Lipid Composition and Thermotropic Properties of Meibum of Animal Models and Humans with Meibomian Gland Dysfunction
Abstract
1. Introduction
2. Results
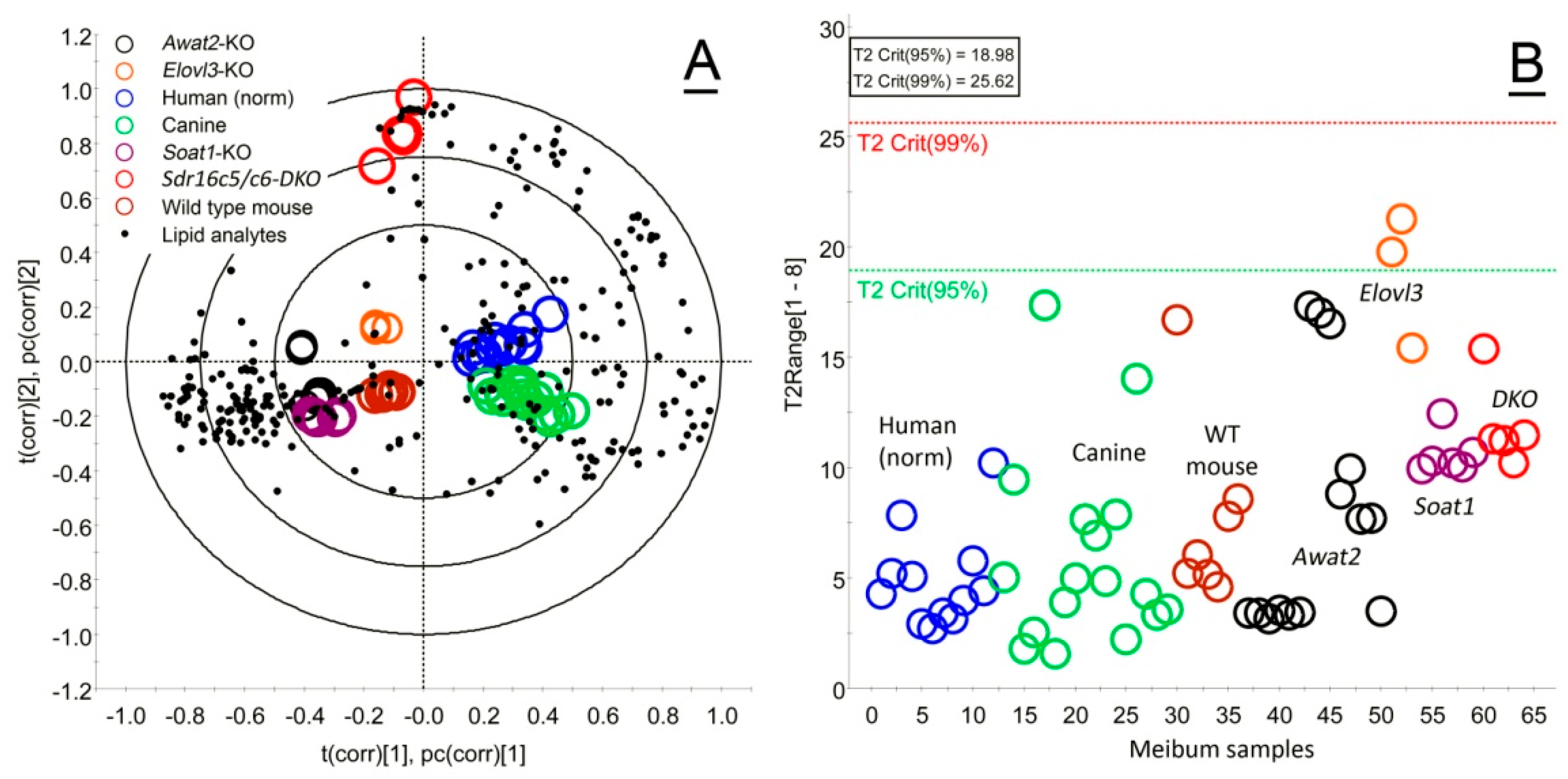
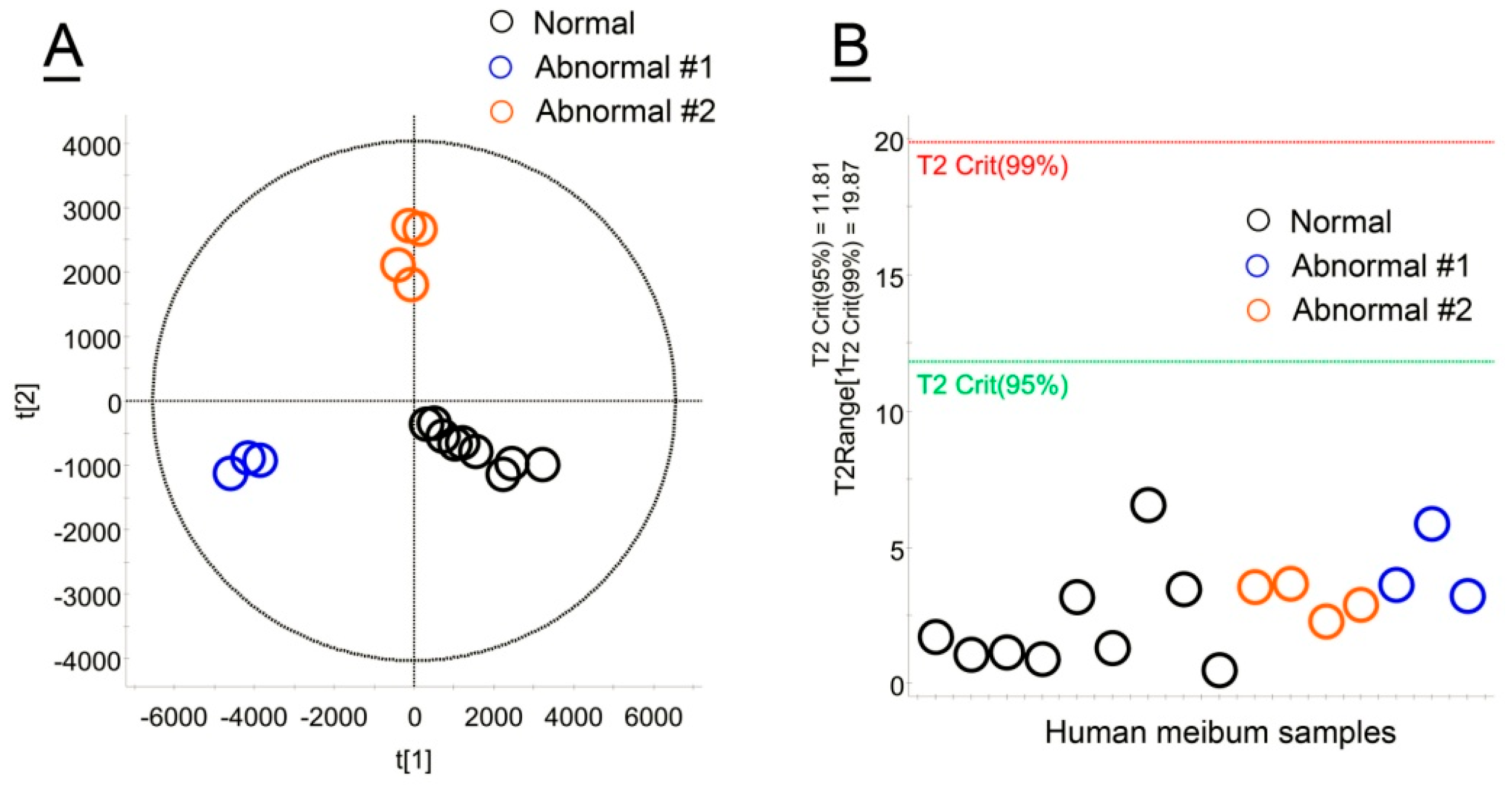
2.1. Untargeted, Unsupervised LC/MS and Multivariate Statistical Analyses of Human and Animal Meibum
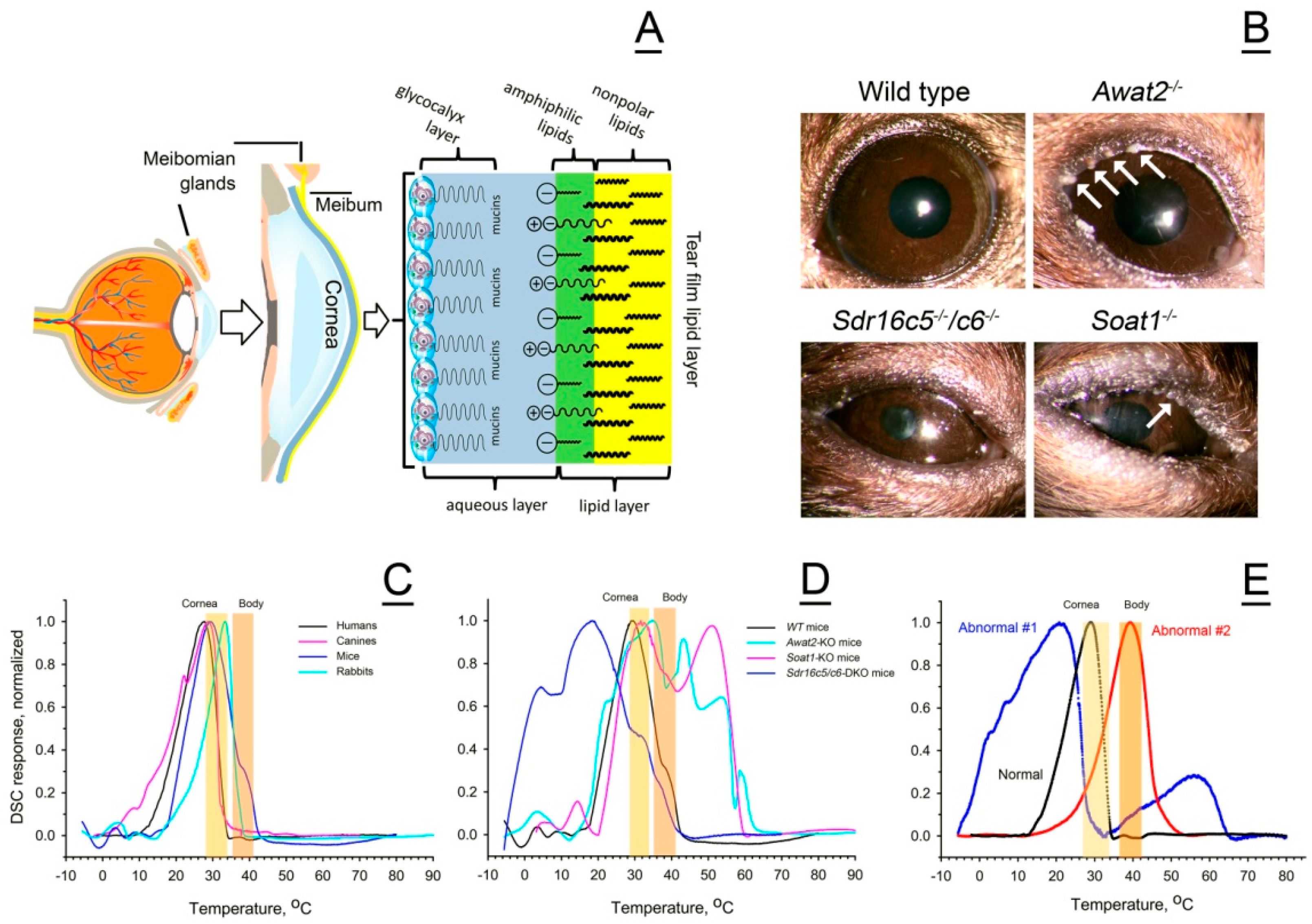
2.2. Mouse Meibum
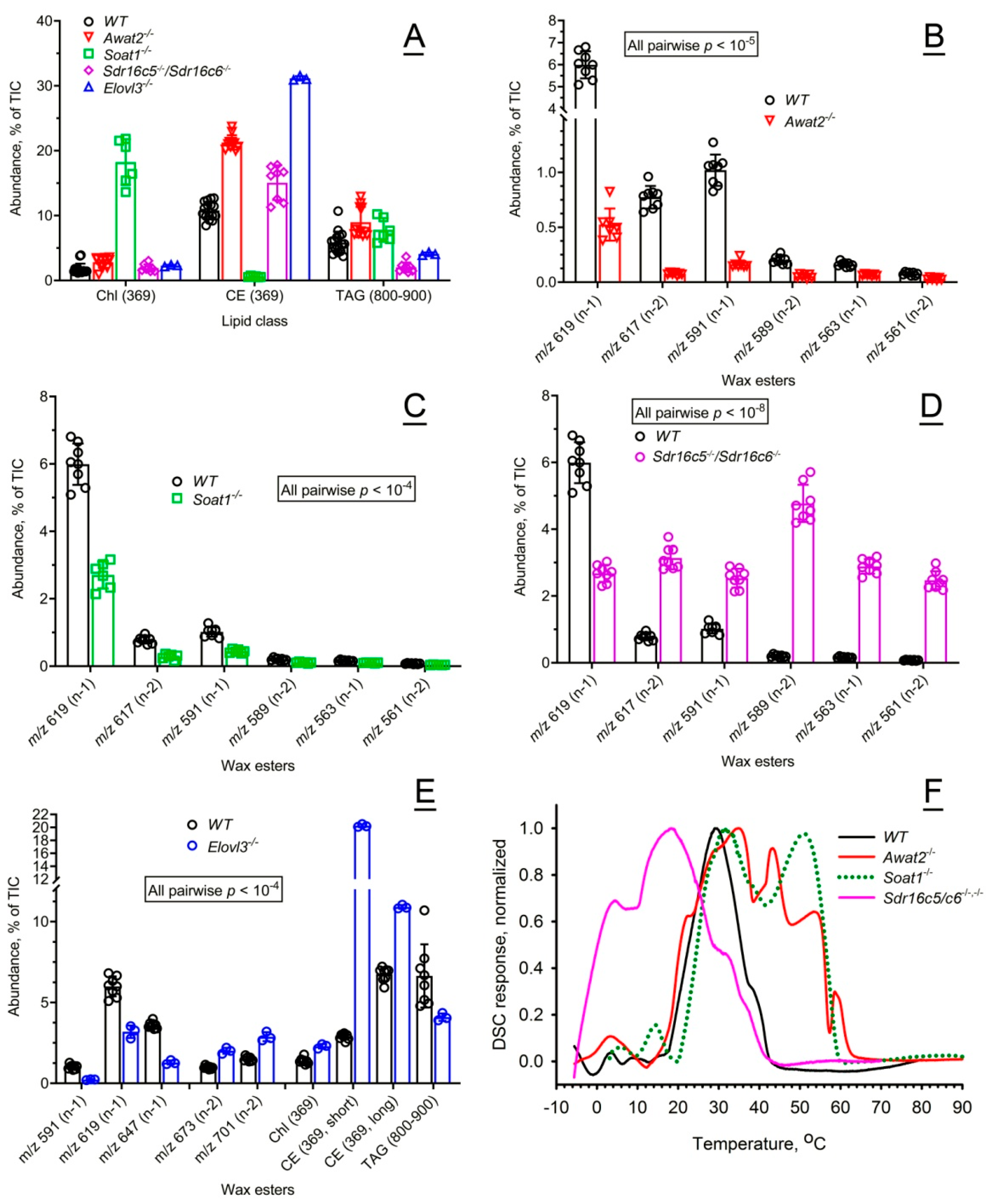
2.2.1. Characterization of the Chemical Composition of Wild-Type and Mutant Mouse Meibum
2.2.2. Effects of the Mutations on Thermotropic Properties of Wild-Type and Mutant Mouse Meibum
2.3. Canine Meibum
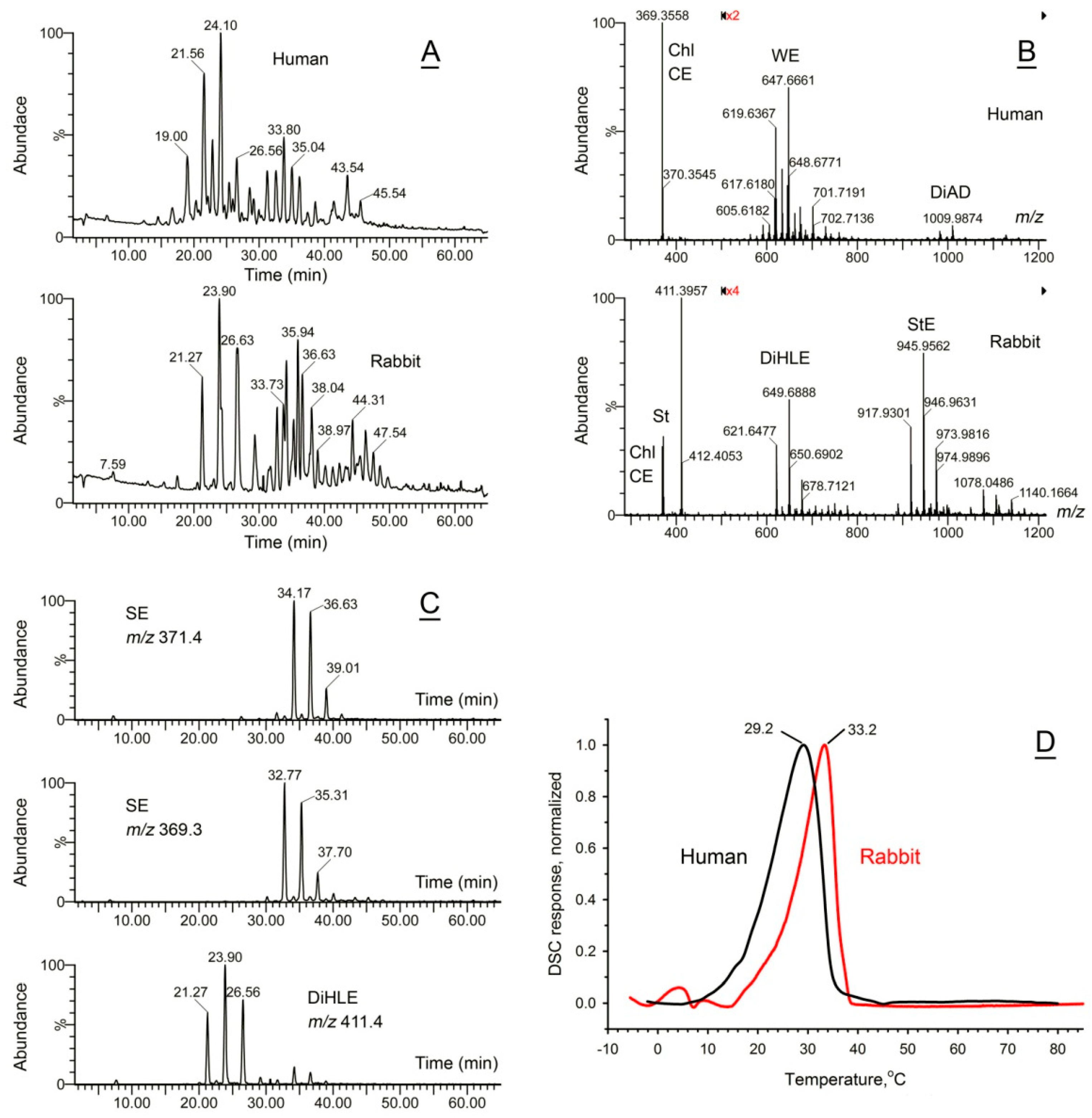
2.4. Rabbit Meibum
2.5. Normal and Abnormal Human Meibum
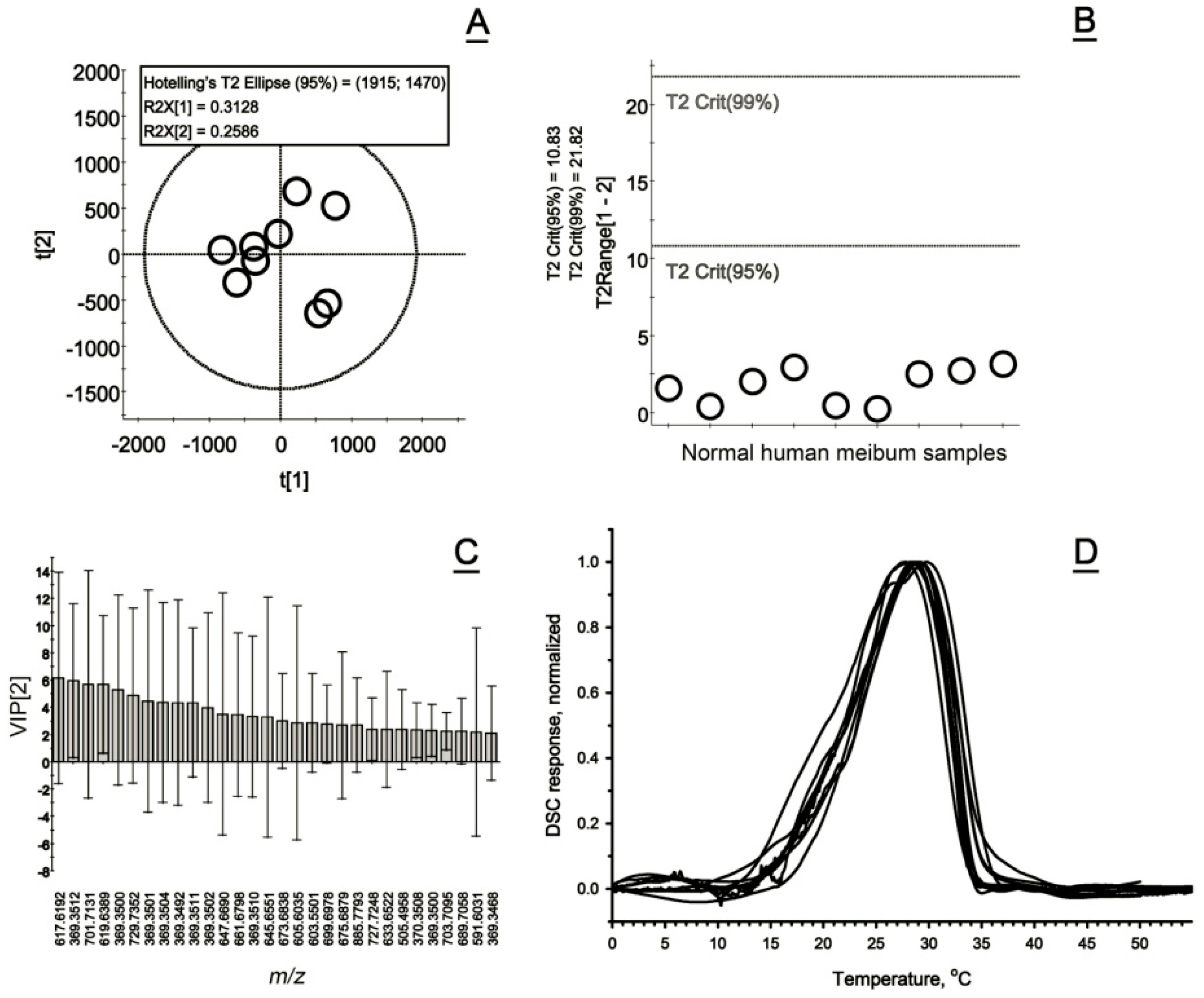
2.5.1. Normal Human Meibum
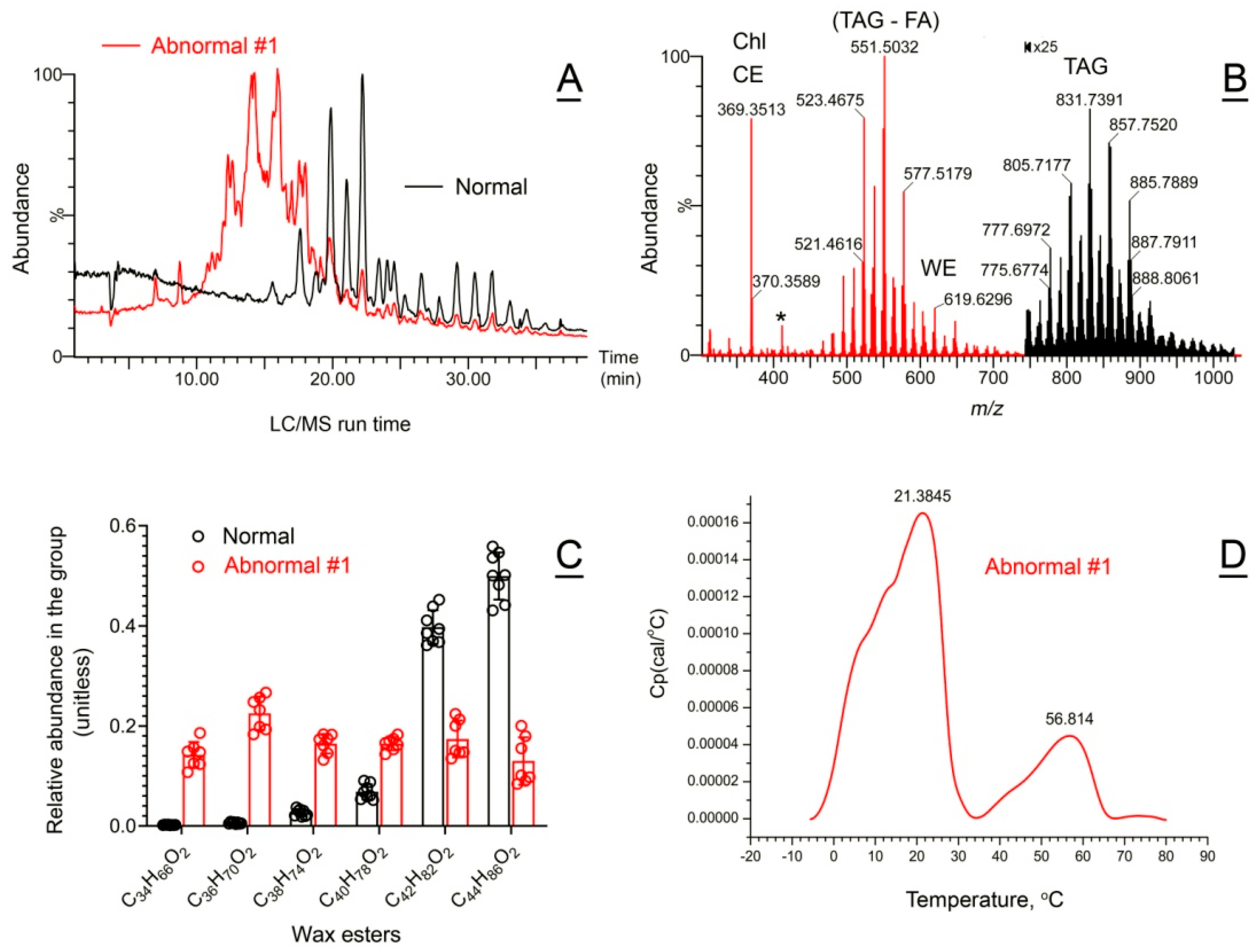
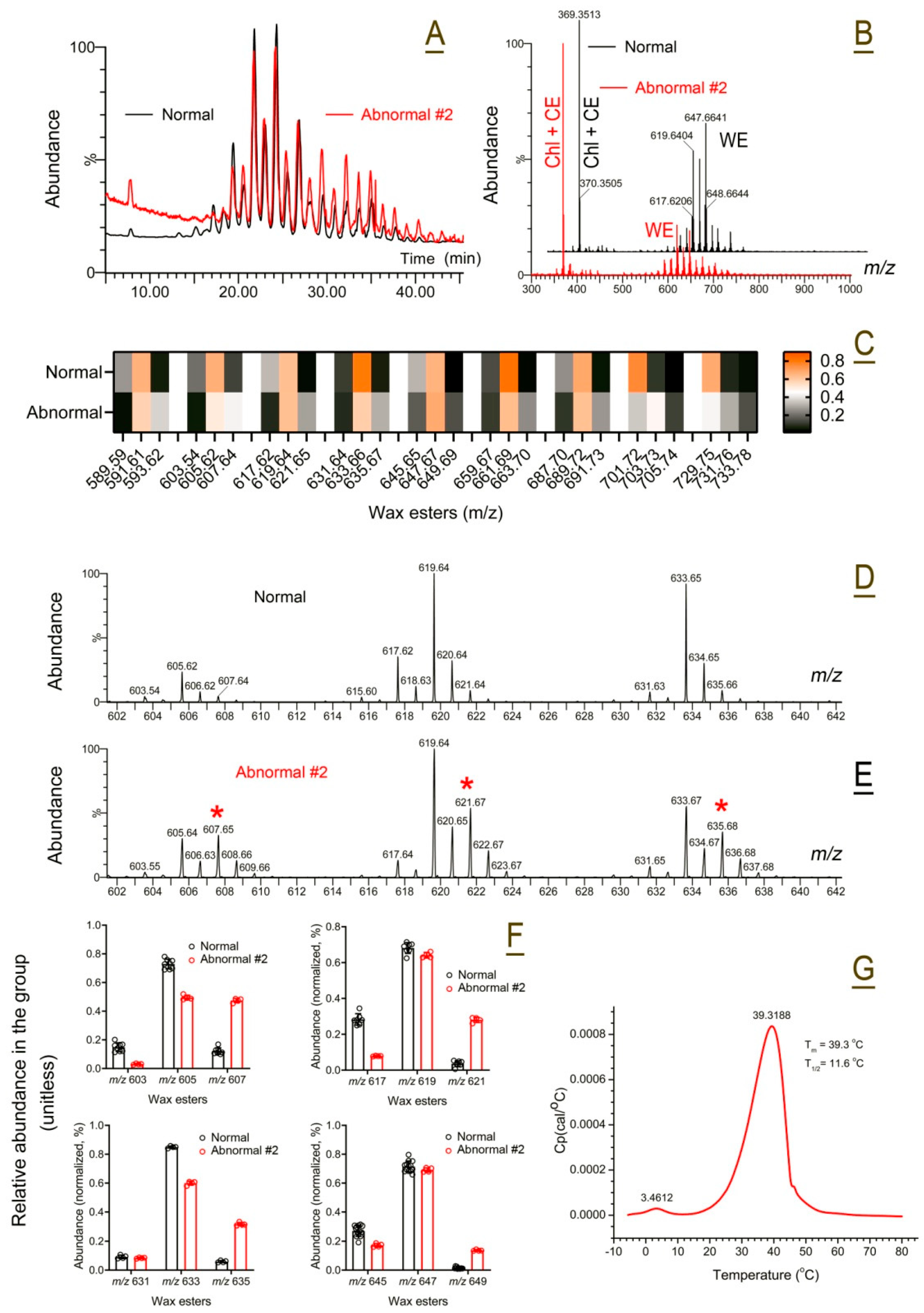
2.5.2. Abnormal Human Meibum
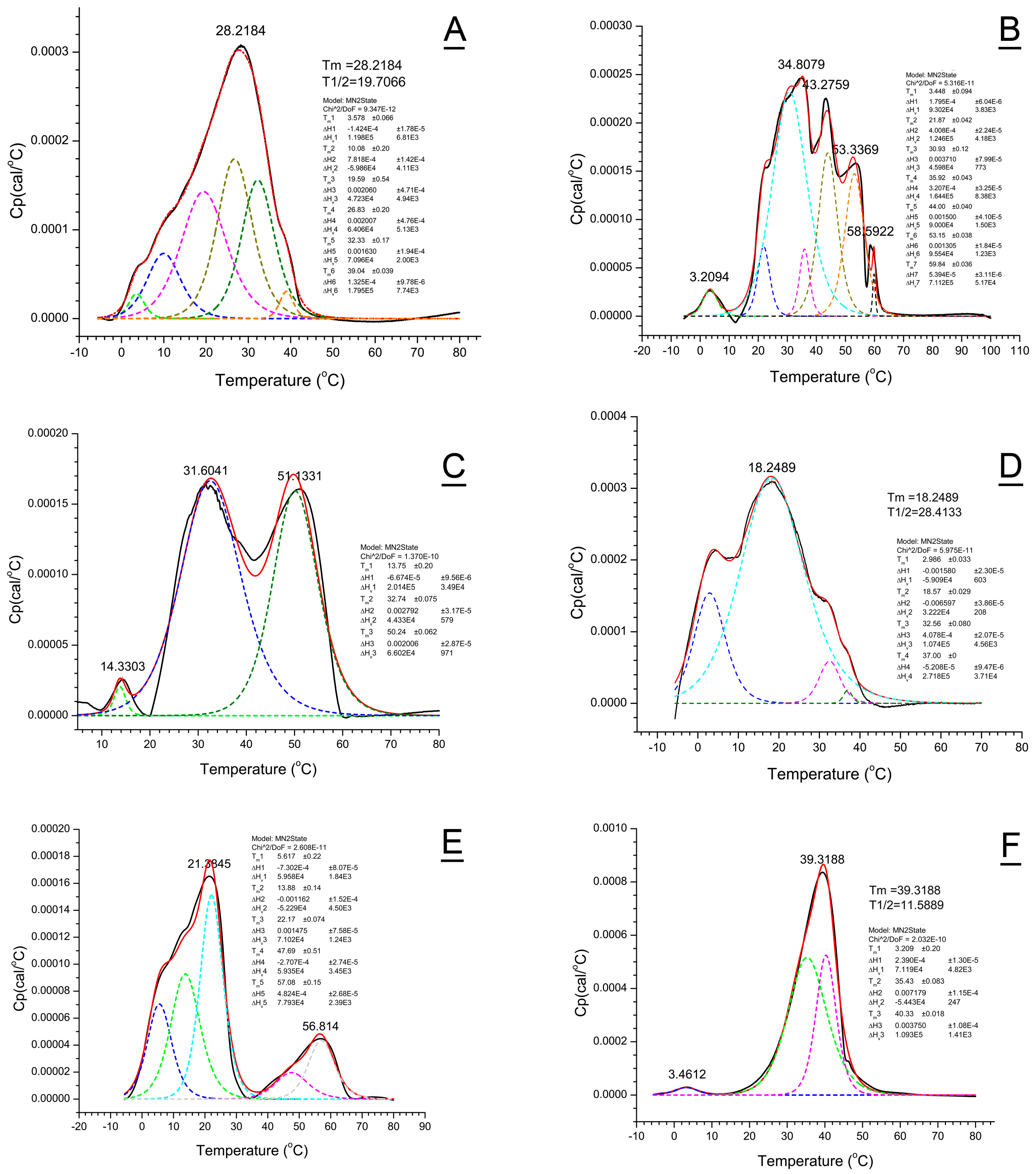
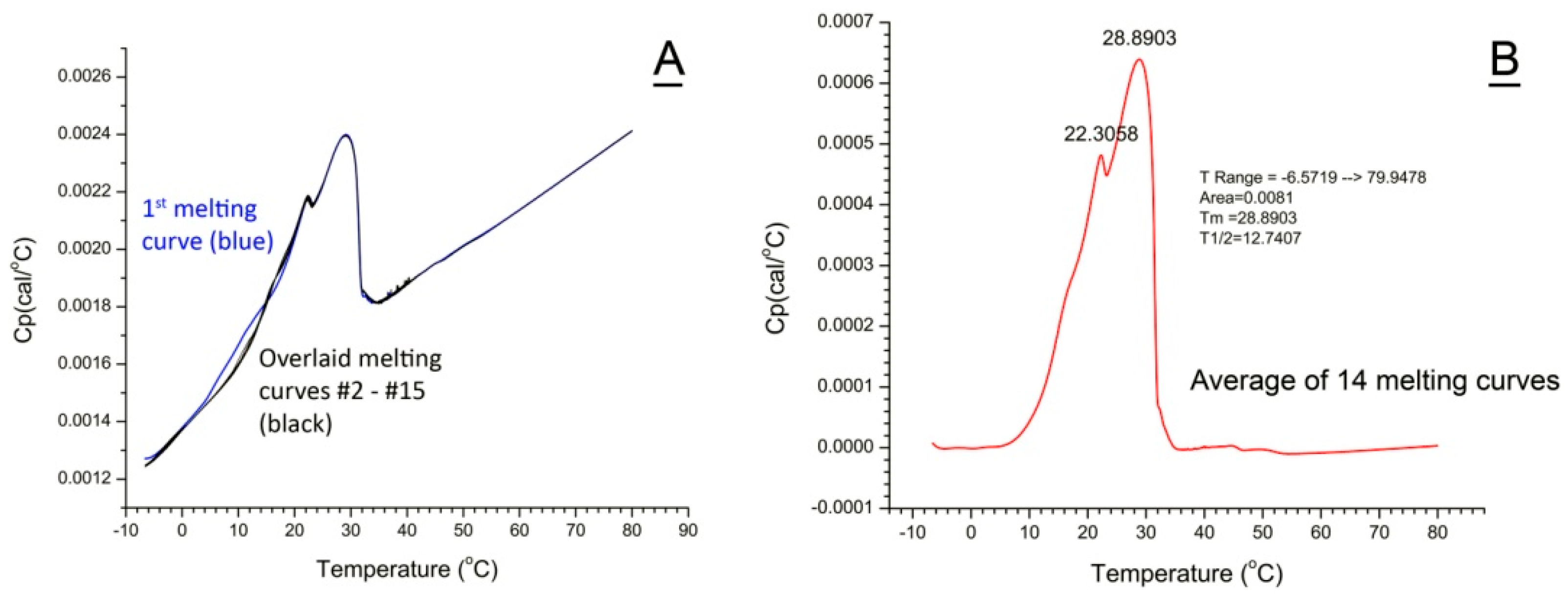
2.6. Deconvolution of Thermograms
2.6.1. WT Mouse Meibum
2.6.2. Awat2−/− Mouse Meibum
2.6.3. Soat1−/− Meibum
2.6.4. DKO Meibum
2.6.5. Canine Meibum
2.6.6. Human Meibum
2.7. Transcriptomic Analysis
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Animal and Human Meibum Samples
4.3. Liquid Chromatography–Mass Spectrometry
4.4. Differential Scanning Microcalorimetry
4.5. Transcriptomic Analysis of Mouse Tarsal Plates and Corneas
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APCI PIM | atmospheric pressure chemical ionization positive ion mode |
| Chl | free cholesterol |
| CE | cholesteryl ester(s) |
| DE | dry eye |
| DKO | Sdr16c5−/−/Sdr16c6−/− mice |
| ΔH | calorimetric transition enthalpies |
| ΔHv | van’t Hoff enthalpy changes |
| EDE | evaporative dry eye |
| DiAD | α,ω-diacylated diols |
| DiHLE | dihydrolanosteryl ester(s) |
| DSC | differential scanning microcalorimetry |
| DUWE | diunsaturated wax ester(s) |
| FA | fatty acid(s) |
| LC | liquid chromatography |
| MS | high resolution time-of-flight mass spectrometry |
| m/z | mass-to-charge ratio (unitless) |
| MG | Meibomian gland(s) |
| MGD | Meibomian gland dysfunction |
| MUWE | monounsaturated wax ester(s) |
| OST | ocular surface temperature |
| PCA | Principal Component Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| SE | steryl ester(s) (non-cholesterol-based) |
| SG | sebaceous gland(s) |
| SWE | saturated wax ester(s) |
| T1/2 | the width of the transition at half-height of the melting peak |
| TAG | triacylglycerol(s) |
| TF | tear film |
| TFLL | tear film lipid layer |
| Tm | transition temperature |
| WE | wax ester(s) |
| WT | wild type |
References
- Vanderwolf, K.; Kyle, C.; Davy, C. A review of sebum in mammals in relation to skin diseases, skin function, and the skin microbiome. PeerJ 2023, 11, e16680. [Google Scholar] [CrossRef]
- Nicolaides, N.; Kaitaranta, J.K.; Rawdah, T.N.; Macy, J.I.; Boswell, F.M., 3rd; Smith, R.E. Meibomian gland studies: Comparison of steer and human lipids. Investig. Ophthalmol. Vis. Sci. 1981, 20, 522–536. [Google Scholar]
- Tiffany, J.M. The normal tear film. Dev. Ophthalmol. 2008, 41, 1–20. [Google Scholar]
- Driver, P.J.; Lemp, M.A. Meibomian gland dysfunction. Surv. Ophthalmol. 1996, 40, 343–367. [Google Scholar] [CrossRef]
- Nicolaides, N.; Santos, E.C.; Smith, R.E.; Jester, J.V. Meibomian gland dysfunction. III. Meibomian gland lipids. Investig. Ophthalmol. Vis. Sci. 1989, 30, 946–951. [Google Scholar]
- Zhao, H.; Wu, S.N.; Shao, Y.; Xiao, D.; Tang, L.Y.; Cheng, Z.; Peng, J. Lipidomics Profiles Revealed Alterations in Patients with Meibomian Gland Dysfunction After Exposure to Intense Pulsed Light. Front. Neurol. 2022, 13, 827544. [Google Scholar] [CrossRef]
- Butovich, I.A.; McMahon, A.; Wojtowicz, J.C.; Lin, F.; Mancini, R.; Itani, K. Dissecting lipid metabolism in meibomian glands of humans and mice: An integrative study reveals a network of metabolic reactions not duplicated in other tissues. Biochim. Biophys. Acta 2016, 1861, 538–553. [Google Scholar] [CrossRef]
- Butovich, I.A.; Wilkerson, A.; Bhat, N.; McMahon, A.; Yuksel, S. On the pivotal role of Elovl3/ELOVL3 in meibogenesis and ocular physiology of mice. FASEB J. 2019, 33, 10034–10048. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, A.; Bhat, N.; Quoc Hai Pham, H.; Yuksel, S.; Butovich, I. Physiological effects of inactivation and the roles of Elovl3/ELOVL3 in maintaining ocular homeostasis. FASEB J. 2021, 35, e21327. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Yuksel, S.; Bhat, N.; Pham, H.; Wilkerson, A.; Butovich, I.A. Inactivation of Awat2 in mice causes loss of wax ester lipids from meibum. Investig. Ophthalmol. Vis. Sci. 2020, 61, 2632. [Google Scholar]
- Hisey, E.A.; Wong, S.; Park, S.; Gamarra, K.A.; Adelman, S.A.; Knickelbein, K.E.; Quan, M.; Ferneding, M.H.; McCorkell, M.; Daley, N.; et al. Meibomian gland lipid alterations and ocular surface sequela in Awat2 knockout murine model of meibomian gland dysfunction and evaporative dry eye disease. Ocul. Surf. 2024, 34, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Wilkerson, A.; Yuksel, S. Depletion of Cholesteryl Esters Causes Meibomian Gland Dysfunction-Like Symptoms in a Soat1-Null Mouse Model. Int. J. Mol. Sci. 2021, 22, 1583. [Google Scholar] [CrossRef]
- Wu, L.; Belyaeva, O.V.; Adams, M.K.; Klyuyeva, A.V.; Lee, S.A.; Goggans, K.R.; Kesterson, R.A.; Popov, K.M.; Kedishvili, N.Y. Mice lacking the epidermal retinol dehydrogenases SDR16C5 and SDR16C6 display accelerated hair growth and enlarged meibomian glands. J. Biol. Chem. 2019, 294, 17060–17074. [Google Scholar] [CrossRef]
- Miyamoto, M.; Sassa, T.; Sawai, M.; Kihara, A. Lipid polarity gradient formed by omega-hydroxy lipids in tear film prevents dry eye disease. elife 2020, 9, e53582. [Google Scholar] [CrossRef]
- Miyazaki, M.; Man, W.C.; Ntambi, J.M. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J. Nutr. 2001, 131, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Russell, D.W. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 2004, 279, 37789–37797. [Google Scholar] [CrossRef]
- Butovich, I.A.; Lu, H.; McMahon, A.; Eule, J.C. Toward an animal model of the human tear film: Biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6881–6896. [Google Scholar] [CrossRef]
- Sawai, M.; Watanabe, K.; Tanaka, K.; Kinoshita, W.; Otsuka, K.; Miyamoto, M.; Sassa, T.; Kihara, A. Diverse meibum lipids produced by Awat1 and Awat2 are important for stabilizing tear film and protecting the ocular surface. iScience 2021, 24, 102478. [Google Scholar] [CrossRef]
- Butovich, I.A.; Wilkerson, A.; Goggans, K.R.; Belyaeva, O.V.; Kedishvili, N.Y.; Yuksel, S. Sdr16c5 and Sdr16c6 control a dormant pathway at a bifurcation point between meibogenesis and sebogenesis. J. Biol. Chem. 2023, 299, 104725. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.K.; Silvaroli, J.A.; Chelstowska, S.; Trischman, T.; Bederman, I.; Sayegh, R.; Golczak, M. Deficiency in Acyl-CoA:Wax Alcohol Acyltransferase 2 causes evaporative dry eye disease by abolishing biosynthesis of wax esters. FASEB J. 2020, 34, 13792–13808. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, S.; Butovich, I.A. Biosynthesis of fatty aldehydes and alcohols in the eye and their role in meibogenesis. J. Biol. Chem. 2025, 301, 110330. [Google Scholar] [CrossRef]
- Kaswan, R.; Pappas, C., Jr.; Wall, K.; Hirsh, S.G. Survey of canine tear deficiency in veterinary practice. Adv. Exp. Med. Biol. 1998, 438, 931–939. [Google Scholar] [PubMed]
- Ofri, R.; Orgad, K.; Kass, P.H.; Dikstein, S. Canine meibometry: Establishing baseline values for meibomian gland secretions in dogs. Vet. J. 2007, 174, 536–540. [Google Scholar] [CrossRef]
- Vinas, M.; Maggio, F.; D’Anna, N.; Rabozzi, R.; Peruccio, C. Meibomian gland dysfunction (MGD), as diagnosed by non-contact infrared Meibography, in dogs with ocular surface disorders (OSD): A retrospective study. BMC Vet. Res. 2019, 15, 443. [Google Scholar] [CrossRef]
- Sebbag, L.; Mochel, J.P. An eye on the dog as the scientist’s best friend for translational research in ophthalmology: Focus on the ocular surface. Med. Res. Rev. 2020, 40, 2566–2604. [Google Scholar] [CrossRef]
- Sun, M.; Moreno, I.Y.; Dang, M.; Coulson-Thomas, V.J. Meibomian Gland Dysfunction: What Have Animal Models Taught Us? Int. J. Mol. Sci. 2020, 21, 8822. [Google Scholar] [CrossRef]
- Butovich, I.A.; Borowiak, A.M.; Eule, J.C. Comparative HPLC-MS analysis of canine and human meibomian lipidomes: Many similarities, a few differences. Sci. Rep. 2011, 1, 24. [Google Scholar] [CrossRef]
- Huang, W.; Tourmouzis, K.; Perry, H.; Honkanen, R.A.; Rigas, B. Animal models of dry eye disease: Useful, varied and evolving (Review). Exp. Ther. Med. 2021, 22, 1394. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Mircheff, A.K.; Geerling, G. Animal models of dry eye. Dev. Ophthalmol. 2008, 41, 298–312. [Google Scholar] [PubMed]
- Prasad, D.; Salman, M.; Reddy, A.A.; Jaffet, J.; Sahoo, A.; Jakati, S.; Bokara, K.K.; Singh, S.; Basu, S.; Singh, V.; et al. A review of rabbit models of meibomian gland dysfunction and scope for translational research. Indian. J. Ophthalmol. 2023, 71, 1227–1236. [Google Scholar] [CrossRef]
- Butovich, I.A. The Meibomian puzzle: Combining pieces together. Prog. Retin. Eye Res. 2009, 28, 483–498. [Google Scholar] [CrossRef]
- Butovich, I.A. Tear film lipids. Exp. Eye Res. 2013, 117, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Widjaja-Adhi, M.A.K.; Chao, K.; Golczak, M. Mouse models in studies on the etiology of evaporative dry eye disease. Exp. Eye Res. 2022, 219, 109072. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Madigan, M.C.; Stapleton, F.; Willcox, M.; Golebiowski, B. Human meibomian gland epithelial cell culture models: Current progress, challenges, and future directions. Ocul. Surf. 2022, 23, 96–113. [Google Scholar] [CrossRef]
- Butovich, I.A. Meibomian glands, meibum, and meibogenesis. Exp. Eye Res. 2017, 163, 2–16. [Google Scholar] [CrossRef]
- Pucker, A.D.; Nichols, J.J. Analysis of meibum and tear lipids. Ocul. Surf. 2012, 10, 230–250. [Google Scholar] [CrossRef]
- Lu, H.; Wojtowicz, J.C.; Butovich, I.A. Differential scanning calorimetric evaluation of human meibomian gland secretions and model lipid mixtures: Transition temperatures and cooperativity of melting. Chem. Phys. Lipids 2013, 170–171, 55–64. [Google Scholar] [CrossRef]
- Efron, N.; Young, G.; Brennan, N.A. Ocular surface temperature. Curr. Eye Res. 1989, 8, 901–906. [Google Scholar]
- Morgan, P.B.; Tullo, A.B.; Efron, N. Infrared thermography of the tear film in dry eye. Eye 1995, 9 Pt 5, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Moussa, S.; Eppig, T.; Pattmoller, J.; Zemova, E.; Seitz, B.; Langenbucher, A.; Szentmary, N. Diurnal and zonal analysis of corneal surface temperature in young healthy adults. Eur. J. Ophthalmol. 2013, 23, 641–645. [Google Scholar] [CrossRef]
- de Ortueta, D.; Magnago, T.; Arba-Mosquera, S. Thermodynamic measurement after cooling the cornea with intact epithelium and lid manipulation. J. Optom. 2015, 8, 170–173. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Zhang, L. Association of Tear Film Stability and Corneal Surface Temperature in Pudong Patients. Curr. Eye Res. 2017, 42, 655–660. [Google Scholar] [CrossRef]
- Butovich, I.A.; Suzuki, T. Delineating a novel metabolic high triglycerides-low waxes syndrome that affects lipid homeostasis in meibomian and sebaceous glands. Exp. Eye Res. 2020, 199, 108189. [Google Scholar] [CrossRef]
- Butovich, I.A.; Schatz, M.; Saboo, U.S.; Wojtowicz, J.C.; Johnson, D.A. Abnormal meibum is associated with SREBF1 mutation and IFAP syndrome 2. Exp. Eye Res. 2025, 262, 110703. [Google Scholar] [CrossRef]
- Kupcu, S.; Lohner, K.; Mader, C.; Sleytr, U.B. Microcalorimetric study on the phase behaviour of S-layer coated liposomes. Mol. Membr. Biol. 1998, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, A.; Pitto, M.; Palestini, P.; Masserini, M. Lipid domains in the membrane: Thermotropic properties of sphingomyelin vesicles containing GM1 ganglioside and cholesterol. Biochemistry 1997, 36, 9232–9236. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.; Levanon, E.Y. Human housekeeping genes, revisited. Trends Genet. 2013, 29, 569–574, Erratum in Trends Genet. 2014, 30, 119–120. [Google Scholar] [CrossRef]
- Nagymihalyi, A.; Dikstein, S.; Tiffany, J.M. The influence of eyelid temperature on the delivery of meibomian oil. Exp. Eye Res. 2004, 78, 367–370. [Google Scholar] [CrossRef]
- Blackie, C.A.; Solomon, J.D.; Greiner, J.V.; Holmes, M.; Korb, D.R. Inner eyelid surface temperature as a function of warm compress methodology. Optom. Vis. Sci. 2008, 85, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kremers, I.; Hohberger, B.; Bergua, A. Infrared thermography: Different options of thermal eyelid warming. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1515–1522. [Google Scholar] [CrossRef]
- Cohen, G.Y.; Ben-David, G.; Singer, R.; Benyosef, S.; Shemesh, R.; Leshno, A.; Barkana, Y.; Skaat, A. Ocular Surface Temperature: Characterization in a Large Cohort of Healthy Human Eyes and Correlations to Systemic Cardiovascular Risk Factors. Diagnostics 2021, 11, 1877. [Google Scholar] [CrossRef]
- Ding, J.E.; Kim, Y.H.; Yi, S.M.; Graham, A.D.; Li, W.; Lin, M.C. Ocular surface cooling rate associated with tear film characteristics and the maximum interblink period. Sci. Rep. 2021, 11, 15030. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Wagner, H.; Gmoser, J.; Worner, A.; Loschberger, A.; Peters, L.; Frey, A.; Hofmann, U.; Frantz, S. Touch-free measurement of body temperature using close-up thermography of the ocular surface. MethodsX 2016, 3, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Graham, A.D.; Selvin, S.; Lin, M.C. Ocular Surface Cooling Corresponds to Tear Film Thinning and Breakup. Optom. Vis. Sci. 2015, 92, e248–e256. [Google Scholar] [CrossRef]
- Craig, J.P.; Singh, I.; Tomlinson, A.; Morgan, P.B.; Efron, N. The role of tear physiology in ocular surface temperature. Eye 2000, 14 Pt 4, 635–641. [Google Scholar] [CrossRef]
- Matteoli, S.; Favuzza, E.; Mazzantini, L.; Aragona, P.; Cappelli, S.; Corvi, A.; Mencucci, R. Ocular surface temperature in patients with evaporative and aqueous-deficient dry eyes: A thermographic approach. Physiol. Meas. 2017, 38, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Arciniega, J.C.; Wojtowicz, J.C. Meibomian lipid films and the impact of temperature. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5508–5518. [Google Scholar] [CrossRef]
- Wilkerson, A.; Yuksel, S.; Acharya, R.; Butovich, I.A. Physiological Effects of Soat1 Inactivation on Homeostasis of the Mouse Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2024, 65, 2. [Google Scholar] [CrossRef]
- Reitman, M.L. Of mice and men—Environmental temperature, body temperature, and treatment of obesity. FEBS Lett. 2018, 592, 2098–2107. [Google Scholar] [CrossRef]
- Sealander, J.A. Body temperatures of white-footed mice in relation to environmental temperature and heat and cold stress. Biol. Bull. 1953, 104, 87–99. [Google Scholar] [CrossRef]
- Weir, J.A. The temperature of the Mouse in Health and Disease. Proc. Iowa Acad. Sci. USA 1946, 54, 383–388. [Google Scholar]
- Spinella, G.; Galimberti, A.; Casagrande, G.; Maffi, S.; Musella, V.; Valentini, S. Ocular and Superficial Body Thermographic Findings in Sled Dogs before and after Competition. Animals 2023, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Biondi, F.; Dornbusch, P.T.; Sampaio, M.; Montiani-Ferreira, F. Infrared ocular thermography in dogs with and without keratoconjunctivitis sicca. Vet. Ophthalmol. 2015, 18, 28–34. [Google Scholar] [CrossRef]
- Zanghi, B.M. Eye and Ear Temperature Using Infrared Thermography Are Related to Rectal Temperature in Dogs at Rest or With Exercise. Front. Vet. Sci. 2016, 3, 111. [Google Scholar] [CrossRef] [PubMed]
- Hisey, E.A.; Galor, A.; Leonard, B.C. A comparative review of evaporative dry eye disease and meibomian gland dysfunction in dogs and humans. Vet. Ophthalmol. 2023, 26 (Suppl. 1), 16–30. [Google Scholar] [CrossRef]
- Butovich, I.A.; Bhat, N.; Wojtowicz, J.C. Comparative Transcriptomic and Lipidomic Analyses of Human Male and Female Meibomian Glands Reveal Common Signature Genes of Meibogenesis. Int. J. Mol. Sci. 2019, 20, 4539. [Google Scholar] [CrossRef]
- Butovich, I.A.; McMahon, A.; Wojtowicz, J.C.; Bhat, N.; Wilkerson, A. Effects of sex (or lack thereof) on meibogenesis in mice (Mus musculus): Comparative evaluation of lipidomes and transcriptomes of male and female tarsal plates. Ocul. Surf. 2019, 17, 793–808. [Google Scholar] [CrossRef]
- Spink, C.H. The deconvolution of differential scanning calorimetry unfolding transitions. Methods 2015, 76, 78–86. [Google Scholar] [CrossRef]
- Chiu, M.H.; Prenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied Sci. 2011, 3, 39–59. [Google Scholar] [CrossRef]


| Animals and Human Subjects | Ocular and Skin Features and Abnormalities | Major Features of MG Lipidomes | Tm, °C (Mean ± SD) * | T1/2, °C (Mean ± SD) * |
|---|---|---|---|---|
| C57BL6/J mice | None | Similar to human | 28 ± 1 | 19 ± 3 |
| Awat2-knockout mice | Severe ocular and Meibomian gland phenotype; solid meibum | WE ↓, free Chl ↑, and CE ↑ | Multiple melting peaks between 20 °C and 70 °C | Extremely wide, >40 °C |
| Soat1-knockout mice | Severe ocular and Meibomian gland phenotype; solid meibum | Lacks CE, free Chl ↑↑↑ | Biphasic melting curve; 30 ± 2 (1st) 50 ± 1 (2nd) | Each peak wider than >20 |
| Elovl3-knockout mice | Severe ocular and Meibomian gland phenotype; liquefied meibum | Abnormal WE and CE elongation and unsaturation patterns; ELC WE ↓ | Very low, <0 | n/d |
| Sdr16c5/Sdr16c6 double knockout mice | Severe ocular and Meibomian gland phenotype; semi-liquefied meibum | Transformed into a sebum-like lipidome; TAG ↑ and short WE ↑ | 19 ± 1 | 30 ± 5 |
| Healthy canines | None | Similar to human | 29 ± 1 | 13 ± 2 |
| White New Zealand rabbits | Extremely stable tear film | Based primarily on DiHL, DiHLE, DiAD, steryl esters (not CE), and OAHFA | 33 ± 1 | 8 ± 1 |
| Normal human subjects | None | Based primarily on ELC saturated and mono-unsaturated WE, CE, Chl, TAG, OAHFA, Chl-OAHFA, and DiAD | 29 ± 1 | 10 ± 1 |
| Abnormal human Subject #1 | Lard-like meibum; abnormal tear film with lipid rafts | TAG ↑, short WE ↑, and ELC WE ↓ | Biphasic melting curve; 21 ± 2 (1st) 57 ± 2 (2nd) | ~17 (1st) ~15 (2nd) |
| Abnormal human Subject #2 | Dry eye, skin, and solid meibum | Saturated WE ↑↑↑ | 39 ± 1 | 12 ± 1 |
| Normal Human Subject | Gender (Age, in Years) | Meibum Tm, °C, * | Meibum T1/2, °C * |
|---|---|---|---|
| N-1 | M (41) | 29.2 | 10.9 |
| N-2 | M (56) | 28.6 | 9.9 |
| N-3 | F (48) | 30.9 | 10.2 |
| N-4 | M (25) | 29.1 | 9.3 |
| N-5 | F (41) | 27.8 | 11.2 |
| N-6 | not recorded | 29.6 | 10.7 |
| N-7 | F (42) | 28.5 | 9.3 |
| N-8 | F (27) | 28.3 | 9.9 |
| N-9 | F (28) | 27.7 | 11.0 |
| N-10 | F (40) | 28.6 | 9.62 |
| N-11 | F (60) | 28.1 | 10.6 |
| Average temperature (Mean ± SD) | 28.8 ± 0.9 | 10.2 ± 0.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butovich, I.A.; Wojtowicz, J.C.; Wilkerson, A.; Yuksel, S. Lipid Composition and Thermotropic Properties of Meibum of Animal Models and Humans with Meibomian Gland Dysfunction. Int. J. Mol. Sci. 2025, 26, 11434. https://doi.org/10.3390/ijms262311434
Butovich IA, Wojtowicz JC, Wilkerson A, Yuksel S. Lipid Composition and Thermotropic Properties of Meibum of Animal Models and Humans with Meibomian Gland Dysfunction. International Journal of Molecular Sciences. 2025; 26(23):11434. https://doi.org/10.3390/ijms262311434
Chicago/Turabian StyleButovich, Igor A., Jadwiga C. Wojtowicz, Amber Wilkerson, and Seher Yuksel. 2025. "Lipid Composition and Thermotropic Properties of Meibum of Animal Models and Humans with Meibomian Gland Dysfunction" International Journal of Molecular Sciences 26, no. 23: 11434. https://doi.org/10.3390/ijms262311434
APA StyleButovich, I. A., Wojtowicz, J. C., Wilkerson, A., & Yuksel, S. (2025). Lipid Composition and Thermotropic Properties of Meibum of Animal Models and Humans with Meibomian Gland Dysfunction. International Journal of Molecular Sciences, 26(23), 11434. https://doi.org/10.3390/ijms262311434








