The Double Life of Plant-Based Food Waste: A Source of Phenolic Acids and a Carrier for Immobilization of Lipases Capable of Their Lipophilization
Abstract
1. Introduction
2. Results
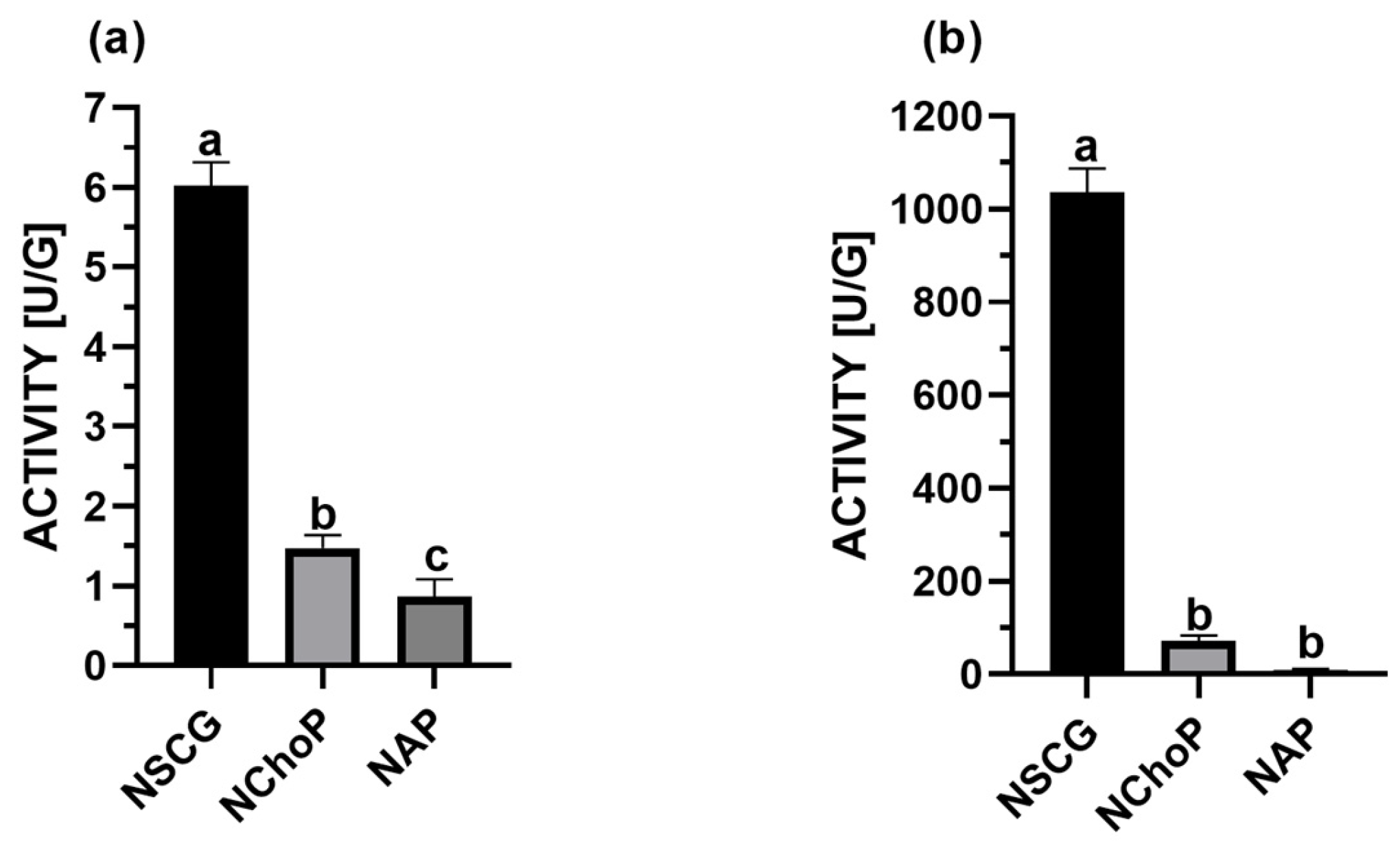
2.1. Activity Evaluation of Immobilized Lipase
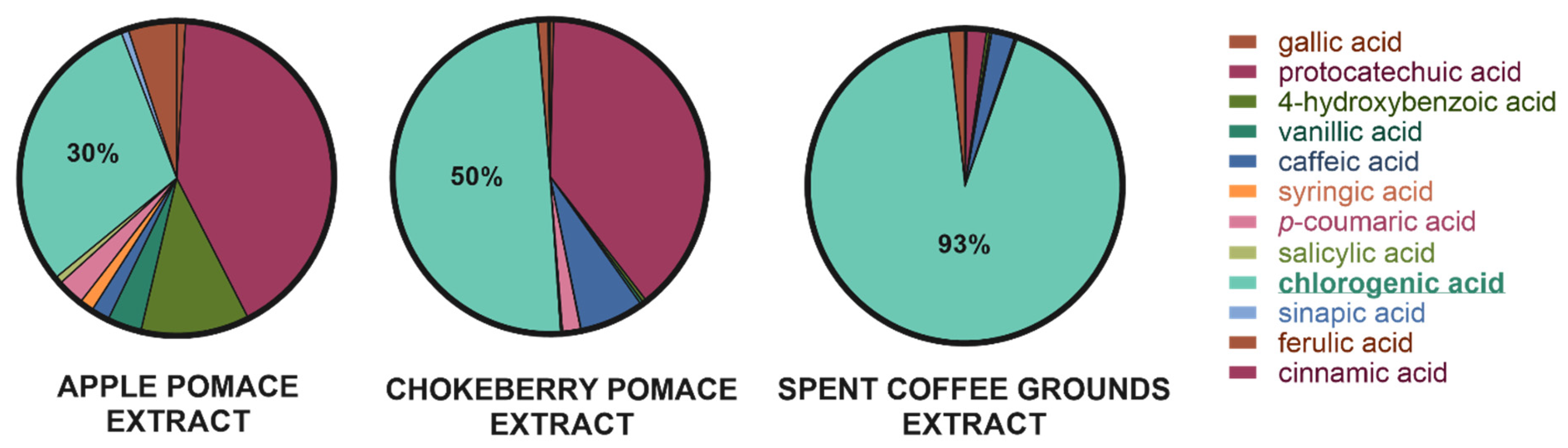
2.2. Content of Selected Phenolic Acids in Lyophilized Extracts
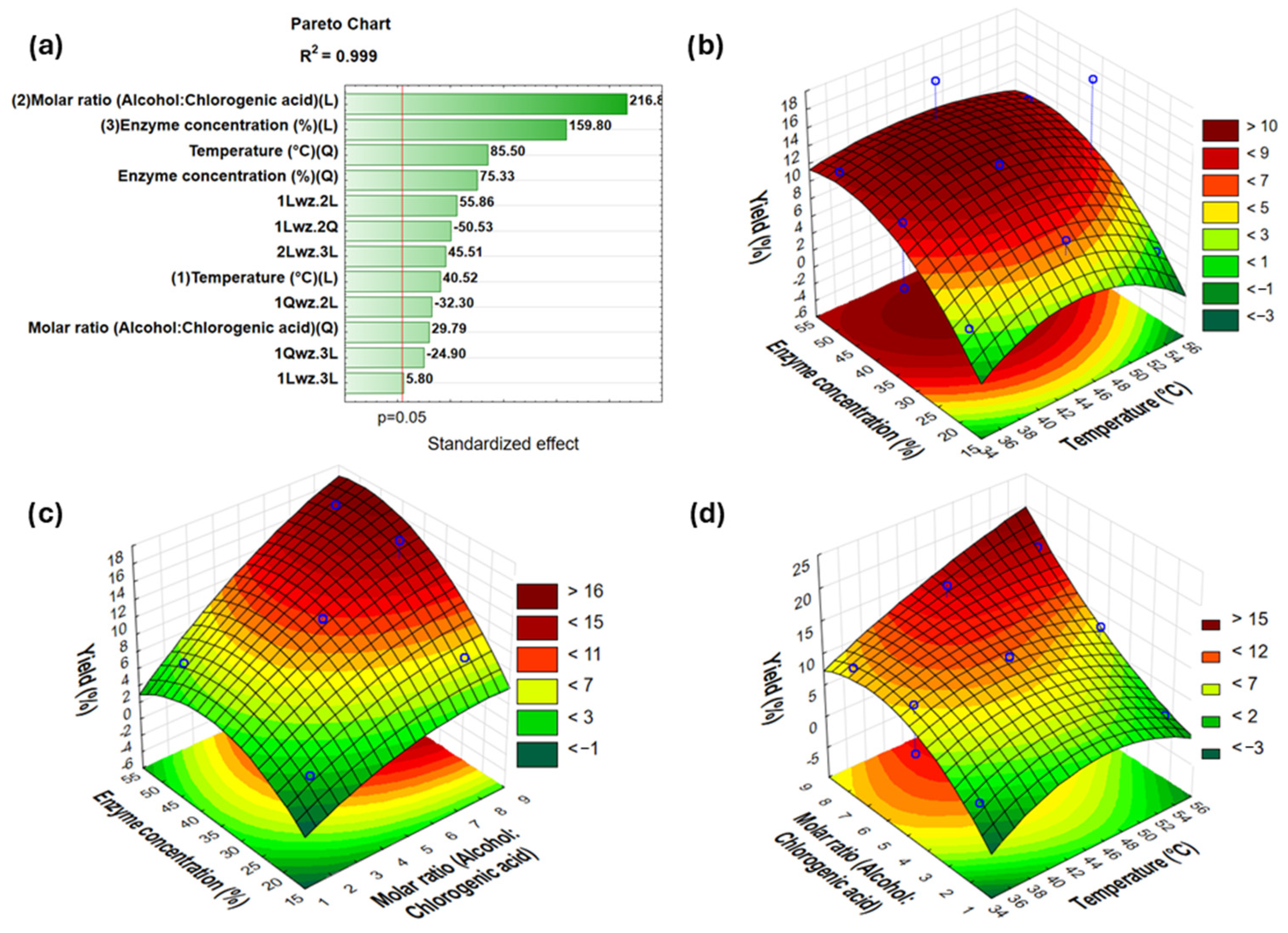
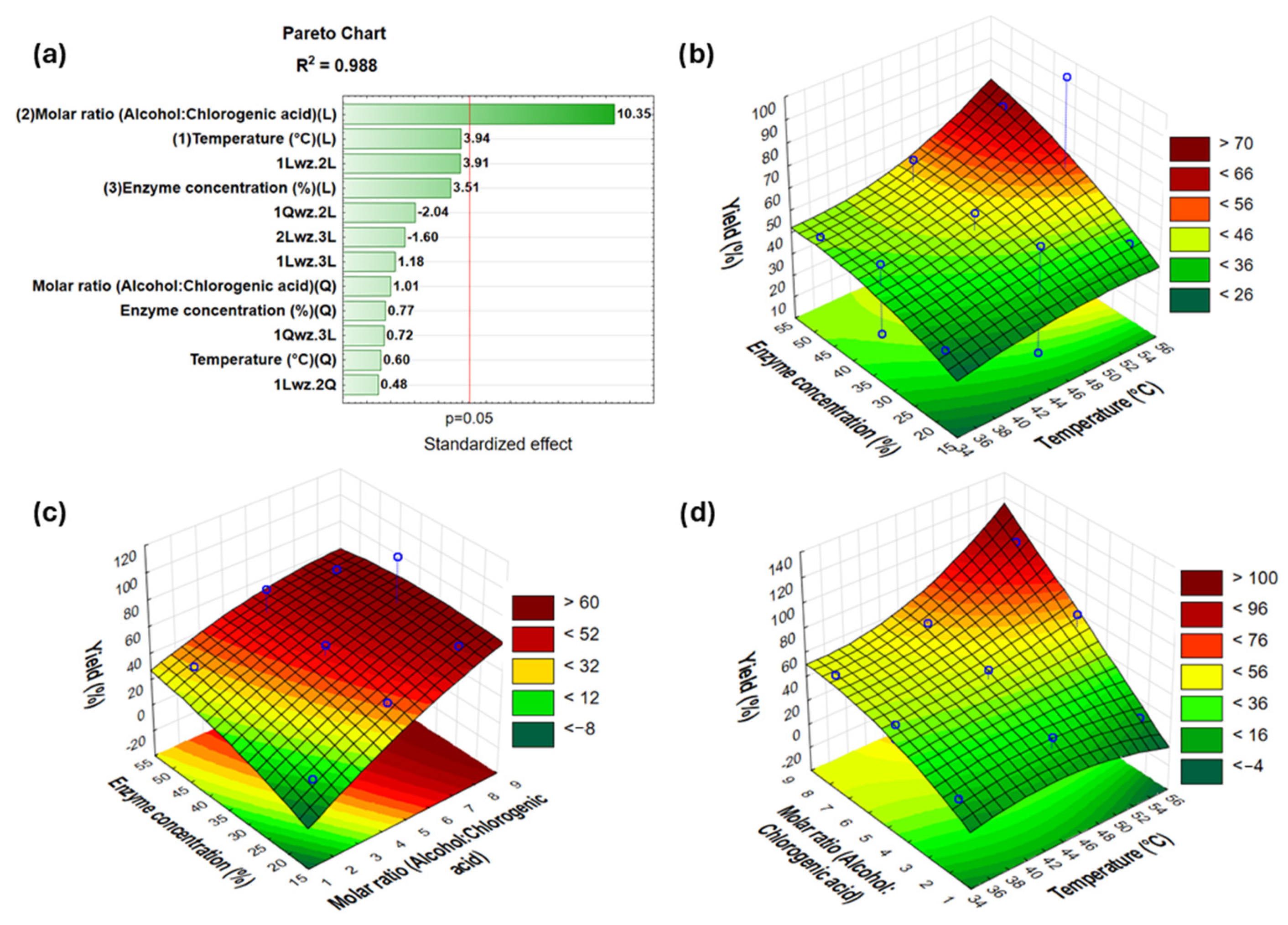
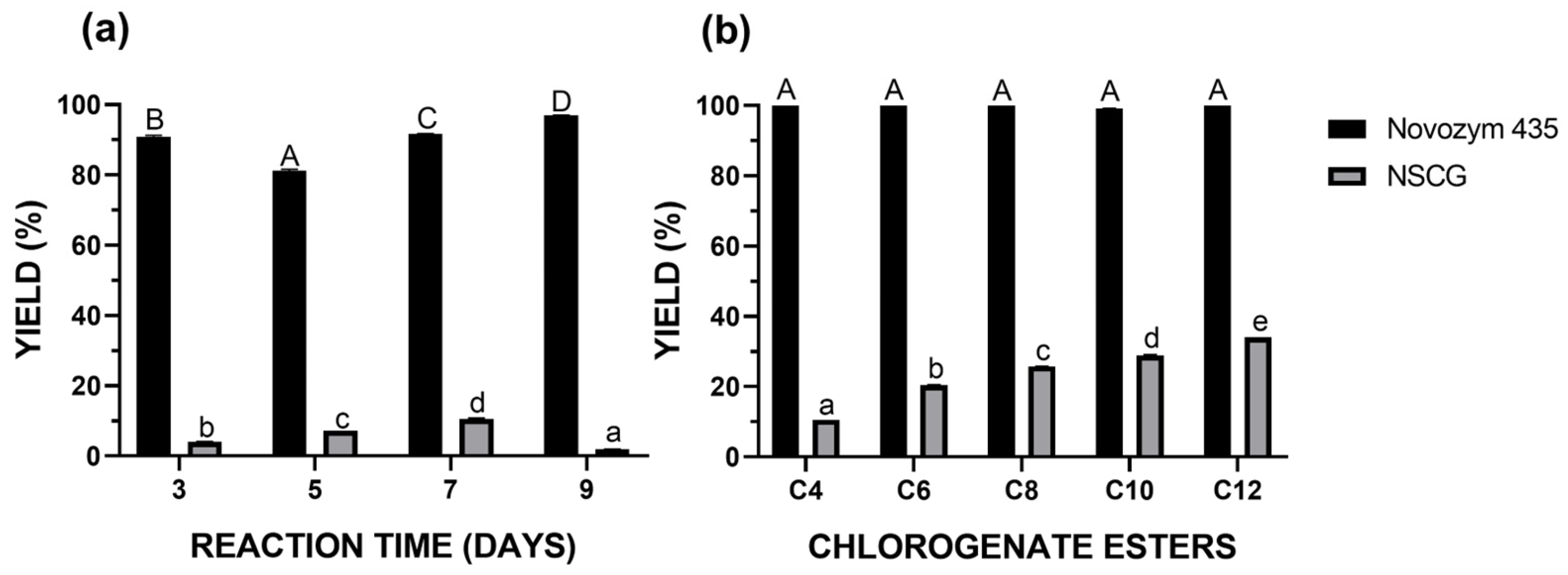
2.3. Optimization of Synthesis Reaction
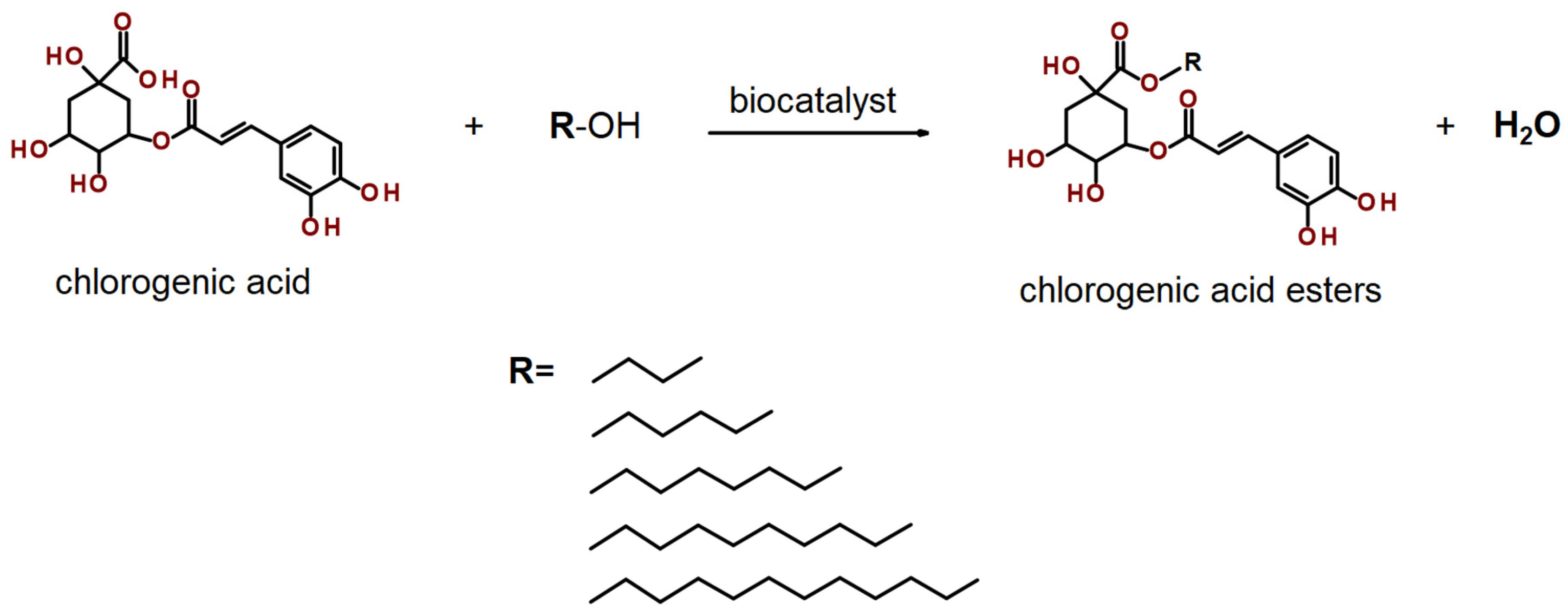
2.4. Extracts Lipophilization
2.5. Properties of Obtained Esters of Chlorogenic Acid and Pre- and Postmodified Extracts
3. Discussion
3.1. Activity of Biocatalysts Immobilized on Food Waste Materials
3.2. Enzymatic Modification of Chlorogenic Acid and Food Waste Extracts
3.3. Properties of Obtained Esters of Chlorogenic Acid and Pre- and Postmodified Extracts
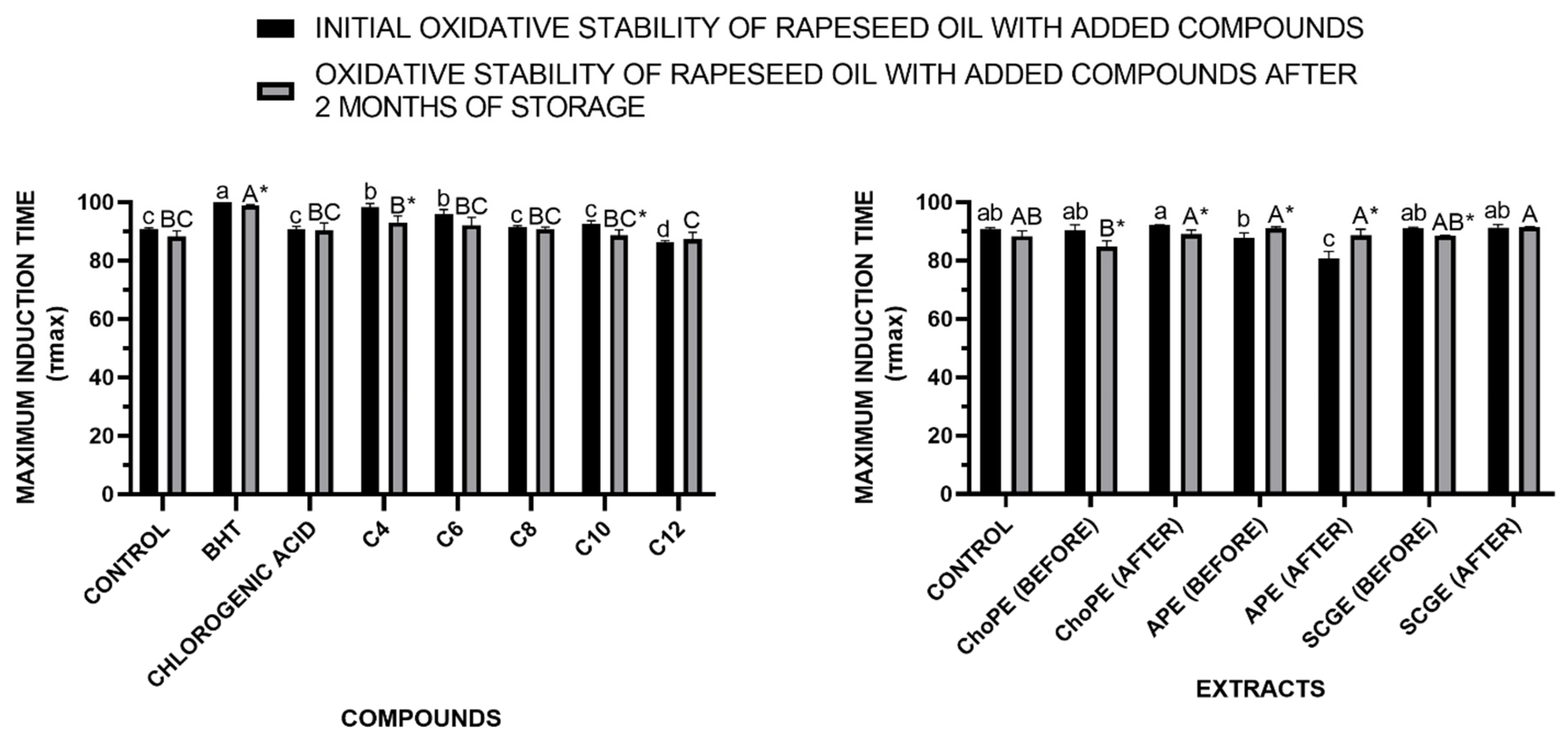
3.3.1. Antioxidant Properties
3.3.2. Antimicrobial Properties
3.3.3. Oxidative Stability in Rapeseed Oil
4. Materials and Methods
4.1. Materials
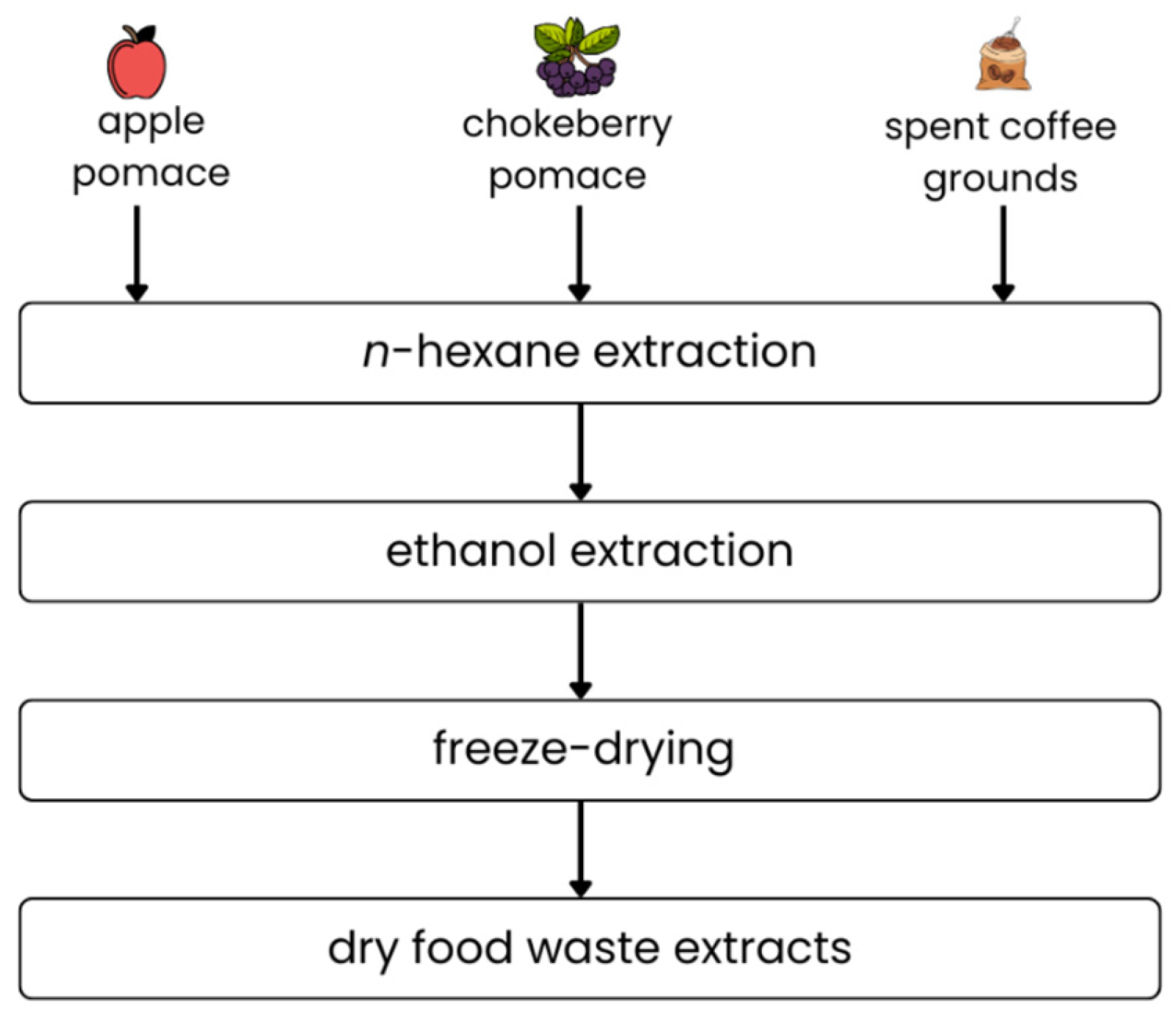
4.2. Preparation of Food Waste
4.3. Lipase Immobilization Procedure
4.4. Lipase Activity Assay—Hydrolytic and Synthetic Activities
4.5. Elemental Compositions of Biocatalysts and Their Supports
4.6. Optimization of Synthesis Reaction—Box–Behnken Design
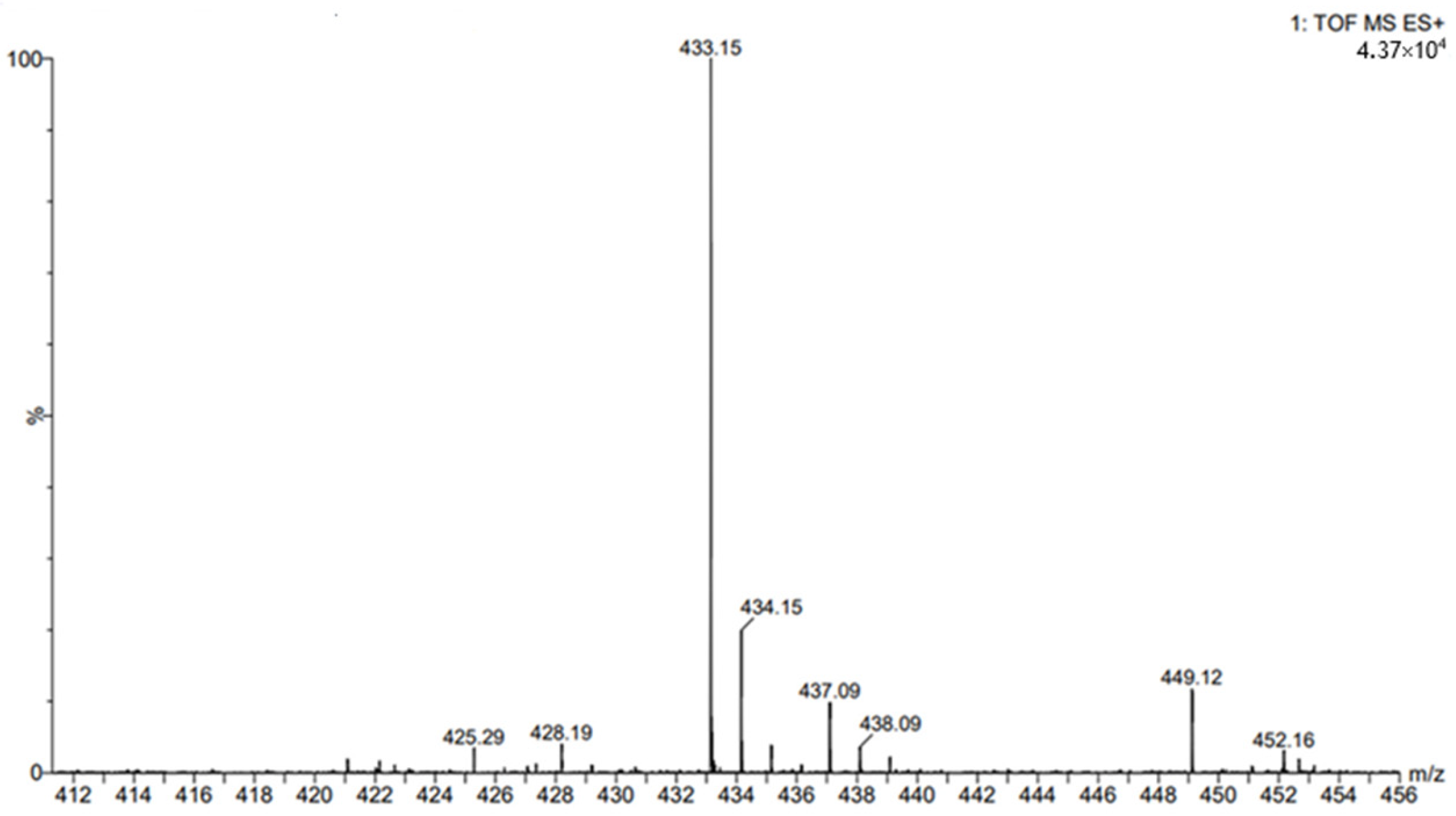
4.7. Chromatographic Determination of Esterified Post-Reaction Mixtures
4.8. Column Chromatography and NMR Analysis
- Butyl chlorogenate
- 13C NMR (126 MHz, DMSO-d6) δ 173.19, 165.43, 148.61, 145.70, 145.17, 125.34, 121.34, 115.85, 114.55, 113.81, 73.12, 71.09, 69.40, 66.91, 64.12, 37.23, 35.09, 30.03, 18.54, 13.56.
- Hexyl chlorogenate
- 13C NMR (126 MHz, DMSO-d6) δ 173.16, 165.35, 148.54, 145.66, 145.15, 125.32, 121.27, 115.79, 114.55, 113.75, 73.01, 71.07, 69.27, 66.75, 64.37, 37.22, 34.97, 30.80, 27.88, 24.90, 21.97, 13.78.
- Octyl chlorogenate
- 13C NMR (126 MHz, DMSO-d6) δ 173.16, 165.35, 148.53, 145.65, 145.15, 125.31, 121.26, 115.77, 114.53, 113.73, 73.01, 71.07, 69.28, 66.77, 64.36, 37.22, 34.99, 31.11, 28.57, 27.92, 25.24, 22.05, 13.94.
- Decyl chlorogenate
- 13C NMR (126 MHz, DMSO-d6) δ 173.16, 165.36, 148.57, 145.67, 145.16, 125.29, 121.27, 115.77, 114.51, 113.71, 73.04, 71.06, 69.29, 66.80, 64.37, 37.22, 35.03, 31.31, 29.12, 29.00, 28.97, 28.92, 28.85, 28.73, 22.10, 13.97.
- Dodecyl chlorogenate
- 13C NMR (126 MHz, DMSO-d6) δ 173.15, 165.35, 148.54, 145.65, 145.14, 125.30, 121.25, 115.76, 114.51, 113.73, 73.04, 71.05, 69.31, 66.80, 64.35, 37.21, 35.03, 31.30, 29.02, 28.99, 28.91, 28.89, 28.72, 28.60, 27.92, 25.24, 22.11, 13.97.
4.9. ESI- MS Experiments
4.10. Lipophilization Process
4.11. LC-MS Assay of the Obtained Extracts
4.12. Total Polyphenol Content
4.13. Evaluation of Antioxidant Properties
4.13.1. The DPPH Assay
4.13.2. CUPRAC Method
4.14. Evaluation of Antimicrobial Properties
4.15. PDSC Measurements
4.16. Lipophilicity Analysis
4.17. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Apple pomace |
| NAP | Lipase from A.oryzae (Novozym 51032) immobilized on apple pomace |
| CGA | Chlorogenic acid |
| ChoP | Chokeberry pomace |
| NChoP | Lipase from A.oryzae (Novozym 51032) immobilized on chokeberry pomace |
| NSCG | Lipase from A.oryzae (Novozym 51032) immobilized on spent coffee grounds |
| SCG | Spent coffee grounds |
References
- UN Environment Programme. UNEP Food Waste Index Report 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 29 April 2025).
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992, Erratum in Science 2019, 363, 6429. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; R, R.; Sirohi, R. Remodeling Agro-Industrial and Food Wastes into Value-Added Bioactives and Biopolymers. Ind. Crops Prod. 2020, 154, 112621. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry, 2006th ed.; Springer: New York, NY, USA, 2008; ISBN 9781402051630. [Google Scholar]
- Durand, E.; Bayrasy, C.; Laguerre, M.; Barouh, N.; Lecomte, J.; Durand, T.; Balas, L.; Wrutniak-Cabello, C.; Cabello, G.; Villeneuve, P. Regioselective Synthesis of Diacylglycerol Rosmarinates and Evaluation of Their Antioxidant Activity in Fibroblasts: Diacylglycerol Rosmarinates as Novel Antioxidants. Eur. J. Lipid Sci. Technol. 2015, 117, 1159–1170. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Villeneuve, P. The Biological and Antimicrobial Activities of Phenolipids. Lipid Technol. 2017, 29, 67–70. [Google Scholar] [CrossRef]
- Ramadan, M.F. Pheno-Phospholipids and Lipo-Phenolics: Novel Structured Antioxidants, 1st ed.; Springer Nature: Cham, Switzerland, 2022; ISBN 9783030674014. [Google Scholar]
- Laguerre, M.; Bayrasy, C.; Lecomte, J.; Chabi, B.; Decker, E.A.; Wrutniak-Cabello, C.; Cabello, G.; Villeneuve, P. How to Boost Antioxidants by Lipophilization? Biochimie 2013, 95, 20–26. [Google Scholar] [CrossRef]
- Mardani, M.; Badakné, K.; Farmani, J.; Shahidi, F. Enzymatic Lipophilization of Bioactive Compounds with High Antioxidant Activity: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 4977–4994. [Google Scholar] [CrossRef]
- Alemán, M.; Bou, R.; Guardiola, F.; Durand, E.; Villeneuve, P.; Jacobsen, C.; Sørensen, A.-D.M. Antioxidative Effect of Lipophilized Caffeic Acid in Fish Oil Enriched Mayonnaise and Milk. Food Chem. 2015, 167, 236–244. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Laguerre, M.; Villeneuve, P.; Lecomte, J. From Phenolics to Phenolipids: Optimizing Antioxidants in Lipid Dispersions. Lipid Technol. 2013, 25, 131–134. [Google Scholar] [CrossRef]
- de Araújo, M.E.M.B.; Franco, Y.E.M.; Messias, M.C.F.; Longato, G.B.; Pamphile, J.A.; Carvalho, P.d.O. Biocatalytic Synthesis of Flavonoid Esters by Lipases and Their Biological Benefits. Planta Med. 2017, 83, 7–22. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial Lipases and Their Industrial Applications: A Comprehensive Review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized Microbial Lipases in the Food Industry: A Systematic Literature Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Y.; Simpson, B. Food Enzymes Immobilization: Novel Carriers, Techniques and Applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase Immobilization with Support Materials, Preparation Techniques, and Applications: Present and Future Aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized Lipases-Based Nano-Biocatalytic Systems—A Versatile Platform with Incredible Biotechnological Potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, Immobilization Methods, and Industrial Applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Palma, B.G.; Leão, R.A.C.; M.A. de Souza, R.O.; G. Pandoli, O. Immobilization of Lipases on Lignocellulosic Bamboo Powder for Biocatalytic Transformations in Batch and Continuous Flow. Catal. Today 2021, 381, 280–287. [Google Scholar] [CrossRef]
- de França Serpa, J.; Matias, G.A.B.; Fechine, P.B.A.; da Costa, V.M.; Freire, R.M.; Denardin, J.C.; Gonçalves, L.R.B.; de Macedo, A.C.; Rocha, M.V.P. New Nanocomposite Made of Cashew Apple Bagasse Lignin and Fe3O4 for Immobilizing of Lipase B from Candida antarctica Aiming at Esterification. J. Chem. Technol. Biotechnol. 2021, 96, 2472–2487. [Google Scholar] [CrossRef]
- Oliveira-Ribeiro, L.M.; Meili, L.; Silva-Belo-Gois, G.N.; Rosas-Garcia-Almeida, R.M.; da Silva-Duarte, J.L. Immobilization of lipase in biochar obtained from Manihot esculenta Crantz. Rev. Ion Investig. Optim. Nuevos Procesos Ing. 2019, 32, 7–13. [Google Scholar] [CrossRef]
- de S. Lira, R.K.; Zardini, R.T.; de Carvalho, M.C.C.; Wojcieszak, R.; Leite, S.G.F.; Itabaiana, I., Jr. Agroindustrial Wastes as a Support for the Immobilization of Lipase from Thermomyces lanuginosus: Synthesis of Hexyl Laurate. Biomolecules 2021, 11, 445. [Google Scholar] [CrossRef]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of Agriculture Waste as a Support for Lipase Immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Carvalho, A.K.F.; Souza, C.R.F.; De Castro, H.F.; Said, S.; Oliveira, W.P. Enzymatic Synthesis of Biodiesel Using Immobilized Lipase on a Non-Commercial Support. Energy Fuels 2016, 30, 4820–4824. [Google Scholar] [CrossRef]
- Nájera-Martínez, E.F.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; Levin, L.N.; Parra-Saldívar, R.; Iqbal, H.M.N. Lignocellulosic Residues as Supports for Enzyme Immobilization, and Biocatalysts with Potential Applications. Int. J. Biol. Macromol. 2022, 208, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Maravić, N.; Stanojev, J.; Hessel, V.; Strelec, I. Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization. Foods 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Mak, S.L.; Wu, M.Y.T.; Chak, W.Y.; Kwong, W.K.; Tang, W.F.; Li, C.H.; Lee, C.C.; Li, C.Y. A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground. Sustainability 2023, 15, 13498. [Google Scholar] [CrossRef]
- Atabani, A.E.; Ali, I.; Naqvi, S.R.; Badruddin, I.A.; Aslam, M.; Mahmoud, E.; Almomani, F.; Juchelková, D.; Atelge, M.R.; Khan, T.M.Y. A State-of-the-Art Review on Spent Coffee Ground (SCG) Pyrolysis for Future Biorefinery. Chemosphere 2022, 286, 131730. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-Industrial Wastes as Potential Carriers for Enzyme Immobilization: A Review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- Chen, K.-I.; Lo, Y.-C.; Liu, C.-W.; Yu, R.-C.; Chou, C.-C.; Cheng, K.-C. Enrichment of Two Isoflavone Aglycones in Black Soymilk by Using Spent Coffee Grounds as an Immobiliser for β-Glucosidase. Food Chem. 2013, 139, 79–85. [Google Scholar] [CrossRef]
- Buntić, A.; Pavlović, M.; Antonović, D.; Pavlović, V.; Vrućinić, D.; Šiler-Marinković, S.; Dimitrijević-Branković, S. Customizing the Spent Coffee for Trichoderma Reesei Cellulase Immobilization by Modification with Activating Agents. Int. J. Biol. Macromol. 2018, 107, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, K.; Nowosad, M.; Perzyna, A.; Bielacki, A.; Dziwiński, S.; Zieniuk, B.; Fabiszewska, A. Sustainable Lipase Immobilization: Chokeberry and Apple Waste as Carriers. Biomolecules 2024, 14, 1564. [Google Scholar] [CrossRef] [PubMed]
- Jasińska, K.; Zieniuk, B.; Piasek, A.M.; Wysocki, Ł.; Sobiepanek, A.; Fabiszewska, A. Obtaining a Biodegradable Biocatalyst—Study on Lipase Immobilization on Spent Coffee Grounds as Potential Carriers. Biocatal. Agric. Biotechnol. 2024, 59, 103255. [Google Scholar] [CrossRef]
- Girelli, A.M.; Chiappini, V.; Amadoro, P. Immobilization of Lipase on Spent Coffee Grounds by Physical and Covalent Methods: A Comparison Study. Biochem. Eng. J. 2023, 192, 108827. [Google Scholar] [CrossRef]
- Guerrero, M.R.B.; Marques da Silva Paula, M.; Zaragoza, M.M.; Gutiérrez, J.S.; Velderrain, V.G.; Ortiz, A.L.; Collins-Martínez, V. Thermogravimetric Study on the Pyrolysis Kinetics of Apple Pomace as Waste Biomass. Int. J. Hydrogen Energy 2014, 39, 16619–16627. [Google Scholar] [CrossRef]
- Osman, A.I.; Young, T.J.; Farrell, C.; Harrison, J.; Al-Muhtaseb, A.H.; Rooney, D.W. Physicochemical Characterization and Kinetic Modeling Concerning Combustion of Waste Berry Pomace. ACS Sustain. Chem. Eng. 2020, 8, 17573–17586. [Google Scholar] [CrossRef]
- Jasińska, K.; Fabiszewska, A.; Białecka-Florjańczyk, E.; Zieniuk, B. Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins. Antioxidants 2022, 11, 1528. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Bebak, A. Aktywność Biologiczna Wybranych Wytłoków Owocowych Oraz Warzywnych. Żywność Nauka Technologia Jakość 2012, 4, 55–65. [Google Scholar]
- Mokhtar, N.F.; Rahman, R.N.Z.R.A.; Muhd Noor, N.D.; Mohd Shariff, F.; Mohamad Ali, M.S. The Immobilization of Lipases on Porous Support by Adsorption and Hydrophobic Interaction Method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Román-Martínez, M.D.C.; Lillo-Ródenas, M.Á. Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider? Molecules 2022, 27, 1630. [Google Scholar] [CrossRef] [PubMed]
- Girelli, A.M.; Chiappini, V. Renewable, Sustainable, and Natural Lignocellulosic Carriers for Lipase Immobilization: A Review. J. Biotechnol. 2023, 365, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Manoel, E.A.; Dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports Involves the Open Form of the Enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Abreu Silveira, E.; Moreno-Perez, S.; Basso, A.; Serban, S.; Pestana Mamede, R.; Tardioli, P.W.; Sanchez Farinas, C.; Rocha-Martin, J.; Fernandez-Lorente, G.; Guisan, J.M. Modulation of the Regioselectivity of Thermomyces lanuginosus Lipase via Biocatalyst Engineering for the Ethanolysis of Oil in Fully Anhydrous Medium. BMC Biotechnol. 2017, 17, 88. [Google Scholar] [CrossRef]
- Brabcová, J.; Demianová, Z.; Vondrášek, J.; Jágr, M.; Zarevúcka, M.; Palomo, J.M. Highly Selective Purification of Three Lipases from Geotrichum Candidum 4013 and Their Characterization and Biotechnological Applications. J. Mol. Catal. B Enzym. 2013, 98, 62–72. [Google Scholar] [CrossRef]
- López Giraldo, L.J.; Laguerre, M.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Barouh, N.; Baréa, B.; Villeneuve, P. Lipase-Catalyzed Synthesis of Chlorogenate Fatty Esters in Solvent-Free Medium. Enzyme Microb. Technol. 2007, 41, 721–726. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Meng, X.; Chen, S.; Huang, D.; Xia, Y.; Zhu, S. Antioxidant Activities of Chlorogenic Acid Derivatives with Different Acyl Donor Chain Lengths and Their Stabilities during in Vitro Simulated Gastrointestinal Digestion. Food Chem. 2021, 357, 129904. [Google Scholar] [CrossRef]
- Gil, M.; Wianowska, D. Chlorogenic Acids-Their Properties, Occurrence and Analysis. Ann. Univ. Mariae Curie-Skłodowska Sect. AA-Chem. 2017, 72, 61. [Google Scholar] [CrossRef]
- Guyot, B.; Gueule, D.; Pina, M.; Graille, J.; Farines, V.; Farines, M. Enzymatic Synthesis of Fatty Esters in 5-Caffeoyl Quinic Acid. Eur. J. Lipid Sci. Technol. 2000, 102, 93–95. [Google Scholar] [CrossRef]
- Lorentz, C.; Dulac, A.; Pencreac’h, G.; Ergan, F.; Richomme, P.; Soultani-Vigneron, S. Lipase-Catalyzed Synthesis of Two New Antioxidants: 4-O- and 3-O-Palmitoyl Chlorogenic Acids. Biotechnol. Lett. 2010, 32, 1955–1960. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, S.; Chen, S.; Xia, Y.; Li, Y. Lipase-Catalyzed Highly Regioselective Synthesis of Acylated Chlorogenic Acid. Food Biosci. 2020, 37, 100706. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple Pomace as Food Fortification Ingredient: A Systematic Review and Meta-Analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Oancea, A.G.; Varzaru, I.; Vlaicu, P.A. Comparative Analysis of Black Chokeberry (Aronia melanocarpa L.) Fruit, Leaves, and Pomace for Their Phytochemical Composition, Antioxidant Potential, and Polyphenol Bioaccessibility. Foods 2024, 13, 1856. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioproc. Tech. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the Polar Paradox Theory: A Critical Overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Wrutniak-Cabello, C.; Chabi, B.; López Giraldo, L.J.; Lecomte, J.; Villeneuve, P.; Cabello, G. Does Hydrophobicity Always Enhance Antioxidant Drugs? A Cut-off Effect of the Chain Length of Functionalized Chlorogenate Esters on ROS-Overexpressing Fibroblasts: Cut-off Effect of Phenolipid Antioxidants. J. Pharm. Pharmacol. 2011, 63, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Relationship between Hydrophobicity and Antioxidant Ability of “Phenolipids” in Emulsion: A Parabolic Effect of the Chain Length of Rosmarinate Esters. J. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef]
- López-Giraldo, L.J.; Laguerre, M.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Kinetic and Stoichiometry of the Reaction of Chlorogenic Acid and Its Alkyl Esters against the DPPH Radical. J. Agric. Food Chem. 2009, 57, 863–870. [Google Scholar] [CrossRef]
- Laguerre, M.; Giraldo, L.J.L.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain Length Affects Antioxidant Properties of Chlorogenate Esters in Emulsion: The Cutoff Theory behind the Polar Paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef]
- Pappalardo, V.; Ravasio, N.; Falletta, E.; De Rosa, M.C.; Zaccheria, F. A Green Lipophilization Reaction of a Natural Antioxidant. Antioxidants 2023, 12, 218. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Nielsen, N.S.; Yang, Z.; Xu, X.; Jacobsen, C. Lipophilization of Dihydrocaffeic Acid Affects Its Antioxidative Properties in Fish-oil-enriched Emulsions. Eur. J. Lipid Sci. Technol. 2012, 114, 134–145. [Google Scholar] [CrossRef]
- Suárez-Quiroz, M.L.; Taillefer, W.; López Méndez, E.M.; González-Ríos, O.; Villeneuve, P.; Figueroa-Espinoza, M.C. Antibacterial Activity and Antifungal and Anti-mycotoxigenic Activities against Aspergillus Flavus and A. Ochraceus of Green Coffee Chlorogenic Acids and Dodecyl Chlorogenates: Antimicrobial Chlorogenic Acids and Chlorogenates. J. Food Saf. 2013, 33, 360–368. [Google Scholar] [CrossRef]
- Ma, C.-M.; Kully, M.; Khan, J.K.; Hattori, M.; Daneshtalab, M. Synthesis of Chlorogenic Acid Derivatives with Promising Antifungal Activity. Bioorg. Med. Chem. 2007, 15, 6830–6833. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.; Niehaus, K.; Bednarz, H.; Thiyam-Hollander, U.; Fehling, E.; Matthäus, B. Enzymatic Lipophilization of Phenolic Extract from Rowanberry (Sorbus aucuparia) and Evaluation of Antioxidative Activity in Edible Oil. Lebenson. Wiss. Technol. 2015, 60, 56–62. [Google Scholar] [CrossRef]
- Aladedunye, F.; Matthäus, B. Phenolic Extracts from Sorbus aucuparia (L.) and Malus baccata (L.) Berries: Antioxidant Activity and Performance in Rapeseed Oil during Frying and Storage. Food Chem. 2014, 159, 273–281. [Google Scholar] [CrossRef]
- Jasińska, K.; Zieniuk, B.; Jankiewicz, U.; Fabiszewska, A. Bio-Based Materials versus Synthetic Polymers as a Support in Lipase Immobilization: Impact on Versatile Enzyme Activity. Catalysts 2023, 13, 395. [Google Scholar] [CrossRef]
- Głowacka, R.; Górska, A.; Wirkowska-Wojdyła, M.; Wołosiak, R.; Majewska, E.; Derewiaka, D. The Influence of Brewing Method on Bioactive Compounds Residues in Spent Coffee Grounds of Different Roasting Degree and Geographical Origin. Int. J. Food Sci. Technol. 2019, 54, 3008–3014. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Valério, A.; de Oliveira, J.V.; de Oliveira, D.; de Araújo, P.H.H.; Riella, H.G.; Fiori, M.A. Synthesis of Geranyl Cinnamate by Lipase-Catalyzed Reaction and Its Evaluation as an Antimicrobial Agent: Lipase-Catalyzed Synthesis of Geranyl Cinnamate. J. Chem. Technol. Biotechnol. 2017, 92, 115–121. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The Main and Modified CUPRAC Methods of Antioxidant Measurement. Trends Analyt. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Regulation-1333/2008-EN-Additives-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj/eng (accessed on 20 November 2025).

| Source | Percentage Content (%) | ||
|---|---|---|---|
| C | N | S | |
| Spent coffee grounds (SCG) | 48.41 ± 0.01 | 2.23 ± 0.01 | 0.12 ± 0.00 |
| Novozym 51032® immobilized on spent coffee grounds (NSCG) | 50.07 ± 0.02 * | 2.37 ± 0.00 * | 0.12 ± 0.00 |
| Chokeberry pomace (ChoP) | 50.31 ± 0.01 | 1.87 ± 0.02 | 0.09 ± 0.00 |
| Novozym 51032® immobilized on chokeberry pomace (NChoP) | 50.49 ± 0.42 | 2.17 ± 0.01 * | 0.10 ± 0.00 * |
| Apple pomace (AP) | 44.62 ± 0.06 | 0.99 ± 0.02 | 0.07 ± 0.00 |
| Novozym 51032® immobilised on apple pomace (NAP) | 44.65 ± 0.09 | 1.26 ± 0.01 * | 0.07 ± 0.00 |
| Exp. No. | Temperature (°C) | Substrate Molar Ratio (Alcohol: Chlorogenic Acid) | Enzyme Concentration Relative to the Total Mass of Substrates (%) | Yield (%) NSCG | Yield (%) Novozym 435 |
|---|---|---|---|---|---|
| 1 | 35 | 2:1 | 35 | 1.78 | 17.73 |
| 2 | 55 | 2:1 | 35 | 1.67 | 12.81 |
| 3 | 35 | 8:1 | 35 | 9.24 | 49.12 |
| 4 | 55 | 8:1 | 35 | 16.02 | 95.67 |
| 5 | 35 | 5:1 | 20 | 2.40 | 30.61 |
| 6 | 55 | 5:1 | 20 | 0.96 | 39.21 |
| 7 | 35 | 5:1 | 50 | 10.11 | 42.32 |
| 8 | 55 | 5:1 | 50 | 9.39 | 66.42 |
| 9 | 45 | 2:1 | 20 | 1.91 | 8.45 |
| 10 | 45 | 8:1 | 20 | 7.20 | 57.10 |
| 11 | 45 | 2:1 | 50 | 5.00 | 31.72 |
| 12 | 45 | 8:1 | 50 | 15.91 | 59.32 |
| 13 | 45 | 5:1 | 35 | 10.94 | 52.81 |
| 14 | 45 | 5:1 | 35 | 10.88 | 40.81 |
| 15 | 45 | 5:1 | 35 | 10.82 | 42.11 |
| Conversion Rate of Butyl Chlorogenate in Extracts After Lipophilization (%) | |||||
|---|---|---|---|---|---|
| Chokeberry Pomace Extract | Apple Pomace Extract | Spent Coffee Grounds Extract | |||
| Novozym 435 | NSCG | Novozym 435 | NSCG | Novozym 435 | NSCG |
| 90.9% | 92.0% | 91.5% | 87.6% | 47.7% | 15.2% |
| Compound | Antioxidant Properties | Lipophilicity | ||
|---|---|---|---|---|
| CUPRAC— TEAC | DPPH— IC50 (mM) | logP | logS | |
| BHT | 3.14 ± 0.22 a | 0.35 ± 0.02 a b | 4.964 | −4.909 |
| Chlorogenic acid | 3.49 ± 0.32 a | 0.26 ± 0.00 a | 0.415 | −1.489 |
| Butyl chlorogenate | 3.26 ± 0.17 a | 0.34 ± 0.00 a b | 2.076 | −2.945 |
| Hexyl chlorogenate | 1.15 ± 0.15 b | 0.40 ± 0.01 b | 2.960 | −3.976 |
| Octyl chlorogenate | 0.80 ± 0.05 b c | 0.58 ± 0.01 c | 3.844 | −5.007 |
| Decyl chlorogenate | 0.64 ± 0.03 c | 0.57 ± 0.01 c | 4.728 | −6.037 |
| Dodecyl chlorogenate | 0.45 ± 0.02 c | 0.91 ± 0.12 d | 5.612 | −7.067 |
| Type of Extract | Lipophilization | Antioxidant Properties | Total Content of Phenolic Compounds | |
|---|---|---|---|---|
| CUPRAC— TEAC | DPPH— IC50 (mg/mL) | Average CGA Content (mg/mL) | ||
| Chokeberry pomace extract | BEFORE | 2.00 ± 0.17 b | 0.26 ± 0.02 a | 0.26 ± 0.03 b |
| AFTER | 0.85 ± 0.07 B * | 0.90 ± 0.02 B * | n.a. | |
| Apple pomace extract | BEFORE | 0.23 ± 0.01 c | 4.55 ± 5.97 b | 0.03 ± 0.01 c |
| AFTER | 0.23 ± 0.01 C | 5.97 ± 0.02 C * | n.a. | |
| Spent coffee grounds extract | BEFORE | 2.50 ± 0.10 a | 0.13 ± 0.09 a | 0.36 ± 0.00 a |
| AFTER | 1.60 ± 0.07 A * | 0.42 ± 0.04 A * | n.a. | |
| Compound | Inhibition Zone Diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| B. cereus PCM 482 | B. subtilis PCM 486 | E. faecalis PCM 2909 | L. monocytogenes PCM 2191 | S. aureus PCM 2054 | E. cloacae PCM 2848 | E. coli PCM 2057 | S. marcescens PCM 549 | |
| BHT | 6 * | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Chlorogenic acid | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Butyl chlorogenate | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Hexyl chlorogenate | 10 | 8 | 10 | 6 | 12 | 6 | 6 | 6 |
| Octyl chlorogenate | 10 | 8 | 6 | 6 | 10 | 6 | 6 | 6 |
| Decyl chlorogenate | 8 | 6 | 6 | 9 | 9 | 6 | 6 | 6 |
| Dodecyl chlorogenate | 7 | 6 | 6 | 7 | 6 | 6 | 6 | 6 |
| Type of Extract | Lipophilization | Inhibition Zone Diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B. cereus PCM 482 | B. subtilis PCM 486 | E. faecalis PCM 2909 | L. monocytogenes PCM 2191 | S. aureus PCM 2054 | E. cloacae PCM 2848 | E. coli PCM 2057 | S. marcescens PCM 549 | ||
| Chokeberry pomace extract | BEFORE | 6 * | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| AFTER | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Apple pomace extract | BEFORE | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| AFTER | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Spent coffee grounds extract | BEFORE | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| AFTER | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Food Waste | Unit | Cellulose (ADF-ADL) | Hemicellulose (NDF-ADF) | Lignin (ADL) | |
|---|---|---|---|---|---|
| Native Apple Pomace | %DM | 20.99 ± 0.07 | 5.87 ± 0.97 | 9.46 ± 0.20 | [35] |
| Native Chokeberry Pomace | 18.87 ± 0.32 | 3.53 ± 1.30 | 32.76 ± 0.08 | [35] | |
| Native Spent Coffee Grounds | 21.06 ± 0.41 | 23.69 ± 0.97 | 16.87 ± 0.48 | [36] |
| Factors | Name | Units | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|---|---|
| 1 | Temperature | °C | 35 | 45 | 55 |
| 2 | Substrate molar ratio | Alcohol:chlorogenic acid | 2:1 | 5:1 | 8:1 |
| 3 | Enzyme concentration | % | 20 | 35 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska, K.; Zieniuk, B.; Bryła, M.; Padewska, D.; Brzezińska, R.; Kruszewski, B.; Nowak, D.; Fabiszewska, A. The Double Life of Plant-Based Food Waste: A Source of Phenolic Acids and a Carrier for Immobilization of Lipases Capable of Their Lipophilization. Int. J. Mol. Sci. 2025, 26, 11400. https://doi.org/10.3390/ijms262311400
Jasińska K, Zieniuk B, Bryła M, Padewska D, Brzezińska R, Kruszewski B, Nowak D, Fabiszewska A. The Double Life of Plant-Based Food Waste: A Source of Phenolic Acids and a Carrier for Immobilization of Lipases Capable of Their Lipophilization. International Journal of Molecular Sciences. 2025; 26(23):11400. https://doi.org/10.3390/ijms262311400
Chicago/Turabian StyleJasińska, Karina, Bartłomiej Zieniuk, Marcin Bryła, Daria Padewska, Rita Brzezińska, Bartosz Kruszewski, Dorota Nowak, and Agata Fabiszewska. 2025. "The Double Life of Plant-Based Food Waste: A Source of Phenolic Acids and a Carrier for Immobilization of Lipases Capable of Their Lipophilization" International Journal of Molecular Sciences 26, no. 23: 11400. https://doi.org/10.3390/ijms262311400
APA StyleJasińska, K., Zieniuk, B., Bryła, M., Padewska, D., Brzezińska, R., Kruszewski, B., Nowak, D., & Fabiszewska, A. (2025). The Double Life of Plant-Based Food Waste: A Source of Phenolic Acids and a Carrier for Immobilization of Lipases Capable of Their Lipophilization. International Journal of Molecular Sciences, 26(23), 11400. https://doi.org/10.3390/ijms262311400









