Genetic Polymorphisms and Predisposition to Peri-Implantitis: A Systematic Review
Abstract
1. Introduction
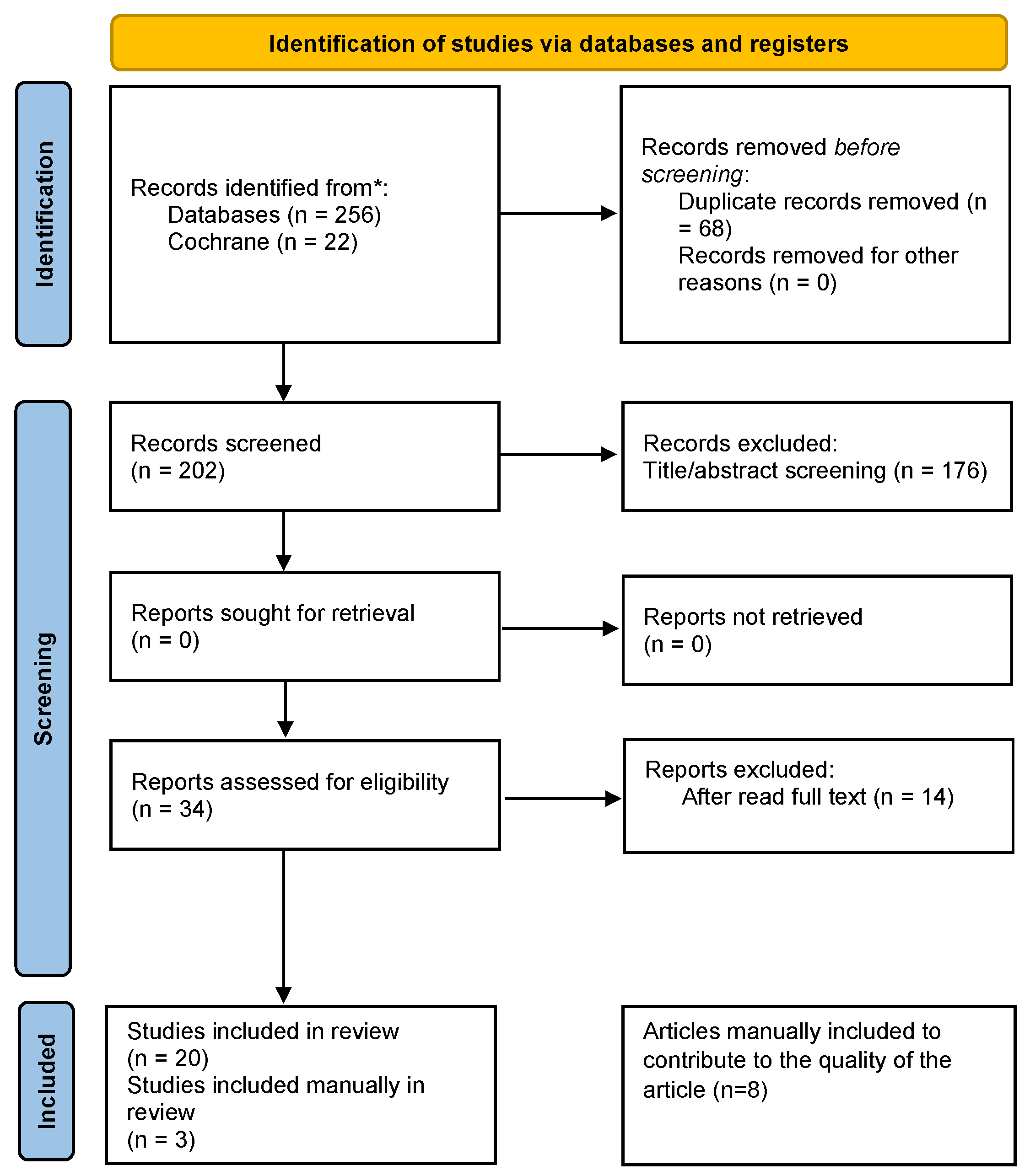
2. Materials and Methods
Strategy and Sources
3. Results
Summary of Evidence Synthesis
4. Discussion
4.1. Polymorphisms
4.1.1. The Interleukins
4.1.2. The RANKL
4.1.3. The Matrix Metalloproteinases
4.1.4. The Tumor Necrosis Factor
4.1.5. The Bone Morphogenetic Proteins
4.1.6. Other Genes
4.2. Association of Genetic Polymorphisms with Peri-Implantitis
4.3. Integrated Scope Analysis
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | Aryl Hydrocarbon Receptor |
| BMP | Bone Morphogenetic Protein |
| BRIMP | Bone Morphogenetic/Retinoic Acid-Inducible Neural-Specific Protein |
| CD | Differentiation Cluster |
| CRP | C-reactive Protein |
| CXCR | C-X-C Chemokine Receptor |
| DNase I | Deoxyribonuclease I |
| EGF | Epidermal Growth Factor |
| ELISA | Enzyme-linked Immunosorbent Assay |
| ET-1 | Endothelin 1 |
| FGF | Fibroblast Growth Factor |
| FGFR | Fibroblast Growth Factor Receptor |
| fMLP | N-Formyl-Methionyl-Leucyl-Phenylalanine |
| FPR | Formyl Peptide Receptor |
| GBP | Guanylate-Binding Proteins |
| GPCR | G protein-coupled Receptor |
| GWAS | Genome-wide Association Study |
| IL | Interleukin |
| IP | Peri-implantitis |
| JBI | Joanna Briggs Institute |
| MMP | Matrix Metalloproteinase |
| ORs | Odds Ratios |
| OPG | Osteoprotegerin |
| PCR | C-reactive Protein Test |
| PICO | Patient, Intervention, Comparison, Outcome |
| RANK | Receptor Activator of Nuclear Factor xB |
| RANKL | Receptor Activator of Nuclear Factor xB Ligand |
| RT-qPCR | Real-time Reverse Transcription Reaction |
| RUNX | Runt-related Transcription Factor |
| SNP | Single-Nucleotide Polymorphism |
| TGF | Tumor Growth Factor |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| TNF | Tumor Necrosis Factor |
| WBC | White Blood Cell |
References
- Ribeiro, R.; Melo, R.; Tortamano Neto, P.; Vajgel, A.; Souza, P.R.; Cimões, R. Polymorphisms of Il-10 (−1082) and RANKL (−438) Genes and the Failure of Dental Implants. Int. J. Dent. 2017, 2017, 3901368. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Taira, J.; Andriani, F.; Watinaga, S.; Bastos, M.F.; Shibli, J.A. Evaluation of IL-4, MIP-1α, and MMP-9 gene expression levels in peri-implant tissues in peri-implantitis. Braz. Dent. J. 2023, 34, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, M.; Firatli, E. The study of genetic predisposition on periodontitis and peri-implantitis. Niger. J. Clin. Pract. 2022, 25, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Y. Osteoprotegerin Gene (OPG) Polymorphisms Associated with Peri-implantitis Susceptibility in a Chinese Han Population. Med. Sci. Monit. 2016, 22, 4271–4276. [Google Scholar] [CrossRef]
- Santostasi, N.; Gerardi, D.; Rinaldi, F.; Bernardi, S.; Bianchi, I.; Pinchi, V.; Piattelli, M.; Varvara, G. Relationship between interleukin 1 (IL-1) genetic polymorphism and periimplantitis: Systematic literature review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 3566–3582. [Google Scholar] [CrossRef]
- He, K.; Jian, F.; He, T.; Tang, H.; Huang, B.; Wei, N. Analysis of the association of TNF-α, IL-1A, and IL-1B polymorphisms with peri-implantitis in a Chinese non-smoking population. Clin. Oral Investig. 2020, 24, 693–699. [Google Scholar] [CrossRef]
- Junior, R.G.; Pinheiro, A.d.R.; Schoichet, J.J.; Nunes, C.H.R.; Gonçalves, R.; Bonato, L.L.; Quinelato, V.; Antunes, L.S.; Küchler, E.C.; Lobo, J.; et al. MMP13, TIMP2 and TGFB3 Gene Polymorphisms in Brazilian Chronic Periodontitis and Periimplantitis Subjects. Braz. Dent. J. 2016, 27, 128–134. [Google Scholar] [CrossRef]
- Petkovic-Curcin, A.; Zeljic, K.; Cikota-Aleksic, B.; Dakovic, D.; Tatic, Z.; Magic, Z. Association of Cytokine Gene Polymorphism with Peri-implantitis Risk. Int. J. Oral Maxillofac. Implant. 2017, 32, e241–e248. [Google Scholar] [CrossRef]
- Rakic, M.; Petkovic-Curcin, A.; Struillou, X.; Matic, S.; Stamatovic, N.; Vojvodic, D. CD14 and TNFα single nucleotide polymorphisms are candidates for genetic biomarkers of peri-implantitis. Clin. Oral Investig. 2015, 19, 791–801. [Google Scholar] [CrossRef]
- Li, J.; Qiao, X.; Shang, J. Association analysis between CD14 gene polymorphisms and peri-implantitis susceptibility in a Chinese population. Immun. Inflamm. Dis. 2024, 12, e1230. [Google Scholar] [CrossRef]
- Chang, Z.; Jiang, D.; Zhang, S.; Pei, D.; Zhang, Z.; Zhang, L.; Cai, J.; Cao, J. Genetic association of the epidermal growth factor gene polymorphisms with peri-implantitis risk in Chinese population. Bioengineered 2021, 12, 8468–8475. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Ibáñez-de-Mendoza, I.; Marichalar-Mendia, X.; Setién-Olarra, A.; García-de-la-Fuente, A.M.; Martínez-Conde-Llamosas, R.; Aguirre-Urizar, J.M. Genetic polymorphisms of inflammatory and bone metabolism related proteins in a population with dental implants of the Basque Country. A case-control study. BMC Oral Health 2024, 24, 659. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.E.; Reis, M.B.L.; Arid, J.; Flores, E.K.B.; Cruz, G.V.; Marañón-Vásquez, G.A.; Souza, L.K.F.; Novaes, A.B.; Queiroz, A.M., Jr.; Küchler, E.C. Association between Genetic Polymorphisms in RANK, RANKL and OPG and Peri-Implant Diseases in Patients from the Amazon Region. Braz. Dent. J. 2020, 31, 63–68. [Google Scholar] [CrossRef]
- Coelho, R.B.; Gonçalves, R.; Junior Villas-Boas, R.d.e.M.; Bonato, L.L.; Quinelato, V.; Pinheiro, A.d.a.R.; Machado, A.; Nunes, C.H.; Gonçalves, R.; Vieira, A.R.; et al. Haplotypes in BMP4 and FGF Genes Increase the Risk of Peri-Implantitis. Braz. Dent. J. 2016, 27, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.; Ribeiro, A.C.; Palos, C.; Proença, L.; Noronha, S.; Alves, R.C. Association between IL-1A and IL-1B gene polymorphisms with peri-implantitis in a Portuguese population—A pilot study. PeerJ 2022, 10, e13729. [Google Scholar] [CrossRef]
- Saremi, L.; Shafizadeh, M.; Esmaeilzadeh, E.; Ghaffari, M.E.; Mahdavi, M.H.; Amid, R.; Kadkhodazadeh, M. Assessment of IL-10, IL-1ß and TNF-α gene polymorphisms in patients with peri-implantitis and healthy controls. Mol. Biol. Rep. 2021, 48, 2285–2290. [Google Scholar] [CrossRef]
- Talib, E.Q.; Taha, G.I. Involvement of interlukin-17A (IL-17A) gene polymorphism and interlukin-23 (IL-23) level in the development of peri-implantitis. BDJ Open 2024, 10, 12. [Google Scholar] [CrossRef]
- Gao, X.; Ha, Y. Correlation Between miR-27a-3p Polymorphisms and Peri-Implantitis Susceptibility: A Case-Control Study. Int. Dent. J. 2025, 75, 1921–1928. [Google Scholar] [CrossRef]
- Qi, Y.; Li, C.; Du, Y.; Lin, J.; Li, N.; Yu, Y. Chemokine Receptor 2 (CXCR2) Gene Polymorphisms and Their Association with the Risk of Developing Peri-Implantitis in Chinese Han Population. J. Inflamm. Res. 2021, 14, 1625–1631. [Google Scholar] [CrossRef]
- Saremi, L.; Shahbazi, S.; Ghaffari, M.E.; Esmaeili, S.; Lotfipanah, S.; Amid, R.; Kadkhodazadeh, M. The Association of Matrix Metalloproteinase-1, -2, -3, -7, and -13 Gene Polymorphisms with Peri-Implantitis in an Iranian Population: A Case-Control Study. Clin. Exp. Dent. Res. 2024, 10, e70049. [Google Scholar] [CrossRef]
- Martins, L.R.L.; Grzech-Leśniak, K.; Castro Dos Santos, N.; Suárez, L.J.; Giro, G.; Bastos, M.F.; Shibli, J.A. Transcription Factor AhR, Cytokines IL-6 and IL-22 in Subjects with and without Peri-Implantitis: A Case Control-Study. Int. J. Environ. Res. Public Health 2022, 19, 7434. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Fu, Y.; Zhao, D.; Wang, L.; Wang, Y.; Hu, R.; Fu, R. Association of MMP-8 rs11225395 Polymorphism with the Susceptibility of Peri-Implantitis. Oral Health Prev. Dent. 2025, 23, 427–433. [Google Scholar] [CrossRef]
- Fragkioudakis, I.; Kottaridi, C.; Doufexi, A.E.; Papadimitriou, K.; Batas, L.; Sakellari, D. Association of MMP-8 -799C/T Polymorphism with Peri-Implantitis: A Cross-Sectional Study. J. Pers. Med. 2025, 15, 182. [Google Scholar] [CrossRef]
- Saito, Y.; Nodai, T.; Munemasa, T.; Mukaibo, T.; Kondo, Y.; Masaki, C.; Hosokawa, R. Diagnostic potential of endothelin-1 in peri-implant diseases: A cross-sectional study. Int. J. Implant Dent. 2024, 10, 32. [Google Scholar] [CrossRef]
- Wang, D.; Tan, J.; Zhu, H.; Mei, Y.; Liu, X. Biomedical Implants with Charge-Transfer Monitoring and Regulating Abilities. Adv. Sci. 2021, 8, e2004393. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60, Erratum in J. Clin. Periodontol. 2021, 48, 163. [Google Scholar] [CrossRef]
- Pimentel, S.P.; Shiota, R.; Cirano, F.R.; Casarin, R.C.V.; Pecorari, V.G.A.; Casati, M.Z.; Haas, A.N.; Ribeiro, F.V. Occurrence of peri-implant diseases and risk indicators at the patient and implant levels: A multilevel cross-sectional study. J. Periodontol. 2018, 89, 1091–1100. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.; Sculean, A. Concepts for prevention of complications in implant therapy. Periodontol. 2000 2019, 81, 7–17. [Google Scholar] [CrossRef]
- Chmielewski, M.; Pilloni, A. Current Molecular, Cellular and Genetic Aspects of Peri-Implantitis Disease: A Narrative Review. Dent. J. 2023, 11, 134. [Google Scholar] [CrossRef]
- Razzouk, S. Regulatory elements and genetic variations in periodontal diseases. Arch. Oral Biol. 2016, 72, 106–115. [Google Scholar] [CrossRef] [PubMed]
- AlMoharib, H.S.; AlRowis, R.; AlMubarak, A.; Waleed Almadhoon, H.; Ashri, N. The relationship between matrix metalloproteinases-8 and peri-implantitis: A systematic review and meta-analysis. Saudi Dent. J. 2023, 35, 283–293. [Google Scholar] [CrossRef] [PubMed]
| Patient | Individuals receiving dental implants |
| Intervention | Dental implant placement |
| Comparison | Genetic alterations vs. absence associated with peri-implantitis |
| Outcome | Susceptibility to developing peri-implantitis |
| Author/Year | Study Type | Population (Country) | Gene/Polymorphism | Main Finding | Effect |
|---|---|---|---|---|---|
| Gonçalves Junior et al., 2016 [7] | Prospective | Brazil | MMP13, TIMP2, TGFB3 | No significant associations found | No association |
| He K. et al., 2020 [6] | Case–control | China | IL-1A (−889 C/T), IL-1B (+3954 C/T), TNF-α (−308 G/A) | IL-1A and IL-1B polymorphisms increase risk; TNF-α not significant | Increased risk |
| Zhou et al., 2016 [4] | Case–control | China | OPG rs2073617, rs2073618 | CC genotype (rs2073618) linked to higher IP risk; rs2073617 not significant | Increased risk |
| Ribeiro et al., 2017 [1] | Case–control | Brazil | IL-10 (−1082 A/G), RANKL (−438 A/G) | No association with implant failure | No association |
| Silva et al., 2020 [13] | Case–control | Brazil | RANK, RANKL, OPG | No significant associations; environmental factors more influential | No association |
| Rakic et al., 2015 [9] | Population-based | Southeastern Europe | CD14-159 C/T, TNFα (−308 A/G) | CC (CD14) and AG (TNFα) genotypes associated with 5-fold higher risk of IP | Increased risk |
| Coelho et al., 2016 [14] | Prospective | Brazil | BMP4, FGF3, FGF10, FGFR1 | BMP4 and FGF10 haplotypes associated with IP; FGF3 TT + CT genotypes linked to healthy implants | Increased risk |
| Chang et al., 2021 [11] | Case–control | China | EGF rs2237051, rs4444903 | GG genotype (rs2237051) protective; (rs4444903) not significant | Protective effect |
| Cardoso et al., 2022 [15] | Case–control | Portugal | IL-1A (−889), IL-1B (+3954) | Mutated alleles more frequent in IP group | Increased risk |
| Petkovic-Curcin et al., 2017 [8] | Case–control | Serbia | CD14, TNFα, IL6, IL10, IL1ra | TNFα-308 GA/AA increase risk; CD14-159 CT/TT protective | Mixed (Risk and Protective) |
| Saremi et al., 2021 [16] | Case–control | Iran | IL-10 (−819 C/T, −592 C/A), IL-1ß (+3954 C/T), TNF-α (−857 G/A, −308 G/A) | IL-10 and IL-1ß variants increase risk; TNF-α not associated | Increased risk |
| Turkmen et al., 2022 [3] | Prospective | Turkey | FPR1 | G/C genotype associated with higher IP risk | Increased risk |
| Li et al., 2024 [10] | Case–control | China | CD14 rs2569190 | GG genotype and G allele increase risk | Increased risk |
| Talib et al., 2024 [17] | Cross sectional | Iraq | IL-17A rs2275913, IL-23 | A/A and G/A genotypes increase risk; IL-23 elevated in IP | Increased risk |
| Giro et al., 2023 [2] | Case–control | Brazil | IL-4, MIP-1α, MMP-9 | IL-4 expression 18× higher in IP tissues | Increased risk |
| Gao et al., 2025 [18] | Case–control | China | miR-27a-3p rs895819 | GG genotype and G allele increase IP susceptibility | Increased risk |
| Qi et al., 2021 [19] | Prospective | China | CXCR2 rs2230054, rs1126580 | CT and AG genotypes increase risk of IP | Increased risk |
| Saremi et al., 2024 [20] | Case–control | Iran | MMP-1, -2, -3, -7, -13 | MMP-3 and MMP-7 variants increase risk | Increased risk |
| Martins et al., 2022 [21] | Case–control | Brazil | AhR, IL-22, IL-6 | Higher AhR and IL-6 expression in IP tissues | Increased risk |
| Jin et al., 2025 [22] | Case–control | China | MMP-8 rs11225395 | T allele (TC/TT) increases risk of IP susceptibility; higher MMP-8 expression | Increased risk |
| Fragkioudakis et al., 2025 [23] | Cross sectional | Greece | MMP-8 (799C/T, −381A/G, +17C/G) | T allele (−799C/T) associated with IP | Increased risk |
| Lafuente Ibañez de Mendoza et al., 2024 [12] | Case–control | Spain | GBP1 rs7911, BRINP3 rs1935881, OPG rs2073617, BMP-4 rs17563, FGF-3 rs1893047 | SNPs linked to IP, especially in smokers and diabetics | Increased risk |
| Saito et al., 2024 [24] | Cross sectional | Japan | ET-1, IL-1β | ET-1 elevated in IP/mucositis; potential diagnostic biomarker | Increased risk |
| Author/Year | Type of Study | Objective | Population | Results | Conclusions |
| Rakic et al., 2015 [9] | Population Study | To investigate whether the SNPs CD14-159 C/T and TNFα −308 A/G are associated with IP. | Southeastern European Caucasians | Analysis of the CD14-159 C/T polymorphism showed that the CC genotype was associated with IP, with a five-fold increased risk in these carriers. For the TNFα−308 A/G polymorphism, the AG genotype was also associated with IP, with a five-fold increased risk. | The CD14-159 C/T and TNFα −308 A/G polymorphisms are associated with IP and may represent biomarkers for peri-implant disease. |
| Coelho et al., 2016 [14] | Prospective Study | Analyzing the correlation between the BMP4, FGF3, FGF10 and FGFR1 genes and peri-implant bone loss. | Brazilian population | The TT polymorphic genotype for BMP4 rs2761884 was associated with healthy peri-implants. The FGF3 rs4631909 genotype (TT + CT genotypes) also showed an association with the control group. The frequency of the C allele for FGF3 rs4631909 showed a trend towards association with IP. The haplotypes FGF10 CCTG (p = 0.03), BMP4 GAAA (p = 0.05), and GGGA (p = 0.02) were associated with IP. | The BMP4 and FGF10 haplotypes are associated with IP. |
| Gonçalves Junior et al., 2016 [7] | Prospective Study | To evaluate possible associations between polymorphisms in the MMP13, TIMP2, and TGFB3 genes and IP. | Brazilian population | No significant associations were found between the polymorphisms in the genes studied and the development of IP. | The polymorphisms studied showed no direct relationship with IP. |
| Zhou et al., 2016 [4] | Case–control Study | To evaluate the association between the T950C (rs2073617) and G1181C (rs2073618) polymorphisms of the OPG gene and susceptibility to IP | Chinese Han population | The results of the study showed that people with the CC genotype of rs2073618 are more likely to have IP than carriers of the GG genotype. In addition, patients with the C allele have a 1.47 times greater risk of developing IP, but there was no association with the rs2073617 polymorphism. The frequency of the G-C haplotype of rs2073618-rs2073617 was significantly correlated with greater susceptibility to IP. | The OPG rs2073618 polymorphism may be related to a risk of IP, but not rs2073617. |
| Petkovic-Curcin et al., 2017 [8] | Case–control study | Analyze the association of polymorphisms in cytokine genes (CD14, TNFα, IL6, IL10, IL1ra) with the risk of IP. | Serbian population | Smokers (OR 3.28) and history of periodontitis (OR 6.33) increased the risk of IP; TNFα-308 GA/AA associated with higher risk (OR 8.89) and CD14-159 CT/TT protective genotype (OR 0.059). | Smoking and the TNFα-308 GA/AA genotype increase risk, while CD14-159 CT/TT reduces susceptibility to peri-implantitis. |
| Ribeiro R. et al., 2017 [1] | Case–control Study | Investigate the association between IL-10 (−1082 A/G) and RANKL (−438 A/G) genetic polymorphisms and dental implant failure. | Brazilian population | No statistically significant difference was found between implant failure and the genotypes or allele frequencies of IL-10 (−1082) or RANKL (−438). | No association was found between the RANKL (−438) and IL-10 (−1082) genetic polymorphisms and implant failure. |
| He K. et al., 2020 [6] | Case–control Study | To ascertain the association between genetic polymorphisms in the TNF-α (−308 G/A), IL-1A (−889 C/T), and IL-1B (+3954 C/T) genes and the risk and severity of peri-implantitis in a non-smoking population. | Chinese population | Genotypes with the T allele in IL-1A (−889 C/T) and IL-1B (+3954 C/T) are significantly associated with an increased risk of peri-implantitis (ORs: 2.0–2.5 for CT and TT vs. CC). The TNF-α (−308 G/A) polymorphism showed no significant association. | The IL-1A −889C/T and IL-1B +3954C/T polymorphisms appear to be genetic markers of susceptibility to peri-implantitis, also influencing clinical severity; meanwhile, the TNF-α −308G/A polymorphism does not appear to be relevant. |
| Silva et al., 2020 [13] | Case–control Study | Investigate polymorphisms in RANK (rs3826620), RANKL (rs9594738), and OPG (rs2073618) and their relationship with mucositis and peri-implantitis. | Brazilian population | There was no significant association between polymorphisms and peri-implantitis/mucositis. | In this population, clinical and environmental factors have a greater influence than genetic polymorphisms on the development of peri-implantitis. |
| Qi et al., 2021 [19] | Prospective study | To investigate the role of chemokine receptor 2 (CXCR2) gene polymorphisms in IP susceptibility. | Chinese Han population | The CT genotype of rs2230054 and the AG genotype and G allele of rs1126580 significantly increased in IP patients compared with healthy implants. | The CXCR2 gene rs220054 and rs1126580 polymorphisms were associated with the IP susceptibility. The CT genotype of rs2230054 and the AG genotype and G allele of rs1126580 serve as risk factors for the occurrence of IP. |
| Saremi et al., 2021 [16] | Case–control Study | Evaluate the frequency of SNPs in the IL-10, IL-1ß, and TNF-α genes in patients with IP and healthy controls. | Iranian population | The analysis revealed that the allele and genotype frequencies of IL-10 −819 C/T, IL-10 −592 C/A, and IL-1ß + 3954 C/T differed significantly between patients with IP and healthy controls. However, no significant association was observed between the TNF-α −857 G/A and TNF-α −308 G/A polymorphisms and IP. | The genetic polymorphisms IL-10 −819 C/T, IL-10 −592 C/A, and IL-1ß + 3954 C/T may play a role in the pathogenesis of IP and increase the risk of its occurrence. |
| Chang et al., 2021 [11] | Case–control Study | The association of epidermal growth factor (EGF) gene polymorphisms with susceptibility to IP. | Chinese population | The GG genotype and the G allele of rs2237051 proved to be more frequent in the IP group compared to the healthy implant group. Compared to carriers of the AA genotype, carriers of the GG genotype of rs2237051 had a lower risk of IP. There was no significant difference in the rs4444903 genotype between cases and controls. | The EGF rs2237051 polymorphism showed a significant association with IP. The GG genotype of rs2237051 and the G allele may be protective factors for the development of IP. |
| Martins et al., 2022 [21] | Case–control Study | It evaluated the expression of AhR, IL-22, and IL-6 in the peri-implant tissues of healthy patients and those with IP. | Brazilian population | Higher levels of AhR and IL-6 expression were observed in the IP tissues. IL-22 expression levels did not differ between the groups. | Higher levels of AhR and IL-6 expression were detected in the soft tissues of patients with IP. |
| Turkmen et al., 2022 [3] | Prospective study | To assess whether there is a solid genetic predisposition that causes the formation of IP. | Turkish population | The polymorphism of the fMLP receptor gene (FPR1) creates a significant difference in individuals with a higher risk of IP. | The results showed that individuals with the G/C genotype have a higher risk of IP. |
| Cardoso et al., 2022 [15] | Case–control Study | To assess whether individuals with SNPs in the IL-1A (rs1800587) and IL-1B (rs1143634) genes are more susceptible to developing IP. | Portuguese population | For the IL-1A polymorphism (−889), it was observed that the mutated allele was present in a higher percentage in the IP group compared to the control group. For the IL-1B polymorphism (+3954), the altered allele was also present in a higher percentage in the IP group than in the control group. | There is an association between the IL-1A (−889) and IL-1B (+3954) polymorphisms and IP. |
| Giro et al., 2023 [2] | Case–control Study | Evaluate the expression levels of IL-4, MIP-1α, and MMP-9 in healthy peri-implant tissue and in IP. | Brazilian Population | IL-4 expression showed higher values (18×) in the group of patients with IP compared to the healthy group. | In the tissues affected by IP, only IL-4 levels were increased when compared to the tissues in the control group. |
| Li et al., 2024 [10] | Case–control Study | To assess the genetic correlation of CD14 gene polymorphisms with predisposition to IP. | Chinese population | A high percentage of carriers of the GG rs2569190 genotype or G allele was observed in the IP group compared to the control group. Carriers of the GG rs2569190 genotype had a higher risk of developing IP. | The rs2569190 polymorphism of the CD14 gene was associated with a predisposition to IP in the Chinese population, with the GG genotype and the G allele being risk factors for the development of IP. |
| Lafuente Ibañez de Mendoza et al., 2024 [12] | Case–control Study | To evaluate whether certain single-nucleotide polymorphisms (SNPs) of genes related to inflammation and bone metabolism are associated with the presence of peri-implantitis. | Spanish population | The GBP1 rs7911 and BRINP3 rs1935881 SNPs were significantly more frequent in patients with IP. In patients with IP who smoked >10 cigarettes/day, a higher prevalence of the OPG rs2073617 SNP was observed. In patients with IP and type II diabetes mellitus, the BMP-4 rs17563 and FGF-3 rs1893047 SNPs were more frequent. | SNPs in GBP1, BRINP3, OPG, BMP-4, and FGF-3 could serve as risk markers, especially in subgroups with comorbidities (diabetes) or habits (smoking). |
| Saremi et al., 2024 [20] | Case–control Study | Evaluating the association between genetic polymorphisms of matrix metalloproteinases (MMP-) 1, -2, -3, -7, and -13 with IP. | Iranian population | The MMP-3 (−1171 5A/6A) and MMP-7 (−181 A/G) polymorphisms showed significant differences between patients with IP and healthy controls. However, the genetic polymorphisms of MMP-1 (−1607 1G/2G), MMP-2 (−1306 C/T), and MMP-13 (−77 A/G) showed no differences in prevalence between the two groups. | The MMP-3 (−1171 5A/6A) and MMP-7 (−181 A/G) polymorphisms showed differences when comparing patients with IP and healthy controls from the study population. |
| Talib et al., 2024 [17] | Cross-sectional study | To identify the role of the immune-genetic variation in IL-17A and related inflammatory cytokine (IL-23) in the initiation and progression of IP. | Iraqi population | A significant elevation in the mean level of IL-23 in the IP patient group, higher than its level in the successful implant and control groups, was observed. The A/A and G/A genotypes were significantly associated with IP increased risk. | IL-17A gene polymorphism may play a role in IP disease susceptibility, especially in persons carrying the rs2275913 A allele at a higher risk of developing IP as compared with those carrying the G allele. |
| Saito et al., 2024 [24] | Cross-sectional Study | To evaluate the potential of Endothelin-1 (ET-1) as a biomarker for diagnosing peri-implant diseases. | Japanese population | ET-1 levels were significantly elevated in the IP compared with the healthy group, and highest in the IP mucositis group. IL-1β levels were significantly higher in the IP group than in the healthy group. ET-1 exhibited superior area under the curve values, sensitivity, and specificity compared to those of IL-1β. | The presence of ET-1 plays a role in IP diseases. Its significantly increased expression IP mucositis indicates its potential for enabling earlier and more accurate assessments of IP inflammation when combined with conventional examination methods. |
| Fragkioudakis et al., 2025 [23] | Cross-sectional study | To explore the relationship between matrix metalloproteinase−8 (MMP−8) gene polymorphisms (−799C/T, −381A/G, and +17C/G) and IP. | Greek population | The −799C/T polymorphism significantly associated with IP, with T allele carriers having a higher diagnosis rate. Although T allele carriers exhibited higher mean values for the probing depth, clinical attachment level, and bleeding on probing, these differences were not statistically significant across genotypes. No associations found between the −381A/G and +17C/G polymorphisms and IP clinical parameters. | The −799C/T polymorphism, specifically the T allele, is strongly linked to IP, indicating its potential as a genetic marker for disease susceptibility. |
| Jin et al., 2025 [22] | Case–control study | To investigate the association between the MMP-8 rs11225395 polymorphism and IP in the Chinese Han population. | Chinese Han population | Rs11225395 T allele was significantly associated with an increased risk of IP, with the TC/TT genotype exhibiting higher susceptibility. MMP-8 expressions were upregulated in IP patients. The rs11225395 locus, individuals with the TC/TT genotype showed a significantly higher relative expression level of MMP-8. | The MMP-8 rs11225395 polymorphism was significantly associated with genetic susceptibility to IP. |
| Gao et al., 2025 [18] | Case–control study | To investigate the relationship between miR-27a-3p rs895819 polymorphism with IP susceptibility. | Chinese population | GG genotype and G allele of rs895819 demonstrate a significant association with an enhanced IP susceptibility. | The rs895819 of miR-27a-3p serves as a significant risk predictor for IP patients. |
| Author and Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Score | Quality |
| Rakic et al., 2015 [9] | Y | Y | N | Y | Y | ? | ? | Y | N/A | Y | 6 | Moderate |
| Coelho et al., 2016 [14] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Gonçalves Junior et al., 2016 [7] | Y | Y | N | Y | Y | ? | ? | Y | N/A | Y | 6 | Moderate |
| Zhou et al., 2016 [4] | Y | Y | Y | ? | Y | ? | ? | Y | ? | Y | 6 | Moderate |
| Petkovic-Curcin et al., 2017 [8] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 | High |
| Ribeiro R et al., 2017 [1] | Y | P | ? | Y | Y | ? | P | ? | Y | P | 5.5 | Moderate |
| He K. et al., 2020 [6] | Y | Y | Y | Y | Y | N | ? | Y | ? | Y | 7 | High |
| Silva et al., 2020 [13] | Y | Y | Y | Y | Y | P | Y | Y | Y | Y | 9.5 | High |
| Chang et al., 2021 [11] | Y | Y | N | Y | Y | ? | ? | Y | N/A | Y | 6 | Moderate |
| Qui et al., 2021 [19] | Y | Y | N | Y | Y | ? | ? | Y | N/A | Y | 6 | Moderate |
| Saremi et al., 2021 [16] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Martins et al., 2022 [20,21] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Turkmen et al., 2022 [3] | Y | Y | N | Y | Y | ? | ? | Y | N/A | Y | 6 | Moderate |
| Cardoso et. al., 2022 [15] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Giro et al., 2023 [2] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Li et al., 2024 [10] | Y | Y | N | Y | Y | ? | Y | Y | N/A | Y | 7 | High |
| Saremi et al., 2024 [20] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Talib et al., 2024 [17] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
| Lafuente Ibañez et al., 2024 [12] | Y | Y | ? | Y | Y | Y | Y | ? | Y | Y | 8 | High |
| Saito I. et al., 2024 [24] | Y | Y | Y | P | Y | N | N | Y | ? | Y | 6.5 | Moderate |
| Jin H. et al., 2025 [22] | Y | Y | Y | Y | Y | Y | Y | N | ? | Y | 8.5 | High |
| Fragkioudakis I. et al., 2025 [23] | Y | Y | ? | Y | Y | Y | ? | N | Y | Y | 8 | High |
| Gao et al., 2025 [18] | Y | Y | Y | Y | Y | ? | ? | Y | N/A | Y | 7 | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, F.; Alvarez, M.B.; Relvas, M.; Pacheco, J.J.; Câmara, M.I.d.; Costa, J.A. Genetic Polymorphisms and Predisposition to Peri-Implantitis: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 11461. https://doi.org/10.3390/ijms262311461
Salazar F, Alvarez MB, Relvas M, Pacheco JJ, Câmara MId, Costa JA. Genetic Polymorphisms and Predisposition to Peri-Implantitis: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(23):11461. https://doi.org/10.3390/ijms262311461
Chicago/Turabian StyleSalazar, Filomena, María Belén Alvarez, Marta Relvas, José Julio Pacheco, Marco Infante da Câmara, and José Adriano Costa. 2025. "Genetic Polymorphisms and Predisposition to Peri-Implantitis: A Systematic Review" International Journal of Molecular Sciences 26, no. 23: 11461. https://doi.org/10.3390/ijms262311461
APA StyleSalazar, F., Alvarez, M. B., Relvas, M., Pacheco, J. J., Câmara, M. I. d., & Costa, J. A. (2025). Genetic Polymorphisms and Predisposition to Peri-Implantitis: A Systematic Review. International Journal of Molecular Sciences, 26(23), 11461. https://doi.org/10.3390/ijms262311461







