Untargeted Metabolomic Study for Urinary Characterization of Adult Patients with Phenylketonuria
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Population
| Analysis Cohort | PKU (n = 36) | Controls (n = 34) | p-Value 3 |
|---|---|---|---|
| Anthropometric Data and Demographic Factors | |||
| Age [mean (SD)], y | 35.17 (9.77) | 33.11 (9.44) | 0.373 |
| Female [n (%)] | 15 (41.7) | 21 (61.8) | 0.093 |
| BMI [mean (SD)], kg/m2 | 24.83 (4.27) | 24.13 (3.35) | 0.513 |
| Classical PKU 1 | - | ||
| Early dx, yes [n (%)] | 28 (77.8) | - | |
| Late infant dx, yes [n (%)] | 4 (11.1) | - | |
| Late adult dx, yes [n (%)] | 4 (11.1) | - | |
| Adequate metabolic control 2 | 14 (39) | - | - |
| Dietary habits | |||
| Energy intake [mean (SD)], kcal/day | 1882.34 (501.03) | 1859.02 (357.71) | 0.827 |
| PS energy intake [mean (SD)], kcal/day | 453.27 (187.90) | - | - |
| Total protein intake [median (IQR)], g/day | 78.97 (22.76) | 78.16 (24.24) | 0.224 |
| Natural protein intake [mean (SD)], g/day | 22.15 (12.12) | 75.73 (18.90) | <0.001 |
| Total protein intake [mean (SD)], % | 17.93 (4.82) | 16.61 (3.44) | 0.202 |
| Total fat intake [mean (SD)], % | 28.81 (6.86) | 44.57 (6.21) | <0.001 |
| Total carbohydrate intake [median (IQR)], % | 53.64 (11.03) | 40.31 (9.18) | <0.001 |
| Vitamin B6 intake [median (IQR)], mg/day | 3.10 (1.41) | 1.35 (1.34) | <0.001 |
| Vitamin E intake [median (IQR)], mg/day | 17.05 (8.35) | 9.59 (4.89) | <0.001 |
| Meat consumption, Yes [n (%)] | 5 (13.9) | 32 (94.1) | <0.001 |
| L-carnitine supplementation | 5 (13.9) | - | - |
| Biochemical parameters | |||
| Phe [median (IQR)], µmol/L | 847.60 (519.0) | 55.95 (15.7) | <0.001 |
| Tyr [median (IQR)], µmol/L | 47.35 (26.9) | 53.65 (28.1) | 0.143 |
| Total carnitine [median (IQR)], µmol/L | 40.35 (11.7) | 40 (12) | 0.855 |
| Free carnitine [mean (SD)], µmol/L | 33.96 (8.72) | 33.90 (7.54) | 0.890 |
| Leucine [median (IQR)], µmol/L | 66.87 (12.88) | 79.75 (23.65) | <0.001 |
| Isoleucine [median (IQR)], µmol/L | 28.70 (13.07) | 31.05 (11.73) | 0.062 |
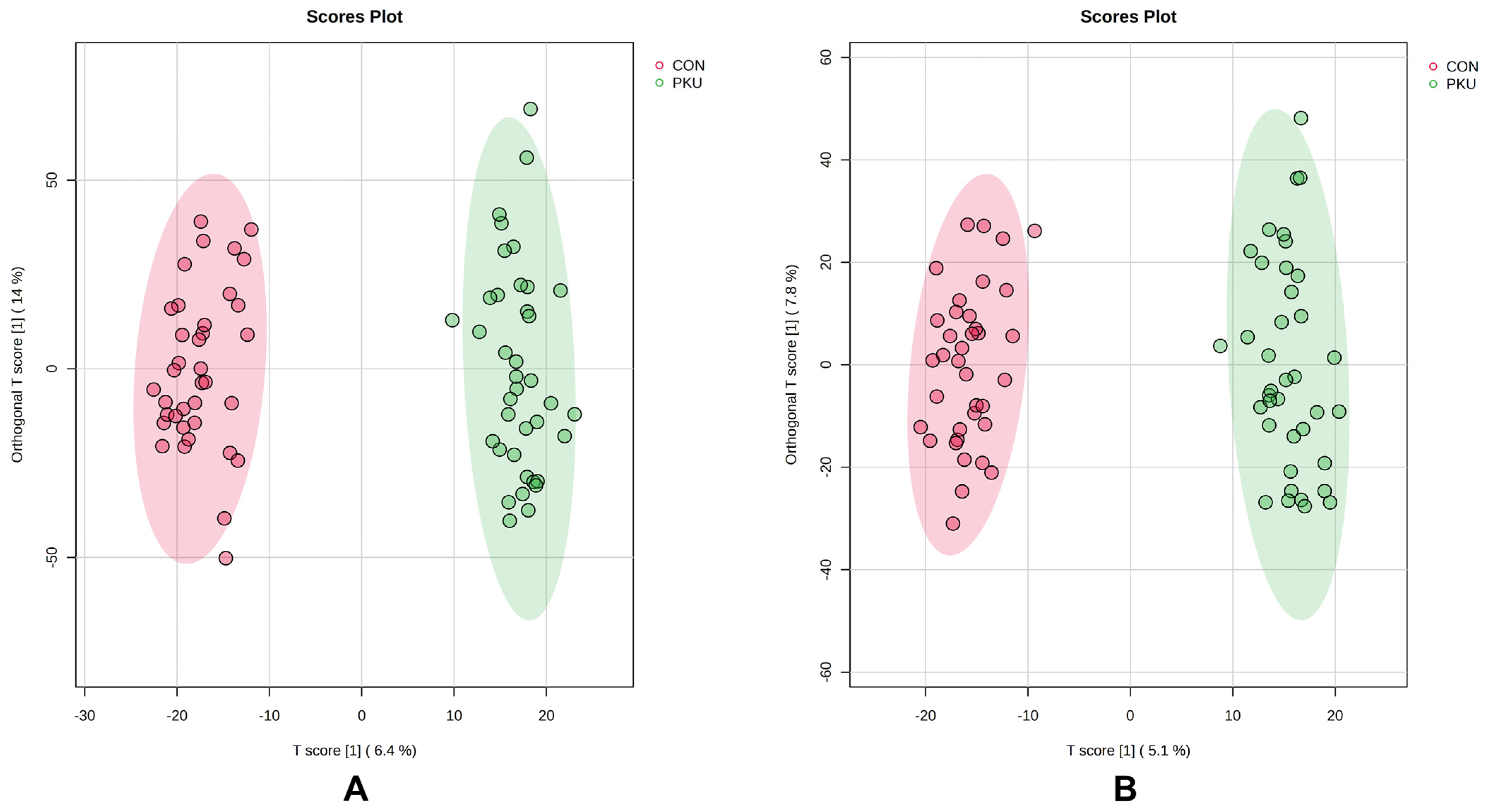
2.2. Multivariate Analysis of Urine Samples
2.3. Identification of Differential Metabolites
2.3.1. Phenylalanine and Phenylalanine Metabolism Metabolites
2.3.2. Nucleoside Compounds
2.3.3. Pteridine Compounds
2.3.4. Tryptophan and Tryptophan Metabolism Compounds
2.3.5. Leucine-Derived Compounds
2.3.6. Carnitine Metabolites
2.3.7. Micronutrients and Dietary Metabolites
2.3.8. Glycine Metabolites and Other Amino Acid Compounds
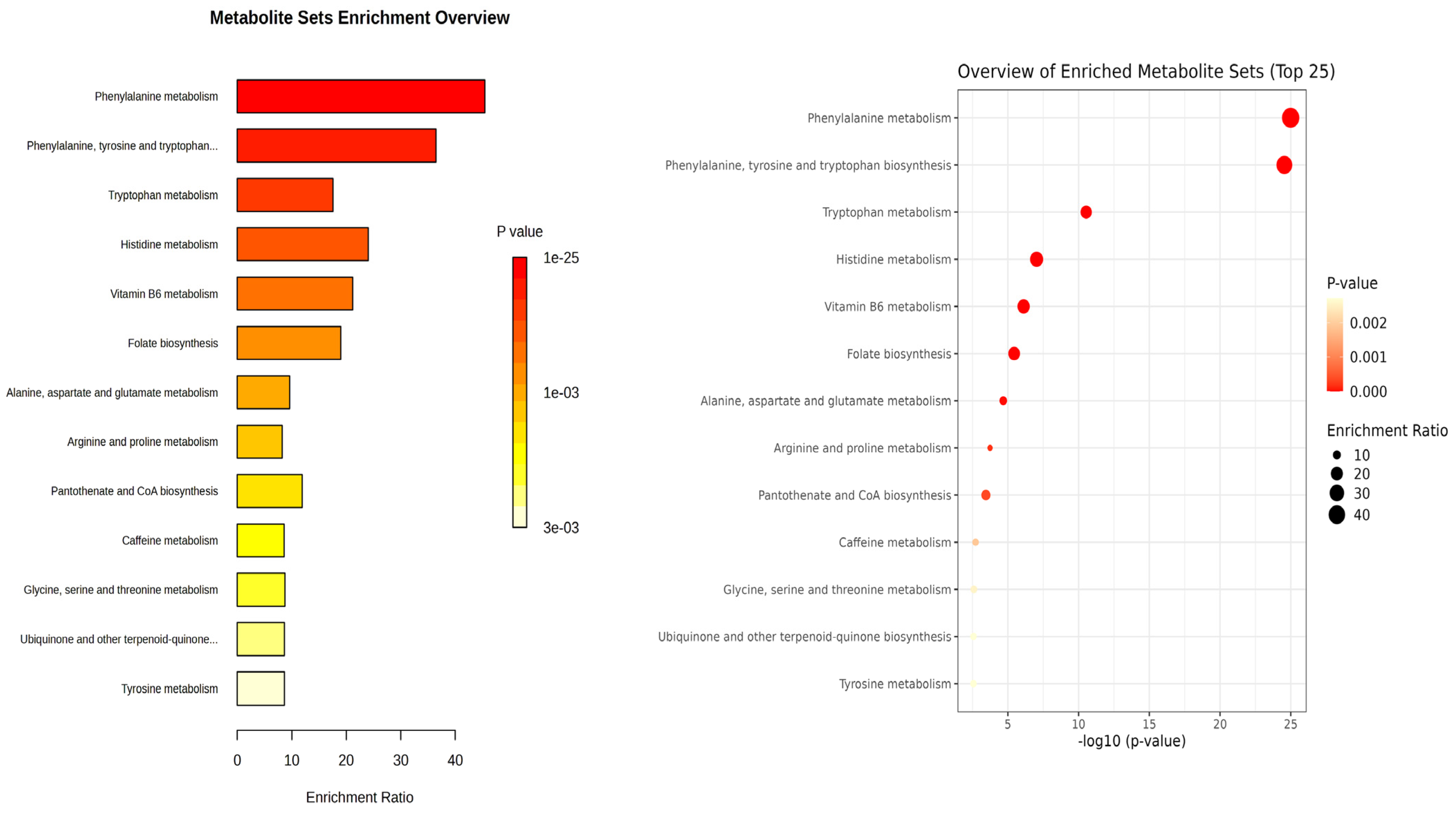
2.4. Enrichment Analysis of Differential Metabolites
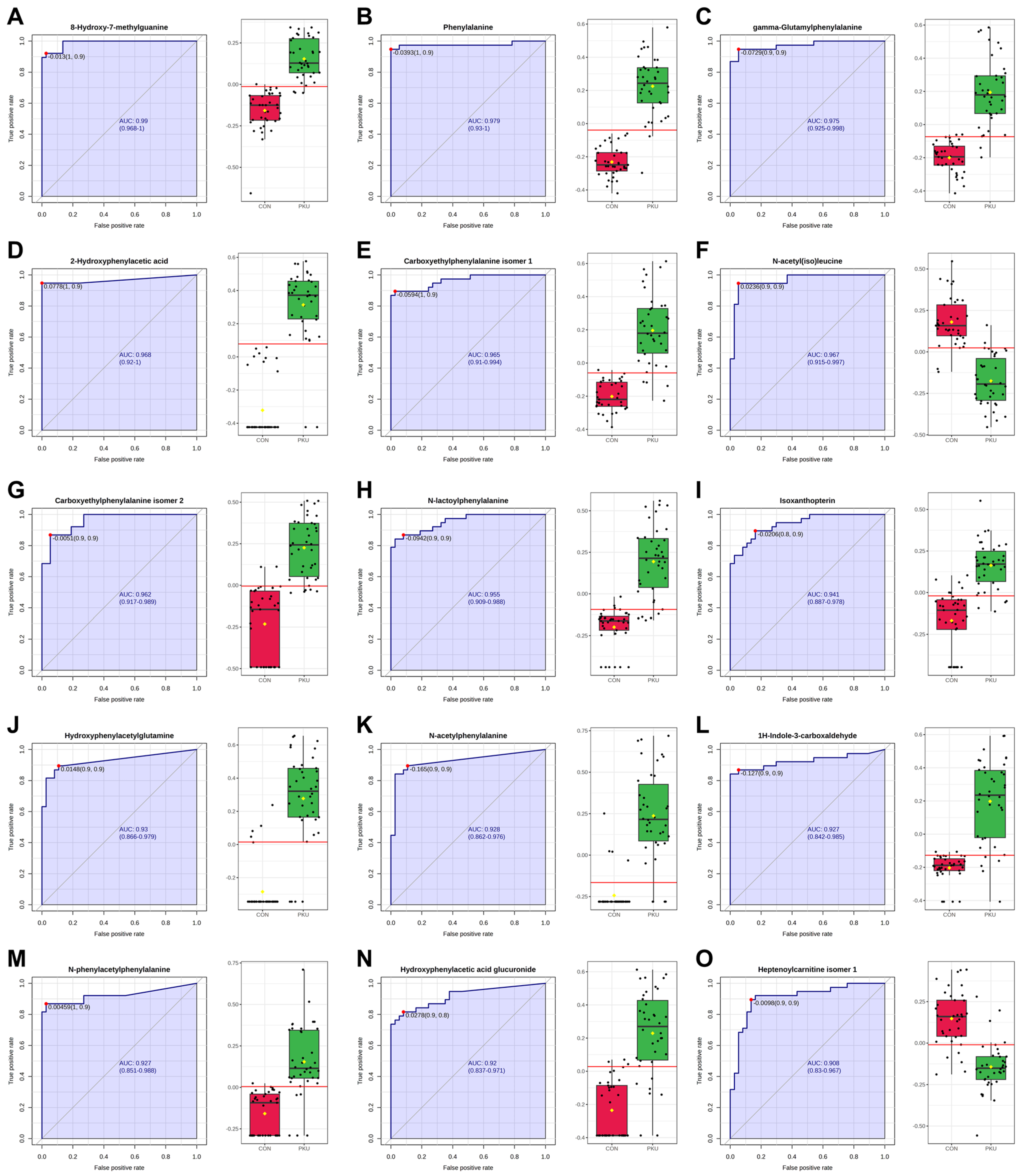
2.5. ROC Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Sample Preparation
4.3. HPLC-QTOF-MS Analysis
4.4. Data Processing and Statistical Analysis
4.5. Metabolite Identification
4.6. Enrichment and Biomarker Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-MG | 7-Methylguanine |
| AUC | Area Under the Curve |
| BH4 | Tetrahydrobiopterin |
| BMI | Body Mass Index |
| ESI | Electrospray Ionization |
| FDR | False Discovery Rate |
| GMP | Glycomacropeptide |
| HMDB | Human Metabolome DataBase |
| HPA | Hyperphenylalaninemia |
| HPLC-QTOF-MS | High-Performance Liquid Chromatography coupled to an Ion Mobility Quadrupole Time-Of-Flight Mass Spectrometer |
| IEM | Inborn Error of Metabolism |
| Ile | Isoleucine |
| IQR | Interquartile Range |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Leu | Leucine |
| LNAAs | Large Neutral Amino Acids |
| MAIT | Metabolite Automatic Identification Toolkit |
| MS | Mass Spectrometry |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PAH | Phenylalanine Hydroxylase |
| PCA | Principal Component Analysis |
| Phe | Phenylalanine |
| PKU | Phenylketonuria |
| PS | Protein Substitutes |
| QC | Quality Control |
| RF | Random Forest |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| VIP | Variable Importance in Projection |
References
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Beblo, S.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Coşkun, T.; et al. European Guidelines on Diagnosis and Treatment of Phenylketonuria: First Revision. Mol. Genet. Metab. 2025, 145, 109125. [Google Scholar] [CrossRef] [PubMed]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; Van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef]
- MacDonald, A.; Van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU Dietary Handbook to Accompany PKU Guidelines. Orphanet J. Rare Dis. 2020, 15, 171, Correction in Orphanet J. Rare Dis. 2020, 15, 230. https://doi.org/10.1186/s13023-020-01486-6. [Google Scholar] [CrossRef]
- Rondanelli, M.; Porta, F.; Gasparri, C.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Patelli, Z.; Peroni, G.; Pirola, M.; et al. A Food Pyramid for Adult Patients with Phenylketonuria and a Systematic Review on the Current Evidences Regarding the Optimal Dietary Treatment of Adult Patients with PKU. Clin. Nutr. 2023, 42, 732–763. [Google Scholar] [CrossRef]
- Mordaunt, D.; Cox, D.; Fuller, M. Metabolomics to Improve the Diagnostic Efficiency of Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 1195. [Google Scholar] [CrossRef]
- Miller, M.J.; Kennedy, A.D.; Eckhart, A.D.; Burrage, L.C.; Wulff, J.E.; Miller, L.A.D.; Milburn, M.V.; Ryals, J.A.; Beaudet, A.L.; Sun, Q.; et al. Untargeted Metabolomic Analysis for the Clinical Screening of Inborn Errors of Metabolism. J. Inherit. Metab. Dis. 2015, 38, 1029–1039, Erratum in J. Inherit. Metab. Dis. 2016, 39, 757. https://doi.org/10.1007/s10545-016-9944-y. [Google Scholar] [CrossRef]
- Bonte, R.; Bongaerts, M.; Demirdas, S.; Langendonk, J.G.; Huidekoper, H.H.; Williams, M.; Onkenhout, W.; Jacobs, E.H.; Blom, H.J.; Ruijter, G.J.G. Untargeted Metabolomics-Based Screening Method for Inborn Errors of Metabolism Using Semi-Automatic Sample Preparation with an UHPLC-Orbitrap-MS Platform. Metabolites 2019, 9, 289. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.; Shanmuganathan, M.; Hayashi, M.; Potter, M.; Britz-Mckibbin, P. Metabolomics for Improved Treatment Monitoring of Phenylketonuria: Urinary Biomarkers for Non-Invasive Assessment of Dietary Adherence and Nutritional Deficiencies. Analyst 2019, 144, 6595–6608. [Google Scholar] [CrossRef]
- Sailer, M.; Elizondo, G.; Martin, J.; Harding, C.O.; Gillingham, M.B. Nutrient Intake, Body Composition, and Blood Phenylalanine Control in Children with Phenylketonuria Compared to Healthy Controls. Mol. Genet. Metab. Rep. 2020, 23, 100599. [Google Scholar] [CrossRef]
- Evans, S.; Daly, A.; Wildgoose, J.; Cochrane, B.; Chahal, S.; Ashmore, C.; Loveridge, N.; Macdonald, A. Growth, Protein and Energy Intake in Children with PKU Taking a Weaning Protein Substitute in the First Two Years of Life: A Case-Control Study. Nutrients 2019, 11, 552. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The Complete European Guidelines on Phenylketonuria: Diagnosis and Treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Xiong, X.; Sheng, X.; Liu, D.; Zeng, T.; Peng, Y.; Wang, Y. A GC/MS-Based Metabolomic Approach for Reliable Diagnosis of Phenylketonuria. Anal. Bioanal. Chem. 2015, 407, 8825–8833. [Google Scholar] [CrossRef]
- Li, V.L.; Xiao, S.; Schlosser, P.; Scherer, N.; Wiggenhorn, A.L.; Spaas, J.; Tung, A.S.H.; Karoly, E.D.; Köttgen, A.; Long, J.Z. SLC17A1/3 Transporters Mediate Renal Excretion of Lac-Phe in Mice and Humans. Nat. Commun. 2024, 15, 6895. [Google Scholar] [CrossRef]
- ChemSpider N-(Ethoxyacetyl)Phenylalanine. Available online: https://www.chemspider.com/Chemical-Structure.239993.html (accessed on 21 March 2025).
- Fismen, L.; Eide, T.; Djurhuus, R.; Svardal, A.M. Simultaneous Quantification of Tetrahydrobiopterin, Dihydrobiopterin, and Biopterin by Liquid Chromatography Coupled Electrospray Tandem Mass Spectrometry. Anal. Biochem. 2012, 430, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Öktem, R.M.; İnci, A.; Bayrak, H.; Demir, F.; Biberoǧlu, G.; Maviş, M.E.; Gürsu, G.G.; Yllmaz, H.; Okur, İ.; Ezgü, F.S.; et al. Pterin Profiling in Serum, Dried Blood Spot, and Urine Samples Using LC-MS/MS in Patients with Inherited Hyperphenylalaninemia. Mol. Syndromol. 2024, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Murray, P.J. Tryptophan and Indole Metabolism in Immune Regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef] [PubMed]
- van der Weerd, J.C.; van Wegberg, A.M.J.; Boer, T.S.; Engelke, U.F.H.; Coene, K.L.M.; Wevers, R.A.; Bakker, S.J.L.; de Blaauw, P.; Groen, J.; van Spronsen, F.J.; et al. Impact of Phenylketonuria on the Serum Metabolome and Plasma Lipidome: A Study in Early-Treated Patients. Metabolites 2024, 14, 479. [Google Scholar] [CrossRef]
- Schoen, M.S.; Singh, R.H. Plasma Metabolomic Profile Changes in Females with Phenylketonuria Following a Camp Intervention. Am. J. Clin. Nutr. 2022, 115, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.O.; Wu, B.T.F.; Li, S.S.J.; Monga, V.; Innis, S.M. Hypaphorine Is Present in Human Milk in Association with Consumption of Legumes. J. Agric. Food Chem. 2013, 61, 7654–7660. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Ulaszewska, M.; Franceschi, P.; Estruel-Amades, S.; Weinert, C.H.; Tor-Roca, A.; Urpi-Sarda, M.; Mattivi, F.; Andres-Lacueva, C. Discovery of Intake Biomarkers of Lentils, Chickpeas, and White Beans by Untargeted LC–MS Metabolomics in Serum and Urine. Mol. Nutr. Food Res. 2020, 64, 1901137. [Google Scholar] [CrossRef]
- Fischer, G.M.; Nemeti, B.; Farkas, V.; Debreceni, B.; Laszlo, A.; Schuler, A.; Somogyi, C.; Sandor, A. Metabolism of Carnitine in Phenylacetic Acid-Treated Rats and in Patients with Phenylketonuria. Biochim. Biophys. Acta 2000, 1501, 200–210, Correction in Biochim. Biophys. Acta 2000, 1502, 515. https://doi.org/10.1016/S0925-4439(00)00073-9. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small Molecule Metabolites: Discovery of Biomarkers and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Václavík, J.; Coene, K.L.M.; Vrobel, I.; Najdekr, L.; Friedecký, D.; Karlíková, R.; Mádrová, L.; Petsalo, A.; Engelke, U.F.H.; van Wegberg, A.; et al. Structural Elucidation of Novel Biomarkers of Known Metabolic Disorders Based on Multistage Fragmentation Mass Spectra. J. Inherit. Metab. Dis. 2018, 41, 407–414. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; van der Weerd, J.C.; Engelke, U.F.H.; Coene, K.L.M.; Jahja, R.; Bakker, S.J.L.; Huijbregts, S.C.J.; Wevers, R.A.; Heiner-Fokkema, M.R.; van Spronsen, F.J. The Clinical Relevance of Novel Biomarkers as Outcome Parameter in Adults with Phenylketonuria. J. Inherit. Metab. Dis. 2024, 47, 624–635. [Google Scholar] [CrossRef]
- Peck, H.; Pollitt, R.J. The Occurrence of γ-Glutamylphenylalanine in the Urine of Newborn Phenylketonurics. Clin. Chim. Acta 1979, 94, 237–240. [Google Scholar] [CrossRef]
- Andrade, F.; Cano, A.; Unceta Suarez, M.; Arza, A.; Vinuesa, A.; Ceberio, L.; López-Oslé, N.; de Frutos, G.; López-Oceja, R.; Aznal, E.; et al. Urine Phenylacetylglutamine Determination in Patients with Hyperphenylalaninemia. J. Clin. Med. 2021, 10, 3674. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Addie, R.; Merkx, R.; Fish, A.; Mahakena, S.; Bleijerveld, O.B.; Altelaar, M.; IJlst, L.; Wanders, R.J.; Borst, P.; et al. N-Lactoyl-Amino Acids Are Ubiquitous Metabolites That Originate from CNDP2-Mediated Reverse Proteolysis of Lactate and Amino Acids. Proc. Natl. Acad. Sci. USA 2015, 112, 6601–6606. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, X.; Qinghong, S.; Sun, H.; Jing, L.; Tang, X.; Guo, Z.; Liu, Y.; Wang, Y.; Ma, J.; et al. Characterization of LC-MS Based Urine Metabolomics in Healthy Children and Adults. PeerJ 2022, 10, e13545. [Google Scholar] [CrossRef]
- Skupp, S.; Ayvazian, J.H. Oxidation of 7-Methylguanine by Human Xanthine Oxidase. J. Lab. Clin. Med. 1969, 73, 909–916. [Google Scholar]
- Altakroni, B.; Nevin, C.; Carroll, M.; Murgatroyd, C.; Horne, G.; Brison, D.R.; Povey, A.C. The Marker of Alkyl DNA Base Damage, N7-Methylguanine, Is Associated with Semen Quality in Men. Sci. Rep. 2021, 11, 3121. [Google Scholar] [CrossRef]
- Dobrowolski, S.F.; Lyons-Weiler, J.; Spridik, K.; Biery, A.; Breck, J.; Vockley, J.; Yatsenko, S.; Sultana, T. Altered DNA Methylation in PAH Deficient Phenylketonuria. Mol. Genet. Metab. 2015, 115, 72–77. [Google Scholar] [CrossRef]
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Lenchner, J.R.; Santos, C. Biochemistry, 5 Hydroxyindoleacetic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551684/ (accessed on 28 October 2024).
- Fila, M.; Chojnacki, J.; Derwich, M.; Chojnacki, C.; Pawlowska, E.; Blasiak, J. Urine 5-Hydroxyindoleacetic Acid Negatively Correlates with Migraine Occurrence and Characteristics in the Interictal Phase of Episodic Migraine. Int. J. Mol. Sci. 2024, 25, 5471. [Google Scholar] [CrossRef]
- Davis, I.; Liu, A. What Is the Tryptophan Kynurenine Pathway and Why Is It Important to Neurotherapeutics? Expert. Rev. Neurother. 2015, 15, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.; Besson, G.; van Noolen, L.; Faure, P.; ECOPHEN Study Group; Maillot, F.; Corne, C. Tryptophan Metabolism in Phenylketonuria: A French Adult Cohort Study. J. Inherit. Metab. Dis. 2020, 43, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, F.J.; De Groot, M.J.; Hoeksma, M.; Reijngoud, D.J.; Van Rijn, M. Large Neutral Amino Acids in the Treatment of PKU: From Theory to Practice. J. Inherit. Metab. Dis. 2010, 33, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Veyrat-Durebex, C.; Bertrand, M.; Patin, F.; Labarthe, F.; Henique, H.; Emond, P.; Andres, C.R.; Antar, C.; Landon, C.; et al. A Multiplatform Metabolomics Approach to Characterize Plasma Levels of Phenylalanine and Tyrosine in Phenylketonuria. JIMD Rep. 2017, 32, 69–79. [Google Scholar] [CrossRef]
- Firman, S.J.; Ramachandran, R.; Whelan, K.; Witard, O.C.; O’Keeffe, M. Protein Status in Phenylketonuria: A Scoping Review. Clin. Nutr. 2022, 41, 894–922. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Sitta, A.; Wajner, M.; Vargas, C.R. Oxidative Stress in Phenylketonuria: What Is the Evidence? Cell. Mol. Neurobiol. 2011, 31, 653–662. [Google Scholar] [CrossRef]
- Sitta, A.; Barschak, A.G.; Deon, M.; De Mari, J.F.; Barden, A.T.; Vanzin, C.S.; Biancini, G.B.; Schwartz, I.V.D.; Wajner, M.; Vargas, C.R. L-Carnitine Blood Levels and Oxidative Stress in Treated Phenylketonuric Patients. Cell. Mol. Neurobiol. 2009, 29, 211–218. [Google Scholar] [CrossRef]
- Dobrowolski, S.F.; Phua, Y.L.; Vockley, J.; Goetzman, E.; Blair, H.C. Phenylketonuria Oxidative Stress and Energy Dysregulation: Emerging Pathophysiological Elements Provide Interventional Opportunity. Mol. Genet. Metab. 2022, 136, 111–117. [Google Scholar] [CrossRef]
- Weigel, C.; Kiener, C.; Meier, N.; Schmid, P.; Rauh, M.; Rascher, W.; Knerr, I. Carnitine Status in Early-Treated Children, Adolescents and Young Adults with Phenylketonuria on Low Phenylalanine Diets. Ann. Nutr. Metab. 2008, 53, 91–95. [Google Scholar] [CrossRef]
- Mütze, U.; Beblo, S.; Kortz, L.; Matthies, C.; Koletzko, B.; Bruegel, M.; Rohde, C.; Thiery, J.; Kiess, W.; Ceglarek, U. Metabolomics of Dietary Fatty Acid Restriction in Patients with Phenylketonuria. PLoS ONE 2012, 7, e43021. [Google Scholar] [CrossRef]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in Brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef]
- Bokayeva, K.; Jamka, M.; Walkowiak, D.; Duś-Żuchowska, M.; Herzig, K.H.; Walkowiak, J. Vitamin Status in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 5065. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; Urpi-Sarda, M.; Jáuregui, O.; Needs, P.W.; Kroon, P.A.; Andrés-Lacueva, C. Quantitative Dietary Fingerprinting (QDF)—A Novel Tool for Comprehensive Dietary Assessment Based on Urinary Nutrimetabolomics. J. Agric. Food Chem. 2020, 68, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Castaldo, D.; Iannaccone, F.; Neglia, G.; Campanile, G.; Balestrieri, M.L. Ruminant Meat and Milk Contain δ-Valerobetaine, Another Precursor of Trimethylamine N-Oxide (TMAO) like γ-Butyrobetaine. Food Chem. 2018, 260, 193–199. [Google Scholar] [CrossRef]
- Tuomainen, M.; Kärkkäinen, O.; Leppänen, J.; Auriola, S.; Lehtonen, M.; Savolainen, M.J.; Hermansen, K.; Risérus, U.; Åkesson, B.; Thorsdottir, I.; et al. Quantitative Assessment of Betainized Compounds and Associations with Dietary and Metabolic Biomarkers in the Randomized Study of the Healthy Nordic Diet (SYSDIET). Am. J. Clin. Nutr. 2019, 110, 1108–1118. [Google Scholar] [CrossRef]
- Kärkkäinen, O.; Tuomainen, T.; Koistinen, V.; Tuomainen, M.; Leppänen, J.; Laitinen, T.; Lehtonen, M.; Rysä, J.; Auriola, S.; Poso, A.; et al. Whole Grain Intake Associated Molecule 5-Aminovaleric Acid Betaine Decreases β-Oxidation of Fatty Acids in Mouse Cardiomyocytes. Sci. Rep. 2018, 8, 13036. [Google Scholar] [CrossRef]
- del Pozo de la Calle, S.; García Iglesias, V.; Cuadrado Vives, C.; Ruiz Moreno, E.; Valero Gaspar, T.; Ávila Torres, J.M.; Varela Moreiras, G. Valoración Nutricional de La Dieta Española de Acuerdo al Panel de Consumo Alimentario; Fundación Española de La Nutrición (FEN): Madrid, Spain, 2012; pp. 1–140. [Google Scholar]
- Ruiz, E.; Ávila, J.M.; Valero, T.; del Pozo, S.; Rodriguez, P.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; et al. Macronutrient Distribution and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2016, 8, 177. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Korovljev, D.; Stajer, V. Dietary Intake of Creatine and Risk of Medical Conditions in U.S. Older Men and Women: Data from the 2017–2018 National Health and Nutrition Examination Survey. Food Sci. Nutr. 2021, 9, 5746–5754. [Google Scholar] [CrossRef]
- Cannet, C.; Bayat, A.; Frauendienst-Egger, G.; Freisinger, P.; Spraul, M.; Himmelreich, N.; Kockaya, M.; Ahring, K.; Godejohann, M.; MacDonald, A.; et al. Phenylketonuria (PKU) Urinary Metabolomic Phenotype Is Defined by Genotype and Metabolite Imbalance: Results in 51 Early Treated Patients Using Ex Vivo 1H-NMR Analysis. Molecules 2023, 28, 4916. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and Spectroscopic Characterization of Urolithins for Their Determination in Biological Samples after the Intake of Foods Containing Ellagitannins and Ellagic Acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef]
- Tulipani, S.; Urpi-Sarda, M.; García-Villalba, R.; Rabassa, M.; López-Uriarte, P.; Bulló, M.; Jáuregui, O.; Tomás-Barberán, F.; Salas-Salvadó, J.; Espín, J.C.; et al. Urolithins Are the Main Urinary Microbial-Derived Phenolic Metabolites Discriminating a Moderate Consumption of Nuts in Free-Living Subjects with Diagnosed Metabolic Syndrome. J. Agric. Food Chem. 2012, 60, 8930–8940. [Google Scholar] [CrossRef]
- Ostrowska, M.; Nowosad, K.; Mikoluc, B.; Szczerba, H.; Komon-Janczara, E. Changes in the Gut and Oral Microbiome in Children with Phenylketonuria in the Context of Dietary Restrictions—A Preliminary Study. Nutrients 2024, 16, 3915. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.P.; Mendes, R.H.; Dobbler, P.T.; Mai, V.; Pylro, V.S.; Waugh, S.G.; Vairo, F.; Refosco, L.F.; Roesch, L.F.W.; Schwartz, I.V.D. Phenylketonuria and Gut Microbiota: A Controlled Study Based on next-Generation Sequencing. PLoS ONE 2016, 11, e0157513. [Google Scholar] [CrossRef] [PubMed]
- Şahiner, B.Y.; Dursun, A.; Gülbakan, B. Metabolomics and Lipidomics Explore Phenotype-Specific Molecular Signatures for Phenylketonuria. Int. J. Mol. Sci. 2025, 26, 7171. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.Z.; Ye, J.; Han, L.S.; Qiu, W.J.; Zhang, H.W.; Yu, Y.G.; Wang, J.G.; Gu, X.F. Application of Isoxanthopterin as a New Pterin Marker in the Differential Diagnosis of Hyperphenylalaninemia. World J. Pediatr. 2019, 15, 66–71. [Google Scholar] [CrossRef]
- Llorach, R.; Urpi-Sarda, M.; Jauregui, O.; Monagas, M.; Andres-Lacueva, C. An LC-MS-Based Metabolomics Approach for Exploring Urinary Metabolome Modifications after Cocoa Consumption. J. Proteome Res. 2009, 8, 5060–5068. [Google Scholar] [CrossRef]
- Tulipani, S.; Llorach, R.; Urpi-Sarda, M.; Andres-Lacueva, C. Comparative Analysis of Sample Preparation Methods to Handle the Complexity of the Blood Fluid Metabolome: When Less Is More. Anal. Chem. 2013, 85, 341–348. [Google Scholar] [CrossRef]
- Tulipani, S.; Mora-Cubillos, X.; Jáuregui, O.; Llorach, R.; García-Fuentes, E.; Tinahones, F.J.; Andres-Lacueva, C. New and Vintage Solutions to Enhance the Plasma Metabolome Coverage by LC-ESI-MS Untargeted Metabolomics: The Not-so-Simple Process of Method Performance Evaluation. Anal. Chem. 2015, 87, 2639–2647. [Google Scholar] [CrossRef]
- Tomás-Navarro, M.; Navarro, J.L.; Vallejo, F.; Tomás-Barberán, F.A. Novel Urinary Biomarkers of Orange Juice Consumption, Interindividual Variability, and Differences with Processing Methods. J. Agric. Food Chem. 2021, 69, 4006–4017. [Google Scholar] [CrossRef]
- Benito, S.; Sánchez-Ortega, A.; Unceta, N.; Andrade, F.; Aldámiz-Echevarria, L.; Goicolea, M.A.; Barrio, R.J. Untargeted Metabolomics for Plasma Biomarker Discovery for Early Chronic Kidney Disease Diagnosis in Pediatric Patients Using LC-QTOF-MS. Analyst 2018, 143, 4448–4458. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; Macdonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Caraux, G.; Pinloche, S. PermutMatrix: A Graphical Environment to Arrange Gene Expression Profiles in Optimal Linear Order. Bioinformatics 2005, 21, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Fernández-Albert, F.; Llorach, R.; Andrés-Lacueva, C.; Perera, A. An R Package to Analyse LC/MS Metabolomic Data: MAIT (Metabolite Automatic Identification Toolkit). Bioinformatics 2014, 30, 1937–1939. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Almanza-Aguilera, E.; Llorach, R.; Vázquez-Fresno, R.; Estruch, R.; Corella, D.; Sorli, J.V.; Carmona, F.; Sanchez-Pla, A.; Salas-Salvadó, J.; et al. Non-Targeted Metabolomic Biomarkers and Metabotypes of Type 2 Diabetes: A Cross-Sectional Study of PREDIMED Trial Participants. Diabetes Metab. 2019, 45, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Akond, M.; Babar, M.A.; Beecher, C.; Erickson, J.; Thomason, K.; De Jong, F.A.; Mason, R.E. LC-HRMS Based Non-Targeted Metabolomic Profiling of Wheat (Triticum aestivum L.) under Post-Anthesis Drought Stress. Am. J. Plant Sci. 2017, 8, 3024–3061. [Google Scholar] [CrossRef]

| M | Metabolite | VIP | Biological Source/Pathway |
|---|---|---|---|
| M01 | Phenylalanine | 3.75 | Phenylalanine metabolism |
| M02 | 2-Hydroxyphenylacetic acid | 3.69 | Phenylalanine metabolism |
| M03 | Hydroxyphenylacetylglutamine | 3.47 | Phenylalanine metabolism |
| M18 | 8-Hydroxy-7-methylguanine | 3.25 | Purine metabolism |
| M04 | Hydroxyphenylacetic acid glucuronide | 3.20 | Phenylalanine metabolism |
| M05 | γ-Glutamylphenylalanine | 3.14 | Phenylalanine metabolism |
| M06 | Carboxyethylphenylalanine isomer 1 | 3.09 | Phenylalanine metabolism |
| M07 | Carboxyethylphenylalanine isomer 2 | 3.07 | Phenylalanine metabolism |
| M08 | N-lactoylphenylalanine | 3.02 | Phenylalanine metabolism |
| M09 | Phenyllactic acid | 3.01 | Phenylalanine metabolism |
| M10 | N-phenylacetylphenylalanine | 3.00 | Phenylalanine metabolism |
| M11 | N-acetylphenylalanine | 2.97 | Phenylalanine metabolism |
| M19 | Isoxanthopterin | 2.97 | Pteridine pathway |
| M21 | 1H-Indole-3-carboxaldehyde | 2.96 | Tryptophan metabolism |
| M12 | N-(ethoxyacetyl)phenylalanine isomer 1 | 2.93 | Phenylalanine metabolism |
| M22 | Indolelactic acid | 2.75 | Tryptophan metabolism |
| M13 | N-(ethoxyacetyl)phenylalanine isomer 2 | 2.73 | Phenylalanine metabolism |
| M23 | Indoleacetic acid | 2.42 | Tryptophan metabolism |
| M14 | Hydroxyphenylacetic acid sulfate | 2.36 | Phenylalanine metabolism |
| M24 | Phenylacetylcarnitine | 2.30 | Acylcarnitine pathway |
| M25 | 4-Pyridoxic acid | 2.10 | Vitamin B6 metabolism |
| M20 | Dihydrobiopterin | 2.04 | Pteridine pathway |
| M26 | α-CEHC glucuronide | 2.04 | Vitamin E metabolism |
| M15 | N-phenylacetylglutamic acid | 2.02 | Phenylalanine metabolism |
| M27 | α-CEHC | 1.93 | Vitamin E metabolism |
| M28 | Pantothenic acid | 1.71 | Pantothenate biosynthesis |
| M16 | Phe-hexose | 1.64 | Phenylalanine metabolism |
| M29 | 1-Pyrroline-5-carboxylic acid | 1.39 | Glutamate metabolism |
| M17 | Phenylacetylglutamine | 1.35 | Phenylalanine metabolism |
| M | Metabolite | VIP | Biological Source/Pathway |
|---|---|---|---|
| M30 | N-acetyl(iso)leucine | 3.40 | Leucine, isoleucine and valine metabolism |
| M33 | Heptenoylcarnitine isomer 1 | 2.77 | Acylcarnitine pathway |
| M46 | N,N,N-trimethyltryptophan betaine | 2.71 | Tryptophan metabolism |
| M53 | 1-Methylhistidine | 2.64 | Animal protein consumption |
| M57 | Dihydroxybenzoic acid isomer | 2.60 | Drug-topical agent |
| M34 | Hydroxyundecanoylcarnitine isomer | 2.57 | Acylcarnitine pathway |
| M58 | Urolithin B glucuronide | 2.48 | Phenolic compound |
| M35 | Octanoylcarnitine or methylheptanoylcarnitine or valproylcarnitine | 2.47 | Acylcarnitine pathway |
| M36 | Undecanoylcarnitine or dimethylnonanoylcarnitine or methyldecanoylcarnitine | 2.36 | Acylcarnitine pathway |
| M37 | Heptenoylcarnitine isomer 2 | 2.36 | Acylcarnitine pathway |
| M54 | 5-Aminovaleric acid betaine | 2.35 | Animal protein consumption |
| M63 | Hepteneoylglycine isomer | 2.33 | Glycine compound |
| M55 | 3-Methylhistidine | 2.30 | Animal protein consumption |
| M38 | Octanoylcarnitine or methylheptanoylcarnitine or valproylcarnitine | 2.24 | Acylcarnitine pathway |
| M39 | Heptanoylcarnitine or methylhexanoylcarnitine | 2.20 | Acylcarnitine pathway |
| M40 | Decanoylcarnitine or methylnonanoylcarnitine | 2.17 | Acylcarnitine pathway |
| M59 | Dihydroxy-H-indole glucuronide isomer 1 | 2.09 | Phenolic compound |
| M65 | Pyrraline | 2.09 | Food component |
| M60 | Enterolactone glucuronide | 2.03 | Phenolic compound |
| M47 | Tryptophan | 1.99 | Tryptophan metabolism |
| M48 | Kynurenine | 1.98 | Tryptophan metabolism |
| M61 | Urolithin A glucuronide | 1.96 | Phenolic compound |
| M62 | Dihydroxy-H-indole glucuronide isomer 2 | 1.96 | Phenolic compound |
| M41 | Dodecenoylcarnitine | 1.93 | Acylcarnitine pathway |
| M42 | Oxononanoylcarnitine or hydroxynonenoylcarnitine isomers | 1.92 | Acylcarnitine pathway |
| M43 | Oxononanoylcarnitine or hydroxynonenoylcarnitine isomers | 1.90 | Acylcarnitine pathway |
| M49 | C-Glycosyltryptophan | 1.81 | Tryptophan metabolism |
| M64 | Methylbutyrylglycine or isovalerylglycine or valerylglycine | 1.72 | Glycine compound |
| M66 | N2,N5-Diacetylornithine | 1.71 | Urea cycle, arginine and proline metabolism |
| M50 | Indoleacetyl glutamine | 1.65 | Tryptophan metabolism |
| M67 | 1,7-Dimethyluric acid | 1.64 | Caffeine metabolism |
| M71 | N-acetylaspartylglutamic acid | 1.54 | Alanine, aspartate and glutamate metabolism |
| M68 | 1,3,7-Trimethyluric acid | 1.53 | Caffeine metabolism |
| M72 | Hexanoylglutamine | 1.51 | Glutamine metabolism |
| M44 | Decanoylcarnitine or methylnonanoylcarnitine | 1.49 | Acylcarnitine pathway |
| M45 | Nonenedioylcarnitine isomer | 1.44 | Acylcarnitine pathway |
| M31 | γ-Glutamyl(iso)leucine | 1.44 | Leucine, isoleucine and valine metabolism |
| M73 | Tyrosine | 1.33 | Tyr metabolism |
| M69 | Caffeine | 1.27 | Caffeine metabolism |
| M56 | Creatine | 1.26 | Animal protein consumption |
| M32 | N-lactoyl(iso)leucine | 1.22 | Leucine, isoleucine and valine metabolism |
| M51 | Kynurenic acid | 1.18 | Tryptophan metabolism |
| M52 | 5-Hydroxyindoleacetic acid | 1.12 | Tryptophan metabolism |
| M70 | Paraxanthine | 1.07 | Caffeine metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Rodriguez, A.; Barrau-Martinez, B.; Pané, A.; López Galera, R.M.; Tobias, E.; Montserrat-Carbonell, C.; Guitart-Mampel, M.; Jáuregui, O.; Roca-Vives, R.; Garcia-Villoria, J.; et al. Untargeted Metabolomic Study for Urinary Characterization of Adult Patients with Phenylketonuria. Int. J. Mol. Sci. 2025, 26, 11808. https://doi.org/10.3390/ijms262411808
Gonzalez-Rodriguez A, Barrau-Martinez B, Pané A, López Galera RM, Tobias E, Montserrat-Carbonell C, Guitart-Mampel M, Jáuregui O, Roca-Vives R, Garcia-Villoria J, et al. Untargeted Metabolomic Study for Urinary Characterization of Adult Patients with Phenylketonuria. International Journal of Molecular Sciences. 2025; 26(24):11808. https://doi.org/10.3390/ijms262411808
Chicago/Turabian StyleGonzalez-Rodriguez, Arnau, Blanca Barrau-Martinez, Adriana Pané, Rosa Maria López Galera, Ester Tobias, Cristina Montserrat-Carbonell, Mariona Guitart-Mampel, Olga Jáuregui, Regina Roca-Vives, Judit Garcia-Villoria, and et al. 2025. "Untargeted Metabolomic Study for Urinary Characterization of Adult Patients with Phenylketonuria" International Journal of Molecular Sciences 26, no. 24: 11808. https://doi.org/10.3390/ijms262411808
APA StyleGonzalez-Rodriguez, A., Barrau-Martinez, B., Pané, A., López Galera, R. M., Tobias, E., Montserrat-Carbonell, C., Guitart-Mampel, M., Jáuregui, O., Roca-Vives, R., Garcia-Villoria, J., Milisenda, J. C., Matas-Garcia, A., Forga Visa, M. d. T., Moreno Lozano, P. J., Garrabou, G., Urpi-Sarda, M., & Llorach, R., on behalf of the Consortium PKU.cat. (2025). Untargeted Metabolomic Study for Urinary Characterization of Adult Patients with Phenylketonuria. International Journal of Molecular Sciences, 26(24), 11808. https://doi.org/10.3390/ijms262411808







