Targeting Molecular Dysregulation in Ulcerative Colitis: A Paired Cellular Perspective on CD4+, CD8+, and IL-6 Immunohistochemistry
Abstract
1. Introduction
1.1. Cellular and Molecular Mechanism
1.1.1. Local Immunity: Macrophages, Mast Cells, and Eosinophils
1.1.2. Cell–Cell Interactions and the Role of the Mesenchymal Compartment
1.1.3. Cytokines and Molecular Signaling
1.1.4. Immunologic Receptors and Cellular Responses
1.1.5. Microbiota and Receptor-Mediated Signaling
2. Results
2.1. Histological Disease Activity
2.2. Distribution of T Lymphocyte Subsets
2.2.1. Cd4+ Lymphocytes
2.2.2. CD8+ Lymphocytes
2.3. Il-6 Expression
2.4. Correlation Analysis
- In the active phase, correlations between intraepithelial CD4+ and CD8+ counts were weak (Spearman’s ρ ≈ 0.21, p = 0.35), suggesting independent recruitment during inflammation.
- During histologic healing, the correlation strengthened significantly (ρ ≈ 0.62, p < 0.01), reflecting a more coordinated immune cell presence consistent with restoration of mucosal homeostasis.
- IL-6 expression correlated positively with intraepithelial CD4+ density (ρ = 0.55, p < 0.05) during active disease but lost significance in remission, highlighting its role in inflammation-driven immune activation.
3. Discussion
3.1. Histologic and Immunologic Correlates of Mucosal Healing
3.2. CD8+ Lymphocyte Dynamics
3.3. IL-6 Expression and Cytokine Modulation
3.4. Clinical Implications
3.5. Study Limitations
3.6. Future Perspectives and Research Directions
- Targeted therapeutics: Development and refinement of inhibitors for Smad7, MEK [63], and specific cytokines, with careful evaluation of safety and efficacy in defined patient subpopulations.
- Microbiota modulation: Exploration of bile acid receptor agonists and microbiome-targeted therapies to restore immune homeostasis and reduce mucosal inflammation [53].
- Single-cell and spatial omics: Mapping novel cellular subtypes and their interactions to identify predictive biomarkers of disease progression and therapeutic response [64].
- Integrative approaches: Consideration of social determinants of health to guide personalized preventive and therapeutic strategies [65].
- Translational models: Use of patient-derived organoids, co-culture systems, and humanized mouse models for mechanistic studies and preclinical drug testing [66].
4. Materials and Methods
4.1. Study Design and Population
4.2. Inclusion and Exclusion Criteria
4.3. Study Groups and Sampling Strategy
- Active phase samples, obtained during a documented clinical and endoscopic flare (Mayo endoscopic sub-score 2–3).
- Histologic healing samples, collected later from the same colonic segments once patients achieved endoscopic remission (Mayo sub-score 0) and were clinically asymptomatic.
4.4. Clinical and Endoscopic Assessment
4.5. Biopsy Collection and Processing
4.6. Histopathological Evaluation
4.7. Immunohistochemical Analysis
- CD3 (pan–T-cell marker)—to evaluate the entire T lymph cell population;
- CD4 (helper T-cell marker);
- CD8 (cytotoxic T-cell marker);
- IL-6 (proinflammatory cytokine).
4.8. Ethical Considerations
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirică, R.E.; Matură, T.F.; Craciun, E.; Pavel, D. The Importance of Magnetic Resonance Enterography in Monitoring Inflammatory Bowel Disease: A Review of Clinical Significance and Current Challenges. Diagnostics 2025, 15, 1540. [Google Scholar] [CrossRef]
- Coward, S.; Clement, F.; Benchimol, E.I.; Bernstein, C.N.; Avina-Zubieta, J.A.; Bitton, A.; Carroll, M.W.; Hazlewood, G.; Jacobson, K.; Jelinski, S.; et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019, 156, 1345–1353.e4. [Google Scholar] [CrossRef]
- Jucan, A.E.; Gavrilescu, O.; Dranga, M.; Popa, I.V.; Mihai, I.-R.; Mihai, V.-C.; Stefanescu, G.; Drug, V.L.; Prelipcean, C.C.; Vulpoi, R.-A. Evaluation of Disease Activity in Inflammatory Bowel Disease: Diagnostic Tools in the Assessment of Histological Healing. Biomedicines 2023, 11, 3090. [Google Scholar] [CrossRef] [PubMed]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Bin Emran, T.; Rabaan, A.A.; Al Mutair, A.; Al Alawi, Z.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccines Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef] [PubMed]
- Jacobse, J.; Li, J.; Rings, E.H.H.M.; Samsom, J.N.; Goettel, J.A. Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease. Front. Immunol. 2021, 12, 716499. [Google Scholar] [CrossRef]
- Van Den Broek, T.; Borghans, J.A.M.; Van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 2018, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Vebr, M.; Pomahačová, R.; Sýkora, J.; Schwarz, J. A Narrative Review of Cytokine Networks: Pathophysiological and Therapeutic Implications for Inflammatory Bowel Disease Pathogenesis. Biomedicines 2023, 11, 3229. [Google Scholar] [CrossRef]
- Pawłowska-Kamieniak, A.; Krawiec, P.; Pac-Kożuchowska, E. Interleukin 6: Biological significance and role in inflammatory bowel diseases. Adv. Clin. Exp. Med. 2021, 30, 465–469. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Hirano, T.; Matsuda, T.; Hosoi, K.; Okano, A.; Matsui, H.; Kishimoto, T. Absence of antiviral activity in recombinant B cell stimulatory factor 2 (BSF-2). Immunol. Lett. 1988, 17, 41–45. [Google Scholar] [CrossRef]
- Kusugami, K.; Fukatsu, A.; Tanimoto, M.; Shinoda, M.; Haruta, J.; Kuroiwa, A.; Ina, K.; Kanayama, K.; Ando, T.; Matsuura, T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig. Dis. Sci. 1995, 40, 949–959. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.-F.; Liang, Y.-J.; Xu, J.; Xu, H.-M.; Nie, Y.-Q.; Wang, L.-S.; Yao, J.; Li, D.-F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal. 2023, 17, 55. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, S. PANoptosis in intestinal epithelium: Its significance in inflammatory bowel disease and a potential novel therapeutic target for natural products. Front. Immunol. 2025, 15, 1507065. [Google Scholar] [CrossRef]
- Travassos, L.H.; Carneiro, L.A.M.; Ramjeet, M.; Hussey, S.; Kim, Y.-G.; Magalhães, J.G.; Yuan, L.; Soares, F.; Chea, E.; Le Bourhis, L.; et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 2010, 11, 55–62. [Google Scholar] [CrossRef]
- Hoefkens, E.; Nys, K.; John, J.M.; Van Steen, K.; Arijs, I.; Van der Goten, J.; Van Assche, G.; Agostinis, P.; Rutgeerts, P.; Vermeire, S.; et al. Genetic association and functional role of Crohn disease risk alleles involved in microbial sensing, autophagy, and endoplasmic reticulum (ER) stress. Autophagy 2013, 9, 2046–2055. [Google Scholar] [CrossRef]
- Goto, Y.; Kiyono, H. Epithelial barrier: An interface for the cross-communication between gut flora and immune system. Immunol. Rev. 2012, 245, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Park, H.; Choe, B.-H.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 848344. [Google Scholar] [CrossRef]
- Larsson, J.M.H.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.V.; Eklund, L.; Sjövall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Gersemann, M.; Becker, S.; Kübler, I.; Koslowski, M.; Wang, G.; Herrlinger, K.R.; Griger, J.; Fritz, P.; Fellermann, K.; Schwab, M.; et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009, 77, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Li, R.; Gao, T. The Intestinal Macrophage-Intestinal Stem Cell Axis in Inflammatory Bowel Diseases: From Pathogenesis to Therapy. Int. J. Mol. Sci. 2025, 26, 2855. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, S.; Chen, S.; Zhang, F.; Li, N.; Yin, J.; Peng, Y.; Wu, L.; Liu, G.; Yin, Y.; et al. Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 2013, 45, 947–955. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Frei, S.M.; Stevens, R.L. The Multifaceted Mast Cell in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 2364–2378. [Google Scholar] [CrossRef]
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 1999, 103, 1719–1727. [Google Scholar] [CrossRef]
- Rothenberg, M.E. Gastrointestinal eosinophils. Immunol. Rev. 2001, 179, 139–155. [Google Scholar] [CrossRef]
- Fagerholm, S.C. Integrins in Health and Disease. N. Engl. J. Med. 2022, 387, 1519–1521. [Google Scholar] [CrossRef]
- Ito, T.; Kayama, H. Roles of fibroblasts in the pathogenesis of inflammatory bowel diseases and IBD-associated fibrosis. Int. Immunol. 2025, 37, 377–392. [Google Scholar] [CrossRef]
- McLean, L.P.; Shea-Donohue, T.; Cross, R.K. Vedolizumab for the treatment of ulcerative colitis and Crohn’s disease. Immunotherapy 2012, 4, 883–898. [Google Scholar] [CrossRef]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Biancheri, P.; Di Sabatino, A.; Corazza, G.R.; MacDonald, T.T. Proteases and the gut barrier. Cell Tissue Res. 2013, 351, 269–280. [Google Scholar] [CrossRef]
- Schreurs, R.; Baumdick, M.E.; Sagebiel, A.F.; Kaufmann, M.; Mokry, M.; Klarenbeek, P.L.; Schaltenberg, N.; Steinert, F.L.; van Rijn, J.M.; Drewniak, A.; et al. Human fetal TNF-alpha-cytokine-producing CD4+ effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 2019, 50, 462–476.e8. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Brodzicki, J.; Liberek, A.; Korzon, M. TGF-beta (transforming growth factor-beta) in chronic inflammatory conditions—A new diagnostic and prognostic marker? Med. Sci. Monit. 2002, 8, RA145–RA151. [Google Scholar] [PubMed]
- Monteleone, G.; Kumberova, A.; Croft, N.M.; McKenzie, C.; Steer, H.W.; MacDonald, T.T. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J. Clin. Investig. 2001, 108, 601–609. [Google Scholar] [CrossRef]
- Boirivant, M.; Pallone, F.; Di Giacinto, C.; Fina, D.; Monteleone, I.; Marinaro, M.; Caruso, R.; Colantoni, A.; Palmieri, G.; Sanchez, M.; et al. Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis. Gastroenterology 2006, 131, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, Y.; Nakase, H.; Shiokawa, M.; Yoshino, T.; Imaeda, H.; Matsuura, M.; Kodama, Y.; Ikeuchi, H.; Andoh, A.; Sakai, Y.; et al. Involvement of interleukin-17A-induced expression of heat shock protein 47 in intestinal fibrosis in Crohn’s disease. Gut 2014, 63, 1902–1912. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhao, Q.; Chen, M. Role of interleukin-17 in pathogenesis of intestinal fibrosis in mice. Dig. Dis. Sci. 2020, 65, 1971–1979. [Google Scholar] [CrossRef]
- Drygiannakis, I.; Valatas, V.; Sfakianaki, O.; Bourikas, L.; Manousou, P.; Kambas, K.; Ritis, K.; Kolios, G.; Kouroumalis, E. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: Implication in intestinal fibrosis. J. Crohns Colitis 2013, 7, 286–300. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Olson, A.; Travers, S.; Diamond, R.H.; Chen, D.M.; Pritchard, M.L.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Hanauer, S.B.; et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am. J. Gastroenterol. 2006, 101, 1030–1038. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Gurujeyalakshmi, G.; Giri, S.N. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: Downregulation of TGF-beta and procollagen I and III gene expression. Exp. Lung Res. 1995, 21, 791–808. [Google Scholar] [CrossRef]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Weichenthal, M.; Schwab, M.; Schäffeler, E.; Schlee, M.; Herrlinger, K.R.; Stallmach, A.; Noack, F.; Fritz, P.; et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 2004, 53, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Chamaillard, M.; Girardin, S.E.; Viala, J.; Philpott, D.J. Nods, Nalps and Naip: Intracellular regulators of bacterial-induced inflammation. Cell. Microbiol. 2003, 5, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Hardenberg, G.; Yao, Y.; Piccirillo, C.A.; Levings, M.K.; Steiner, T.S. Toll-like receptor 5 deficiency protects from wasting disease in a T cell transfer colitis model in T cell receptor-β-deficient mice. Inflamm. Bowel Dis. 2012, 18, 85–93. [Google Scholar] [CrossRef]
- Sato, T.; Nakai, T.; Tamura, N.; Okamoto, S.; Matsuoka, K.; Sakuraba, A.; Fukushima, T.; Uede, T.; Hibi, T. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut 2005, 54, 1254–1262. [Google Scholar] [CrossRef]
- Masuda, H.; Takahashi, Y.; Asai, S.; Takayama, T. Distinct gene expression of osteopontin in patients with ulcerative colitis. J. Surg. Res. 2003, 111, 85–90. [Google Scholar] [CrossRef]
- Agnholt, J.; Kelsen, J.; Schack, L.; Hvas, C.L.; Dahlerup, J.F.; Sorensen, E.S. Osteopontin, a protein with cytokine-like properties, is associated with inflammation in Crohn’s disease. Scand. J. Immunol. 2007, 65, 453–460. [Google Scholar] [CrossRef]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Renga, B.; Migliorati, M.; Mencarelli, A.; Fiorucci, S. Reciprocal regulation of the bile acid-activated receptor FXR and the interferon-gamma-STAT-1 pathway in macrophages. Biochim. Biophys. Acta 2009, 1792, 564–573. [Google Scholar] [CrossRef]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.; Riddell, R.; Öst, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef]
- Rosenberg, L.; Nanda, K.S.; Zenlea, T.; Gifford, A.; Lawlor, G.O.; Falchuk, K.R.; Wolf, J.L.; Cheifetz, A.S.; Goldsmith, J.D.; Moss, A.C. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin. Gastroenterol. Hepatol. 2013, 11, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Bessissow, T.; Lemmens, B.; Ferrante, M.; Bisschops, R.; Van Steen, K.; Geboes, K.; Van Assche, G.; Vermeire, S.; Rutgeerts, P.; De Hertogh, G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am. J. Gastroenterol. 2012, 107, 1684–1692. [Google Scholar] [CrossRef]
- Boland, B.S.; Sandborn, W.J.; Chang, J.T. Update on Janus kinase antagonists in inflammatory bowel disease. Gastroenterol. Clin. North Am. 2014, 43, 603–617. [Google Scholar] [CrossRef]
- Digby-Bell, J.L.; Atreya, R.; Monteleone, G.; Powell, N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 9–20. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S., Jr.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Loftus, E.V.; Peyrin-Biroulet, L., Jr.; Van Assche, G.; D’Haens, G.; Schreiber, S.; Colombel, J.F.; Lewis, J.D.; Ghosh, S.; et al. Efficacy and safety of upadacitinib in a randomized trial of patients with crohn’s disease. Gastroenterology 2020, 158, 2123–2138.e8. [Google Scholar] [CrossRef] [PubMed]
- Stankey, C.T.; Bourges, C.; Haag, L.M.; Turner-Stokes, T.; Piedade, A.P.; Palmer-Jones, C.; Papa, I.; dos Santos, M.S.; Zhang, Q.; Cameron, A.J.; et al. A disease-associated gene desert directs macrophage inflammation through ETS2. Nature 2024, 630, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Gudiño, V.; Bartolomé-Casado, R.; Salas, A. Single-cell omics in inflammatory bowel disease: Recent insights and future clinical applications. Gut 2025, 74, 1335–1345. [Google Scholar] [CrossRef]
- Shah, R.; Kelley, J.; Amundsen, T.; Coggins, K.; Edwards, A.; Johnson, C.M. Medical and social determinants of health as predictors of adverse outcomes in patients with inflammatory bowel disease. Bayl. Univ. Med. Cent. Proc. 2023, 36, 165–170. [Google Scholar] [CrossRef]
- Gonzalez-Acera, M.; Patankar, J.V.; Erkert, L.; Cineus, R.; Gamez-Belmonte, R.; Leupold, T. Integrated multimodel analysis of intestinal inflammation exposes key molecular features of preclinical and clinical IBD. Gut 2025, 74, 1602–1615. [Google Scholar] [CrossRef]

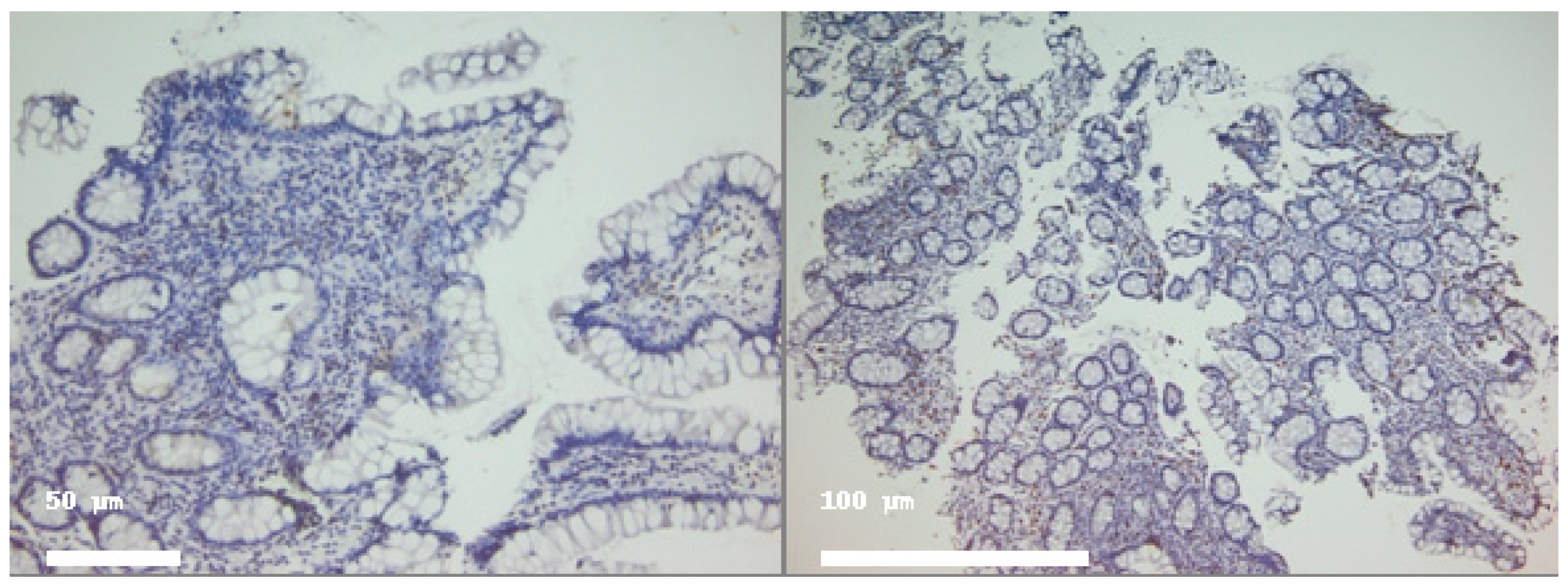

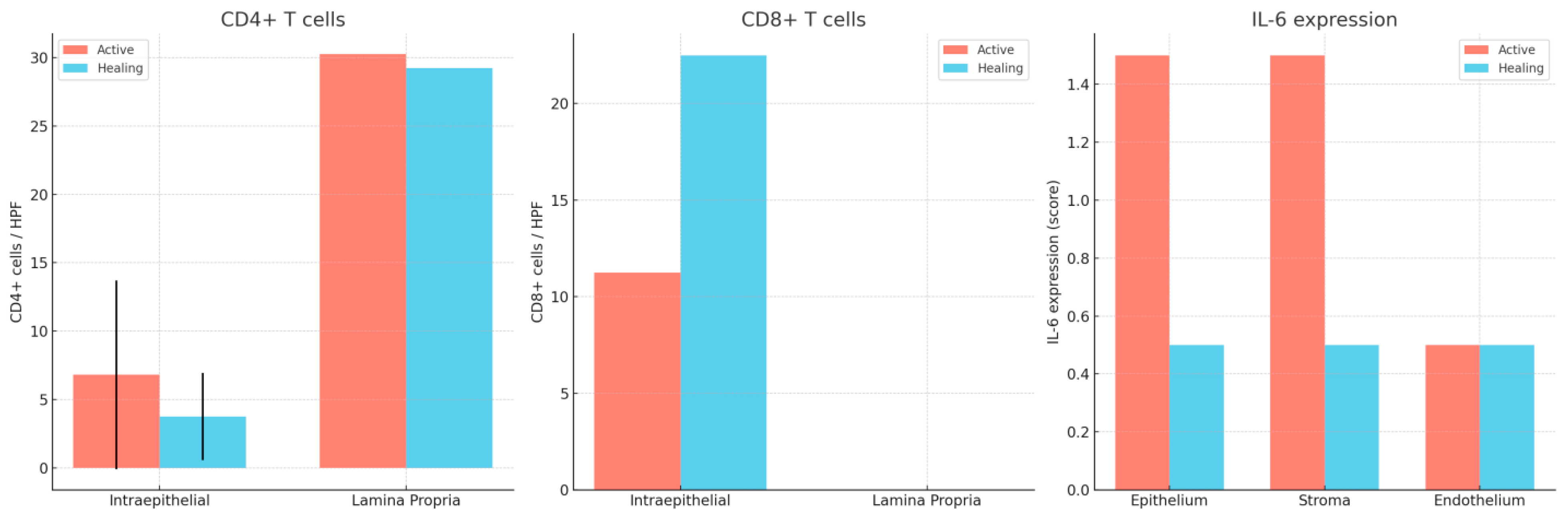
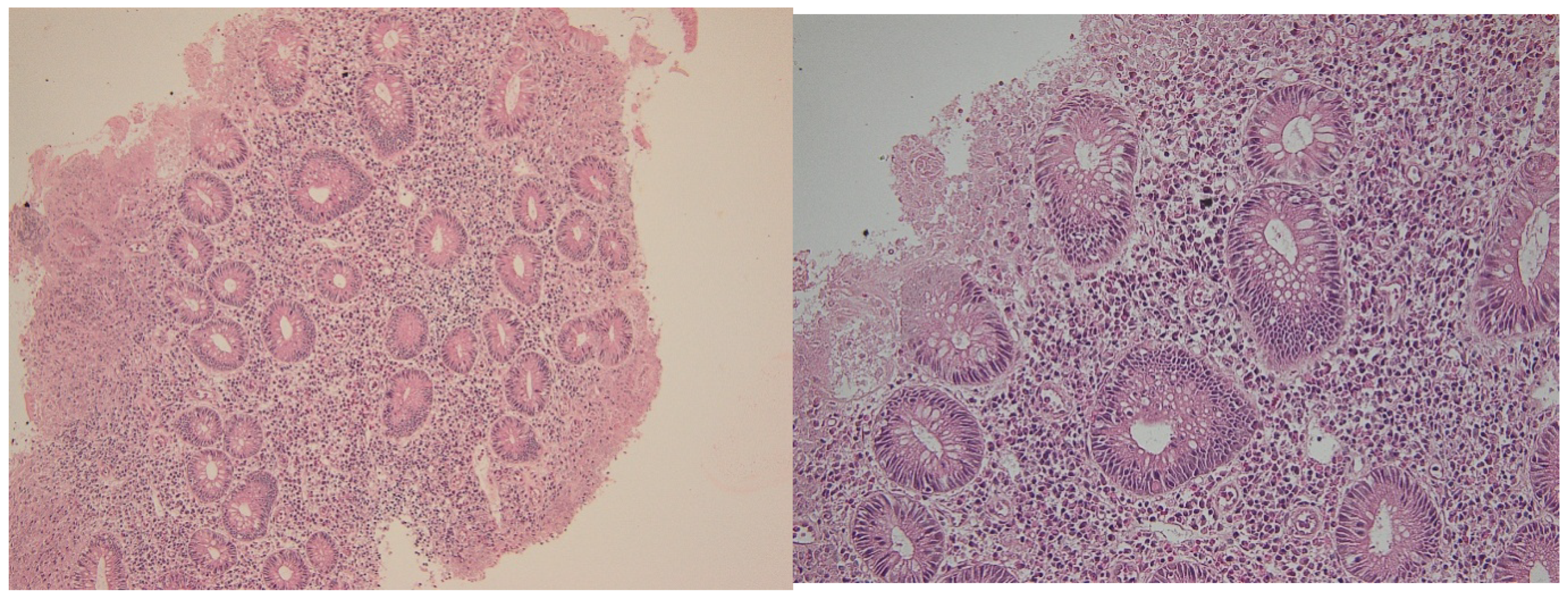
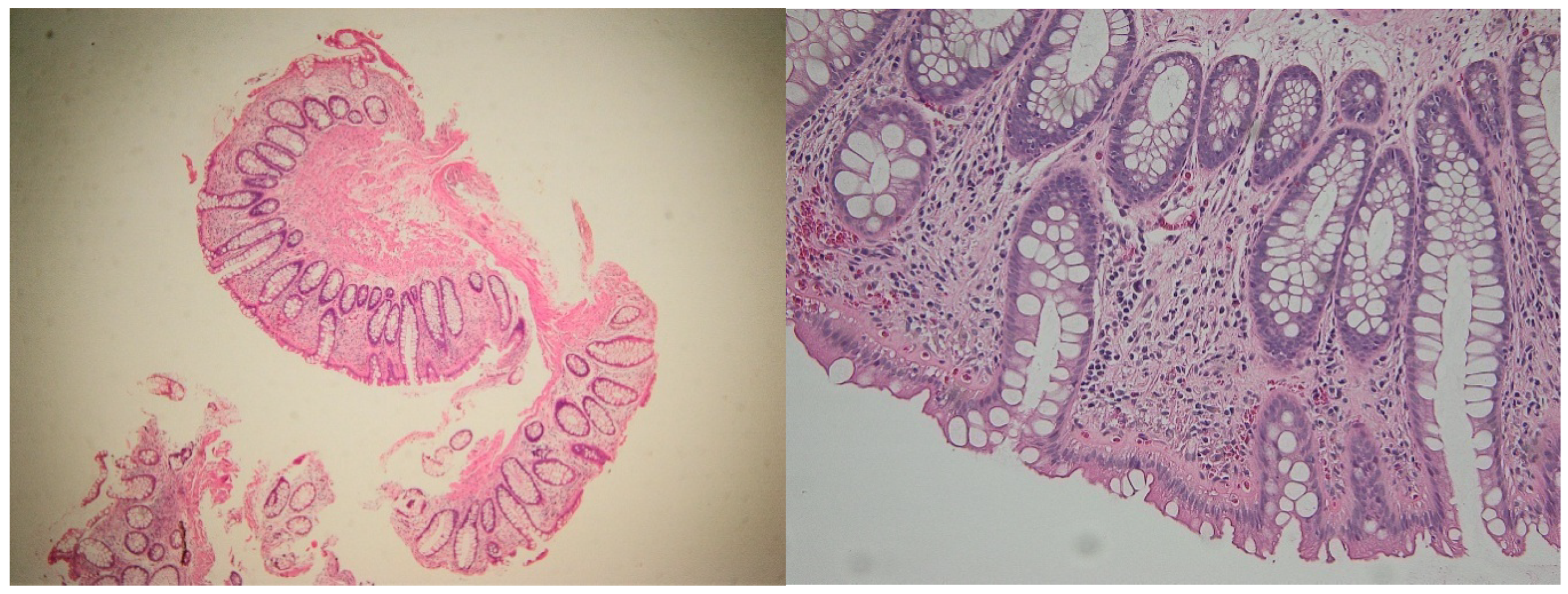
| Marker | Compartment | Active Disease (Mean ± SD) | Median (IQR) | Histologic Healing (Mean ± SD) | Median (IQR) |
|---|---|---|---|---|---|
| CD4+ | Intraepithelial | 6.8 ± 6.9 | 5 (3–9) | 3.75 ± 3.2 | 4 (2–6) |
| Lamina propria | 30.25 ± — | 10 (6–25) | 29.25 ± — | 30 (20–40) | |
| CD8+ | Intraepithelial | 11.25 ± — | 10 (7–15) | 22.5 ± — | 16 (12–25) |
| Lamina propria | 72.5± | 71 (5–125) | 82± | 75 (5–175) | |
| IL-6 | Epithelium | 1.5 ± — | 1–2 | 0.5 ± — | 0–1 |
| Stroma | 1.5 ± — | 1–2 | 0.5 ± — | 0–1 | |
| Endothelium | 0.5 ± — | — | 0.5 ± — | No significant change between phases |
| Antigen | Clone | Vendor | Catalog No. | Use | Retrieval Buffer/Pretreatment | Incubation/Dilution | Detection system |
|---|---|---|---|---|---|---|---|
| CD3 | LN10 | Leica Biosystems | PA0553 | Ready-to-Use | BOND ER | BOND | BOND Polymer Refine Detection |
| CD4 | 4B12 | Leica Biosystems | PA0427 | Ready-to-Use | HIER | BOND | BOND |
| CD8 | 4B11 | Leica Biosystems | PA0191 | Ready-to-Use | HIER | BOND | BOND |
| IL-6 | 10C12 | Leica Biosystems | NCL-L-IL6 | Liquid concentrate | HIER | BOND | BOND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirica, R.E.; Coman, A.; State, M.; Popp, C. Targeting Molecular Dysregulation in Ulcerative Colitis: A Paired Cellular Perspective on CD4+, CD8+, and IL-6 Immunohistochemistry. Int. J. Mol. Sci. 2025, 26, 11773. https://doi.org/10.3390/ijms262411773
Mirica RE, Coman A, State M, Popp C. Targeting Molecular Dysregulation in Ulcerative Colitis: A Paired Cellular Perspective on CD4+, CD8+, and IL-6 Immunohistochemistry. International Journal of Molecular Sciences. 2025; 26(24):11773. https://doi.org/10.3390/ijms262411773
Chicago/Turabian StyleMirica, Roxana Elena, Andrei Coman, Monica State, and Cristiana Popp. 2025. "Targeting Molecular Dysregulation in Ulcerative Colitis: A Paired Cellular Perspective on CD4+, CD8+, and IL-6 Immunohistochemistry" International Journal of Molecular Sciences 26, no. 24: 11773. https://doi.org/10.3390/ijms262411773
APA StyleMirica, R. E., Coman, A., State, M., & Popp, C. (2025). Targeting Molecular Dysregulation in Ulcerative Colitis: A Paired Cellular Perspective on CD4+, CD8+, and IL-6 Immunohistochemistry. International Journal of Molecular Sciences, 26(24), 11773. https://doi.org/10.3390/ijms262411773






