Recurrent Limitations of CAR-T Therapy in Gliomas: Evidence from Preclinical and Phase I Clinical Studies
Abstract
1. Introduction
2. CAR-T Design, Cell Persistence, and Trafficking
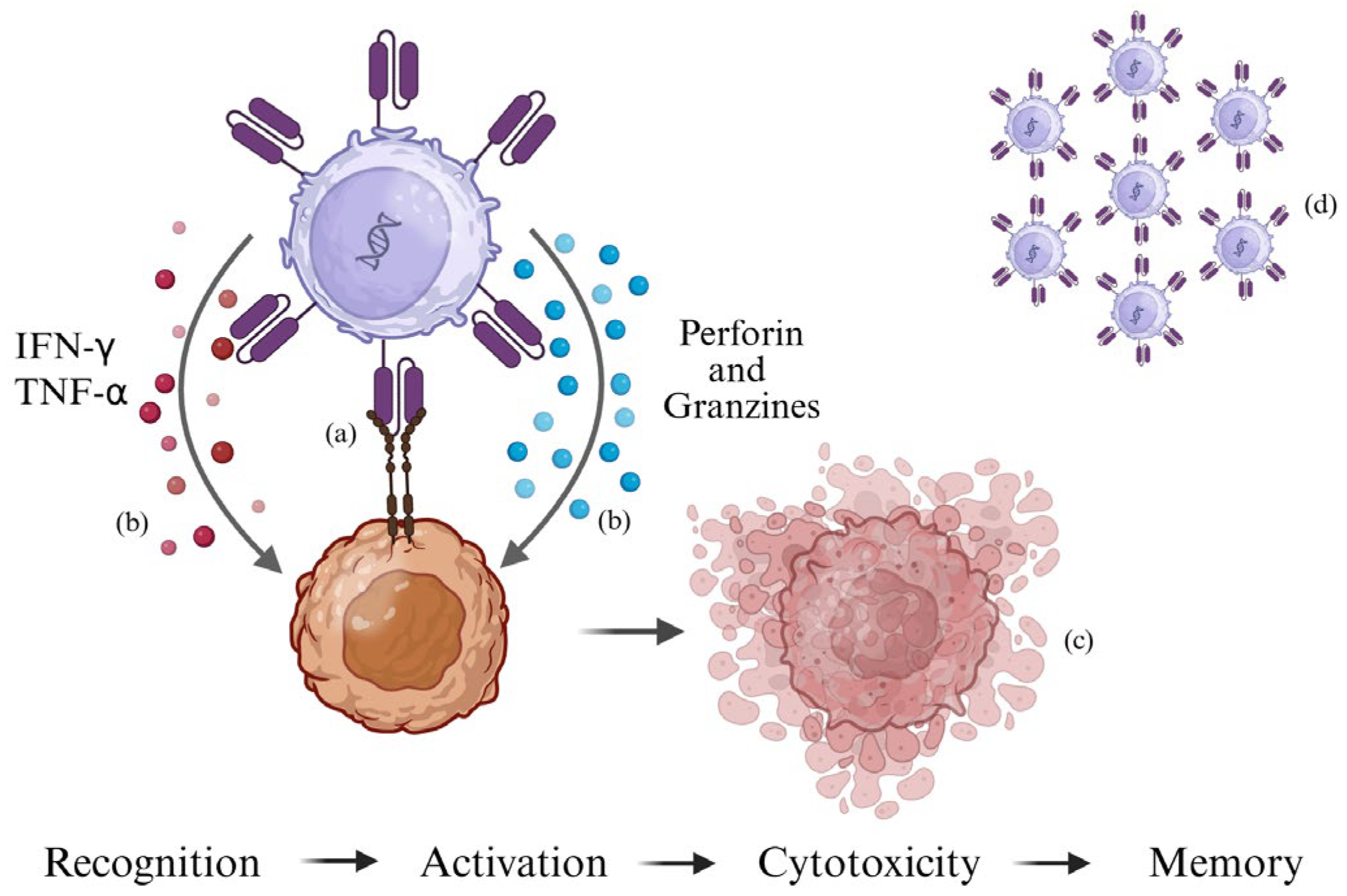
2.1. Mechanism of Action of CAR-T Cells
2.2. Structure of the CAR
- The extracellular domain, which is derived from a single-chain variable fragment (scFv) of an antibody. This fragment binds to a specific tumor antigen (e.g., EGFRvIII in gliomas or CD19 in lymphomas) in an MHC-independent manner [21].
- The transmembrane domain, whose purpose is to anchor the receptor to the T-cell membrane.
- The intracellular domain, which is responsible for transmitting the activation signal. The composition of the intracellular domain determines the generation of the CAR. To improve the efficacy and persistence of CAR-T cells after infusion, several modifications have been made to the intracellular domain, leading to the creation of five generations of CAR-T cells [22,23].
2.3. Activation, Cytotoxicity, and Persistence
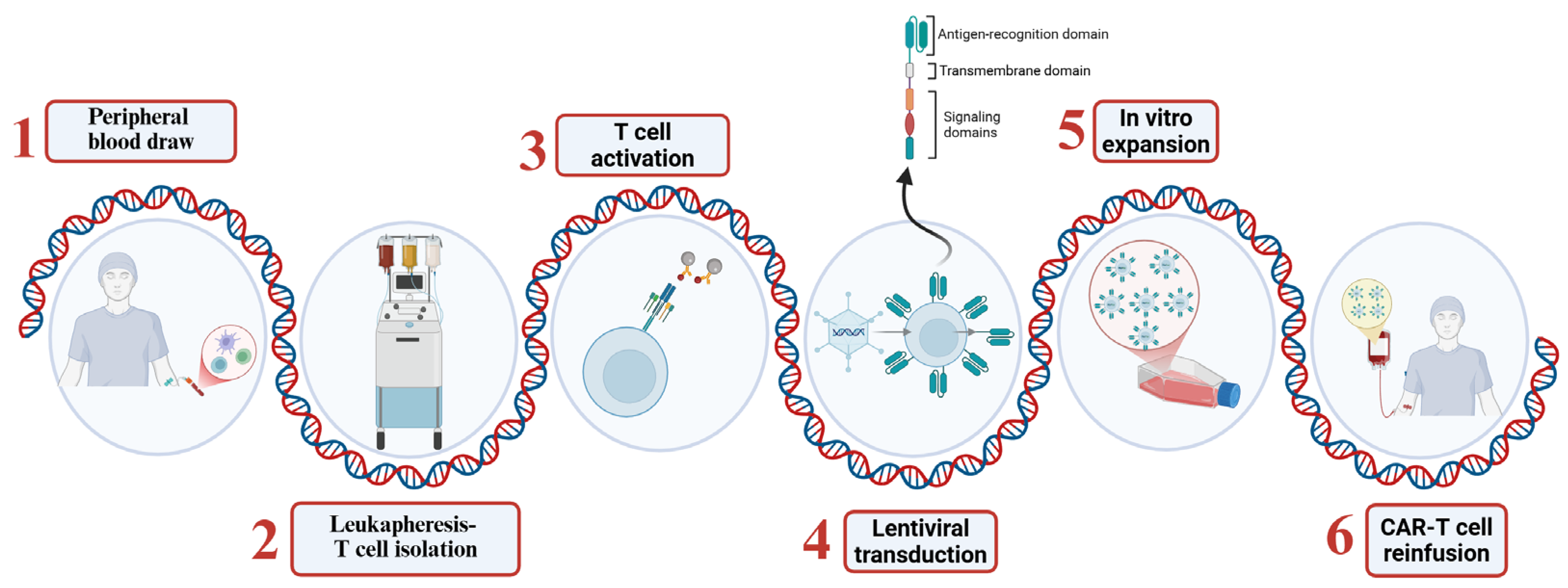
2.4. Production and Administration of CAR-T Cells
- T cell isolation: T lymphocytes are collected from the patient’s peripheral blood through a process known as leukapheresis.
- Genetic modification: The isolated T cells are activated and genetically modified to express the CAR, typically through transduction with viral vectors such as lentiviruses or retroviruses [25].
- Expansion: Once the CAR-T cells have been genetically modified, they are expanded to obtain a therapeutic dose of cells.
2.5. Routes of Administration in Gliomas
3. The Dilemma of CAR-T-Cell-Based Therapy in Glioma
3.1. Study Design: Small Cohorts and Limited Experimental Design
3.2. Target Antigen Selection
- IL-13Rα2: Overexpressed in over 50% of GBMs, with minimal expression in healthy brain tissue [16].
- HER2: Expressed in a subset of gliomas and other solid tumors, offering an additional target [18].
- B7-H3: Expressed in a wide range of pediatric and adult solid tumors, with limited healthy tissue expression [47].
- ECM: A complex network composed of several multidomain macromolecules arranged in a tissue-specific manner, present in both normal and tumor tissues but differing in composition and function. The tumor ECM supports the aggressive biology of brain tumors, representing a potential strategy for GBM therapy [48].
3.2.1. IL-13Rα2: A Selective Target for Glioblastoma
3.2.2. HER2: A Target for Midline and Hemispheric Pediatric Gliomas
3.2.3. EGFRvIII: A Relevant but Challenging Target
3.2.4. GD2: An Emerging Target in Midline Gliomas
3.2.5. B7-H3: A Promising Immunoregulatory Target with Limitations
3.2.6. The Extracellular Matrix: Beyond Structural Support
| Target | Expression and Function in GBM | Clinical/ Preclinical Evidence | Key Limitations | Challenges/ Notes | Refs. |
|---|---|---|---|---|---|
| IL-13Rα2 | Overexpressed in >50% of GBMs - Associated with poor diagnosis - Found in stem-like and differentiated tumor cells - Minimal expression in healthy brain | - First gen CAR-T: median OS ~11 months; - Second gen with E12Y + 4-1BB trial showed safety but limited benefit | - Limited clinical efficacy - Antigen escape with single-target CAR-T | - Development of multi-target strategies (e.g., IL-13Rα2 + B7-H3 or GD2/HER2) | [30,32,49] |
| HER2 | - Highly expressed in pediatric DMGs and G34R-mutant gliomas - Also expressed in some GBMs and other solid tumors | - Pediatric trial locoregional delivery of balanced CD4:CD8 CAR-T showed safety but no strong efficacy data | - Very small sample size - Lack of long-term efficacy data | - Validation in larger cohorts - Demonstrated durable clinical benefit | [41] |
| EGFRvIII | - Tumor-specific but heterogeneously expressed | - Preclinical and early clinical studies showed feasibility and specificity | - High heterogeneity - Loss of antigen over time | - Strategies to overcome antigen escape - Combine with other targets | [19] |
| GD2 | - Highly expressed in DMGs and some GBMs | - Lentiviral CAR-T: persistent expression but local inflammatory toxicity - mRNA CAR-T: dose-dependent anti-tumor effect; improved safety but transient persistence | - Toxicity in early studies - Short persistence with mRNA CAR-T | - Improve persistence while maintaining safety - Optimize dosing/repeat infusion schedules | [52] |
| B7-H3 (CD276) | - Overexpressed in GBMs and many solid tumors - Minimal expression in healthy tissue and heterogeneous within tumors | - Preclinical xenograft studies: tumor suppression with B7-H3 CAR-T - No major off-tumor toxicity observed | - Heterogeneous expression - Suboptimal persistence - Potential off-target effects if upregulated in inflamed tissues | - Enhance CAR-T persistence - Validate antigen density threshold -Humanized models | [55] |
| ECM | - Promotes tumor aggressiveness, immune evasion, and therapy resistance | - In vitro studies: targeting ECM molecules enhances T cell entry and boosts therapy | - Still in early stages - ECM present in both normal and tumor tissue | - Identify tumor-specific ECM components - Translate findings into clinical strategies | [57] |
3.3. Tumor Microenvironment
3.4. Delivery and Access
3.5. Toxicity
3.5.1. Cytokine Release Syndrome (CRS)
3.5.2. Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS)
3.5.3. Tumor Inflammation-Associated Neurotoxicity (TIAN)
- Type 1—mechanical effects caused by edema, such as increased intracranial pressure or hydrocephalus, requiring urgent intervention [73];
4. Future Perspectives
4.1. Multitargeting CAR-T Cells: A Strategy Against Tumor Heterogeneity
4.2. Patient-Derived Organoids
5. Conclusions and Future Perspectives
- Multi-targeted CARs, designed to reduce antigen escape by targeting multiple tumor antigens simultaneously;
- “Armored” CARs, designed to resist the immunosuppressive tumor microenvironment; for example, by secreting immunostimulatory cytokines such as IL-12 [81];
- Combination therapies, encompassing the use of CAR-T cells in combination with immune checkpoint inhibitors or radiotherapy to improve their efficacy;
- “Standard” CAR-T cells, encompassing the development of allogeneic CAR-T cells from healthy donors to reduce production times and costs [25].
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Di Vito, A.; Donato, A.; Bria, J.; Conforti, F.; La Torre, D.; Malara, N.; Donato, G. Extracellular Matrix Structure and Interaction with Immune Cells in Adult Astrocytic Tumors. Cell. Mol. Neurobiol. 2024, 44, 54. [Google Scholar] [CrossRef]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Zhong, J.; Cheng, W.; Qi, Y. Global, Regional, and National Burden of Brain and Central Nervous System Cancer: A Systematic Analysis of Incidence, Deaths, and DALYS with Predictions to 2040. Int. J. Surg. 2025, 111, 4033–4038. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- GBD 2016 Brain and Other CNS Cancer Collaborators. Global, Regional, and National Burden of Brain and Other CNS Cancer, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef]
- Guzzi, G.; Ricciuti, R.A.; Della Torre, A.; Lo Turco, E.; Lavano, A.; Longhini, F.; La Torre, D. Intraoperative Neurophysiological Monitoring in Neurosurgery. J. Clin. Med. 2024, 13, 2966. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, F.; Aguennouz, M.; La Torre, D.; Sfacteria, A.; Grasso, G. Role of Erythropoietin in Cerebral Glioma: An Innovative Target in Neuro-Oncology. World Neurosurg. 2019, 131, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Mukasa, A.; Narita, Y.; Tabei, Y.; Shinoura, N.; Shibui, S.; Saito, N. Toxicity and Outcome of Radiotherapy with Concomitant and Adjuvant Temozolomide in Elderly Patients with Glioblastoma: A Retrospective Study. Neurol. Med. Chir. 2014, 54, 272–279. [Google Scholar] [CrossRef]
- Tao, Q.; Zhu, T.; Ge, X.; Gong, S.; Guo, J. The Efficacy and Safety of Low-Dose Temozolomide Maintenance Therapy in Elderly Patients with Glioblastoma: A Retrospective Cohort Study. Ann. Palliat. Med. 2022, 11, 3513–3519. [Google Scholar] [CrossRef]
- van Hout, L.; Borgo, A.D.; Grun, N.; Schuur, M.; Broen, M.P.G.; Westerman, B.A.; Bartelink, I.; Vandertop, W.P.; Lissenberg-Witte, B.I.; Kouwenhoven, M.C.M. Severe Temozolomide-Induced Thrombocytopenia Is Linked to Increased Healthcare Utilization in Glioblastoma and Disproportionally Impacts Female Patients. Neurooncol. Pract. 2025, 12, 678–690. [Google Scholar] [CrossRef]
- Zeiner, P.S.; Filipski, K.; Filmann, N.; Forster, M.-T.; Voss, M.; Fokas, E.; Herrlinger, U.; Harter, P.N.; Steinbach, J.P.; Ronellenfitsch, M.W. Sex-Dependent Analysis of Temozolomide-Induced Myelosuppression and Effects on Survival in a Large Real-Life Cohort of Patients with Glioma. Neurology 2022, 98, e2073–e2083. [Google Scholar] [CrossRef]
- Bae, S.H.; Park, M.-J.; Lee, M.M.; Kim, T.M.; Lee, S.-H.; Cho, S.Y.; Kim, Y.-H.; Kim, Y.J.; Park, C.-K.; Kim, C.-Y. Toxicity Profile of Temozolomide in the Treatment of 300 Malignant Glioma Patients in Korea. J. Korean Med. Sci. 2014, 29, 980–984. [Google Scholar] [CrossRef]
- Dada, O.E.; Chisango, Z.; Nkansah-Poku, K.A.B.; Sowah, M.N.; Rodrigues, A.C.L.F.; Duru, O.; Myers, M.; Williams, S.T.; Ushewokunze, S.; Collis, S.J.; et al. Race and “Omic” Data in Glioma: A Systematic Review of Contemporary Research to Explore the Digital Divide. Neuro-Oncol. Pract. 2025, 12, 585–599. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Feldman, L.; Brown, C.; Badie, B. Chimeric Antigen Receptor (CAR) T Cell Therapy for Glioblastoma. Neuromol. Med. 2022, 24, 35–40. [Google Scholar] [CrossRef]
- Coluccio, M.L.; Presta, I.; Greco, M.; Gervasi, R.; La Torre, D.; Renne, M.; Voci, C.P.; Lunelli, L.; Donato, G.; Malara, N. Microenvironment Molecular Profile Combining Glycation Adducts and Cytokines Patterns on Secretome of Short-Term Blood-Derived Cultures during Tumour Progression. Int. J. Mol. Sci. 2020, 21, 4711. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.H.; et al. Tandem CAR T Cells Targeting HER2 and IL13Rα2 Mitigate Tumor Antigen Escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A Single Dose of Peripherally Infused EGFRvIII-Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Ramesh, P.; Hui, H.Y.L.; Brownrigg, L.M.; Fuller, K.A.; Erber, W.N. Chimeric Antigen Receptor T-Cells: Properties, Production, and Quality Control. Int. J. Lab. Hematol. 2023, 45, 425–435. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor-Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. “Off-the-Shelf” Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR-T Cells of Defined CD4+:CD8+ Composition in Adult B Cell ALL Patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef]
- Ayala Ceja, M.; Khericha, M.; Harris, C.M.; Puig-Saus, C.; Chen, Y.Y. CAR-T Cell Manufacturing: Major Process Parameters and next-Generation Strategies. J. Exp. Med. 2024, 221, e20230903. [Google Scholar] [CrossRef]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal Bivalent CAR T Cells Targeting EGFR and IL13Rα2 in Recurrent Glioblastoma: Phase 1 Trial Interim Results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Barish, M.E.; Aftabizadeh, M.; Hibbard, J.; Blanchard, M.S.; Ostberg, J.R.; Wagner, J.R.; Manchanda, M.; Paul, J.; Stiller, T.; Aguilar, B.; et al. Chlorotoxin-Directed CAR T Cell Therapy for Recurrent Glioblastoma: Interim Clinical Experience Demonstrating Feasibility and Safety. Cell Rep. Med. 2025, 6, 102302. [Google Scholar] [CrossRef]
- Brown, C.E.; Hibbard, J.C.; Alizadeh, D.; Blanchard, M.S.; Natri, H.M.; Wang, D.; Ostberg, J.R.; Aguilar, B.; Wagner, J.R.; Paul, J.A.; et al. Locoregional Delivery of IL-13Rα2-Targeting CAR-T Cells in Recurrent High-Grade Glioma: A Phase 1 Trial. Nat. Med. 2024, 30, 1001–1012. [Google Scholar] [CrossRef]
- Brown, C.E.; Rodriguez, A.; Palmer, J.; Ostberg, J.R.; Naranjo, A.; Wagner, J.R.; Aguilar, B.; Starr, R.; Weng, L.; Synold, T.W.; et al. Off-the-Shelf, Steroid-Resistant, IL13Rα2-Specific CAR T Cells for Treatment of Glioblastoma. Neuro-Oncology 2022, 24, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rui, W.; Zhao, X.; Lin, X. Enhancing CAR-T Cell Efficacy in Solid Tumors by Targeting the Tumor Microenvironment. Cell. Mol. Immunol. 2021, 18, 1085–1095. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T Cell Therapy for H3K27M-Mutated Diffuse Midline Gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Monje, M.; Mahdi, J.; Majzner, R.; Yeom, K.W.; Schultz, L.M.; Richards, R.M.; Barsan, V.; Song, K.-W.; Kamens, J.; Baggott, C.; et al. Intravenous and Intracranial GD2-CAR T Cells for H3K27M+ Diffuse Midline Gliomas. Nature 2025, 637, 708–715. [Google Scholar] [CrossRef]
- Vitanza, N.A.; Ronsley, R.; Choe, M.; Seidel, K.; Huang, W.; Rawlings-Rhea, S.D.; Beam, M.; Steinmetzer, L.; Wilson, A.L.; Brown, C.; et al. Intracerebroventricular B7-H3-Targeting CAR T Cells for Diffuse Intrinsic Pontine Glioma: A Phase 1 Trial. Nat. Med. 2025, 31, 861–868. [Google Scholar] [CrossRef]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A Promising Approach for Glioma Treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef]
- Sampath, P.; Sengupta, S.; Sengupta, S.; Junghans, R. IMMU-01. Temozolomide-Resistant Car-T Enhances Glioblastoma Clearance by Concurrent Chemotherapy and Immunotherapy. Neuro-Oncology 2017, 19, vi112. [Google Scholar] [CrossRef]
- Suryadevara, C.M.; Desai, R.; Abel, M.L.; Riccione, K.A.; Batich, K.A.; Shen, S.H.; Chongsathidkiet, P.; Gedeon, P.C.; Elsamadicy, A.A.; Snyder, D.J.; et al. Temozolomide Lymphodepletion Enhances CAR Abundance and Correlates with Antitumor Efficacy against Established Glioblastoma. Oncoimmunology 2018, 7, e1434464. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Johnson, A.J.; Wilson, A.L.; Brown, C.; Yokoyama, J.K.; Künkele, A.; Chang, C.A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; et al. Locoregional Infusion of HER2-Specific CAR T Cells in Children and Young Adults with Recurrent or Refractory CNS Tumors: An Interim Analysis. Nat. Med. 2021, 27, 1544–1552. [Google Scholar] [CrossRef]
- Marcuello, C.; Lim, K.; Nisini, G.; Pokrovsky, V.S.; Conde, J.; Ruggeri, F.S. Nanoscale Analysis beyond Imaging by Atomic Force Microscopy: Molecular Perspectives on Oncology and Neurodegeneration. Small Sci. 2025, 5, 2500351. [Google Scholar] [CrossRef]
- Andolfi, L.; Bourkoula, E.; Migliorini, E.; Palma, A.; Pucer, A.; Skrap, M.; Scoles, G.; Beltrami, A.P.; Cesselli, D.; Lazzarino, M. Investigation of Adhesion and Mechanical Properties of Human Glioma Cells by Single Cell Force Spectroscopy and Atomic Force Microscopy. PLoS ONE 2014, 9, e112582. [Google Scholar] [CrossRef] [PubMed]
- Najera, J.; Rosenberger, M.R.; Datta, M. Atomic Force Microscopy Methods to Measure Tumor Mechanical Properties. Cancers 2023, 15, 3285. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent Antitumor Efficacy of Anti-GD2 CAR T Cells in H3-K27M+ Diffuse Midline Gliomas. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Mintz, A.; Gibo, D.M.; Slagle-Webb, B.; Christensen, N.D.; Debinski, W. IL-13Rα2 Is a Glioma-Restricted Receptor for Interleukin-13. Neoplasia 2002, 4, 388. [Google Scholar] [CrossRef]
- Fatehi, D.; Baral, T.N.; Abulrob, A. In Vivo Imaging of Brain Cancer Using Epidermal Growth Factor Single Domain Antibody Bioconjugated to Near-Infrared Quantum Dots. J. Nanosci. Nanotechnol. 2014, 14, 5355–5362. [Google Scholar] [CrossRef]
- Wang, S.S.; Davenport, A.J.; Iliopoulos, M.; Hughes-Parry, H.E.; Watson, K.A.; Arcucci, V.; Mulazzani, M.; Eisenstat, D.D.; Hansford, J.R.; Cross, R.S.; et al. HER2 Chimeric Antigen Receptor T Cell Immunotherapy Is an Effective Treatment for Diffuse Intrinsic Pontine Glioma. Neuro-Oncol. Adv. 2023, 5, vdad024. [Google Scholar] [CrossRef]
- Foster, J.B.; Madsen, P.J.; Harvey, K.; Griffin, C.; Stern, A.; Patterson, L.; Joshi, N.; Dickson, C.; McManus, O.; Beaubien, E.; et al. Transient mRNA CAR T Cells Targeting GD2 Provide Dose-Adjusted Efficacy against Diffuse Midline Glioma and High Grade Glioma Models. Neuro-Oncology 2025, noaf115. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, X.; Zhang, C.; Chen, W.; Fu, Y.; Yu, Y.; Chen, Y.; Shao, T.; Zhang, J.; Ding, G. Tumor Immunotherapy Targeting B7-H3: From Mechanisms to Clinical Applications. Immunotargets Ther. 2025, 14, 291–320. [Google Scholar] [CrossRef] [PubMed]
- Inthanachai, T.; Boonkrai, C.; Phakham, T.; Pisitkun, T.; Thaiwong, R.; Chuthaphakdikun, V.; Sakunrangsit, N.; Limprasutr, V.; Chinsuwan, T.; Hirankarn, N.; et al. Novel B7-H3 CAR T Cells Show Potent Antitumor Effects in Glioblastoma: A Preclinical Study. J. Immunother. Cancer 2025, 13, e010083. [Google Scholar] [CrossRef]
- Nehama, D.; Ianni, N.D.; Musio, S.; Du, H.; Patané, M.; Pollo, B.; Finocchiaro, G.; Park, J.J.; Dunn, D.E.; Edwards, D.S.; et al. B7-H3-Redirected Chimeric Antigen Receptor T Cells Target Glioblastoma and Neurospheres. EBioMedicine 2019, 47, 33. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.D.; Lozada, J.R.; Zorko, N.A.; Elliott, A.; Makovec, A.; Radovich, M.; Heath, E.I.; Agarwal, N.; Mckay, R.R.; Garje, R.; et al. Pan-Cancer Interrogation of B7-H3 (CD276) as an Actionable Therapeutic Target Across Human Malignancies. Cancer Res. Commun. 2024, 4, 1369–1379. [Google Scholar] [CrossRef]
- Mohiuddin, E.; Wakimoto, H. Extracellular Matrix in Glioblastoma: Opportunities for Emerging Therapeutic Approaches. Am. J. Cancer Res. 2021, 11, 3742–3754. [Google Scholar]
- Ma, Q.; Long, W.; Xing, C.; Chu, J.; Luo, M.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Cancer Stem Cells and Immunosuppressive Microenvironment in Glioma. Front. Immunol. 2018, 9, 2924. [Google Scholar] [CrossRef]
- Ma, T.; Su, G.; Wu, Q.; Shen, M.; Feng, X.; Zhang, Z. Tumor-Derived Extracellular Vesicles: How They Mediate Glioma Immunosuppression. Mol. Biol. Rep. 2024, 51, 235. [Google Scholar] [CrossRef]
- Ravi, V.M.; Neidert, N.; Will, P.; Joseph, K.; Maier, J.P.; Kückelhaus, J.; Vollmer, L.; Goeldner, J.M.; Behringer, S.P.; Scherer, F.; et al. T-Cell Dysfunction in the Glioblastoma Microenvironment Is Mediated by Myeloid Cells Releasing Interleukin-10. Nat. Commun. 2022, 13, 925. [Google Scholar] [CrossRef]
- Yin, B.; Cai, Y.; Chen, L.; Li, Z.; Li, X. Immunosuppressive MDSC and Treg Signatures Predict Prognosis and Therapeutic Response in Glioma. Int. Immunopharmacol. 2024, 141, 112922. [Google Scholar] [CrossRef]
- Flies, D.B.; Langermann, S.; Jensen, C.; Karsdal, M.A.; Willumsen, N. Regulation of Tumor Immunity and Immunotherapy by the Tumor Collagen Extracellular Matrix. Front. Immunol. 2023, 14, 1199513. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Y. Unraveling the Immunosuppressive Microenvironment of Glioblastoma and Advancements in Treatment. Front. Immunol. 2025, 16, 1590781. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, L.; Guo, Z.; Zhou, M. Optimizing CAR-T Cell Function in Solid Tumor Microenvironment: Insights from Culture Media Additives. Curr. Res. Transl. Med. 2025, 73, 103491. [Google Scholar] [CrossRef]
- Tsai, H.-F.; Trubelja, A.; Shen, A.Q.; Bao, G. Tumour-on-a-Chip: Microfluidic Models of Tumour Morphology, Growth and Microenvironment. J. R. Soc. Interface 2017, 14, 20170137. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Z.; Zhou, X.; Khoo, B.L.; Gunawan, R.; Chin, Y.R.; Zhang, L.; Yi, C.; Guan, X.; Yang, M. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Adv. Healthc. Mater. 2023, 12, e2202609. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.-Y.; Chen, T.; Chang, L.-J.; et al. Safety and Antitumor Activity of GD2-Specific 4SCAR-T Cells in Patients with Glioblastoma. Mol. Cancer 2023, 22, 3. [Google Scholar] [CrossRef]
- Rubin, D.B.; Al Jarrah, A.; Li, K.; LaRose, S.; Monk, A.D.; Ali, A.B.; Spendley, L.N.; Nikiforow, S.; Jacobson, C.; Vaitkevicius, H. Clinical Predictors of Neurotoxicity After Chimeric Antigen Receptor T-Cell Therapy. JAMA Neurol. 2020, 77, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.R.; Tozlu, C.; Gordillo, C.A.; Chan, H.T.; Reshef, R.; Wesley, S.F. Novel Risk Factors for Predicting Immune Effector Cell-Associated Neurotoxicity Syndrome. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, H.; Xu, C.; Hu, M.; Li, J.; Chang, W. Systemic Toxicity of CAR-T Therapy and Potential Monitoring Indicators for Toxicity Prevention. Front. Immunol. 2024, 15, 1422591. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef]
- Ma, K.; Hu, P. Chimeric Antigen Receptor T-Cell Therapy for Glioblastoma. Cancers 2023, 15, 5652. [Google Scholar] [CrossRef]
- Mahdi, J.; Dietrich, J.; Straathof, K.; Roddie, C.; Scott, B.J.; Davidson, T.B.; Prolo, L.M.; Batchelor, T.T.; Campen, C.J.; Davis, K.L.; et al. Tumor Inflammation-Associated Neurotoxicity. Nat. Med. 2023, 29, 803–810. [Google Scholar] [CrossRef]
- Logun, M.; Wang, X.; Sun, Y.; Bagley, S.J.; Li, N.; Desai, A.; Zhang, D.Y.; Nasrallah, M.P.; Pai, E.L.-L.; Oner, B.S.; et al. Patient-Derived Glioblastoma Organoids as Real-Time Avatars for Assessing Responses to Clinical CAR-T Cell Therapy. Cell Stem Cell 2025, 32, 181–190.e4. [Google Scholar] [CrossRef]
- Khamis, Z.I.; Sarker, D.B.; Xue, Y.; Al-Akkary, N.; James, V.D.; Zeng, C.; Li, Y.; Sang, Q.-X.A. Modeling Human Brain Tumors and the Microenvironment Using Induced Pluripotent Stem Cells. Cancers 2023, 15, 1253. [Google Scholar] [CrossRef] [PubMed]
- Vandecandelaere, G.; Ramapriyan, R.; Gaffey, M.; Richardson, L.G.; Steuart, S.J.; Tazhibi, M.; Kalaw, A.; Grewal, E.P.; Sun, J.; Curry, W.T.; et al. Pre-Clinical Models for CAR T-Cell Therapy for Glioma. Cells 2024, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
- Linkous, A.; Balamatsias, D.; Snuderl, M.; Edwards, L.; Miyaguchi, K.; Milner, T.; Reich, B.; Cohen-Gould, L.; Storaska, A.; Nakayama, Y.; et al. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 2019, 26, 3203–3211.e5. [Google Scholar] [CrossRef]
- Wen, J.; Liu, F.; Cheng, Q.; Weygant, N.; Liang, X.; Fan, F.; Li, C.; Zhang, L.; Liu, Z. Applications of Organoid Technology to Brain Tumors. CNS Neurosci. Ther. 2023, 29, 2725–2743. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-Armed Oncolytic Adenoviruses Enhance CAR-T Cell Therapeutic Efficacy and Reprogram Tumor Microenvironment in Glioblastoma. Mol. Ther. 2023, 31, 134–153. [Google Scholar] [CrossRef]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-Tumoral Heterogeneity. Cell 2020, 180, 188–204.e22. [Google Scholar] [CrossRef]
- Zarychta, J.; Kowalczyk, A.; Marszołek, A.; Zawitkowska, J.; Lejman, M. Strategies to Overcome Tumor Microenvironment Immunosuppressive Effect on the Functioning of CAR-T Cells in High-Grade Glioma. Ther. Adv. Med. Oncol. 2024, 16, 17588359241266140. [Google Scholar] [CrossRef] [PubMed]
| Category | Limitations |
|---|---|
| Study Design | - Early-phase, low-powered trials - Lack of biomarker-driven patient selection - Clinical endpoints and follow-up duration |
| Target antigen selection | - Single antigen CAR-T therapies can promote antigen escape, making them ineffective |
| Tumor Microenvironment (TME) | - Profound immunosuppression (Tregs, MDSCs, TAMs) - Hypoxia and acidic pH - Barrier to CAR-T infiltration |
| Delivery and Access | - Blood–brain barrier (BBB) limits systemic CAR-T entry - Locoregional (Ommaya/intracerebral) preferred |
| Toxicity | - Neurotoxicity - Potential for on-target/off-tumor effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bria, J.; Filardo, A.; Di Vito, A.; Della Torre, A.; Lavano, A.; Coscarella, I.; Chiarella, E.; Procopio, E.; Egiziano, M.T.; Longo, P.; et al. Recurrent Limitations of CAR-T Therapy in Gliomas: Evidence from Preclinical and Phase I Clinical Studies. Int. J. Mol. Sci. 2025, 26, 11435. https://doi.org/10.3390/ijms262311435
Bria J, Filardo A, Di Vito A, Della Torre A, Lavano A, Coscarella I, Chiarella E, Procopio E, Egiziano MT, Longo P, et al. Recurrent Limitations of CAR-T Therapy in Gliomas: Evidence from Preclinical and Phase I Clinical Studies. International Journal of Molecular Sciences. 2025; 26(23):11435. https://doi.org/10.3390/ijms262311435
Chicago/Turabian StyleBria, Jessica, Andrea Filardo, Anna Di Vito, Attilio Della Torre, Angelo Lavano, Isabella Coscarella, Emanuela Chiarella, Emanuela Procopio, Maria Teresa Egiziano, Prospero Longo, and et al. 2025. "Recurrent Limitations of CAR-T Therapy in Gliomas: Evidence from Preclinical and Phase I Clinical Studies" International Journal of Molecular Sciences 26, no. 23: 11435. https://doi.org/10.3390/ijms262311435
APA StyleBria, J., Filardo, A., Di Vito, A., Della Torre, A., Lavano, A., Coscarella, I., Chiarella, E., Procopio, E., Egiziano, M. T., Longo, P., & La Torre, D. (2025). Recurrent Limitations of CAR-T Therapy in Gliomas: Evidence from Preclinical and Phase I Clinical Studies. International Journal of Molecular Sciences, 26(23), 11435. https://doi.org/10.3390/ijms262311435










