Exercise and Epigenetic Regulation in COPD: Current Evidence and Potential Mechanistic Pathways
Abstract
1. Introduction
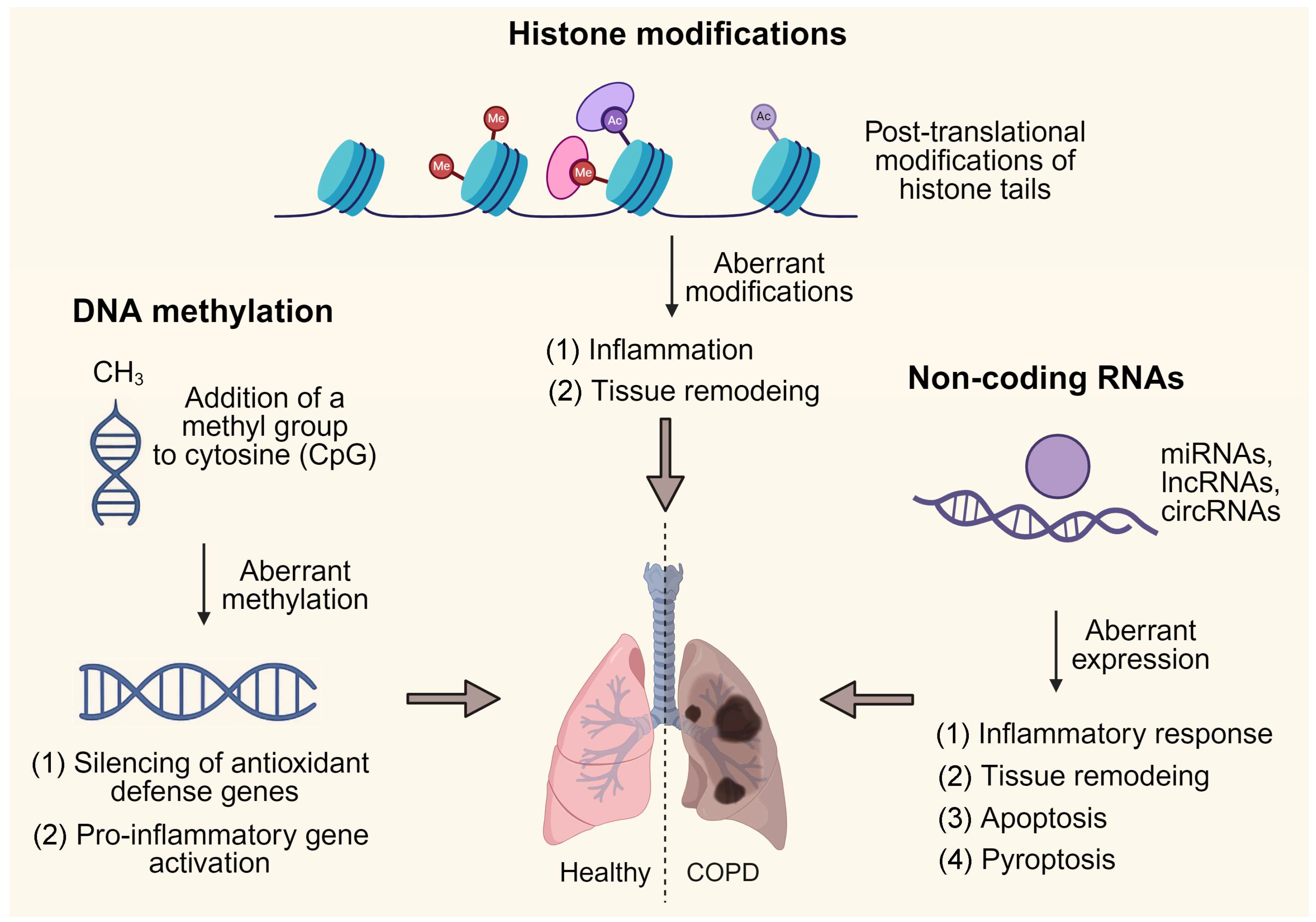
2. Epigenetic Landscape of COPD Pathogenesis
2.1. DNA Methylation in COPD Pathogenesis
2.2. Histone Modifications in COPD Pathogenesis
2.3. Non-Coding RNAs as Epigenetic Regulators in COPD Pathogenesis
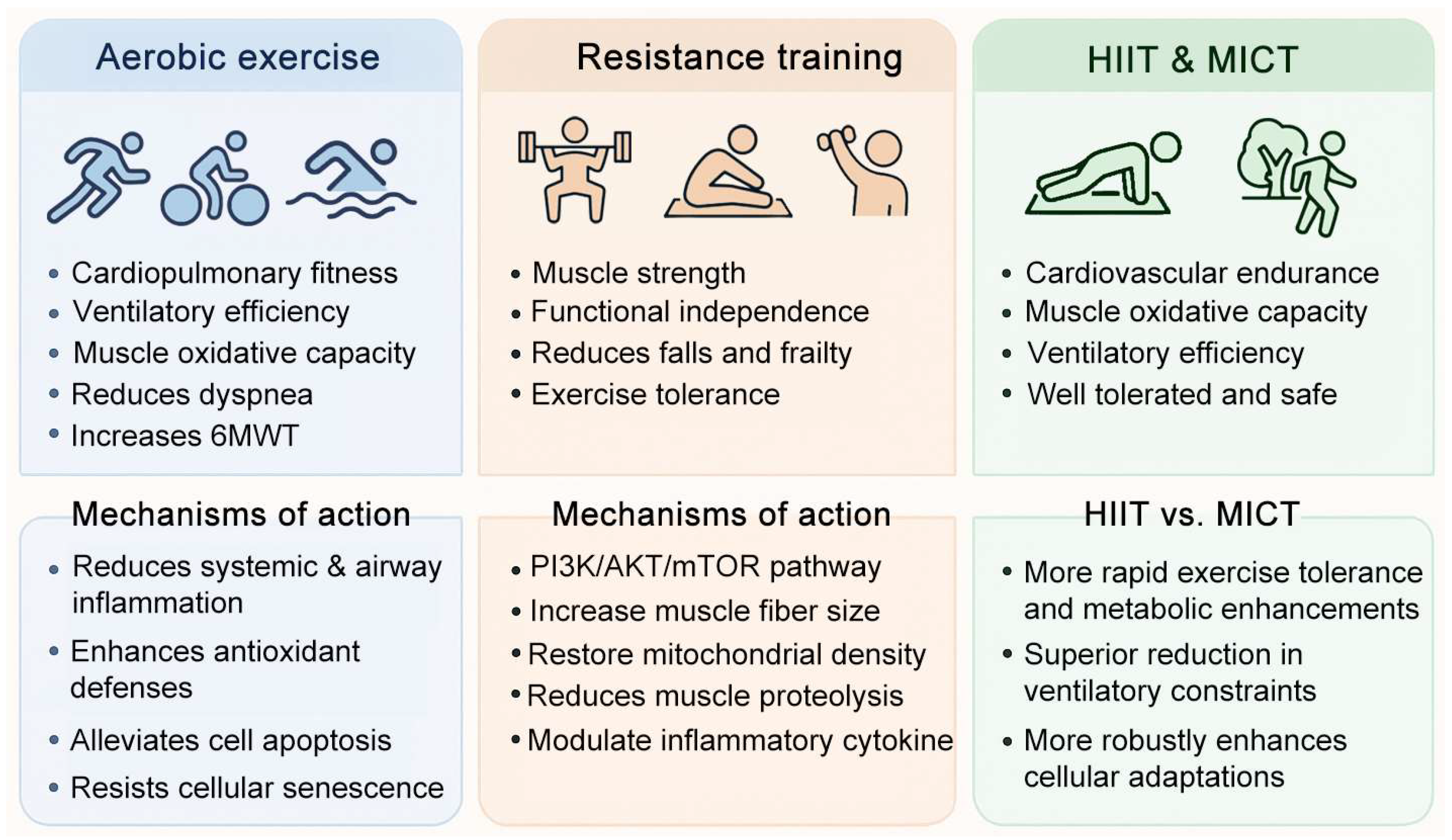
3. Exercise in COPD: Benefits and Mechanisms
3.1. Aerobic Exercise–Mediated Mechanisms in COPD
3.2. Mechanistic Role of Resistance Training in COPD
3.3. Comparative Efficacy of HIIT and MICT in COPD Rehabilitation
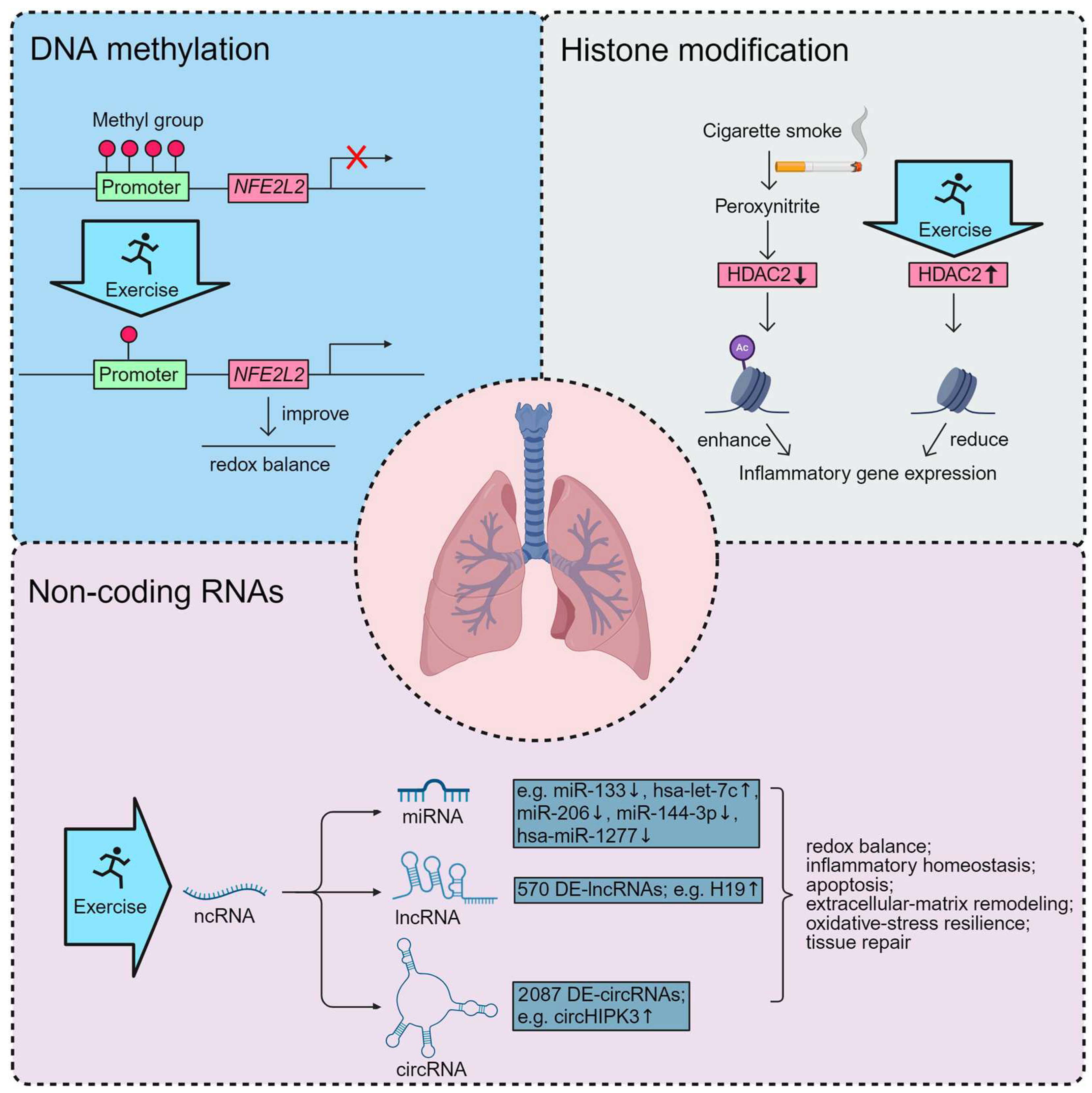
4. Epigenetic Basis of Exercise Benefits in COPD
4.1. Exercise-Induced DNA Methylation in COPD
4.2. Exercise-Induced Histone Modification in COPD
4.3. Exercise-Induced ncRNAs in COPD
5. Discussion
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| circRNAs | Circular RNAs |
| COPD | Chronic Obstructive Pulmonary Disease |
| CS | Cigarette Smoke |
| DNMT | DNA Methyltransferase |
| EMT | Epithelial–Mesenchymal Transition |
| EWAS | Epigenome-Wide Association Studies |
| HDACs | Histone Deacetylases |
| HIIT | High-Intensity Interval Training |
| lncRNAs | Long Non-Coding RNAs |
| MICT | Moderate-Intensity Continuous Training |
| miRNAs | MicroRNAs |
| ncRNAs | Non-Coding RNAs |
References
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic Obstructive Pulmonary Disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- Berg, K.; Wright, J.L. The Pathology of Chronic Obstructive Pulmonary Disease: Progress in the 20th and 21st Centuries. Arch. Pathol. Lab. Med. 2016, 140, 1423–1428. [Google Scholar] [CrossRef]
- Lareau, S.C.; Fahy, B.; Meek, P.; Wang, A. Chronic Obstructive Pulmonary Disease (Copd). Am. J. Respir. Crit. Care Med. 2019, 199, P1–P2. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.C.; Subhankar, S.; Mohanta, P.C.; Jagaty, S.K.; Dutta, P.; Pothal, S. Prevalence of Metabolic Syndrome in Chronic Obstructive Pulmonary Disease and Its Correlation with Severity of Disease. J. Fam. Med. Prim. Care. 2022, 11, 2094–2098. [Google Scholar] [CrossRef]
- Barreiro, E.; Jaitovich, A. Muscle Atrophy in Chronic Obstructive Pulmonary Disease: Molecular Basis and Potential Therapeutic Targets. J. Thorac. Dis. 2018, 10, S1415–S1424. [Google Scholar] [CrossRef]
- Franssen, F.M.; Rochester, C.L. Comorbidities in Patients with Copd and Pulmonary Rehabilitation: Do They Matter? Eur. Respir. Rev. 2014, 23, 131–141. [Google Scholar] [CrossRef]
- Halpin, D.M. Systemic Effects of Chronic Obstructive Pulmonary Disease. Expert. Rev. Respir. Med. 2007, 1, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Vucic, E.A.; Chari, R.; Thu, K.L.; Wilson, I.M.; Cotton, A.M.; Kennett, J.Y.; Zhang, M.; Lonergan, K.M.; Steiling, K.; Brown, C.J.; et al. DNA Methylation Is Globally Disrupted and Associated with Expression Changes in Chronic Obstructive Pulmonary Disease Small Airways. Am. J. Respir. Cell Mol. Biol. 2014, 50, 912–922. [Google Scholar] [CrossRef]
- Shakespear, M.R.; Halili, M.A.; Irvine, K.M.; Fairlie, D.P.; Sweet, M.J. Histone Deacetylases as Regulators of Inflammation and Immunity. Trends Immunol. 2011, 32, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Masjedi, M.R.; Barnes, P.J.; Adcock, I.M. Epigenetics and Chromatin Remodeling Play a Role in Lung Disease. Tanaffos 2011, 10, 7–16. [Google Scholar]
- Tuncel, O.; Kara, M.; Yaylak, B.; Erdogan, I.; Akgul, B. Noncoding Rnas in Apoptosis: Identification and Function. Turk. J. Biol. 2022, 46, 1–40. [Google Scholar] [PubMed]
- Wang, H.; Sun, K.; Peng, H.; Wang, Y.; Zhang, L. Emerging Roles of Noncoding Rnas in Idiopathic Pulmonary Fibrosis. Cell Death Discov. 2024, 10, 443. [Google Scholar] [CrossRef]
- Xie, N.; Liu, G. Ncrna-Regulated Immune Response and Its Role in Inflammatory Lung Diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1076–L1087. [Google Scholar] [CrossRef]
- Jiang, M.; Li, P.; Wang, Y.; Cao, Y.; Han, X.; Jiang, L.; Liu, X.; Wu, W. Role of Nrf2 and Exercise in Alleviating Copd-Induced Skeletal Muscle Dysfunction. Ther. Adv. Respir. Dis. 2023, 17, 17534666231208633. [Google Scholar] [CrossRef]
- Frinchi, M.; Morici, G.; Mudo, G.; Bonsignore, M.R.; Di Liberto, V. Beneficial Role of Exercise in the Modulation of Mdx Muscle Plastic Remodeling and Oxidative Stress. Antioxidants. 2021, 10, 558. [Google Scholar] [CrossRef]
- Han, L.; Li, P.; He, Q.; Yang, C.; Jiang, M.; Wang, Y.; Cao, Y.; Han, X.; Liu, X.; Wu, W. Revisiting Skeletal Muscle Dysfunction and Exercise in Chronic Obstructive Pulmonary Disease: Emerging Significance of Myokines. Aging Dis. 2023, 15, 2453–2469. [Google Scholar] [CrossRef]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomies, P.; Berger, P. Main Pathogenic Mechanisms and Recent Advances in Copd Peripheral Skeletal Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar] [CrossRef]
- Sellami, M.; Bragazzi, N.; Prince, M.S.; Denham, J.; Elrayess, M. Regular, Intense Exercise Training as a Healthy Aging Lifestyle Strategy: Preventing DNA Damage, Telomere Shortening and Adverse DNA Methylation Changes over a Lifetime. Front. Genet. 2021, 12, 652497. [Google Scholar] [CrossRef] [PubMed]
- Swiatowy, W.J.; Drzewiecka, H.; Kliber, M.; Sasiadek, M.; Karpinski, P.; Plawski, A.; Jagodzinski, P.P. Physical Activity and DNA Methylation in Humans. Int. J. Mol. Sci. 2021, 22, 12989. [Google Scholar] [CrossRef]
- Widmann, M.; Niess, A.M.; Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med. 2019, 49, 509–523. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Guo, Y.; Lv, Y.; Lin, C.; Wang, D.; Wang, S.; Liu, Y.; Hu, X. Physical Exercise and Epigenetic Modifications in Skeletal Muscle, Brain, and Heart. Epigenetics Chromatin. 2025, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Takai, D. The Role of DNA Methylation in Mammalian Epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, C.; Liu, J.; Sai, X.; Qin, C.; Di, T.; Yang, Y.; Wu, Y.; Bian, T. Hypermethylation of the Nrf2 Promoter Induces Ferroptosis by Inhibiting the Nrf2-Gpx4 Axis in Copd. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 3347–3362. [Google Scholar] [CrossRef]
- He, L.X.; Tang, Z.H.; Huang, Q.S.; Li, W.H. DNA Methylation: A Potential Biomarker of Chronic Obstructive Pulmonary Disease. Front. Cell Dev. Biol. 2020, 8, 585. [Google Scholar] [CrossRef]
- Gao, X.; Jia, M.; Zhang, Y.; Breitling, L.P.; Brenner, H. DNA Methylation Changes of Whole Blood Cells in Response to Active Smoking Exposure in Adults: A Systematic Review of DNA Methylation Studies. Clin. Epigenetics 2015, 7, 113. [Google Scholar] [CrossRef]
- Guida, F.; Sandanger, T.M.; Castagne, R.; Campanella, G.; Polidoro, S.; Palli, D.; Krogh, V.; Tumino, R.; Sacerdote, C.; Panico, S.; et al. Dynamics of Smoking-Induced Genome-Wide Methylation Changes with Time since Smoking Cessation. Hum. Mol. Genet. 2015, 24, 2349–2359. [Google Scholar] [CrossRef]
- Dogan, M.V.; Shields, B.; Cutrona, C.; Gao, L.; Gibbons, F.X.; Simons, R.; Monick, M.; Brody, G.H.; Tan, K.; Beach, S.R.; et al. The Effect of Smoking on DNA Methylation of Peripheral Blood Mononuclear Cells from African American Women. BMC Genom. 2014, 15, 151. [Google Scholar] [CrossRef]
- Elliott, H.R.; Tillin, T.; McArdle, W.L.; Ho, K.; Duggirala, A.; Frayling, T.M.; Davey Smith, G.; Hughes, A.D.; Chaturvedi, N.; Relton, C.L. Differences in Smoking Associated DNA Methylation Patterns in South Asians and Europeans. Clin. Epigenetics 2014, 6, 4. [Google Scholar] [CrossRef]
- Shenker, N.S.; Ueland, P.M.; Polidoro, S.; van Veldhoven, K.; Ricceri, F.; Brown, R.; Flanagan, J.M.; Vineis, P. DNA Methylation as a Long-Term Biomarker of Exposure to Tobacco Smoke. Epidemiology 2013, 24, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, S.; Kuhnel, B.; Klopp, N.; Baurecht, H.; Kleinschmidt, A.; Gieger, C.; Weidinger, S.; Lattka, E.; Adamski, J.; Peters, A.; et al. Tobacco Smoking Leads to Extensive Genome-Wide Changes in DNA Methylation. PLoS ONE 2013, 8, e63812. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.V.; Smith, A.K.; Conneely, K.N.; Chang, Q.; Li, W.; Lazarus, A.; Smith, J.A.; Almli, L.M.; Binder, E.B.; Klengel, T.; et al. Epigenomic Association Analysis Identifies Smoking-Related DNA Methylation Sites in African Americans. Hum. Genet. 2013, 132, 1027–1037. [Google Scholar] [CrossRef]
- Zong, D.; Liu, X.; Li, J.; Ouyang, R.; Chen, P. The Role of Cigarette Smoke-Induced Epigenetic Alterations in Inflammation. Epigenetics Chromatin. 2019, 12, 65. [Google Scholar] [CrossRef]
- Beetch, M.; Harandi-Zadeh, S.; Shen, K.; Lubecka, K.; Kitts, D.D.; O’Hagan, H.M.; Stefanska, B. Dietary Antioxidants Remodel DNA Methylation Patterns in Chronic Disease. Br. J. Pharmacol. 2020, 177, 1382–1408. [Google Scholar] [CrossRef]
- Morrow, J.D.; Glass, K.; Cho, M.H.; Hersh, C.P.; Pinto-Plata, V.; Celli, B.; Marchetti, N.; Criner, G.; Bueno, R.; Washko, G.; et al. Human Lung DNA Methylation Quantitative Trait Loci Colocalize with Chronic Obstructive Pulmonary Disease Genome-Wide Association Loci. Am. J. Respir. Crit. Care Med. 2018, 197, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.L.; Fishbane, N.; Patel, J.; MacIsaac, J.L.; McEwen, L.M.; Fisher, A.J.; Brandsma, C.A.; Nair, P.; Kobor, M.S.; Hackett, T.L.; et al. Altered DNA Methylation Is Associated with Aberrant Gene Expression in Parenchymal but Not Airway Fibroblasts Isolated from Individuals with Copd. Clin. Epigenetics 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Casas-Recasens, S.; Cassim, R.; Mendoza, N.; Agusti, A.; Lodge, C.; Li, S.; Bui, D.; Martino, D.; Dharmage, S.C.; Faner, R. Epigenome-Wide Association Studies of Chronic Obstructive Pulmonary Disease and Lung Function: A Systematic Review. Am. J. Respir. Crit. Care Med. 2024, 210, 766–778. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tsai, Y.H.; Wang, C.C.; Liu, S.F.; Chen, T.W.; Fang, W.F.; Lee, C.P.; Hsu, P.Y.; Chao, T.Y.; Wu, C.C.; et al. Epigenome-Wide Association Study on Asthma and Chronic Obstructive Pulmonary Disease Overlap Reveals Aberrant DNA Methylations Related to Clinical Phenotypes. Sci. Rep. 2021, 11, 5022. [Google Scholar] [CrossRef]
- Lee, M.K.; Hong, Y.; Kim, S.Y.; Kim, W.J.; London, S.J. Epigenome-Wide Association Study of Chronic Obstructive Pulmonary Disease and Lung Function in Koreans. Epigenomics 2017, 9, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Daujat, S.; Schneider, R. Lateral Thinking: How Histone Modifications Regulate Gene Expression. Trends Genet. 2016, 32, 42–56. [Google Scholar] [CrossRef]
- Ito, K.; Ito, M.; Elliott, W.M.; Cosio, B.; Caramori, G.; Kon, O.M.; Barczyk, A.; Hayashi, S.; Adcock, I.M.; Hogg, J.C.; et al. Decreased Histone Deacetylase Activity in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2005, 352, 1967–1976. [Google Scholar] [CrossRef]
- Barnes, P.J.; Ito, K.; Adcock, I.M. Corticosteroid Resistance in Chronic Obstructive Pulmonary Disease: Inactivation of Histone Deacetylase. Lancet 2004, 363, 731–733. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.R.; Edirisinghe, I.; Yao, H.; Adenuga, D.; Rahman, I. Deacetylases and Nf-Kappab in Redox Regulation of Cigarette Smoke-Induced Lung Inflammation: Epigenetics in Pathogenesis of Copd. Antioxid. Redox Signal. 2008, 10, 799–811. [Google Scholar] [CrossRef]
- Sundar, I.K.; Yao, H.; Rahman, I. Oxidative Stress and Chromatin Remodeling in Chronic Obstructive Pulmonary Disease and Smoking-Related Diseases. Antioxid. Redox Signal. 2013, 18, 1956–1971. [Google Scholar] [CrossRef]
- Bartling, T.R.; Drumm, M.L. Oxidative Stress Causes Il8 Promoter Hyperacetylation in Cystic Fibrosis Airway Cell Models. Am. J. Respir. Cell Mol. Biol. 2009, 40, 58–65. [Google Scholar] [CrossRef]
- Ito, K.; Hanazawa, T.; Tomita, K.; Barnes, P.J.; Adcock, I.M. Oxidative Stress Reduces Histone Deacetylase 2 Activity and Enhances Il-8 Gene Expression: Role of Tyrosine Nitration. Biochem. Biophys. Res. Commun. 2004, 315, 240–245. [Google Scholar] [CrossRef]
- Trejo-Villegas, O.A.; Heijink, I.H.; Avila-Moreno, F. Preclinical Evidence in the Assembly of Mammalian Swi/Snf Complexes: Epigenetic Insights and Clinical Perspectives in Human Lung Disease Therapy. Mol. Ther. 2024, 32, 2470–2488. [Google Scholar] [CrossRef]
- Xie, L.; Wu, M.; Lin, H.; Liu, C.; Yang, H.; Zhan, J.; Sun, S. An Increased Ratio of Serum Mir-21 to Mir-181a Levels Is Associated with the Early Pathogenic Process of Chronic Obstructive Pulmonary Disease in Asymptomatic Heavy Smokers. Mol. Biosyst. 2014, 10, 1072–1081. [Google Scholar] [CrossRef]
- Zeng, Z.; He, S.; Lu, J.; Liu, C.; Lin, H.; Xu, C.; Xie, L.; Sun, S. Microrna-21 Aggravates Chronic Obstructive Pulmonary Disease by Promoting Autophagy. Exp. Lung Res. 2018, 44, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liu, X.; Xiang, F.; He, X.; Li, J.; Liu, H.; Xie, L. Microrna-21 Plays a Role in Exacerbating Chronic Obstructive Pulmonary Disease by Regulating Necroptosis and Apoptosis in Bronchial Epithelial Cells. Tob. Induc. Dis. 2025, 23. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Liu, X.; Nelson, A.; Nakanishi, M.; Kanaji, N.; Wang, X.; Kim, M.; Li, Y.; Sun, J.; Michalski, J.; et al. Reduced Mir-146a Increases Prostaglandin E(2)in Chronic Obstructive Pulmonary Disease Fibroblasts. Am. J. Respir. Crit. Care Med. 2010, 182, 1020–1029. [Google Scholar] [CrossRef]
- Schembri, F.; Sridhar, S.; Perdomo, C.; Gustafson, A.M.; Zhang, X.; Ergun, A.; Lu, J.; Liu, G.; Zhang, X.; Bowers, J.; et al. Micrornas as Modulators of Smoking-Induced Gene Expression Changes in Human Airway Epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 2319–2324. [Google Scholar] [CrossRef]
- Ezzie, M.E.; Crawford, M.; Cho, J.H.; Orellana, R.; Zhang, S.; Gelinas, R.; Batte, K.; Yu, L.; Nuovo, G.; Galas, D.; et al. Gene Expression Networks in Copd: Microrna and Mrna Regulation. Thorax 2012, 67, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Riddoch-Contreras, J.; Natanek, S.A.; Donaldson, A.; Man, W.D.; Moxham, J.; Hopkinson, N.S.; Polkey, M.I.; Kemp, P.R. Downregulation of the Serum Response Factor/Mir-1 Axis in the Quadriceps of Patients with Copd. Thorax 2012, 67, 26–34. [Google Scholar] [CrossRef]
- Donaldson, A.; Natanek, S.A.; Lewis, A.; Man, W.D.; Hopkinson, N.S.; Polkey, M.I.; Kemp, P.R. Increased Skeletal Muscle-Specific Microrna in the Blood of Patients with Copd. Thorax 2013, 68, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Brandsma, C.A.; Campbell, J.D.; Knight, D.A.; Pechkovsky, D.V.; Hogg, J.C.; Timens, W.; Postma, D.S.; Lenburg, M.; Spira, A. Mir-638 Regulates Gene Expression Networks Associated with Emphysematous Lung Destruction. Genome Med. 2013, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Sai, X.; Qin, C.; Zhang, Z.; Yu, H.; Bian, T. A Mirna-21-Mediated Pten/Akt/Nf-Kappab Axis Promotes Chronic Obstructive Pulmonary Disease Pathogenesis. Int. J. Chron. Obstruct. Pulmon. Dis. 2024, 19, 1141–1151. [Google Scholar] [CrossRef]
- Geisler, S.; Coller, J. Rna in Unexpected Places: Long Non-Coding Rna Functions in Diverse Cellular Contexts. Nat. Rev. Mol. Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef]
- Ge, J.; Geng, S.; Jiang, H. Long Noncoding Rnas Antisense Noncoding Rna in the Ink4 Locus (Anril) Correlates with Lower Acute Exacerbation Risk, Decreased Inflammatory Cytokines, and Mild Gold Stage in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Lab. Anal. 2019, 33, e22678. [Google Scholar] [CrossRef]
- Tang, W.; Shen, Z.; Guo, J.; Sun, S. Screening of Long Non-Coding Rna and Tug1 Inhibits Proliferation with Tgf-Beta Induction in Patients with Copd. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 2951–2964. [Google Scholar] [CrossRef]
- Mo, R.; Li, J.; Chen, Y.; Ding, Y. Lncrna Gas5 Promotes Pyroptosis in Copd by Functioning as a Cerna to Regulate the Mir-223-3p/Nlrp3 Axis. Mol. Med. Rep. 2022, 26, 219. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Y.; Tao, Q.; Liu, H.; Wang, J.; Zhang, C.; Zhou, Y.; Wei, C.; Chang, Y.; Jin, Y.; et al. The Role of Lncrna in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Heliyon 2023, 9, e22460. [Google Scholar] [CrossRef]
- Manevski, M.; Devadoss, D.; Long, C.; Singh, S.P.; Nasser, M.W.; Borchert, G.M.; Nair, M.N.; Rahman, I.; Sopori, M.; Chand, H.S. Increased Expression of Lasi Lncrna Regulates the Cigarette Smoke and Copd Associated Airway Inflammation and Mucous Cell Hyperplasia. Front. Immunol. 2022, 13, 803362, Erratum in: Front Immunol. 2022, 13, 988069. [Google Scholar]
- Bamodu, O.A.; Wu, S.M.; Feng, P.H.; Sun, W.L.; Lin, C.W.; Chuang, H.C.; Ho, S.C.; Chen, K.Y.; Chen, T.T.; Tseng, C.H.; et al. Lnc-Il7r Expression Reflects Physiological Pulmonary Function and Its Aberration Is a Putative Indicator of Copd. Biomedicines 2022, 10, 786. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Hu, D.; Zhang, X. Linc00612/Mir-31-5p/Notch1 Axis Regulates Apoptosis, Inflammation, and Oxidative Stress in Human Pulmonary Microvascular Endothelial Cells Induced by Cigarette Smoke Extract. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Y.; Zhao, Y.Y.; Zhou, Z.J.; Duan, J.X.; Zhu, Y.Z.; Cai, S.; Chen, P. Microarray Analysis of Long Non-Coding Rnas in Lung Tissues of Patients with Copd and Hoxa-As2 Promotes Hpmecs Proliferation Via Notch1. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 2449–2460. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, R.; Zhang, Z.; Li, D.; Xu, J.; Gong, Y.; Chen, X.; Lu, W. Lncrna Nqo1-As1 Attenuates Cigarette Smoke-Induced Oxidative Stress by Upregulating Its Natural Antisense Transcript Nqo1. Front. Pharmacol. 2021, 12, 729062. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, L.; Deng, G.; Gu, X.; Tang, Z.; Li, S.; Jin, W.; Yang, J.; Guo, X.; Li, Q. Knockdown of Long Noncoding Rna Miat Attenuates Cigarette Smoke-Induced Airway Remodeling by Downregulating Mir-29c-3p-Hif3a Axis. Toxicol. Lett. 2022, 357, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Lin, F.; Shi, H.; Guan, Z.; Jiang, Y. Long Non-Coding Rna Oip5-As1 Regulates Smoke-Related Chronic Obstructive Pulmonary Disease Via Targeting Micro Rna -410-3p/Il-13. Bioengineered 2021, 12, 11664–11676. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Zeng, H.; Meng, Y.; Gao, H.; Zhang, M.; Zhao, L. Lncrna Casc2 Is Involved in the Development of Chronic Obstructive Pulmonary Disease Via Targeting Mir-18a-5p/Igf1 Axis. Ther. Adv. Respir. Dis. 2021, 15, 17534666211028072. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient MicroRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Li, X.; Jin, S.H.; Wang, H.; Gaipl, U.S.; Ma, H.; Wang, S.; Zhou, J.G. Circrnas: Functions and Emerging Roles in Cancer and Immunotherapy. BMC Med. 2025, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Niu, H.; Yu, T.; Cui, H.; Yang, T.; Hao, K.; Wang, C. Identification and Bioinformatic Analysis of Circular Rna Expression in Peripheral Blood Mononuclear Cells from Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Yang, Z.; Xian, S.; Lin, Q.; Huang, L.; Ding, Y. Hsa_Circ_0008833 Promotes Copd Progression Via Inducing Pyroptosis in Bronchial Epithelial Cells. Exp. Lung Res. 2024, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Zhang, N.; Zhao, X.; Li, R.; Wang, Y.; Chen, C.; Wang, D.; Zhang, X.; Chen, L.; et al. Comprehensive Identification of Rna Transcripts and Construction of Rna Network in Chronic Obstructive Pulmonary Disease. Respir. Res. 2022, 23, 154. [Google Scholar] [CrossRef]
- Shen, X.R.; Liu, Y.Y.; Qian, R.Q.; Zhang, W.Y.; Huang, J.A.; Zhang, X.Q.; Zeng, D.X. Circular Rna Expression of Peripheral Blood Mononuclear Cells Associated with Risk of Acute Exacerbation in Smoking Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2024, 19, 789–797. [Google Scholar] [CrossRef]
- Tang, S.; Ding, Y.; Zhou, Z.; Yang, W. Identification and Bioinformatic Analysis of Circrnas in the Plasma of Patients with Very Severe Chronic Obstructive Pulmonary Disease. BMC Pulm. Med. 2023, 23, 211. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, S.; Kang, X. Circrna Circ_0006892 Regulates Mir-24/Phlpp2 Axis to Mitigate Cigarette Smoke Extract-Induced Bronchial Epithelial Cell Injury. Biotechnol. Appl. Biochem. 2022, 69, 735–748. [Google Scholar] [CrossRef]
- Wang, Z.; Zuo, Y.; Gao, Z. Circankrd11 Knockdown Protects Hpmecs from Cigarette Smoke Extract-Induced Injury by Regulating Mir-145-5p/Brd4 Axis. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 887–899. [Google Scholar] [CrossRef]
- Hansen, E.S.H.; Pitzner-Fabricius, A.; Toennesen, L.L.; Rasmusen, H.K.; Hostrup, M.; Hellsten, Y.; Backer, V.; Henriksen, M. Effect of Aerobic Exercise Training on Asthma in Adults: A Systematic Review and Meta-Analysis. Eur. Respir. J. 2020, 56, 2000146. [Google Scholar] [CrossRef]
- Armstrong, M.; Vogiatzis, I. Personalized Exercise Training in Chronic Lung Diseases. Respirology 2019, 24, 854–862. [Google Scholar] [CrossRef]
- Troosters, T.; Janssens, W.; Demeyer, H.; Rabinovich, R.A. Pulmonary Rehabilitation and Physical Interventions. Eur. Respir. Rev. 2023, 32, 220222. [Google Scholar] [CrossRef]
- Gloeckl, R.; Marinov, B.; Pitta, F. Practical Recommendations for Exercise Training in Patients with Copd. Eur. Respir. Rev. 2013, 22, 178–186. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef]
- Alcazar, J.; Losa-Reyna, J.; Rodriguez-Lopez, C.; Navarro-Cruz, R.; Alfaro-Acha, A.; Ara, I.; Garcia-Garcia, F.J.; Alegre, L.M.; Guadalupe-Grau, A. Effects of Concurrent Exercise Training on Muscle Dysfunction and Systemic Oxidative Stress in Older People with Copd. Scand. J. Med. Sci. Sports 2019, 29, 1591–1603. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of Physical Activities in Daily Life in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Burtin, C.; De Boever, P.; Langer, D.; Vogiatzis, I.; Wouters, E.F.; Franssen, F.M. Copd and Exercise: Does It Make a Difference? Breathe 2016, 12, e38–e49. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X. Clinical Effects of Exercise Combined with Respiratory Training in the Rehabilitation Treatment of Patients with Chronic Obstructive Pulmonary Disease. Altern. Ther. Health Med. 2024, 30, 188–194. [Google Scholar]
- Kantorowski, A.; Wan, E.S.; Homsy, D.; Kadri, R.; Richardson, C.R.; Moy, M.L. Determinants and Outcomes of Change in Physical Activity in Copd. ERJ Open Res. 2018, 4, 00054–2018. [Google Scholar] [CrossRef]
- Marquez-Martin, E.; Ruiz, F.O.; Ramos, P.C.; Lopez-Campos, J.L.; Azcona, B.V.; Cortes, E.B. Randomized Trial of Non-Invasive Ventilation Combined with Exercise Training in Patients with Chronic Hypercapnic Failure Due to Chronic Obstructive Pulmonary Disease. Respir. Med. 2014, 108, 1741–1751. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Exercise Alleviates Depression Related Systemic Inflammation in Chronic Obstructive Pulmonary Disease Patients. Afr. Health Sci. 2016, 16, 1078–1088. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H.; Al-Shreef, F.M. Plasma Inflammatory Biomarkers Response to Aerobic Versus Resisted Exercise Training for Chronic Obstructive Pulmonary Disease Patients. Afr. Health Sci. 2016, 16, 507–515. [Google Scholar] [CrossRef]
- Menegali, B.T.; Nesi, R.T.; Souza, P.S.; Silva, L.A.; Silveira, P.C.; Valenca, S.S.; Pinho, R.A. The Effects of Physical Exercise on the Cigarette Smoke-Induced Pulmonary Oxidative Response. Pulm. Pharmacol. Ther. 2009, 22, 567–573. [Google Scholar] [CrossRef]
- Nesi, R.T.; de Souza, P.S.; Dos Santos, G.P.; Thirupathi, A.; Menegali, B.T.; Silveira, P.C.; da Silva, L.A.; Valenca, S.S.; Pinho, R.A. Physical Exercise Is Effective in Preventing Cigarette Smoke-Induced Pulmonary Oxidative Response in Mice. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 603–610. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Tang, D. Aerobic Exercise Alleviates Inflammation, Oxidative Stress, and Apoptosis in Mice with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 1369–1379, Erratum in: Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 3201–3202. [Google Scholar] [CrossRef]
- Liu, P.; Gao, H.; Wang, Y.; Li, Y.; Zhao, L. Lncrna H19 Contributes to Smoke-Related Chronic Obstructive Pulmonary Disease by Targeting Mir-181/Pdcd4 Axis. COPD 2023, 20, 119–125. [Google Scholar] [CrossRef]
- Denham, J.; Sellami, M. Exercise Training Increases Telomerase Reverse Transcriptase Gene Expression and Telomerase Activity: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2021, 70, 101411. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef]
- Pellegrino, D.; Casas-Recasens, S.; Faner, R.; Palange, P.; Agusti, A. When Getomics Meets Aging and Exercise in Copd. Respir. Med. 2023, 216, 107294. [Google Scholar] [CrossRef] [PubMed]
- So, B.; Park, J.; Jang, J.; Lim, W.; Imdad, S.; Kang, C. Effect of Aerobic Exercise on Oxidative Stress and Inflammatory Response During Particulate Matter Exposure in Mouse Lungs. Front. Physiol. 2021, 12, 773539. [Google Scholar] [CrossRef] [PubMed]
- To, M.; Swallow, E.B.; Akashi, K.; Haruki, K.; Natanek, S.A.; Polkey, M.I.; Ito, K.; Barnes, P.J. Reduced Hdac2 in Skeletal Muscle of Copd Patients. Respir. Res. 2017, 18, 99. [Google Scholar] [CrossRef]
- Simpson, K.; Killian, K.; McCartney, N.; Stubbing, D.G.; Jones, N.L. Randomised Controlled Trial of Weightlifting Exercise in Patients with Chronic Airflow Limitation. Thorax 1992, 47, 70–75. [Google Scholar] [CrossRef]
- Spruit, M.A.; Gosselink, R.; Troosters, T.; De Paepe, K.; Decramer, M. Resistance Versus Endurance Training in Patients with Copd and Peripheral Muscle Weakness. Eur. Respir. J. 2002, 19, 1072–1078. [Google Scholar] [CrossRef]
- Iepsen, U.W.; Jorgensen, K.J.; Ringbaek, T.; Hansen, H.; Skrubbeltrang, C.; Lange, P. A Systematic Review of Resistance Training Versus Endurance Training in Copd. J. Cardiopulm. Rehabil. Prev. 2015, 35, 163–172. [Google Scholar] [CrossRef]
- Klijn, P.; van Keimpema, A.; Legemaat, M.; Gosselink, R.; van Stel, H. Nonlinear Exercise Training in Advanced Chronic Obstructive Pulmonary Disease Is Superior to Traditional Exercise Training. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2013, 188, 193–200. [Google Scholar] [CrossRef]
- Frykholm, E.; Klijn, P.; Saey, D.; van Hees, H.W.H.; Stal, P.; Sandstrom, T.; Sorlin, A.; Maltais, F.; Nyberg, A. Effect and Feasibility of Non-Linear Periodized Resistance Training in People with Copd: Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 6. [Google Scholar] [CrossRef]
- Loughran, K.J.; Emerson, J.; Avery, L.; Suri, S.; Flynn, D.; Kaner, E.; Rapley, T.; Martin, D.; McPhee, J.; Fernandes-James, C.; et al. Exercise-Based Interventions Targeting Balance and Falls in People with Copd: A Systematic Review and Meta-Analysis. Eur. Respir. Rev. 2024, 33, 240003. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.K.; Houchen, L.; Harrison, S.; Singh, S.J.; Morgan, M.D.; Steiner, M.C. Ultrasound Assessment of Lower Limb Muscle Mass in Response to Resistance Training in Copd. Respir. Res. 2012, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, S.D.; Taylor, N.F.; Paratz, J.D. Progressive Resistance Exercise Improves Muscle Strength and May Improve Elements of Performance of Daily Activities for People with Copd: A Systematic Review. Chest 2009, 136, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Nunes, E.A.; Currier, B.S.; McLeod, J.C.; Thomas, A.C.Q.; Phillips, S.M. An Evidence-Based Narrative Review of Mechanisms of Resistance Exercise-Induced Human Skeletal Muscle Hypertrophy. Med. Sci. Sports Exerc. 2022, 54, 1546–1559. [Google Scholar] [CrossRef]
- Paoli, A.; Cerullo, G.; Bianco, A.; Neri, M.; Gennaro, F.; Charrier, D.; Moro, T. Not Only Protein: Dietary Supplements to Optimize the Skeletal Muscle Growth Response to Resistance Training: The Current State of Knowledge. J. Hum. Kinet. 2024, 91, 225–244. [Google Scholar] [CrossRef]
- Figueiredo, V.C. Revisiting the Roles of Protein Synthesis During Skeletal Muscle Hypertrophy Induced by Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R709–R718. [Google Scholar] [CrossRef]
- Deldicque, L. Protein Intake and Exercise-Induced Skeletal Muscle Hypertrophy: An Update. Nutrients 2020, 12, 2023. [Google Scholar] [CrossRef]
- Marillier, M.; Bernard, A.C.; Verges, S.; Neder, J.A. Locomotor Muscles in Copd: The Rationale for Rehabilitative Exercise Training. Front. Physiol. 2019, 10, 1590. [Google Scholar] [CrossRef]
- Lourenço, Í.; Neto, W.K.; Amorim, L.d.S.P.; Ortiz, V.M.M.; Geraldo, V.L.; Ferreira, G.H.d.S.; Caperuto, É.C.; Gama, E.F. Muscle Hypertrophy and Ladder-Based Resistance Training for Rodents: A Systematic Review and Meta-Analysis. Physiol. Rep. 2020, 8, e14502. [Google Scholar] [CrossRef]
- Krause Neto, W.; Silva, W.; Oliveira, T.; Vilas Boas, A.; Ciena, A.; Caperuto, E.C.; Gama, E.F. Ladder-Based Resistance Training with the Progression of Training Load Altered the Tibial Nerve Ultrastructure and Muscle Fiber Area without Altering the Morphology of the Postsynaptic Compartment. Front. Physiol. 2024, 15, 1371839. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ahn, N.; Jung, S.; Park, S. Effects of Intermittent Ladder-Climbing Exercise Training on Mitochondrial Biogenesis and Endoplasmic Reticulum Stress of the Cardiac Muscle in Obese Middle-Aged Rats. Korean J. Physiol. Pharmacol. 2017, 21, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.B.T.; Guzzoni, V.; Hord, J.M.; Lopes, G.N.; Marqueti, R.C.; de Andrade, R.V.; Selistre-de-Araujo, H.S.; Durigan, J.L.Q. Resistance Training Regulates Gene Expression of Molecules Associated with Intramyocellular Lipids, Glucose Signaling and Fiber Size in Old Rats. Sci. Rep. 2017, 7, 8593, Erratum in: Sci. Rep. 2019, 9, 6383. [Google Scholar] [CrossRef]
- Ramirez-Venegas, A.; Ward, J.L.; Olmstead, E.M.; Tosteson, A.N.; Mahler, D.A. Effect of Exercise Training on Dyspnea Measures in Patients with Chronic Obstructive Pulmonary Disease. J. Cardiopulm. Rehabil. 1997, 17, 103–109. [Google Scholar] [CrossRef]
- Strasser, B.; Siebert, U.; Schobersberger, W. Effects of Resistance Training on Respiratory Function in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Sleep Breath. 2013, 17, 217–226. [Google Scholar] [CrossRef]
- Nejatian Hoseinpour, A.; Bassami, M.; Ahmadizad, S.; Donath, L.; Setayesh, S.; Mirzaei, M.; Mohammad Rahimi, G.R. The Influence of Resistance Training on Inflammatory Markers, Body Composition and Functional Capacity in Healthy Older Adults: A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2025, 130, 105731. [Google Scholar] [CrossRef]
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. The Anti-Inflammatory Actions of Exercise Training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Calle, M.C.; Fernandez, M.L. Effects of Resistance Training on the Inflammatory Response. Nutr. Res. Pract. 2010, 4, 259–269. [Google Scholar] [CrossRef]
- Okamura, M.; Shimizu, M.; Yamamoto, S.; Nishie, K.; Konishi, M. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Heart Fail. Rev. 2023, 28, 1113–1128. [Google Scholar] [CrossRef]
- Atakan, M.M.; Li, Y.; Kosar, S.N.; Turnagol, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public. Health 2021, 18, 7201. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Yang, Q. Effects of High-Intensity Interval Training and Moderate-Intensity Continuous Training on Mitochondrial Dynamics in Human Skeletal Muscle. Front. Physiol. 2025, 16, 1554222. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Liu, L.; Cao, J.; Liang, Q.; Zhang, X. High-Intensity Interval Training Improves the Outcomes of Patients with Chronic Obstructive Pulmonary Disease: A Meta-Analysis of Randomized Controlled Trials. Respir. Med. 2023, 208, 107128. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Niu, J.; Zhang, X.; Li, M. Effectiveness of High-Intensity Interval Training in Rehabilitation Nursing for Mild-to-Moderate Stable Copd Patients: A Randomized Controlled Clinical Trial. BMC Sports Sci. Med. Rehabil. 2025, 17, 28. [Google Scholar] [CrossRef]
- Liu, S.; Yang, A.; Yu, Y.; Xu, B.; Yu, G.; Wang, H. Exercise Prescription Training in Chronic Obstructive Pulmonary Disease: Benefits and Mechanisms. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 1071–1082. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Cao, Y.; Liu, C.; Wang, J.; Wu, W. Skeletal Muscle Mitochondrial Dysfunction in Chronic Obstructive Pulmonary Disease: Underlying Mechanisms and Physical Therapy Perspectives. Aging Dis. 2023, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A Practical Model of Low-Volume High-Intensity Interval Training Induces Mitochondrial Biogenesis in Human Skeletal Muscle: Potential Mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Ryrso, C.K.; Thaning, P.; Siebenmann, C.; Lundby, C.; Lange, P.; Pedersen, B.K.; Hellsten, Y.; Iepsen, U.W. Effect of Endurance Versus Resistance Training on Local Muscle and Systemic Inflammation and Oxidative Stress in Copd. Scand. J. Med. Sci. Sports 2018, 28, 2339–2348. [Google Scholar] [CrossRef]
- Han, C.; Lu, P.; Yan, S.Z. Effects of High-Intensity Interval Training on Mitochondrial Supercomplex Assembly and Biogenesis, Mitophagy, and the Amp-Activated Protein Kinase Pathway in the Soleus Muscle of Aged Female Rats. Exp. Gerontol. 2022, 158, 111648. [Google Scholar] [CrossRef]
- Pengam, M.; Goanvec, C.; Moisan, C.; Simon, B.; Albacete, G.; Feray, A.; Guernec, A.; Amerand, A. Moderate Intensity Continuous Versus High Intensity Interval Training: Metabolic Responses of Slow and Fast Skeletal Muscles in Rat. PLoS ONE 2023, 18, e0292225. [Google Scholar] [CrossRef]
- Torma, F.; Gombos, Z.; Jokai, M.; Takeda, M.; Mimura, T.; Radak, Z. High Intensity Interval Training and Molecular Adaptive Response of Skeletal Muscle. Sports Med. Health Sci. 2019, 1, 24–32. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New Insights into the Immunology of Chronic Obstructive Pulmonary Disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants. 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Barres, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute Exercise Remodels Promoter Methylation in Human Skeletal Muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef]
- Turner, D.C.; Seaborne, R.A.; Sharples, A.P. Comparative Transcriptome and Methylome Analysis in Human Skeletal Muscle Anabolism, Hypertrophy and Epigenetic Memory. Sci. Rep. 2019, 9, 4251. [Google Scholar] [CrossRef] [PubMed]
- Seaborne, R.A.; Strauss, J.; Cocks, M.; Shepherd, S.; O’Brien, T.D.; Someren, K.A.V.; Bell, P.G.; Murgatroyd, C.; Morton, J.P.; Stewart, C.E.; et al. Methylome of Human Skeletal Muscle after Acute & Chronic Resistance Exercise Training, Detraining & Retraining. Sci. Data 2018, 5, 180213. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z. Pgc-1 Coactivators and Skeletal Muscle Adaptations in Health and Disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Furrer, R.; Hawley, J.A.; Handschin, C. The Molecular Athlete: Exercise Physiology from Mechanisms to Medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef] [PubMed]
- Seaborne, R.A.; Strauss, J.; Cocks, M.; Shepherd, S.; O’Brien, T.D.; van Someren, K.A.; Bell, P.G.; Murgatroyd, C.; Morton, J.P.; Stewart, C.E.; et al. Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Sci. Rep. 2018, 8, 1898. [Google Scholar] [CrossRef]
- Schenk, A.; Koliamitra, C.; Bauer, C.J.; Schier, R.; Schweiger, M.R.; Bloch, W.; Zimmer, P. Impact of Acute Aerobic Exercise on Genome-Wide DNA-Methylation in Natural Killer Cells-a Pilot Study. Genes 2019, 10, 380. [Google Scholar]
- Robson-Ansley, P.J.; Saini, A.; Toms, C.; Ansley, L.; Walshe, I.H.; Nimmo, M.A.; Curtin, J.A. Dynamic Changes in Dna Methylation Status in Peripheral Blood Mononuclear Cells Following an Acute Bout of Exercise: Potential Impact of Exercise-Induced Elevations in Interleukin-6 Concentration. J. Biol. Regul. Homeost. Agents. 2014, 28, 407–417. [Google Scholar]
- da Silva, I.R.V.; de Araujo, C.L.P.; Dorneles, G.P.; Peres, A.; Bard, A.L.; Reinaldo, G.; Teixeira, P.J.Z.; Lago, P.D.; Elsner, V.R. Exercise-Modulated Epigenetic Markers and Inflammatory Response in Copd Individuals: A Pilot Study. Respir. Physiol. Neurobiol. 2017, 242, 89–95. [Google Scholar] [CrossRef]
- Kubo, H.; Asai, K.; Kojima, K.; Sugitani, A.; Kyomoto, Y.; Okamoto, A.; Yamada, K.; Ijiri, N.; Watanabe, T.; Hirata, K.; et al. Exercise Ameliorates Emphysema of Cigarette Smoke-Induced Copd in Mice through the Exercise-Irisin-Nrf2 Axis. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2507–2516. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Wei, A.; Chen, F.; Gao, Q.; Lu, K.; Jiang, Q.; Cao, W. Nrf2 Epigenetic Derepression Induced by Running Exercise Protects against Osteoporosis. Bone Res. 2021, 9, 15. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadottir, T. Nrf2 Mediates Redox Adaptations to Exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef]
- Barnes, P.J. Reduced Histone Deacetylase in Copd: Clinical Implications. Chest 2006, 129, 151–155. [Google Scholar] [CrossRef]
- Barnes, P.J. Role of Hdac2 in the Pathophysiology of Copd. Annu. Rev. Physiol. 2009, 71, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Papaioannou, A.I.; Papaporfyriou, A.; Baker, J.R.; Vuppusetty, C.; Loukides, S.; Barnes, P.J.; Ito, K. Decreased Serum Sirtuin-1 in Copd. Chest 2017, 152, 343–352. [Google Scholar] [CrossRef]
- Lim, C.; Shimizu, J.; Kawano, F.; Kim, H.J.; Kim, C.K. Adaptive Responses of Histone Modifications to Resistance Exercise in Human Skeletal Muscle. PLoS ONE 2020, 15, e0231321. [Google Scholar] [CrossRef]
- McGee, S.L.; Fairlie, E.; Garnham, A.P.; Hargreaves, M. Exercise-Induced Histone Modifications in Human Skeletal Muscle. J. Physiol. 2009, 587, 5951–5958. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Li, C.; Zhang, X.; Shan, Y.; Zhang, Z.; Bo, H.; Zhang, Y. Endurance Exercise-Induced Histone Methylation Modification Involved in Skeletal Muscle Fiber Type Transition and Mitochondrial Biogenesis. Sci. Rep. 2024, 14, 21154. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, M.; Gao, H.; Han, S.; Liu, J.; Sun, X.; Zhao, L. Regulation of Whole-Transcriptome Sequencing Expression in Copd after Personalized Precise Exercise Training: A Pilot Study. Respir. Res. 2023, 24, 156. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Shen, W.; Wu, Y.; Bian, T. Microrna Let-7 Induces M2 Macrophage Polarization in Copd Emphysema through the Il-6/Stat3 Pathway. Int. J. Chron. Obs. Pulm. Dis. 2023, 18, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Li, W.; Shi, Z.; Du, W.; Xu, H.; Liu, Z.; Wu, Y. The Circrere/Mir-144-3p/Tlr2/Mmp9 Signaling Axis in Copd Pulmonary Monocytes Promotes the Emt of Pulmonary Epithelial Cells. Biochem. Biophys. Res. Commun. 2022, 625, 1–8. [Google Scholar] [CrossRef]
- Sweef, O.; Mahfouz, R.; Tascioglu, T.; Albowaidey, A.; Abdelmonem, M.; Asfar, M.; Zaabout, E.; Corcino, Y.L.; Thomas, V.; Choi, E.S.; et al. Decoding Lncrna in Copd: Unveiling Prognostic and Diagnostic Power and Their Driving Role in Lung Cancer Progression. Int. J. Mol. Sci. 2024, 25, 9001. [Google Scholar] [CrossRef]
- Liu, X.; Ali, M.K.; Dua, K.; Mao, Y.; Liu, J. Circular Rnas: Emerging Players in Asthma and Copd. Front. Cell Dev. Biol. 2023, 11, 1267792. [Google Scholar] [CrossRef]
- Dey, T.; Kalita, J.; Weldon, S.; Taggart, C.C. Proteases and Their Inhibitors in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2018, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Betsuyaku, T.; Ito, Y.; Nagai, K.; Nasuhara, Y.; Kaga, K.; Kondo, S.; Nishimura, M. Down-Regulated Nf-E2-Related Factor 2 in Pulmonary Macrophages of Aged Smokers and Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2008, 39, 673–682. [Google Scholar] [CrossRef]
- de Sousa Neto, I.V.; Tibana, R.A.; da Cunha Nascimento, D.; Vieira, D.C.; Durigan, J.L.; Pereira, G.B.; Navalta, J.W.; de Cassia Marqueti, R.; Prestes, J. Effects of Resistance Training Volume on Mmps in Circulation, Muscle and Adipose Tissue. Int. J. Sports Med. 2017, 38, 307–313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Xu, J.; Chen, X.; Lv, Y.; Zheng, X. Exercise and Epigenetic Regulation in COPD: Current Evidence and Potential Mechanistic Pathways. Int. J. Mol. Sci. 2025, 26, 11392. https://doi.org/10.3390/ijms262311392
Zhong Y, Xu J, Chen X, Lv Y, Zheng X. Exercise and Epigenetic Regulation in COPD: Current Evidence and Potential Mechanistic Pathways. International Journal of Molecular Sciences. 2025; 26(23):11392. https://doi.org/10.3390/ijms262311392
Chicago/Turabian StyleZhong, Yuanming, Jianhua Xu, Xia Chen, Yi Lv, and Xi Zheng. 2025. "Exercise and Epigenetic Regulation in COPD: Current Evidence and Potential Mechanistic Pathways" International Journal of Molecular Sciences 26, no. 23: 11392. https://doi.org/10.3390/ijms262311392
APA StyleZhong, Y., Xu, J., Chen, X., Lv, Y., & Zheng, X. (2025). Exercise and Epigenetic Regulation in COPD: Current Evidence and Potential Mechanistic Pathways. International Journal of Molecular Sciences, 26(23), 11392. https://doi.org/10.3390/ijms262311392




