Fueling the Seed: Growth Factors and Cytokines Driving Cancer Stem Cells in Gynecological Malignancies
Abstract
1. Introduction
2. Gynecological Cancers
2.1. Breast Cancer
2.2. Ovarian Cancer
2.3. Cervical Cancer
2.4. Endometrial Cancer
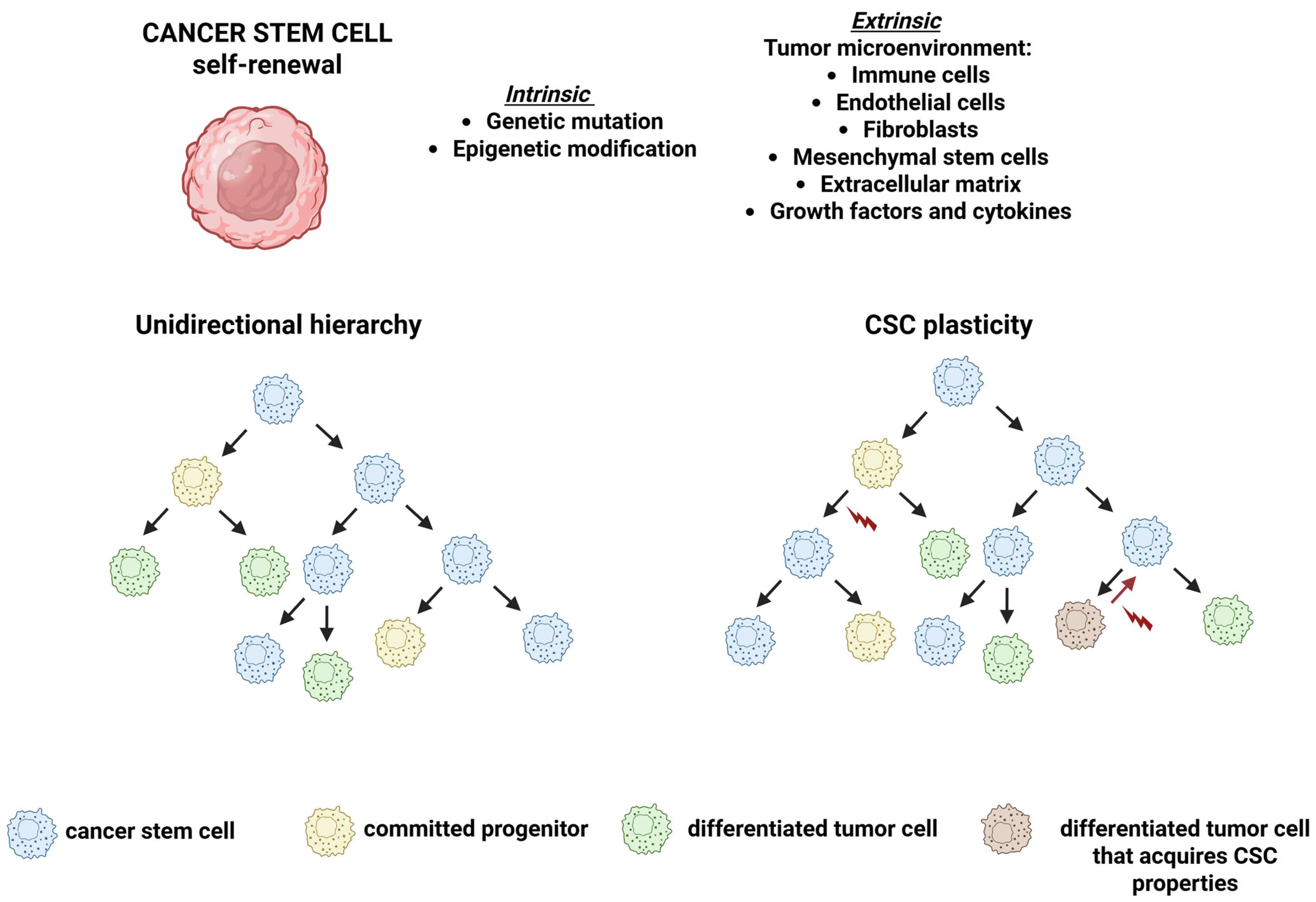
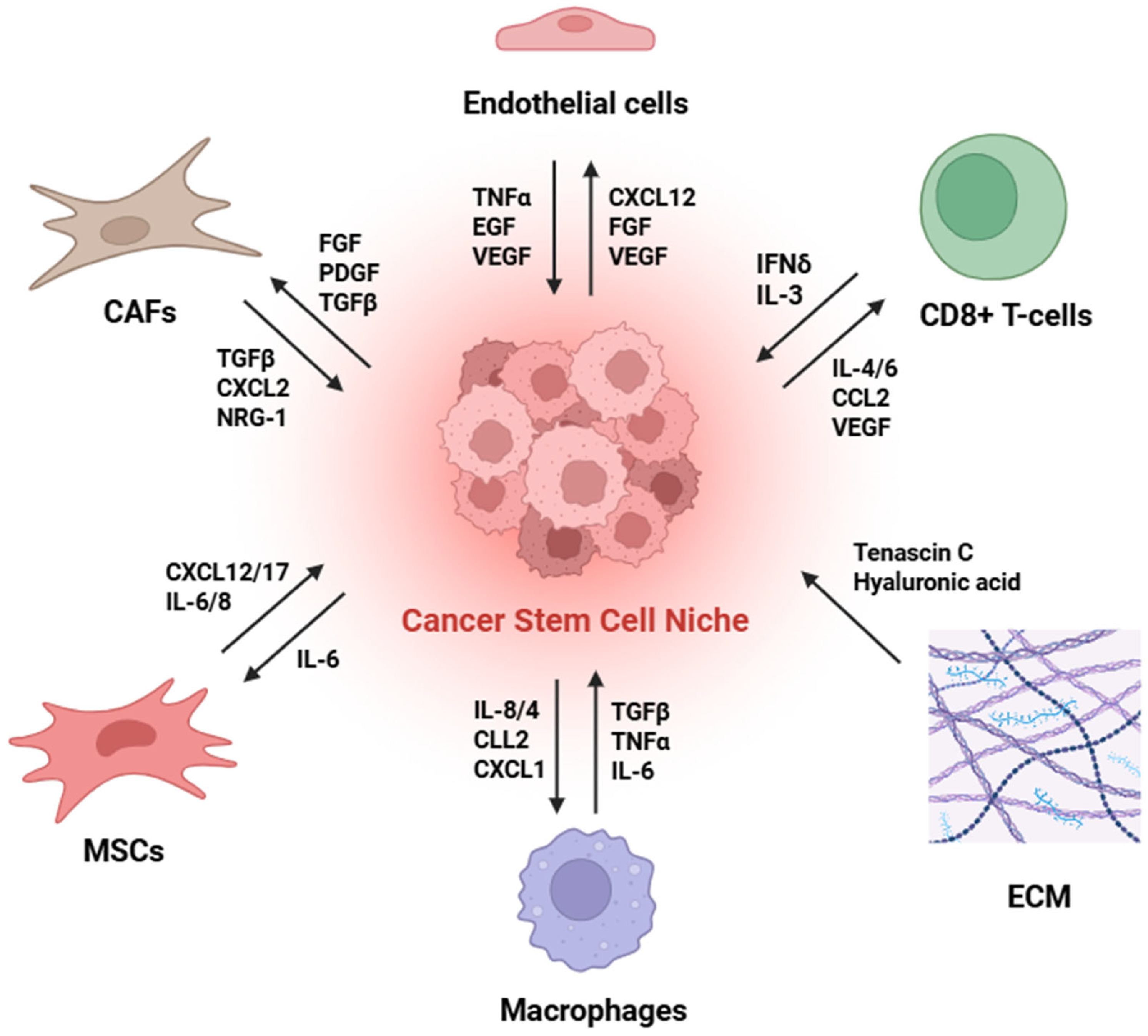
3. The CSC Niche
De-Differentiation: Back to the Future
4. CSCs in Gynecological Tumors
4.1. Breast CSCs
4.2. Ovarian CSCs
4.3. Cervical CSCs
4.4. Endometrial CSCs
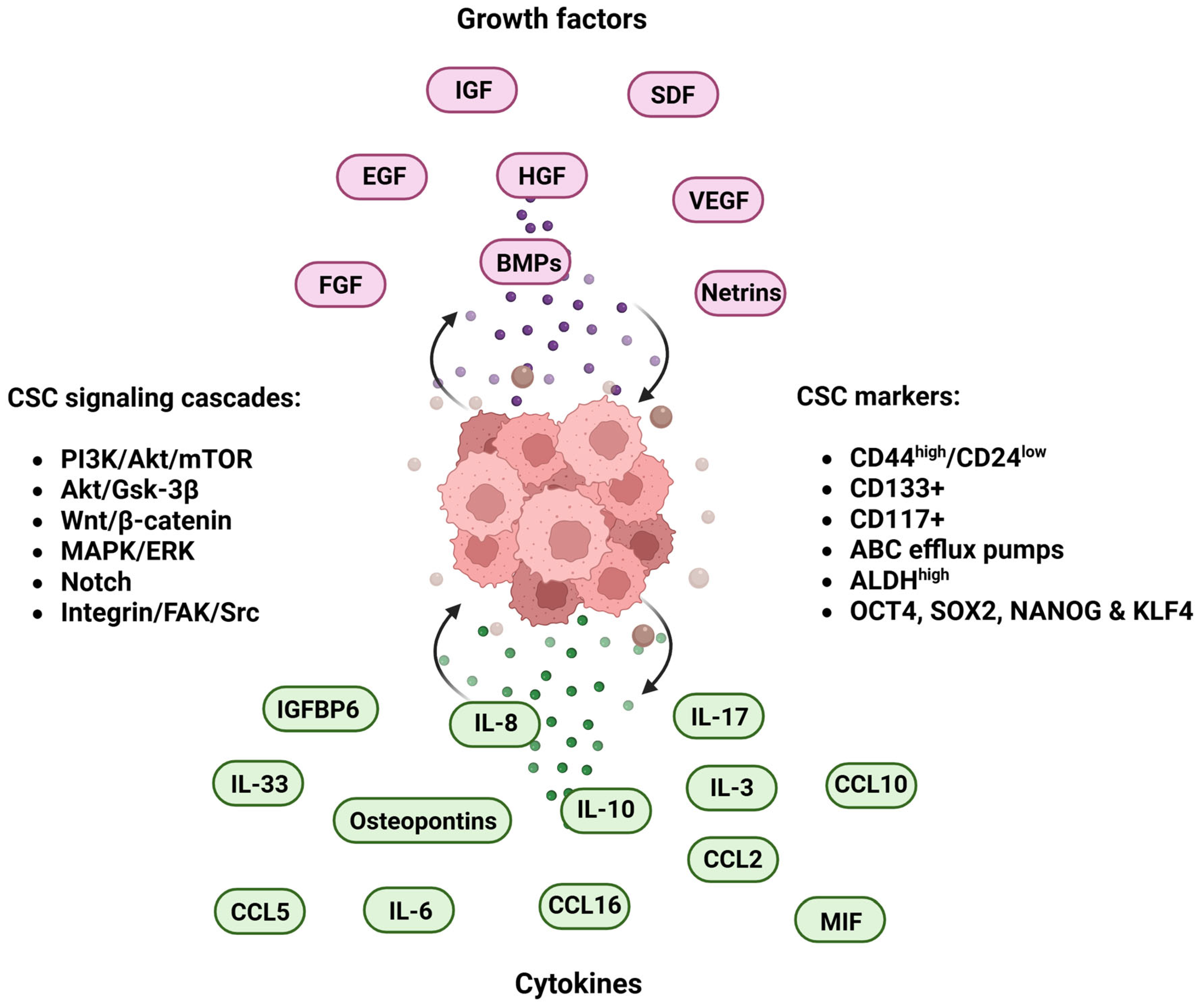
5. Microenvironmental Cues: Growth Factors and Cytokines Impact on Cancer Stemness
5.1. Growth Factors
5.2. Growth Factor and CSCs Crosstalk in Gynecological Cancer
5.3. Cytokines
5.4. Cytokines and CSCs Crosstalk in Gynecological Cancer
6. Growth Factors and Cytokines as Potential Targets for CSC Therapy
6.1. Growth Factor-Targeted Therapies
6.2. Cytokine-Targeted Therapies
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aravantinou-Fatorou, A.; Georgakopoulou, V.E.; Dimopoulos, M.A.; Liontos, M. Precision Medicine in Gynecological Cancer (Review). Biomed. Rep. 2025, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gu, H.; Mao, Z.; Beeraka, N.M.; Zhao, X.; Anand, M.P.; Zheng, Y.; Zhao, R.; Li, S.; Manogaran, P.; et al. Global Burden of Gynaecological Cancers in 2022 and Projections to 2050. J. Glob. Health 2024, 14, 04155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted Therapies in Gynecological Cancers: A Comprehensive Review of Clinical Evidence. Signal Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef] [PubMed]
- Keyvani, V.; Riahi, E.; Yousefi, M.; Esmaeili, S.-A.; Shafabakhsh, R.; Moradi Hasan-Abad, A.; Mahjoubin-Tehran, M.; Hamblin, M.R.; Mollazadeh, S.; Mirzaei, H. Gynecologic Cancer, Cancer Stem Cells, and Possible Targeted Therapies. Front. Pharmacol. 2022, 13, 823572. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer Stem Cells--Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer Stem Cells: An Evolving Concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Mehrgou, A.; Akouchekian, M. The Importance of BRCA1 and BRCA2 Genes Mutations in Breast Cancer Development. Med. J. Islam. Repub. Iran. 2016, 30, 369. [Google Scholar]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key Steps for Effective Breast Cancer Prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef]
- Tečić Vuger, A.; Šeparović, R.; Vazdar, L.; Pavlović, M.; Lepetić, P.; Šitić, S.; Bajić, Ž.; Šarčević, B.; Vrbanec, D. Characteristics and Prognosis of Triple-Negative Breast Cancer Patients: A Croatian Single Institution Retrospective Cohort Study. Acta Clin. Croat. 2020, 59, 97–108. [Google Scholar] [CrossRef]
- Al-thoubaity, F.K. Molecular Classification of Breast Cancer: A Retrospective Cohort Study. Ann. Med. Surg. 2020, 49, 44–48. [Google Scholar] [CrossRef]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Cao, L.; Xiang, G.; Liu, F.; Xu, C.; Liu, J.; Meng, Q.; Lyu, S.; Wang, S.; Niu, Y. A High AR:ERα or PDEF:ERα Ratio Predicts a Sub-Optimal Response to Tamoxifen Therapy in ERα-Positive Breast Cancer. Cancer Chemother. Pharmacol. 2019, 84, 609–620. [Google Scholar] [CrossRef]
- Biancolella, M.; Testa, B.; Baghernajad Salehi, L.; D’Apice, M.R.; Novelli, G. Genetics and Genomics of Breast Cancer: Update and Translational Perspectives. Semin. Cancer Biol. 2021, 72, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, J.P.M.; Verma, S.; Verma, S. Advances in the Management of Metastatic Breast Cancer: Options Beyond First-Line Chemotherapy. Curr. Oncol. 2012, 19, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Astone, A.; Botticelli, A.; Carbognin, L.; Cassano, A.; D’Auria, G.; Fabbri, A.; Fabi, A.; Gamucci, T.; Krasniqi, E.; et al. CDK4/6 Inhibitor Treatments in Patients with Hormone Receptor Positive, Her2 Negative Advanced Breast Cancer: Potential Molecular Mechanisms, Clinical Implications and Future Perspectives. Cancers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Sareyeldin, R.M.; Gupta, I.; Al-Hashimi, I.; Al-Thawadi, H.A.; al Farsi, H.F.; Vranic, S.; al Moustafa, A.-E. Cancers Gene Expression and MiRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers 2019, 11, 646. [Google Scholar] [CrossRef]
- Wolff, A.C.; McShane, L.M.; Hammond, M.E.H.; Allison, K.H.; Fitzgibbons, P.; Press, M.F.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef]
- Cesca, M.G.; Vian, L.; Cristóvão-Ferreira, S.; Pondé, N.; de Azambuja, E. HER2-Positive Advanced Breast Cancer Treatment in 2020. Cancer Treat. Rev. 2020, 88, 102033. [Google Scholar] [CrossRef]
- Gradishar, W.; Salerno, K.E. NCCN Guidelines Update: Breast Cancer. J. Natl. Compr. Cancer Netw. 2016, 14, 641–644. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bang, Y.-J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Choong, G.M.; Cullen, G.D.; O’Sullivan, C.C. Evolving Standards of Care and New Challenges in the Management of HER2-positive Breast Cancer. CA Cancer J. Clin. 2020, 70, 355–374. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743, Correction in N. Engl. J. Med. 2007, 356, 1487. [Google Scholar] [CrossRef]
- Guo, L.; Shao, W.; Zhou, C.; Yang, H.; Yang, L.; Cai, Q.; Wang, J.; Shi, Y.; Huang, L.; Zhang, J. Neratinib for HER2-positive breast cancer with an overlooked option. Mol. Med. 2023, 29, 134. [Google Scholar] [CrossRef]
- Pommier, R.M.; Sanlaville, A.; Tonon, L.; Kielbassa, J.; Thomas, E.; Ferrari, A.; Sertier, A.S.; Hollande, F.; Martinez, P.; Tissier, A.; et al. Comprehensive Characterization of Claudin-Low Breast Tumors Reflects the Impact of the Cell-of-Origin on Cancer Evolution. Nat. Commun. 2020, 11, 3431. [Google Scholar] [CrossRef]
- Borri, F.; Granaglia, A. Pathology of Triple Negative Breast Cancer. Semin. Cancer Biol. 2020, 72, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Viale, G.; Curigliano, G. Recent Advances in Triple Negative Breast Cancer: The Immunotherapy Era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zuo, W.-J.; Shao, Z.-M.; Jiang, Y.-Z. Molecular Subtypes and Precision Treatment of Triple-Negative Breast Cancer. Ann. Transl. Med. 2020, 8, 499. [Google Scholar] [CrossRef]
- Michel, L.L.; von Au, A.; Mavratzas, A.; Smetanay, K.; Schütz, F.; Schneeweiss, A.; von Au Alexandravonau, A. Immune Checkpoint Blockade in Patients with Triple-Negative Breast Cancer. Target. Oncol. 2020, 15, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.C.; Pareja, F.; Weigelt, B.; Rakha, E.; Ellis, I.O.; Schnitt, S.J.; Reis-Filho, J.S. The Spectrum of Triple-Negative Breast Disease: High- and Low-Grade Lesions. Am. J. Pathol. 2017, 187, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 Pathway: Basic Biology and Role in Cancer Immunotherapy. J. Cell Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as Regulators of DNA Repair, Transcription, and Cell Cycle in Response to DNA Damage. Cancer Sci. 2004, 95, 866–871. [Google Scholar] [CrossRef]
- Caparica, R.; Lambertini, M.; de Azambuja, E. How I Treat Metastatic Triple-Negative Breast Cancer. ESMO Open 2019, 4, e000504. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- Kalimutho, M.; Parsons, K.; Mittal, D.; López, J.A.; Srihari, S.; Khanna, K.K. Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol. Sci. 2015, 36, 822–846. [Google Scholar] [CrossRef]
- Herschkowitz, J.I.; Simin, K.; Weigman, V.J.; Mikaelian, I.; Usary, J.; Hu, Z.; Rasmussen, K.E.; Jones, L.P.; Assefnia, S.; Chandrasekharan, S.; et al. Identification of Conserved Gene Expression Features between Murine Mammary Carcinoma Models and Human Breast Tumors. Genome Biol. 2007, 8, R76. [Google Scholar] [CrossRef]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I.; et al. Residual Breast Cancers after Conventional Therapy Display Mesenchymal as Well as Tumor-Initiating Features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Gonzalez-Angulo, A.-M.; Stemke-Hale, K.; Gilcrease, M.Z.; Krishnamurthy, S.; Lee, J.-S.; Fridlyand, J.; Sahin, A.; Agarwal, R.; Joy, C.; et al. Characterization of a Naturally Occurring Breast Cancer Subset Enriched in Epithelial-to-Mesenchymal Transition and Stem Cell Characteristics. Cancer Res. 2009, 69, 4116–4124. [Google Scholar] [CrossRef]
- Fougner, C.; Bergholtz, H.; Norum, J.H.; Sørlie, T. Re-Definition of Claudin-Low as a Breast Cancer Phenotype. Nat. Commun. 2020, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and Molecular Characterization of the Claudin-Low Intrinsic Subtype of Breast Cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, R.; Finetti, P.; Guille, A.; Adelaide, J.; Chaffanet, M.; Viens, P.; Birnbaum, D.; Bertucci, F. Claudin-Low Breast Cancers: Clinical, Pathological, Molecular and Prognostic Characterization. Mol. Cancer 2014, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Fougner, C.; Bergholtz, H.; Kuiper, R.; Norum, J.H.; Sørlie, T. Claudin-Low-like Mouse Mammary Tumors Show Distinct Transcriptomic Patterns Uncoupled from Genomic Drivers. Breast Cancer Res. 2019, 21, 85. [Google Scholar] [CrossRef]
- Murphy, A.D.; Morgan, R.D.; Clamp, A.R.; Jayson, G.C. The Role of Vascular Endothelial Growth Factor Inhibitors in the Treatment of Epithelial Ovarian Cancer. Br. J. Cancer 2022, 126, 851–864. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Momenimovahed, Z.; Allahqoli, L.; Tiznobaik, A.; Hajinasab, N.; Salehiniya, H.; Alkatout, I. The Global, Regional and National Epidemiology, Incidence, Mortality, and Burden of Ovarian Cancer. Health Sci. Rep. 2022, 5, e936. [Google Scholar] [CrossRef]
- Ducie, J.; Dao, F.; Considine, M.; Olvera, N.; Shaw, P.A.; Kurman, R.J.; Shih, I.M.; Soslow, R.A.; Cope, L.; Levine, D.A. Molecular Analysis of High-Grade Serous Ovarian Carcinoma with and without Associated Serous Tubal Intra-Epithelial Carcinoma. Nat. Commun. 2017, 8, 990. [Google Scholar] [CrossRef]
- Wang, Y.; Farnell, D.; Farahani, H.; Nursey, M.; Tessier-Cloutier, B.; Jones, S.J.M.; Huntsman, D.G.; Gilks, C.B.; Bashashati, A. Classification of Epithelial Ovarian Carcinoma Whole-Slide Pathology Images Using Deep Transfer Learning. arXiv 2020, arXiv:2005.10957. [Google Scholar] [CrossRef]
- De Leo, A.; Santini, D.; Ceccarelli, C.; Santandrea, G.; Palicelli, A.; Acquaviva, G.; Chiarucci, F.; Rosini, F.; Ravegnini, G.; Pession, A.; et al. What Is New on Ovarian Carcinoma: Integrated Morphologic and Molecular Analysis Following the New 2020 World Health Organization Classification of Female Genital Tumors. Diagnostics 2021, 11, 697. [Google Scholar] [CrossRef]
- Salvati, A.; Carnevali, I.; Alexandrova, E.; Facchi, S.; Ronchi, S.; Libera, L.; Sahnane, N.; Memoli, D.; Lamberti, J.; Amabile, S.; et al. Targeted Molecular Profiling of Epithelial Ovarian Cancer from Italian BRCA Wild-Type Patients with a BRCA and PARP Pathways Gene Panel. Exp. Mol. Pathol. 2022, 128, 104833. [Google Scholar] [CrossRef]
- Cont, N.T.; Ferrero, A.; Peccatori, F.A.; D’Alonzo, M.; Codacci-Pisanelli, G.; Colombo, N.; Biglia, N. Medical Treatment of Early Stage and Rare Histological Variants of Epithelial Ovarian Cancer. Ecancermedicalscience 2015, 9, 584. [Google Scholar] [CrossRef]
- van Roon, Y.; Vetter, M. Advancing Ovarian Cancer Treatment: The Latest Insights on PARP Inhibitors from ASCO 2023. Heal. TIMES Oncol. Hematol. 2023, 18, 28–31. [Google Scholar] [CrossRef]
- Perez-Fidalgo, J.A.; Gálvez-Montosa, F.; Guerra, E.M.; Madariaga, A.; Manzano, A.; Martin-Lorente, C.; Rubio-Pérez, M.J.; Alarcón, J.; Barretina-Ginesta, M.P.; Gaba, L. SEOM–GEICO Clinical Guideline on Epithelial Ovarian Cancer (2023). Clin. Transl. Oncol. 2024, 26, 2758–2770. [Google Scholar] [CrossRef]
- Zolotareva, O.; Legler, K.; Tsoy, O.; Esteve, A.; Sergushichev, A.; Sukhov, V.; Baumbach, J.; Eylmann, K.; Qi, M.; Alawi, M.; et al. Transcriptome Signature for the Identification of Bevacizumab Responders in Ovarian Cancer. arXiv 2025, arXiv:2501.04869. [Google Scholar] [CrossRef]
- Garg, V.; Oza, A.M. Treatment of Ovarian Cancer Beyond PARP Inhibition: Current and Future Options. Drugs 2023, 83, 1365–1385. [Google Scholar] [CrossRef]
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020, 10, 570. [Google Scholar] [CrossRef]
- Wilson, E.M.; Eskander, R.N.; Binder, P.S. Recent Therapeutic Advances in Gynecologic Oncology: A Review. Cancers 2024, 16, 770. [Google Scholar] [CrossRef]
- Yang, M.; Du, J.; Lu, H.; Xiang, F.; Mei, H.; Xiao, H. Global Trends and Age-Specific Incidence and Mortality of Cervical Cancer from 1990 to 2019: An International Comparative Study Based on the Global Burden of Disease. BMJ Open 2022, 12, 055470. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Mazidimoradi, A.; Maroofi, P.; Allahqoli, L.; Salehiniya, H.; Alkatout, I. Global, Regional and National Burden, Incidence, and Mortality of Cervical Cancer. Cancer Rep. 2023, 6, e1756. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Progress in the Pathological Arena of Gynecological Cancers. Int. J. Gynecol. Obstet. 2021, 155, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, C.; Weng, L. Exploring Cervical Adenocarcinoma: Epidemiological Insights, Diagnostic and Therapeutic Challenges, and Pathogenetic Mechanisms. Cancer Med. 2025, 14, 70620. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the Cervix Uteri: 2021 Update. Int. J. Gynecol. Obstet. 2021, 155, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Podwika, S.E.; Duska, L.R. Top Advances of the Year: Cervical Cancer. Cancer 2023, 129, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, A.; Hasegawa, K. Recent Advances in Immunotherapy for Cervical Cancer. Int. J. Clin. Oncol. 2025, 30, 434–448. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Tewari, K.S.; Dubot, C.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Salman, P.; Yañez, E.; Gümüş, M.; et al. First-Line Pembrolizumab + Chemotherapy Versus Placebo + Chemotherapy for Persistent, Recurrent, or Metastatic Cervical Cancer: Final Overall Survival Results of KEYNOTE-826. J. Clin. Oncol. 2023, 41, 5505–5511. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Vergote, I.; González-Martín, A.; Fujiwara, K.; Kalbacher, E.; Bagaméri, A.; Ghamande, S.; Lee, J.-Y.; Banerjee, S.; Maluf, F.C.; Lorusso, D.; et al. Tisotumab Vedotin as Second- or Third-Line Therapy for Recurrent Cervical Cancer. N. Engl. J. Med. 2024, 391, 44–55. [Google Scholar] [CrossRef]
- Giudice, E.; Mirza, M.R.; Lorusso, D. Advances in the Management of Recurrent Cervical Cancer: State of the Art and Future Perspectives. Curr. Oncol. Rep. 2023, 25, 1307–1326. [Google Scholar] [CrossRef]
- Anakwenze, C.P.; Ewongwo, A.; Onyewadume, L.; Oyekan, A.; Chigbo, C.O.; Valle, L.; Geng, Y.; Olapade, P.; Okwunze, K.; Lasebikan, N.; et al. A Systematic Review of Endometrial Cancer Clinical Research in Africa. Infect. Agents Cancer 2024, 19, 2. [Google Scholar] [CrossRef]
- Dörk, T.; Hillemanns, P.; Tempfer, C.; Breu, J.; Fleisch, M.C. Genetic Susceptibility to Endometrial Cancer: Risk Factors and Clinical Management. Cancers 2020, 12, 2407. [Google Scholar] [CrossRef]
- Terzic, M.; Aimagambetova, G.; Kunz, J.; Bapayeva, G.; Aitbayeva, B.; Terzic, S.; Laganà, A.S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9274. [Google Scholar] [CrossRef] [PubMed]
- eBioMedicine. Endometrial Cancer: Improving Management among Increasing Incidence Rates. eBioMedicine 2024, 103, 105159. [Google Scholar]
- Yasuda, M. New Clinicopathological Concept of Endometrial Carcinoma with Integration of Histological Features and Molecular Profiles. Pathol. Int. 2024, 74, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers 2023, 15, 4101. [Google Scholar] [CrossRef]
- Kumar, H.; Deshwal, A.; Datwani, S.; Li, Z. Decoding High-Grade Endometrial Cancer: A Molecular-Histologic Integration Using the Cancer Genome Atlas Framework. J. Clin. Transl. Pathol. 2025, 5, 104–113. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients with Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Somasegar, S.; MSN, B.S.; Jairam-Thodla, A.; Dorigo, O. Long Term Treatment of Advanced Endometrial Cancer with Lenvatinib and Pembrolizumab. Gynecol. Oncol. Rep. 2025, 58, 101717. [Google Scholar] [CrossRef]
- Keller, P.J.; Adams, E.J.; Wu, R.; Côté, A.; Arora, S.; Cantone, N.; Meyer, R.; Mertz, J.A.; Gehling, V.; Cui, J.; et al. Comprehensive Target Engagement by the EZH2 Inhibitor Tulmimetostat Allows for Targeting of ARID1A Mutant Cancers. Cancer Res. 2024, 84, 2501–2517. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-Tumor Heterogeneity from a Cancer Stem Cell Perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Takahashi, R.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Haddadin, L.; Sun, X. Stem Cells in Cancer: From Mechanisms to Therapeutic Strategies. Cells 2025, 14, 538. [Google Scholar] [CrossRef]
- Rotar, I.C.; Bernad, E.; Moraru, L.; Ivan, V.; Apostol, A.; Bernad, S.I.; Muresan, D.; Mitranovici, M.-I. Therapeutic and Prognostic Relevance of Cancer Stem Cell Populations in Endometrial Cancer: A Narrative Review. Diagnostics 2025, 15, 1872. [Google Scholar] [CrossRef]
- Zeimet, A.G.; Reimer, D.; Sopper, S.; Boesch, M.; Martowicz, A.; Roessler, J.; Wiedemair, A.M.; Rumpold, H.; Untergasser, G.; Concin, N.; et al. Ovarian Cancer Stem Cells. Neoplasma 2012, 59, 747–755. [Google Scholar] [CrossRef]
- Frąszczak, K.; Barczyński, B. Characteristics of Cancer Stem Cells and Their Potential Role in Endometrial Cancer. Cancers 2024, 16, 1083. [Google Scholar] [CrossRef]
- Sun, H.R.; Wang, S.; Yan, S.C.; Zhang, Y.; Nelson, P.J.; Jia, H.L.; Qin, L.X.; Dong, Q.Z. Therapeutic Strategies Targeting Cancer Stem Cells and Their Microenvironment. Front. Oncol. 2019, 9, 01104. [Google Scholar] [CrossRef]
- Jain, S.; Annett, S.L.; Morgan, M.P.; Robson, T. The Cancer Stem Cell Niche in Ovarian Cancer and Its Impact on Immune Surveillance. Int. J. Mol. Sci. 2021, 22, 4091. [Google Scholar] [CrossRef]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.Z.; Ren, N. Characteristics of the Cancer Stem Cell Niche and Therapeutic Strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, Z.; Li, G.; Zhang, Y.; Liu, X.; Li, B.; Wang, J.; Li, X. Cancer Stem Cells and Their Niche in Cancer Progression and Therapy. Cancer Cell Int. 2023, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.-J.; Ma, S. Hallmarks of Cancer Stemness. Cell Stem Cell 2024, 31, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Hallmarks of Stemness in Mammalian Tissues. Cell Stem Cell 2024, 31, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 Is a Marker of Constitutive and Reprogrammed Cancer Stem Cells Driving Colon Cancer Metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, S.; Fingerle, A.A.; Cammareri, P.; Nebelsiek, T.; Göktuna, S.I.; Ziegler, P.K.; Canli, O.; Heijmans, J.; Huels, D.J.; Moreaux, G.; et al. Intestinal Tumorigenesis Initiated by Dedifferentiation and Acquisition of Stem-Cell-like Properties. Cell 2013, 152, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Sousa E Melo, F.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt Activity Defines Colon Cancer Stem Cells and Is Regulated by the Microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e16. [Google Scholar] [CrossRef]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-Associated Mesothelial Cells Promote Ovarian Cancer Chemoresistance through Paracrine Osteopontin Signaling. J. Clin. Investig. 2021, 131, 146186. [Google Scholar] [CrossRef]
- Lenos, K.J.; Miedema, D.M.; Lodestijn, S.C.; Nijman, L.E.; van den Bosch, T.; Romero Ros, X.; Lourenço, F.C.; Lecca, M.C.; van der Heijden, M.; van Neerven, S.M.; et al. Stem Cell Functionality Is Microenvironmentally Defined during Tumour Expansion and Therapy Response in Colon Cancer. Nat. Cell Biol. 2018, 20, 1193–1202. [Google Scholar] [CrossRef]
- Li, G.; Choi, J.E.; Kryczek, I.; Sun, Y.; Liao, P.; Li, S.; Wei, S.; Grove, S.; Vatan, L.; Nelson, R.; et al. Intersection of Immune and Oncometabolic Pathways Drives Cancer Hyperprogression during Immunotherapy. Cancer Cell 2023, 41, 304–322.e7. [Google Scholar] [CrossRef]
- Beziaud, L.; Young, C.M.; Alonso, A.M.; Norkin, M.; Minafra, A.R.; Huelsken, J. IFNγ-Induced Stem-like State of Cancer Cells as a Driver of Metastatic Progression Following Immunotherapy. Cell Stem Cell 2023, 30, 818–831.e6. [Google Scholar] [CrossRef]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. CCL5 Derived from Tumor-Associated Macrophages Promotes Prostate Cancer Stem Cells and Metastasis via Activating β-Catenin/STAT3 Signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef]
- Sharma, V.P.; Tang, B.; Wang, Y.; Duran, C.L.; Karagiannis, G.S.; Xue, E.A.; Entenberg, D.; Borriello, L.; Coste, A.; Eddy, R.J.; et al. Live Tumor Imaging Shows Macrophage Induction and TMEM-Mediated Enrichment of Cancer Stem Cells during Metastatic Dissemination. Nat. Commun. 2021, 12, 7300. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-Y.; Ham, S.W.; Kim, J.-K.; Jin, X.; Lee, S.Y.; Shin, Y.J.; Choi, C.-Y.; Sa, J.K.; Kim, S.H.; Chun, T.; et al. Ly6G+ Inflammatory Cells Enable the Conversion of Cancer Cells to Cancer Stem Cells in an Irradiated Glioblastoma Model. Cell Death Differ. 2019, 26, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.-L.; Lan, H.-Y.; Cheng, W.-C.; Huang, S.-C.; Yang, M.-H. Tumor Stem-like Cell-Derived Exosomal RNAs Prime Neutrophils for Facilitating Tumorigenesis of Colon Cancer. J. Hematol. Oncol. 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dong, X.; Qi, P.; Ye, Y.; Shen, W.; Leng, L.; Wang, L.; Li, X.; Luo, X.; Chen, Y.; et al. Sox2 Communicates with Tregs Through CCL1 to Promote the Stemness Property of Breast Cancer Cells. Stem Cells 2017, 35, 2351–2365. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Wang, B.; Zhang, H.; Qin, G.; Li, C.; Cao, L.; Gao, Q.; Ping, Y.; Zhang, K.; et al. Regulatory T Cells Promote Glioma Cell Stemness through TGF-β–NF-ΚB–IL6–STAT3 Signaling. Cancer Immunol. Immunother. 2021, 70, 2601–2616. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Lu, H.; Xie, Y.; Tran, L.; Lan, J.; Yang, Y.; Murugan, N.L.; Wang, R.; Wang, Y.J.; Semenza, G.L. Chemotherapy-Induced S100A10 Recruits KDM6A to Facilitate OCT4-Mediated Breast Cancer Stemness. J. Clin. Investig. 2020, 130, 4607–4623. [Google Scholar] [CrossRef]
- Wu, C.; Rakhshandehroo, T.; Wettersten, H.I.; Campos, A.; von Schalscha, T.; Jain, S.; Yu, Z.; Tan, J.; Mose, E.; Childers, B.G.; et al. Pancreatic Cancer Cells Upregulate LPAR4 in Response to Isolation Stress to Promote an ECM-Enriched Niche and Support Tumour Initiation. Nat. Cell Biol. 2023, 25, 309–322. [Google Scholar] [CrossRef]
- Solé, L.; Lobo-Jarne, T.; Álvarez-Villanueva, D.; Alonso-Marañón, J.; Guillén, Y.; Guix, M.; Sangrador, I.; Rozalén, C.; Vert, A.; Barbachano, A.; et al. P53 Wild-Type Colorectal Cancer Cells That Express a Fetal Gene Signature Are Associated with Metastasis and Poor Prognosis. Nat. Commun. 2022, 13, 2866. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Huang, Y.-H.; Luo, M.-H.; Ni, Y.-B.; Chan, S.-K.; Lui, P.C.W.; Yu, A.M.C.; Tan, P.H.; Tse, G.M. Cancer Stem Cell Markers Are Associated with Adverse Biomarker Profiles and Molecular Subtypes of Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Escudero Mendez, L.; Srinivasan, M.; Hamouda, R.K.; Ambedkar, B.; Arzoun, H.; Sahib, I.; Fondeur, J.; Mohammed, L. Evaluation of CD44+/CD24- and Aldehyde Dehydrogenase Enzyme Markers in Cancer Stem Cells as Prognostic Indicators for Triple-Negative Breast Cancer. Cureus 2022, 14, 28056. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Su, Y.; Zhang, X. ALDH1+ Stem Cells Demonstrate More Stem Cell-like Characteristics than CD44+/CD24–/Low Stem Cells in Different Molecular Subtypes of Breast Cancer. Transl. Cancer Res. 2020, 9, 1652–1659. [Google Scholar] [CrossRef]
- Zhong, Y.; Shen, S.; Zhou, Y.; Mao, F.; Guan, J.; Lin, Y.; Xu, Y.; Sun, Q. ALDH1 Is a Better Clinical Indicator for Relapse of Invasive Ductal Breast Cancer than the CD44+/CD24− Phenotype. Med. Oncol. 2014, 31, 864. [Google Scholar] [CrossRef]
- Chute, J.P.; Muramoto, G.G.; Whitesides, J.; Colvin, M.; Safi, R.; Chao, N.J.; McDonnell, D.P. Inhibition of Aldehyde Dehydrogenase and Retinoid Signaling Induces the Expansion of Human Hematopoietic Stem Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11707–11712. [Google Scholar] [CrossRef]
- Dionísio, M.R.; Vieira, A.F.; Carvalho, R.; Conde, I.; Oliveira, M.; Gomes, M.; Pinto, M.T.; Pereira, P.; Pimentel, J.; Souza, C.; et al. BR-BCSC Signature: The Cancer Stem Cell Profile Enriched in Brain Metastases That Predicts a Worse Prognosis in Lymph Node-Positive Breast Cancer. Cells 2020, 9, 2442. [Google Scholar] [CrossRef]
- Zhang, X.; Powell, K.; Li, L. Breast Cancer Stem Cells: Biomarkers, Identification and Isolation Methods, Regulating Mechanisms, Cellular Origin, and Beyond. Cancers 2020, 12, 3765. [Google Scholar] [CrossRef]
- Pinto, C.A.; Widodo, E.; Waltham, M.; Thompson, E.W. Breast Cancer Stem Cells and Epithelial Mesenchymal Plasticity—Implications for Chemoresistance. Cancer Lett. 2013, 341, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk-Drapała, A.; Totoń, E.; Taube, M.; Idzik, M.; Rubiś, B.; Lisiak, N. Breast Cancer Stem Cells and Tumor Heterogeneity: Characteristics and Therapeutic Strategies. Cancers 2024, 16, 2481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Huang, Z.; Li, X.; Xiao, J.; Qu, Y.; Huang, L.; Wang, Y. Identification of Prognosis Biomarkers for High-Grade Serous Ovarian Cancer Based on Stemness. Front. Genet. 2022, 13, 861954. [Google Scholar] [CrossRef] [PubMed]
- Sriramkumar, S.; Metcalfe, T.X.; Lai, T.; Zong, X.; Fang, F.; O’Hagan, H.M.; Nephew, K.P. Single-Cell Analysis of a High-Grade Serous Ovarian Cancer Cell Line Reveals Transcriptomic Changes and Cell Subpopulations Sensitive to Epigenetic Combination Treatment. PLoS ONE 2022, 17, e0271584. [Google Scholar] [CrossRef]
- Dinh, H.Q.; Lin, X.; Abbasi, F.; Nameki, R.; Haro, M.; Olingy, C.E.; Chang, H.; Hernandez, L.; Gayther, S.A.; Wright, K.N.; et al. Single-Cell Transcriptomics Identifies Gene Expression Networks Driving Differentiation and Tumorigenesis in the Human Fallopian Tube. Cell Rep. 2021, 35, 108978. [Google Scholar] [CrossRef]
- Kan, T.; Zhang, S.; Zhou, S.; Zhang, Y.; Zhao, Y.; Gao, Y.; Zhang, T.; Gao, F.; Wang, X.; Zhao, L.; et al. Single-Cell RNA-Seq Recognized the Initiator of Epithelial Ovarian Cancer Recurrence. Oncogene 2022, 41, 895–906. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.H.; Park, E.; Myung, J.K.; Park, J.H.; Kim, D.I.; Kim, S.I.; Lee, M.; Kim, Y.; Park, C.M.; et al. Differential Epithelial and Stromal LGR5 Expression in Ovarian Carcinogenesis. Sci. Rep. 2022, 12, 11200. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Ingram, P.N.; Yang, K.; Coffman, L.; Iyengar, M.; Bai, S.; Thomas, D.G.; Yoon, E.; Buckanovich, R.J. Identifying an Ovarian Cancer Cell Hierarchy Regulated by Bone Morphogenetic Protein 2. Proc. Natl. Acad. Sci. USA 2015, 112, 6882–6888, Erratum in Proc. Natl. Acad. Sci. USA 2021, 118, e2117843118. [Google Scholar] [CrossRef]
- Lin, J.; Ding, D. The Prognostic Role of the Cancer Stem Cell Marker CD44 in Ovarian Cancer: A Meta-Analysis. Cancer Cell Int. 2017, 17, 8. [Google Scholar] [CrossRef]
- Sun, Y.; Yoshida, T.; Okabe, M.; Zhou, K.; Wang, F.; Soko, C.; Saito, S.; Nikaido, T. Isolation of Stem-Like Cancer Cells in Primary Endometrial Cancer Using Cell Surface Markers CD133 and CXCR4. Transl. Oncol. 2017, 10, 976–987. [Google Scholar] [CrossRef]
- Meng, E.; Mitra, A.; Tripathi, K.; Finan, M.A.; Scalici, J.; McClellan, S.; da Silva, L.M.; Reed, E.; Shevde, L.A.; Palle, K.; et al. ALDH1A1 Maintains Ovarian Cancer Stem Cell-Like Properties by Altered Regulation of Cell Cycle Checkpoint and DNA Repair Network Signaling. PLoS ONE 2014, 9, e107142. [Google Scholar] [CrossRef] [PubMed]
- Frąszczak, K.; Barczyński, B. The Role of Cancer Stem Cell Markers in Ovarian Cancer. Cancers 2023, 16, 40. [Google Scholar] [CrossRef]
- Burgos-Ojeda, D.; Wu, R.; McLean, K.; Chen, Y.-C.; Talpaz, M.; Yoon, E.; Cho, K.R.; Buckanovich, R.J. CD24+ Ovarian Cancer Cells Are Enriched for Cancer-Initiating Cells and Dependent on JAK2 Signaling for Growth and Metastasis. Mol. Cancer Ther. 2015, 14, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Choi, Y.P.; Gao, M.-Q.; Kang, S.; Kim, B.G.; Lee, J.H.; Kwon, M.J.; Shin, Y.K.; Cho, N.H. CD24+ Ovary Cancer Cells Exhibit an Invasive Mesenchymal Phenotype. Biochem. Biophys. Res. Commun. 2013, 432, 333–338. [Google Scholar] [CrossRef]
- Yang, B.; Yan, X.; Liu, L.; Jiang, C.; Hou, S. Overexpression of the Cancer Stem Cell Marker CD117 Predicts Poor Prognosis in Epithelial Ovarian Cancer Patients: Evidence from Meta-Analysis. Onco Targets Ther. 2017, 10, 2951–2961. [Google Scholar] [CrossRef]
- Tayama, S.; Motohara, T.; Narantuya, D.; Li, C.; Fujimoto, K.; Sakaguchi, I.; Tashiro, H.; Saya, H.; Nagano, O.; Katabuchi, H. The Impact of EpCAM Expression on Response to Chemotherapy and Clinical Outcomes in Patients with Epithelial Ovarian Cancer. Oncotarget 2017, 8, 44312–44325. [Google Scholar] [CrossRef]
- Parte, S.C.; Batra, S.K.; Kakar, S.S. Characterization of Stem Cell and Cancer Stem Cell Populations in Ovary and Ovarian Tumors. J. Ovarian Res. 2018, 11, 69. [Google Scholar] [CrossRef]
- Abubaker, K.; Latifi, A.; Luwor, R.; Nazaretian, S.; Zhu, H.; Quinn, M.A.; Thompson, E.W.; Findlay, J.K.; Ahmed, N. Short-Term Single Treatment of Chemotherapy Results in the Enrichment of Ovarian Cancer Stem Cell-like Cells Leading to an Increased Tumor Burden. Mol. Cancer 2013, 12, 24. [Google Scholar] [CrossRef]
- Robinson, M.; Gilbert, S.F.; Waters, J.A.; Lujano-Olazaba, O.; Lara, J.; Alexander, L.J.; Green, S.E.; Burkeen, G.A.; Patrus, O.; Sarwar, Z.; et al. Characterization of SOX2, OCT4 and NANOG in Ovarian Cancer Tumor-Initiating Cells. Cancers 2021, 13, 262. [Google Scholar] [CrossRef]
- Xie, W.; Yu, J.; Yin, Y.; Zhang, X.; Zheng, X.; Wang, X. OCT4 Induces EMT and Promotes Ovarian Cancer Progression by Regulating the PI3K/AKT/MTOR Pathway. Front. Oncol. 2022, 12, 876257. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Teng, Y.; Hua, R.; Hu, Y.; Li, X. KLF4 Promotes Cisplatin Resistance by Activating MTORC1 Signaling in Ovarian Cancer. Discov. Oncol. 2024, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Peng, C.; Li, C.; Zhou, Y.; Li, M.; Ling, B.; Wei, H.; Tian, Z. Identification and Characterization of Cancer Stem-like Cells from Primary Carcinoma of the Cervix Uteri. Oncol. Rep. 2009, 22, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Lu, R.; Zhang, Y.; Zhang, Y.; Zhao, C.; Lin, R.; Lin, Z. Cervical Cancer Stem Cells. Cell Prolif. 2015, 48, 611–625. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Poitevin, A.; Mendoza-Martínez, V.; Pérez-Plasencia, C.; García-Carrancá, A. Cancer-Initiating Cells Derived from Established Cervical Cell Lines Exhibit Stem-Cell Markers and Increased Radioresistance. BMC Cancer 2012, 12, 48. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, E.; Santiago-López, L.; Cruz-Domínguez, V.B.; Toledo-Guzmán, M.E.; Hernández-Cueto, D.; Muñiz-Hernández, S.; Garrido, E.; De León, D.C.; García-Carrancá, A. Characterization of Cervical Cancer Stem Cell-like Cells: Phenotyping, Stemness, and Human Papilloma Virus Co-Receptor Expression. Oncotarget 2016, 7, 31943–31954. [Google Scholar] [CrossRef]
- Tyagi, A.; Vishnoi, K.; Mahata, S.; Verma, G.; Srivastava, Y.; Masaldan, S.; Roy, B.G.; Bharti, A.C.; Das, B.C. Cervical Cancer Stem Cells Selectively Overexpress HPV Oncoprotein E6 That Controls Stemness and Self-Renewal through Upregulation of HES1. Clin. Cancer Res. 2016, 22, 4170–4184. [Google Scholar] [CrossRef]
- Ji, J.; Zheng, P.-S. Expression of Sox2 in Human Cervical Carcinogenesis. Hum. Pathol. 2010, 41, 1438–1447. [Google Scholar] [CrossRef]
- Sato, A.; Ishiwata, T.; Matsuda, Y.; Yamamoto, T.; Asakura, H.; Takeshita, T.; Naito, Z. Expression and Role of Nestin in Human Cervical Intraepithelial Neoplasia and Cervical Cancer. Int. J. Oncol. 2012, 41, 441–448. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Zheng, P.-S. High Aldehyde Dehydrogenase Activity Identifies Cancer Stem Cells in Human Cervical Cancer. Oncotarget 2013, 4, 2462–2475. [Google Scholar] [CrossRef]
- Cao, H.-Z.; Liu, X.-F.; Yang, W.-T.; Chen, Q.; Zheng, P.-S. LGR5 Promotes Cancer Stem Cell Traits and Chemoresistance in Cervical Cancer. Cell Death Dis. 2017, 8, e3039. [Google Scholar] [CrossRef]
- Gopalan, V.; Islam, F.; Lam, A.K. Surface Markers for the Identification of Cancer Stem Cells. In Cancer Stem Cells: Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 17–29. [Google Scholar]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; Corallo, M.; Prisco, M.G.; Eramo, A.; Napoletano, C.; Gallo, D.; Perillo, A.; et al. Cells with Characteristics of Cancer Stem/Progenitor Cells Express the CD133 Antigen in Human Endometrial Tumors. Clin. Cancer Res. 2009, 15, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Liu, H.-W.; Chang, Y.-H.; Chu, T.-Y. Expression of CD133 in Endometrial Cancer Cells and Its Implications. J. Cancer 2017, 8, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yamawaki, K.; Ishiguro, T.; Yoshihara, K.; Ueda, H.; Sato, A.; Ohata, H.; Yoshida, Y.; Minamino, T.; Okamoto, K.; et al. ALDH-Dependent Glycolytic Activation Mediates Stemness and Paclitaxel Resistance in Patient-Derived Spheroid Models of Uterine Endometrial Cancer. Stem Cell Rep. 2019, 13, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Attademo, L.; Scotto, G.; Genta, S.; Ghisoni, E.; Tuninetti, V.; Aglietta, M.; Pignata, S.; Valabrega, G. Endometrial Cancer Stem Cells: Role, Characterization and Therapeutic Implications. Cancers 2019, 11, 1820. [Google Scholar] [CrossRef]
- Saygin, C.; Wiechert, A.; Rao, V.S.; Alluri, R.; Connor, E.; Thiagarajan, P.S.; Hale, J.S.; Li, Y.; Chumakova, A.; Jarrar, A.; et al. CD55 Regulates Self-Renewal and Cisplatin Resistance in Endometrioid Tumors. J. Exp. Med. 2017, 214, 2715–2732. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B.; Yin, S.; Li, S.; Guo, Y.; Wang, M.; Wang, K.; Wan, X. Hypoxia Induces an Endometrial Cancer Stem-like Cell Phenotype via HIF-Dependent Demethylation of SOX2 MRNA. Oncogenesis 2020, 9, 81. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Jiang, W.; Zhang, R.; Zhang, B.; Silayiding, A.; Duan, X. MicroRNA-135a Promotes Proliferation, Migration, Invasion and Induces Chemoresistance of Endometrial Cancer Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2020, 5, 100103. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Liu, T.; Kong, J. MicroRNA-423 Promotes Proliferation, Migration and Invasion and Induces Chemoresistance of Endometrial Cancer Cells. Exp. Ther. Med. 2018, 16, 4213–4224. [Google Scholar] [CrossRef]
- Konno, Y.; Dong, P.; Xiong, Y.; Suzuki, F.; Lu, J.; Cai, M.; Watari, H.; Mitamura, T.; Hosaka, M.; Hanley, S.J.B.; et al. MicroRNA-101 Targets EZH2, MCL-1 and FOS to Suppress Proliferation, Invasion and Stem Cell-like Phenotype of Aggressive Endometrial Cancer Cells. Oncotarget 2014, 5, 6049–6062. [Google Scholar] [CrossRef]
- Murgo, E.; Falco, G.; Serviddio, G.; Mazzoccoli, G.; Colangelo, T. Circadian Patterns of Growth Factor Receptor-Dependent Signaling and Implications for Carcinogenesis. Cell Commun. Signal. 2024, 22, 319. [Google Scholar] [CrossRef]
- Wiedlocha, A.; Haugsten, E.M.; Zakrzewska, M. Roles of the Fgf-Fgfr Signaling System in Cancer Development and Inflammation. Cells 2021, 10, 2231. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, H.; Jia, Z.; Cui, M.; Tian, J. Chemoresistance and Targeting of Growth Factors/Cytokines Signalling Pathways: Towards the Development of Effective Therapeutic Strategy for Endometrial Cancer. Am. J. Cancer Res. 2018, 8, 1317. [Google Scholar] [PubMed]
- Wong, S.K.; Mohamad, N.V.; Jayusman, P.A.; Ibrahim, N.I. A Review on the Crosstalk between Insulin and Wnt/β-Catenin Signalling for Bone Health. Int. J. Mol. Sci. 2023, 24, 12441. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, C.; Morrione, A.; Scotlandi, K. Extracellular Interactors of the IGF System: Impact on Cancer Hallmarks and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 5915. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, X.; He, X. Platelet-Derived Growth Factors and Their Receptors: Structural and Functional Perspectives. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2176–2186. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor Specificity of the Fibroblast Growth Factor Family: The Complete Mammalian FGF Family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Z.; Chen, Z.; Xu, G.; Chen, Y. Fibroblast Growth Factor Receptors (Fgfrs): Structures and Small Molecule Inhibitors. Cells 2019, 8, 614. [Google Scholar] [CrossRef]
- Józefiak, A.; Larska, M.; Pomorska-Mól, M.; Ruszkowski, J.J. The Igf-1 Signaling Pathway in Viral Infections. Viruses 2021, 13, 1488. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming Growth Factor-β in Myocardial Disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Yan, X.; Fu, C.; Chen, L.; Qin, J.; Zeng, Q.; Yuan, H.; Nan, X.; Chen, H.; Zhou, J.; Lin, Y.; et al. Mesenchymal Stem Cells from Primary Breast Cancer Tissue Promote Cancer Proliferation and Enhance Mammosphere Formation Partially via EGF/EGFR/Akt Pathway. Breast Cancer Res. Treat. 2012, 132, 153–164. [Google Scholar] [CrossRef]
- Fillmore, C.M.; Gupta, P.B.; Rudnick, J.A.; Caballero, S.; Keller, P.J.; Lander, E.S.; Kuperwasser, C. Estrogen Expands Breast Cancer Stem-like Cells through Paracrine FGF/Tbx3 Signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 21737–21742. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. JNCI J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Lero, M.W.; Mercado-Matos, J.; Zhu, S.; Jo, M.; Tocheny, C.E.; Morgan, J.S.; Shaw, L.M. The Insulin and IGF Signaling Pathway Sustains Breast Cancer Stem Cells by IRS2/PI3K-Mediated Regulation of MYC. Cell Rep. 2022, 41, 111759. [Google Scholar] [CrossRef] [PubMed]
- Eterno, V.; Zambelli, A.; Pavesi, L.; Villani, L.; Zanini, V.; Petrolo, G.; Manera, S.; Tuscano, A.; Amato, A. Adipose-Derived Mesenchymal Stem Cells (ASCs) May Favour Breast Cancer Recurrence via HGF/c-Met Signaling. Oncotarget 2014, 5, 613–633. [Google Scholar] [CrossRef]
- Lv, F.; Si, W.; Xu, X.; He, X.; Wang, Y.; Li, Y.; Li, F. RUNX2 Prompts Triple Negative Breast Cancer Drug Resistance through TGF-β Pathway Regulating Breast Cancer Stem Cells. Neoplasia 2024, 48, 100967. [Google Scholar] [CrossRef]
- Choi, S.; Yu, J.; Park, A.; Dubon, M.J.; Do, J.; Kim, Y.; Nam, D.; Noh, J.; Park, K.-S. BMP-4 Enhances Epithelial Mesenchymal Transition and Cancer Stem Cell Properties of Breast Cancer Cells via Notch Signaling. Sci. Rep. 2019, 9, 11724. [Google Scholar] [CrossRef]
- Hassan, A.A.; Artemenko, M.; Tang, M.K.S.; Shi, Z.; Chen, L.-Y.; Lai, H.-C.; Yang, Z.; Shum, H.-C.; Wong, A.S.T. Ascitic Fluid Shear Stress in Concert with Hepatocyte Growth Factor Drive Stemness and Chemoresistance of Ovarian Cancer Cells via the C-Met-PI3K/Akt-MiR-199a-3p Signaling Pathway. Cell Death Dis. 2022, 13, 537. [Google Scholar] [CrossRef]
- Hsu, C.-F.; Huang, H.-S.; Chen, P.-C.; Ding, D.-C.; Chu, T.-Y. IGF-Axis Confers Transformation and Regeneration of Fallopian Tube Fimbria Epithelium upon Ovulation. EBioMedicine 2019, 41, 597–609. [Google Scholar] [CrossRef]
- McLean, K.; Gong, Y.; Choi, Y.; Deng, N.; Yang, K.; Bai, S.; Cabrera, L.; Keller, E.; McCauley, L.; Cho, K.R.; et al. Human Ovarian Carcinoma–Associated Mesenchymal Stem Cells Regulate Cancer Stem Cells and Tumorigenesis via Altered BMP Production. J. Clin. Investig. 2011, 121, 3206–3219. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, T.; Zhao, Y.; Huang, Y.; Gao, Y. Cisplatin Targets the Stromal Cell-Derived Factor-1-CXC Chemokine Receptor Type 4 Axis to Suppress Metastasis and Invasion of Ovarian Cancer-Initiating Cells. Tumor Biol. 2014, 35, 4637–4644. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Kim, M.; Gilbert, C.A.; Simpkins, F.; Ince, T.A.; Slingerland, J.M. VEGFA Activates an Epigenetic Pathway Upregulating Ovarian Cancer-initiating Cells. EMBO Mol. Med. 2017, 9, 304–318. [Google Scholar] [CrossRef] [PubMed]
- García-Rocha, R.; Monroy-García, A.; Carrera-Martínez, M.; Hernández-Montes, J.; Don-López, C.A.; Weiss-Steider, B.; Monroy-Mora, K.A.; Ponce-Chavero, M.d.l.Á.; Montesinos-Montesinos, J.J.; Escobar-Sánchez, M.L.; et al. Evidence That Cervical Cancer Cells Cultured as Tumorspheres Maintain High CD73 Expression and Increase Their Protumor Characteristics through TGF-β Production. Cell Biochem. Funct. 2022, 40, 760–772, Erratum in Cell Biochem. Funct. 2022, 40, 960. https://doi.org/10.1002/cbf.3761. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, L.; Zhou, C.; Wei, W.; Chen, X.; Yi, H.; Wu, X.; Bai, X.; Guo, S.; Yu, Y.; et al. TGF-β1-induced CK 17 Enhances Cancer Stem Cell-like Properties Rather than EMT in Promoting Cervical Cancer Metastasis via the ERK 1/2- MZF 1 Signaling Pathway. FEBS J. 2017, 284, 3000–3017. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.-M.; Zheng, P.-S.; Zhang, P. GDF15 Promotes the Proliferation of Cervical Cancer Cells by Phosphorylating AKT1 and Erk1/2 through the Receptor ErbB2. J. Exp. Clin. Cancer Res. 2018, 37, 80. [Google Scholar] [CrossRef]
- Javed, S.; Bhattacharyya, S.; Bagga, R.; Srinivasan, R. Insulin Growth Factor-1 Pathway in Cervical Carcinoma Cancer Stem Cells. Mol. Cell Biochem. 2020, 473, 51–62. [Google Scholar] [CrossRef]
- Dong, Z.; Yu, C.; Rezhiya, K.; Gulijiahan, A.; Wang, X. Downregulation of MiR-146a Promotes Tumorigenesis of Cervical Cancer Stem Cells via VEGF/CDC42/PAK1 Signaling Pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3711–3719. [Google Scholar] [CrossRef]
- Sato, M.; Kawana, K.; Fujimoto, A.; Yoshida, M.; Nakamura, H.; Nishida, H.; Inoue, T.; Taguchi, A.; Takahashi, J.; Adachi, K.; et al. Clinical Significance of Gremlin 1 in Cervical Cancer and Its Effects on Cancer Stem Cell Maintenance. Oncol. Rep. 2016, 35, 391–397. [Google Scholar] [CrossRef]
- Dong, W.; Sun, S.; Cao, X.; Cui, Y.; Chen, A.; Li, X.; Zhang, J.; Cao, J.; Wang, Y. Exposure to TNF-α Combined with TGF-β Induces Carcinogenesis in Vitro via NF-ΚB/Twist Axis. Oncol. Rep. 2017, 37, 1873–1882. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, W.-L.; Wang, P.-H.; Lee, H.-T.; Chang, W.-W. EXOSC5 Maintains Cancer Stem Cell Activity in Endometrial Cancer by Regulating the NTN4/Integrin Β1 Signalling Axis. Int. J. Biol. Sci. 2024, 20, 265–279. [Google Scholar] [CrossRef]
- Fukuda, T.; Fukuda, R.; Miyazono, K.; Heldin, C.-H. Tumor Promoting Effect of BMP Signaling in Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 7882. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; McGrail, D.J.; Dai, H.; Li, K.; Lin, S.-Y. CHD4 Mutations Promote Endometrial Cancer Stemness by Activating TGF-Beta Signaling. Am. J. Cancer Res. 2018, 8, 903–914. [Google Scholar] [PubMed]
- Lee, C.-J.; Sung, P.-L.; Kuo, M.-H.; Tsai, M.-H.; Wang, C.-K.; Pan, S.-T.; Chen, Y.-J.; Wang, P.-H.; Wen, K.-C.; Chou, Y.-T. Crosstalk between SOX2 and Cytokine Signaling in Endometrial Carcinoma. Sci. Rep. 2018, 8, 17550. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, F.; Li, Q.; Ma, H.; Zhong, J.; Zhang, H.; Cheng, S.; Wu, H.; Zhao, Y.; Wang, N.; et al. CytoSIP: An Annotated Structural Atlas for Interactions Involving Cytokines or Cytokine Receptors. Commun. Biol. 2024, 7, 630. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Stanilov, N.; Velikova, T.; Stanilova, S. Navigating the Cytokine Seas: Targeting Cytokine Signaling Pathways in Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 1009. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical Insights into Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef]
- Jones, S.A.; Bryant, C.; Lloyd, C.M.; Mcinnes, I.; O’neill, L. A Vision for Cytokine Biology with 20/20 Clarity. Function 2021, 2, zqaa042. [Google Scholar] [CrossRef]
- Wang, X.; Lupardus, P.; LaPorte, S.L.; Garcia, K.C. Structural Biology of Shared Cytokine Receptors. Annu. Rev. Immunol. 2009, 27, 29–60. [Google Scholar] [CrossRef]
- Baldo, B.A. Cytokines. In Safety of Biologics Therapy; Springer International Publishing: Cham, Switzerland, 2016; pp. 217–261. [Google Scholar]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Hobbs, K.J.; Bayless, R.; Sheats, M.K. A Comparative Review of Cytokines and Cytokine Targeting in Sepsis: From Humans to Horses. Cells 2024, 13, 1489. [Google Scholar] [CrossRef]
- Cascetta, G.; Colombo, G.; Eremita, G.; Garcia, J.G.N.; Lenti, M.V.; Di Sabatino, A.; Travelli, C. Pro- and Anti-Inflammatory Cytokines: The Hidden Keys to Autoimmune Gastritis Therapy. Front. Pharmacol. 2024, 15, 1450558. [Google Scholar] [CrossRef]
- Dentelli, P.; Rosso, A.; Calvi, C.; Ghiringhello, B.; Garbarino, G.; Camussi, G.; Pegoraro, L.; Brizzi, M.F. IL-3 Affects Endothelial Cell-Mediated Smooth Muscle Cell Recruitment by Increasing TGFβ Activity: Potential Role in Tumor Vessel Stabilization. Oncogene 2004, 23, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Wimperis, J.Z.; Niemeyer, C.M.; Sieff, C.A.; Mathey-Prevot, B.; Nathan, D.G.; Arceci, R.J. Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-3 MRNAs Are Produced by a Small Fraction of Blood Mononuclear Cells. Blood 1989, 74, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Owen, W.F.; Silberstein, D.S.; Woods, J.; Soberman, R.J.; Austen, K.F.; Stevens, R.L. Human Eosinophils Have Prolonged Survival, Enhanced Functional Properties, and Become Hypodense When Exposed to Human Interleukin 3. J. Clin. Investig. 1988, 81, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Ihle, J.N. Interleukin-3 and Hematopoiesis. Chem. Immunol. 1992, 51, 65–106. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Doublier, S.; Brizzi, M.F.; Deambrosis, I.; Albini, A.; Camussi, G. HIV-1-Tat Protein Activates Phosphatidylinositol 3-Kinase/AKT-Dependent Survival Pathways in Kaposi’s Sarcoma Cells. J. Biol. Chem. 2002, 277, 25195–25202. [Google Scholar] [CrossRef]
- Lombardo, G.; Dentelli, P.; Togliatto, G.; Rosso, A.; Gili, M.; Gallo, S.; Deregibus, M.C.; Camussi, G.; Brizzi, M.F. Activated Stat5 Trafficking Via Endothelial Cell-Derived Extracellular Vesicles Controls IL-3 Pro-Angiogenic Paracrine Action. Sci. Rep. 2016, 6, 25689. [Google Scholar] [CrossRef]
- Dentelli, P.; Rosso, A.; Olgasi, C.; Camussi, G.; Brizzi, M.F. IL-3 Is a Novel Target to Interfere with Tumor Vasculature. Oncogene 2011, 30, 4930–4940. [Google Scholar] [CrossRef]
- Uberti, B.; Dentelli, P.; Rosso, A.; Defilippi, P.; Brizzi, M.F. Inhibition of Β1 Integrin and IL-3Rβ Common Subunit Interaction Hinders Tumour Angiogenesis. Oncogene 2010, 29, 6581–6590. [Google Scholar] [CrossRef]
- Zeoli, A.; Dentelli, P.; Rosso, A.; Togliatto, G.; Trombetta, A.; Damiano, L.; di Celle, P.F.; Pegoraro, L.; Altruda, F.; Brizzi, M.F. Interleukin-3 Promotes Expansion of Hemopoietic-Derived CD45+ Angiogenic Cells and Their Arterial Commitment via STAT5 Activation. Blood 2008, 112, 350–361. [Google Scholar] [CrossRef]
- Koni, M.; Lopatina, T.; Grange, C.; Sarcinella, A.; Cedrino, M.; Bruno, S.; Buffolo, F.; Femminò, S.; Camussi, G.; Brizzi, M.F. Circulating Extracellular Vesicles Derived from Tumor Endothelial Cells Hijack the Local and Systemic Anti-Tumor Immune Response: Role of MTOR/G-CSF Pathway. Pharmacol. Res. 2023, 195, 106871. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.J.; Escarbe, S.; Tvorogov, D.; Farshid, G.; Gregory, P.A.; Khew-Goodall, Y.; Madden, S.; Ingman, W.V.; Lindeman, G.J.; Lim, E.; et al. Interleukin-3 Production by Basal-like Breast Cancer Cells Is Associated with Poor Prognosis. Growth Factors 2024, 42, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Shigdar, S.; Li, Y.; Bhattacharya, S.; O’Connor, M.; Pu, C.; Lin, J.; Wang, T.; Xiang, D.; Kong, L.; Wei, M.Q.; et al. Inflammation and Cancer Stem Cells. Cancer Lett. 2014, 345, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zuo, S.; Wang, Z.; Zhang, S.; Liu, L.; Luo, H.; Xie, Y.; Zhang, Y.; Li, D. Targeting of the IL-33/Wnt Axis Restricts Breast Cancer Stemness and Metastasis. Sci. Rep. 2025, 15, 18172. [Google Scholar] [CrossRef]
- Chen, X.; Yang, M.; Yin, J.; Li, P.; Zeng, S.; Zheng, G.; He, Z.; Liu, H.; Wang, Q.; Zhang, F.; et al. Tumor-Associated Macrophages Promote Epithelial–Mesenchymal Transition and the Cancer Stem Cell Properties in Triple-Negative Breast Cancer through CCL2/AKT/β-Catenin Signaling. Cell Commun. Signal. 2022, 20, 92. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, M.; Tu, J.; Li, F.; Deng, Q.; Xu, J.; He, X.; Ding, J.; Xia, J.; Sheng, D.; et al. PMN-MDSCs Modulated by CCL20 from Cancer Cells Promoted Breast Cancer Cell Stemness through CXCL2-CXCR2 Pathway. Signal Transduct. Target. Ther. 2023, 8, 97. [Google Scholar] [CrossRef]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.-P. Senescence-Associated IL-6 and IL-8 Cytokines Induce a Self- and Cross-Reinforced Senescence/Inflammatory Milieu Strengthening Tumorigenic Capabilities in the MCF-7 Breast Cancer Cell Line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Tang, J.; Zhang, Z.; Du, R.; Luo, D.; Liu, X.; Xia, Y.; Li, Y.; Wang, S.; et al. CCL16 Maintains Stem Cell-like Properties in Breast Cancer by Activating CCR2/GSK3β/β-Catenin/OCT4 Axis. Theranostics 2021, 11, 2297–2317, Erratum in Theranostics 2021, 11, 7128–7129. [Google Scholar] [CrossRef]
- Sarcinella, A.; Kholia, S.; Femminò, S.; Cedrino, M.; Tapparo, M.; Wen, X.; De Miglio, M.R.; Salemme, V.; Scavuzzo, A.; Poncina, M.; et al. IL-3/STAT5/MiR-155-5p Axis Supports Stem-Related Pathway Reprogramming in TNBC. Breast Cancer Res. 2025, 27, 195. [Google Scholar] [CrossRef]
- Gening, S.O.; Abakumova, T.V.; Antoneeva, I.I.; Rizvanov, A.A.; Gening, T.P.; Gafurbaeva, D.U. Stem-like Tumor Cells and Proinflammatory Cytokines in the Ascitic Fluid of Ovarian Cancer Patients. Russ. Clin. Lab. Diagn. 2021, 66, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zong, X.; Mitra, S.; Mitra, A.K.; Matei, D.; Nephew, K.P. IL-6 Mediates Platinum-Induced Enrichment of Ovarian Cancer Stem Cells. JCI Insight 2018, 3, 122360. [Google Scholar] [CrossRef]
- Ning, Y.; Cui, Y.; Li, X.; Cao, X.; Chen, A.; Xu, C.; Cao, J.; Luo, X. Co-Culture of Ovarian Cancer Stem-like Cells with Macrophages Induced SKOV3 Cells Stemness via IL-8/STAT3 Signaling. Biomed. Pharmacother. 2018, 103, 262–271. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Li, Y.; Li, M.; Lei, M.; Wu, M.; Qu, Y.; Yuan, Y.; Chen, T.; Jiang, H. Ovarian Cancer Stem Cells Promote Tumour Immune Privilege and Invasion via CCL5 and Regulatory T Cells. Clin. Exp. Immunol. 2017, 191, 60–73. [Google Scholar] [CrossRef]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 Produced by Tumor Microenvironment Promotes Self-Renewal of CD133+ Cancer Stem-like Cells in Ovarian Cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef]
- Castro-Oropeza, R.; Vazquez-Santillan, K.; Díaz-Gastelum, C.; Melendez-Zajgla, J.; Zampedri, C.; Ferat-Osorio, E.; Rodríguez-González, A.; Arriaga-Pizano, L.; Maldonado, V. Adipose-Derived Mesenchymal Stem Cells Promote the Malignant Phenotype of Cervical Cancer. Sci. Rep. 2020, 10, 14205. [Google Scholar] [CrossRef]
- Sato, M.; Kawana, K.; Adachi, K.; Fujimoto, A.; Yoshida, M.; Nakamura, H.; Nishida, H.; Inoue, T.; Taguchi, A.; Ogishima, J.; et al. Regeneration of Cervical Reserve Cell-like Cells from Human Induced Pluripotent Stem Cells (IPSCs): A New Approach to Finding Targets for Cervical Cancer Stem Cell Treatment. Oncotarget 2017, 8, 40935–40945. [Google Scholar] [CrossRef]
- Wang, K.-H.; Chang, Y.-H.; Harnod, T.; Ding, D.-C. Endometrial Cancer-Infiltrating Mesenchymal Stem Cells Exhibit Immunosuppressive Effects. Cell Transplant. 2022, 31, 9636897221104452. [Google Scholar] [CrossRef]
- Xu, Z.; Goel, H.L.; Burkart, C.; Burman, L.; Chong, Y.E.; Barber, A.G.; Geng, Y.; Zhai, L.; Wang, M.; Kumar, A.; et al. Inhibition of VEGF Binding to Neuropilin-2 Enhances Chemosensitivity and Inhibits Metastasis in Triple-Negative Breast Cancer. Sci. Transl. Med. 2023, 15, eadf1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Mi, D.; Ling, J.; Li, H.; He, P.; Liu, N.; Chen, Q.; Chen, Y.; Huang, L. Discovery of YH677 as a Cancer Stemness Inhibitor That Suppresses Triple-Negative Breast Cancer Growth and Metastasis by Regulating the TGFβ Signaling Pathway. Cancer Lett. 2023, 560, 216142, Erratum in Cancer Lett. 2023, 567, 216195. [Google Scholar] [CrossRef]
- Kar, T.; Dugam, P.; Shivhare, S.; Shetty, S.R.; Choudhury, S.; Sen, D.; Deb, B.; Majumdar, S.; Debnath, S.; Das, A. Epidermal Growth Factor Receptor Inhibition Potentiates Chemotherapeutics-mediated Sensitization of Metastatic Breast Cancer Stem Cells. Cancer Rep. 2024, 7, 2049. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, Y.-T.; Hsieh, H.-C.; Yu, Y.; Yu, A.L.; Chang, W.-W. Epidermal Growth Factor/Heat Shock Protein 27 Pathway Regulates Vasculogenic Mimicry Activity of Breast Cancer Stem/Progenitor Cells. Biochimie 2014, 104, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.W.; Choi, D.K.; Yu, J.H.; Kim, J.H.; Lee, D.-S.; Min, S.-H. Poziotinib Suppresses Ovarian Cancer Stem Cell Growth via Inhibition of HER4-Mediated STAT5 Pathway. Biochem. Biophys. Res. Commun. 2020, 526, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Yang, K.; Taylor-Harding, B.; Wiedemeyer, W.R.; Buckanovich, R.J. VEGFR3 Inhibition Chemosensitizes Ovarian Cancer Stemlike Cells through Down-Regulation of BRCA1 and BRCA2. Neoplasia 2014, 16, 343–353.e2. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Kim, J.Y.; Song, C.H.; Kim, M.; Do, Y.T.; Vo, T.T.L.; Choi, E.; Ha, E.; Seo, J.H.; Shin, S.-J. The FGFR Family Inhibitor AZD4547 Exerts an Antitumor Effect in Ovarian Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10817. [Google Scholar] [CrossRef]
- Wen, H.; Qian, M.; He, J.; Li, M.; Yu, Q.; Leng, Z. Inhibiting of Self-Renewal, Migration and Invasion of Ovarian Cancer Stem Cells by Blocking TGF-β Pathway. PLoS ONE 2020, 15, e0230230. [Google Scholar] [CrossRef]
- Chhabra, R. Let-7i-5p, MiR-181a-2-3p and EGF/PI3K/SOX2 Axis Coordinate to Maintain Cancer Stem Cell Population in Cervical Cancer. Sci. Rep. 2018, 8, 7840. [Google Scholar] [CrossRef]
- Lv, Y.; Cang, W.; Li, Q.; Liao, X.; Zhan, M.; Deng, H.; Li, S.; Jin, W.; Pang, Z.; Qiu, X.; et al. Erlotinib Overcomes Paclitaxel-Resistant Cancer Stem Cells by Blocking the EGFR-CREB/GRβ-IL-6 Axis in MUC1-Positive Cervical Cancer. Oncogenesis 2019, 8, 70. [Google Scholar] [CrossRef]
- Qu, N.; Li, Z.; Wei, J.; Yang, Y.; Meng, Y.; Gao, Y. Bevacizumab Increases Cisplatin Efficacy by Inhibiting Epithelial–Mesenchymal Transition via ALDH1 in Cervical Carcinoma. Int. Immunopharmacol. 2025, 145, 113736. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Tang, X.; Du, X.; Wang, G.; Zhang, H.; Ye, F.; Liu, T. Superparamagnetic Iron Oxide Nanoparticle-Mediated Expression of miR-326 Inhibits Human Endometrial Carcinoma Stem Cell Growth. Int. J. Nanomed. 2019, 14, 2719–2731. [Google Scholar] [CrossRef]
- Shang, C.; Lang, B.; Meng, L.-R. Blocking NOTCH Pathway Can Enhance the Effect of EGFR Inhibitor through Targeting CD133+ Endometrial Cancer Cells. Cancer Biol. Ther. 2018, 19, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, L.; Peng, X.; Tu, J.; Li, S.; He, X.; Li, F.; Qiang, J.; Dong, H.; Deng, Q.; et al. IL1R2 Blockade Alleviates Immunosuppression and Potentiates Anti-PD-1 Efficacy in Triple-Negative Breast Cancer. Cancer Res. 2024, 84, 2282–2296. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K.; Das, S.K.; Azab, B.; Menezes, M.E.; Dent, P.; Wang, X.-Y.; Sarkar, D.; Fisher, P.B. Targeting Breast Cancer-Initiating/Stem Cells with Melanoma Differentiation-Associated Gene-7/Interleukin-24. Int. J. Cancer 2013, 133, 2726–2736, Erratum in Int. J. Cancer 2016, 139, E3. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Bhatia, S.; Bhoopathi, P.; Das, S.K.; Emdad, L.; Dasgupta, S.; Dent, P.; Wang, X.-Y.; Sarkar, D.; Fisher, P.B. MDA-7/IL-24: Multifunctional Cancer Killing Cytokine. Adv. Exp. Med. Biol. 2014, 818, 127–153. [Google Scholar]
- Tang, S.; Xiang, T.; Huang, S.; Zhou, J.; Wang, Z.; Xie, R.; Long, H.; Zhu, B. Ovarian Cancer Stem-like Cells Differentiate into Endothelial Cells and Participate in Tumor Angiogenesis through Autocrine CCL5 Signaling. Cancer Lett. 2016, 376, 137–147. [Google Scholar] [CrossRef]
- Gil, M.; Komorowski, M.P.; Seshadri, M.; Rokita, H.; McGray, A.J.R.; Opyrchal, M.; Odunsi, K.O.; Kozbor, D. CXCL12/CXCR4 Blockade by Oncolytic Virotherapy Inhibits Ovarian Cancer Growth by Decreasing Immunosuppression and Targeting Cancer-Initiating Cells. J. Immunol. 2014, 193, 5327–5337. [Google Scholar] [CrossRef]
- Liao, T.; Kaufmann, A.M.; Qian, X.; Sangvatanakul, V.; Chen, C.; Kube, T.; Zhang, G.; Albers, A.E. Susceptibility to Cytotoxic T Cell Lysis of Cancer Stem Cells Derived from Cervical and Head and Neck Tumor Cell Lines. J. Cancer Res. Clin. Oncol. 2013, 139, 159–170. [Google Scholar] [CrossRef]
- Wang, D.; Upadhyaya, B.; Liu, Y.; Knudsen, D.; Dey, M. Phenethyl Isothiocyanate Upregulates Death Receptors 4 and 5 and Inhibits Proliferation in Human Cancer Stem-like Cells. BMC Cancer 2014, 14, 591. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Lv, Q.; Shi, C.; Qiu, M.; Xie, L.; Liu, W.; Yang, B.; Shan, W.; Cheng, Y.; et al. Endometrium-Derived Mesenchymal Stem Cells Suppress Progression of Endometrial Cancer via the DKK1-Wnt/β-Catenin Signaling Pathway. Stem Cell Res. Ther. 2023, 14, 159. [Google Scholar] [CrossRef]
- van der Zee, M.; Sacchetti, A.; Cansoy, M.; Joosten, R.; Teeuwssen, M.; Heijmans-Antonissen, C.; Ewing-Graham, P.C.; Burger, C.W.; Blok, L.J.; Fodde, R. IL6/JAK1/STAT3 Signaling Blockade in Endometrial Cancer Affects the ALDHhi/CD126+ Stem-like Component and Reduces Tumor Burden. Cancer Res. 2015, 75, 3608–3622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarcinella, A.; Guerra Villacis, J.S.; Brizzi, M.F. Fueling the Seed: Growth Factors and Cytokines Driving Cancer Stem Cells in Gynecological Malignancies. Int. J. Mol. Sci. 2025, 26, 11462. https://doi.org/10.3390/ijms262311462
Sarcinella A, Guerra Villacis JS, Brizzi MF. Fueling the Seed: Growth Factors and Cytokines Driving Cancer Stem Cells in Gynecological Malignancies. International Journal of Molecular Sciences. 2025; 26(23):11462. https://doi.org/10.3390/ijms262311462
Chicago/Turabian StyleSarcinella, Alessandro, Juan Sebastian Guerra Villacis, and Maria Felice Brizzi. 2025. "Fueling the Seed: Growth Factors and Cytokines Driving Cancer Stem Cells in Gynecological Malignancies" International Journal of Molecular Sciences 26, no. 23: 11462. https://doi.org/10.3390/ijms262311462
APA StyleSarcinella, A., Guerra Villacis, J. S., & Brizzi, M. F. (2025). Fueling the Seed: Growth Factors and Cytokines Driving Cancer Stem Cells in Gynecological Malignancies. International Journal of Molecular Sciences, 26(23), 11462. https://doi.org/10.3390/ijms262311462






