Electronic Cigarette Exposure Induces Adverse Cellular Alterations in Skeletal Muscle in Male Mice Subjected to a High-Fat Diet
Abstract
1. Introduction
2. Results
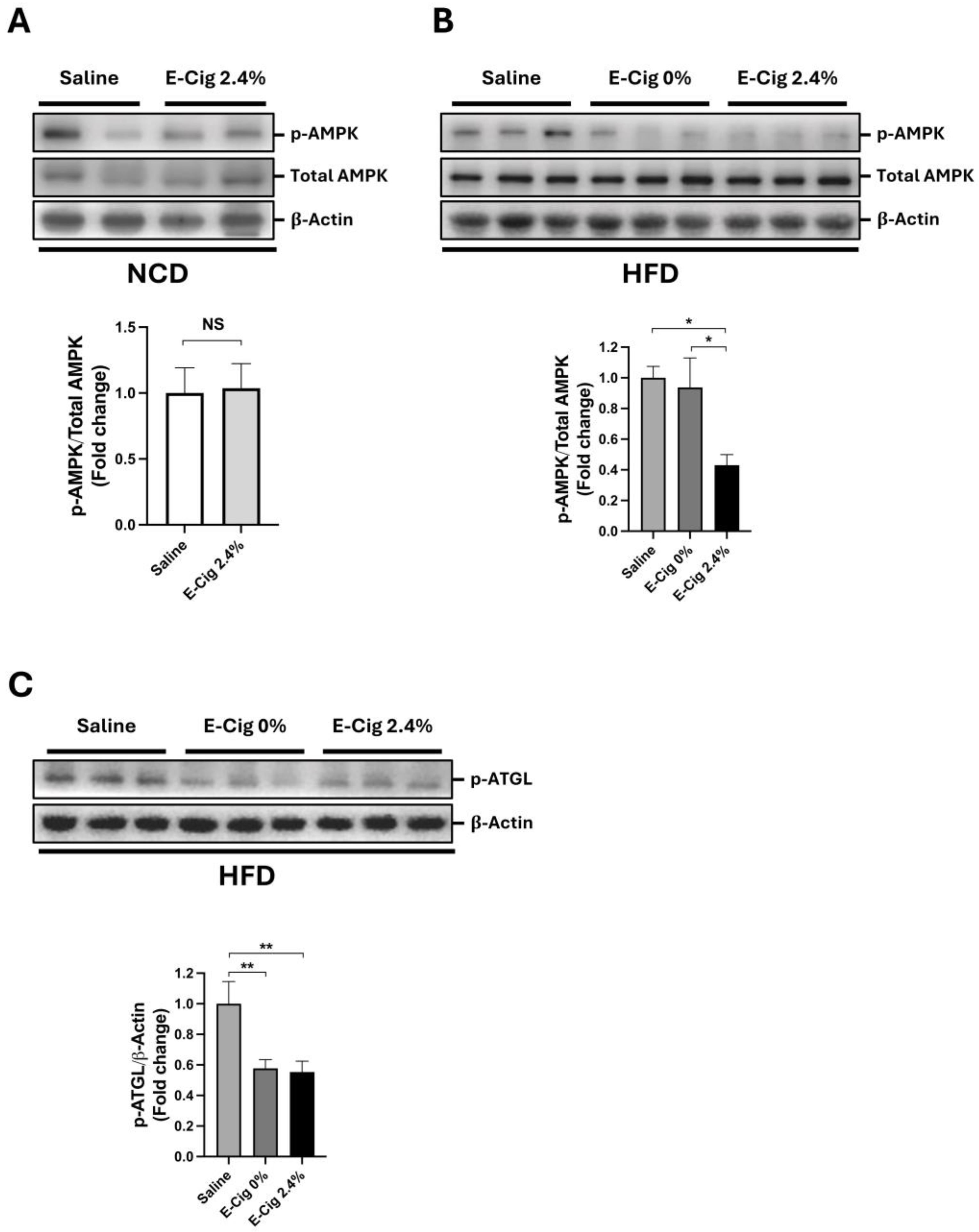
2.1. Effects of E-Cig Exposure on AMPK and ATGL Phosphorylation
2.2. Effects of E-Cig Exposure on Oxidative Stress Markers
2.3. Effects of E-Cig on Cellular Stress Pathway
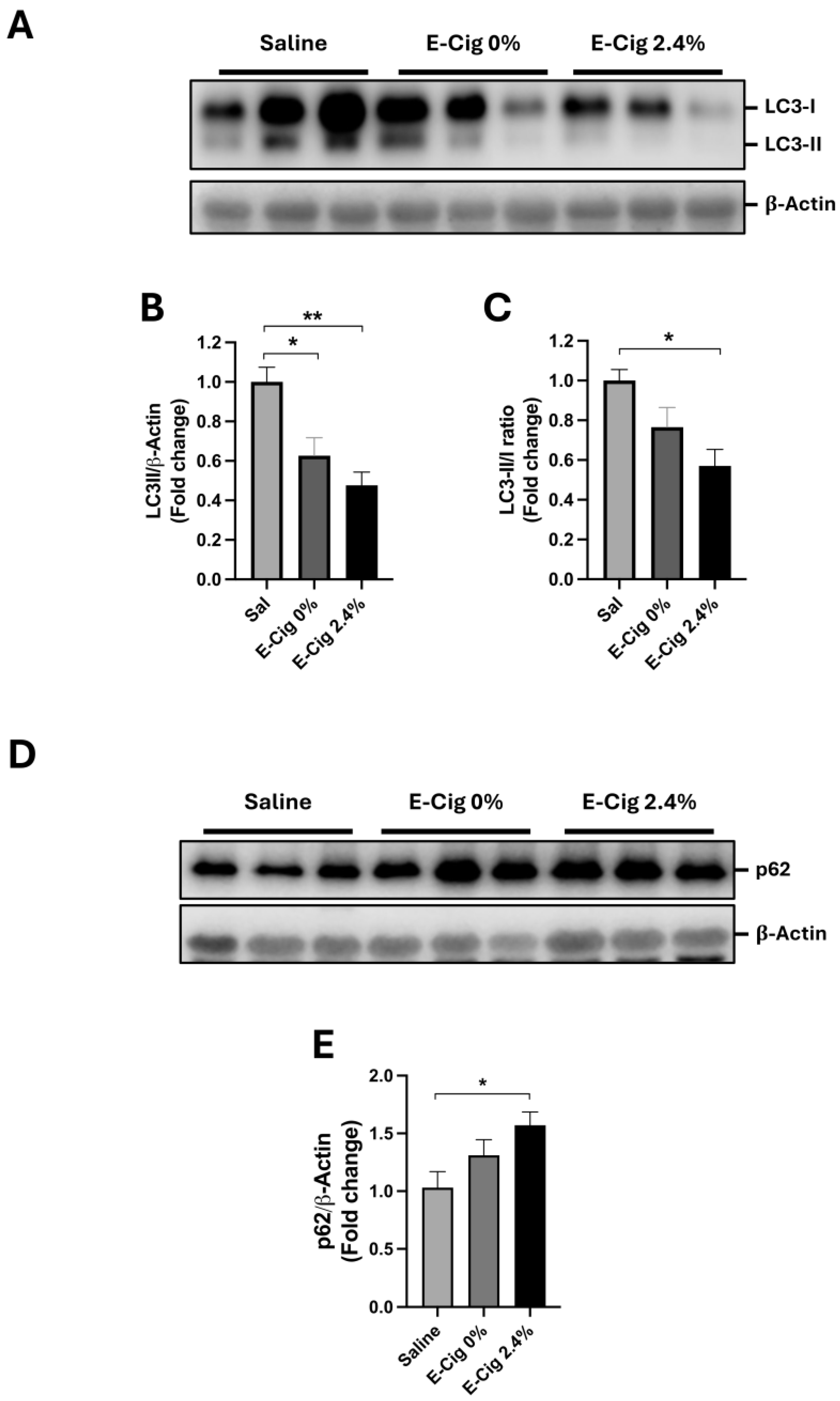
2.4. Effects of E-Cig Exposure on Autophagic Proteins LC3B
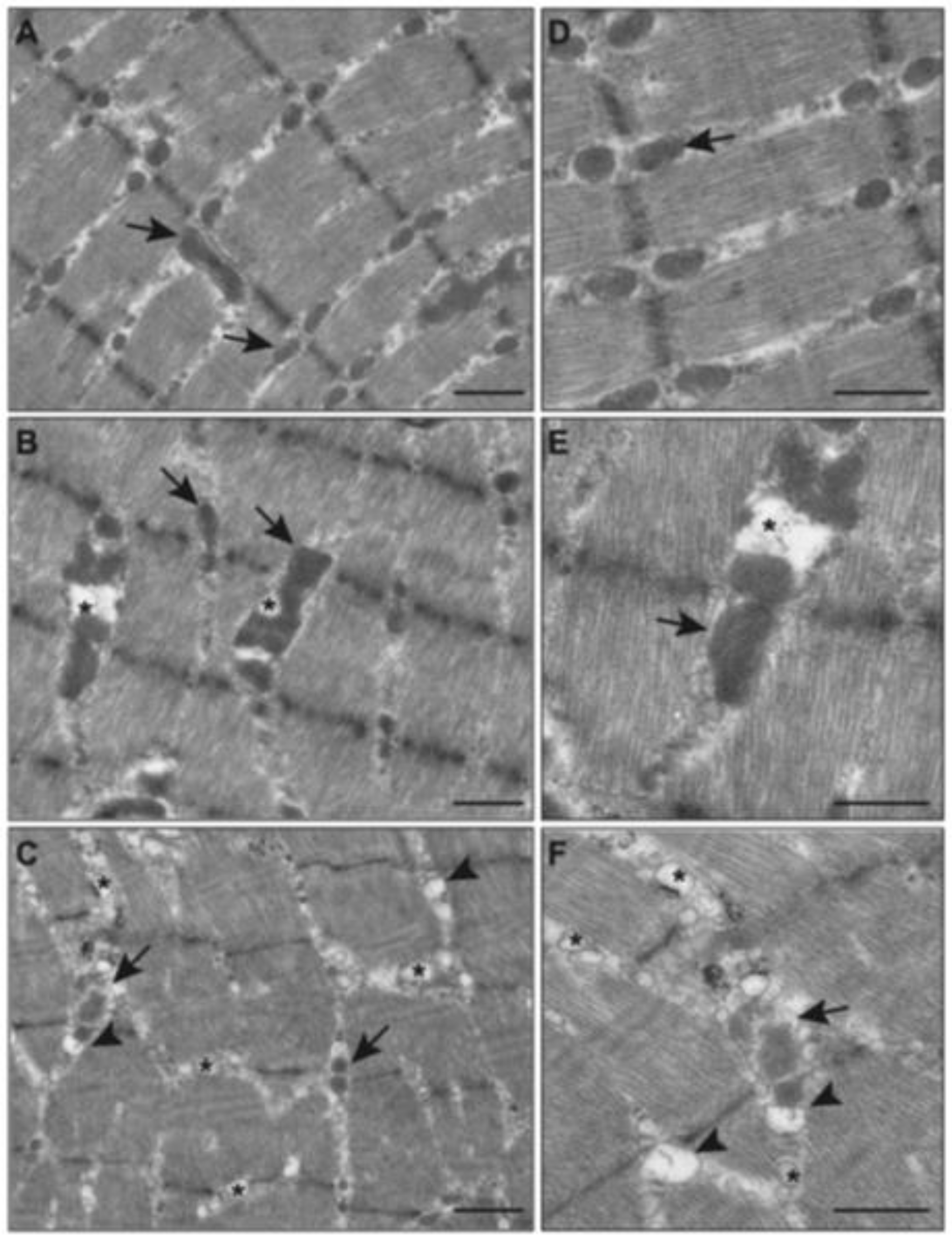
2.5. Effects of E-Cig on Mitochondrial Morphology (TEM)
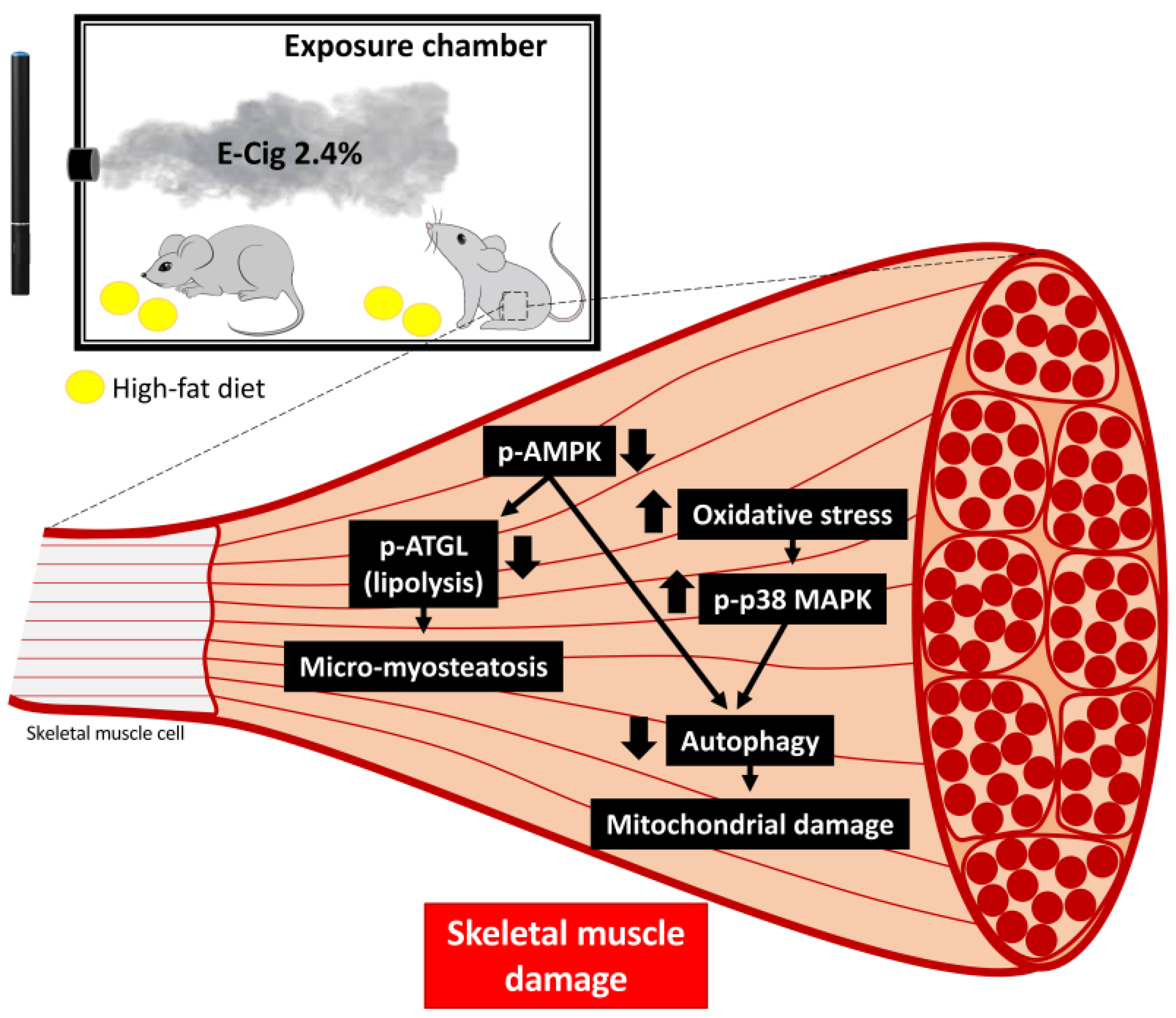
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Western Blot Analyses
4.3. Skeletal Muscle Triglyceride Quantification
4.4. Transmission Electron Microscopy Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- Cooper, M.; Park-Lee, E.; Ren, C.; Cornelius, M.; Jamal, A.; Cullen, K.A. Notes from the Field: E-cigarette Use Among Middle and High School Students—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1283–1285. [Google Scholar] [CrossRef]
- Gentzke, A.S.; Wang, T.W.; Jamal, A.; Park-Lee, E.; Ren, C.; Cullen, K.A.; Neff, L. Tobacco Product Use Among Middle and High School Students—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1881–1888. [Google Scholar] [CrossRef]
- Jamal, A.; Park-Lee, E.; Birdsey, J.; West, A.; Cornelius, M.; Cooper, M.R.; Cowan, H.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; et al. Tobacco Product Use Among Middle and High School Students—National Youth Tobacco Survey, United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 917–924. [Google Scholar] [CrossRef]
- Arrazola, R.A.; Husten, C.G.; Cornelius, M.E.; Armour, B.S. Notes from the Field: Tobacco Product Use Among Adults—United States, 2017–2023. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 118–121. [Google Scholar] [CrossRef]
- CDC. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Available online: https://e-cigarettes.surgeongeneral.gov/documents/2016_SGR_Full_Report_non-508.pdf (accessed on 18 November 2025).
- Hasan, K.M.; Friedman, T.C.; Shao, X.; Parveen, M.; Sims, C.; Lee, D.L.; Espinoza-Derout, J.; Sinha-Hikim, I.; Sinha-Hikim, A.P. E-cigarettes and Western Diet: Important Metabolic Risk Factors for Hepatic Diseases. Hepatology 2019, 69, 2442–2454. [Google Scholar] [CrossRef]
- Espinoza-Derout, J.; Arambulo, J.M.L.; Ramirez-Trillo, W.; Rivera, J.C.; Hasan, K.M.; Lao, C.J.; Jordan, M.C.; Shao, X.M.; Roos, K.P.; Sinha-Hikim, A.P.; et al. The lipolysis inhibitor acipimox reverses the cardiac phenotype induced by electronic cigarettes. Sci. Rep. 2023, 13, 18239. [Google Scholar] [CrossRef]
- Thomas-Eapen, N. Childhood Obesity. Prim. Care 2021, 48, 505–515. [Google Scholar] [CrossRef]
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. Natl. Health Stat. Rep. 2021, 158, 10-15620. [Google Scholar] [CrossRef]
- Delk, J.; Creamer, M.R.; Perry, C.L.; Harrell, M.B. Weight Status and Cigarette and Electronic Cigarette Use in Adolescents. Am. J. Prev. Med. 2018, 54, e31–e35. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, M.; Wu, J.; Xu, X.; Yin, P.; Huang, Z.; Zhang, X.; Zhou, Y.; Zhang, X.; Li, C.; et al. E-cigarette use among adults in China: Findings from repeated cross-sectional surveys in 2015–16 and 2018–19. Lancet Public Health 2020, 5, e639–e649. [Google Scholar] [CrossRef]
- Hochgraf, A.K.; Fosco, G.M.; Lanza, S.T. Age-Varying Associations Between Attempts to Lose Weight and Nicotine Vaping Across Adolescence: Results From a Nationally Representative Sample. J. Adolesc. Health 2023, 72, 352–358. [Google Scholar] [CrossRef]
- Mohapatra, S.; Wisidagama, S.; Schifano, F. Exploring Vaping Patterns and Weight Management-Related Concerns among Adolescents and Young Adults: A Systematic Review. J. Clin. Med. 2024, 13, 2896. [Google Scholar] [CrossRef]
- Hills, A.P.; Andersen, L.B.; Byrne, N.M. Physical activity and obesity in children. Br. J. Sports Med. 2011, 45, 866–870. [Google Scholar] [CrossRef]
- Stankov, I.; Olds, T.; Cargo, M. Overweight and obese adolescents: What turns them off physical activity? Int. J. Behav. Nutr. Phys. Act. 2012, 9, 53. [Google Scholar] [CrossRef]
- Miller, C.; Smith, D.M.; Goniewicz, M.L. Physical activity among adolescent tobacco and electronic cigarette users: Cross-sectional findings from the Population Assessment of Tobacco and Health study. Prev. Med. Rep. 2019, 15, 100897. [Google Scholar] [CrossRef]
- Santana, E.E.S.; Neves, L.M.; Souza, K.C.; Mendes, T.B.; Rossi, F.E.; Silva, A.A.D.; Oliveira, R.; Perilhao, M.S.; Roschel, H.; Gil, S. Physically Inactive Undergraduate Students Exhibit More Symptoms of Anxiety, Depression, and Poor Quality of Life than Physically Active Students. Int. J. Environ. Res. Public Health 2023, 20, 4494. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef]
- De Mario, A.; Gherardi, G.; Rizzuto, R.; Mammucari, C. Skeletal muscle mitochondria in health and disease. Cell Calcium 2021, 94, 102357. [Google Scholar] [CrossRef]
- von Haehling, S.; Garfias Macedo, T.; Valentova, M.; Anker, M.S.; Ebner, N.; Bekfani, T.; Haarmann, H.; Schefold, J.C.; Lainscak, M.; Cleland, J.G.F.; et al. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J. Cachexia Sarcopenia Muscle 2020, 11, 1242–1249. [Google Scholar] [CrossRef]
- Degens, H.; Gayan-Ramirez, G.; van Hees, H.W. Smoking-induced skeletal muscle dysfunction: From evidence to mechanisms. Am. J. Respir. Crit. Care Med. 2015, 191, 620–625. [Google Scholar] [CrossRef]
- Faisal, A.G.A.; Junejo, R.; Jones, S.; Phillips, O.; Vadher, J.; Henthorn, L.; Morse, C. Detrimental effects of electronic cigarettes on vascular function and ventilatory efficiency during exercise. Eur. Respir. Soc. 2024, 64 (Suppl. S68), OA1954. [Google Scholar]
- Nogueira, L.; Zemljic-Harpf, A.E.; Yusufi, R.; Ranjbar, M.; Susanto, C.; Tang, K.; Mahata, S.K.; Jennings, P.A.; Breen, E.C. E-cigarette aerosol impairs male mouse skeletal muscle force development and prevents recovery from injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R849–R860. [Google Scholar] [CrossRef]
- Chen, Y.M.; Huang, C.C.; Sung, H.C.; Lee, M.C.; Hsiao, C.Y. Electronic cigarette exposure reduces exercise performance and changes the biochemical profile of female mice. Biosci. Biotechnol. Biochem. 2019, 83, 2318–2326. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Friedman, T.C.; Shin, C.S.; Lee, D.; Ivey, R.; Sinha-Hikim, A.P. Nicotine in combination with a high-fat diet causes intramyocellular mitochondrial abnormalities in male mice. Endocrinology 2014, 155, 865–872. [Google Scholar] [CrossRef]
- Kjobsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmoller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Ahmadian, M.; Abbott, M.J.; Tang, T.; Hudak, C.S.; Kim, Y.; Bruss, M.; Hellerstein, M.K.; Lee, H.Y.; Samuel, V.T.; Shulman, G.I.; et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011, 13, 739–748. [Google Scholar] [CrossRef]
- Knapp, M.; Gorski, J. The skeletal and heart muscle triacylglycerol lipolysis revisited. J. Physiol. Pharmacol. 2017, 68, 3–11. [Google Scholar]
- Alves de Souza, R.W.; Gallo, D.; Lee, G.R.; Katsuyama, E.; Schaufler, A.; Weber, J.; Csizmadia, E.; Tsokos, G.C.; Koch, L.G.; Britton, S.L.; et al. Skeletal muscle heme oxygenase-1 activity regulates aerobic capacity. Cell Rep. 2021, 35, 109018. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Caron, M.A.; Morissette, M.C.; Theriault, M.E.; Nikota, J.K.; Stampfli, M.R.; Debigare, R. Alterations in skeletal muscle cell homeostasis in a mouse model of cigarette smoke exposure. PLoS ONE 2013, 8, e66433. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Espinoza-Derout, J.; Hasan, K.M.; Shao, X.M.; Jordan, M.C.; Sims, C.; Lee, D.L.; Sinha, S.; Simmons, Z.; Mtume, N.; Liu, Y.; et al. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H445–H459. [Google Scholar] [CrossRef]
- Shao, X.M.; Lopez, B.; Nathan, D.; Wilson, J.; Bankole, E.; Tumoyan, H.; Munoz, A.; Espinoza-Derout, J.; Hasan, K.M.; Chang, S.; et al. A mouse model for chronic intermittent electronic cigarette exposure exhibits nicotine pharmacokinetics resembling human vapers. J. Neurosci. Methods 2019, 326, 108376. [Google Scholar] [CrossRef]
- Hasan, K.M.; Friedman, T.C.; Parveen, M.; Espinoza-Derout, J.; Bautista, F.; Razipour, M.M.; Shao, X.M.; Jordan, M.C.; Roos, K.P.; Mahata, S.K.; et al. Electronic cigarettes cause alteration in cardiac structure and function in diet-induced obese mice. PLoS ONE 2020, 15, e0239671. [Google Scholar] [CrossRef]
- Friedman, T.C.; Sinha-Hikim, I.; Parveen, M.; Najjar, S.M.; Liu, Y.; Mangubat, M.; Shin, C.S.; Lyzlov, A.; Ivey, R.; Shaheen, M.; et al. Additive effects of nicotine and high-fat diet on hepatic steatosis in male mice. Endocrinology 2012, 153, 5809–5820. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Friedman, T.C.; Falz, M.; Chalfant, V.; Hasan, M.K.; Espinoza-Derout, J.; Lee, D.L.; Sims, C.; Tran, P.; Mahata, S.K.; et al. Nicotine plus a high-fat diet triggers cardiomyocyte apoptosis. Cell Tissue Res. 2017, 368, 159–170. [Google Scholar] [CrossRef]
- Espinoza-Derout, J.; Shao, X.M.; Lao, C.J.; Hasan, K.M.; Rivera, J.C.; Jordan, M.C.; Echeverria, V.; Roos, K.P.; Sinha-Hikim, A.P.; Friedman, T.C. Electronic Cigarette Use and the Risk of Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 879726. [Google Scholar] [CrossRef]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef]
- McClung, J.M.; Judge, A.R.; Powers, S.K.; Yan, Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am. J. Physiol. Cell Physiol. 2010, 298, C542–C549. [Google Scholar] [CrossRef]
- Mano, Y.; Tsukamoto, M.; Wang, K.Y.; Nabeshima, T.; Kosugi, K.; Tajima, T.; Yamanaka, Y.; Suzuki, H.; Kawasaki, M.; Nakamura, E.; et al. Oxidative stress causes muscle structural alterations via p38 MAPK signaling in COPD mouse model. J. Bone Miner. Metab. 2022, 40, 927–939. [Google Scholar] [CrossRef]
- Brennan, C.M.; Emerson, C.P., Jr.; Owens, J.; Christoforou, N. p38 MAPKs—Roles in skeletal muscle physiology, disease mechanisms, and as potential therapeutic targets. JCI Insight 2021, 6, e149915. [Google Scholar] [CrossRef]
- Grumati, P.; Bonaldo, P. Autophagy in skeletal muscle homeostasis and in muscular dystrophies. Cells 2012, 1, 325–345. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.H. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Shao, X.M.; Friedman, T.C. Pod-mod vs. conventional e-cigarettes: Nicotine chemistry, pH, and health effects. J. Appl. Physiol. 2020, 128, 1056–1058. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Ogunwale, M.A.; Li, M.; Ramakrishnam Raju, M.V.; Chen, Y.; Nantz, M.H.; Conklin, D.J.; Fu, X.A. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega 2017, 2, 1207–1214. [Google Scholar] [CrossRef]
- Khlystov, A.; Samburova, V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Marques, P.; Piqueras, L.; Sanz, M.J. An updated overview of e-cigarette impact on human health. Respir. Res. 2021, 22, 151. [Google Scholar] [CrossRef]
- Chen, H.J.; Wang, C.C.; Chan, D.C.; Chiu, C.Y.; Yang, R.S.; Liu, S.H. Adverse effects of acrolein, a ubiquitous environmental toxicant, on muscle regeneration and mass. J. Cachexia Sarcopenia Muscle 2019, 10, 165–176. [Google Scholar] [CrossRef]
- Alam, F.; Silveyra, P. Sex Differences in E-Cigarette Use and Related Health Effects. Int. J. Environ. Res. Public. Health 2023, 20, 7079. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Denies Marketing Applications for Flavored blu E-Cigarette Products. Available online: https://www.fda.gov/tobacco-products/ctp-newsroom/fda-denies-marketing-applications-flavored-blu-e-cigarette-products#:~:text=FDA%20Denies%20Marketing%20Applications%20for%20Flavored%20blu%20E%2Dcigarette%20Products,-CTP%20Newsroom&text=On%20Feb.,several%20flavored%20disposable%20e%2Dcigarettes (accessed on 18 November 2025).
- Food and Drug Administration. FDA Authorizes Marketing of Tobacco- and Menthol-Flavored JUUL E-Cigarette Products. Available online: https://www.fda.gov/tobacco-products/ctp-newsroom/fda-authorizes-marketing-tobacco-and-menthol-flavored-juul-e-cigarette-products (accessed on 18 November 2025).
- Chen, C.N.; Chuang, L.M.; Wu, Y.T. Clinical measures of physical fitness predict insulin resistance in people at risk for diabetes. Phys. Ther. 2008, 88, 1355–1364. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Rivera, J.C.; Abrigo, J.; Tacchi, F.; Simon, F.; Brandan, E.; Santos, R.A.; Bader, M.; Chiong, M.; Cabello-Verrugio, C. Angiotensin-(1-7) Prevents Lipopolysaccharide-Induced Autophagy via the Mas Receptor in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 9344. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, J.C.; Espinoza-Derout, J.; Hasan, K.; Lao, C.J.; Wilson, J.; Tintut, Y.; Shao, X.M.; Jordan, M.C.; Roos, K.P.; Liu, Y.; et al. Electronic Cigarette Exposure Induces Adverse Cellular Alterations in Skeletal Muscle in Male Mice Subjected to a High-Fat Diet. Int. J. Mol. Sci. 2025, 26, 11491. https://doi.org/10.3390/ijms262311491
Rivera JC, Espinoza-Derout J, Hasan K, Lao CJ, Wilson J, Tintut Y, Shao XM, Jordan MC, Roos KP, Liu Y, et al. Electronic Cigarette Exposure Induces Adverse Cellular Alterations in Skeletal Muscle in Male Mice Subjected to a High-Fat Diet. International Journal of Molecular Sciences. 2025; 26(23):11491. https://doi.org/10.3390/ijms262311491
Chicago/Turabian StyleRivera, Juan Carlos, Jorge Espinoza-Derout, Kamrul Hasan, Candice J. Lao, Julian Wilson, Yin Tintut, Xuesi M. Shao, Maria C. Jordan, Kenneth P. Roos, Yanjun Liu, and et al. 2025. "Electronic Cigarette Exposure Induces Adverse Cellular Alterations in Skeletal Muscle in Male Mice Subjected to a High-Fat Diet" International Journal of Molecular Sciences 26, no. 23: 11491. https://doi.org/10.3390/ijms262311491
APA StyleRivera, J. C., Espinoza-Derout, J., Hasan, K., Lao, C. J., Wilson, J., Tintut, Y., Shao, X. M., Jordan, M. C., Roos, K. P., Liu, Y., Sinha-Hikim, A. P., Puri, V., & Friedman, T. C. (2025). Electronic Cigarette Exposure Induces Adverse Cellular Alterations in Skeletal Muscle in Male Mice Subjected to a High-Fat Diet. International Journal of Molecular Sciences, 26(23), 11491. https://doi.org/10.3390/ijms262311491





