Significance of Vitamins A and E in Cancer Progression and Prevention
Abstract
1. Introduction
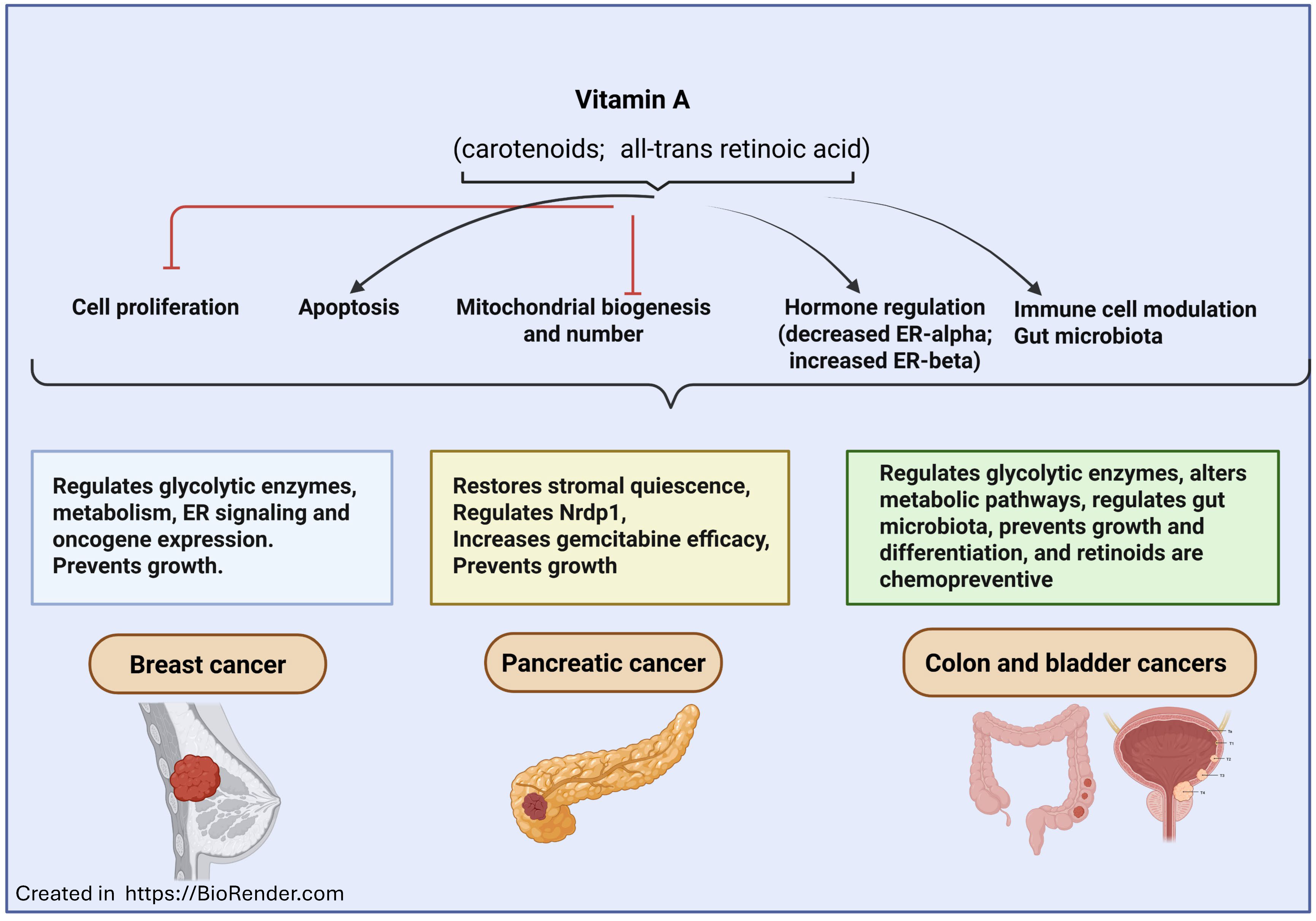
2. Vitamin A in Cancer Prevention and Treatment
2.1. Vitamin A in Acute Promyelocytic Leukemia
2.2. Vitamin A in Non-Melanoma Skin Cancers
2.3. Vitamin A in Melanoma
2.4. Vitamin A in Breast, Lung, and Head and Neck Cancers
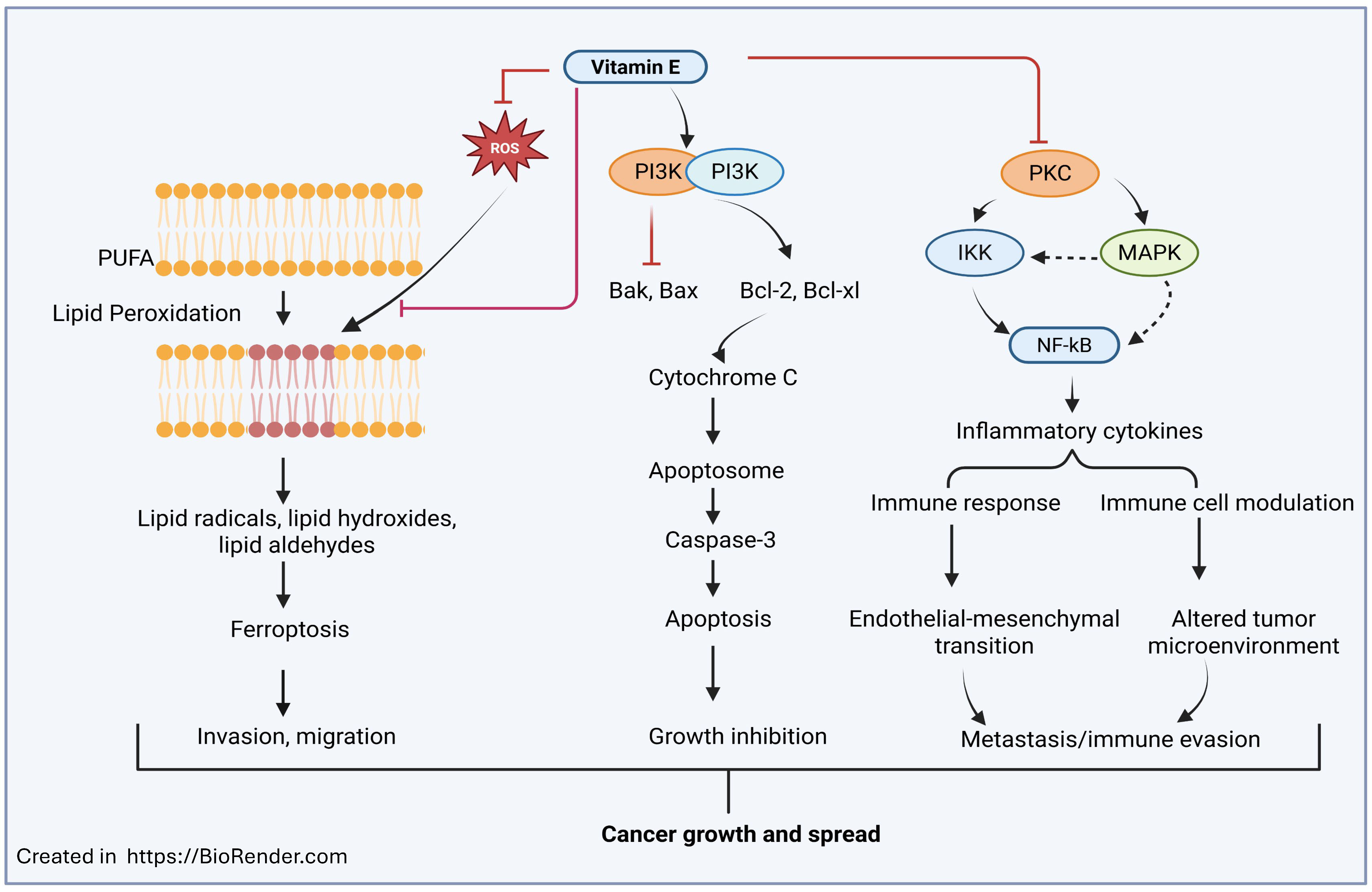
3. Vitamin E in Cancer Prevention and Treatment
3.1. Vitamin E in Colorectal Cancer
3.2. Vitamin E in Lung Cancer
3.3. Vitamin E in Prostate Cancer
3.4. Vitamin E in Breast Cancer
3.5. Vitamin E in Pancreatic Cancer
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrès, E.; Lorenzo-Villalba, N.; Terrade, J.E.; Méndez-Bailon, M. Fat-Soluble Vitamins A, D, E, and K: Review of the Literature and Points of Interest for the Clinician. J. Clin. Med. 2024, 13, 3641. [Google Scholar] [CrossRef]
- Siener, R.; Machaka, I.; Alteheld, B.; Bitterlich, N.; Metzner, C. Effect of Fat-Soluble Vitamins A, D, E and K on Vitamin Status and Metabolic Profile in Patients with Fat Malabsorption with and without Urolithiasis. Nutrients 2020, 12, 3110. [Google Scholar] [CrossRef]
- English, K.; Uwibambe, C.; Daniels, P.; Dzukey, E. Scoping Review of Micronutrient Imbalances, Clinical Manifestations, and Interventions. World J. Methodol. 2025, 15, 107664. [Google Scholar] [CrossRef]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient Balance and Micronutrient Amounts through Growth and Development. Ital. J. Pediatr. 2021, 47, 109. [Google Scholar] [CrossRef]
- Omer, E.; Chiodi, C. Fat Digestion and Absorption: Normal Physiology and Pathophysiology of Malabsorption, Including Diagnostic Testing. Nutr. Clin. Pract. 2024, 39 (Suppl. S1), S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Mârza, S.M.; Papuc, I. The Immunomodulatory Effects of Vitamins in Cancer. Front. Immunol. 2024, 15, 1464329. [Google Scholar] [CrossRef]
- Panda, P.K.; Saraf, S.; Verma, A.; Jain, A.; Bidla, P.D.; Raikwar, S.; Kumari, P.; Jain, S.K. Role of Vitamins in Therapeutic and Targeting Approaches for Prostate Cancer: An Overview. Curr. Drug Targets 2024, 25, 934–952. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.V.T.; Nguyen, Y.T.; Kim, N.; Lee, H.J. Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity. Int. J. Mol. Sci. 2023, 24, 17038. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, K.; Kiślak, J.; Orywal, K.; Zajkowska, M. Vitamins in the Pathogenesis of Prostate Cancer: Implications for Prevention and Therapeutic Support. Int. J. Mol. Sci. 2025, 26, 4336. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Tokumura, K.; Yoshimoto, M.; Hinoi, E. Association between Fat-Soluble Vitamin Metabolic Process and Glioma Progression. Biol. Pharm. Bull. 2024, 47, 1682–1689. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.; Zhang, T.; Pu, X. Fat-Soluble Vitamins and Lung Cancer: Where We Are? Recent Pat. Anticancer Drug Discov. 2024, 20, 521–531. [Google Scholar] [CrossRef]
- Mekky, R.Y.; Elemam, N.M.; Eltahtawy, O.; Zeinelabdeen, Y.; Youness, R.A. Evaluating Risk: Benefit Ratio of Fat-Soluble Vitamin Supplementation to SARS-CoV-2-Infected Autoimmune and Cancer Patients: Do Vitamin-Drug Interactions Exist? Life 2022, 12, 1654. [Google Scholar] [CrossRef]
- Palanca, A.; Ampudia-Blasco, F.J.; Real, J.T. The Controversial Role of Vitamin D in Thyroid Cancer Prevention. Nutrients 2022, 14, 2593. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Jang, J.H.; Lee, S.Y. An Updated Comprehensive Review on Vitamin A and Carotenoids in Breast Cancer: Mechanisms, Genetics, Assessment, Current Evidence, and Future Clinical Implications. Nutrients 2021, 13, 3162. [Google Scholar] [CrossRef]
- De Flora, S.; Bagnasco, M.; Vainio, H. Modulation of Genotoxic and Related Effects by Carotenoids and Vitamin A in Experimental Models: Mechanistic Issues. Mutagenesis 1999, 14, 153–172. [Google Scholar] [CrossRef]
- Talib, W.H.; Ahmed Jum’AH, D.A.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: Therapeutic potentials and mechanisms of action. Front Nutr. 2024, 10, 1281879. [Google Scholar] [CrossRef]
- Misotti, A.M.; Gnagnarella, P. Vitamin Supplement Consumption and Breast Cancer Risk: A Review. Ecancermedicalscience 2013, 7, 365. [Google Scholar] [CrossRef]
- Darouei, B.; Bohn, T.; Vahid, F.; Amani-Beni, R.; Haghjooy Javanmard, S.; Zendehdel, K.; Abdollahpour, I. Dietary Carotenoids and Breast Cancer Risk: Evidence from a Large Population-Based Incident Case-Control Study. Nutr. Metab. 2025, 22, 107. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, X.; Huang, Y. Associations between vitamins intake and risk of cancer in United States adults: 2003 to 2016 National Health and Nutrition Examination Survey. Front. Nutr. 2025, 12, 1561251. [Google Scholar] [CrossRef] [PubMed]
- Frost, Z.; Bakhit, S.; Amaefuna, C.N.; Powers, R.V.; Ramana, K.V. Recent Advances on the Role of B Vitamins in Cancer Prevention and Progression. Int. J. Mol. Sci. 2025, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Li, T.; Li, X.; Hou, L.; Sun, W. Vitamin A and Its Related Diseases. Food Sci. Nutr. 2025, 13, e70630. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Židzik, J.; Mičková, H. Therapeutic Uses of Retinol and Retinoid-Related Antioxidants. Molecules 2025, 30, 2191. [Google Scholar] [CrossRef]
- Dawson, M.I. The Importance of Vitamin A in Nutrition. Curr. Pharm. Des. 2000, 6, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Carotenoids, β-Apocarotenoids, and Retinoids: The Long and the Short of It. Nutrients 2022, 14, 1411. [Google Scholar] [CrossRef]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef]
- Swigris, J.; Widjaja-Adhi, M.A.K.; Golczak, M. Retinoid Dynamics in Vision: From Visual Cycle Biology to Retina Disease Treatments. Pharmacol. Ther. 2025, 273, 108902. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.D.; Salom, D.; Kochman, M.A.; Kubas, A.; Kiser, P.D.; Palczewski, K. Chromophore Hydrolysis and Release from Photoactivated Rhodopsin in Native Membranes. Proc. Natl. Acad. Sci. USA 2022, 119, e2213911119. [Google Scholar] [CrossRef]
- Abadie, R.B.; Staples, A.A.; Lauck, L.V.; Dautel, A.D.; Spillers, N.J.; Klapper, R.J.; Hirsch, J.D.; Varrassi, G.; Ahmadzadeh, S.; Shekoohi, S.; et al. Vitamin A-Mediated Birth Defects: A Narrative Review. Cureus 2023, 15, e50513. [Google Scholar] [CrossRef]
- Shoeibi, N.; Khazaei, S.; Motamed Shariati, M. Xerophthalmia and Nyctalopia as Presenting Signs of Vitamin A Deficiency in a Patient with Rapid Intentional Weight Loss: A Case Report and Literature Review. Clin. Case Rep. 2025, 13, e70896. [Google Scholar] [CrossRef]
- Medina-García, M.; Baeza-Morales, A.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. Carotenoids and Their Interaction with the Immune System. Antioxidants 2025, 14, 1111. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; Gil, J.V.; Genovés, S.; Ramón, D.; Zacarías, L.; Rodrigo, M.J. Evaluation of Carotenoids Protection Against Oxidative Stress in the Animal Model Caenorhabditis elegans. Methods Mol. Biol. 2020, 2083, 387–401. [Google Scholar] [CrossRef]
- Yang, H.; Gu, J.; Zhu, Q.; Lu, H.; Wang, K.; Ni, X.; Lu, Y.; Lu, L. Protection of Acute GVHD by All-Trans Retinoic Acid through Suppression of T Cell Expansion and Induction of Regulatory T Cells through IL-2 Signaling. Int. Immunopharmacol. 2015, 28, 911–916. [Google Scholar] [CrossRef]
- Jeong, M.; Cortopassi, F.; See, J.X.; De La Torre, C.; Cerwenka, A.; Stojanovic, A. Vitamin A-Treated Natural Killer Cells Reduce Interferon-Gamma Production and Support Regulatory T-Cell Differentiation. Eur. J. Immunol. 2024, 54, e2250342. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins A and D Take Centre Stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Giordano, E.; Hammerling, U.; Champaneri, D.; von Lintig, J.; Hussain, M.M.; Quadro, L. The Intestine-Specific Homeobox (ISX) Modulates β-Carotene-Dependent Regulation of Microsomal Triglyceride Transfer Protein (MTP) in a Tissue-Specific Manner. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159584. [Google Scholar] [CrossRef]
- Kołodziejczyk, A.M.; Karwowski, B. Anti-DNA Damage Mechanisms and the Role of Carotenoids, Vitamin A, and Its Derivatives. Nutrients 2025, 17, 2721. [Google Scholar] [CrossRef]
- Xie, G.; Cao, S.; Wang, G.; Zhang, X.; Zhang, Y.; Wu, H.; Shen, S.; Le, J.; Li, K.; Huang, Z. Vitamin A and Its Influence on Tumour Extracellular Matrix. Discov. Oncol. 2025, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Everts, H.B.; Akuailou, E.N. Retinoids in Cutaneous Squamous Cell Carcinoma. Nutrients 2021, 13, 153. [Google Scholar] [CrossRef]
- Karthik, N.; Sharma, S. Vitamin A Deficiency Masquerading as Cancer-Associated Retinopathy. Can. J. Ophthalmol. 2025, 60, e779–e781. [Google Scholar] [CrossRef]
- Ocadiz-Delgado, R.; Serafin-Higuera, N.; Alvarez-Rios, E.; García-Villa, E.; Tinajero-Rodríguez, M.; Rodríguez-Uribe, G.; Escobar-Wilches, D.C.; Estela Albino-Sánchez, M.; Ramírez-Rosas, A.; Sierra-Santoyo, A.; et al. Vitamin A deficiency in K14E7HPV expressing transgenic mice facilitates the formation of malignant cervical lesions. APMIS 2021, 129, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Damaj, N.; Elias, N.; Zeidan, T.; Kattan, J. Understanding the differentiation syndrome in acute promyelocytic leukemia: A comprehensive updated review. Invest. New Drugs 2025, 43, 750–756. [Google Scholar] [CrossRef]
- Zhang, A.; Qiu, S. Advances in RARα fusion genes in acute promyelocytic leukemia. Exp. Hematol. 2025, 149, 104822. [Google Scholar] [CrossRef]
- Liu, S.; Zhan, W.; He, X.; Hao, M.; Shen, W.; Zhang, X.; Wang, M.; Li, Z.; Hou, R.; Ou, Z.; et al. ATPR induces acute promyelocytic leukemia cells differentiation and cycle arrest via the lncRNA CONCR/DDX11/PML-RARα signaling axis. Gene 2024, 917, 148443. [Google Scholar] [CrossRef]
- Zhou, G.B.; Zhang, J.; Wang, Z.Y.; Chen, S.J.; Chen, Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: A paradigm of synergistic molecular targeting therapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 959–971. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.Y.; Pereira-Martins, D.A.; Weinhäuser, I.; Ortiz, C.; Cândido, L.A.; Lange, A.P.; De Abreu, N.F.; Mendonza, S.E.S.; de Deus Wagatsuma, V.M.; Do Nascimento, M.C.; et al. The combination of gefitinib with ATRA and ATO induces myeloid differentiation in acute promyelocytic leukemia resistant cells. Front. Oncol. 2021, 11, 686445. [Google Scholar] [CrossRef]
- Li, L.; Xi, H.M.; Lu, H.; Cai, X. Combination of ethacrynic acid and ATRA triggers differentiation and/or apoptosis of acute myeloid leukemia cells through ROS. Anticancer Agents Med. Chem. 2024, 24, 412–422. [Google Scholar] [CrossRef]
- Hu, L.; Li, Q.; Wang, J.; Wang, H.; Ren, X.; Huang, K.; Wang, Y.; Liang, X.; Pu, L.; Xiong, S.; et al. The CDK4/6 inhibitor palbociclib synergizes with ATRA to induce differentiation in AML. Mol. Cancer Ther. 2024, 23, 961–972. [Google Scholar] [CrossRef]
- Xi, H.M.; Lu, H.; Weng, X.Q.; Sheng, Y.; Wu, J.; Li, L.; Cai, X. Combined application of salinomycin and ATRA induces apoptosis and differentiation of acute myeloid leukemia cells by inhibiting WNT/β-catenin pathway. Anticancer Agents Med. Chem. 2023, 23, 1074–1084. [Google Scholar] [CrossRef]
- Parsa, L.; Motafakkerazad, R.; Soheyli, S.T.; Haratian, A.; Kosari-Nasab, M.; Mahdavi, M. Silymarin in combination with ATRA enhances apoptosis induction in human acute promyelocytic NB4 cells. Toxicon 2023, 228, 107127. [Google Scholar] [CrossRef] [PubMed]
- Samarkhazan, N.S.; Yekta, R.; Sayadi, M.; Tackallou, S.H.; Safaralizadeh, R.; Mahdavi, M. 2-NDC from dithiocarbamates improves ATRA efficiency and ROS-induced apoptosis via downregulation of Bcl2 and Survivin in human acute promyelocytic NB4 cells. Hum. Exp. Toxicol. 2020, 39, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Liu, J.; Sato, Y.; Miyake, R.; Suzuki, S.; Okitsu, Y.; Fukuda, T.; Isaji, T.; Gu, J.; Takahashi, S. Fucosylation inhibitor 6-alkynylfucose enhances the ATRA-induced differentiation effect on acute promyelocytic leukemia cells. Biochem. Biophys. Res. Commun. 2024, 710, 149541. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Moseley, A.B.; Coutre, S.E.; DeAngelo, D.J.; Othus, M.; Tallman, M.S.; Litzow, M.R.; Komrokji, R.S.; Erba, H.P.; Appelbaum, F.R. A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535). Blood Adv. 2020, 4, 1683–1689. [Google Scholar] [CrossRef]
- Jen, W.Y.; Marvin-Peek, J.; Kantarjian, H.M.; Alvarado, Y.; Borthakur, G.; Jabbour, E.; Wierda, W.; Kadia, T.M.; Daver, N.G.; DiNardo, C.D.; et al. Long-term follow-up of a phase 2 study of all-trans retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin in acute promyelocytic leukemia. Cancer 2025, 131, e35662. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gong, S.; Li, G.H.; Yao, Y.Z.; Zheng, Y.S.; Lu, X.H.; Wei, S.H.; Qin, W.W.; Liu, H.B.; Wang, M.C.; et al. An effective and chemotherapy-free strategy of all-trans retinoic acid and arsenic trioxide for acute promyelocytic leukemia in all risk groups (APL15 trial). Blood Cancer J. 2022, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Ramchatesingh, B.; Martínez Villarreal, A.; Arcuri, D.; Lagacé, F.; Setah, S.A.; Touma, F.; Al-Badarin, F.; Litvinov, I.V. The use of retinoids for the prevention and treatment of skin cancers: An updated review. Int. J. Mol. Sci. 2022, 23, 12622. [Google Scholar] [CrossRef]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef]
- Siddikuzzaman; Grace, V.M. Anti-metastatic study of liposome-encapsulated all-trans retinoic acid (ATRA) in B16F10 melanoma cells-implanted C57BL/6 mice. Cancer Investig. 2014, 32, 507–517. [Google Scholar] [CrossRef]
- Melnik, B.C. p53: Key conductor of all anti-acne therapies. J. Transl. Med. 2017, 15, 195. [Google Scholar] [CrossRef]
- Du, Y.; Li, L.L.; Chen, H.; Wang, C.; Qian, X.W.; Feng, Y.B.; Zhang, L.; Chen, F.H. A novel all-trans retinoic acid derivative inhibits proliferation and induces apoptosis of myelodysplastic syndromes cell line SKM-1 cells via up-regulating p53. Int. Immunopharmacol. 2018, 65, 561–570. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Hu, H.; Cai, Y.; Yang, T.; Liu, O. All-trans retinoic acid exacerbates Stevens–Johnson syndrome and toxic epidermal necrolysis via TNF signaling pathways: A network toxicology, molecular docking, and experimental study. Toxicol. Appl. Pharmacol. 2025, 504, 117542. [Google Scholar] [CrossRef]
- Ianhez, M.; Fleury, L.F., Jr.; Miot, H.A.; Bagatin, E. Retinoids for prevention and treatment of actinic keratosis. An. Bras. Dermatol. 2013, 88, 585–593. [Google Scholar] [CrossRef]
- Shalinsky, D.R.; Bischoff, E.D.; Gregory, M.L.; Gottardis, M.M.; Hayes, J.S.; Lamph, W.W.; Heyman, R.A.; Shirley, M.A.; Cooke, T.A.; Davies, P.J.; et al. Retinoid-induced suppression of squamous cell differentiation in human oral squamous cell carcinoma xenografts (line 1483) in athymic nude mice. Cancer Res. 1995, 55, 3183–3191. [Google Scholar]
- Shapiro, S.S.; Seiberg, M.; Cole, C.A. Vitamin A and its derivatives in experimental photocarcinogenesis: Preventive effects and relevance to humans. J. Drugs Dermatol. 2013, 12, 458–463. [Google Scholar]
- Kraemer, K.H.; DiGiovanna, J.J.; Moshell, A.N.; Tarone, R.E.; Peck, G.L. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N. Engl. J. Med. 1988, 318, 1633–1637. [Google Scholar] [CrossRef]
- Mahamat-Saleh, Y.; Savoye, I.; Cervenka, I.; Al-Rahmoun, M.; Cadeau, C.; Boutron-Ruault, M.C.; Kvaskoff, M. Dietary antioxidant supplements and risk of keratinocyte cancers in women: A prospective cohort study. Eur. J. Nutr. 2022, 61, 2825–2836. [Google Scholar] [CrossRef]
- El Hajj, H.; Khalil, B.; Ghandour, B.; Nasr, R.; Shahine, S.; Ghantous, A.; Abdel-Samad, R.; Sinjab, A.; Hasegawa, H.; Jabbour, M.; et al. Preclinical efficacy of the synthetic retinoid ST1926 for treating adult T-cell leukemia/lymphoma. Blood 2014, 124, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Goli, M.; Sandilya, V.; Ghandour, B.; Hajj, H.E.; Kobeissy, F.; Darwiche, N.; Mechref, Y. Exploring the anti-leukemic effect of the synthetic retinoid ST1926 on malignant T cells: A comprehensive proteomics approach. Int. J. Mol. Sci. 2025, 26, 4651. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Galvan, M.; Santoni, F.; Alvarez, S.; de Lera, A.R.; Ivanova, D.; Gronemeyer, H.; Caruso, A.; Guidoboni, M.; Cassai, E.; et al. Retinoic acid analogues inhibit human herpesvirus 8 replication. Antivir. Ther. 2008, 13, 199–209. [Google Scholar] [CrossRef] [PubMed]
- González de Arriba, A.; Pérez-Gala, S.; Goiriz-Valdés, R.; Ríos-Buceta, L.; García-Díez, A. Sarcoma de Kaposi clásico tratado con alitretinoína tópica [Kaposi’s sarcoma treated with topical alitretinoin]. Actas Dermosifiliogr. 2007, 98, 50–53. [Google Scholar] [CrossRef]
- Oliveira, S.; Costa, J.; Faria, I.; Guerreiro, S.G.; Fernandes, R. Vitamin A enhances macrophages activity against B16-F10 malignant melanocytes: A new player for cancer immunotherapy? Medicina 2019, 55, 604. [Google Scholar] [CrossRef]
- Jobani, B.M.; Najafzadeh, N.; Mazani, M.; Arzanlou, M.; Vardin, M.M. Molecular mechanism and cytotoxicity of allicin and all-trans retinoic acid against CD44+ versus CD117+ melanoma cells. Phytomedicine 2018, 48, 161–169. [Google Scholar] [CrossRef]
- Kanai, M.; Shinagawa, A.; Ota, M.; Virgona, N.; Yano, T. Resveratrol can differentiate human melanoma stem-like cells from spheroids treated with all-trans retinoic acid. Anticancer Res. 2024, 44, 5283–5292. [Google Scholar] [CrossRef]
- Wang, Y.B.; Sun, B.; Han, B. Retinoic acid increases the anticancer effect of paclitaxel by inducing differentiation of cancer stem cells in melanoma. Pharmazie 2018, 73, 729–732. [Google Scholar] [CrossRef]
- Kim, G.; Bhattarai, P.Y.; Oh, C.H.; Choi, H.S. All-trans retinoic acid overcomes acquired resistance to PLX4032 via inhibition of PIN1 in melanoma cells. Anticancer Res. 2019, 39, 6537–6546. [Google Scholar] [CrossRef]
- Grace, V.M.B.; Saranya, S.; Wilson, D.D. Protective role of all-trans retinoic acid on B16F10 melanoma cell line metastasis in C57BL/6 mice by enhancing RAR-β protein and homeostasis maintenance. J. Histotechnol. 2021, 44, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Song, Y.; Liu, Q.; Wu, Y.; He, R. Topical treatment of all-trans retinoic acid inhibits murine melanoma partly by promoting CD8+ T-cell immunity. Immunology 2017, 152, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, X.; An, Q.; Zhang, Y.; Li, K.; Yao, W.; Shi, F.; Pan, Y.; Jia, Q.; Zhou, W.; et al. Inhibition of cancer stem cell like cells by a synthetic retinoid. Nat. Commun. 2018, 9, 1406. [Google Scholar] [CrossRef] [PubMed]
- Tobin, R.P.; Cogswell, D.T.; Cates, V.M.; Davis, D.M.; Borgers, J.S.W.; Van Gulick, R.J.; Katsnelson, E.; Couts, K.L.; Jordan, K.R.; Gao, D.; et al. Targeting MDSC differentiation using ATRA: A phase I/II clinical trial combining pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin. Cancer Res. 2023, 29, 1209–1219. [Google Scholar] [CrossRef]
- Tobin, R.P.; Jordan, K.R.; Robinson, W.A.; Davis, D.; Borges, V.F.; Gonzalez, R.; Lewis, K.D.; McCarter, M.D. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with ipilimumab. Int. Immunopharmacol. 2018, 63, 282–291. [Google Scholar] [CrossRef]
- Mittal, V.; So, J.Y.; Li, S.; Swetter, S.M.; Linos, E.; Van Horn, L.; Neuhouser, M.L.; Stefanick, M.L.; Tang, J.Y. Associations between dietary and supplemental vitamin A intake and melanoma and non-melanoma skin cancer. Skin Health Dis. 2024, 4, e462. [Google Scholar] [CrossRef]
- Aborode, A.T.; Onifade, I.A.; Olorunshola, M.M.; Adenikinju, G.O.; Aruorivwooghene, I.J.; Femi, A.C.; Osayawe, O.J.; Osinuga, A.; Omojowolo, E.A.; Adeoye, A.F.; et al. Biochemical mechanisms and molecular interactions of vitamins in cancer therapy. Cancer Pathog. Ther. 2024, 3, 3–15. [Google Scholar] [CrossRef]
- Manoochehri, H.; Farrokhnia, M.; Sheykhhasan, M.; Mahaki, H.; Tanzadehpanah, H. Key target genes related to anti-breast cancer activity of ATRA: A network pharmacology, molecular docking, and experimental investigation. Heliyon 2024, 10, e34300. [Google Scholar] [CrossRef]
- Peng, C.; Zeleznik, O.A.; Shutta, K.H.; Rosner, B.A.; Kraft, P.; Clish, C.B.; Stampfer, M.J.; Willett, W.C.; Tamimi, R.M.; Eliassen, A.H. A metabolomics analysis of circulating carotenoids and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2022, 31, 85–96. [Google Scholar] [CrossRef]
- Dehnavi, M.K.; Ebrahimpour-Koujan, S.; Lotfi, K.; Azadbakht, L. The association between circulating carotenoids and risk of breast cancer: A systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2024, 15, 100135. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Lu, M.S.; Wang, L.; Mo, X.F.; Luo, W.P.; Du, Y.F.; Zhang, C.X. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: A case–control study. Br. J. Nutr. 2016, 115, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 1197–1205. [Google Scholar] [CrossRef]
- El Habre, R.; Aoun, R.; Tahtouh, R.; Hilal, G. All-trans-retinoic acid modulates glycolysis via H19 and telomerase: The role of miR-let-7a in estrogen receptor-positive breast cancer cells. BMC Cancer 2024, 24, 615. [Google Scholar] [CrossRef] [PubMed]
- Caricasulo, M.A.; Zanetti, A.; Terao, M.; Garattini, E.; Paroni, G. Cellular and micro-environmental responses influencing the antitumor activity of all-trans retinoic acid in breast cancer. Cell Commun. Signal. 2024, 22, 127. [Google Scholar] [CrossRef]
- Byun, S.; Shin, S.H.; Lee, E.; Lee, J.; Lee, S.Y.; Farrand, L.; Jung, S.K.; Cho, Y.Y.; Um, S.J.; Sin, H.S.; et al. The retinoic acid derivative, ABPN, inhibits pancreatic cancer through induction of Nrdp1. Carcinogenesis 2015, 36, 1580–1589. [Google Scholar] [CrossRef][Green Version]
- Chronopoulos, A.; Robinson, B.; Sarper, M.; Cortes, E.; Auernheimer, V.; Lachowski, D.; Attwood, S.; García, R.; Ghassemi, S.; Fabry, B.; et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 2016, 7, 12630. [Google Scholar] [CrossRef]
- Kuroda, H.; Tachikawa, M.; Uchida, Y.; Inoue, K.; Ohtsuka, H.; Ohtsuki, S.; Unno, M.; Terasaki, T. All-trans retinoic acid enhances gemcitabine cytotoxicity in human pancreatic cancer cell line AsPC-1 by up-regulating protein expression of deoxycytidine kinase. Eur. J. Pharm. Sci. 2017, 103, 116–121. [Google Scholar] [CrossRef]
- Wang, K.; Baldwin, G.S.; Nikfarjam, M.; He, H. Antitumor effects of all-trans retinoic acid and its synergism with gemcitabine are associated with downregulation of p21-activated kinases in pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G632–G640. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Qin, S.; Wang, M.; Wang, X.; Zhang, X.; Liu, F.; Zhang, S. The association between dietary vitamin A intake and pancreatic cancer risk: A meta-analysis of 11 studies. Biosci. Rep. 2016, 36, e00414. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, Y.; Zhi, X.; Ta, N.; Jiang, H.; Zheng, J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016, 6, 38936. [Google Scholar] [CrossRef] [PubMed]
- Kocher, H.M.; Basu, B.; Froeling, F.E.M.; Sarker, D.; Slater, S.; Carlin, D.; deSouza, N.M.; De Paepe, K.N.; Goulart, M.R.; Hughes, C.; et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11, 4841. [Google Scholar] [CrossRef]
- Xue, Y.; Harris, E.; Wang, W.; Baybutt, R.C. Vitamin A depletion induced by cigarette smoke is associated with an increase in lung cancer-related markers in rats. J. Biomed Sci. 2015, 22, 84. [Google Scholar] [CrossRef]
- Okayasu, I.; Hana, K.; Nemoto, N.; Yoshida, T.; Saegusa, M.; Yokota-Nakatsuma, A.; Song, S.Y.; Iwata, M. Vitamin A inhibits development of dextran sulfate sodium-induced colitis and colon cancer in a mouse model. Biomed Res. Int. 2016, 2016, 4874809. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zheng, L.; Zou, J.; Chen, T.; Zou, J.; Li, W.; Chen, Q.; Qian, B. Insights into vitamin A in bladder cancer, lack of attention to gut microbiota? Front. Immunol. 2023, 14, 1252616. [Google Scholar] [CrossRef]
- Tratnjek, L.; Jeruc, J.; Romih, R.; Zupančič, D. Vitamin A and retinoids in bladder cancer chemoprevention and treatment: A narrative review of current evidence, challenges and future prospects. Int. J. Mol. Sci. 2021, 22, 3510. [Google Scholar] [CrossRef]
- Mere Del Aguila, E.; Tang, X.H.; Gudas, L.J. Pancreatic ductal adenocarcinoma: New insights into the actions of vitamin A. Oncol. Res. Treat. 2022, 45, 291–298. [Google Scholar] [CrossRef]
- Hrabak, P.; Zelenkova, M.; Krechler, T.; Soupal, J.; Vocka, M.; Hanus, T.; Petruzelka, L.; Svacina, S.; Zak, A.; Zima, T.; et al. Levels of retinol and retinoic acid in pancreatic cancer, type-2 diabetes and chronic pancreatitis. Biomed Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2024, 168, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bazhin, A.V.; Bleul, T.; de Lera, A.R.; Werner, J.; Rühl, R. Relationship between all-trans-13,14-dihydro retinoic acid and pancreatic adenocarcinoma. Pancreas 2016, 45, e29–e31. [Google Scholar] [CrossRef]
- Groener, J.B.; Gelen, D.; Mogler, C.; Herpel, E.; Toth, C.; Kender, Z.; Peichl, M.; Haufe, S.; Haberkorn, U.; Sulaj, A.; et al. BRAF V600E and retinoic acid in radioiodine-refractory papillary thyroid cancer. Horm. Metab. Res. 2019, 51, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, Y.; Peng, B.; Luo, N.; Zhang, Y.; Zhu, W.; Yang, F.; Chen, Z.; Zhang, Q.; Li, Q.; et al. All-trans retinoic acid inhibits glioblastoma progression and attenuates radiation-induced brain injury. JCI Insight 2024, 9, e179530. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Zhang, B.; Major, S.; Webb, A. All-trans retinoic acid eluting poly(diol citrate) wafers for treatment of glioblastoma. J. Biomed Mater. Res. B Appl. Biomater. 2020, 108, 619–628. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, L.; Li, R.; Pan, X.; Li, J.; Dou, S.; Jiang, W.; Wang, C.; Chen, W.; Zhu, G. Combined all-trans retinoic acid with low-dose apatinib in treatment of recurrent/metastatic head and neck adenoid cystic carcinoma: A single-center, secondary analysis of a phase II study. Cancer Med. 2023, 12, 9144–9155. [Google Scholar] [CrossRef]
- Işlek Köklü, Z.; Şanverdi, E.L.; Karadağ, B.; Üçişik, M.H.; Taşkan, E.; Şahin, F. Combinational therapy of all-trans retinoic acid (ATRA) and sphingomyelin induces apoptosis and cell cycle arrest in B16F10 melanoma cancer cells. Turk. J. Biol. 2024, 48, 401–413. [Google Scholar] [CrossRef]
- Younes, M.; Loubnane, G.; Sleiman, C.; Rizk, S. Tocotrienol isoforms: The molecular mechanisms underlying their effects in cancer therapy and their implementation in clinical trials. J. Integr. Med. 2024, 22, 1–11. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Sauro, G.; Giannandrea, D.; Limonta, P.; Casati, L. Molecular insights in the anticancer activity of natural tocotrienols: Targeting mitochondrial metabolism and cellular redox homeostasis. Antioxidants 2025, 14, 115. [Google Scholar] [CrossRef]
- Kaye, A.D.; Thomassen, A.S.; Mashaw, S.A.; MacDonald, E.M.; Waguespack, A.; Hickey, L.; Singh, A.; Gungor, D.; Kallurkar, A.; Kaye, A.M.; et al. Vitamin E (α-tocopherol): Emerging clinical role and adverse risks of supplementation in adults. Cureus 2025, 17, e78679. [Google Scholar] [CrossRef] [PubMed]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-tocopherol: A comprehensive review of its antioxidant, anti-inflammatory, and anticancer properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Pierpaoli, E.; Viola, V.; Pilolli, F.; Piroddi, M.; Galli, F.; Provinciali, M. Gamma- and delta-tocotrienols exert a more potent anticancer effect than alpha-tocopheryl succinate on breast cancer cell lines irrespective of HER-2/neu expression. Life Sci. 2010, 86, 668–675. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E as effective agents for cancer prevention and therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef]
- Khalid, A.Q.; Bhuvanendran, S.; Magalingam, K.B.; Ramdas, P.; Radhakrishnan, A.K. Inhibition of proliferation and induction of apoptosis by gamma- or delta-tocotrienols in human colorectal carcinoma cells. Biomed Res. Int. 2025, 2025, 4421336. [Google Scholar] [CrossRef]
- Jang, Y.; Park, N.Y.; Rostgaard-Hansen, A.L.; Huang, J.; Jiang, Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic. Biol. Med. 2016, 95, 190–199. [Google Scholar] [CrossRef]
- Guan, F.; Li, G.; Liu, A.B.; Lee, M.J.; Yang, Z.; Chen, Y.K.; Lin, Y.; Shih, W.; Yang, C.S. δ- and γ-tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev. Res. 2012, 5, 644–654. [Google Scholar] [CrossRef]
- Falsetti, I.; Palmini, G.; Zonefrati, R.; Vasa, K.; Donati, S.; Aurilia, C.; Baroncelli, A.; Viglianisi, C.; Ranaldi, F.; Iantomasi, T.; et al. Antiproliferative role of natural and semi-synthetic tocopherols on colorectal cancer cells overexpressing the estrogen receptor β. Int. J. Mol. Sci. 2025, 26, 2305. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.Q.; Zaidan, T.N.; Bhuvanendran, S.; Magalingam, K.B.; Mohamedahmed, S.M.; Ramdas, P.; Radhakrishnan, A.K. Insights into the anticancer mechanisms modulated by gamma and delta tocotrienols in colorectal cancers. Nutr. Rev. 2025, 83, e1295–e1310. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Coppola, D.; Yang, C.S.; Malafa, M.P. Effect of vitamin E δ-tocotrienol and aspirin on Wnt signaling in human colon cancer stem cells and in adenoma development in APCmin/+ mice. Carcinogenesis 2024, 45, 881–892. [Google Scholar] [CrossRef]
- Schlörmann, W.; Liao, S.; Dinc, T.; Lorkowski, S.; Wallert, M.; Glei, M. Chemopreventive effects of α-tocopherol and its long-chain metabolites α-13′-hydroxy- and α-13′-carboxychromanol in LT97 colon adenoma cells. Food Funct. 2024, 15, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Wang, Q.; Nakatsu, C.H.; Jones-Hall, Y.; Jiang, Q. Combining gamma-tocopherol and aspirin synergistically suppresses colitis-associated colon tumorigenesis and modulates the gut microbiota in mice, and inhibits the growth of human colon cancer cells. Eur. J. Pharmacol. 2023, 946, 175656. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, R.; Bijanpour, H.; Pirouzpanah, S.M.B.; Yaghmaei, P.; Rashtchizadeh, N. Adjuvant therapy with γ-tocopherol induces apoptosis in HT-29 colon cancer via cyclin-dependent cell cycle arrest mechanism. J. Biochem. Mol. Toxicol. 2019, 33, e22399. [Google Scholar] [CrossRef]
- Chen, J.X.; Liu, A.; Lee, M.J.; Wang, H.; Yu, S.; Chi, E.; Reuhl, K.; Suh, N.; Yang, C.S. δ- and γ-tocopherols inhibit PhIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages. Mol. Carcinog. 2017, 56, 172–183. [Google Scholar] [CrossRef]
- Yoon, H.S.; Wu, J.; Shidal, C.; Sun, Y.; Franke, A.A.; Yang, J.J.; Braithwaite, D.; Courtney, R.; Cai, H.; Blot, W.J.; et al. Associations between plasma tocopherols and lung cancer risk: Results from the Southern Community Cohort Study. Cancer Epidemiol. Biomark. Prev. 2024, 33, 480–488. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. A prospective study of serum vitamin E and 28-year risk of lung cancer. J. Natl. Cancer Inst. 2020, 112, 191–199. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Bo, Y.C.; Liu, X.X.; Qiu, C.G. Association of dietary vitamin E intake with risk of lung cancer: A dose-response meta-analysis. Asia Pac. J. Clin. Nutr. 2017, 26, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef]
- Rajasinghe, L.D.; Hutchings, M.; Gupta, S.V. Delta-tocotrienol modulates glutamine dependence by inhibiting ASCT2 and LAT1 transporters in non-small cell lung cancer (NSCLC) cells: A metabolomic approach. Metabolites 2019, 9, 50. [Google Scholar] [CrossRef]
- Rajasinghe, L.D.; Pindiprolu, R.H.; Gupta, S.V. Delta-tocotrienol inhibits non-small-cell lung cancer cell invasion via the inhibition of NF-κB, uPA activator, and MMP-9. Onco Targets Ther. 2018, 11, 4301–4314. [Google Scholar] [CrossRef]
- Uchihara, Y.; Kidokoro, T.; Tago, K.; Mashino, T.; Tamura, H.; Funakoshi-Tago, M. A major component of vitamin E, α-tocopherol, inhibits the anti-tumor activity of crizotinib against cells transformed by EML4-ALK. Eur. J. Pharmacol. 2018, 825, 1–9. [Google Scholar] [CrossRef]
- Daifuku, R.; Koratich, M.; Stackhouse, M. Vitamin E phosphate nucleoside prodrugs: A platform for intracellular delivery of monophosphorylated nucleosides. Pharmaceuticals 2018, 11, 16. [Google Scholar] [CrossRef]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Metabolomic analysis of prostate cancer risk in a prospective cohort: The alpha-tocopherol, beta-carotene cancer prevention (ATBC) study. Int. J. Cancer 2015, 137, 2124–2132. [Google Scholar] [CrossRef]
- Antwi, S.O.; Steck, S.E.; Zhang, H.; Stumm, L.; Zhang, J.; Hurley, T.G.; Hebert, J.R. Plasma carotenoids and tocopherols in relation to prostate-specific antigen (PSA) levels among men with biochemical recurrence of prostate cancer. Cancer Epidemiol. 2015, 39, 752–762. [Google Scholar] [CrossRef]
- Wang, H.; Hong, J.; Yang, C.S. δ-Tocopherol inhibits receptor tyrosine kinase-induced AKT activation in prostate cancer cells. Mol. Carcinog. 2016, 55, 1728–1738. [Google Scholar] [CrossRef]
- Yeganehjoo, H.; DeBose-Boyd, R.; McFarlin, B.K.; Mo, H. Synergistic impact of d-δ-tocotrienol and geranylgeraniol on the growth and HMG-CoA reductase of human DU145 prostate carcinoma cells. Nutr. Cancer 2017, 69, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.M.; MacKenzie, D.A.; Olguin, S.L.; Scariano, J.K.; Rabinowitz, I.; Thompson, T.A. Antioxidants abrogate alpha-tocopherylquinone-mediated down-regulation of the androgen receptor in androgen-responsive prostate cancer cells. PLoS ONE 2016, 11, e0151525. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Liu, A.; Wang, G.; Bosland, M.C.; Yang, C.S. δ-Tocopherol inhibits the development of prostate adenocarcinoma in prostate-specific Pten-/- mice. Carcinogenesis 2018, 39, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, W.; Sun, Y.; Yang, C.S. δ-Tocotrienol is the most potent vitamin E form in inhibiting prostate cancer cell growth and inhibits prostate carcinogenesis in Ptenp-/- mice. Cancer Prev. Res. 2022, 15, 233–245. [Google Scholar] [CrossRef]
- Sato, C.; Kaneko, S.; Sato, A.; Virgona, N.; Namiki, K.; Yano, T. Combination effect of δ-tocotrienol and γ-tocopherol on prostate cancer cell growth. J. Nutr. Sci. Vitaminol. 2017, 63, 349–354. [Google Scholar] [CrossRef]
- Fontana, F.; Moretti, R.M.; Raimondi, M.; Marzagalli, M.; Beretta, G.; Procacci, P.; Sartori, P.; Montagnani Marelli, M.; Limonta, P. δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019, 52, e12576. [Google Scholar] [CrossRef]
- Moore, C.; Palau, V.E.; Mahboob, R.; Lightner, J.; Stone, W.; Krishnan, K. Upregulation of pERK and c-JUN by γ-tocotrienol and not α-tocopherol are essential to the differential effect on apoptosis in prostate cancer cells. BMC Cancer 2020, 20, 428. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.D.; Liu, J.; Russell, P.J.; Clements, J.A.; Ling, M.T. Gamma-tocotrienol induces apoptosis in prostate cancer cells by targeting the Ang-1/Tie-2 signalling pathway. Int. J. Mol. Sci. 2019, 20, 1164. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, R.; Su, Z.Y.; Guo, Y.; Zheng, X.; Yang, C.S.; Kong, A.N. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. J. Nutr. Biochem. 2017, 40, 155–163. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, X.; Li, J.; Fan, L.; Zhao, C.; Yin, S.; Hu, H. δ-Tocotrienol potentiates breast and prostate cancer cells to paclitaxel via suppressing PD-L1-mediated cancer-promoting signaling. Chem. Biol. Drug Des. 2025, 105, e70143. [Google Scholar] [CrossRef]
- Helzlsouer, K.J.; Huang, H.Y.; Alberg, A.J.; Hoffman, S.; Burke, A.; Norkus, E.P.; Morris, J.S.; Comstock, G.W. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J. Natl. Cancer Inst. 2000, 92, 2018–2023. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Drotleff, A.M.; Büsing, A.; Willenberg, I.; Empl, M.T.; Steinberg, P.; Ternes, W. HPLC separation of vitamin E and its oxidation products and effects of oxidized tocotrienols on the viability of MCF-7 breast cancer cells in vitro. J. Agric. Food Chem. 2015, 63, 8930–8939. [Google Scholar] [CrossRef]
- Alawin, O.A.; Ahmed, R.A.; Ibrahim, B.A.; Briski, K.P.; Sylvester, P.W. Antiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cells. J. Nutr. Biochem. 2016, 27, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.A.; Alawin, O.A.; Sylvester, P.W. γ-Tocotrienol reversal of epithelial-to-mesenchymal transition in human breast cancer cells is associated with inhibition of canonical Wnt signalling. Cell Prolif. 2016, 49, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.X.; Zhang, J.Z.; Zhao, T.; Xue, F.; Gao, F.; Ma, S.M.; Wang, Y. Vitamin E promotes breast cancer cell proliferation by reducing ROS production and p53 expression. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2710–2717. [Google Scholar] [PubMed]
- Ding, Y.; Fan, J.; Fan, Z.; Zhang, K. γ-Tocotrienol reverses multidrug resistance of breast cancer cells through the regulation of the γ-tocotrienol–NF-κB–P-gp axis. J. Steroid Biochem. Mol. Biol. 2021, 209, 105835. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, Y.; Deng, L.; Fan, J.; Huang, B. Gamma-tocotrienol reverses multidrug resistance of breast cancer cells with a mechanism distinct from that of atorvastatin. J. Steroid Biochem. Mol. Biol. 2017, 167, 67–77. [Google Scholar] [CrossRef]
- Bak, M.J.; Das Gupta, S.; Wahler, J.; Lee, H.J.; Li, X.; Lee, M.J.; Yang, C.S.; Suh, N. Inhibitory effects of γ- and δ-tocopherols on estrogen-stimulated breast cancer in vitro and in vivo. Cancer Prev. Res. 2017, 10, 188–197. [Google Scholar] [CrossRef]
- Ye, J.; Dong, W.; Yang, Y.; Hao, H.; Liao, H.; Wang, B.; Han, X.; Jin, Y.; Xia, X.; Liu, Y. Vitamin E-rich nanoemulsion enhances the antitumor efficacy of low-dose paclitaxel by driving Th1 immune response. Pharm. Res. 2017, 34, 1244–1254. [Google Scholar] [CrossRef]
- Sailo, B.L.; Chauhan, S.; Hegde, M.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Goel, A.; Sethi, G.; Kunnumakkara, A.B. Therapeutic potential of tocotrienols as chemosensitizers in cancer therapy. Phytother. Res. 2025, 39, 1694–1720. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Q.; Wang, J.; Zhang, B.; Hao, T.; Chen, Q.; Li, L.; Chen, L.; Cui, H.; Li, Z. PEGylated phospholipid micelles containing D-α-tocopheryl succinate as multifunctional nanocarriers for enhancing the antitumor efficacy of doxorubicin. Int. J. Pharm. 2021, 607, 120979. [Google Scholar] [CrossRef]
- Opoku-Damoah, Y.; Zhang, R.; Ta, H.T.; Xu, Z.P. Vitamin E-facilitated carbon monoxide pro-drug nanomedicine for efficient light-responsive combination cancer therapy. Biomater. Sci. 2021, 9, 6086–6097. [Google Scholar] [CrossRef]
- Queiroz Schmidt, F.M.; Serna González, C.V.; Mattar, R.C.; Lopes, L.B.; Santos, M.F.; Santos, V.L.C.G. Topical application of a cream containing nanoparticles with vitamin E for radiodermatitis prevention in women with breast cancer: A randomized, triple-blind, controlled pilot trial. Eur. J. Oncol. Nurs. 2022, 61, 102230. [Google Scholar] [CrossRef]
- Long, X.; Guo, J.; Yin, Y.; Cheng, M.; Zhang, X.; Zhang, J.; Wang, P.; Zang, J.; Zhao, L. A blinded-endpoint, randomized controlled trial of Sanyrene with natural active ingredient for prophylaxis of radiation dermatitis in patients receiving radiotherapy. Radiat. Oncol. 2023, 18, 174. [Google Scholar] [CrossRef]
- Moustafa, I.; Connolly, C.; Anis, M.; Mustafa, H.; Oosthuizen, F.; Viljoen, M. A prospective study to evaluate the efficacy and safety of vitamin E and levocarnitine prophylaxis against doxorubicin-induced cardiotoxicity in adult breast cancer patients. J. Oncol. Pharm. Pract. 2024, 30, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Kjær, I.M.; Kahns, S.; Timm, S.; Andersen, R.F.; Madsen, J.S.; Jakobsen, E.H.; Tabor, T.P.; Jakobsen, A.; Bechmann, T. Phase II trial of delta-tocotrienol in neoadjuvant breast cancer with evaluation of treatment response using ctDNA. Sci. Rep. 2023, 13, 8419. [Google Scholar] [CrossRef]
- Husain, K.; Centeno, B.A.; Coppola, D.; Trevino, J.; Sebti, S.M.; Malafa, M.P. δ-Tocotrienol, a natural form of vitamin E, inhibits pancreatic cancer stem-like cells and prevents pancreatic cancer metastasis. Oncotarget 2017, 8, 31554–31567. [Google Scholar] [CrossRef] [PubMed]
- Palau, V.E.; Chakraborty, K.; Wann, D.; Lightner, J.; Hilton, K.; Brannon, M.; Stone, W.; Krishnan, K. γ-Tocotrienol induces apoptosis in pancreatic cancer cells by upregulation of ceramide synthesis and modulation of sphingolipid transport. BMC Cancer 2018, 18, 564. [Google Scholar] [CrossRef]
- Francois, R.A.; Zhang, A.; Husain, K.; Wang, C.; Hutchinson, S.; Kongnyuy, M.; Batra, S.K.; Coppola, D.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-tocotrienol sensitizes human pancreatic cancer cells to TRAIL-induced apoptosis through proteasome-mediated down-regulation of c-FLIPs. Cancer Cell Int. 2019, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Kapoor, E.; Ding, L.; Yu, A.; Tang, W.; Hang, Y.; Smith, L.M.; Sil, D.; Oupický, D. Effect of tocopherol conjugation on polycation-mediated siRNA delivery to orthotopic pancreatic tumors. Biomater. Adv. 2023, 145, 213236. [Google Scholar] [CrossRef]
- Behera, C.; Kaur Sandha, K.; Banjare, N.; Kumar Shukla, M.; Mudassir Ali, S.; Singh, M.; Gupta, P.N. Biodegradable nanocarrier of gemcitabine and tocopherol succinate synergistically ameliorates anti-proliferative response in MIA PaCa-2 cells. Int. J. Pharm. 2024, 649, 123599. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Miranda-Pastoriza, D.; Diaz-Gomez, L.; Sotelo, E.; Paiva-Santos, A.C.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Gemcitabine–vitamin E prodrug-loaded micelles for pancreatic cancer therapy. Pharmaceutics 2024, 16, 95. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Diaz-Gomez, L.; Blanco-Fernandez, B.; Paiva-Santos, A.C.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Biomimetic cancer cell membrane-enriched vitamin E-stapled gemcitabine-loaded TPGS micelles for pancreatic cancer therapy. Drug Deliv. 2025, 32, 2527759. [Google Scholar] [CrossRef]
- Springett, G.M.; Husain, K.; Neuger, A.; Centeno, B.; Chen, D.T.; Hutchinson, T.Z.; Lush, R.M.; Sebti, S.; Malafa, M.P. A phase I safety, pharmacokinetic, and pharmacodynamic presurgical trial of vitamin E δ-tocotrienol in patients with pancreatic ductal neoplasia. EBioMedicine 2015, 2, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Mahipal, A.; Klapman, J.; Vignesh, S.; Yang, C.S.; Neuger, A.; Chen, D.T.; Malafa, M.P. Pharmacokinetics and safety of vitamin E δ-tocotrienol after single and multiple doses in healthy subjects with measurement of vitamin E metabolites. Cancer Chemother. Pharmacol. 2016, 78, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, H.; Wei, P.; Zheng, J.; Daniel, C.R.; Hassan, M.M. Vitamin C and vitamin E mitigate the risk of pancreatic ductal adenocarcinoma from meat-derived mutagen exposure in adults in a case-control study. J. Nutr. 2019, 149, 1443–1450. [Google Scholar] [CrossRef]
- Zhao, M.; Ye, M.; Zhao, Y. Causal link between dietary antioxidant vitamins intake, oxidative stress injury biomarkers and colorectal cancer: A Mendelian randomization study. Medicine 2025, 104, e41531. [Google Scholar] [CrossRef] [PubMed]
- Raunkilde, L.; Hansen, T.F.; Havelund, B.M.; Thomsen, C.B.; Rafaelsen, S.R.; Lindebjerg, J.; Jensen, L.H. Delta tocotrienol as a supplement to FOLFOXIRI in first-line treatment of metastatic colorectal cancer: A randomized, double-blind, placebo-controlled phase II study. Acta Oncol. 2023, 62, 1066–1075. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Teixeira, F.M.E.; Sato, M.N. Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef]
- Snyder, L.M.; Arora, J.; Kennett, M.J.; Weaver, V.; Cantorna, M.T. Retinoid signaling in intestinal epithelial cells is essential for early survival from gastrointestinal infection. Front. Immunol. 2020, 11, 559635. [Google Scholar] [CrossRef]
- Lavudi, K.; Nuguri, S.M.; Olverson, Z.; Dhanabalan, A.K.; Patnaik, S.; Kokkanti, R.R. Targeting the retinoic acid signaling pathway as a modern precision therapy against cancers. Front. Cell Dev. Biol. 2023, 11, 1254612. [Google Scholar] [CrossRef]
- Nagai, Y.; Ambinder, A.J. The promise of retinoids in the treatment of cancer: Neither burnt out nor fading away. Cancers 2023, 15, 3535. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tong, X.; Lu, R.; Zhang, Z.; Ma, T. All-trans retinoic acid in hematologic disorders: Not just acute promyelocytic leukemia. Front. Pharmacol. 2024, 15, 1404092. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Michael, H.; Chepngeno, J.; Raev, S.A.; Saif, L.J.; Vlasova, A.N. Immune impairment associated with vitamin A deficiency: Insights from clinical studies and animal model research. Nutrients 2022, 14, 5038. [Google Scholar] [CrossRef]
- Bastos Maia, S.; Rolland Souza, A.S.; Costa Caminha, M.F.; Lins da Silva, S.; Callou Cruz, R.S.B.L.; Carvalho Dos Santos, C.; Batista Filho, M. Vitamin A and pregnancy: A narrative review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef]
- Esposito, M.; Amory, J.K.; Kang, Y. The pathogenic role of retinoid nuclear receptor signaling in cancer and metabolic syndromes. J. Exp. Med. 2024, 221, e20240519. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Revisiting the therapeutic potential of tocotrienol. Biofactors 2022, 48, 813–856. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef]
- Niki, E. Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E. Free Radic. Biol. Med. 2021, 176, 1–15. [Google Scholar] [CrossRef]
- Moses, G. The safety of commonly used vitamins and minerals. Aust Prescr. 2021, 44, 119–123. [Google Scholar] [CrossRef]
- Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Francomano, D.; Sciarretta, S.; Pastori, D.; Peruzzi, M.; Cavarretta, E.; D’Amico, A.; et al. The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants 2021, 10, 146. [Google Scholar] [CrossRef]

| Vitamin | Dietary Sources | Key Biochemical Roles | Deficiency | Overdose/Toxicity |
|---|---|---|---|---|
| Vitamin A (Retinoids; Carotenoids) |
|
|
|
|
| Vitamin E (Tocopherols; Tocotrienols) |
|
|
|
|
| Cancer Type | Mechanisms | Key Results | References |
|---|---|---|---|
| Acute promyelocytic leukemia | ATRA overcomes PML-RARα differentiation block, enhances apoptosis, decreases MMP and increases caspase-3/7, sensitizes CDK4/6 inhibition, and modulates WNT/β-catenin with salinomycin | ATRA induces promyelocyte differentiation; combining it with ATO, GO, gefitinib, ethacrynic acid, palbociclib, and salinomycin could improve its efficacy | [42,43,44,45,46,47,48,49,50,51,52,53,54,55] |
| Non-melanoma skin cancers (BCC/SCC), CTCL; Kaposi’s sarcoma | Increases retinoid receptor/transport signaling, decreases keratinocyte proliferation, increases p53 and pro-apoptotic caspases, causes cell cycle arrest, and inhibits angiogenesis | Chemoprevention in high-risk patients; mixed epidemiological data (may be associated with BCC/SCC risk); clinical use in CTCL/KS | [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Melanoma | ATRA can cause apoptosis and G2/M cell cycle arrest; decrease PD-L1, PIN1, and stemness markers; increase differentiation, CD8+ T-cell responses, and activate RAR and caspase-3 | ATRA synergizes with SM, allicin, and resveratrol, and enhances docetaxel, dacarbazine, and paclitaxel; WYC-209 reduces metastasis; ATRA plus pembrolizumab increases ORR; and ATRA plus ipilimumab reduces MDSC function | [71,72,73,74,75,76,77,78,79,80,81] |
| Breast cancer | Endogenous ATRA is anti-proliferative, decreases metabolic reprogramming, regulates ER signaling, and increases RARβ | Higher carotenoids/vitamin A are associated with lower risk of BC; ATRA reduces proliferation and survival | [82,83,84,85,86,87,88,89] |
| Pancreatic ductal adenocarcinoma | RAR-β activation restores PSC quiescence, decreases cancer cell invasion, increases chemo-sensitivity to gemcitabine, and regulates the PAK pathway | ATRA plus gemcitabine–nab-paclitaxel is safe with stromal modulation; meta-analysis studies link higher dietary vitamin A/β-carotene to lower risk of pancreatic cancer | [90,91,92,93,94,95,96] |
| Other cancers (colon, lung, thyroid and glioblastoma) | Increases cell differentiation and apoptosis, and decreases cell proliferation, immune cell modulation, and the AKT/mTOR/PPARγ/Plin4 axis in glioblastoma | Prevents DSS-colon cancer in mice; deficiency increases smoke-induced lung cancer and promotes gut microbiota-mediated bladder cancer protection; RA redifferentiation benefits the thyroid; ATRA-eluting wafers prevent glioblastoma | [97,98,99,100,101,102,103,104,105,106,107] |
| Cancer Type | Mechanism of Action | Key Results | References |
|---|---|---|---|
| Colorectal Cancer γ-, δ-tocopherol; γ-, δ-tocotrienol; vitamin E metabolites (α-13′-OH, α-13′-COOH) | Inhibits cell proliferation and adenoma formation, upregulates ER-β expression, inhibits Wnt/β-catenin signaling along with aspirin, regulates telomerase activity and immune responses, induces reactive oxygen species (ROS) scavenging and caspase-independent cell death, and suppresses oxidative and nitrosative stress. | δ- and γ-tocopherols prevent colon tumors, γ-tocopherol plus aspirin reduces inflammation and tumor burden, δ-tocotrienol modulates the gut microbiota and reduces colitis-associated cancer, vitamin E metabolites protect DNA from ROS, and its combination with 5-FU enhances apoptosis. | [117,118,119,120,121,122,123,124,125,126] |
| Lung Cancer α-, β-, γ-, δ-tocopherol; δ-tocotrienol | Protects against ROS damage and downregulates KRAS-driven metastasis; δ-tocotrienol inhibits glutamine metabolism and mTOR pathway, increases miR-451, and reduces metastasis; and vitamin E phosphate prodrugs such as NUC050/NUC052 enhance gemcitabine efficacy. | Higher plasma tocopherol levels reduce lung cancer risk in smokers and men, δ-tocotrienol induces apoptosis and reduces NSCLC growth, α-tocopherol could interfere with crizotinib efficacy, and NUC050/052 prodrugs prolong survival of NSCLC mice. | [127,128,129,130,131,132,133,134] |
| Prostate Cancer α-, γ-, δ-tocopherol; δ-, γ-tocotrienol | Induces apoptosis and cell cycle arrest (G1/G2-M) via AKT inhibition, downregulates HMG-CoA reductase and K-RAS, suppresses androgen receptor (AR) signaling, activates ER stress and JNK/p38, inhibits angiopoietin-1/Tie-2 and HDAC expression, and enhances chemotherapy sensitivity through PD-L1 suppression. | δ-Tocopherol and δ-tocotrienol are most potent in reducing AKT activity and inducing apoptosis, γ-tocopherol promotes apoptosis via caspase-9 and -3, and δ-T3 + γ-tocopherol synergistically inhibit LNCaP growth. Some clinical trials (SELECT 2009, 2011) show no prevention benefit. | [135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150] |
| Breast Cancer γ-, δ-tocotrienol; γ-, δ-tocopherol; α-TOS | Inhibits HER2 signaling and lipid raft formation, suppresses Wnt/β-catenin and reverses EMT, induces apoptosis and cell cycle arrest, overcomes multidrug resistance, increases Th1 and decreases Th2 cytokines, improves paclitaxel efficacy via nano-formulations, and protects against radiodermatitis and doxorubicin-induced cardiotoxicity. | γ- and δ-tocotrienols inhibit breast cancer growth and metastasis, vitamin E nano-emulsions and creams improve chemo/radiotherapy tolerance, and some oxidized tocotrienols show anti-proliferative activity. | [151,152,153,154,155,156,157,158,159,160,161,162,163,164,165] |
| Pancreatic Cancer γ-, δ-tocotrienol; α-, δ-tocopherol succinate; α-tocopherol conjugates | Targets cancer stem-like cells; inhibits migration, invasion, and angiogenesis; modulates ceramide metabolism; promotes TRAIL-induced apoptosis via c-FLIP degradation; enhances siRNA or gemcitabine delivery through tocopherol-based nanocarriers; and induces tumor apoptosis in pre-surgical patients. | δ-Tocotrienol suppresses PDAC stemness and metastasis, γ-tocotrienol promotes apoptosis via ceramide signaling, α-tocopherol succinate nanocarriers potentiate gemcitabine, δ-tocotrienol was safe and pro-apoptotic in a phase-I trial, and high vitamin E intake inversely correlates with PDAC risk. | [166,167,168,169,170,171,172,173,174,175] |
| Others α-, γ-, δ-tocopherol; α-, γ-, δ-tocotrienols | Antioxidant and anti-inflammatory effects reduce ROS and NF-κB activation, and also modulate immune responses and cell differentiation, and these effects are form-dependent (non-α forms > α-tocopherol). | γ- and δ-isomers exhibit stronger anticancer and anti-inflammatory properties, and α-tocopherol is sometimes neutral or adverse; combination of tocopherols and tocotrienols shows additive chemopreventive benefit. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupfer, J.T.; Boekweg, N.; Zheng, H.; Puckett, J.; Ramana, K.V. Significance of Vitamins A and E in Cancer Progression and Prevention. Int. J. Mol. Sci. 2025, 26, 11588. https://doi.org/10.3390/ijms262311588
Kupfer JT, Boekweg N, Zheng H, Puckett J, Ramana KV. Significance of Vitamins A and E in Cancer Progression and Prevention. International Journal of Molecular Sciences. 2025; 26(23):11588. https://doi.org/10.3390/ijms262311588
Chicago/Turabian StyleKupfer, Jesse T., Noah Boekweg, Hailiang Zheng, John Puckett, and Kota V. Ramana. 2025. "Significance of Vitamins A and E in Cancer Progression and Prevention" International Journal of Molecular Sciences 26, no. 23: 11588. https://doi.org/10.3390/ijms262311588
APA StyleKupfer, J. T., Boekweg, N., Zheng, H., Puckett, J., & Ramana, K. V. (2025). Significance of Vitamins A and E in Cancer Progression and Prevention. International Journal of Molecular Sciences, 26(23), 11588. https://doi.org/10.3390/ijms262311588






