Pan-Cancer Analysis Reveals AEBP1-Collagen Co-Expression and Its Potential Role in CAF-Mediated Tumor Stiffness
Abstract
1. Introduction
2. Results
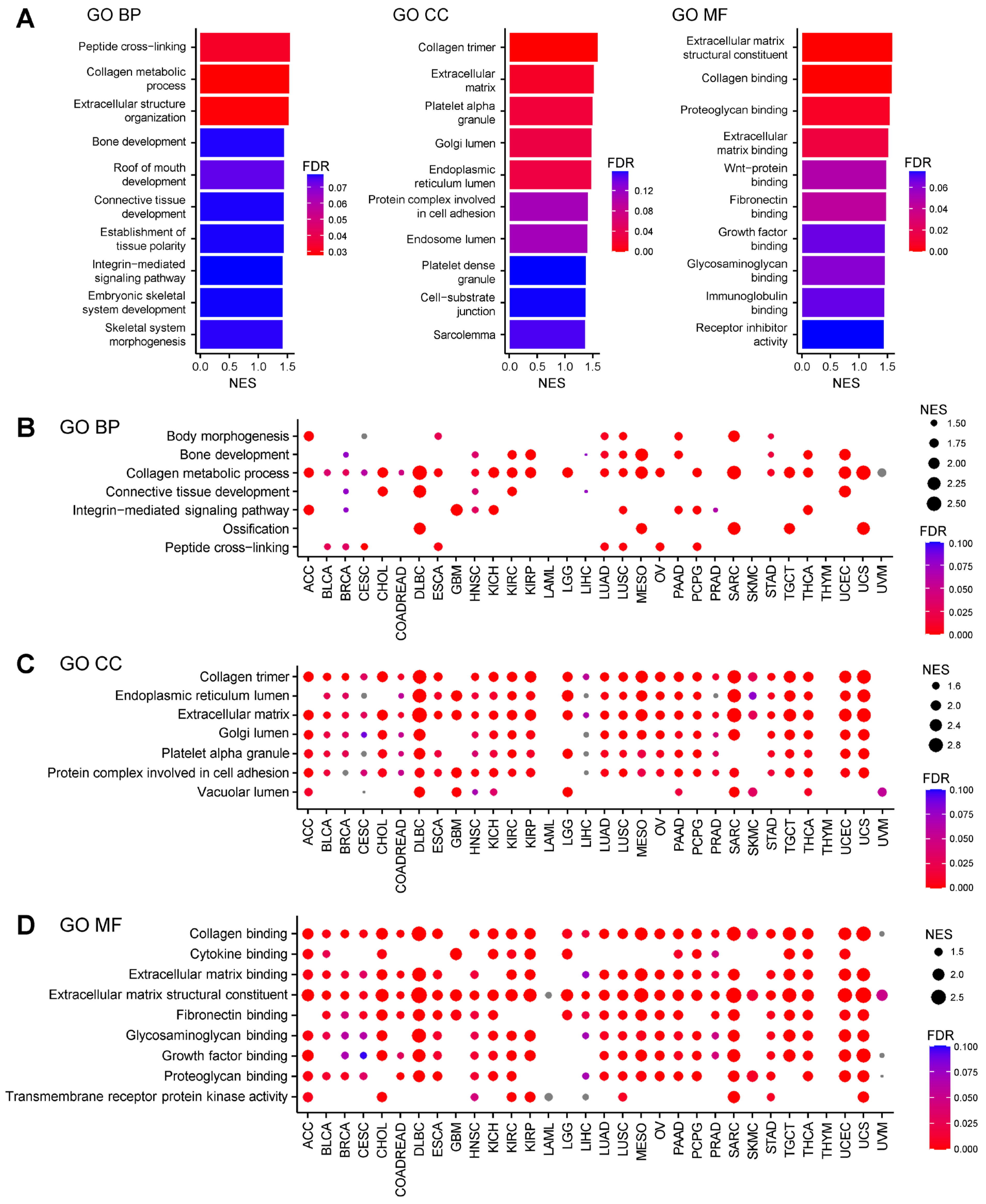
2.1. AEBP1-Associated Gene Expression Signatures Across Cancers
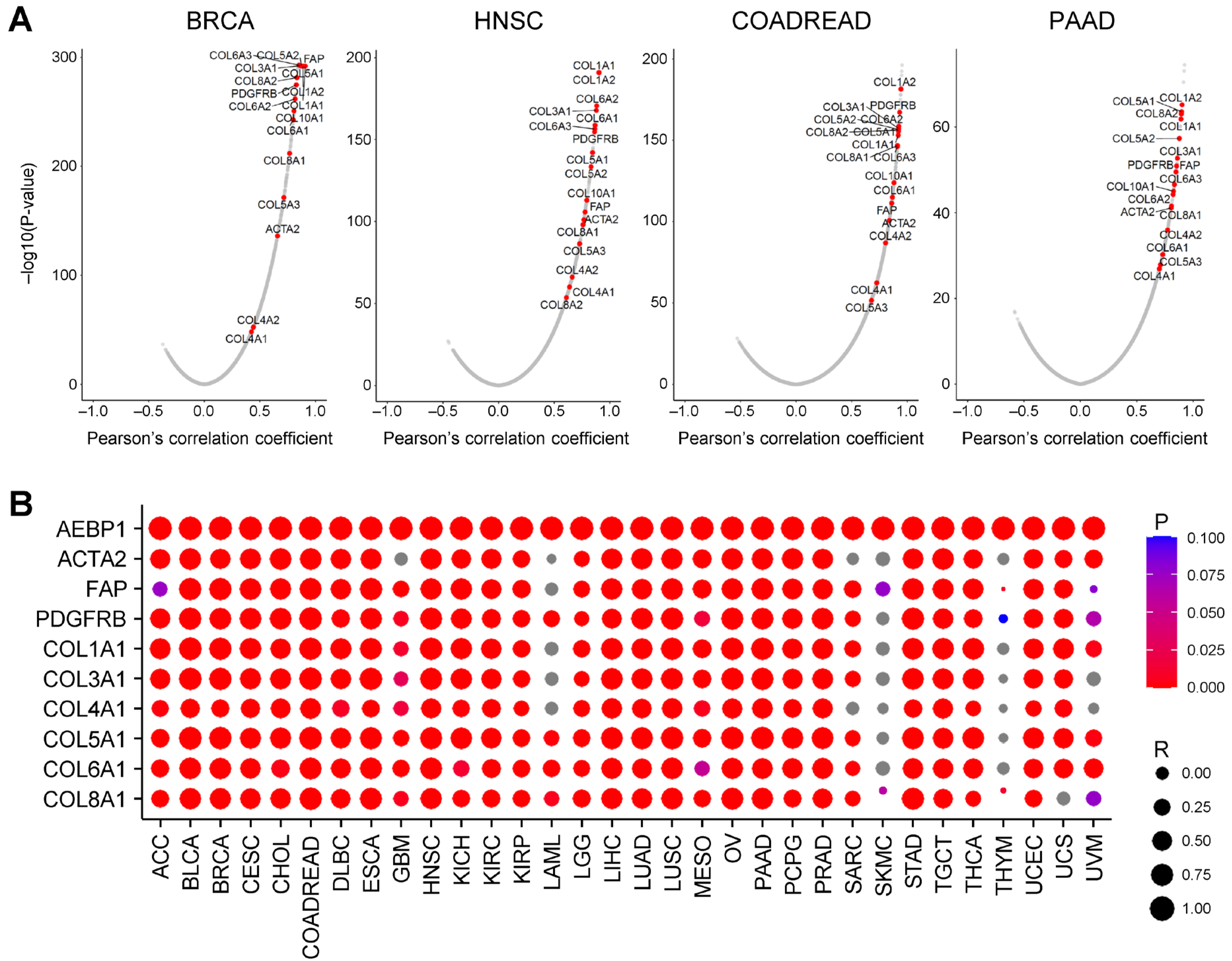
2.2. AEBP1 Expression Strongly Correlates with Collagen and CAF Marker Gene Expression
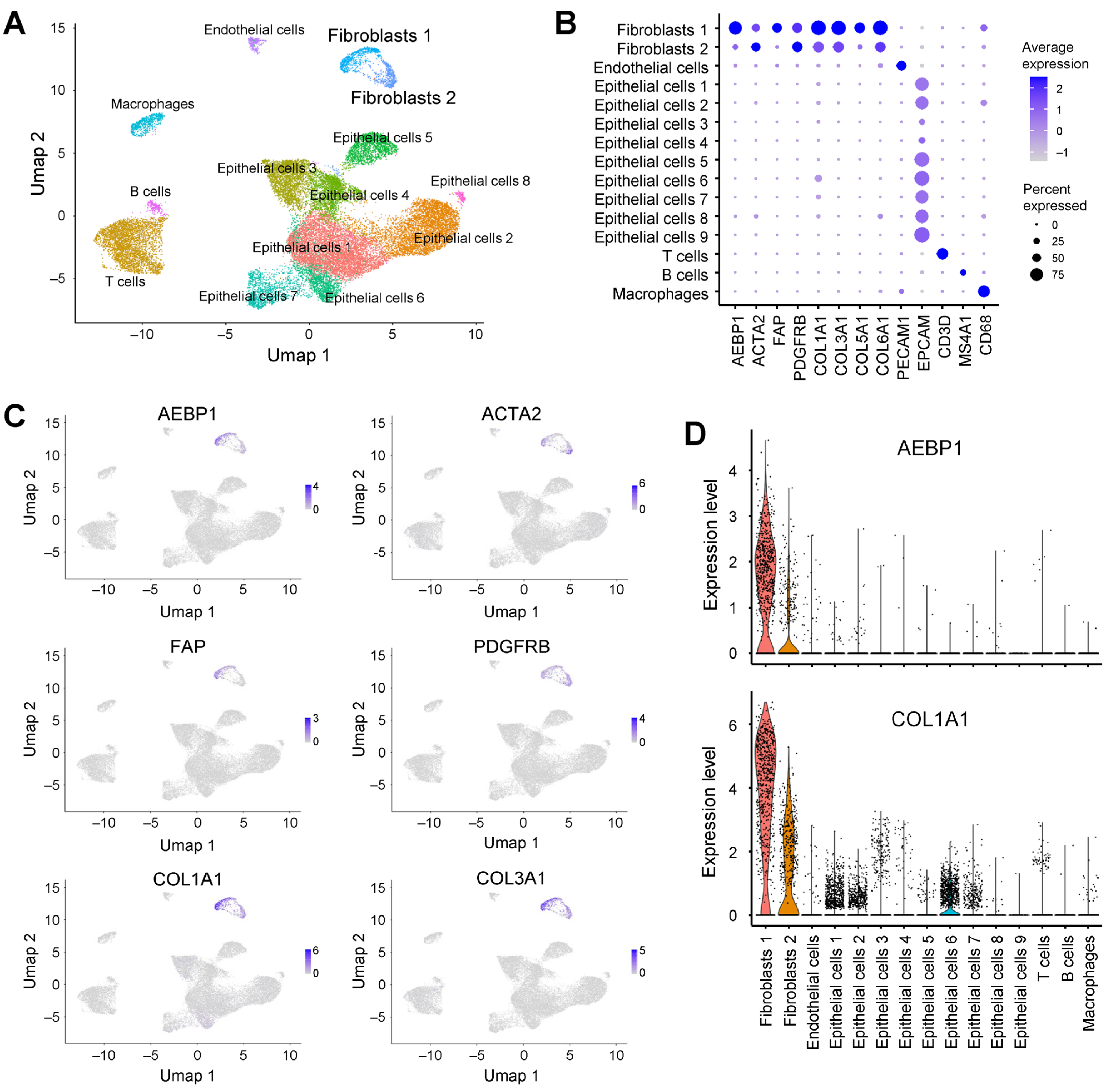
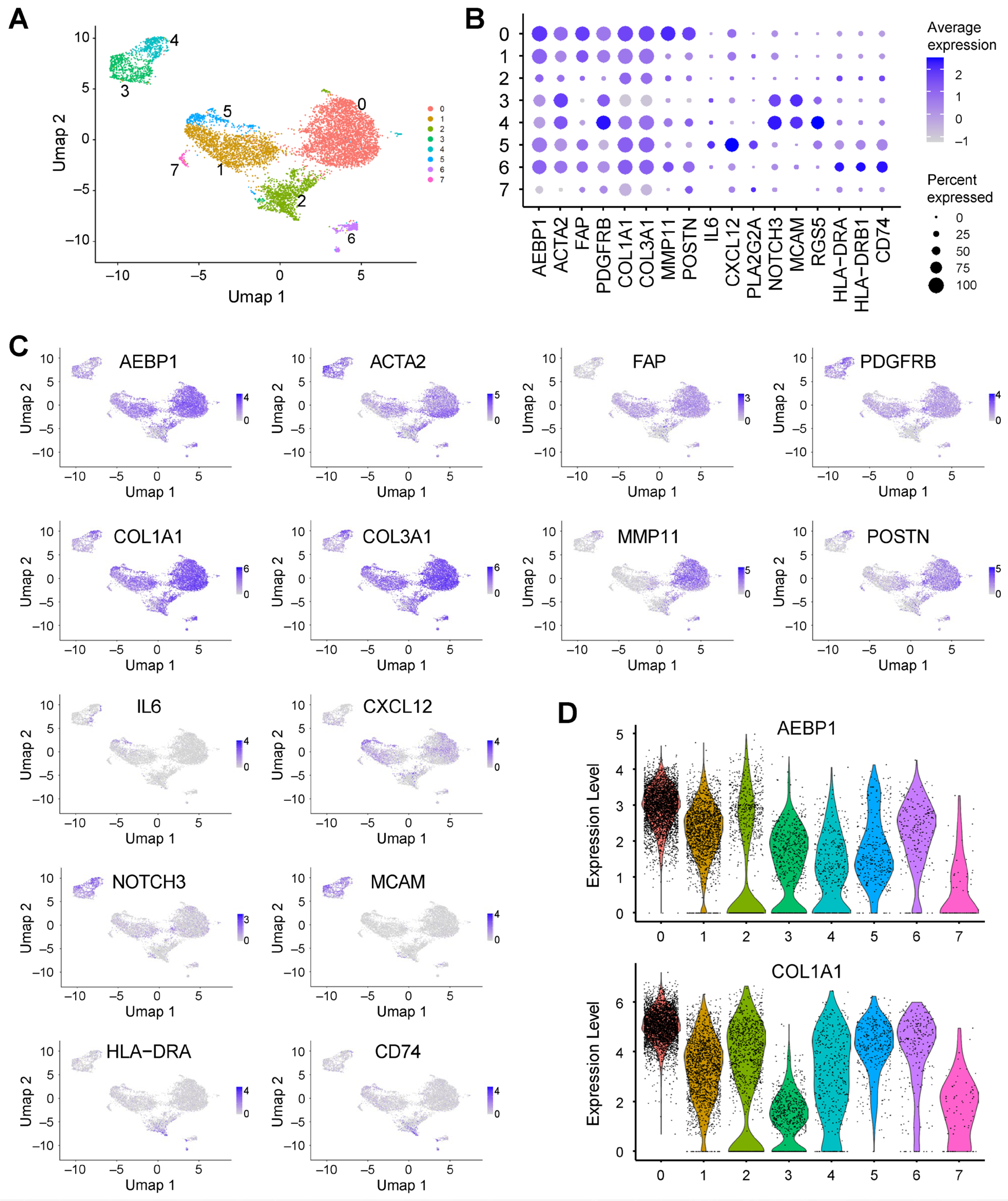
2.3. AEBP1 and Collagen Genes Are Co-Expressed in CAFs from Breast Cancer
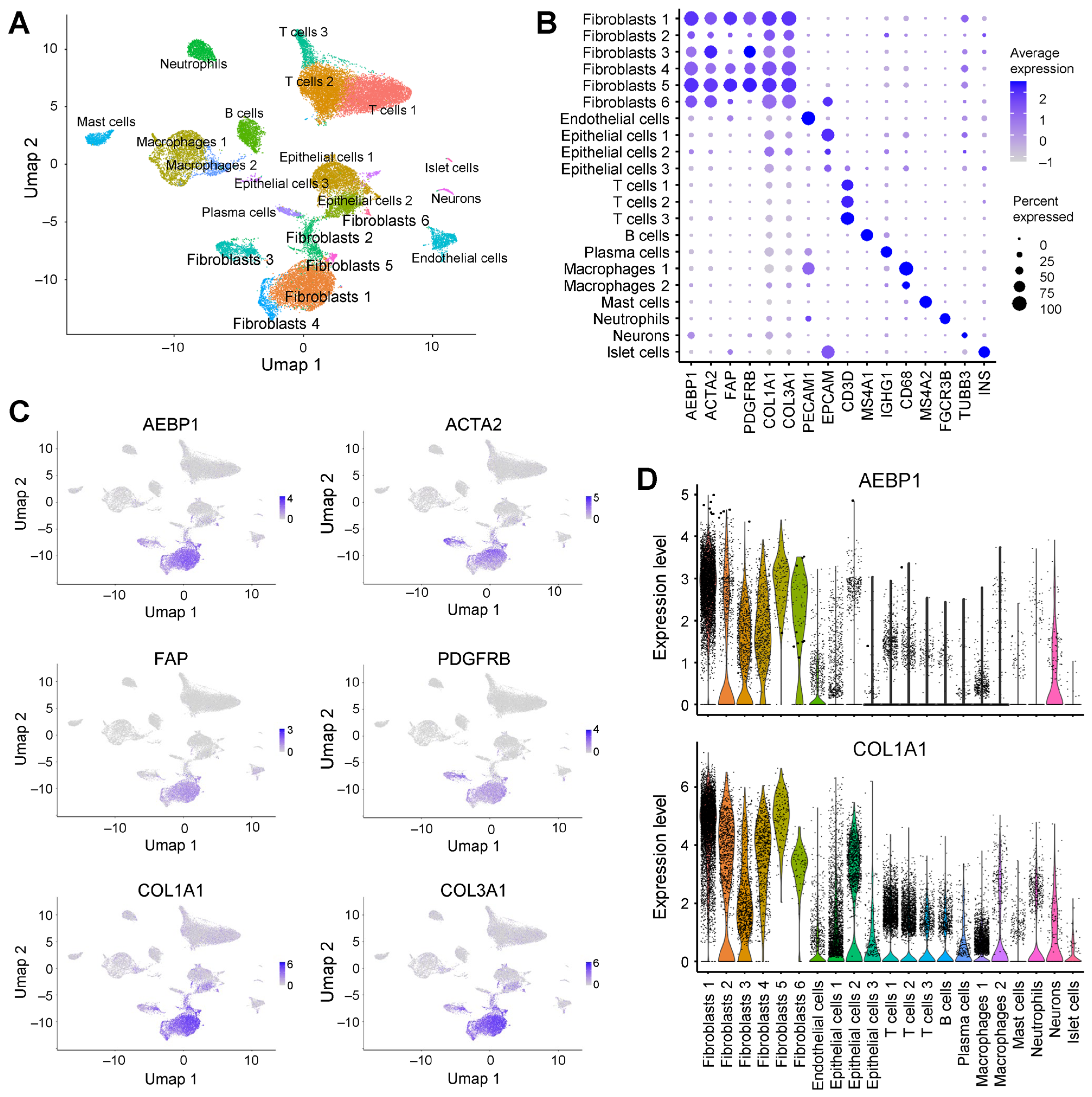
2.4. AEBP1 and Collagen Genes Are Co-Expressed in CAFs from Pancreatic Cancer
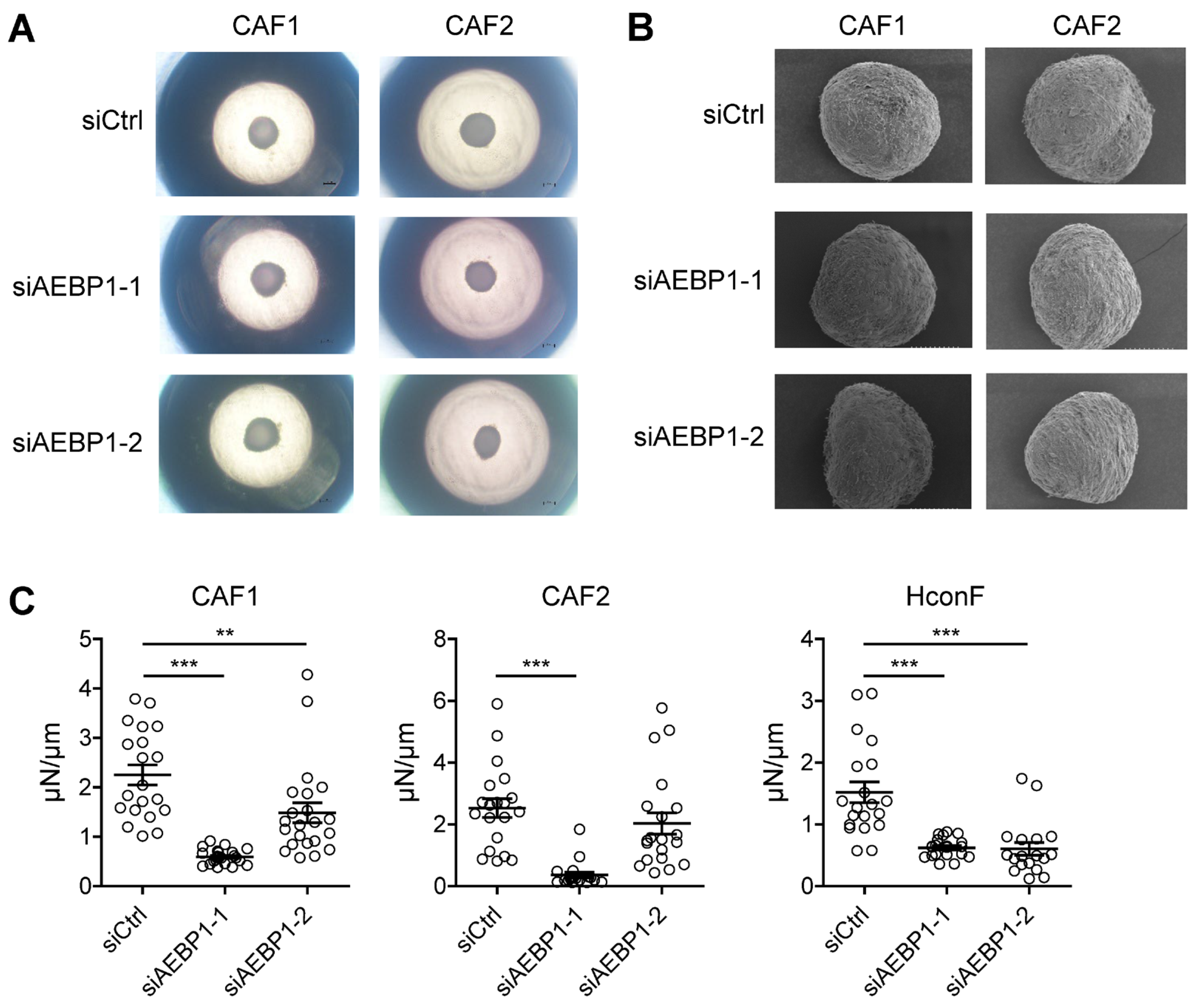
2.5. AEBP1 Enhances the Mechanical Stiffness of CAF-Derived 3D Spheroids
3. Discussion
4. Materials and Methods
4.1. The Cancer Genome Atlas Data Analysis
4.2. Single-Cell RNA-Sequencing Data Analysis
4.3. Three Dimensional Cell Culture Experiments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Adrenocortical carcinoma |
| ACLP | Aortic carboxypeptidase-like protein |
| ACTA2 | Actin alpha 2, smooth muscle |
| AEBP1 | Adipocyte enhancer-binding protein 1 |
| apCAF | Antigen-presenting cancer-associated fibroblast |
| BLCA | Bladder urothelial carcinoma |
| BRCA | Breast invasive carcinoma |
| CAF | Cancer-associated fibroblast |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma |
| CHOL | Cholangiocarcinoma |
| COADREAD | Colorectal adenocarcinoma |
| DLBC | Diffuse large B-cell lymphoma |
| ECM | Extracellular matrix |
| ESCA | Esophageal carcinoma |
| FAP | Fibroblast activation protein |
| FDR | False discovery rate |
| GBM | Glioblastoma multiforme |
| GO-BP | Gene ontology-biological process |
| GO-CC | Gene ontology-cellular component |
| GO-MF | Gene ontology-molecular function |
| HNSC | Head and neck squamous cell carcinoma |
| iCAF | Inflammatory cancer-associated fibroblast |
| KICH | Kidney chromophobe |
| KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma |
| LAML | Acute myeloid leukemia |
| LGG | Lower grade glioma |
| LIHC | Liver hepatocellular carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| mCAF | Matrix cancer-associated fibroblast |
| MESO | Mesothelioma |
| myCAF | Myofibroblastic cancer-associated fibroblast |
| NES | Normalized enrichment score |
| OSCC | Oral squamous cell carcinoma |
| OV | Ovarian serous cystadenocarcinoma |
| PAAD | Pancreatic adenocarcinoma |
| PCPG | Pheochromocytoma and paraganglioma |
| PDGFRB | Platelet-derived growth factor receptor β |
| PRAD | Prostate adenocarcinoma |
| SARC | Sarcoma |
| SKCM | Skin cutaneous melanoma |
| scRNA-seq | Single-cell RNA-sequencing |
| STAD | Stomach adenocarcinoma |
| TCGA | The Cancer Genome Atlas |
| TGCT | Testicular germ cell tumors |
| THCA | Thyroid carcinoma |
| THYM | Thymoma |
| UCEC | Uterine corpus endometrial carcinoma |
| UCS | Uterine carcinosarcoma |
| UMAP | Uniform manifold approximation and projection |
| UVM | Uveal melanoma |
| vCAF | Vascular cancer-associated fibroblast |
References
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, G.; Tuveson, D.A. Activated fibroblasts in cancer: Perspectives and challenges. Cancer Cell 2023, 41, 434–449. [Google Scholar] [CrossRef]
- Naito, Y. How Do Cancer Cells Create Cancer-Associated Fibroblast Subtypes? Impacts of Extracellular Vesicles on Stromal Diversity. Cancer Sci. 2025, 116, 2347–2361. [Google Scholar] [CrossRef] [PubMed]
- Arpinati, L.; Carradori, G.; Scherz-Shouval, R. CAF-induced physical constraints controlling T cell state and localization in solid tumours. Nat. Rev. Cancer 2024, 24, 676–693. [Google Scholar] [CrossRef]
- Yamazaki, M.; Ishimoto, T. Targeting Cancer-Associated Fibroblasts: Eliminate or Reprogram? Cancer Sci. 2025, 116, 613–621. [Google Scholar] [CrossRef]
- Yorozu, A.; Yamamoto, E.; Niinuma, T.; Tsuyada, A.; Maruyama, R.; Kitajima, H.; Numata, Y.; Kai, M.; Sudo, G.; Kubo, T.; et al. Upregulation of adipocyte enhancer-binding protein 1 in endothelial cells promotes tumor angiogenesis in colorectal cancer. Cancer Sci. 2020, 111, 1631–1644. [Google Scholar] [CrossRef]
- Layne, M.D.; Endege, W.O.; Jain, M.K.; Yet, S.F.; Hsieh, C.M.; Chin, M.T.; Perrella, M.A.; Blanar, M.A.; Haber, E.; Lee, M.E. Aortic carboxypeptidase-like protein, a novel protein with discoidin and carboxypeptidase-like domains, is up-regulated during vascular smooth muscle cell differentiation. J. Biol. Chem. 1998, 273, 15654–15660. [Google Scholar] [CrossRef]
- Layne, M.D.; Yet, S.F.; Maemura, K.; Hsieh, C.M.; Bernfield, M.; Perrella, M.A.; Lee, M.E. Impaired abdominal wall development and deficient wound healing in mice lacking aortic carboxypeptidase-like protein. Mol. Cell. Biol. 2001, 21, 5256–5261. [Google Scholar] [CrossRef]
- Ro, H.S.; Kim, S.W.; Wu, D.; Webber, C.; Nicholson, T.E. Gene structure and expression of the mouse adipocyte enhancer-binding protein. Gene 2001, 280, 123–133. [Google Scholar] [CrossRef]
- Zhang, R.; Han, W.; Shen, Z.; Wang, T.; Zhong, J. AEBP1 or ACLP, which is the key factor in inflammation and fibrosis? Int. J. Biol. Macromol. 2025, 310, 143554. [Google Scholar] [CrossRef] [PubMed]
- Schissel, S.L.; Dunsmore, S.E.; Liu, X.; Shine, R.W.; Perrella, M.A.; Layne, M.D. Aortic carboxypeptidase-like protein is expressed in fibrotic human lung and its absence protects against bleomycin-induced lung fibrosis. Am. J. Pathol. 2009, 174, 818–828. [Google Scholar] [CrossRef]
- Tumelty, K.E.; Smith, B.D.; Nugent, M.A.; Layne, M.D. Aortic carboxypeptidase-like protein (ACLP) enhances lung myofibroblast differentiation through transforming growth factor beta receptor-dependent and -independent pathways. J. Biol. Chem. 2014, 289, 2526–2536. [Google Scholar] [CrossRef]
- Wang, D.; Rabhi, N.; Yet, S.F.; Farmer, S.R.; Layne, M.D. Aortic carboxypeptidase-like protein regulates vascular adventitial progenitor and fibroblast differentiation through myocardin related transcription factor A. Sci. Rep. 2021, 11, 3948. [Google Scholar] [CrossRef]
- Sekiguchi, S.; Yorozu, A.; Okazaki, F.; Niinuma, T.; Takasawa, A.; Yamamoto, E.; Kitajima, H.; Kubo, T.; Hatanaka, Y.; Nishiyama, K.; et al. ACLP Activates Cancer-Associated Fibroblasts and Inhibits CD8+ T-Cell Infiltration in Oral Squamous Cell Carcinoma. Cancers 2023, 15, 4303. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhu, X.X.; Wu, X.; Li, J.H.; Ni, X.H.; Li, S.J.; Zhao, W.; Yin, X.Y. ACLP promotes activation of cancer-associated fibroblasts and tumor metastasis via ACLP-PPARgamma-ACLP feedback loop in pancreatic cancer. Cancer Lett. 2022, 544, 215802. [Google Scholar] [CrossRef]
- Vishwanath, N.; Monis, W.J.; Hoffmann, G.A.; Ramachandran, B.; DiGiacomo, V.; Wong, J.Y.; Smith, M.L.; Layne, M.D. Mechanisms of aortic carboxypeptidase-like protein secretion and identification of an intracellularly retained variant associated with Ehlers-Danlos syndrome. J. Biol. Chem. 2020, 295, 9725–9735. [Google Scholar] [CrossRef]
- Joo, E.H.; Kim, S.; Park, D.; Lee, T.; Park, W.Y.; Han, K.Y.; Lee, J.E. Migratory Tumor Cells Cooperate with Cancer Associated Fibroblasts in Hormone Receptor-Positive and HER2-Negative Breast Cancer. Int. J. Mol. Sci. 2024, 25, 5876. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Q.; Liu, X.; Tian, X.; Dong, A.; Yang, Y. Immune profiling and prognostic model of pancreatic cancer using quantitative pathology and single-cell RNA sequencing. J. Transl. Med. 2023, 21, 210. [Google Scholar] [CrossRef]
- Cords, L.; Tietscher, S.; Anzeneder, T.; Langwieder, C.; Rees, M.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 2023, 14, 4294. [Google Scholar] [CrossRef]
- Nishikiori, N.; Takada, K.; Sato, T.; Miyamoto, S.; Watanabe, M.; Hirakawa, Y.; Sekiguchi, S.; Furuhashi, M.; Yorozu, A.; Takano, K.; et al. Physical Properties and Cellular Metabolic Characteristics of 3D Spheroids Are Possible Definitive Indices for the Biological Nature of Cancer-Associated Fibroblasts. Cells 2023, 12, 2160. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jiang, L.; Liu, J.J.; He, T.; Cui, Y.H.; Qian, F.; Yu, P.W. AEBP1 promotes epithelial-mesenchymal transition of gastric cancer cells by activating the NF-kappaB pathway and predicts poor outcome of the patients. Sci. Rep. 2018, 8, 11955. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, Z.; Chi, F.; Zhou, Y.; Ren, S.; Zhao, Z.; Zhu, Y.; Piao, D. AEBP1, a prognostic indicator, promotes colon adenocarcinoma cell growth and metastasis through the NF-kappaB pathway. Mol. Carcinog. 2019, 58, 1795–1808. [Google Scholar] [CrossRef]
- Li, J.; Ruan, Y.; Zheng, C.; Pan, Y.; Lin, B.; Chen, Q.; Zheng, Z. AEBP1 Contributes to Breast Cancer Progression by Facilitating Cell Proliferation, Migration, Invasion, and Blocking Apoptosis. Discov. Med. 2023, 35, 45–56. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Zhang, Y.; Wang, L.; Chen, Z. Inhibition of AEBP1 predisposes cisplatin-resistant oral cancer cells to ferroptosis. BMC Oral Health 2022, 22, 478. [Google Scholar] [CrossRef]
- Ju, G.; Xing, T.; Xu, M.; Zhang, X.; Sun, Y.; Mu, Z.; Sun, D.; Miao, S.; Li, L.; Liang, J.; et al. AEBP1 promotes papillary thyroid cancer progression by activating BMP4 signaling. Neoplasia 2024, 49, 100972. [Google Scholar] [CrossRef]
- Ladha, J.; Sinha, S.; Bhat, V.; Donakonda, S.; Rao, S.M. Identification of genomic targets of transcription factor AEBP1 and its role in survival of glioma cells. Mol. Cancer Res. 2012, 10, 1039–1051. [Google Scholar] [CrossRef]
- Liu, S.; Gu, Y.; Shi, Y.; Yu, S.; Li, W.; Lv, W. AEBP1 upregulation contributes to cervical cancer progression by facilitating cell proliferation, migration, and invasion. J. Obstet. Gynaecol. Res. 2024, 50, 1166–1174. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamada, N.; Nakamura, R.; Ito, H.; Ohnishi, K.; Inoko, A.; Riku, M.; Muramatsu, T.; Sassa, N.; Kasai, K. AEBP1-GLI1 pathway attenuates the FACT complex dependency of bladder cancer cell survival. Biochem. Biophys. Rep. 2025, 43, 102101. [Google Scholar] [CrossRef]
- Jia, C.; Gong, Z.; Zhang, L. Silencing of AEBP1 inhibits proliferation and promotes apoptosis via the AKT signaling pathway in osteosarcoma. Biomed. Rep. 2025, 23, 128. [Google Scholar] [CrossRef]
- Blackburn, P.R.; Xu, Z.; Tumelty, K.E.; Zhao, R.W.; Monis, W.J.; Harris, K.G.; Gass, J.M.; Cousin, M.A.; Boczek, N.J.; Mitkov, M.V.; et al. Bi-allelic Alterations in AEBP1 Lead to Defective Collagen Assembly and Connective Tissue Structure Resulting in a Variant of Ehlers-Danlos Syndrome. Am. J. Hum. Genet. 2018, 102, 696–705. [Google Scholar] [CrossRef]
- Syx, D.; De Wandele, I.; Symoens, S.; De Rycke, R.; Hougrand, O.; Voermans, N.; De Paepe, A.; Malfait, F. Bi-allelic AEBP1 mutations in two patients with Ehlers-Danlos syndrome. Hum. Mol. Genet. 2019, 28, 1853–1864. [Google Scholar] [CrossRef]
- Hebebrand, M.; Vasileiou, G.; Krumbiegel, M.; Kraus, C.; Uebe, S.; Ekici, A.B.; Thiel, C.T.; Reis, A.; Popp, B. A biallelic truncating AEBP1 variant causes connective tissue disorder in two siblings. Am. J. Med. Genet. A 2019, 179, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sanai, H.; Nakamura, Y.; Koike, Y.; Murota, H.; Kosho, T.; Sase, M. The detailed obstetric course of the first Japanese patient with AEBP1-related Ehlers-Danlos syndrome (classical-like EDS, type 2). J. Obstet. Gynaecol. Res. 2023, 49, 1043–1047. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Song, D.; Wu, Y.; Liu, J.; Yi, Z.; Sun, J.; Huang, J.; Wu, L.; Zhang, X.; et al. AEBP1 drives fibroblast-mediated T cell dysfunction in tumors. Nat. Commun. 2025, 16, 8171. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.R.; Raychaudhuri, S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-beta2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2alpha agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekiguchi, S.; Yorozu, A.; Watanabe, M.; Okazaki, F.; Ohwada, S.; Yamamoto, E.; Niinuma, T.; Kitajima, H.; Ishiguro, K.; Saito, M.; et al. Pan-Cancer Analysis Reveals AEBP1-Collagen Co-Expression and Its Potential Role in CAF-Mediated Tumor Stiffness. Int. J. Mol. Sci. 2025, 26, 11474. https://doi.org/10.3390/ijms262311474
Sekiguchi S, Yorozu A, Watanabe M, Okazaki F, Ohwada S, Yamamoto E, Niinuma T, Kitajima H, Ishiguro K, Saito M, et al. Pan-Cancer Analysis Reveals AEBP1-Collagen Co-Expression and Its Potential Role in CAF-Mediated Tumor Stiffness. International Journal of Molecular Sciences. 2025; 26(23):11474. https://doi.org/10.3390/ijms262311474
Chicago/Turabian StyleSekiguchi, Shohei, Akira Yorozu, Megumi Watanabe, Fumika Okazaki, Satoshi Ohwada, Eiichiro Yamamoto, Takeshi Niinuma, Hiroshi Kitajima, Kazuya Ishiguro, Mitsunobu Saito, and et al. 2025. "Pan-Cancer Analysis Reveals AEBP1-Collagen Co-Expression and Its Potential Role in CAF-Mediated Tumor Stiffness" International Journal of Molecular Sciences 26, no. 23: 11474. https://doi.org/10.3390/ijms262311474
APA StyleSekiguchi, S., Yorozu, A., Watanabe, M., Okazaki, F., Ohwada, S., Yamamoto, E., Niinuma, T., Kitajima, H., Ishiguro, K., Saito, M., Kai, M., Idogawa, M., Takano, K., Miyazaki, A., Ohguro, H., & Suzuki, H. (2025). Pan-Cancer Analysis Reveals AEBP1-Collagen Co-Expression and Its Potential Role in CAF-Mediated Tumor Stiffness. International Journal of Molecular Sciences, 26(23), 11474. https://doi.org/10.3390/ijms262311474





