Integrative Assessment of Glycyrrhiza uralensis Extract in Cosmetics Using HPLC Analysis, Network Pharmacology, and Computational Threshold of Toxicological Concern-Based Safety Evaluation
Abstract
1. Introduction
2. Results
2.1. Quantitative Analysis of Liquiritin and Glycyrrhizin
2.2. Quantitative Validation of the Analytical Method
2.3. Physicochemical Descriptors of Liquiritin and Glycyrrhizin
2.4. Identification of Targets of Liquiritin- and Glycyrrhizin-Induced Skin Sensitivity and Toxicology
2.5. Target Function and Pathway Enrichment of Core Pathway
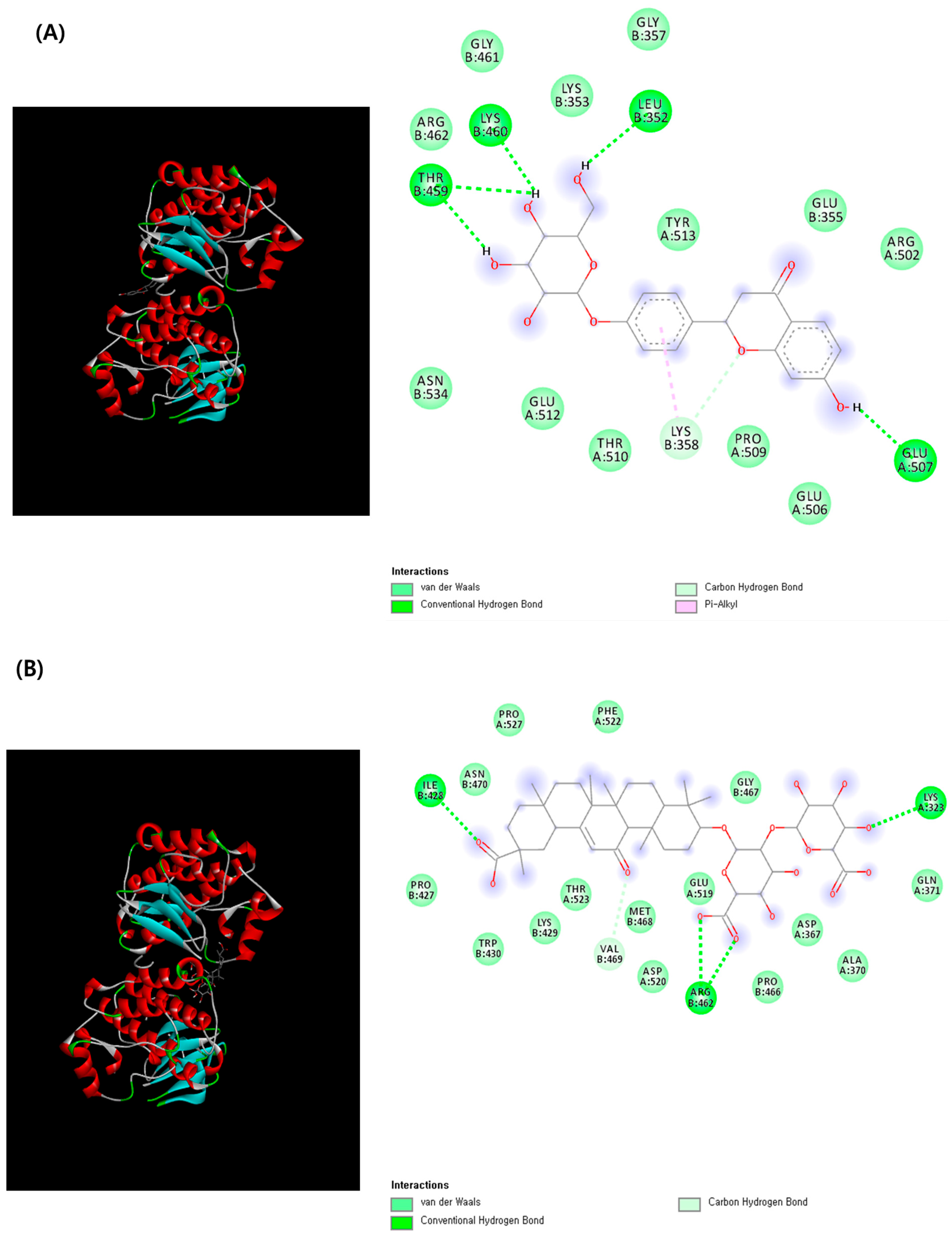
2.6. Prediction of Toxic Targets of Liquiritin and Glycyrrhizin and Molecular Docking Analysis
2.7. In Silico Skin Sensitivity and Irritation Prediction
2.8. Cramer Classification and Safety Assessment Using the Threshold of Toxicological Concern Approach
3. Discussion
4. Materials and Methods
4.1. Preparation of Glycyrrhiza uralensis (GU) Root Extract and Reagents
4.2. Identification and Quantification of Chemicals Contained in G. uralensis Root Extract
4.3. Analytical Method Validation
4.4. Structural Data Acquisition, Physicochemical Description, and In Silico Target Prediction
4.5. Network Construction and Analysis
4.6. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
4.7. Prediction of Toxic Targets and Molecular Docking of Selected Compounds and Targets
4.8. Prediction of In Silico Skin Sensitivity and Irritation
4.9. Cramer Class Assignment and Application for Threshold of Toxicological Concern (TTC) Thresholds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar]
- Wang, Z.Y.; Nixon, D.W. Licorice and cancer. Nutr. Cancer 2001, 39, 1–11. [Google Scholar] [CrossRef]
- Nazari, S.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review. Phytother. Res. 2017, 31, 1635–1650. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Ahmad, K.; Lim, J.H.; Ahmad, S.S.; Lee, E.J.; Choi, I. Biological insights and therapeutic potential of Glycyrrhiza uralensis and its bioactive compounds: An updated review. Arch. Pharm. Res. 2024, 47, 871–892. [Google Scholar] [CrossRef]
- Wu, L.; Ma, T.; Zang, C.; Xu, Z.; Sun, W.; Luo, H.; Yang, M.; Song, J.; Chen, S.; Yao, H. Glycyrrhiza, a commonly used medicinal herb: Review of species classification, pharmacology, active ingredient biosynthesis, and synthetic biology. J. Adv. Res. 2024, 75, 249–270. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.Y.; Liu, Y.Q.; Shi, H.M.; Li, X.B. Quality analysis of raw and honey-processed licorice of glycyrrhiza uralensls Fisch. and G. glabra L. by simultaneous determination of five bioactive components using RP-HPLC/DAD method. J. Food Drug Anal. 2011, 19, 131–138. [Google Scholar]
- Yu, P.; Li, Q.; Feng, Y.; Ma, S.N.; Chen, Y.Y.; Li, G.C. Extraction and Analysis of Six Effective Components in Glycyrrhiza uralensis Fisch by Deep Eutectic Solvents (DES) Combined with Quantitative Analysis of Multi-Components by Single Marker (QAMS) Method. Molecules 2021, 26, 1310. [Google Scholar] [CrossRef]
- Qin, J.Y.; Chen, J.R.; Peng, F.; Sun, C.; Lei, Y.; Chen, G.R.; Li, G.M.; Yin, Y.P.; Lin, Z.W.; Wu, L.J.; et al. Pharmacological activities and pharmacokinetics of liquiritin: A review. J. Ethnopharmacol. 2022, 293, 115257. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds, Glycyrrhizic Acid, Liquiritin and Liquiritigenin, in BV2 Cells and Mice Liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Ceccuzzi, G.; Rapino, A.; Perna, B.; Costanzini, A.; Farinelli, A.; Fiorica, I.; Marziani, B.; Cianci, A.; Rossin, F.; Cesaro, A.E.; et al. Liquorice Toxicity: A Comprehensive Narrative Review. Nutrients 2023, 15, 3866. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Y.-S.; Jiang, Y.; Peng, Y.-F.; Sun, Z.; Dai, X.-N.; Cao, Q.-T.; Sun, Y.-M.; Han, J.-C.; Gao, Y.-J. Targeted metabolomic study indicating glycyrrhizins protection against acetaminophen-induced liver damage through reversing fatty acid metabolism. Phytother. Res. 2014, 28, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, K.I.; Morinaga, O.; Yoshino, T.; Mitamura, M.; Hirasawa, A.; Maki, Y.; Tashita, Y.; Kondo, T.; Ogawa, K.; Lian, F.; et al. Identification of an Alternative Glycyrrhizin Metabolite Causing Liquorice-Induced Pseudohyperaldosteronism and the Development of ELISA System to Detect the Predictive Biomarker. Front. Pharmacol. 2021, 12, 688508, Erratum in Front. Pharmacol. 2022, 13, 1090327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Du, Q.; Zhu, Z.; Chen, T.; Xue, Y.; Wang, Y.; Zeng, Q.; Shen, C.; Jiang, C.; et al. Pharmacological Effects and Underlying Mechanisms of Licorice-Derived Flavonoids. Evid.-Based Complement. Altern. Med. 2022, 2022, 9523071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Peng, Q.; Shen, J.; Liu, H. Efficient analysis of toxicity and mechanisms of Acetyl tributyl citrate on aging with network toxicology and molecular docking strategy. Toxicology 2025, 510, 154009. [Google Scholar] [CrossRef]
- Bueso-Bordils, J.I.; Anton-Fos, G.M.; Martin-Algarra, R.; Aleman-Lopez, P.A. Overview of Computational Toxicology Methods Applied in Drug and Green Chemical Discovery. J. Xenobiotics 2024, 14, 1901–1918. [Google Scholar] [CrossRef]
- Vasiljev, T.G.; Salvioni, L.; Colombo, M.; Galli, P.; Greselin, F. From animal testing to in Silico models: A systematic review and practical guide to cosmetic assessment. Stat. Methods Appl. 2025, 34, 895–937. [Google Scholar] [CrossRef]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârțu, I.; Agoroaei, L. Current Trends in Toxicity Assessment of Herbal Medicines: A Narrative Review. Processes 2023, 11, 83. [Google Scholar] [CrossRef]
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol. 1978, 16, 255–276. [Google Scholar] [CrossRef]
- Munro, I.C.; Ford, R.A.; Kennepohl, E.; Sprenger, J.G. Correlation of structural class with no-observed-effect levels: A proposal for establishing a threshold of concern. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1996, 34, 829–867. [Google Scholar] [CrossRef] [PubMed]
- Kroes, R.; Renwick, A.G.; Cheeseman, M.; Kleiner, J.; Mangelsdorf, I.; Piersma, A.; Schilter, B.; Schlatter, J.; van Schothorst, F.; Vos, J.G.; et al. Structure-based thresholds of toxicological concern (TTC): Guidance for application to substances present at low levels in the diet. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2004, 42, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Reilly, K.; Ellis, L.J.A.; Davoudi, H.H.; Supian, S.; Maia, M.T.; Silva, G.H.; Guo, Z.L.; Martinez, D.S.T.; Lynch, I. Daphnia as a model organism to probe biological responses to nanomaterials-from individual to population effects via adverse outcome pathways. Front. Toxicol. 2023, 5. [Google Scholar] [CrossRef]
- Patlewicz, G.; Jeliazkova, N.; Safford, R.J.; Worth, A.P.; Aleksiev, B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ. Res. 2008, 19, 495–524. [Google Scholar] [CrossRef]
- Simon, T.W.; Ryman, J.; Becker, R.A. Commentary: Value of information case study strongly supports use of the Threshold of Toxicological Concern (TTC). Regul. Toxicol. Pharmacol. 2024, 149, 28. [Google Scholar] [CrossRef]
- Patlewicz, G.; Wambaugh, J.F.; Felter, S.P.; Simon, T.W.; Becker, R.A. Utilizing Threshold of Toxicological Concern (TTC) with High Throughput Exposure Predictions (HTE) as a Risk-Based Prioritization Approach for thousands of chemicals. Comput. Toxicol. 2018, 7, 58–67. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, E.Y.; Nam, S.J.; Lim, K.M. Safety assessment of Paeonia lactiflora root extract for a cosmetic ingredient employing the threshold of toxicological concern (TTC) approach. Regul. Toxicol. Pharmacol. 2024, 149, 12. [Google Scholar] [CrossRef]
- Lancia, P.; Louazzani, M.; Gros, L.; Ginestar, J.; Fioravanzo, E.; Baleydier, A. Overview of In Silico Tools to Evaluate Human Health Toxicity, Ecotoxicity, and Toxicokinetic Profiles in the Hazard Assessment of Chemicals Used in Cosmetics. Chem. Res. Toxicol. 2025, 38, 1652–1680. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat. Rev. Cancer 2006, 6, 803–812. [Google Scholar] [CrossRef]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zheng, Y.X.; Xu, F.; Cui, Y.Z.; Chen, X.Y.; Chen, S.Q.; Yan, B.X.; Zhou, Y.; Zheng, M.; Man, X.Y. Epidermal keratinocyte-specific STAT3 deficiency aggravated atopic dermatitis-like skin inflammation in mice through TSLP upregulation. Front. Immunol. 2023, 14, 1273182. [Google Scholar]
- Szilveszter, K.P.; Németh, T.; Mócsai, A. Tyrosine Kinases in Autoimmune and Inflammatory Skin Diseases. Front. Immunol. 2019, 10, 1862. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.J.; Kim, J.M.; Kang, H.J.; Cho, S.H.; Chang, S.E. Epidermal Growth Factor Relieves Inflammatory Signals in Staphylococcus aureus-Treated Human Epidermal Keratinocytes and Atopic Dermatitis-Like Skin Lesions in Nc/Nga Mice. Biomed. Res. Int. 2018, 15, 9439182. [Google Scholar]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar] [CrossRef]
- Devillers, J.; Mombelli, E. Evaluation of the OECD QSAR Application Toolbox and Toxtree for estimating the mutagenicity of chemicals. Part 2. alpha-beta unsaturated aliphatic aldehydes. SAR QSAR Environ. Res. 2010, 21, 771–783. [Google Scholar] [CrossRef]
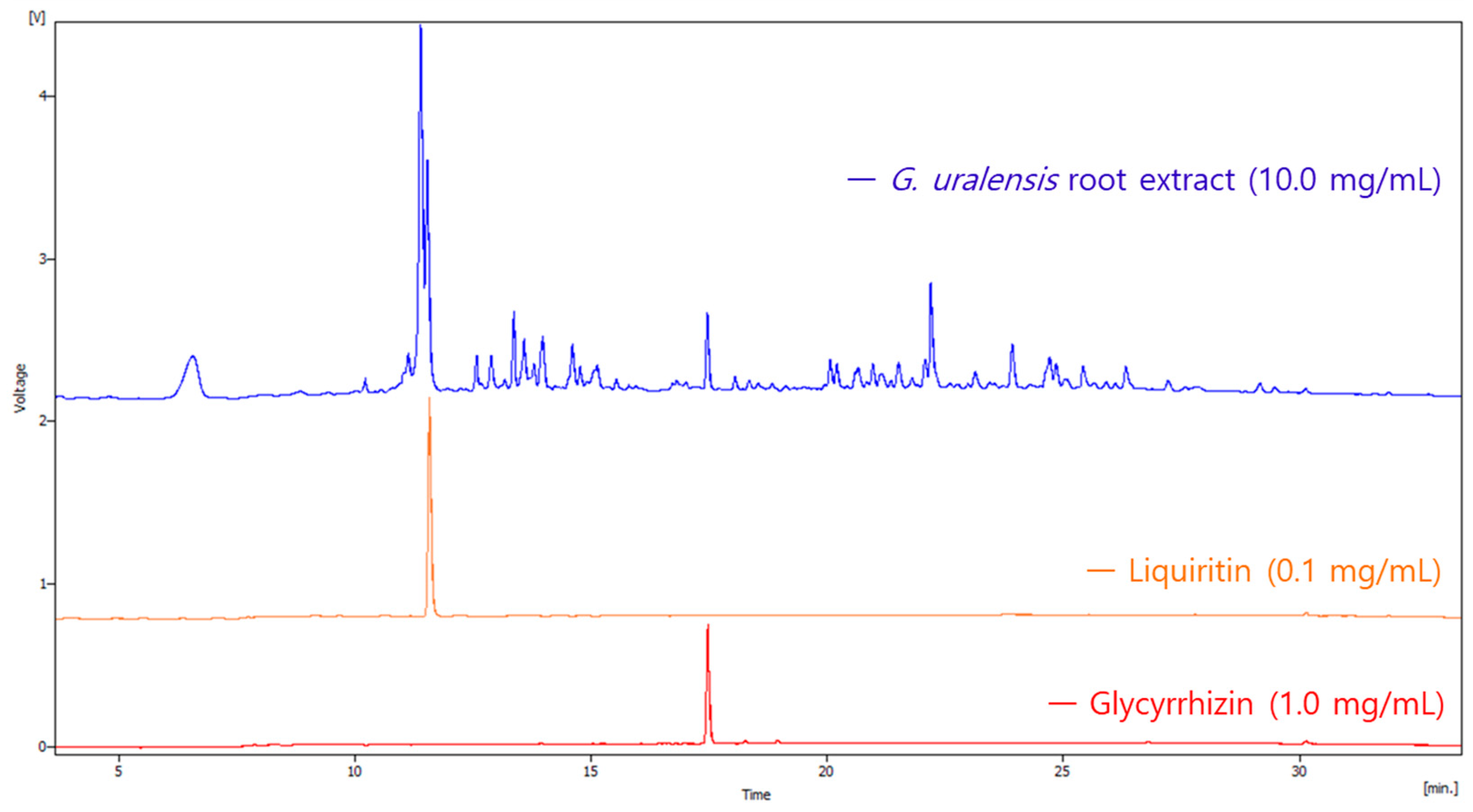
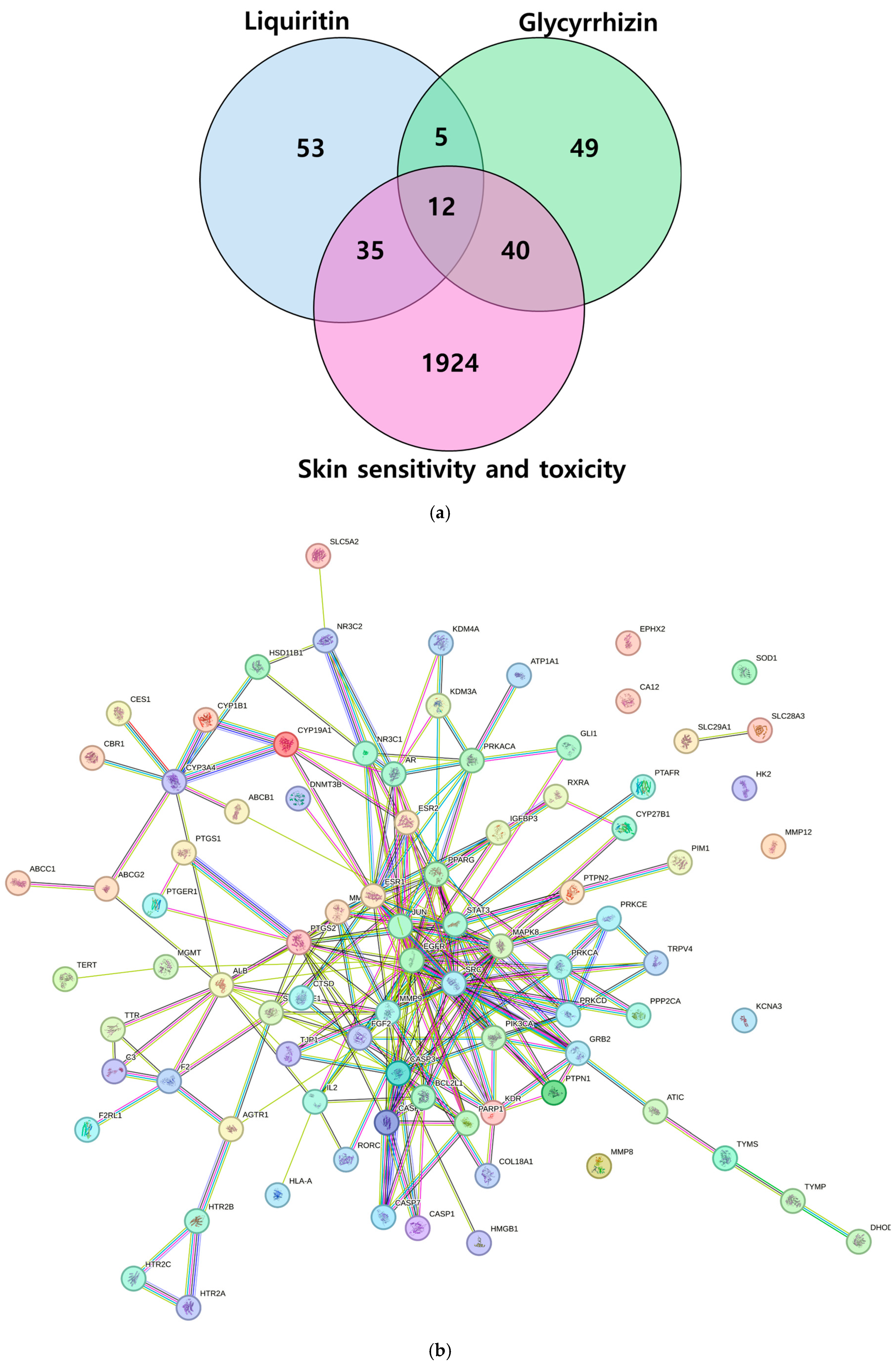
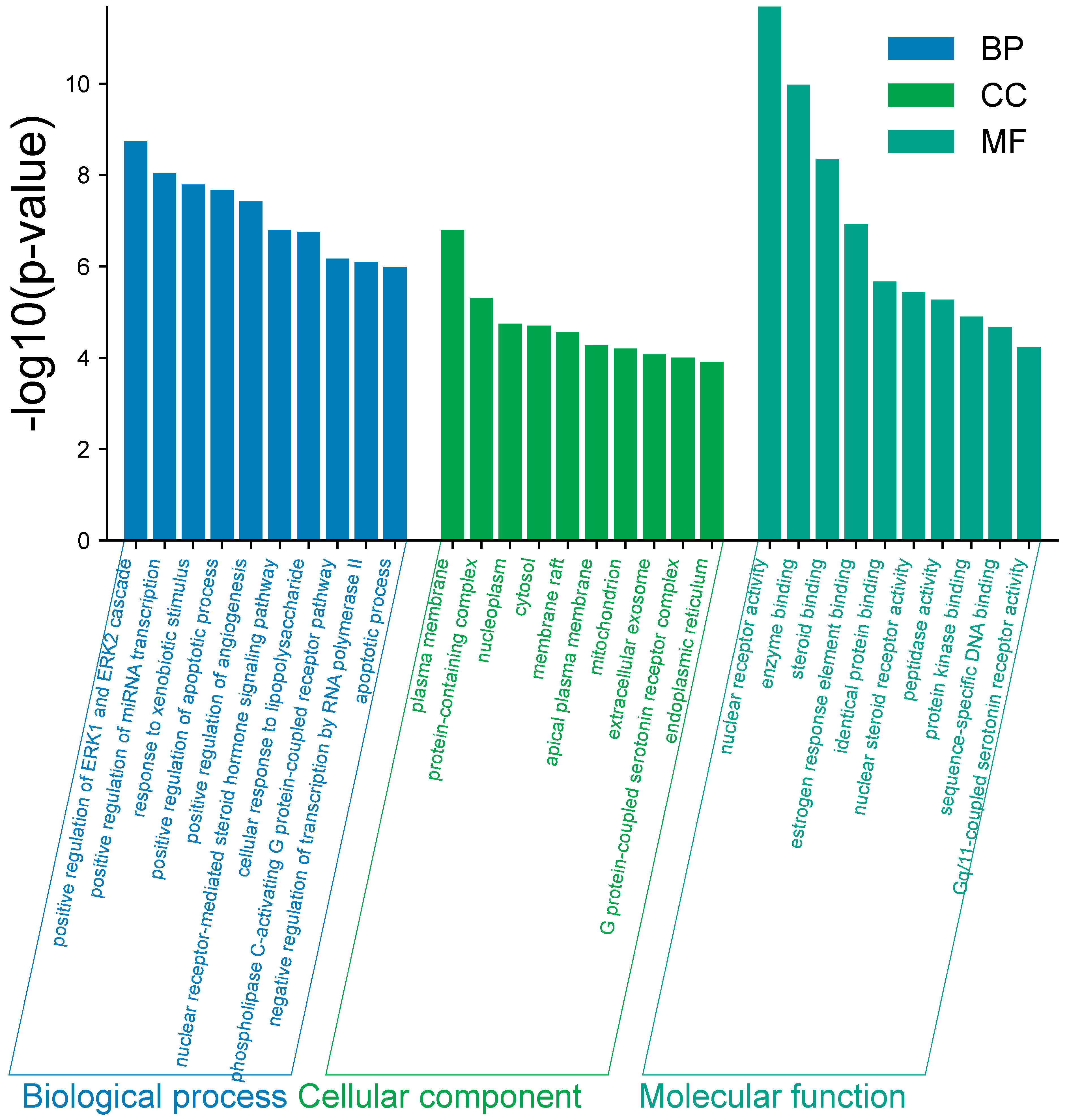
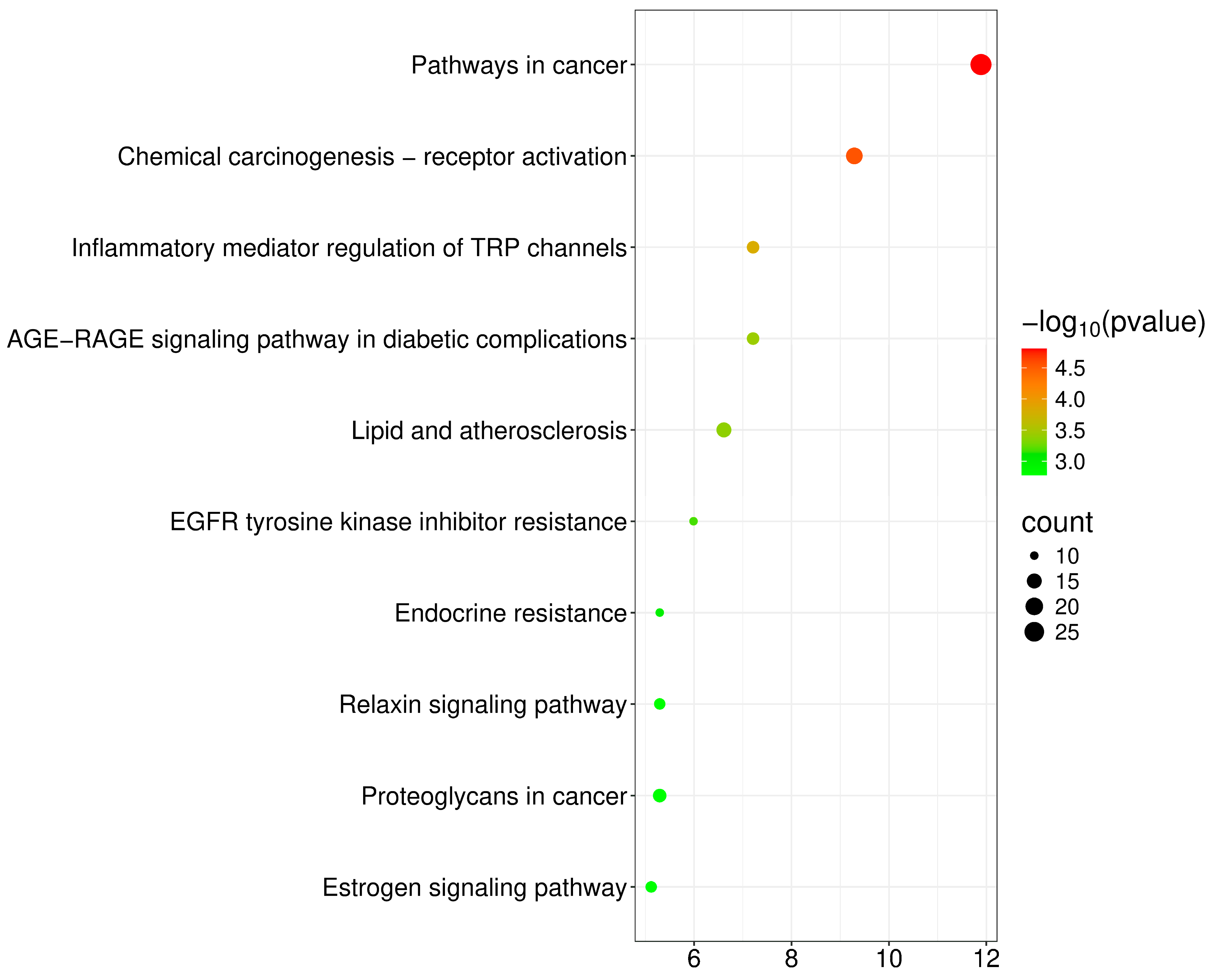
| Analyte | Concentration Range (μg/mL) | Linear Regression Equation | r2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Liquiritin | 6.25–1250 | y = 573.21x − 1.3025 | 0.9994 | 1.5 | 6.25 |
| Glycyrrhizin | 12.5–2500 | y = 388.75x + 0.5123 | 0.9997 | 3.1 | 12.5 |
| Compound | MW (g/mol) | n-ROTB | HBA | HBD | MR | cLogP | TPSA |
|---|---|---|---|---|---|---|---|
| Liquiritin | 418.4 | 4 | 16 | 5 | 101.67 | 0.4 | 145.91 |
| Glycyrrhizin | 822.93 | 7 | 9 | 8 | 202.84 | 1.49 | 267.04 |
| Compound (Ligand) | Target (PDB ID) | Binding Affinity (kcal/mol) |
|---|---|---|
| Liquiritin | EGFR (4I23) | −9.7 |
| STAT3 (6TLC) | −8.1 | |
| SRC (1YOJ) | −9.8 | |
| Glycyrrhizin | EGFR (4I23) | −9.0 |
| STAT3 (6TLC) | −9.5 | |
| SRC (1YOJ) | −9.6 |
| Toxicity | Liquiritin | Glycyrrhizin |
|---|---|---|
| Skin sensitization | Inactive | Inactive |
| Skin irritation | Non-irritating | Non-irritating |
| Compound/ Product Type | A = Use Amount (g/day) | Cprod (%) | SED (µg/kg bw/day) | TTC (µg/kg bw/day) | Cmax (%) | Risk Characterization |
|---|---|---|---|---|---|---|
| Liquiritin /Face cream (leave-on) | 1.54 | 0.011 | 1.41 | 1.5 | 0.0117 | SED < TTC |
| Glycyrrhizin/ Face cream (leave-on) | 1.54 | 0.015 | 1.93 | 1.5 | 0.0117 | SED > TTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Park, K.; Kim, Y.B.; Kim, M. Integrative Assessment of Glycyrrhiza uralensis Extract in Cosmetics Using HPLC Analysis, Network Pharmacology, and Computational Threshold of Toxicological Concern-Based Safety Evaluation. Int. J. Mol. Sci. 2025, 26, 11677. https://doi.org/10.3390/ijms262311677
Kim H, Park K, Kim YB, Kim M. Integrative Assessment of Glycyrrhiza uralensis Extract in Cosmetics Using HPLC Analysis, Network Pharmacology, and Computational Threshold of Toxicological Concern-Based Safety Evaluation. International Journal of Molecular Sciences. 2025; 26(23):11677. https://doi.org/10.3390/ijms262311677
Chicago/Turabian StyleKim, Hiyoung, Kihoon Park, Young Bong Kim, and Minjee Kim. 2025. "Integrative Assessment of Glycyrrhiza uralensis Extract in Cosmetics Using HPLC Analysis, Network Pharmacology, and Computational Threshold of Toxicological Concern-Based Safety Evaluation" International Journal of Molecular Sciences 26, no. 23: 11677. https://doi.org/10.3390/ijms262311677
APA StyleKim, H., Park, K., Kim, Y. B., & Kim, M. (2025). Integrative Assessment of Glycyrrhiza uralensis Extract in Cosmetics Using HPLC Analysis, Network Pharmacology, and Computational Threshold of Toxicological Concern-Based Safety Evaluation. International Journal of Molecular Sciences, 26(23), 11677. https://doi.org/10.3390/ijms262311677






