limbs in a Murine Diabetic Model. Front. Pharmacol. 2021, 12, 666334. [Google Scholar] [CrossRef]
- Liu, C.C.; Karimi Galougahi, K.; Weisbrod, R.M.; Hansen, T.; Ravaie, R.; Nunez, A.; Liu, Y.B.; Fry, N.; Garcia, A.; Hamilton, E.J.; et al. Oxidative inhibition of the vascular Na+-K+ pump via NADPH oxidase-dependent beta1-subunit glutathionylation: Implications for angiotensin II-induced vascular dysfunction. Free Radic. Biol. Med. 2013, 65, 563–572. [Google Scholar] [CrossRef]
- Karimi Galougahi, K.; Liu, C.C.; Garcia, A.; Fry, N.A.; Hamilton, E.J.; Figtree, G.A.; Rasmussen, H.H. beta3-Adrenoceptor activation relieves oxidative inhibition of the cardiac Na+-K+ pump in hyperglycemia induced by insulin receptor blockade. Am. J. Physiol. Cell Physiol. 2015, 309, C286–C295. [Google Scholar] [CrossRef]
- Coats, A.; Jain, S. Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. J. Hum. Hypertens. 2017, 31, 376–381. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X. Nebivolol ameliorates asymmetric dimethylarginine-induced vascular response in rat aorta via beta3 adrenoceptor-mediated mechanism. Clin. Exp. Hypertens. 2016, 38, 252–259. [Google Scholar] [CrossRef]
- Alpoim, P.N.; Sousa, L.P.; Mota, A.P.; Rios, D.R.; Dusse, L.M. Asymmetric Dimethylarginine (ADMA) in cardiovascular and renal disease. Clin. Chim. Acta 2015, 440, 36–39. [Google Scholar] [CrossRef]
- Gao, J.; Xie, Q.; Wei, T.; Huang, C.; Zhou, W.; Shen, W. Nebivolol Improves Obesity-Induced Vascular Remodeling by Suppressing NLRP3 Activation. J. Cardiovasc. Pharmacol. 2019, 73, 326–333. [Google Scholar] [CrossRef]
- Mollnau, H.; Schulz, E.; Daiber, A.; Baldus, S.; Oelze, M.; August, M.; Wendt, M.; Walter, U.; Geiger, C.; Agrawal, R.; et al. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Sisodia, M.; Trinh, K.; Bedrood, S.; Wu, G.; Wei, L.H.; Buga, G.M. Nebivolol inhibits vascular smooth muscle cell proliferation by mechanisms involving nitric oxide but not cyclic GMP. Nitric Oxide 2002, 7, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fratta Pasini, A.; Garbin, U.; Nava, M.C.; Stranieri, C.; Davoli, A.; Sawamura, T.; Lo Cascio, V.; Cominacini, L. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J. Hypertens. 2005, 23, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Hadi, T.; Douhard, R.; Dias, A.M.M.; Wendremaire, M.; Pezze, M.; Bardou, M.; Sagot, P.; Garrido, C.; Lirussi, F. Beta3 adrenergic receptor stimulation in human macrophages inhibits NADPHoxidase activity and induces catalase expression via PPARgamma activation. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1769–1784. [Google Scholar] [CrossRef]
- Farmer, P.; Pugin, J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L675–L682. [Google Scholar] [CrossRef]
- Tan, K.S.; Nackley, A.G.; Satterfield, K.; Maixner, W.; Diatchenko, L.; Flood, P.M. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell. Signal. 2007, 19, 251–260. [Google Scholar] [CrossRef]
- Sanaee, F.; Jamali, F. Action and disposition of the beta3-agonist nebivolol in the presence of inflammation; an alternative to conventional beta1-blockers. Curr. Pharm. Des. 2014, 20, 1311–1317. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef]
- van der Vorst, E.P.C. High-Density Lipoproteins and Apolipoprotein A1. Subcell. Biochem. 2020, 94, 399–420. [Google Scholar] [CrossRef]
- Shi, S.T.; Li, Y.F.; Guo, Y.Q.; Wang, Z.H. Effect of beta-3 adrenoceptor stimulation on the levels of ApoA-I, PPARalpha, and PPARgamma in apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 2014, 64, 407–411. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, Y.F.; Guo, Y.Q. beta3-Adrenoceptor activation attenuates atherosclerotic plaque formation in ApoE(−/−) mice through lowering blood lipids and glucose. Acta Pharmacol. Sin. 2013, 34, 1156–1163. [Google Scholar] [CrossRef][Green Version]
- Gao, X.Q.; Li, Y.F.; Jiang, Z.L. beta3-Adrenoceptor activation upregulates apolipoprotein A-I expression in HepG2 cells, which might further promote cholesterol efflux from macrophage foam cells. Drug Des. Dev. Ther. 2017, 11, 617–627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, X.; Li, Y. beta3-Adrenergic receptor regulates hepatic apolipoprotein A-I gene expression. J. Clin. Lipidol. 2017, 11, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Moughaizel, M.; Dagher, E.; Bouhsina, N.; Lalanne, V.; Thorin, C.; Desfontis, J.C.; Mallem, M.Y. Effects of chronic mirabegron treatment on metabolic and cardiovascular parameters as well as on atherosclerotic lesions of WHHL rabbits with high-fructose high-fat diet-induced insulin resistance. Eur. J. Pharmacol. 2022, 921, 174870. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.T.; Tisdale, M.J. Role of β-adrenergic receptors in the anti-obesity and anti-diabetic effects of zinc-α2-glycoprotien (ZAG). Biochim. Biophys. Acta 2012, 1821, 590–599. [Google Scholar] [CrossRef]
- Huang, D.; Mao, X.; Peng, J.; Cheng, M.; Bai, T.; Du, M.; Huang, K.; Liu, B.; Yang, L.; Huang, K.; et al. Role of adipokine zinc-alpha2-glycoprotein in coronary heart disease. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1055–E1062. [Google Scholar] [CrossRef]
- Dutta, P.; Courties, G.; Wei, Y.; Leuschner, F.; Gorbatov, R.; Robbins, C.S.; Iwamoto, Y.; Thompson, B.; Carlson, A.L.; Heidt, T.; et al. Myocardial infarction accelerates atherosclerosis. Nature 2012, 487, 325–329. [Google Scholar] [CrossRef]
- Tjandra, P.M.; Paralkar, M.P.; Osipov, B.; Chen, Y.J.; Zhao, F.; Ripplinger, C.M.; Christiansen, B.A. Systemic bone loss following myocardial infarction in mice. J. Orthop. Res. 2021, 39, 739–749. [Google Scholar] [CrossRef]
- Bubb, K.J.; Harmer, J.A.; Finemore, M.; Aitken, S.J.; Ali, Z.S.; Billot, L.; Chow, C.; Golledge, J.; Mister, R.; Gray, M.P.; et al. Protocol for the Stimulating beta3-Adrenergic Receptors for Peripheral Artery Disease (STAR-PAD) trial: A double-blinded, randomised, placebo- controlled study evaluating the effects of mirabegron on functional performance in patients with peripheral arterial disease. BMJ Open 2021, 11, e049858. [Google Scholar] [CrossRef]
- Manek, G.; Bhardwaj, A. Pulmonary Hypertension. In StatPearls [Internet]; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rozec, B.; Gauthier, C. beta3-adrenoceptors in the cardiovascular system: Putative roles in human pathologies. Pharmacol. Ther. 2006, 111, 652–673. [Google Scholar] [CrossRef]
- Garcia-Lunar, I.; Blanco, I.; Fernandez-Friera, L.; Prat-Gonzalez, S.; Jorda, P.; Sanchez, J.; Pereda, D.; Pujadas, S.; Rivas, M.; Sole-Gonzalez, E.; et al. Design of the beta3-Adrenergic Agonist Treatment in Chronic Pulmonary Hypertension Secondary to Heart Failure Trial. JACC Basic Transl. Sci. 2020, 5, 317–327. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, A.; Blanco, I.; Garcia-Lunar, I.; Jorda, P.; Rodriguez-Arias, J.J.; Fernandez-Friera, L.; Zegri, I.; Nuche, J.; Gomez-Bueno, M.; Prat, S.; et al. beta3 adrenergic agonist treatment in chronic pulmonary hypertension associated with heart failure (SPHERE-HF): A double blind, placebo- controlled, randomized clinical trial. Eur. J. Heart Fail. 2023, 25, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cheng, J.; Ding, X.; Chi, J.; Yang, J.; Li, W. beta3 adrenergic receptor antagonist SR59230A exerts beneficial effects on right ventricular performance in monocrotaline-induced pulmonary arterial hypertension. Exp. Ther. Med. 2020, 19, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Axelsson Raja, A.; Iversen, K.; Valeur, N.; Tonder, N.; Schou, M.; Christensen, A.H.; Bruun, N.E.; Soholm, H.; Ghanizada, M.; et al. Hemodynamic Effects of Cyclic Guanosine Monophosphate-Dependent Signaling Through beta3 Adrenoceptor Stimulation in Patients With Advanced Heart Failure: A Randomized Invasive Clinical Trial. Circ. Heart Fail. 2022, 15, e009120. [Google Scholar] [CrossRef]
- Aragon, J.P.; Condit, M.E.; Bhushan, S.; Predmore, B.L.; Patel, S.S.; Grinsfelder, D.B.; Gundewar, S.; Jha, S.; Calvert, J.W.; Barouch, L.A.; et al. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia- reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J. Am. Coll. Cardiol. 2011, 58, 2683–2691. [Google Scholar] [CrossRef]
- Xu, D.Q.; Luo, Y.; Liu, Y.; Wang, J.; Zhang, B.; Xu, M.; Wang, Y.X.; Dong, H.Y.; Dong, M.Q.; Zhao, P.T.; et al. Beta-estradiol attenuates hypoxic pulmonary hypertension by stabilizing the expression of p27kip1 in rats. Respir. Res. 2010, 11, 182. [Google Scholar] [CrossRef]
- Jordan, J.; Kurschat, C.; Reuter, H. Arterial Hypertension. Dtsch. Arztebl. Int. 2018, 115, 557–568. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef]
- Yang, L.K.; Tao, Y.X. Physiology and pathophysiology of the beta3-adrenergic receptor. Prog. Mol. Biol. Transl. Sci. 2019, 161, 91–112. [Google Scholar] [CrossRef]
- Tavernier, G.; Toumaniantz, G.; Erfanian, M.; Heymann, M.F.; Laurent, K.; Langin, D.; Gauthier, C. beta3-Adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human beta3-adrenergic receptor. Cardiovasc. Res. 2003, 59, 288–296. [Google Scholar] [CrossRef]
- Donckier, J.E.; Massart, P.E.; Van Mechelen, H.; Heyndrickx, G.R.; Gauthier, C.; Balligand, J.L. Cardiovascular effects of beta 3-adrenoceptor stimulation in perinephritic hypertension. Eur. J. Clin. Investig. 2001, 31, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Trochu, J.N.; Leblais, V.; Rautureau, Y.; Beverelli, F.; Le Marec, H.; Berdeaux, A.; Gauthier, C. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br. J. Pharmacol. 1999, 128, 69–76. [Google Scholar] [CrossRef]
- Berg, T.; Piercey, B.W.; Jensen, J. Role of beta1-3-adrenoceptors in blood pressure control at rest and during tyramine-induced norepinephrine release in spontaneously hypertensive rats. Hypertension 2010, 55, 1224–1230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saunders, S.L.; Hutchinson, D.S.; Britton, F.C.; Liu, L.; Markus, I.; Sandow, S.L.; Murphy, T.V. Effect of beta1/beta2 -adrenoceptor blockade on beta3 -adrenoceptor activity in the rat cremaster muscle artery. Br. J. Pharmacol. 2021, 178, 1789–1804. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.J.; Ruan, C.C.; Ma, Y.; Chen, D.R.; Kong, L.R.; Zhu, D.L.; Gao, P.J. Beta3 adrenergic receptor is involved in vascular injury in deoxycorticosterone acetate-salt hypertensive mice. FEBS Lett. 2016, 590, 769–778. [Google Scholar] [CrossRef]
- Ling, S.; Nanhwan, M.; Qian, J.; Kodakandla, M.; Castillo, A.C.; Thomas, B.; Liu, H.; Ye, Y. Modulation of microRNAs in hypertension-induced arterial remodeling through the beta1 and beta3-adrenoreceptor pathways. J. Mol. Cell. Cardiol. 2013, 65, 127–136. [Google Scholar] [CrossRef]
- Fondjo, L.A.; Awuah, E.O.; Sakyi, S.A.; Senu, E.; Detoh, E. Association between endothelial nitric oxide synthase (eNOS) gene variants and nitric oxide production in preeclampsia: A case-control study in Ghana. Sci. Rep. 2023, 13, 14740. [Google Scholar] [CrossRef]
- Bueno-Pereira, T.O.; Nunes, P.R.; Matheus, M.B.; Vieira da Rocha, A.L.; Sandrim, V.C. Nebivolol Increases Nitric Oxide Synthase via beta3 Adrenergic Receptor in Endothelial Cells Following Exposure to Plasma from Preeclamptic Patients. Cells 2022, 11, 883. [Google Scholar] [CrossRef]
- Ekataksin, W.; Kaneda, K. Liver microvascular architecture: An insight into the pathophysiology of portal hypertension. Semin. Liver Dis. 1999, 19, 359–382. [Google Scholar] [CrossRef]
- Suk, K.T. Hepatic venous pressure gradient: Clinical use in chronic liver disease. Clin. Mol. Hepatol. 2014, 20, 6–14. [Google Scholar] [CrossRef]
- Vasina, V.; Giannone, F.; Domenicali, M.; Latorre, R.; Berzigotti, A.; Caraceni, P.; Zoli, M.; De Ponti, F.; Bernardi, M. Portal hypertension and liver cirrhosis in rats: Effect of the beta3-adrenoceptor agonist SR58611A. Br. J. Pharmacol. 2012, 167, 1137–1147. [Google Scholar] [CrossRef]
- Cirino, G.; Sorrentino, R.; di Villa Bianca, R.; Popolo, A.; Palmieri, A.; Imbimbo, C.; Fusco, F.; Longo, N.; Tajana, G.; Ignarro, L.J.; et al. Involvement of beta 3-adrenergic receptor activation via cyclic GMP- but not NO-dependent mechanisms in human corpus cavernosum function. Proc. Natl. Acad. Sci. USA 2003, 100, 5531–5536. [Google Scholar] [CrossRef]
- Trebicka, J.; Hennenberg, M.; Schulze Probsting, A.; Laleman, W.; Klein, S.; Granzow, M.; Nevens, F.; Zaagsma, J.; Heller, J.; Sauerbruch, T. Role of beta3-adrenoceptors for intrahepatic resistance and portal hypertension in liver cirrhosis. Hepatology 2009, 50, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geng, J.; Liu, Y.; Li, Y.; Shen, J.; Xiao, X.; Sheng, L.; Yang, B.; Cheng, C.; Li, W. beta3-adrenoceptor mediates metabolic protein remodeling in a rabbit model of tachypacing-induced atrial fibrillation. Cell. Physiol. Biochem. 2013, 32, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, W.; Li, Y.; Zhao, J.; Wang, L.; Dong, D.; Pan, Z.; Yang, B. Activation of beta(3)-adrenoceptor promotes rapid pacing-induced atrial electrical remodeling in rabbits. Cell. Physiol. Biochem. 2011, 28, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sartiani, L.; De Paoli, P.; Stillitano, F.; Aimond, F.; Vassort, G.; Mugelli, A.; Cerbai, E. Functional remodeling in post-myocardial infarcted rats: Focus on beta- adrenoceptor subtypes. J. Mol. Cell. Cardiol. 2006, 40, 258–266. [Google Scholar] [CrossRef]
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef]
- Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lopatin, A.N. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997, 59, 171–191. [Google Scholar] [CrossRef]
- Nilius, B.; Droogmans, G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef]
- Scherer, D.; Kiesecker, C.; Kulzer, M.; Gunth, M.; Scholz, E.P.; Kathofer, S.; Thomas, D.; Maurer, M.; Kreuzer, J.; Bauer, A.; et al. Activation of inwardly rectifying Kir2.x potassium channels by beta 3-adrenoceptors is mediated via different signaling pathways with a predominant role of PKC for Kir2.1 and of PKA for Kir2.2. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Schram, G.; Pourrier, M.; Wang, Z.; White, M.; Nattel, S. Barium block of Kir2 and human cardiac inward rectifier currents: Evidence for subunit-heteromeric contribution to native currents. Cardiovasc. Res. 2003, 59, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Niwa, N.; Nerbonne, J.M. Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation. J. Mol. Cell. Cardiol. 2010, 48, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kathofer, S.; Zhang, W.; Karle, C.; Thomas, D.; Schoels, W.; Kiehn, J. Functional coupling of human beta 3-adrenoreceptors to the KvLQT1/MinK potassium channel. J. Biol. Chem. 2000, 275, 26743–26747. [Google Scholar] [CrossRef]
- Bosch, R.F.; Schneck, A.C.; Kiehn, J.; Zhang, W.; Hambrock, A.; Eigenberger, B.W.; Rub, N.; Gogel, J.; Mewis, C.; Seipel, L.; et al. beta3-Adrenergic regulation of an ion channel in the heart-inhibition of the slow delayed rectifier potassium current I(Ks) in guinea pig ventricular myocytes. Cardiovasc. Res. 2002, 56, 393–403. [Google Scholar] [CrossRef]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Benitah, J.P.; Alvarez, J.L.; Gomez, A.M. L-type Ca2+ current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 26–36. [Google Scholar] [CrossRef]
- Anderson, M.E. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc. Med. 2004, 14, 152–161. [Google Scholar] [CrossRef]
- Skeberdis, V.A.; Gendviliene, V.; Zablockaite, D.; Treinys, R.; Macianskiene, R.; Bogdelis, A.; Jurevicius, J.; Fischmeister, R. beta3-adrenergic receptor activation increases human atrial tissue contractility and stimulates the L-type Ca2+ current. J. Clin. Investig. 2008, 118, 3219–3227. [Google Scholar] [CrossRef]
- Christ, T.; Molenaar, P.; Klenowski, P.M.; Ravens, U.; Kaumann, A.J. Human atrial beta1L-adrenoceptor but not beta3-adrenoceptor activation increases force and Ca2+ current at physiological temperature. Br. J. Pharmacol. 2011, 162, 823–839. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.Y.; Lau, C.P.; Chin-Wan Fung, P.; Li, G.R. Evidence for cystic fibrosis transmembrane conductance regulator chloride current in swine ventricular myocytes. J. Mol. Cell. Cardiol. 2007, 42, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Leblais, V.; Demolombe, S.; Vallette, G.; Langin, D.; Baro, I.; Escande, D.; Gauthier, C. beta3-adrenoceptor control the cystic fibrosis transmembrane conductance regulator through a cAMP/protein kinase A-independent pathway. J. Biol. Chem. 1999, 274, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Alhayek, S.; Preuss, C.V. Beta 1 Receptors. In StatPearls [Internet]; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sterin-Borda, L.; Bernabeo, G.; Ganzinelli, S.; Joensen, L.; Borda, E. Role of nitric oxide/cyclic GMP and cyclic AMP in beta3 adrenoceptor- chronotropic response. J. Mol. Cell. Cardiol. 2006, 40, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, G.; Galitzky, J.; Bousquet-Melou, A.; Montastruc, J.L.; Berlan, M. The positive chronotropic effect induced by BRL 37344 and CGP 12177, two beta-3 adrenergic agonists, does not involve cardiac beta adrenoceptors but baroreflex mechanisms. J. Pharmacol. Exp. Ther. 1992, 263, 1083–1090. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, P.; Cheng, Z.; Hao, W.; Wang, R.; Fang, Q.; Cao, J.M. Altered circadian rhythm of cardiac beta3-adrenoceptor activity following myocardial infarction in the rat. Basic Res. Cardiol. 2011, 106, 37–50. [Google Scholar] [CrossRef]
- Stockigt, F.; Brixius, K.; Lickfett, L.; Andrie, R.; Linhart, M.; Nickenig, G.; Schrickel, J.W. Total beta-adrenoceptor knockout slows conduction and reduces inducible arrhythmias in the mouse heart. PLoS ONE 2012, 7, e49203. [Google Scholar] [CrossRef]
- Krauz, K.; Kempinski, M.; Janczak, P.; Momot, K.; Zarebinski, M.; Poprawa, I.; Wojciechowska, M. The Role of Epicardial Adipose Tissue in Acute Coronary Syndromes, Post- Infarct Remodeling and Cardiac Regeneration. Int. J. Mol. Sci. 2024, 25, 3583. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef]
- Babakr, A.A.; Fomison-Nurse, I.C.; van Hout, I.; Aitken-Buck, H.M.; Sugunesegran, R.; Davis, P.J.; Bunton, R.W.; Williams, M.J.A.; Coffey, S.; Stiles, M.K.; et al. Acute interaction between human epicardial adipose tissue and human atrial myocardium induces arrhythmic susceptibility. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E164–E172. [Google Scholar] [CrossRef]
- Samuel, M.; Elsokkari, I.; Sapp, J.L. Ventricular Tachycardia Burden and Mortality: Association or Causality? Can. J. Cardiol. 2022, 38, 454–464. [Google Scholar] [CrossRef]
- Zhou, S.; Tan, A.Y.; Paz, O.; Ogawa, M.; Chou, C.C.; Hayashi, H.; Nihei, M.; Fishbein, M.C.; Chen, L.S.; Lin, S.F.; et al. Antiarrhythmic effects of beta3-adrenergic receptor stimulation in a canine model of ventricular tachycardia. Heart Rhythm. 2008, 5, 289–297. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Huang, H.; Tang, Y.; Yang, B.; Huang, C. Activation of beta3-adrenergic receptor inhibits ventricular arrhythmia in heart failure through calcium handling. Tohoku J. Exp. Med. 2010, 222, 167–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwasaki, Y.K.; Nishida, K.; Kato, T.; Nattel, S. Atrial fibrillation pathophysiology: Implications for management. Circulation 2011, 124, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, R.; Liu, G.; Dong, J.; Zhao, G.; Tian, J.; Sun, J.; Jia, X.; Wei, L.; Wang, Y.; et al. The beta3 Adrenergic Receptor Agonist BRL37344 Exacerbates Atrial Structural Remodeling Through iNOS Uncoupling in Canine Models of Atrial Fibrillation. Cell. Physiol. Biochem. 2016, 38, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.M.; Silva Junior, E.L.D.; Martins, Y.O.; Rocha, H.A.L.; Scanavacca, M.I.; Gutierrez, P.S. Cardiac Autonomic Nervous System Remodeling May Play a Role in Atrial Fibrillation: A Study of the Autonomic Nervous System and Myocardial Receptors. Arq. Bras. Cardiol. 2021, 117, 999–1007. [Google Scholar] [CrossRef]
- van Staveren, L.N.; de Groot, N.M.S. Exploring Refractoriness as an Adjunctive Electrical Biomarker for Staging of Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e018427. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, J.; Zhang, M.; Liu, G.; Wang, X.; Liu, Y.; Yang, N.; Liu, Y.; Zhao, G.; Sun, J.; et al. beta3-Adrenoceptor Impairs Mitochondrial Biogenesis and Energy Metabolism During Rapid Atrial Pacing-Induced Atrial Fibrillation. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 114–126. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, Q.; Huang, K.; Liu, G.; Zhao, J.; Xu, W.; Liu, Y.; Li, W.; Li, Y. Upregulation of beta3-adrenergic receptors contributes to atrial structural remodeling in rapid pacing induced atrial fibrillation canines. Cell. Physiol. Biochem. 2012, 30, 372–381. [Google Scholar] [CrossRef]
- Mulder, B.A.; van Veldhuisen, D.J.; Crijns, H.J.; Bohm, M.; Cohen-Solal, A.; Babalis, D.; Roughton, M.; Flather, M.D.; Coats, A.J.; Van Gelder, I.C. Effect of nebivolol on outcome in elderly patients with heart failure and atrial fibrillation: Insights from SENIORS. Eur. J. Heart Fail. 2012, 14, 1171–1178. [Google Scholar] [CrossRef]
- Dessy, C.; Moniotte, S.; Ghisdal, P.; Havaux, X.; Noirhomme, P.; Balligand, J.L. Endothelial beta3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation 2004, 110, 948–954. [Google Scholar] [CrossRef]
- Togni, M.; Vigorito, F.; Windecker, S.; Abrecht, L.; Wenaweser, P.; Cook, S.; Billinger, M.; Meier, B.; Hess, O.M. Does the beta-blocker nebivolol increase coronary flow reserve? Cardiovasc. Drugs Ther. 2007, 21, 99–108. [Google Scholar] [CrossRef]
- Hung, O.Y.; Molony, D.; Corban, M.T.; Rasoul-Arzrumly, E.; Maynard, C.; Eshtehardi, P.; Dhawan, S.; Timmins, L.H.; Piccinelli, M.; Ahn, S.G.; et al. Comprehensive Assessment of Coronary Plaque Progression With Advanced Intravascular Imaging, Physiological Measures, and Wall Shear Stress: A Pilot Double-Blinded Randomized Controlled Clinical Trial of Nebivolol Versus Atenolol in Nonobstructive Coronary Artery Disease. J. Am. Heart Assoc. 2016, 5, e002764. [Google Scholar] [CrossRef]
- Mercanoglu, G.; Safran, N.; Gungor, M.; Pamukcu, B.; Uzun, H.; Sezgin, C.; Mercanoglu, F.; Fici, F. The effects of nebivolol on apoptosis in a rat infarct model. Circ. J. 2008, 72, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, J.; Garcia-Ruiz, J.M.; Sanz-Rosa, D.; Pun, A.; Garcia-Alvarez, A.; Davidson, S.M.; Fernandez-Friera, L.; Nuno-Ayala, M.; Fernandez-Jimenez, R.; Bernal, J.A.; et al. beta3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res. Cardiol. 2014, 109, 422. [Google Scholar] [CrossRef] [PubMed]
- Salie, R.; Alsalhin, A.K.H.; Marais, E.; Lochner, A. Cardioprotective Effects of Beta3-Adrenergic Receptor (beta3-AR) Pre-, Per-, and Post-treatment in Ischemia-Reperfusion. Cardiovasc. Drugs Ther. 2019, 33, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, L.; Jin, Z.; Gao, G.; Li, H.; Zhang, L.; Zhang, L.; Lu, X.; Hu, L.; Lu, B.; et al. Nebivolol protects against myocardial infarction injury via stimulation of beta 3-adrenergic receptors and nitric oxide signaling. PLoS ONE 2014, 9, e98179. [Google Scholar] [CrossRef]
- Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells 2022, 11, 1553. [Google Scholar] [CrossRef]
- Mendes-Silverio, C.B.; Alexandre, E.M.; Lescano, C.H.; Antunes, E.; Monica, F.Z. Mirabegron, a beta3-adrenoceptor agonist reduced platelet aggregation through cyclic adenosine monophosphate accumulation. Eur. J. Pharmacol. 2018, 829, 79–84. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Wang, Z.; Zhang, J.; Zhou, Z. Myocardial ischemia-reperfusion injury; Molecular mechanisms and prevention. Microvasc. Res. 2023, 149, 104565. [Google Scholar] [CrossRef]
- Calvert, J.W.; Condit, M.E.; Aragon, J.P.; Nicholson, C.K.; Moody, B.F.; Hood, R.L.; Sindler, A.L.; Gundewar, S.; Seals, D.R.; Barouch, L.A.; et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: Role of nitrite and nitrosothiols. Circ. Res. 2011, 108, 1448–1458. [Google Scholar] [CrossRef]
- Barr, L.A.; Lambert, J.P.; Shimizu, Y.; Barouch, L.A.; Naqvi, N.; Calvert, J.W. Exercise training provides cardioprotection by activating and coupling endothelial nitric oxide synthase via a beta3-adrenergic receptor-AMP- activated protein kinase signaling pathway. Med. Gas. Res. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, A.; Battault, S.; Belaidi, E.; Tanguy, S.; Rosselin, M.; Boulghobra, D.; Meyer, G.; Gayrard, S.; Walther, G.; Geny, B.; et al. Exercise does not activate the beta3 adrenergic receptor-eNOS pathway, but reduces inducible NOS expression to protect the heart of obese diabetic mice. Basic. Res. Cardiol. 2016, 111, 40. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.T.; Li, W.M.; Xiu, C.H.; Shen, J.X.; Wang, X.; Wu, S.; Kong, Y.H. Chronic blocking of beta 3-adrenoceptor ameliorates cardiac function in rat model of heart failure. Chin. Med. J. 2007, 120, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Audigane, L.; Persello, A.; Piriou, N.; Ferron, M.; Trochu, J.N.; Lauzier, B.; Gauthier, C.; Rozec, B. Early nebivolol treatment is beneficial in myocardial infarction in rats partly through beta3-adrenoceptor remodelling. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1007–1015. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, X.; Wang, Y.; Wang, F.; Yu, P.; Shen, J.; Fu, L. Effects of carvedilol on ventricular remodeling and the expression of beta3-adrenergic receptor in a diabetic rat model subjected myocardial infarction. Int. J. Cardiol. 2016, 222, 178–184. [Google Scholar] [CrossRef]
- Doughty, R.N.; Whalley, G.A.; Walsh, H.A.; Gamble, G.D.; Lopez-Sendon, J.; Sharpe, N. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: The CAPRICORN Echo Substudy. Circulation 2004, 109, 201–206. [Google Scholar] [CrossRef]
- Woo, A.Y.; Xiao, R.P. β-Adrenergic receptor subtype signaling in heart: From bench to bedside. Acta Pharmacol. Sin. 2012, 33, 335–341. [Google Scholar] [CrossRef]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S.; et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef]
- Bristow, M.R.; Minobe, W.A.; Raynolds, M.V.; Port, J.D.; Rasmussen, R.; Ray, P.E.; Feldman, A.M. Reduced beta 1 receptor messenger RNA abundance in the failing human heart. J. Clin. Investig. 1993, 92, 2737–2745. [Google Scholar] [CrossRef]
- Bristow, M.R.; Hershberger, R.E.; Port, J.D.; Minobe, W.; Rasmussen, R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol. Pharmacol. 1989, 35, 295–303. [Google Scholar] [CrossRef]
- Moniotte, S.; Kobzik, L.; Feron, O.; Trochu, J.N.; Gauthier, C.; Balligand, J.L. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 2001, 103, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Kayki-Mutlu, G.; Karaomerlioglu, I.; Arioglu-Inan, E.; Altan, V.M. Beta-3 adrenoceptors: A potential therapeutic target for heart disease. Eur. J. Pharmacol. 2019, 858, 172468. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.J.; Zhang, Z.S.; Onishi, K.; Ukai, T.; Sane, D.C.; Cheng, C.P. Upregulation of functional beta(3)-adrenergic receptor in the failing canine myocardium. Circ. Res. 2001, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Hasegawa, H.; Cheng, H.J.; Little, W.C.; Cheng, C.P. Endogenous beta3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2425–H2433. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, F.; Liu, J.B.; He, Y.; Li, B.; Jiang, Z.F.; Wu, T.G.; Wang, L.X. Upregulation of beta3-adrenergic receptor expression in the atrium of rats with chronic heart failure. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 133–137. [Google Scholar] [CrossRef]
- Post, S.R.; Hammond, H.K.; Insel, P.A. Beta-adrenergic receptors and receptor signaling in heart failure. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 343–360. [Google Scholar] [CrossRef]
- Spinale, F.G.; Tempel, G.E.; Mukherjee, R.; Eble, D.M.; Brown, R.; Vacchiano, C.A.; Zile, M.R. Cellular and molecular alterations in the beta adrenergic system with cardiomyopathy induced by tachycardia. Cardiovasc. Res. 1994, 28, 1243–1250. [Google Scholar] [CrossRef]
- Zolk, O.; Kouchi, I.; Schnabel, P.; Bohm, M. Heterotrimeric G proteins in heart disease. Can. J. Physiol. Pharmacol. 2000, 78, 187–198. [Google Scholar] [CrossRef]
- Perrone, M.G.; Scilimati, A. beta(3)-Adrenoceptor ligand development history through patent review. Expert Opin. Ther. Pat. 2011, 21, 505–536. [Google Scholar] [CrossRef]
- Angelone, T.; Filice, E.; Quintieri, A.M.; Imbrogno, S.; Recchia, A.; Pulera, E.; Mannarino, C.; Pellegrino, D.; Cerra, M.C. Beta3-adrenoceptors modulate left ventricular relaxation in the rat heart via the NO-cGMP-PKG pathway. Acta Physiol 2008, 193, 229–239. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Cheng, H.J.; Onishi, K.; Ohte, N.; Wannenburg, T.; Cheng, C.P. Enhanced inhibition of L-type Ca2+ current by beta3-adrenergic stimulation in failing rat heart. J. Pharmacol. Exp. Ther. 2005, 315, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Sernia, C.; Newling, R.; Fletcher, P. Cardiac responses after norepinephrine-induced ventricular hypertrophy in rats. J. Cardiovasc. Pharmacol. 1992, 20, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.H.; Figtree, G.A.; Krum, H.; Bundgaard, H. The use of beta3-adrenergic receptor agonists in the treatment of heart failure. Curr. Opin. Investig. Drugs 2009, 10, 955–962. [Google Scholar] [PubMed]
- Pott, C.; Steinritz, D.; Napp, A.; Bloch, W.; Schwinger, R.H.; Brixius, K. [On the function of beta3-adrenoceptors in the human heart: Signal transduction, inotropic effect and therapeutic prospects]. Wien. Med. Wochenschr. 2006, 156, 451–458. [Google Scholar] [CrossRef]
- Skeberdis, V.A. Structure and function of beta3-adrenergic receptors. Medicina 2004, 40, 407–413. [Google Scholar]
- Moens, A.L.; Leyton-Mange, J.S.; Niu, X.; Yang, R.; Cingolani, O.; Arkenbout, E.K.; Champion, H.C.; Bedja, D.; Gabrielson, K.L.; Chen, J.; et al. Adverse ventricular remodeling and exacerbated NOS uncoupling from pressure-overload in mice lacking the beta3-adrenoreceptor. J. Mol. Cell. Cardiol. 2009, 47, 576–585. [Google Scholar] [CrossRef]
- Niu, X.; Watts, V.L.; Cingolani, O.H.; Sivakumaran, V.; Leyton-Mange, J.S.; Ellis, C.L.; Miller, K.L.; Vandegaer, K.; Bedja, D.; Gabrielson, K.L.; et al. Cardioprotective effect of beta-3 adrenergic receptor agonism: Role of neuronal nitric oxide synthase. J. Am. Coll. Cardiol. 2012, 59, 1979–1987, Erratum in J. Am. Coll. Cardiol. 2012, 60, 481. [Google Scholar] [CrossRef]
- Hermida, N.; Michel, L.; Esfahani, H.; Dubois-Deruy, E.; Hammond, J.; Bouzin, C.; Markl, A.; Colin, H.; Steenbergen, A.V.; De Meester, C.; et al. Cardiac myocyte beta3-adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress-dependent paracrine signaling. Eur. Heart J. 2018, 39, 888–898. [Google Scholar] [CrossRef]
- Belge, C.; Hammond, J.; Dubois-Deruy, E.; Manoury, B.; Hamelet, J.; Beauloye, C.; Markl, A.; Pouleur, A.C.; Bertrand, L.; Esfahani, H.; et al. Enhanced expression of beta3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 2014, 129, 451–462. [Google Scholar] [CrossRef]
- Kamiya, M.; Asai, K.; Maejima, Y.; Shirakabe, A.; Murai, K.; Noma, S.; Komiyama, H.; Sato, N.; Mizuno, K.; Shimizu, W. beta 3-Adrenergic Receptor Agonist Prevents Diastolic Dysfunction in an Angiotensin II-Induced Cardiomyopathy Mouse Model. J. Pharmacol. Exp. Ther. 2021, 376, 473–481. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, J.; Xin, J.; Feng, Y.; Hu, G.; Shen, J.; Li, M.; Zhang, Y.; Xiao, H.; Wang, L. beta3-adrenergic receptor activation induces TGFbeta1 expression in cardiomyocytes via the PKG/JNK/c-Jun pathway. Biochem. Biophys. Res. Commun. 2018, 503, 146–151. [Google Scholar] [CrossRef]
- Xie, X.J.; Li, C.Q. Chrysophanol Protects Against Acute Heart Failure by Inhibiting JNK1/2 Pathway in Rats. Med. Sci. Monit. 2020, 26, e926392. [Google Scholar] [CrossRef]
- Lin, J.R.; Ding, L.L.; Xu, L.; Huang, J.; Zhang, Z.B.; Chen, X.H.; Cheng, Y.W.; Ruan, C.C.; Gao, P.J. Brown Adipocyte ADRB3 Mediates Cardioprotection via Suppressing Exosomal iNOS. Circ. Res. 2022, 131, 133–147. [Google Scholar] [CrossRef]
- Wu, Q.Q.; Xiao, Y.; Duan, M.X.; Yuan, Y.; Jiang, X.H.; Yang, Z.; Liao, H.H.; Deng, W.; Tang, Q.Z. Aucubin protects against pressure overload-induced cardiac remodelling via the beta3 -adrenoceptor-neuronal NOS cascades. Br. J. Pharmacol. 2018, 175, 1548–1566. [Google Scholar] [CrossRef]
- Wisenbaugh, T.; Katz, I.; Davis, J.; Essop, R.; Skoularigis, J.; Middlemost, S.; Rothlisberger, C.; Skudicky, D.; Sareli, P. Long-term (3-month) effects of a new beta-blocker (nebivolol) on cardiac performance in dilated cardiomyopathy. J. Am. Coll. Cardiol. 1993, 21, 1094–1100. [Google Scholar] [CrossRef]
- Flather, M.D.; Shibata, M.C.; Coats, A.J.; Van Veldhuisen, D.J.; Parkhomenko, A.; Borbola, J.; Cohen-Solal, A.; Dumitrascu, D.; Ferrari, R.; Lechat, P.; et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2005, 26, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Kamp, O.; Metra, M.; Bugatti, S.; Bettari, L.; Dei Cas, A.; Petrini, N.; Dei Cas, L. Nebivolol: Haemodynamic effects and clinical significance of combined beta-blockade and nitric oxide release. Drugs 2010, 70, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Zhang, K.; Gupta, R.C.; Xu, J.; Singh-Gupta, V.; Ma, M.; Stauber, K.; Nguyen, N.; Adams, J. Intravenous Infusion of the beta3-Adrenergic Receptor Antagonist APD418 Improves Left Ventricular Systolic Function in Dogs With Systolic Heart Failure. J. Card. Fail. 2021, 27, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Napp, A.; Brixius, K.; Pott, C.; Ziskoven, C.; Boelck, B.; Mehlhorn, U.; Schwinger, R.H.; Bloch, W. Effects of the beta3-adrenergic agonist BRL 37344 on endothelial nitric oxide synthase phosphorylation and force of contraction in human failing myocardium. J Card Fail 2009, 15, 57–67. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, T.G.; Jiang, Z.F.; Chen, G.W.; Lin, Y.; Wang, L.X. Effect of beta-blockers on beta3-adrenoceptor expression in chronic heart failure. Cardiovasc. Drugs Ther. 2007, 21, 85–90. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A.; Swedberg, K.; Cleland, J.G.; Di Lenarda, A.; Hanrath, P.; Komajda, M.; Lubsen, J.; Lutiger, B.; Metra, M.; Remme, W.J.; et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): Randomised controlled trial. Lancet 2003, 362, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.H.; Figtree, G. “Don’t flog the heart!”—Development of specific drug therapies for heart failure. Crit. Care Resusc. 2007, 9, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.K.; Liu, C.C.; Hamilton, E.J.; Garcia, A.; Fry, N.A.; Hannam, W.; Figtree, G.A.; Rasmussen, H.H. Stimulation of the cardiac myocyte Na+-K+ pump due to reversal of its constitutive oxidative inhibition. Am. J. Physiol. Cell Physiol. 2015, 309, C239–C250. [Google Scholar] [CrossRef] [PubMed]
- Balligand, J.L. Cardiac beta3-adrenergic receptors in the clinical arena: The end of the beginning. Eur. J. Heart Fail. 2017, 19, 576–578. [Google Scholar] [CrossRef]
- Bundgaard, H.; Liu, C.C.; Garcia, A.; Hamilton, E.J.; Huang, Y.; Chia, K.K.; Hunyor, S.N.; Figtree, G.A.; Rasmussen, H.H. beta(3) adrenergic stimulation of the cardiac Na+-K+ pump by reversal of an inhibitory oxidative modification. Circulation 2010, 122, 2699–2708. [Google Scholar] [CrossRef]
- Maier, L.S. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: Inhibition of late I(Na) using ranolazine. J. Cardiovasc. Pharmacol. 2009, 54, 279–286. [Google Scholar] [CrossRef]
- Kayki Mutlu, G.; Arioglu Inan, E.; Karaomerlioglu, I.; Altan, V.M.; Yersal, N.; Korkusuz, P.; Rocchetti, M.; Zaza, A. Role of the beta3-adrenergic receptor subtype in catecholamine-induced myocardial remodeling. Mol. Cell. Biochem. 2018, 446, 149–160. [Google Scholar] [CrossRef]
- Moniotte, S.; Belge, C.; Sekkali, B.; Massion, P.B.; Rozec, B.; Dessy, C.; Balligand, J.L. Sepsis is associated with an upregulation of functional beta3 adrenoceptors in the myocardium. Eur. J. Heart Fail. 2007, 9, 1163–1171. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Okada, M.; Ijiri, E.; Koga, D.; Watanabe, T.; Hayashi, K.; Kashiwagi, Y.; Fujita, S.; Hasebe, N. beta3-Adrenergic receptor blockade reduces mortality in endotoxin-induced heart failure by suppressing induced nitric oxide synthase and saving cardiac metabolism. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H283–H294. [Google Scholar] [CrossRef]
- Ichinose, F.; Buys, E.S.; Neilan, T.G.; Furutani, E.M.; Morgan, J.G.; Jassal, D.S.; Graveline, A.R.; Searles, R.J.; Lim, C.C.; Kaneki, M.; et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ. Res. 2007, 100, 130–139. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Ma, X.; Bai, K.; Wang, L.; Yan, Z.; Lv, T.; Zhao, Z.; Zhao, R.; Liu, H. Autoantibodies against the beta3-adrenoceptor protect from cardiac dysfunction in a rat model of pressure overload. PLoS ONE 2013, 8, e78207. [Google Scholar] [CrossRef][Green Version]
- Li, M.X.; Wang, X.L.; Tang, J.N.; Liu, X.J.; Tian, J.; Yan, L.; Liu, H.R. Distribution and property of anti-beta3-adrenoceptor autoantibody in patients with heart failure. Zhonghua Xin Xue Guan Bing Za Zhi 2005, 33, 1114–1118. [Google Scholar] [PubMed][Green Version]
- Markousis-Mavrogenis, G.; Minich, W.B.; Al-Mubarak, A.A.; Anker, S.D.; Cleland, J.G.F.; Dickstein, K.; Lang, C.C.; Ng, L.L.; Samani, N.J.; Zannad, F.; et al. Clinical and prognostic associations of autoantibodies recognizing adrenergic/muscarinic receptors in patients with heart failure. Cardiovasc. Res. 2023, 119, 1690–1705. [Google Scholar] [CrossRef] [PubMed]
- Merlet, N.; Piriou, N.; Rozec, B.; Grabherr, A.; Lauzier, B.; Trochu, J.N.; Gauthier, C. Increased beta2-adrenoceptors in doxorubicin-induced cardiomyopathy in rat. PLoS ONE 2013, 8, e64711. [Google Scholar] [CrossRef] [PubMed]
- Freiwan, M.; Kovacs, M.G.; Kovacs, Z.Z.A.; Szucs, G.; Dinh, H.; Losonczi, R.; Siska, A.; Kriston, A.; Kovacs, F.; Horvath, P.; et al. Investigation of the Antiremodeling Effects of Losartan, Mirabegron and Their Combination on the Development of Doxorubicin-Induced Chronic Cardiotoxicity in a Rat Model. Int. J. Mol. Sci. 2022, 23, 2201. [Google Scholar] [CrossRef]
- Baker, A.J.; Redfern, C.H.; Harwood, M.D.; Simpson, P.C.; Conklin, B.R. Abnormal contraction caused by expression of G(i)-coupled receptor in transgenic model of dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1653–H1659. [Google Scholar] [CrossRef]
- Amour, J.; Loyer, X.; Le Guen, M.; Mabrouk, N.; David, J.S.; Camors, E.; Carusio, N.; Vivien, B.; Andriantsitohaina, R.; Heymes, C.; et al. Altered contractile response due to increased beta3-adrenoceptor stimulation in diabetic cardiomyopathy: The role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology 2007, 107, 452–460. [Google Scholar] [CrossRef]
- Sharma, V.; Parsons, H.; Allard, M.F.; McNeill, J.H. Metoprolol increases the expression of beta(3)-adrenoceptors in the diabetic heart: Effects on nitric oxide signaling and forkhead transcription factor-3. Eur. J. Pharmacol. 2008, 595, 44–51. [Google Scholar] [CrossRef]
- Lahaye Sle, D.; Gratas-Delamarche, A.; Malarde, L.; Vincent, S.; Zguira, M.S.; Morel, S.L.; Delamarche, P.; Zouhal, H.; Carre, F.; Bekono, F.R. Intense exercise training induces adaptation in expression and responsiveness of cardiac beta-adrenoceptors in diabetic rats. Cardiovasc. Diabetol. 2010, 9, 72. [Google Scholar] [CrossRef]
- Masutani, S.; Cheng, H.J.; Morimoto, A.; Hasegawa, H.; Han, Q.H.; Little, W.C.; Cheng, C.P. beta3-Adrenergic receptor antagonist improves exercise performance in pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H923–H930. [Google Scholar] [CrossRef]
- Wang, B.; Xu, M.; Li, W.; Li, X.; Zheng, Q.; Niu, X. Aerobic exercise protects against pressure overload-induced cardiac dysfunction and hypertrophy via beta3-AR-nNOS-NO activation. PLoS ONE 2017, 12, e0179648. [Google Scholar] [CrossRef]
- Yamashita, K.; Ito, K.; Endo, J.; Matsuhashi, T.; Katsumata, Y.; Yamamoto, T.; Shirakawa, K.; Isobe, S.; Kataoka, M.; Yoshida, N.; et al. Adrenal cortex hypoxia modulates aldosterone production in heart failure. Biochem. Biophys. Res. Commun. 2020, 524, 184–189. [Google Scholar] [CrossRef]
- Balligand, J.L.; Brito, D.; Brosteanu, O.; Casadei, B.; Depoix, C.; Edelmann, F.; Ferreira, V.; Filippatos, G.; Gerber, B.; Gruson, D.; et al. Repurposing the beta3-Adrenergic Receptor Agonist Mirabegron in Patients With Structural Cardiac Disease: The Beta3-LVH Phase 2b Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 1031–1040. [Google Scholar] [CrossRef]
- Bundgaard, H.; Axelsson, A.; Hartvig Thomsen, J.; Sorgaard, M.; Kofoed, K.F.; Hasselbalch, R.; Fry, N.A.; Valeur, N.; Boesgaard, S.; Gustafsson, F.; et al. The first-in-man randomized trial of a beta3 adrenoceptor agonist in chronic heart failure: The BEAT-HF trial. Eur. J. Heart Fail. 2017, 19, 566–575. [Google Scholar] [CrossRef]
- Bahrami, H.S.Z.; Hasselbalch, R.B.; Soholm, H.; Thomsen, J.H.; Sorgaard, M.; Kofoed, K.F.; Valeur, N.; Boesgaard, S.; Fry, N.A.S.; Moller, J.E.; et al. First-In-Man Trial of beta3-Adrenoceptor Agonist Treatment in Chronic Heart Failure: Impact on Diastolic Function. J. Cardiovasc. Pharmacol. 2024, 83, 466–473. [Google Scholar] [CrossRef]
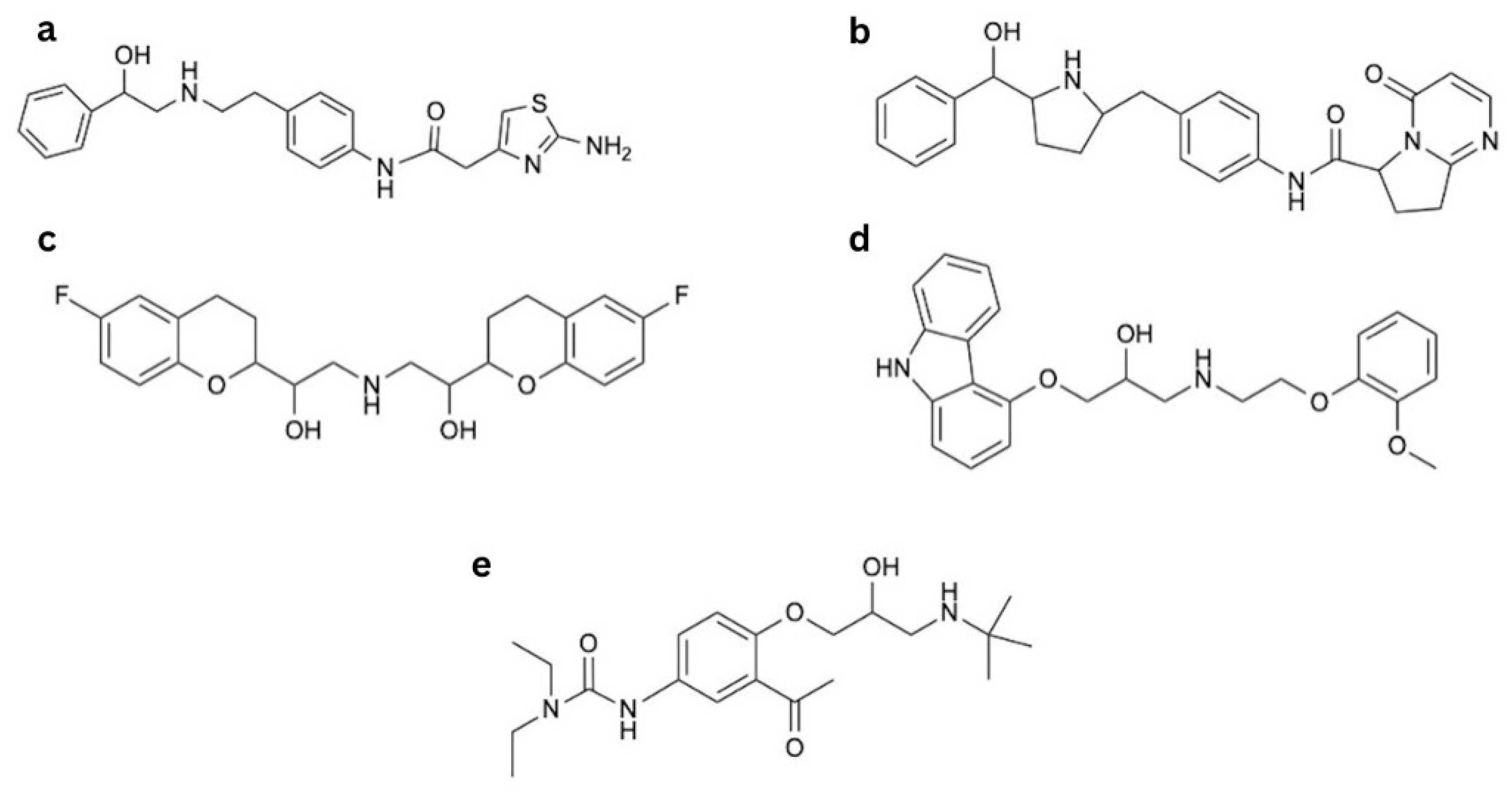
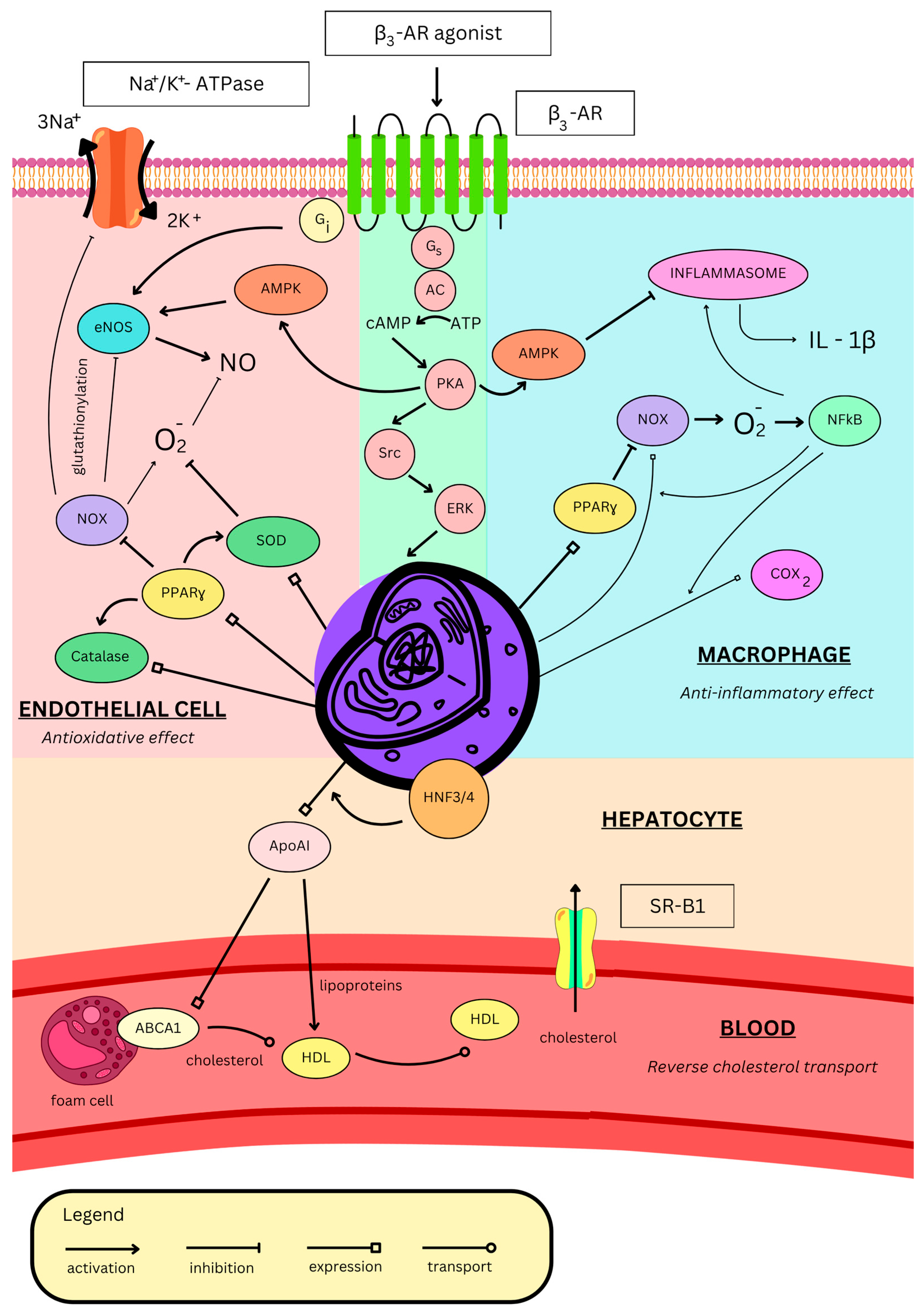
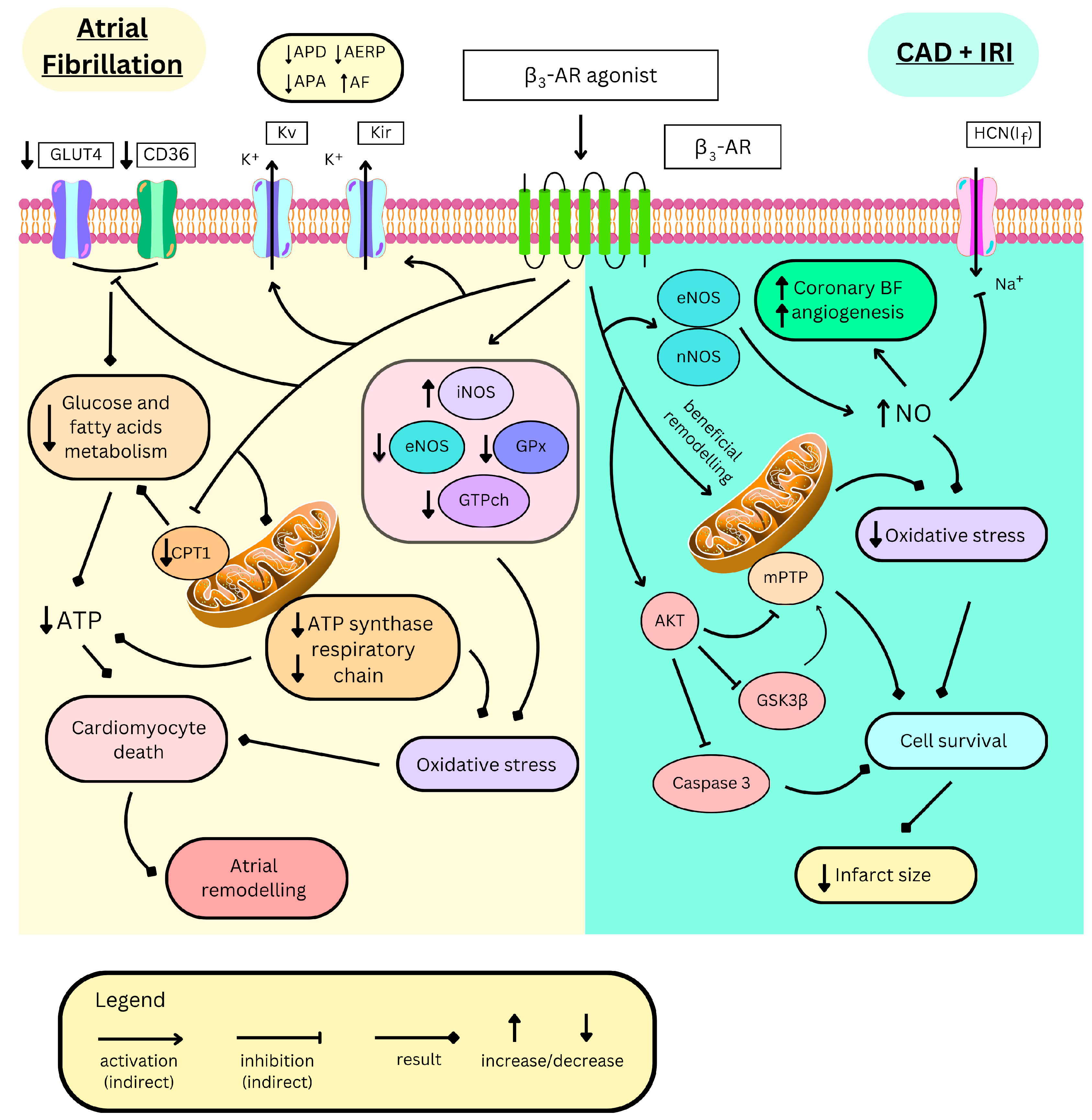

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempiński, M.; Jańczak, P.; Porębska, A.; Zawadzka, P.S.; Jastrzębska, P.; Granat, M.M.; Wojciechowska, M. Therapeutic Potential of the β3-Adrenergic Receptor and Its Ligands in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 11844. https://doi.org/10.3390/ijms262411844
Kempiński M, Jańczak P, Porębska A, Zawadzka PS, Jastrzębska P, Granat MM, Wojciechowska M. Therapeutic Potential of the β3-Adrenergic Receptor and Its Ligands in Cardiovascular Diseases. International Journal of Molecular Sciences. 2025; 26(24):11844. https://doi.org/10.3390/ijms262411844
Chicago/Turabian StyleKempiński, Marcel, Paweł Jańczak, Adrianna Porębska, Patrycja Sandra Zawadzka, Paulina Jastrzębska, Marcin Mateusz Granat, and Małgorzata Wojciechowska. 2025. "Therapeutic Potential of the β3-Adrenergic Receptor and Its Ligands in Cardiovascular Diseases" International Journal of Molecular Sciences 26, no. 24: 11844. https://doi.org/10.3390/ijms262411844
APA StyleKempiński, M., Jańczak, P., Porębska, A., Zawadzka, P. S., Jastrzębska, P., Granat, M. M., & Wojciechowska, M. (2025). Therapeutic Potential of the β3-Adrenergic Receptor and Its Ligands in Cardiovascular Diseases. International Journal of Molecular Sciences, 26(24), 11844. https://doi.org/10.3390/ijms262411844






