IL-15 Complexes Combined with PD-1 Blockade Affect Immune Cell Distribution, Localization, and Immune Signatures in Regressing Versus Non-Regressing Metastatic Breast Tumors
Abstract
1. Introduction
2. Results
2.1. A Single Dose of IL-15 Complex Fails to Enhance the Efficacy of Anti-PD-1 Therapy
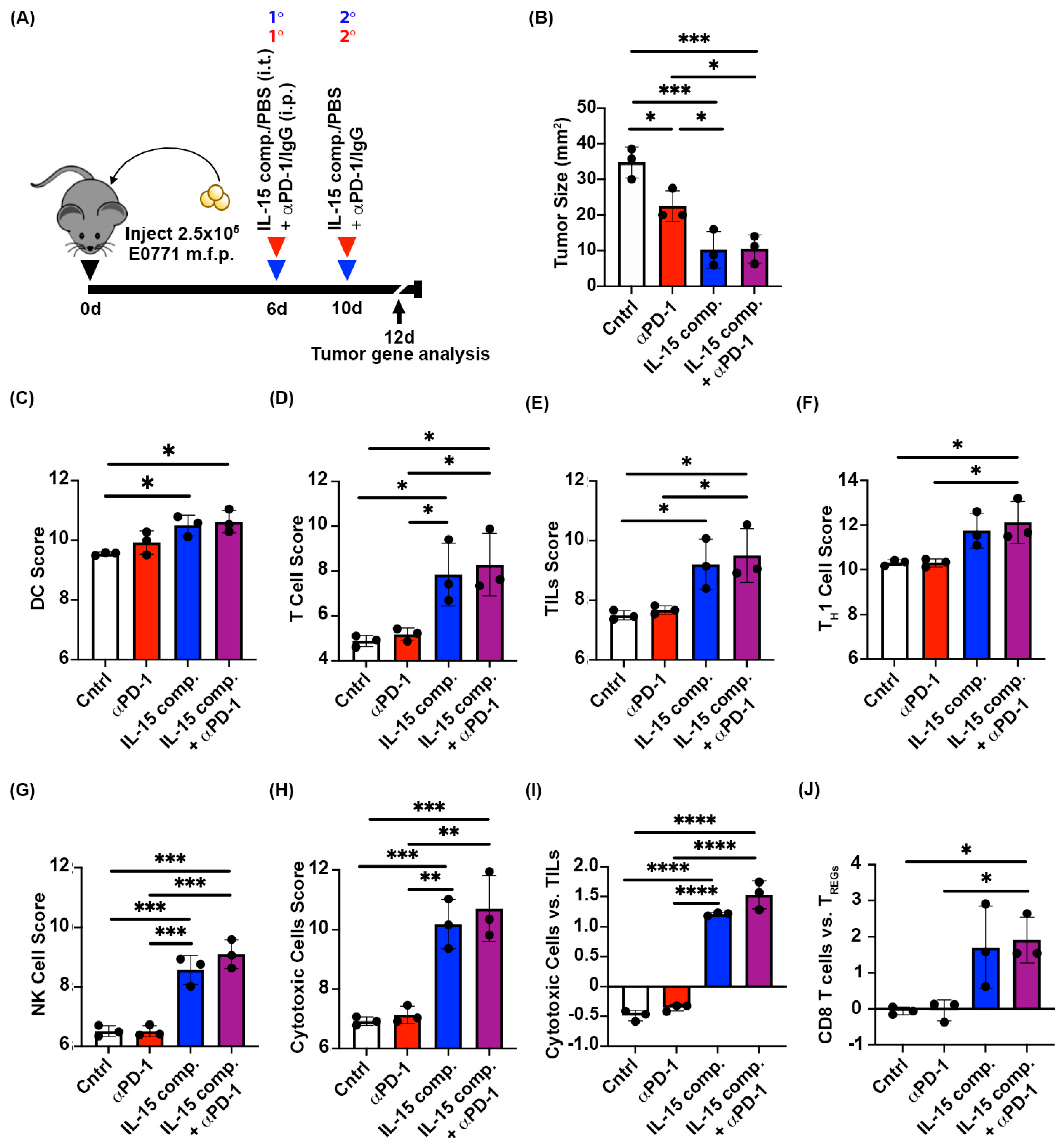
2.2. Two Doses of IL-15 Complex Intratumorally Augment Anti-PD-1 Therapy in Murine EO771 Breast Cancer
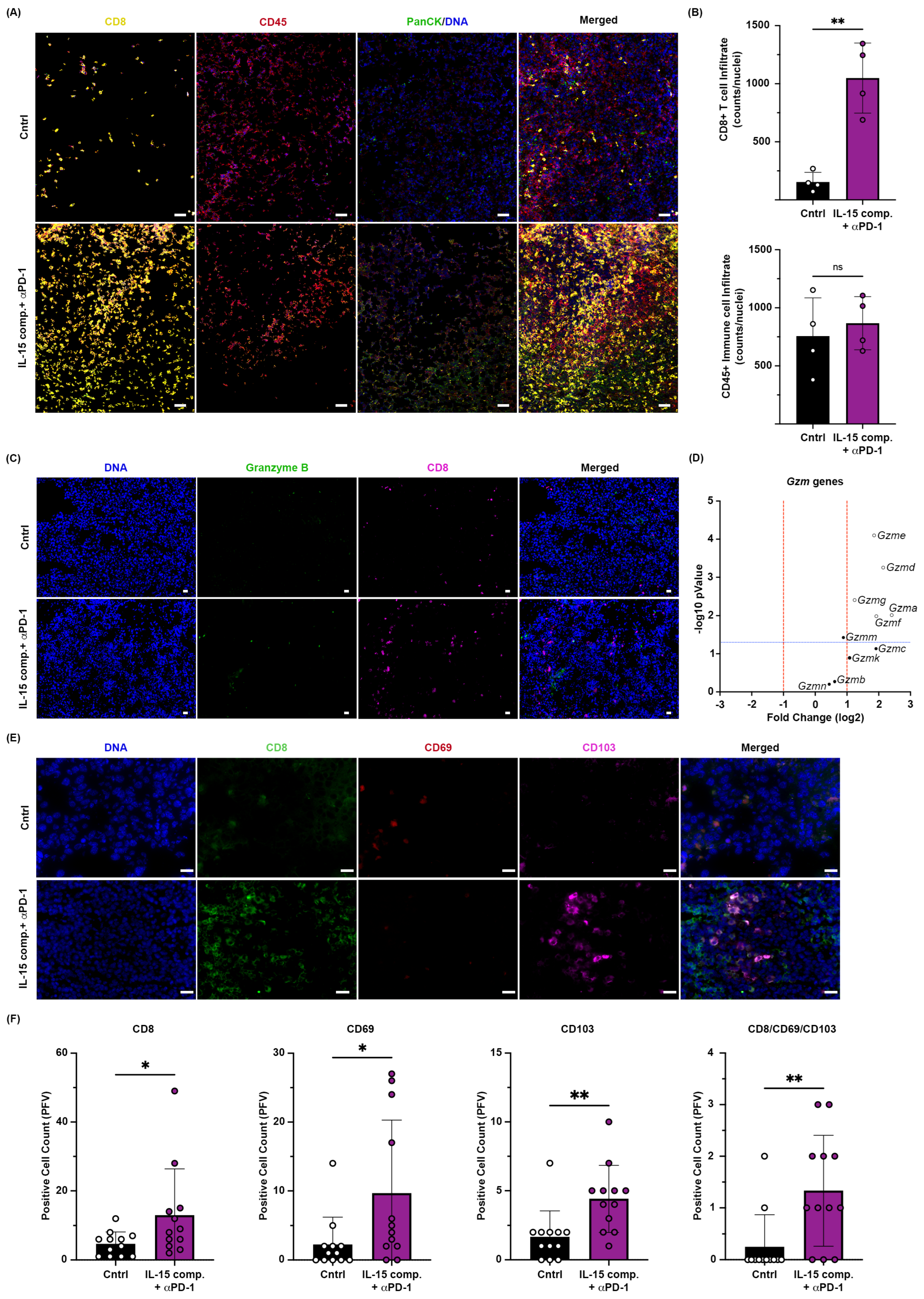
2.3. Intratumoral Treatment with IL-15 Complexes Enhances Cytotoxic Immune Infiltrates in Anti-PD-1 Treatment Within EO771 Tumors, a Murine Luminal B Breast Cancer Model
2.4. Intratumoral Administration of IL-15 Complexes in Combination with Anti-PD-1mAb Therapy Enhances CD8+ T Cell and NK Cell Infiltration and Granzyme Genes Expressed in CD8+ TILs of Murine EO771 Breast Cancer
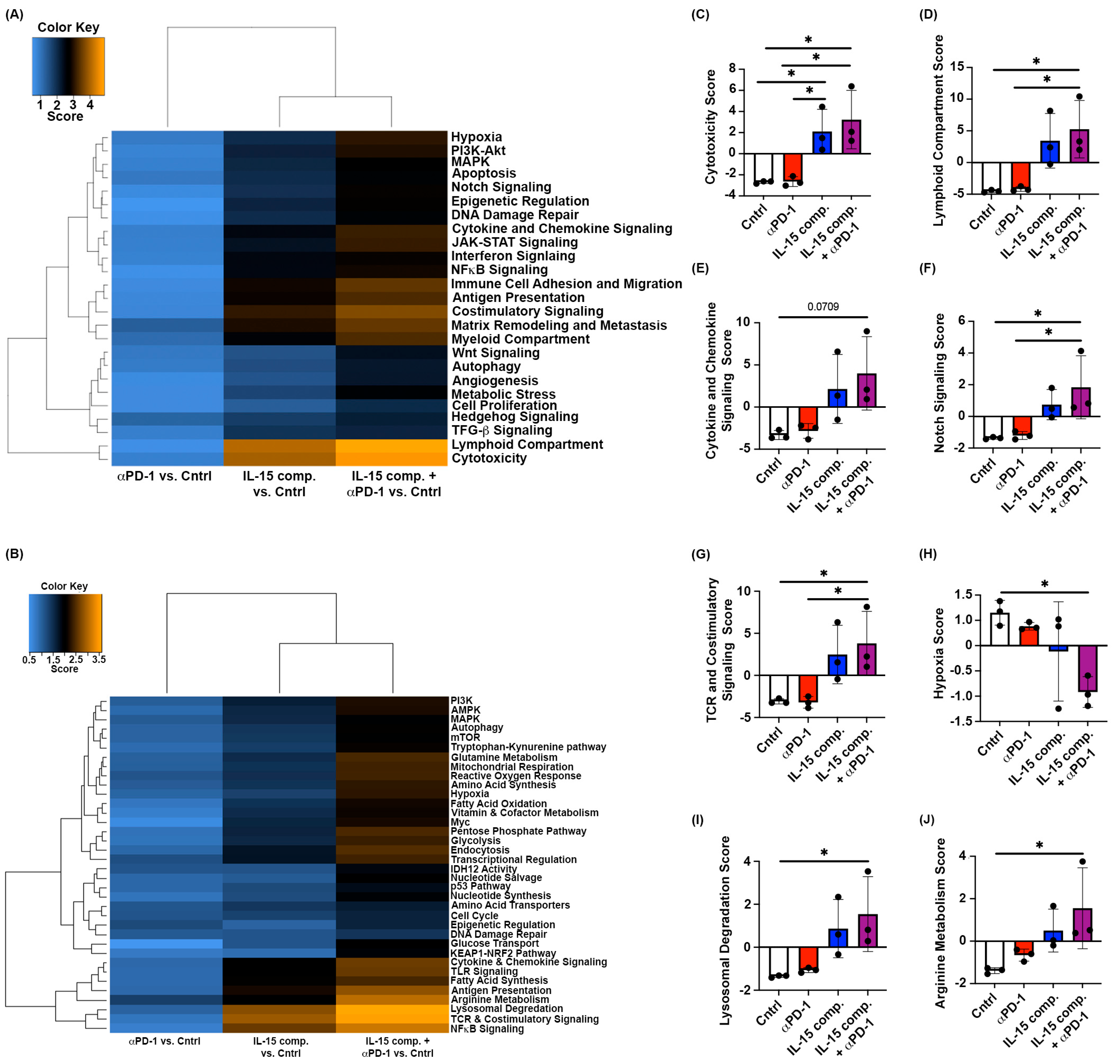
2.5. Intratumoral Treatment with IL-15 Complexes Modulates Immune Activation Signaling and Metabolic Pathways Within Murine EO771 Breast Cancer
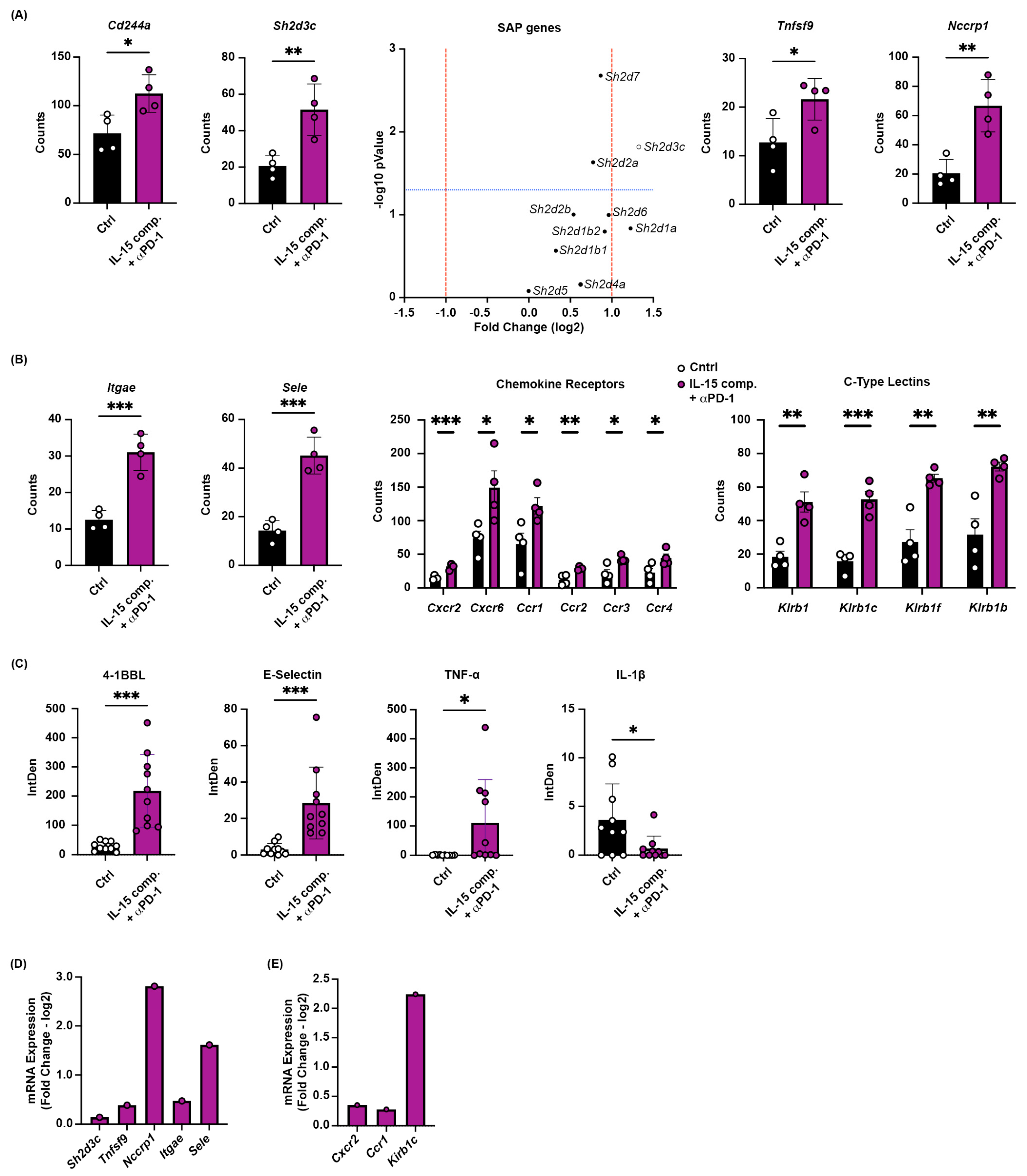
2.6. Increased Gene Expression of Multiple Tumor-Homing Receptors in CD8+ TILs Following Combinatorial Treatment with IL-15 Complexes and PD-1 Blockade
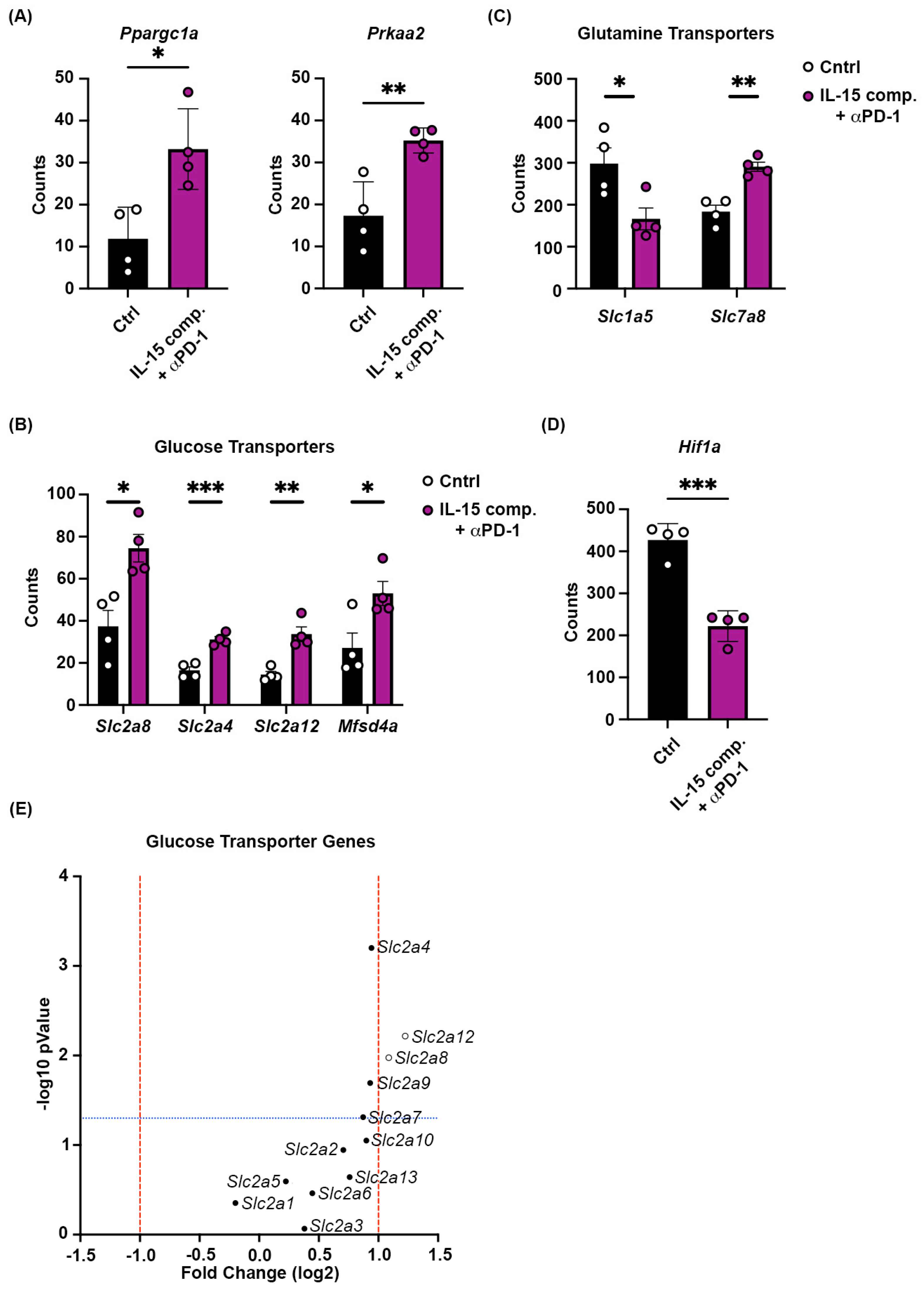
2.7. Combining Intratumoral IL-15 Complexes with PD-1 Blockade Regulates Gene Expression of Critical Mitochondrial Biogenesis Gene, Peroxisome Proliferator-Activated Receptor Gamma Coactivator Alpha (ppargc1a), and Glucose Transport Proteins in CD8+ T Cells to Reduce Hypoxia
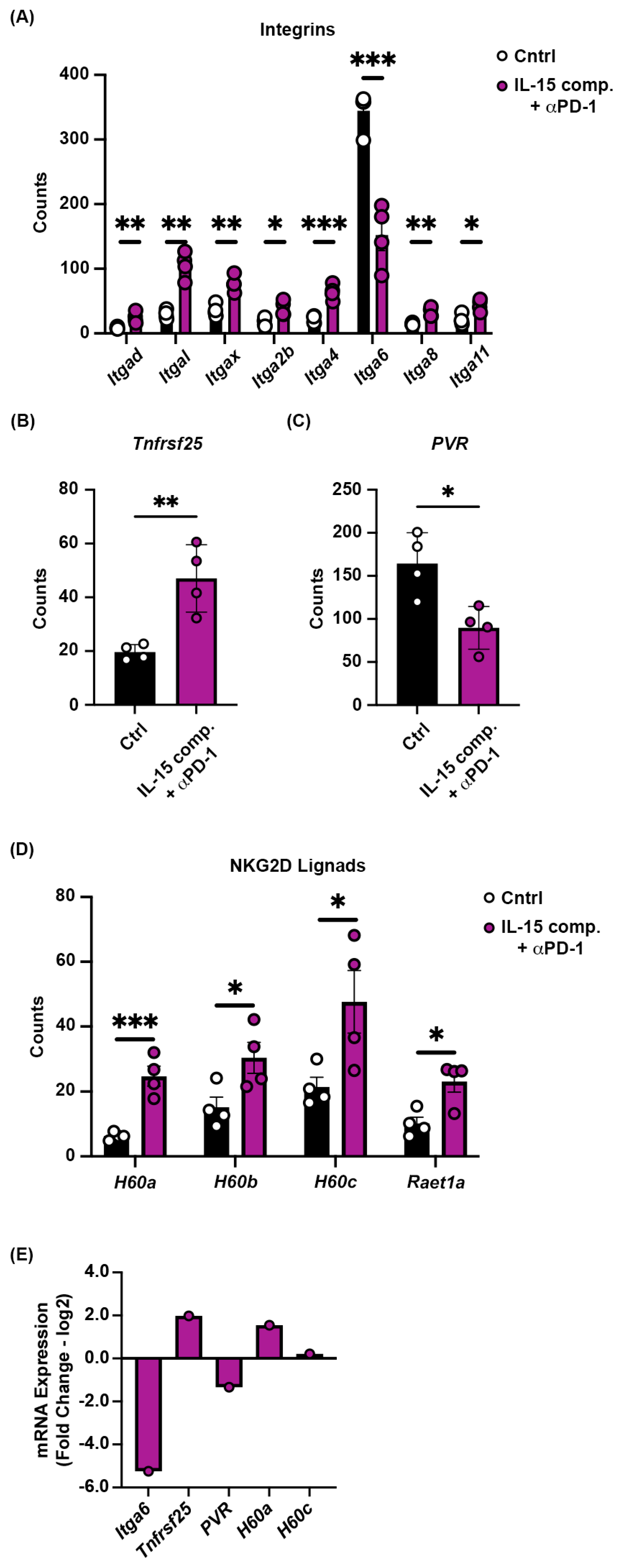
2.8. Receptor and Ligand Expression on Tumors Treated with IL-15 Complexes and Anti-PD-1 mAb
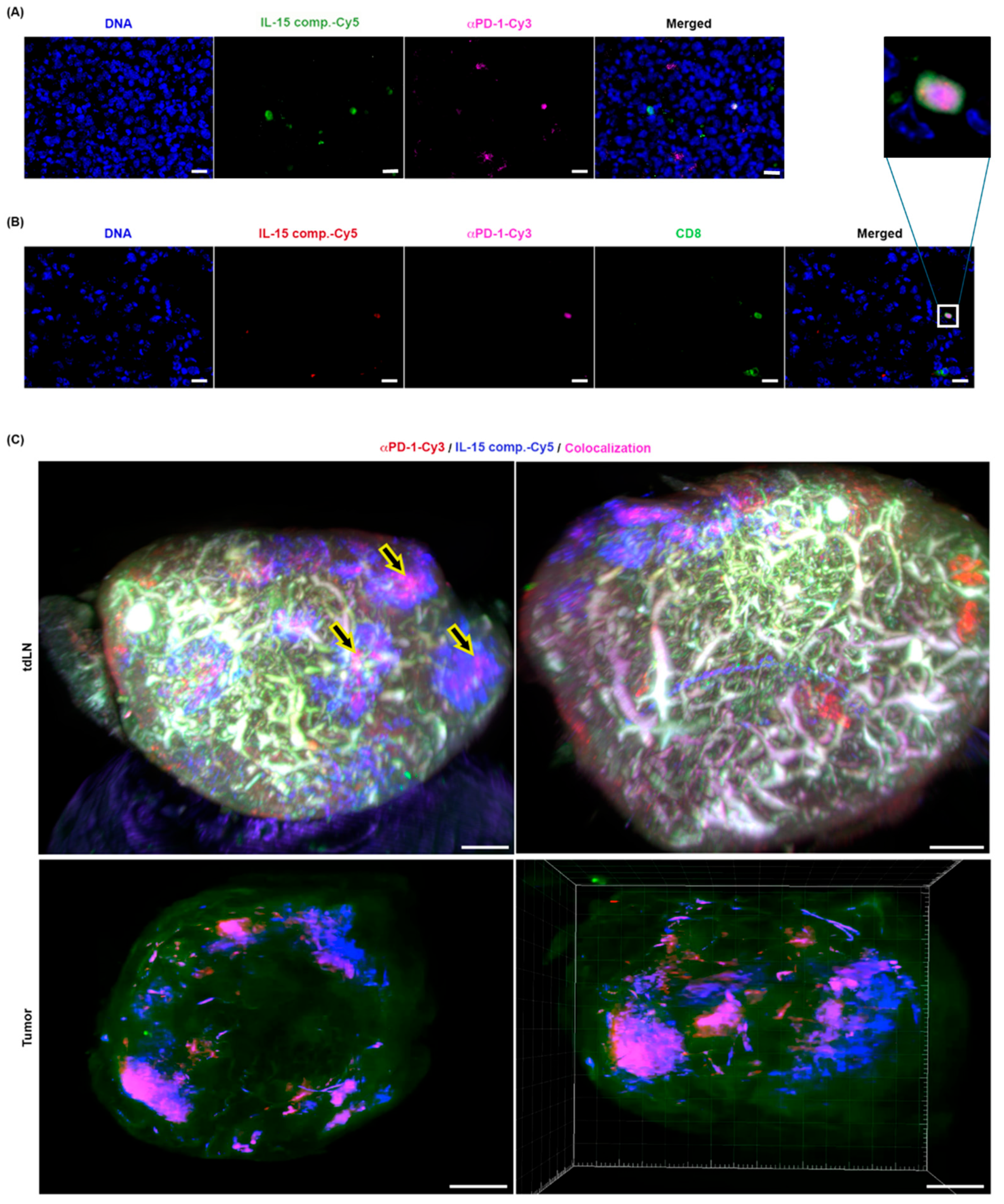
2.9. The Spatial Distribution of IL-15 Complexes and Anti-PD-1 mAb Therapy in the TME and Tumor-Draining Lymph Nodes (TDLN)
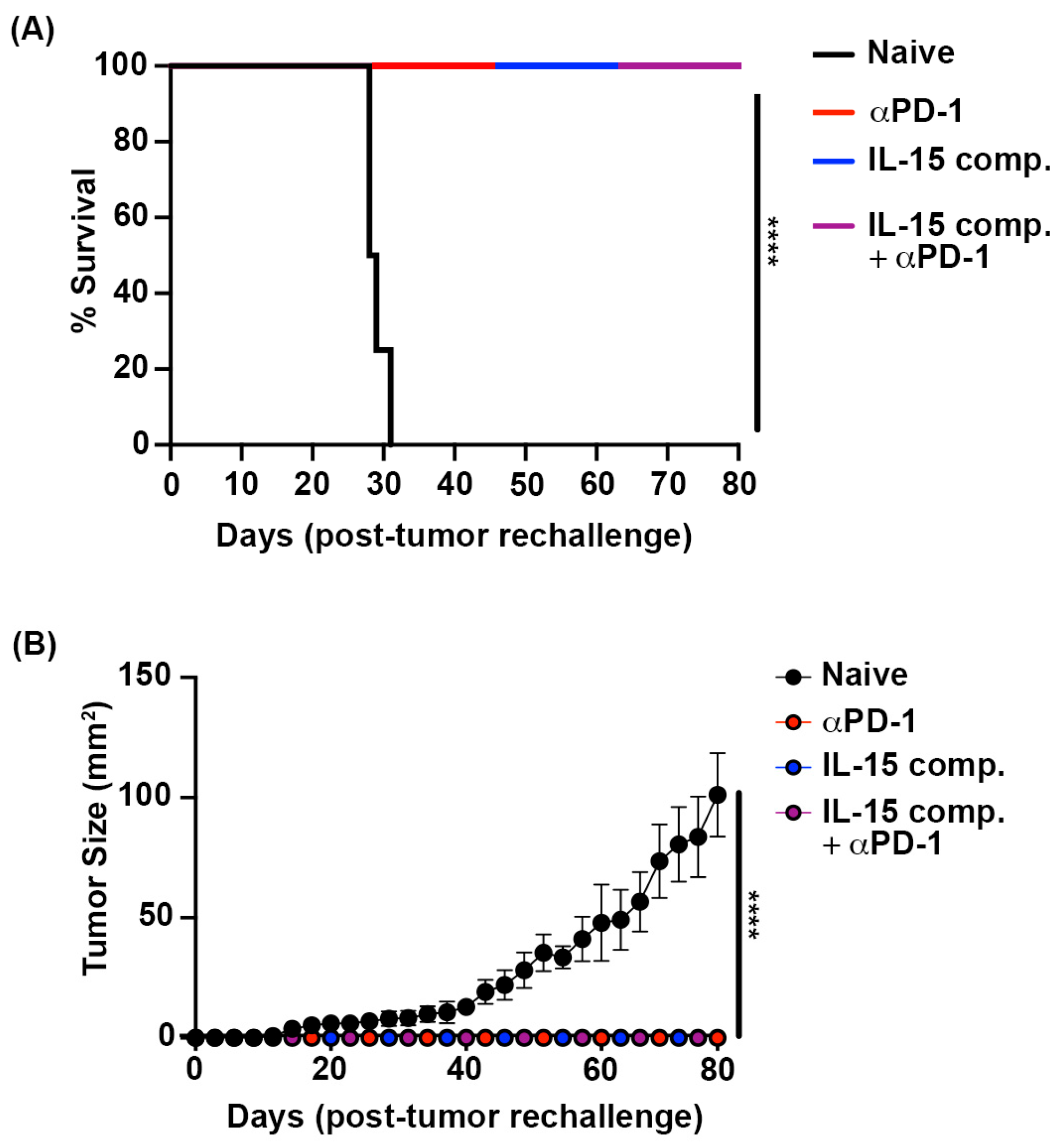
2.10. Complete Responses to Immunotherapy Generate Durable Immune Responses Against Murine Luminal Breast Cancer
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Tumor Challenge and Treatment with Anti-PD-1 mAb Therapy and IL-15 Complexes
4.3. IL-15 Complex Treatment and Protein Labeling
4.4. Nanostring® PanCancer IO 360TM Panel and GeoMx® Digital Spatial Profiling
4.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
4.6. Cell Score and Counts
4.7. Immunofluorescent Staining and Microscopy
4.8. Microscopy and Data Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leidner, R.; Conlon, K.; McNeel, D.G.; Wang-Gillam, A.; Gupta, S.; Wesolowski, R.; Chaudhari, M.; Hassounah, N.; Lee, J.B.; Ho Lee, L.; et al. First-in-Human Phase I/Ib Study of NIZ985, a Recombinant Heterodimer of IL-15 and IL-15Rα, as a Single Agent and in Combination with Spartalizumab in Patients with Advanced and Metastatic Solid Tumors. J. Immunother. Cancer 2023, 11, e007725. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Hu, Y.; Huang, W. Landscape of Immune Microenvironment Under Immune Cell Infiltration Pattern in Breast Cancer. Front. Immunol. 2021, 12, 711433. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-Tumor Genomic Biomarkers for PD-1 Checkpoint Blockade-Based Immunotherapy. Science 2018, 362, eaar3593, Erratum in Science 2019, 363, eaax1384. https://doi.org/10.1126/science.aax1384. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Corrales, L.; Williams, J.; Horton, B.; Sivan, A.; Spranger, S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv. Exp. Med. Biol. 2017, 1036, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ-Related MRNA Profile Predicts Clinical Response to PD-1 Blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Imani, S.; Farghadani, R.; Roozitalab, G.; Maghsoudloo, M.; Emadi, M.; Moradi, A.; Abedi, B.; Jabbarzadeh Kaboli, P. Reprogramming the Breast Tumor Immune Microenvironment: Cold-to-Hot Transition for Enhanced Immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 131. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14, Erratum in Immunity 2019, 51, 411–412. https://doi.org/10.1016/j.immuni.2019.08.004. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H. Introduction to Checkpoint Inhibitors and Cancer Immunotherapy. Immunol. Rev. 2017, 276, 5–8. [Google Scholar] [CrossRef]
- Bu, X.; Mahoney, K.M.; Freeman, G.J. Learning from PD-1 Resistance: New Combination Strategies. Trends Mol. Med. 2016, 22, 448–451. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef]
- Wilkinson, R.W.; Leishman, A.J. Further Advances in Cancer Immunotherapy: Going Beyond Checkpoint Blockade. Front. Immunol. 2018, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, C.; Stravokefalou, V.; Stellas, D.; Karaliota, S.; Felber, B.K.; Pavlakis, G.N. Heterodimeric IL-15 in Cancer Immunotherapy. Cancers 2021, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Husman, T.; Cen, X.; Tsao, T.; Brown, J.; Bajpai, A.; Li, M.; Zhou, K.; Yang, L. Interleukin 15 in Cell-Based Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 7311. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Dubois, S.; Miljkovic, M.D.; Conlon, K.C. IL-15 in the Combination Immunotherapy of Cancer. Front. Immunol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Bai, X.; Handley, M.; Shan, F. Biological Effects of IL-15 on Immune Cells and Its Potential for the Treatment of Cancer. Int. Immunopharmacol. 2021, 91, 107318. [Google Scholar] [CrossRef]
- Peng, Y.; Fu, S.; Zhao, Q. 2022 Update on the Scientific Premise and Clinical Trials for IL-15 Agonists as Cancer Immunotherapy. J. Leukoc. Biol. 2022, 112, 823–834. [Google Scholar] [CrossRef]
- Waldmann, T.A. The Biology of Interleukin-2 and Interleukin-15: Implications for Cancer Therapy and Vaccine Design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Sanjabi, S.; Mosaheb, M.M.; Flavell, R.A. Opposing Effects of TGF-Beta and IL-15 Cytokines Control the Number of Short-Lived Effector CD8+ T Cells. Immunity 2009, 31, 131–144. [Google Scholar] [CrossRef]
- Waldmann, T.A. The Shared and Contrasting Roles of IL2 and IL15 in the Life and Death of Normal and Neoplastic Lymphocytes: Implications for Cancer Therapy. Cancer Immunol. Res. 2015, 3, 219–227. [Google Scholar] [CrossRef]
- Wuest, T.Y.; Willette-Brown, J.; Durum, S.K.; Hurwitz, A.A. The Influence of IL-2 Family Cytokines on Activation and Function of Naturally Occurring Regulatory T Cells. J. Leukoc. Biol. 2008, 84, 973–980. [Google Scholar] [CrossRef]
- Stoklasek, T.A.; Schluns, K.S.; Lefrançois, L. Combined IL-15/IL-15Ralpha Immunotherapy Maximizes IL-15 Activity in Vivo. J. Immunol. 2006, 177, 6072–6080. [Google Scholar] [CrossRef]
- Sarhan, D.; Hippen, K.L.; Lemire, A.; Hying, S.; Luo, X.; Lenvik, T.; Curtsinger, J.; Davis, Z.; Zhang, B.; Cooley, S.; et al. Adaptive NK Cells Resist Regulatory T-Cell Suppression Driven by IL37. Cancer Immunol. Res. 2018, 6, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Knudson, K.M.; Hodge, J.W.; Schlom, J.; Gameiro, S.R. Rationale for IL-15 Superagonists in Cancer Immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 705–709, Erratum in Expert Opin. Biol. Ther. 2021, 21, i. https://doi.org/10.1080/14712598.2020.1826233. [Google Scholar] [CrossRef] [PubMed]
- Rosario, M.; Liu, B.; Kong, L.; Schneider, S.E.; Jeng, E.K.; Rhode, P.R.; Wong, H.C.; Fehniger, T.A. The IL-15 Superagonist ALT-803 Enhances Anti-CD20 Antibody-Directed NK Cell ADCC and in Vivo Clearance of B Cell Lymphomas. J. Immunother. Cancer 2014, 2, P168. [Google Scholar] [CrossRef]
- Knudson, K.M.; Hicks, K.C.; Ozawa, Y.; Schlom, J.; Gameiro, S.R. Functional and Mechanistic Advantage of the Use of a Bifunctional Anti-PD-L1/IL-15 Superagonist. J. Immunother. Cancer 2020, 8, e000493. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, A.; Koffi, Y.; Diab, M.; Le Guennec, D.; Rougé, S.; Aldekwer, S.; Goncalves-Mendes, N.; Talvas, J.; Farges, M.C.; Caldefie-Chezet, F.; et al. EO771, the First Luminal B Mammary Cancer Cell Line from C57BL/6 Mice. Cancer Cell Int. 2020, 20, 328. [Google Scholar] [CrossRef]
- Creighton, C.J. The Molecular Profile of Luminal B Breast Cancer. Biologics 2012, 6, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Walens, A.; Olsson, L.T.; Gao, X.; Hamilton, A.M.; Kirk, E.L.; Cohen, S.M.; Midkiff, B.R.; Xia, Y.; Sherman, M.E.; Nikolaishvili-Feinberg, N.; et al. Protein-Based Immune Profiles of Basal-like vs. Luminal Breast Cancers. Lab. Investig. 2021, 101, 785–793. [Google Scholar] [CrossRef]
- Sowell, R.T.; Goldufsky, J.W.; Rogozinska, M.; Quiles, Z.; Cao, Y.; Castillo, E.F.; Finnegan, A.; Marzo, A.L. IL-15 Complexes Induce Migration of Resting Memory CD8 T Cells into Mucosal Tissues. J. Immunol. 2017, 199, 2536–2546. [Google Scholar] [CrossRef] [PubMed]
- MacNabb, B.W.; Tumuluru, S.; Chen, X.; Godfrey, J.; Kasal, D.N.; Yu, J.; Jongsma, M.L.M.; Spaapen, R.M.; Kline, D.E.; Kline, J. Dendritic Cells Can Prime Anti-Tumor CD8+ T Cell Responses through Major Histocompatibility Complex Cross-Dressing. Immunity 2022, 55, 982–997.e8, Erratum in Immunity 2022, 55, 2206–2208. https://doi.org/10.1016/j.immuni.2022.09.015. [Google Scholar] [CrossRef]
- Gooden, M.J.M.; De Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The Prognostic Influence of Tumour-Infiltrating Lymphocytes in Cancer: A Systematic Review with Meta-Analysis. Br. J. Cancer 2011, 105, 93. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Patel, A.; Wu, R.; Le, L.; Tokumaru, Y.; Yamada, A.; Yan, L.; Matsuyama, R.; Ishikawa, T.; Endo, I.; et al. Enhanced Immune Response Outperform Aggressive Cancer Biology and Is Associated with Better Survival in Triple-Negative Breast Cancer. npj Breast Cancer 2022, 8, 92. [Google Scholar] [CrossRef]
- Solis-Castillo, L.A.; Garcia-Romo, G.S.; Diaz-Rodriguez, A.; Reyes-Hernandez, D.; Tellez-Rivera, E.; Rosales-Garcia, V.H.; Mendez-Cruz, A.R.; Jimenez-Flores, J.R.; Villafana-Vazquez, V.H.; Pedroza-Gonzalez, A. Tumor-Infiltrating Regulatory T Cells, CD8/Treg Ratio, and Cancer Stem Cells Are Correlated with Lymph Node Metastasis in Patients with Early Breast Cancer. Breast Cancer 2020, 27, 837–849. [Google Scholar] [CrossRef]
- Ballabio, A. The Awesome Lysosome. EMBO Mol. Med. 2016, 8, 73–76. [Google Scholar] [CrossRef]
- Tang, T.; Yang, Z.Y.; Wang, D.; Yang, X.Y.; Wang, J.; Li, L.; Wen, Q.; Gao, L.; Bian, X.W.; Yu, S.C. The Role of Lysosomes in Cancer Development and Progression. Cell Biosci. 2020, 10, 131. [Google Scholar] [CrossRef]
- Martí i Líndez, A.A.; Reith, W. Arginine-Dependent Immune Responses. Cell Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Sun, L.; Gang, X.; Li, Z.; Zhao, X.; Zhou, T.; Zhang, S.; Wang, G. Advances in Understanding the Roles of CD244 (SLAMF4) in Immune Regulation and Associated Diseases. Front. Immunol. 2021, 12, 648182. [Google Scholar] [CrossRef]
- Sandigursky, S.; Philips, M.R.; Mor, A. SAP Interacts with CD28 to Inhibit PD-1 Signaling in T Lymphocytes. Clin. Immunol. 2020, 217, 108485. [Google Scholar] [CrossRef]
- Dail, M.; Kalo, M.S.; Seddon, J.A.; Côté, J.F.; Vuori, K.; Pasquale, E.B. SHEP1 Function in Cell Migration Is Impaired by a Single Amino Acid Mutation That Disrupts Association with the Scaffolding Protein Cas but Not with Ras GTPases. J. Biol. Chem. 2004, 279, 41892–41902. [Google Scholar] [CrossRef]
- Salih, H.R.; Kiener, P.A.; Nüssler, V. 4-1 BB Ligand–Just Another Costimulating Molecule? Int. J. Clin. Pharmacol. Ther. 2002, 40, 348–353. [Google Scholar] [CrossRef]
- Corgnac, S.; Boutet, M.; Kfoury, M.; Naltet, C.; Mami-Chouaib, F. The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin. Front. Immunol. 2018, 9, 1904. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Lekas, A.G.; Lazaris, A.C.; Chrisofos, M.; Papatsoris, A.G.; Lappas, D.; Patsouris, E.; Deliveliotis, C. Finasteride Effects on Hypoxia and Angiogenetic Markers in Benign Prostatic Hyperplasia. Urology 2006, 68, 436–441. [Google Scholar] [CrossRef]
- Brooks, D.L.P.; Schwab, L.P.; Krutilina, R.; Parke, D.N.; Sethuraman, A.; Hoogewijs, D.; Schörg, A.; Gotwald, L.; Fan, M.; Wenger, R.H.; et al. ITGA6 Is Directly Regulated by Hypoxia-Inducible Factors and Enriches for Cancer Stem Cell Activity and Invasion in Metastatic Breast Cancer Models. Mol. Cancer 2016, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Khademi, R.; Malekzadeh, H.; Bahrami, S.; Saki, N.; Khademi, R.; Villa-Diaz, L.G. Regulation and Functions of A6-Integrin (CD49f) in Cancer Biology. Cancers 2023, 15, 3466. [Google Scholar] [CrossRef]
- Valatas, V.; Kolios, G.; Bamias, G. TL1A (TNFSF15) and DR3 (TNFRSF25): A Co-Stimulatory System of Cytokines with Diverse Functions in Gut Mucosal Immunity. Front. Immunol. 2019, 10, 583. [Google Scholar] [CrossRef]
- Takai, Y.; Miyoshi, J.; Ikeda, W.; Ogita, H. Nectins and Nectin-like Molecules: Roles in Contact Inhibition of Cell Movement and Proliferation. Nat. Rev. Mol. Cell Biol. 2008, 9, 603–615. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Cancer Exosomes and NKG2D Receptor-Ligand Interactions: Impairing NKG2D-Mediated Cytotoxicity and Anti-Tumour Immune Surveillance. Semin. Cancer Biol. 2014, 28, 24–30. [Google Scholar] [CrossRef]
- Diefenbach, A.; Jensen, E.R.; Jamieson, A.M.; Raulet, D.H. Rae1 and H60 Ligands of the NKG2D Receptor Stimulate Tumour Immunity. Nature 2001, 413, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Connolly, J.; Shimasaki, N.; Mimura, K.; Kono, K.; Campana, D. A Chimeric Receptor with NKG2D Specificity Enhances Natural Killer Cell Activation and Killing of Tumor Cells. Cancer Res. 2013, 73, 1777–1786. [Google Scholar] [CrossRef]
- Isvoranu, G.; Surcel, M.; Munteanu, A.; Bratu, O.; Ionita-Radu, F.; Neagu, M.; Chiritoiu-Butnaru, M. Therapeutic Potential of Interleukin-15 in Cancer (Review). Exp. Ther. Med. 2021, 22, 675. [Google Scholar] [CrossRef]
- Knudson, K.M.; Hicks, K.C.; Alter, S.; Schlom, J.; Gameiro, S.R. Mechanisms Involved in IL-15 Superagonist Enhancement of Anti-PD-L1 Therapy. J. Immunother. Cancer 2019, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60. [Google Scholar] [CrossRef]
- Shi, W.; Liu, N.; Liu, Z.; Yang, Y.; Zeng, Q.; Wang, Y.; Song, L.; Hu, F.; Fu, J.; Chen, J.; et al. Next-Generation Anti-PD-L1/IL-15 Immunocytokine Elicits Superior Antitumor Immunity in Cold Tumors with Minimal Toxicity. Cell Rep. Med. 2024, 5, 101531. [Google Scholar] [CrossRef]
- Xu, Y.; Carrascosa, L.C.; Yeung, Y.A.; Chu, M.L.H.; Yang, W.; Djuretic, I.; Pappas, D.C.; Zeytounian, J.; Ge, Z.; de Ruiter, V.; et al. An Engineered IL15 Cytokine Mutein Fused to an Anti-PD1 Improves Intratumoral t-Cell Function and Antitumor Immunity. Cancer Immunol. Res. 2021, 9, 1141–1157. [Google Scholar] [CrossRef]
- Nagasaki, J.; Inozume, T.; Sax, N.; Ariyasu, R.; Ishikawa, M.; Yamashita, K.; Kawazu, M.; Ueno, T.; Irie, T.; Tanji, E.; et al. PD-1 Blockade Therapy Promotes Infiltration of Tumor-Attacking Exhausted T Cell Clonotypes. Cell Rep. 2022, 38, 110331. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Pandit, H.; Nagy, B.A.; Stellas, D.; Jensen, S.M.; Bear, J.; Cam, M.; Valentin, A.; Fox, B.A.; Felber, B.K.; et al. Heterodimeric IL-15 Delays Tumor Growth and Promotes Intratumoral CTL and Dendritic Cell Accumulation by a Cytokine Network Involving XCL1, IFN-γ, CXCL9 and CXCL10. J. Immunother. Cancer 2020, 8, e000599. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.F.; Goldusky, J.; Bhimalli, P.; Marzo, A.L.; Schneider, J.R. Tracking Fluorescently Labeled IL-15 and Anti-PD-1 in the Tumor Microenvironment and Draining Lymph Nodes. J. Immunol. Methods. 2022, 505, 113253. [Google Scholar] [CrossRef]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The Impact of O2 Availability on Human Cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef]
- Bost, F.; Kaminski, L. The Metabolic Modulator PGC-1α in Cancer. Am. J. Cancer Res. 2019, 9, 198. [Google Scholar] [PubMed]
- Najjar, Y.G.; Menk, A.V.; Sander, C.; Rao, U.; Karunamurthy, A.; Bhatia, R.; Zhai, S.; Kirkwood, J.M.; Delgoffe, G.M. Tumor Cell Oxidative Metabolism as a Barrier to PD-1 Blockade Immunotherapy in Melanoma. JCI Insight 2019, 4, e124989. [Google Scholar] [CrossRef] [PubMed]
- Dumauthioz, N.; Tschumi, B.; Wenes, M.; Marti, B.; Wang, H.; Franco, F.; Li, W.; Lopez-Mejia, I.C.; Fajas, L.; Ho, P.C.; et al. Enforced PGC-1α Expression Promotes CD8 T Cell Fitness, Memory Formation and Antitumor Immunity. Cell. Mol. Immunol. 2020, 18, 1761–1771. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, S.; Hu, X. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha: A Double-Edged Sword in Prostate Cancer. Curr. Cancer Drug Targets 2022, 22, 541–559. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Gromeier, M.; Lachmann, S.; Rosenfeld, M.R.; Gutin, P.H.; Wimmer, E. Intergeneric Poliovirus Recombinants for the Treatment of Malignant Glioma. Proc. Natl. Acad. Sci. USA 2000, 97, 6803–6808. [Google Scholar] [CrossRef]
- Masson, D.; Jarry, A.; Baury, B.; Blanchardie, P.; Laboisse, C.; Lustenberger, P.; Denis, M.G. Overexpression of the CD155 Gene in Human Colorectal Carcinoma. Gut 2001, 49, 236–240. [Google Scholar] [CrossRef]
- Castriconi, R.; Dondero, A.; Corrias, M.V.; Lanino, E.; Pende, D.; Moretta, L.; Bottino, C.; Moretta, A. Natural Killer Cell-Mediated Killing of Freshly Isolated Neuroblastoma Cells: Critical Role of DNAX Accessory Molecule-1-Poliovirus Receptor Interaction. Cancer Res. 2004, 64, 9180–9184. [Google Scholar] [CrossRef]
- Pende, D.; Spaggiari, G.M.; Marcenaro, S.; Martini, S.; Rivera, P.; Capobianco, A.; Falco, M.; Lanino, E.; Pierri, I.; Zambello, R.; et al. Analysis of the Receptor-Ligand Interactions in the Natural Killer-Mediated Lysis of Freshly Isolated Myeloid or Lymphoblastic Leukemias: Evidence for the Involvement of the Poliovirus Receptor (CD155) and Nectin-2 (CD112). Blood 2005, 105, 2066–2073. [Google Scholar] [CrossRef]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Ljunggren, H.-G.; Kiessling, R.; Malmberg, K.-J. Primary Human Tumor Cells Expressing CD155 Impair Tumor Targeting by Down-Regulating DNAM-1 on NK Cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Lepletier, A.; Madore, J.; O’Donnell, J.S.; Johnston, R.L.; Li, X.Y.; McDonald, E.; Ahern, E.; Kuchel, A.; Eastgate, M.; Pearson, S.A.; et al. Tumor CD155 Expression Is Associated with Resistance to Anti-PD1 Immunotherapy in Metastatic Melanoma. Clin. Cancer Res. 2020, 26, 3671–3681. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldufsky, J.W.; Reyes, A.F.; Heller, A.A.; Leifheit, M.E.; Albalawi, M.N.; King, N.T.; Kuzel, T.M.; Schneider, J.R.; Marzo, A.L. IL-15 Complexes Combined with PD-1 Blockade Affect Immune Cell Distribution, Localization, and Immune Signatures in Regressing Versus Non-Regressing Metastatic Breast Tumors. Int. J. Mol. Sci. 2025, 26, 11490. https://doi.org/10.3390/ijms262311490
Goldufsky JW, Reyes AF, Heller AA, Leifheit ME, Albalawi MN, King NT, Kuzel TM, Schneider JR, Marzo AL. IL-15 Complexes Combined with PD-1 Blockade Affect Immune Cell Distribution, Localization, and Immune Signatures in Regressing Versus Non-Regressing Metastatic Breast Tumors. International Journal of Molecular Sciences. 2025; 26(23):11490. https://doi.org/10.3390/ijms262311490
Chicago/Turabian StyleGoldufsky, Josef W., Anjelica F. Reyes, Allie A. Heller, Malia E. Leifheit, Maram N. Albalawi, Noah T. King, Timothy M. Kuzel, Jeffrey R. Schneider, and Amanda L. Marzo. 2025. "IL-15 Complexes Combined with PD-1 Blockade Affect Immune Cell Distribution, Localization, and Immune Signatures in Regressing Versus Non-Regressing Metastatic Breast Tumors" International Journal of Molecular Sciences 26, no. 23: 11490. https://doi.org/10.3390/ijms262311490
APA StyleGoldufsky, J. W., Reyes, A. F., Heller, A. A., Leifheit, M. E., Albalawi, M. N., King, N. T., Kuzel, T. M., Schneider, J. R., & Marzo, A. L. (2025). IL-15 Complexes Combined with PD-1 Blockade Affect Immune Cell Distribution, Localization, and Immune Signatures in Regressing Versus Non-Regressing Metastatic Breast Tumors. International Journal of Molecular Sciences, 26(23), 11490. https://doi.org/10.3390/ijms262311490





