Molecular Mechanisms Driving Metastatic Progression Within the Aged Tumor Microenvironment
Abstract
1. Introduction
2. Aging Hallmarks in the Tumor Microenvironment
3. Molecular Mechanisms and Signaling Pathways
3.1. Extracellular Matrix (ECM) Remodeling
3.2. Inflammation and Cytokine Signaling
3.3. Senescence-Associated Secretory Phenotype (SASP)
3.4. Immune Cell Dysfunction
3.5. Metabolic Reprogramming
4. Discussion of Major Age-Related Cancers
4.1. Breast Cancer in the Aging Population
4.2. Prostate Cancer and Aging
4.3. Lung Cancer in the Elderly
4.4. Bladder Cancer and Age-Related Changes
4.5. Colorectal Cancer in the Aging Population
5. Conclusions and Perspective
Limitations of the Study
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piña-Sánchez, P.; Chávez-González, A.; Ruiz-Tachiquín, M.; Vadillo, E.; Monroy-García, A.; Montesinos, J.J.; Grajales, R.; Gutiérrez de la Barrera, M.; Mayani, H. Cancer Biology, Epidemiology, and Treatment in the 21st Century: Current Status and Future Challenges from a Biomedical Perspective. Cancer Control 2021, 28, 10732748211038736. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on Prostate Cancer: Planning for the Surge in Cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism Insights and Therapeutic Intervention of Tumor Metastasis: Latest Developments and Perspectives. Signal Transduct. Target. Ther. 2024, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Prathap, R.; Kirubha, S.; Rajan, A.T.; Manoharan, S.; Elumalai, K. The Increasing Prevalence of Cancer in the Elderly: An Investigation of Epidemiological Trends. Aging Med. 2024, 7, 516. [Google Scholar] [CrossRef]
- Montégut, L.; López-Otín, C.; Kroemer, G. Aging and Cancer. Mol. Cancer 2024, 23, 106. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Bai, X.; Huang, X.; Wang, Q. Tumor Microenvironment as a Complex Milieu Driving Cancer Progression: A Mini Review. Clin. Transl. Oncol. 2024, 27, 1943. [Google Scholar] [CrossRef]
- Biray Avci, C.; Goker Bagca, B.; Nikanfar, M.; Takanlou, L.S.; Takanlou, M.S.; Nourazarian, A. Tumor Microenvironment and Cancer Metastasis: Molecular Mechanisms and Therapeutic Implications. Front. Pharmacol. 2024, 15, 1442888. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Ali, N.M.; Zhang, B.; Cui, X. The Role of Innate Immune Cells in the Tumor Microenvironment and Research Progress in Anti-Tumor Therapy. Front. Immunol. 2023, 13, 1039260. [Google Scholar] [CrossRef]
- Liu, X.; Kang, X.; Kang, H.; Yan, H. The Immunosuppressive Role of MDSCs in HCC: Mechanisms and Therapeutic Opportunities. Cell Commun. Signal. 2025, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, Y.; Mei, J.; Liu, Y.; Zhou, G. Extracellular Matrix Dynamics in Tumor Immunoregulation: From Tumor Microenvironment to Immunotherapy. J. Hematol. Oncol. 2025, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Sahel, D.; Kubal, B.; Postwala, H.; Shah, Y.; Chavda, V.P.; Fernandes, C.; Khatri, D.K.; Vora, L.K. Role of the Extracellular Matrix in Cancer: Insights into Tumor Progression and Therapy. Adv. Ther. 2025, 8, 2400370. [Google Scholar] [CrossRef]
- Yang, F.; Lee, G.; Fan, Y. Navigating Tumor Angiogenesis: Therapeutic Perspectives and Myeloid Cell Regulation Mechanism. Angiogenesis 2024, 27, 333. [Google Scholar] [CrossRef]
- Kunachowicz, D.; Tomecka, P.; Sędzik, M.; Kalinin, J.; Kuźnicki, J.; Rembiałkowska, N. Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies. Cells 2025, 14, 1057. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Li, D.; Wei, J.; Chen, K.; Zhang, E.; Liu, G.; Chu, X.; Liu, X.; Liu, W.; et al. Cancer Associated Fibroblasts and Metabolic Reprogramming: Unraveling the Intricate Crosstalk in Tumor Evolution. J. Hematol. Oncol. 2024, 17, 80. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.; Jawaid, T.; Jayasingh Chellammal, H.S.; Upadhyay, P. Dual Role of Lactate in Human Health and Disease. Front. Physiol. 2025, 16, 1621358. [Google Scholar] [CrossRef]
- Kimmelman, A.C.; Sherman, M.H. The Role of Stroma in Cancer Metabolism. Cold Spring Harb. Perspect. Med. 2024, 14, a041540. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Cai, Q.; Deng, L.; Ouyang, Q.; Zhang, X.H.F.; Zheng, J. Invasion and Metastasis in Cancer: Molecular Insights and Therapeutic Targets. Signal Transduct. Target. Ther. 2025, 10, 57. [Google Scholar] [CrossRef]
- Upadhyay, P.; Kumar, S.; Chellammal, H.S.J.; Sahu, N.; Srivastava, S.; Kumar, R.; Gasmi, A. Gut Microbiota and Dietary Strategies for Age-Related Diseases. Mol. Nutr. Food Res. 2025, e70308. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, B.; Feng, N.; Zhang, X.; Zhang, X.; Wei, Y.; Zhang, W. Aging Microenvironment and Antitumor Immunity for Geriatric Oncology: The Landscape and Future Implications. J. Hematol. Oncol. 2023, 16, 28. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. Normal Aging and Its Role in Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037341. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef]
- Srinivasan, J.; Vasudev, A.; Shasha, C.; Selden, H.J.; Perez, E.; LaFleur, B.; Sinari, S.A.; Krueger, A.; Richie, E.R.; Ehrlich, L.I.R. The Initial Age-Associated Decline in Early T-Cell Progenitors Reflects Fewer Pre-Thymic Progenitors and Altered Signals in the Bone Marrow and Thymus Microenvironments. Aging Cell 2023, 22, e13870. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.Y.; Jaiswal, S.; Martinez, D.; Yerinde, C.; Ji, K.; Miranda, V.; Fung, M.E.; Weiss, S.A.; Zschummel, M.; Taguchi, K.; et al. The Aged Tumor Microenvironment Limits T Cell Control of Cancer. Nat. Immunol. 2024, 25, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alqahtani, T.; Venkatesan, K.; Sivadasan, D.; Ahmed, R.; Sirag, N.; Elfadil, H.; Abdullah Mohamed, H.; TA, H.; Elsayed Ahmed, R.; et al. SASP Modulation for Cellular Rejuvenation and Tissue Homeostasis: Therapeutic Strategies and Molecular Insights. Cells 2025, 14, 608. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic Reprogramming and Therapeutic Resistance in Primary and Metastatic Breast Cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Admasu, T.D.; Yu, J.S. Harnessing Immune Rejuvenation: Advances in Overcoming T Cell Senescence and Exhaustion in Cancer Immunotherapy. Aging Cell 2025, 24, e70055. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular Matrix Stiffness: Mechanisms in Tumor Progression and Therapeutic Potential in Cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef]
- Colucci, M.; Sarill, M.; Maddalena, M.; Valdata, A.; Troiani, M.; Massarotti, M.; Bolis, M.; Bressan, S.; Kohl, A.; Robesti, D.; et al. Senescence in Cancer. Cancer Cell 2025, 43, 1204–1226. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Buriani, A.; Fortinguerra, S.; Davinelli, S.; Scapagnini, G.; Cassidy, A.; De Vivo, I. Cell Survival, Death, and Proliferation in Senescent and Cancer Cells: The Role of (Poly)Phenols. Adv. Nutr. 2023, 14, 1111. [Google Scholar] [CrossRef]
- Chitty, J.L.; Cox, T.R. The Extracellular Matrix in Cancer: From Understanding to Targeting. Trends Cancer 2025, 11, 839–849. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q.; Sun, W.; Yue, Q.; He, P.; Niu, D.; Zhang, M. Mechanical Forces in the Tumor Microenvironment: Roles, Pathways, and Therapeutic Approaches. J. Transl. Med. 2025, 23, 313. [Google Scholar] [CrossRef]
- Yin, Q.; Qin, F.; Gan, F.; Zhao, G.; Chen, R.; Wen, Y.; Hua, X.; Zeng, F.; Zhang, Y.; Xiao, Y.; et al. Colonic Aging and Colorectal Cancer: An Unignorable Interplay and Its Translational Implications. Biology 2025, 14, 805. [Google Scholar] [CrossRef]
- Agrawal, A.; Javanmardi, Y.; Watson, S.A.; Serwinski, B.; Djordjevic, B.; Li, W.; Aref, A.R.; Jenkins, R.W.; Moeendarbary, E. Mechanical Signatures in Cancer Metastasis. NPJ Biol. Phys. Mech. 2025, 2, 3. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Sig Transduct Target Ther 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sampson, C.; Liu, C.; Piao, H.L.; Liu, H.X. Integrin Signaling in Cancer: Bidirectional Mechanisms and Therapeutic Opportunities. Cell Commun. Signal. 2023, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, Y.; Zhu, J.; Qin, Y.; Wu, H.; Yu, J.; Zhai, Q.; Li, S.; Qin, X.; Wang, D.; et al. Extracellular Matrix Signaling Cues: Biological Functions, Diseases, and Therapeutic Targets. MedComm 2025, 6, e70281. [Google Scholar] [CrossRef]
- Lawson, C.D.; Burridge, K. The On-off Relationship of Rho and Rac during Integrin-Mediated Adhesion and Cell Migration. Small GTPases 2014, 5, e27958. [Google Scholar] [CrossRef]
- Yang, J.; Hawthorne, L.; Stack, S.; Blagg, B.; Ali, A.; Zorlutuna, P. Engineered Age-Mimetic Breast Cancer Models Reveal Differential Drug Responses in Young and Aged Microenvironments. Adv. Healthc. Mater. 2025, 14, 2404461. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F.; Wu, Y.; Zhu, Y.; Jiang, Y.; Wu, Q.; Dong, Z.; Liu, K. Inflammation in Cancer: Therapeutic Opportunities from New Insights. Mol. Cancer 2025, 24, 51. [Google Scholar] [CrossRef]
- Li, Y.; Peng, S.; Xu, J.; Liu, W.; Luo, Q. Integrin Signaling in Tumor Biology: Mechanisms of Intercellular Crosstalk and Emerging Targeted Therapies. PeerJ 2025, 13, e19328. [Google Scholar] [CrossRef]
- Mao, H.; Zhao, X.; Sun, S.C. NF-ΚB in Inflammation and Cancer. Cell Mol. Immunol. 2025, 22, 811. [Google Scholar] [CrossRef]
- Thuya, W.L.; Cao, Y.; Ho, P.C.L.; Wong, A.L.A.; Wang, L.; Zhou, J.; Nicot, C.; Goh, B.C. Insights into IL-6/JAK/STAT3 Signaling in the Tumor Microenvironment: Implications for Cancer Therapy. Cytokine Growth Factor Rev. 2025, 85, 26–42. [Google Scholar] [CrossRef]
- Larson, C.R.; Mandloi, A.; Acharyya, S.; Carstens, J.L. The Tumor Microenvironment across Four Dimensions: Assessing Space and Time in Cancer Biology. Front. Immunol. 2025, 16, 1554114. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Wang, S.Q.; Shang, H.L.; Lv, H.F.; Chen, B.B.; Gao, S.G.; Chen, X.B. Roles and Inhibitors of FAK in Cancer: Current Advances and Future Directions. Front. Pharmacol. 2024, 15, 1274209. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Ashja Ardalan, A.; Dimesa, A.M.; Coban, M.A. Targeting Invasion: The Role of MMP-2 and MMP-9 Inhibition in Colorectal Cancer Therapy. Biomolecules 2024, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Geethadevi, A.; Ku, Z.; Tsaih, S.W.; Parashar, D.; Kadamberi, I.P.; Xiong, W.; Deng, H.; George, J.; Kumar, S.; Mittal, S.; et al. Blocking Oncostatin M Receptor Abrogates STAT3 Mediated Integrin Signaling and Overcomes Chemoresistance in Ovarian Cancer. NPJ Precis. Oncol. 2024, 8, 127. [Google Scholar] [CrossRef]
- Hu, T.; Zhai, J.; Yang, Z.; Peng, J.; Wang, C.; Liu, X.; Li, Y.; Yao, J.; Chen, F.; Li, H.; et al. Myeloid-Derived Suppressor Cells in Cancer: Mechanistic Insights and Targeted Therapeutic Innovations. MedComm 2025, 6, e70231. [Google Scholar] [CrossRef]
- Tylutka, A.; Walas, Ł.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and Age-Related Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef]
- Bayraktar, E.; Rodriguez-Aguayo, C.; Stur, E.; Kumar, S.; Mangala, L.S.; Jennings, N.B.; Bayram, N.N.; Corvigno, S.; Asare, A.; Ivan, C.; et al. Epigenetic Modulation of BARD1 to Enhance Anti-VEGF Therapy. Cell Rep. Med. 2025, 6, 102329. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Vining, D.J.; Arora, S.P.; de Achaval, S.; Larson, J.; Kauh, J.; Cartwright, C.; Avritscher, R.; Alibhai, I.; Tweardy, D.J.; et al. Phase I Trial of TTI-101, a First-in-Class Oral Inhibitor of STAT3, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2025, 31, 965–974. [Google Scholar] [CrossRef]
- Czajkowski, K.; Herbet, M.; Murias, M.; Piątkowska-Chmiel, I. Senolytics: Charting a New Course or Enhancing Existing Anti-Tumor Therapies? Cell. Oncol. 2024, 48, 351. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Bahijri, S.; Tuomilehto, J.; Uversky, V.N.; Ren, J. Hallmarks of Cellular Senescence: Biology, Mechanisms, Regulations. Exp. Mol. Med. 2025, 57, 1482–1491. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular Senescence and SASP in Tumor Progression and Therapeutic Opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef]
- Upadhyay, P.; Suhail, A.; Khanal, P.; Kumar, S. Mechanistic Insights and Biomarker Discovery in Immune Cell Aging and Age-Associated Diseases. Front. Immunol. 2025, 16, 1637191. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, S.; Takahashi, A. Cellular Senescence: Mechanisms and Relevance to Cancer and Aging. J. Biochem. 2025, 177, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Rapamycin Promotes Endothelial-Mesenchymal Transition during Stress-Induced Premature Senescence through the Activation of Autophagy. Cell Commun. Signal. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Saliev, T.; Singh, P.B. Targeting Senescence: A Review of Senolytics and Senomorphics in Anti-Aging Interventions. Biomolecules 2025, 15, 860. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, R.; Fan, H.; Yu, Y.; Jia, H.; Peng, Z.; Zhou, L.; Yu, G.; Zhang, W. Impact and Potential Value of Immunosenescence on Solid Gastrointestinal Tumors. Front. Immunol. 2024, 15, 1375730. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Z.; Zhang, C.; Zhao, Q.; Liu, Z.; Cheng, Q.; Zhang, J.; Lin, A.; Luo, P. NK Cell Senescence in Cancer: From Molecular Mechanisms to Therapeutic Opportunities. Aging Dis, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Wang, Y.; Mohseni, M.; Grauel, A.; Diez, J.E.; Guan, W.; Liang, S.; Choi, J.E.; Pu, M.; Chen, D.; Laszewski, T.; et al. SHP2 Blockade Enhances Anti-Tumor Immunity via Tumor Cell Intrinsic and Extrinsic Mechanisms. Sci. Rep. 2021, 11, 1399. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.F.; Li, S. PD-1-Mediated Inhibition of T Cell Activation: Mechanisms and Strategies for Cancer Combination Immunotherapy. Cell Insight 2024, 3, 100146. [Google Scholar] [CrossRef]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct Functions in Immune Regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Liu, L.; Hao, Z.; Yang, X.; Li, Y.; Wang, S.; Li, L. Metabolic Reprogramming in T Cell Senescence: A Novel Strategy for Cancer Immunotherapy. Cell Death Discov. 2025, 11, 161. [Google Scholar] [CrossRef]
- Marr, B.; Jo, D.; Jang, M.; Lee, S.H. Cytokines in Focus: IL-2 and IL-15 in NK Adoptive Cell Cancer Immunotherapy. Immune Netw. 2025, 25, e17. [Google Scholar] [CrossRef] [PubMed]
- Chambuso, R.; Meena, S.S. Single-Cell Spatial Immune Profiling for Precision Immunotherapy in Lynch Syndrome. J. Natl. Cancer Cent. 2024, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Zhu, B.; Deng, M.; Qiu, J.; Feng, Y.; Ding, N.; Huang, C. Metabolic Reprogramming Induced by Aging Modifies the Tumor Microenvironment. Cells 2024, 13, 1721. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Marafie, S.K.; Al-Mulla, F.; Abubaker, J. MTOR: Its Critical Role in Metabolic Diseases, Cancer, and the Aging Process. Int. J. Mol. Sci. 2024, 25, 6141. [Google Scholar] [CrossRef]
- Yang, M.; Mu, Y.; Yu, X.; Gao, D.; Zhang, W.; Li, Y.; Liu, J.; Sun, C.; Zhuang, J. Survival Strategies: How Tumor Hypoxia Microenvironment Orchestrates Angiogenesis. Biomed. Pharmacother. 2024, 176, 116783. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Maswikiti, E.P.; Yu, Y.; Song, K.; Ma, C.; Han, X.; Ma, H.; Deng, X.; Yu, R.; et al. AMPK–a Key Factor in Crosstalk between Tumor Cell Energy Metabolism and Immune Microenvironment? Cell Death Discov. 2024, 10, 237. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, X.; Han, Q.; Ren, C.; Gao, S.; Liu, Y.; Li, X. Relationship between Breast Tissue Involution and Breast Cancer. Front. Oncol. 2025, 15, 1420350. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, T.; Liu, Z.; Liu, Y.; Liu, J.; Wang, S.; Shi, X.; Zhou, H. Persistent Accumulation of Therapy-Induced Senescent Cells: An Obstacle to Long-Term Cancer Treatment Efficacy. Int. J. Oral. Sci. 2025, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Finger, A.M.; Hendley, A.M.; Figueroa, D.; Gonzalez, H.; Weaver, V.M. Tissue Mechanics in Tumor Heterogeneity and Aggression. Trends Cancer 2025, 11, 806–824. [Google Scholar] [CrossRef]
- Wang, L.; Tang, D. Immunosenescence Promotes Cancer Development: From Mechanisms to Treatment Strategies. Cell Commun. Signal. 2025, 23, 128. [Google Scholar] [CrossRef]
- Khalil, R.; Diab-Assaf, M.; Lemaitre, J.M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023, 12, 915. [Google Scholar] [CrossRef]
- Clark, R.; Vesprini, D.; Narod, S.A. The Effect of Age on Prostate Cancer Survival. Cancers 2022, 14, 4149. [Google Scholar] [CrossRef]
- Rago, V.; Conforti, F.; La Russa, D.; Antonucci, G.; Urlandini, L.; Lofaro, D.; Bossio, S.; Mandalà, M.; Pellegrino, D.; Aversa, A.; et al. The Effects of Caloric Restriction on Inflammatory Targets in the Prostates of Aged Rats. Int. J. Mol. Sci. 2024, 25, 5236. [Google Scholar] [CrossRef]
- Vecchiotti, D.; Clementi, L.; Cornacchia, E.; Di Vito Nolfi, M.; Verzella, D.; Capece, D.; Zazzeroni, F.; Angelucci, A. Evidence of the Link between Stroma Remodeling and Prostate Cancer Prognosis. Cancers 2024, 16, 3215. [Google Scholar] [CrossRef]
- Tufail, M.; Huang, Y.Q.; Hu, J.J.; Liang, J.; He, C.Y.; Wan, W.D.; Jiang, C.H.; Wu, H.; Li, N. Cellular Aging and Senescence in Cancer: A Holistic Review of Cellular Fate Determinants. Aging Dis. 2024, 16, 1483. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, C.M.; Wu, H. Environmental Exposures and Lung Aging: Molecular Mechanisms and Implications for Improving Respiratory Health. Curr. Environ. Health Rep. 2021, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Han, S.J.; Georgiev, P.; Ringel, A.E.; Sharpe, A.H.; Haigis, M.C. Age-Associated Remodeling of T Cell Immunity and Metabolism. Cell Metab. 2022, 35, 36. [Google Scholar] [CrossRef]
- Araghi, M.; Mannani, R.; Heidarnejad maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent Advances in Non-Small Cell Lung Cancer Targeted Therapy; an Update Review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Siegel, F.; Torelli, A.; Mattis, M.; Debatin, J.; Erben, P.; Gumbel, M. Proliferation and Regeneration of the Healthy Human Urothelium: A Multi-Scale Simulation Approach with 16 Hypotheses of Cell Differentiation. PLoS ONE 2025, 20, e0325132. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lam, H.M.; Rosser, C.; Theodorescu, D.; Parks, W.C.; Chan, K.S. The Dynamic Roles of the Bladder Tumour Microenvironment. Nat. Rev. Urol. 2022, 19, 515. [Google Scholar] [CrossRef]
- Aghamir, S.M.K.; Khatami, F.; Farrokhpour, H.; Reis, L.O.; Pishkuhi, M.A.; Mohammadi, A. Oncologic Outcomes of Bacillus Calmette-Guérin Therapy in Elderly Patients with Non-Muscle-Invasive Bladder Cancer: A Meta-Analysis. PLoS ONE 2022, 17, e0267934. [Google Scholar] [CrossRef]
- Godlewski, D.; Czech, S.; Bartusik-Aebisher, D.; Aebisher, D. Bladder Cancer Basic Study and Current Clinical Trials. Uro 2024, 4, 145–196. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal Cancer Incidence Trends in Younger versus Older Adults: An Analysis of Population-Based Cancer Registry Data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Christenson, E.S.; Tsai, H.L.; Le, D.T.; Jaffee, E.M.; Dudley, J.; Xian, R.R.; Gocke, C.D.; Eshleman, J.R.; Lin, M.T. Colorectal Cancer in Patients of Advanced Age Is Associated with Increased Incidence of BRAF p.V600E Mutation and Mismatch Repair Deficiency. Front. Oncol. 2023, 13, 1193259. [Google Scholar] [CrossRef]
- Sminia, P.; Guipaud, O.; Viktorsson, K.; Ahire, V.; Baatout, S.; Boterberg, T.; Cizkova, J.; Dostál, M.; Fernandez-Palomo, C.; Filipova, A.; et al. Clinical Radiobiology for Radiation Oncology. In Radiobiology Textbook; Springer International Publishing: Cham, Switzerland, 2023; pp. 237–309. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular Mechanisms and Diseases. Signal Transduct. Target Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Kao, C.; Charmsaz, S.; Tsai, H.L.; Aziz, K.; Shu, D.H.; Munjal, K.; Griffin, E.; Leatherman, J.M.; Lipson, E.J.; Ged, Y.; et al. Age-Related Divergence of Circulating Immune Responses in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors. Nat. Commun. 2025, 16, 3531. [Google Scholar] [CrossRef]
- Smith, A.; Boby, J.; Benny, S.; Ghazali, N.; Vermeulen, E.; George, M. Immunotherapy in Older Patients with Cancer: A Narrative Review. Int. J. Gen. Med. 2024, 17, 305–313. [Google Scholar] [CrossRef] [PubMed]
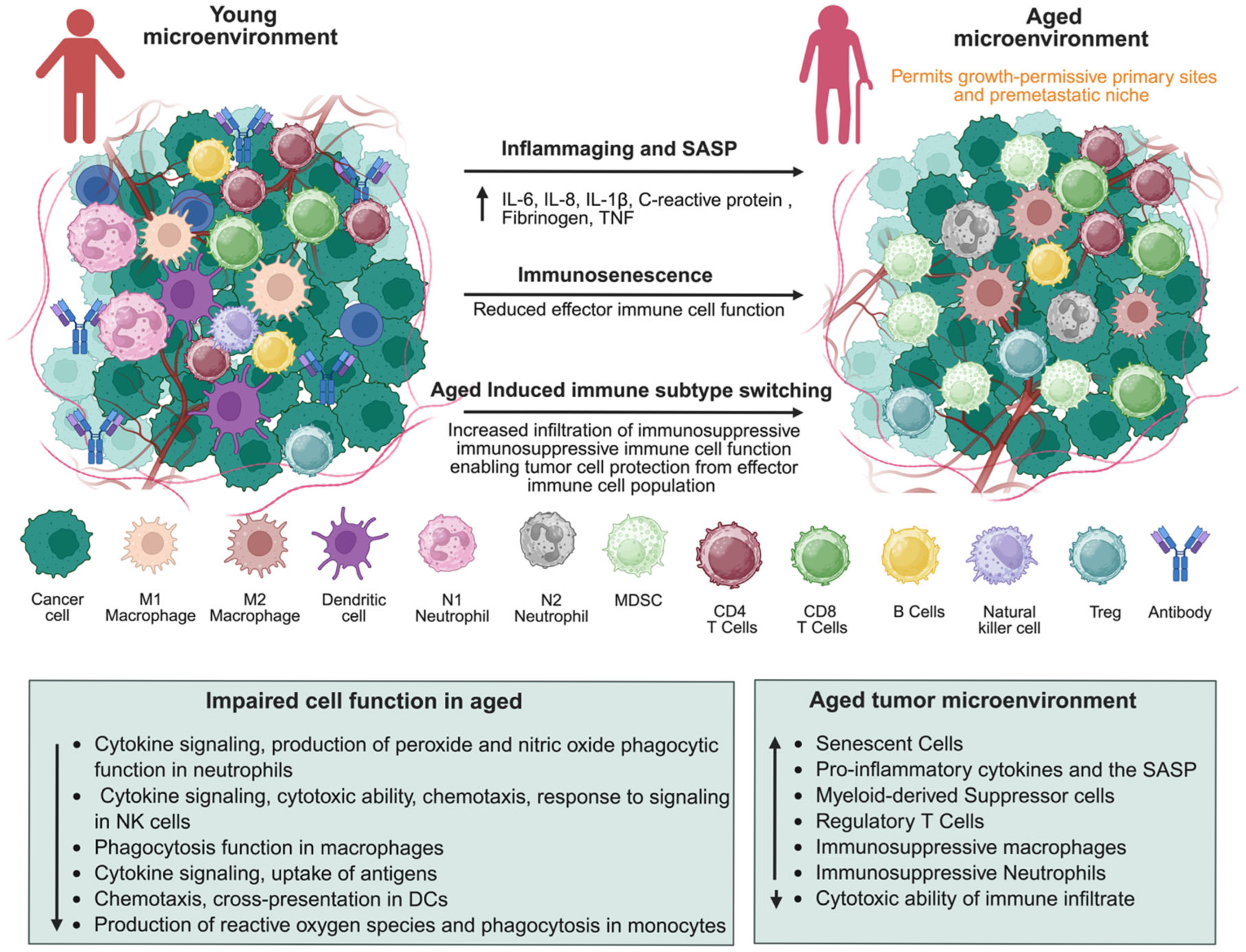
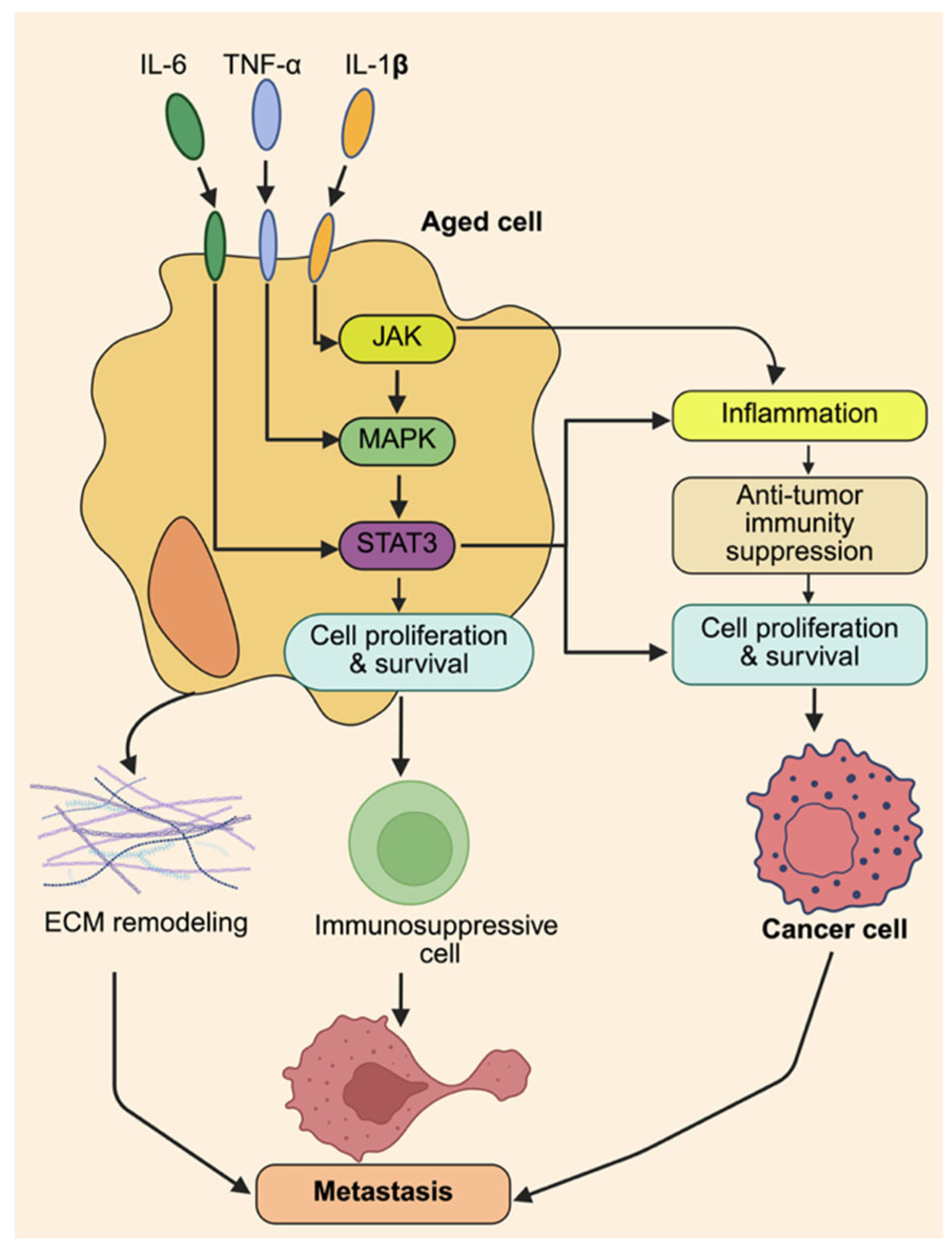
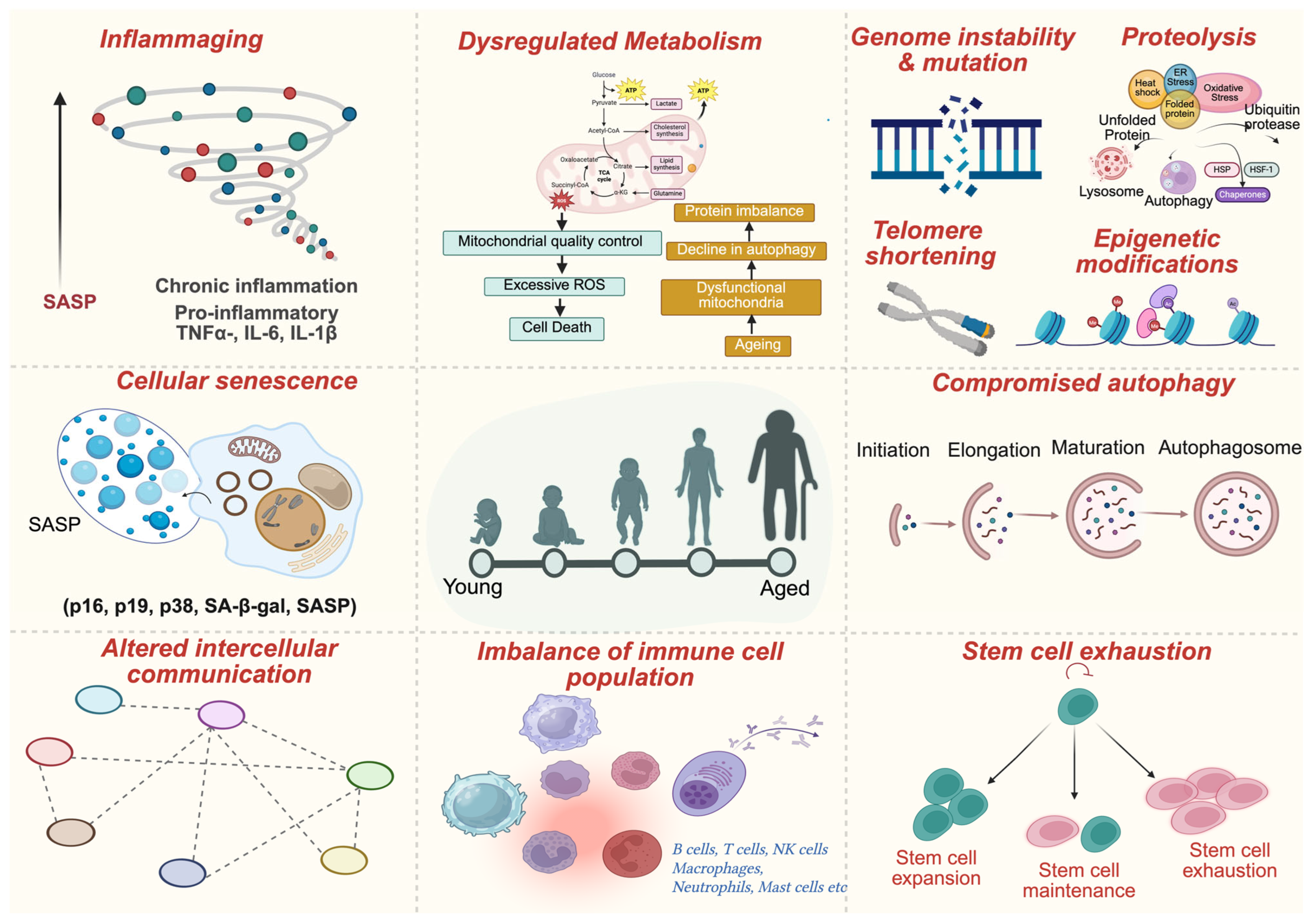
| Cancer Type | TME Feature | Key Pathways | Immune Changes | Clinical/Unique Aspect |
|---|---|---|---|---|
| Breast | Stromal fibrosis, adipose replacement | LOX, YAP/TAZ, TGF-β | ↑Tregs, ↓naive T cells | Hormone receptor-positive, senolytics promising |
| Prostate | Chronic inflammation, senescent fibroblasts | NF-κB, AR dysregulation | ↑Tregs, TAMs | CRPC development; androgen signaling altered |
| Lung | Fibrosis, epithelial senescence | STAT3, HIF-1α, EMT | Exhausted T cells, ↓naive T cells | Reduced immunotherapy efficacy |
| Bladder | ECM stiffening, recurrent inflammation | NF-κB, STAT3, LOX | ↑MDSCs, ↑Tregs | Reduced BCG response in elderly |
| Colorectal | Dysbiosis, stromal fibrosis | TGF-β, IL-6, Wnt | ↓mucosal immunity, ↑Tregs | Microsatellite instability-high tumors, microbiome-targeted therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Chand, J.; Sharma, P.; Singh, S.; Khanal, P.; Singh Jayasingh Chellammal, H.; Suhail, A.; Mittal, S. Molecular Mechanisms Driving Metastatic Progression Within the Aged Tumor Microenvironment. Int. J. Mol. Sci. 2025, 26, 11508. https://doi.org/10.3390/ijms262311508
Kumar S, Chand J, Sharma P, Singh S, Khanal P, Singh Jayasingh Chellammal H, Suhail A, Mittal S. Molecular Mechanisms Driving Metastatic Progression Within the Aged Tumor Microenvironment. International Journal of Molecular Sciences. 2025; 26(23):11508. https://doi.org/10.3390/ijms262311508
Chicago/Turabian StyleKumar, Sudhir, Jagdish Chand, Preeti Sharma, Sudhakar Singh, Pukar Khanal, Hanish Singh Jayasingh Chellammal, Aamir Suhail, and Sonam Mittal. 2025. "Molecular Mechanisms Driving Metastatic Progression Within the Aged Tumor Microenvironment" International Journal of Molecular Sciences 26, no. 23: 11508. https://doi.org/10.3390/ijms262311508
APA StyleKumar, S., Chand, J., Sharma, P., Singh, S., Khanal, P., Singh Jayasingh Chellammal, H., Suhail, A., & Mittal, S. (2025). Molecular Mechanisms Driving Metastatic Progression Within the Aged Tumor Microenvironment. International Journal of Molecular Sciences, 26(23), 11508. https://doi.org/10.3390/ijms262311508







