The Potential of NGTs to Overcome Constraints in Plant Breeding and Their Regulatory Implications
Abstract
1. Introduction
2. Main Section: CRISPR/Cas-Catalyzed Reactions Can Overcome Structural Genomic Elements, Cytogenic Factors, and Mechanisms
2.1. Mode of Action of CRISPR/Cas
2.2. Potential of NGTs to Overcome Structural Genomic Elements, Cytogenic Factors, and Mechanisms
2.2.1. Cytogenic Features
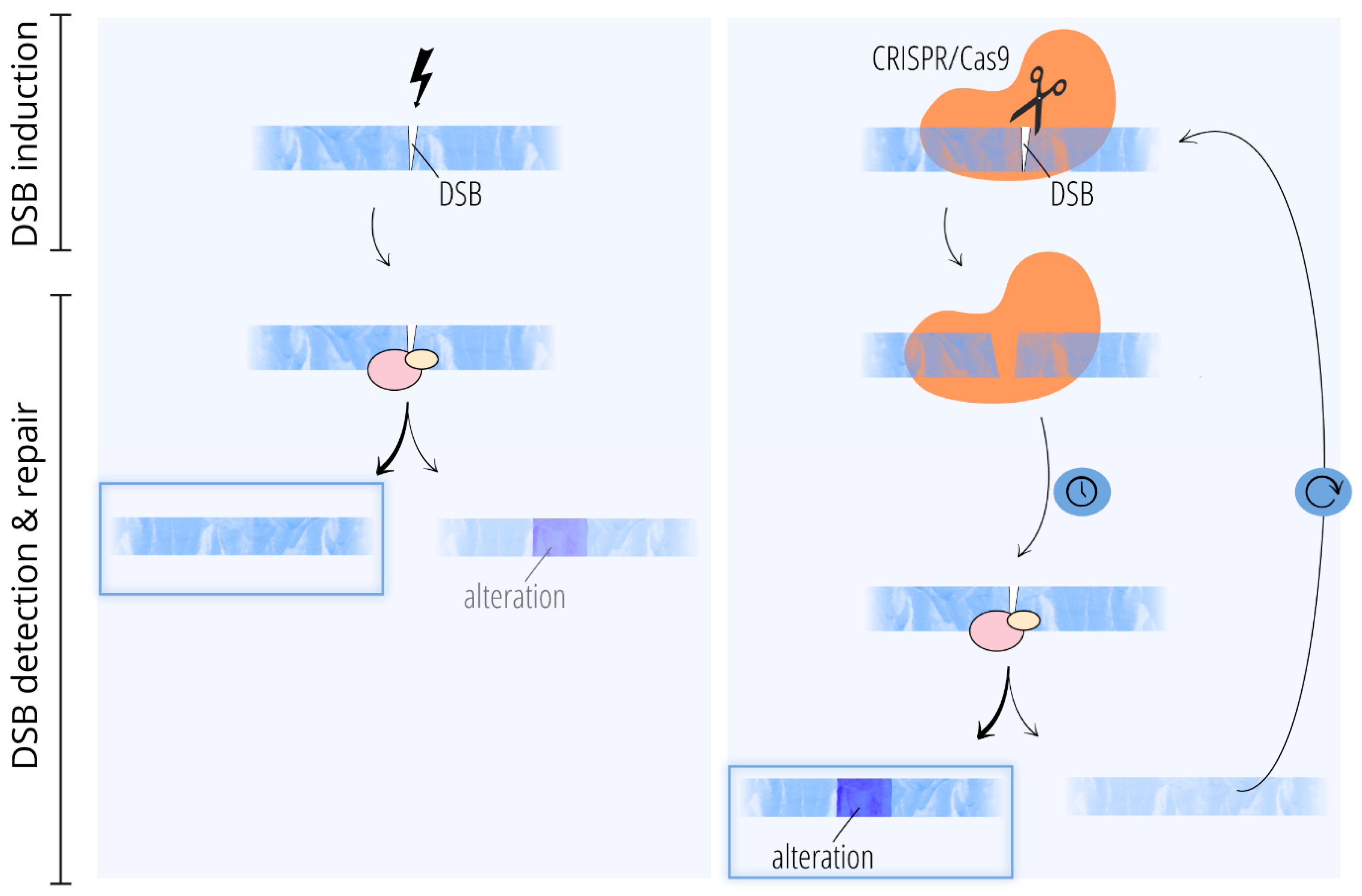
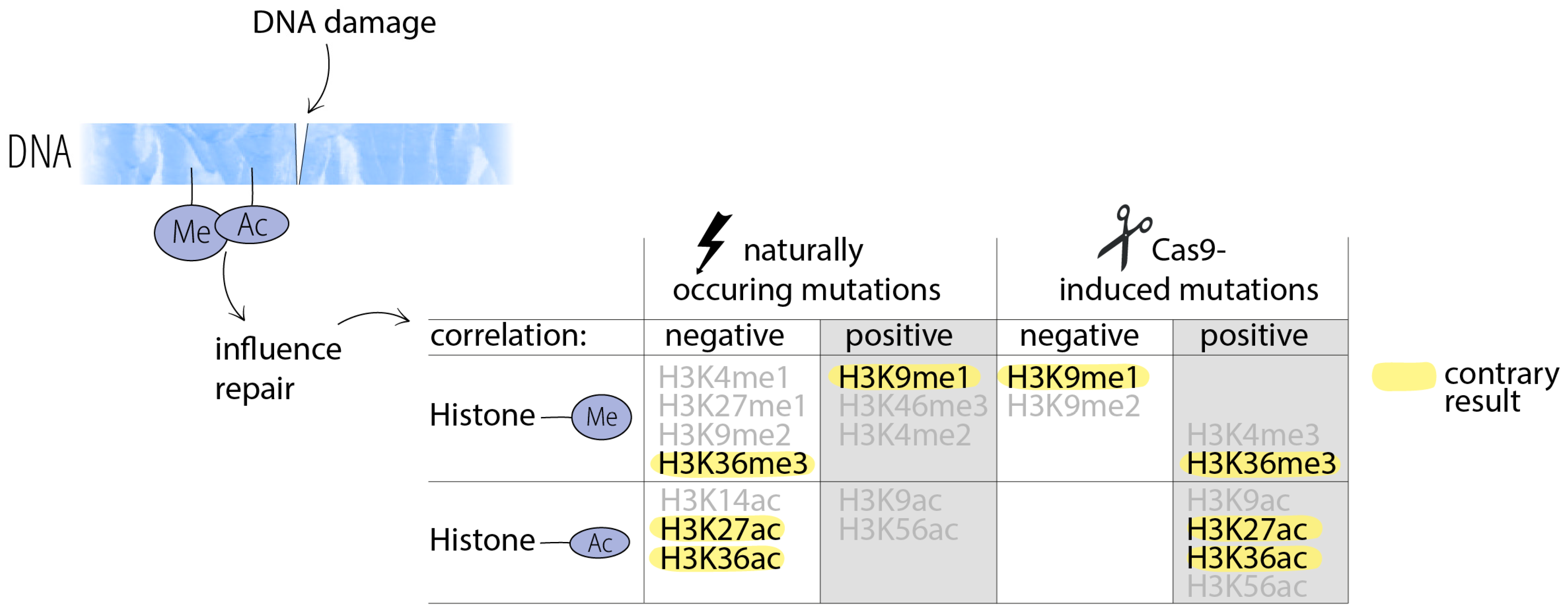
Different Causes of Mutations Can Result in Different Outcomes
2.2.2. Factors Influencing Recombination and Stability of the Genome
Recombinant Enzymatic Mutagens Can Create Novel Patterns of Crossovers and Bypass Genetic Linkage
2.2.3. Gene Copies with and Without Proximity
2.2.4. Other Genomic Features
3. Examples Showing the Potential of NGTs to Overcome the Constraints of Conventional Breeding
3.1. Tomato with Improved Harvesting Properties and Plant Architecture
3.2. De Novo Domesticated Tomato
3.3. Camelina with Altered Fatty Acid Content
3.4. Rice with Modified Flavone Content
3.5. Wheat with Low Gluten or Asparagine Content
3.6. Rice with Low Glutelin Content
3.7. Sugarcane with Less and Modified Lignin
3.8. Switchgrass with Increased Tiller Production
3.9. Tomato with Increased GABA Content
3.10. Early-Flowering Poplar
3.11. Rice with Asexual Reproduction Enabling the Maintenance of Hybrids (Synthetic Apomixis)
3.12. Mustard Greens with Reduced Pungency
3.13. Maize with Increased Drought Tolerance
3.14. Rice with Fine-Tuned Protein Expression
| Traits | Altered Gene(s) | Ploidy Level | Number of Genomic Alterations | Constraints for Conventional Breeding | Reference |
|---|---|---|---|---|---|
| Bread Wheat (Triticum aestivum) | |||||
| Reduction in gluten content | α-gliadins | hexaploid | up to 35 genes simultaneously | gene copies, genetic linkage (gene cluster) | Sánchez-León et al., 2018 [187] |
| Reduction in gluten content | ω- and γ-gliadins | hexaploid | up to 9 ω-gliadin and 12 γ-gliadin genes simultaneously | gene copies, genetic linkage (gene cluster) | Yu et al., 2023; Sánchez-León et al., 2024 [188,189] |
| Reduction in asparagine content | asparagine synthetase (asn2) | hexaploid | 3 genes simultaneously in 6 alleles | gene copies | Raffan et al., 2021 [190] |
| Camelina/false flax (Camelina sativa) | |||||
| Early flowering, shorter stature, and/or basal branching | flowering locus c (flc), short vegetative phase (svp), like heterochromatin protein 1 (lhp1), terminal flower 1 (tfl1) and early flowering locus 3 (elf3) | hexaploid | up to 10 genes simultaneously in up to 20 alleles | gene copies, genetic linkage | Bellec et al., 2022 [176] |
| Reduction in polyunsaturated fatty acids and increase in oleic acid | fatty acid desaturase 2 (fad2) | hexaploid | up to 3 genes simultaneously in up to 6 alleles | gene copies | Morineau et al., 2017 [174] |
| Lettuce (Lactuca sativa L.) | |||||
| Increase in ascorbic acid content and oxidation stress tolerance | GDP-l-galactose phosphorylase (ggp1 and ggp2) | diploid | 1 gene in 2 alleles | rare naturally occurring mutations in gene-regulatory regions | Zhang et al., 2018 [150] |
| Maize (Zea mays) | |||||
| Increase in drought tolerance | argoS8 | diploid | 1 gene in 2 alleles | artificial transfer of cisgenic sequences | Shi et al., 2016 [208] |
| Mustard Greens (Brassica juncea) | |||||
| Reduction in pungency | type-I myrosinase multigene | allotetraploid | 17 genes simultaneously in 34 alleles | gene copies, genetic linkage | Karlson et al., 2022 [206] |
| Poplar (Populus spp.) | |||||
| Early flowering, sex-switch, and hairless seeds | centroradialis (cen1 and cen2), type-A response regulator (arr17), MYB transcription factors (myb186/138/38 (Fuzzy3)) | diploid | up to 3 genes simultaneously in different combination of 1 arr17, 8 myb, and 4 cen1/cen2 alleles | Not known | Ortega et al., 2023 [153] |
| Rice (Oryza sativa) | |||||
| Reduction in glutelin content | glutelins (glua3, glub1a, glub1b, glub2, and gluc) | diploid | 5 genes simultaneously in 10 alleles | genetic linkage (gene cluster) | Wakasa et al., 2024 [191] |
| Synthetic apomixes, maintenance of hybrids | baby boom (bbm1, bbm2 and bbm3) and meiotic genes (rec8, pair1 and osd1) | diploid | up to 6 genes simultaneously | complex multiplexing including expression of male-genome-derived BBM1 in egg cell | Khanday et al., 2019 [203] |
| Fine-tuning of gene expression | uORFs of various genes | diploid | 1 gene | rare naturally occurring mutations in gene-regulatory regions, de novo sequences | Xue et al., 2023 [209] |
| Increase in apigenin content | flavonoid 3′ hydroxylases (cyp75b3 and cyp75b4) | diploid | 2 genes simultaneously in 4 alleles | genetic linkage, centromere/suppressed recombination | Yan et al., 2022 [177] |
| Strawberry (Fragaria vesca) | |||||
| Increase in sugar content | transcription factor basic (region) leucine zipper proteins (FvebZIPs1.1) | diploid | 1 gene in 2 alleles | rare naturally occurring mutations in gene-regulatory regions | Xing et al., 2020 [151] |
| Sugarcane (Saccarum officinarum) | |||||
| Reduction in lignin content and syringyl/guaiacyl (S/G) ratio | caffeic acid O-methyltransferases (comt) | allopolyploid | 107 alleles simultaneously | gene copies | Kannan et al., 2018 [192] |
| Reduction in chlorophyll content | magnesium chelatase subunit I (mgch) | allopolyploid | 49 alleles simultaneously | gene copies | Eid et al., 2021 [194] |
| Herbicide tolerance | acetolactate synthase (als) | allopolyploid | 3 alleles simultaneously | gene copies | Oz et al., 2021 [195] |
| Reduction in lignin content and increase in S/G ratio | transcription factor LIM (lim) | allopolyploid | not specified | gene copies | Laksana et al., 2024 [196] |
| Switchgrass (Panicum virgatum) | |||||
| Increase in tiller production | teosinte branched 1 (tb1a and tbtb) and phosphoglycerate mutase (pgm) | heterozygous polyploid | up to 2 genes simultaneously with multiple alleles for each gene | gene copies, self-incompatible | Liu et al., 2018 [197] |
| Increase in tiller production | tb1/cycloidea/proliferating cell factor (tcp19 and tcp 22) | heterozygous polyploid | 2 genes simultaneously in 4 alleles | gene copies, self-incompatible | Sun et al., 2025 [200] |
| Tomato (Solanum lycopersicum) | |||||
| Increase in GABA content | tomato phytoene desaturase (slyPDS), pyruvate-dependent GABA-T (gaba-tp1, gaba-tp2 and gaba-tp3), transporter cat9 (cat9) and Succinate semialdehyde dehydrogenase (ssadh) | diploid | up to 4 genes simultaneously in up to 8 alleles | genetic linkage | Li et al., 2018 [171] |
| Increase in lycopene content | cyclisation of lycopene (lcy-e, lcy-b1, lcy-b2, and blc) | diploid | up to 4 genes simultaneously in up to 8 alleles | genetic linkage | Li et al., 2018 [172] |
| Accumulation of pigments | phytoene synthase 1 (psy1), R2R3-MYB transcription factor (myb12), stay-green 1 (sgr1) | diploid | 3 genes simultaneously in 6 alleles | genetic linkage | Yang et al., 2023 [173] |
| Increase in γ-aminobutyric acid (GABA) content | glutamate decarboxylase (gad2/3) | diploid | 2 genes simultaneously in 4 alleles | specific alteration in regulatory domain | Nonaka et al., 2017 [152] |
| jointless trait, floral architecture | jointless 2 (j2), weak enhancer of jointless 2 (ej2) | diploid | up to 2 genes simultaneously in up to 4 alleles | genetic linkage, centromere/suppressed recombination | Roldan et al. 2017, Soyk et al., 2017, Klee 2019 [116,165,166] |
| Tomato (S. pimpinellifolium) | |||||
| De novo domestication | self-pruning (sp), ovate (o), fruit weight 2.2 (fw2.2), lycopene beta cyclase (cycb), fasciated (fas)/clavata 3 (clv3), multiflora (mult) | diploid | up to 4 genes simultaneously in different homozygous and heterozygous allele combinations | genetic linkage | Zsögön et al., 2018 [169] |
4. Discussion: The Relevance of Differences Between NGTs and Conventional Breeding for Risk Assessment and Regulation
4.1. Comparison with Conventional Breeding
4.2. Regulatory Implications
4.3. Regulatory Concepts
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Z.; Cui, J.; Wang, L.; Teng, N.; Zhang, S.; Lam, H.-M.; Zhu, Y.; Xiao, S.; Ke, W.; Lin, J.; et al. Genome-wide DNA mutations in Arabidopsis plants after multigenerational exposure to high temperatures. Genome Biol. 2021, 22, 160. [Google Scholar] [CrossRef]
- Schubert, I.; Vu, G.T.H. Genome Stability and Evolution: Attempting a Holistic View. Trends Plant Sci. 2016, 21, 749–757. [Google Scholar] [CrossRef]
- Sall, S.O.; Alioua, A.; Staerck, S.; Graindorge, S.; Pellicioli, M.; Schuler, J.; Galindo, C.; Raffy, Q.; Rousseau, M.; Molinier, J. Characterization of radiations-induced genomic structural variations in Arabidopsis thaliana. Plant J. 2025, 121, e17180. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Maintenance of genome stability in plants: Repairing DNA double strand breaks and chromatin structure stability. Front. Plant Sci. 2014, 5, 487. [Google Scholar] [CrossRef]
- Hu, Z.; Cools, T.; Veylder, L.D. Mechanisms Used by Plants to Cope with DNA Damage. Annu. Rev. Plant Biol. 2016, 67, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, D.; Lensink, M.; Kliebenstein, D.J.; Monroe, J.G. Causes of Mutation Rate Variability in Plant Genomes. Annu. Rev. Plant Biol. 2023, 74, 751–775. [Google Scholar] [CrossRef]
- Kawall, K. The Generic Risks and the Potential of SDN-1 Applications in Crop Plants. Plants 2021, 10, 2259. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.; Heissenberger, A. New Genetic Engineering—Possible Unintended Effects; Verlag Arbeiterkammer Wien: Vienna, Austria, 2023; Volume 208. [Google Scholar]
- ANSES. Risques et Enjeux Socio-Économiques Liés Aux Plantes NTG. 2024. Available online: https://www.anses.fr/fr/system/files/BIORISK2021SA0019Ra.pdf (accessed on 22 November 2025).
- Bohle, F.; Schneider, R.; Mundorf, J.; Zühl, L.; Simon, S.; Engelhard, M. Where does the EU-path on new genomic techniques lead us? Front. Genome Ed. 2024, 6, 1377117. [Google Scholar] [CrossRef]
- Mundorf, J.; Simon, S.; Engelhard, M. The European Commission’s regulatory proposal on new genomic techniques in plants: A focus on equivalence, complexity, and artificial intelligence. Environ. Sci. Eur. 2025, 37, 143. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, J.; Chen, X. A novel and powerful approach for designing future crops—Target editing promoter overcomes tradeoffs caused by gene pleiotropy. Sci. China Life Sci. 2022, 65, 2128–2130. [Google Scholar] [CrossRef]
- Luo, G.; Palmgren, M. Fine-tuning of quantitative traits. Sci. China Life Sci. 2023, 66, 1456–1458. [Google Scholar] [CrossRef]
- Yadav, B.; Majhi, A.; Phagna, K.; Meena, M.K.; Ram, H. Negative regulators of grain yield and mineral contents in rice: Potential targets for CRISPR-Cas9-mediated genome editing. Funct. Integr. Genom. 2023, 23, 317. [Google Scholar] [CrossRef]
- Puchta, H.; Houben, A. Plant chromosome engineering—Past, present and future. New Phytol. 2024, 241, 541–552. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Levy, A.A. How plants make ends meet: DNA double-strand break repair. Trends Plant Sci. 1999, 4, 263–269. [Google Scholar] [CrossRef]
- McVey, M.; Lee, S.E. MMEJ repair of double-strand breaks (director’s cut): Deleted sequences and alternative endings. Trends Genet. 2008, 24, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H.; Fauser, F. Synthetic nucleases for genome engineering in plants: Prospects for a bright future. Plant J. 2014, 78, 727–741. [Google Scholar] [CrossRef]
- Sfeir, A.; Symington, L.S. Microhomology-mediated end joining: A back-up survival mechanism or dedicated pathway? Trends Biochem. Sci. 2015, 40, 701–714. [Google Scholar] [CrossRef]
- Seol, J.-H.; Shim, E.Y.; Lee, S.E. Microhomology-mediated end joining: Good, bad and ugly. Mutat. Res. 2018, 809, 81–87. [Google Scholar] [CrossRef]
- Truong, L.N.; Li, Y.; Shi, L.Z.; Hwang, P.Y.-H.; He, J.; Wang, H.; Razavian, N.; Berns, M.W.; Wu, X. Microhomology-mediated End Joining and Homologous Recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7720–7725. [Google Scholar] [CrossRef]
- Shen, M.W.; Arbab, M.; Hsu, J.Y.; Worstell, D.; Culbertson, S.J.; Krabbe, O.; Cassa, C.A.; Liu, D.R.; Gifford, D.K.; Sherwood, R.I. Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 2018, 563, 646–651, Correction in Nature 2019, 567, E1–E2. https://doi.org/10.1038/s41586-019-0938-4. [Google Scholar] [CrossRef]
- Tan, J.; Zhao, Y.; Wang, B.; Hao, Y.; Wang, Y.; Li, Y.; Luo, W.; Zong, W.; Li, G.; Chen, S.; et al. Efficient CRISPR/Cas9-based plant genomic fragment deletions by microhomology-mediated end joining. Plant Biotechnol. J. 2020, 18, 2161–2163. [Google Scholar] [CrossRef]
- Xue, C.; Greene, E.C. DNA repair pathway choices in CRISPR-Cas9 mediated genome editing. Trends Genet. 2021, 37, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Choulika, A.; Perrin, A.; Dujon, B.; Nicolas, J.-F. Induction of Homologous Recombination in Mammalian Chromosomes by Using the I-SceI System of Saccharomyces cerevisiae. Mol. Cell. Biol. 1995, 15, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Sasaki, M.S.; Sonoda, E.; Morrison, C.; Hashimoto, M.; Utsumi, H.; Yamaguchi-Iwai, Y.; Shinohara, A.; Takeda, S. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998, 17, 5497–5508. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Svitashev, S.; Young, J.K.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted Mutagenesis, Precise Gene Editing, and Site-Specific Gene Insertion in Maize Using Cas9 and Guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Liu, W.; Gao, W.; Liu, C.; Song, G.; Li, W.-X.; Mao, L.; Chen, B.; Xu, Y.; et al. An alternative strategy for targeted gene replacement in plants using a dual-sgRNA/Cas9 design. Sci. Rep. 2016, 6, 23890. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Sharp, P.A.; Langer, R. Altered DNA repair pathway engagement by engineered CRISPR-Cas9 nucleases. Proc. Natl. Acad. Sci. USA 2023, 120, e2300605120. [Google Scholar] [CrossRef] [PubMed]
- Přibylová, A.; Fischer, L.; Pyott, D.E.; Bassett, A.; Molnar, A. DNA methylation can alter CRISPR/Cas9 editing frequency and DNA repair outcome in a target-specific manner. New Phytol. 2022, 235, 2285–2299. [Google Scholar] [CrossRef]
- Stephenson, A.A.; Raper, A.T.; Suo, Z. Bidirectional Degradation of DNA Cleavage Products Catalyzed by CRISPR/Cas9. J. Am. Chem. Soc. 2018, 140, 3743–3750. [Google Scholar] [CrossRef]
- Přibylová, A.; Fischer, L. How to use CRISPR/Cas9 in plants: From target site selection to DNA repair. J. Exp. Bot. 2024, 75, 5325–5343. [Google Scholar] [CrossRef]
- Richardson, C.D.; Ray, G.J.; DeWitt, M.A.; Curie, G.L.; Corn, J.E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 2016, 34, 339–344. [Google Scholar] [CrossRef]
- Brinkman, E.K.; Chen, T.; de Haas, M.; Holland, H.A.; Akhtar, W.; van Steensel, B. Kinetics and Fidelity of the Repair of Cas9-Induced Double-Strand DNA Breaks. Mol. Cell 2018, 70, 801–813.e6. [Google Scholar] [CrossRef]
- Aldag, P.; Welzel, F.; Jakob, L.; Schmidbauer, A.; Rutkauskas, M.; Fettes, F.; Grohmann, D.; Seidel, R. Probing the stability of the SpCas9–DNA complex after cleavage. Nucleic Acids Res. 2021, 49, 12411–12421. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, S.; Chen, R.; Xie, A. Target binding and residence: A new determinant of DNA double-strand break repair pathway choice in CRISPR/Cas9 genome editing. J. Zhejiang Univ. Sci. B 2021, 22, 73–86. [Google Scholar] [CrossRef]
- Clarke, R.; Heler, R.; MacDougall, M.S.; Yeo, N.C.; Chavez, A.; Regan, M.; Hanakahi, L.; Church, G.M.; Marraffini, L.A.; Merrill, B.J. Enhanced bacterial immunity and mammalian genome editing via RNA polymerase-mediated dislodging of Cas9 from double strand DNA breaks. Mol. Cell 2018, 71, 42–55.e8. [Google Scholar] [CrossRef]
- Hall, P.M.; Inman, J.T.; Fulbright, R.M.; Le, T.T.; Brewer, J.J.; Lambert, G.; Darst, S.A.; Wang, M.D. Polarity of the CRISPR roadblock to transcription. Nat. Struct. Mol. Biol. 2022, 29, 1217–1227. [Google Scholar] [CrossRef]
- Reginato, G.; Stritto, M.R.D.; Wang, Y.; Hao, J.; Pavani, R.; Schmitz, M.; Halder, S.; Morin, V.; Cannavo, E.; Ceppi, I.; et al. HLTF disrupts Cas9-DNA post-cleavage complexes to allow DNA break processing. Nat. Commun. 2024, 15, 5789. [Google Scholar] [CrossRef]
- Liu, S.-C.; Feng, Y.-L.; Sun, X.-N.; Chen, R.-D.; Liu, Q.; Xiao, J.-J.; Zhang, J.-N.; Huang, Z.-C.; Xiang, J.-F.; Chen, G.-Q.; et al. Target residence of Cas9-sgRNA influences DNA double-strand break repair pathway choices in CRISPR/Cas9 genome editing. Genome Biol. 2022, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, V.; Zhou, Q.; Kokkonda, P.; Bermudez-Cabrera, H.C.; Lim, D.; Law, B.K.; Holmes, B.R.; Chaudhary, S.K.; Pergu, R.; Leger, B.S.; et al. Chemogenetic System Demonstrates That Cas9 Longevity Impacts Genome Editing Outcomes. ACS Cent. Sci. 2020, 6, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Ben-Tov, D.; Mafessoni, F.; Cucuy, A.; Honig, A.; Melamed-Bessudo, C.; Levy, A.A. Uncovering the dynamics of precise repair at CRISPR/Cas9-induced double-strand breaks. Nat. Commun. 2024, 15, 5096. [Google Scholar] [CrossRef]
- Nambiar, T.S.; Baudrier, L.; Billon, P.; Ciccia, A. CRISPR-Based Genome Editing Through the Lens of DNA Repair. Mol. Cell 2022, 82, 348–388. [Google Scholar] [CrossRef]
- Vítor, A.C.; Huertas, P.; Legube, G.; de Almeida, S.F. Studying DNA Double-Strand Break Repair: An Ever-Growing Toolbox. Front. Mol. Biosci. 2020, 7, 24. [Google Scholar] [CrossRef]
- Ledford, H. CRISPR gene editing in human embryos wreaks chromosomal mayhem. Nature 2020, 583, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.L.; Papathanasiou, S.; Doerfler, P.A.; Blaine, L.J.; Sun, L.; Yao, Y.; Zhang, C.-Z.; Weiss, M.J.; Pellman, D. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 2021, 53, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Amendola, M.; Brusson, M.; Miccio, A. CRISPRthripsis: The Risk of CRISPR/Cas9-induced Chromothripsis in Gene Therapy. Stem Cells Transl. Med. 2022, 11, 1003–1009. [Google Scholar] [CrossRef]
- Lazar, N.H.; Celik, S.; Chen, L.; Fay, M.M.; Irish, J.C.; Jensen, J.; Tillinghast, C.A.; Urbanik, J.; Bone, W.P.; Gibson, C.C.; et al. High-resolution genome-wide mapping of chromosome-arm-scale truncations induced by CRISPR–Cas9 editing. Nat. Genet. 2024, 56, 1482–1493. [Google Scholar] [CrossRef]
- de Groot, D.; Spanjaard, A.; Hogenbirk, M.A.; Jacobs, H. Chromosomal Rearrangements and Chromothripsis: The Alternative End Generation Model. Int. J. Mol. Sci. 2023, 24, 794. [Google Scholar] [CrossRef]
- Cullot, G.; Aird, E.J.; Schlapansky, M.F.; Yeh, C.D.; van de Venn, L.; Vykhlyantseva, I.; Kreutzer, S.; Mailänder, D.; Lewków, B.; Klermund, J.; et al. Genome editing with the HDR-enhancing DNA-PKcs inhibitor AZD7648 causes large-scale genomic alterations. Nat. Biotechnol. 2024, 43, 1778–1782. [Google Scholar] [CrossRef]
- Samach, A.; Mafessoni, F.; Gross, O.; Melamed-Bessudo, C.; Filler-Hayut, S.; Dahan-Meir, T.; Amsellem, Z.; Pawlowski, W.P.; Levy, A.A. CRISPR/Cas9-induced DNA breaks trigger crossover, chromosomal loss, and chromothripsis-like rearrangements. Plant Cell 2023, 35, 3957–3972. [Google Scholar] [CrossRef]
- Koller, F.; Cieslak, M. A perspective from the EU: Unintended genetic changes in plants caused by NGT—Their relevance for a comprehensive molecular characterisation and risk assessment. Front. Bioeng. Biotechnol. 2023, 11, 1276226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Sharma, S.; Bewg, W.P.; Xue, L.-J.; Gizelbach, C.R.; Tsai, C.-J. Multiplex Editing of the Nucleoredoxin1 Tandem Array in Poplar: From Small Indels to Translocations and Complex Inversions. Cris. J. 2023, 6, 339–349. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.-Z.; Li, C.; Li, Y.; Li, J.-F. Hidden prevalence of deletion-inversion bi-alleles in CRISPR-mediated deletions of tandemly arrayed genes in plants. Nat. Commun. 2023, 14, 6787. [Google Scholar] [CrossRef]
- Loewe, L. Genetic mutation. Nat. Educ. 2008, 1, 113. Available online: https://www.nature.com/scitable/topicpage/genetic-mutation-1127/ (accessed on 22 November 2025).
- Monroe, J.G.; Srikant, T.; Carbonell-Bejerano, P.; Becker, C.; Lensink, M.; Exposito-Alonso, M.; Klein, M.; Hildebrandt, J.; Neumann, M.; Kliebenstein, D.; et al. Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 2022, 602, 101–105, Correction in Nature 2023, 620, E13. https://doi.org/10.1038/s41586-023-06387-9. [Google Scholar] [CrossRef]
- Martincorena, I.; Seshasayee, A.S.N.; Luscombe, N.M. Evidence of non-random mutation rates suggests an evolutionary risk management strategy. Nature 2012, 485, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Schuster-Böckler, B.; Lehner, B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature 2012, 488, 504–507. [Google Scholar] [CrossRef]
- Li, F.; Mao, G.; Tong, D.; Huang, J.; Gu, L.; Yang, W.; Li, G.-M. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSα. Cell 2013, 153, 590–600. [Google Scholar] [CrossRef]
- Yazdi, P.G.; Pedersen, B.A.; Taylor, J.F.; Khattab, O.S.; Chen, Y.-H.; Chen, Y.; Jacobsen, S.E.; Wang, P.H. Increasing Nucleosome Occupancy Is Correlated with an Increasing Mutation Rate so Long as DNA Repair Machinery Is Intact. PLoS ONE 2015, 10, e0136574. [Google Scholar] [CrossRef]
- Kawall, K. New Possibilities on the Horizon: Genome Editing Makes the Whole Genome Accessible for Changes. Front. Plant Sci. 2019, 10, 525. [Google Scholar] [CrossRef]
- Arndt, P.F.; Hwa, T.; Petrov, D.A. Substantial regional variation in substitution rates in the human genome: Importance of GC content, gene density, and telomere-specific effects. J. Mol. Evol. 2005, 60, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Mugal, C.F.; Ellegren, H. Substitution rate variation at human CpG sites correlates with non-CpG divergence, methylation level and GC content. Genome Biol. 2011, 12, R58. [Google Scholar] [CrossRef]
- Staunton, P.M.; Peters, A.J.; Seoighe, C. Somatic mutations inferred from RNA-seq data highlight the contribution of replication timing to mutation rate variation in a model plant. Genetics 2023, 225, iyad128, Correction in Genetics 2023, 225, iyad166. https://doi.org/10.1093/genetics/iyad166. [Google Scholar] [CrossRef]
- Huang, Y.; Li, G.-M. DNA mismatch repair preferentially safeguards actively transcribed genes. DNA Repair 2018, 71, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gu, L.; Li, G.-M. H3K36me3-mediated mismatch repair preferentially protects actively transcribed genes from mutation. J. Biol. Chem. 2018, 293, 7811–7823. [Google Scholar] [CrossRef] [PubMed]
- Bergis-Ser, C.; Reji, M.; Latrasse, D.; Bergounioux, C.; Benhamed, M.; Raynaud, C. Chromatin dynamics and RNA metabolism are double-edged swords for the maintenance of plant genome integrity. Nat. Plants 2024, 10, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Y.; Jia, J.; Fang, Y.; Tang, Y.; Wu, H.; Fang, D. H3K36me3, message from chromatin to DNA damage repair. Cell Biosci. 2020, 10, 9. [Google Scholar] [CrossRef]
- Quiroz, D.; Oya, S.; Lopez-Mateos, D.; Zhao, K.; Pierce, A.; Ortega, L.; Ali, A.; Carbonell-Bejerano, P.; Yarov-Yarovoy, V.; Suzuki, S.; et al. H3K4me1 recruits DNA repair proteins in plants. Plant Cell 2024, 36, 2410–2426. [Google Scholar] [CrossRef]
- Belfield, E.J.; Ding, Z.J.; Jamieson, F.J.C.; Visscher, A.M.; Zheng, S.J.; Mithani, A.; Harberd, N.P. DNA mismatch repair preferentially protects genes from mutation. Genome Res. 2018, 28, 66–74. [Google Scholar] [CrossRef]
- Lee, H.; Popodi, E.; Tang, H.; Foster, P.L. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, E2774–E2783. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhang, Z.; Zheng, Y.; Du, L.; Zhu, B. Preferential Protection of Genetic Fidelity within Open Chromatin by the Mismatch Repair Machinery. J. Biol. Chem. 2016, 291, 17692–17705. [Google Scholar] [CrossRef]
- Frigola, J.; Sabarinathan, R.; Mularoni, L.; Muiños, F.; Gonzalez-Perez, A.; López-Bigas, N. Reduced mutation rate in exons due to differential mismatch repair. Nat. Genet. 2017, 49, 1684–1692, Correction in Nat. Genet. 2018, 50, 1196. https://doi.org/10.1038/s41588-018-0123-y. [Google Scholar] [CrossRef]
- Supek, F.; Lehner, B. Clustered Mutation Signatures Reveal that Error-Prone DNA Repair Targets Mutations to Active Genes. Cell 2017, 170, 534–547.e23. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.L.; Niccum, B.A.; Popodi, E.; Townes, J.P.; Lee, H.; MohammedIsmail, W.; Tang, H. Determinants of Base-Pair Substitution Patterns Revealed by Whole-Genome Sequencing of DNA Mismatch Repair Defective Escherichia coli. Genetics 2018, 209, 1029–1042. [Google Scholar] [CrossRef]
- Niccum, B.A.; Lee, H.; MohammedIsmail, W.; Tang, H.; Foster, P.L. The Spectrum of Replication Errors in the Absence of Error Correction Assayed Across the Whole Genome of Escherichia coli. Genetics 2018, 209, 1043–1054. [Google Scholar] [CrossRef]
- Yan, W.; Deng, X.W.; Yang, C.; Tang, X. The Genome-Wide EMS Mutagenesis Bias Correlates With Sequence Context and Chromatin Structure in Rice. Front. Plant Sci. 2021, 12, 579675. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Zhang, S.; Cai, H.; Xu, L.; An, B.; Wang, R.; Liu, G.; He, Y.; Jiao, C.; et al. The Mutational, Epigenetic, and Transcriptional Effects Between Mixed High-Energy Particle Field (CR) and 7Li-Ion Beams (LR) Radiation in Wheat M1 Seedlings. Front. Plant Sci. 2022, 13, 878420. [Google Scholar] [CrossRef] [PubMed]
- van Overbeek, M.; Capurso, D.; Carter, M.M.; Thompson, M.S.; Frias, E.; Russ, C.; Reece-Hoyes, J.S.; Nye, C.; Gradia, S.; Vidal, B.; et al. DNA Repair Profiling Reveals Nonrandom Outcomes at Cas9-Mediated Breaks. Mol. Cell 2016, 63, 633–646. [Google Scholar] [CrossRef]
- Yarrington, R.M.; Verma, S.; Schwartz, S.; Trautman, J.K.; Carroll, D. Nucleosomes inhibit target cleavage by CRISPR-Cas9 in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, 9351–9358. [Google Scholar] [CrossRef]
- Weiss, T.; Crisp, P.A.; Rai, K.M.; Song, M.; Springer, N.M.; Zhang, F. Epigenetic features drastically impact CRISPR–Cas9 efficacy in plants. Plant Physiol. 2022, 190, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Gisler, S.; Gonçalves, J.P.; Akhtar, W.; de Jong, J.; Pindyurin, A.V.; Wessels, L.F.A.; van Lohuizen, M. Multiplexed Cas9 targeting reveals genomic location effects and gRNA-based staggered breaks influencing mutation efficiency. Nat. Commun. 2019, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Schep, R.; Brinkman, E.K.; Leemans, C.; Vergara, X.; van der Weide, R.H.; Morris, B.; van Schaik, T.; Manzo, S.G.; Peric-Hupkes, D.; van den Berg, J.; et al. Impact of chromatin context on Cas9-induced DNA double-strand break repair pathway balance. Mol. Cell 2021, 81, 2216–2230.e10. [Google Scholar] [CrossRef]
- Yu, Z.; Ren, M.; Wang, Z.; Zhang, B.; Rong, Y.S.; Jiao, R.; Gao, G. Highly Efficient Genome Modifications Mediated by CRISPR/Cas9 in Drosophila. Genetics 2013, 195, 289–291. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, J.; Wang, R.; Liu, Y.; Birchler, J.A.; Han, F. Efficient Targeted Genome Modification in Maize Using CRISPR/Cas9 System. J. Genet. Genom. 2016, 43, 37–43. [Google Scholar] [CrossRef]
- Kallimasioti-Pazi, E.M.; Chathoth, K.T.; Taylor, G.C.; Meynert, A.; Ballinger, T.; Kelder, M.J.E.; Lalevée, S.; Sanli, I.; Feil, R.; Wood, A.J. Heterochromatin delays CRISPR-Cas9 mutagenesis but does not influence the outcome of mutagenic DNA repair. PLoS Biol. 2018, 16, e2005595, Correction in PLoS Biol. 2019, 17, e3000160. https://doi.org/10.1371/journal.pbio.3000160. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.S.; Schwarzacher, T. Organisation of the plant genome in chromosomes. Plant J. 2011, 66, 18–33. [Google Scholar] [CrossRef]
- Dluzewska, J.; Szymanska, M.; Ziolkowski, P.A. Where to Cross Over? Defining Crossover Sites in Plants. Front. Genet. 2018, 9, 609. [Google Scholar] [CrossRef]
- Lloyd, A. Crossover patterning in plants. Plant Reprod. 2023, 36, 55–72. [Google Scholar] [CrossRef]
- Choi, K.; Henderson, I.R. Meiotic recombination hotspots—A comparative view. Plant J. 2015, 83, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.B.; Naish, M.; Lian, Q.; Burns, R.; Tock, A.J.; Rabanal, F.A.; Wlodzimierz, P.; Habring, A.; Nicholas, R.E.; Weigel, D.; et al. Structural variation and DNA methylation shape the centromere-proximal meiotic crossover landscape in Arabidopsis. Genome Biol. 2024, 25, 30. [Google Scholar] [CrossRef] [PubMed]
- Termolino, P.; Cremona, G.; Consiglio, M.F.; Conicella, C. Insights into epigenetic landscape of recombination-free regions. Chromosoma 2016, 125, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Copenhaver, G.P. Meiotic Recombination: Mixing It Up in Plants. Annu. Rev. Plant Biol. 2018, 69, 577–609. [Google Scholar] [CrossRef]
- Kejnovsky, E.; Hobza, R.; Cermak, T.; Kubat, Z.; Vyskot, B. The role of repetitive DNA in structure and evolution of sex chromosomes in plants. Heredity 2009, 102, 533–541. [Google Scholar] [CrossRef]
- Hartley, G.; O’Neill, R.J. Centromere Repeats: Hidden Gems of the Genome. Genes 2019, 10, 223. [Google Scholar] [CrossRef]
- Simon, L.; Voisin, M.; Tatout, C.; Probst, A.V. Structure and Function of Centromeric and Pericentromeric Heterochromatin in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1049. [Google Scholar] [CrossRef]
- Mayer, K.F.X.; Waugh, R.; Langridge, P.; Close, T.J.; Wise, R.P.; Graner, A.; Matsumoto, T.; Sato, K.; Schulman, A.; Muehlbauer, G.J.; et al. A physical, genetic and functional sequence assembly of the barley genome. Nature 2012, 491, 711–716. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Zhang, R.; Lin, Z. Genome-wide recombination rate variation in a recombination map of cotton. PLoS ONE 2017, 12, e0188682. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Yan, J. Dissecting meiotic recombination based on tetrad analysis by single-microspore sequencing in maize. Nat. Commun. 2015, 6, 6648. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Bauer, E.; Falque, M.; Walter, H.; Bauland, C.; Camisan, C.; Campo, L.; Meyer, N.; Ranc, N.; Rincent, R.; Schipprack, W.; et al. Intraspecific variation of recombination rate in maize. Genome Biol. 2013, 14, R103. [Google Scholar] [CrossRef]
- Blary, A.; Jenczewski, E. Manipulation of crossover frequency and distribution for plant breeding. Theor. Appl. Genet. 2019, 132, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, M.; Smith, G.R. Repression of harmful meiotic recombination in centromeric regions. Semin. Cell Dev. Biol. 2016, 54, 188. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Séguéla-Arnaud, M.; Larchevêque, C.; Lloyd, A.H.; Mercier, R. Unleashing meiotic crossovers in hybrid plants. Proc. Natl. Acad. Sci. USA 2017, 115, 2431. [Google Scholar] [CrossRef]
- Serra, H.; Lambing, C.; Griffin, C.H.; Topp, S.D.; Nageswaran, D.C.; Underwood, C.J.; Ziolkowski, P.A.; Séguéla-Arnaud, M.; Fernandes, J.B.; Mercier, R.; et al. Massive crossover elevation via combination of HEI10 and recq4a recq4b during Arabidopsis meiosis. Proc. Natl. Acad. Sci. USA 2018, 115, 2437. [Google Scholar] [CrossRef]
- Choi, K.; Zhao, X.; Tock, A.J.; Lambing, C.; Underwood, C.J.; Hardcastle, T.J.; Serra, H.; Kim, J.; Cho, H.S.; Kim, J.; et al. Nucleosomes and DNA methylation shape meiotic DSB frequency in Arabidopsis thaliana transposons and gene regulatory regions. Genome Res. 2018, 28, 532. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.B.; Wlodzimierz, P.; Henderson, I.R. Meiotic recombination within plant centromeres. Curr. Opin. Plant Biol. 2019, 48, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Soyk, S.; Lemmon, Z.H.; Oved, M.; Fisher, J.; Liberatore, K.L.; Park, S.J.; Goren, A.; Jiang, K.; Ramos, A.; van der Knaap, E.; et al. Bypassing Negative Epistasis on Yield in Tomato Imposed by a Domestication Gene. Cell 2017, 169, 1142–1155.e12. [Google Scholar] [CrossRef]
- Rönspies, M.; Schmidt, C.; Schindele, P.; Lieberman-Lazarovich, M.; Houben, A.; Puchta, H. Massive crossover suppression by CRISPR–Cas-mediated plant chromosome engineering. Nat. Plants 2022, 8, 1153–1159. [Google Scholar] [CrossRef]
- Hayut, S.F.; Bessudo, C.M.; Levy, A.A. Targeted recombination between homologous chromosomes for precise breeding in tomato. Nat. Commun. 2017, 8, 15605. [Google Scholar] [CrossRef]
- Kouranov, A.; Armstrong, C.; Shrawat, A.; Sidorov, V.; Huesgen, S.; Lemke, B.; Boyle, T.; Gasper, M.; Lawrence, R.; Yang, S. Demonstration of targeted crossovers in hybrid maize using CRISPR technology. Commun. Biol. 2022, 5, 53. [Google Scholar] [CrossRef]
- Shlush, I.B.; Samach, A.; Melamed-Bessudo, C.; Ben-Tov, D.; Dahan-Meir, T.; Filler-Hayut, S.; Levy, A.A. CRISPR/Cas9 Induced Somatic Recombination at the CRTISO Locus in Tomato. Genes 2020, 12, 59. [Google Scholar] [CrossRef]
- Filler-Hayut, S.; Kniazev, K.; Melamed-Bessudo, C.; Levy, A.A. Targeted Inter-Homologs Recombination in Arabidopsis Euchromatin and Heterochromatin. Int. J. Mol. Sci. 2021, 22, 12096. [Google Scholar] [CrossRef]
- Rönspies, M.; Dorn, A.; Schindele, P.; Puchta, H. CRISPR-Cas-mediated chromosome engineering for crop improvement and synthetic biology. Nat. Plants 2021, 7, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Hinrichs, R.; Rönspies, M.; Haghi, R.; Puchta, H.; Houben, A. Epigenetic state and gene expression remain stable after CRISPR/Cas-mediated chromosomal inversions. New Phytol. 2025, 245, 2527–2539. [Google Scholar] [CrossRef]
- Beying, N.; Schmidt, C.; Pacher, M.; Houben, A.; Puchta, H. CRISPR–Cas9-mediated induction of heritable chromosomal translocations in Arabidopsis. Nat. Plants 2020, 6, 638–645. [Google Scholar] [CrossRef]
- Gehrke, F.; Schindele, A.; Puchta, H. Nonhomologous end joining as key to CRISPR/Cas-mediated plant chromosome engineering. Plant Physiol. 2021, 188, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Fransz, P.; Rönspies, M.; Dreissig, S.; Fuchs, J.; Heckmann, S.; Houben, A.; Puchta, H. Changing local recombination patterns in Arabidopsis by CRISPR/Cas mediated chromosome engineering. Nat. Commun. 2020, 11, 4418. [Google Scholar] [CrossRef]
- Schwartz, C.; Lenderts, B.; Feigenbutz, L.; Barone, P.; Llaca, V.; Fengler, K.; Svitashev, S. CRISPR-Cas9-mediated 75.5-Mb inversion in maize. Nat. Plants 2020, 6, 1427–1431. [Google Scholar] [CrossRef]
- Lee, K.; Wang, K. Level up to chromosome restructuring. Nat. Plants 2020, 6, 600–601. [Google Scholar] [CrossRef]
- Schmidt, C.; Schindele, P.; Puchta, H. From gene editing to genome engineering: Restructuring plant chromosomes via CRISPR/Cas. aBIOTECH 2019, 1, 21–31. [Google Scholar] [CrossRef]
- Huang, K.; Rieseberg, L.H. Frequency, Origins, and Evolutionary Role of Chromosomal Inversions in Plants. Front. Plant Sci. 2020, 11, 296. [Google Scholar] [CrossRef]
- Lucek, K.; Giménez, M.D.; Joron, M.; Rafajlović, M.; Searle, J.B.; Walden, N.; Westram, A.M.; Faria, R. The Impact of Chromosomal Rearrangements in Speciation: From Micro- to Macroevolution. Cold Spring Harb. Perspect. Biol. 2023, 15, a041447. [Google Scholar] [CrossRef] [PubMed]
- Farré, A.; Cuadrado, A.; Lacasa-Benito, I.; Cistué, L.; Schubert, I.; Comadran, J.; Jansen, J.; Romagosa, I. Genetic characterization of a reciprocal translocation present in a widely grown barley variety. Mol. Breed. 2012, 30, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Huettel, B.; Walkemeier, B.; Mayjonade, B.; Lopez-Roques, C.; Gil, L.; Roux, F.; Schneeberger, K.; Mercier, R. A pan-genome of 69 Arabidopsis thaliana accessions reveals a conserved genome structure throughout the global species range. Nat. Genet. 2024, 56, 982–991. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Z.; Chebotarov, D.; Chougule, K.; Lu, Z.; Rivera, L.F.; Kathiresan, N.; Al-Bader, N.; Mohammed, N.; Alsantely, A.; et al. Pan-genome inversion index reveals evolutionary insights into the subpopulation structure of Asian rice. Nat. Commun. 2023, 14, 1567. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Chen, L.; Guo, M.; Liang, R.; Pan, Y. The genomic pattern of insertion/deletion variations during rice improvement. BMC Genom. 2024, 25, 1263. [Google Scholar] [CrossRef]
- Zhou, X.; Qiang, C.; Chen, L.; Qing, D.; Huang, J.; Li, J.; Pan, Y. The Landscape of Presence/Absence Variations during the Improvement of Rice. Genes 2024, 15, 645. [Google Scholar] [CrossRef]
- Escudero, M.; Marques, A.; Lucek, K.; Hipp, A.L. Genomic hotspots of chromosome rearrangements explain conserved synteny despite high rates of chromosome evolution in a holocentric lineage. Mol. Ecol. 2024, 33, e17086. [Google Scholar] [CrossRef]
- Kileeg, Z.; Wang, P.; Mott, G.A. Chromosome-Scale Assembly and Annotation of Eight Arabidopsis thaliana Ecotypes. Genome Biol. Evol. 2024, 16, evae169. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Su, T.; Cheng, G.-Q.; Wang, B.-X.; Li, X.; Deng, C.-L.; Gao, W.-J. Chromosome Evolution in Connection with Repetitive Sequences and Epigenetics in Plants. Genes. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Quadrana, L.; Etcheverry, M.; Gilly, A.; Caillieux, E.; Madoui, M.-A.; Guy, J.; Bortolini Silveira, A.; Engelen, S.; Baillet, V.; Wincker, P.; et al. Transposition favors the generation of large effect mutations that may facilitate rapid adaption. Nat. Commun. 2019, 10, 3421. [Google Scholar] [CrossRef] [PubMed]
- Roquis, D.; Robertson, M.; Yu, L.; Thieme, M.; Julkowska, M.; Bucher, E. Genomic impact of stress-induced transposable element mobility in Arabidopsis. Nucleic Acids Res. 2021, 49, 10431–10447, Correction in Nucleic Acids Res. 2021, 49, 12002–12003. https://doi.org/10.1093/nar/gkab1078. [Google Scholar] [CrossRef]
- Capdeville, N.; Schindele, P.; Puchta, H. Getting better all the time—Recent progress in the development of CRISPR/Cas-based tools for plant genome engineering. Curr. Opin. Biotechnol. 2023, 79, 102854. [Google Scholar] [CrossRef]
- Hanada, K.; Zou, C.; Lehti-Shiu, M.D.; Shinozaki, K.; Shiu, S.-H. Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol. 2008, 148, 993–1003. [Google Scholar] [CrossRef]
- Maher, C.; Stein, L.; Ware, D. Evolution of Arabidopsis microRNA families through duplication events. Genome Res. 2006, 16, 510–519. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Qi, Y.; Li, X.; Zhang, Y.; Starker, C.G.; Baltes, N.J.; Zhang, F.; Sander, J.D.; Reyon, D.; Joung, J.K.; Voytas, D.F. Targeted Deletion and Inversion of Tandemly Arrayed Genes in Arabidopsis thaliana Using Zinc Finger Nucleases. G3 Genes Genomes Genet. 2013, 3, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Capistrano-Gossmann, G.; Braatz, J.; Sashidhar, N.; Melzer, S. Recent developments in genome editing and applications in plant breeding. Plant Breed. 2018, 137, 1–9. [Google Scholar] [CrossRef]
- Waites, J.; Achary, V.M.M.; Syombua, E.D.; Hearne, S.J.; Bandyopadhyay, A. CRISPR-mediated genome editing of wheat for enhancing disease resistance. Front. Genome Ed. 2025, 7, 1542487. [Google Scholar] [CrossRef]
- Rottersman, M.G.; Zhang, W.; Zhang, J.; Grigorean, G.; Burguener, G.; Carter, C.; Vang, T.; Hegarty, J.; Zhang, X.; Finnie, S.; et al. Deletion of wheat alpha-gliadins from chromosome 6D improves gluten strength and reduces immunodominant celiac disease epitopes. Theor. Appl. Genet. 2025, 138, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Si, X.; Ji, X.; Fan, R.; Liu, J.; Chen, K.; Wang, D.; Gao, C. Genome editing of upstream open reading frames enables translational control in plants. Nat. Biotechnol. 2018, 36, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Chen, K.; Zhu, H.; Zhang, R.; Zhang, H.; Li, B.; Gao, C. Fine-tuning sugar content in strawberry. Genome Biol. 2020, 21, 230. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057, Correction in Sci. Rep. 2019, 9, 19822. https://doi.org/10.1038/s41598-019-55119-5. [Google Scholar] [CrossRef]
- Ortega, M.A.; Zhou, R.; Chen, M.S.S.; Bewg, W.P.; Simon, B.; Tsai, C.-J. In vitro floral development in poplar: Insights into seed trichome regulation and trimonoecy. New Phytol. 2023, 237, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, G.; Zhao, Y.; Zhang, R.; Tang, X.; Li, L.; Jia, X.; Guo, Y.; Wu, Y.; Han, Y.; et al. An efficient CRISPR–Cas12a promoter editing system for crop improvement. Nat. Plants 2023, 9, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y. Beyond knockouts: Fine-tuning regulation of gene expression in plants with CRISPR-Cas-based promoter editing. New Phytol. 2023, 239, 868–874. [Google Scholar] [CrossRef]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise plant genome editing using base editors and prime editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef]
- Lam, D.K.; Feliciano, P.R.; Arif, A.; Bohnuud, T.; Fernandez, T.P.; Gehrke, J.M.; Grayson, P.; Lee, K.D.; Ortega, M.A.; Sawyer, C.; et al. Improved cytosine base editors generated from TadA variants. Nat. Biotechnol. 2023, 41, 686–697. [Google Scholar] [CrossRef]
- Lin, Q.; Jin, S.; Zong, Y.; Yu, H.; Zhu, Z.; Liu, G.; Kou, L.; Wang, Y.; Qiu, J.-L.; Li, J.; et al. High-efficiency prime editing with optimized, paired pegRNAs in plants. Nat. Biotechnol. 2021, 39, 923–927. [Google Scholar] [CrossRef]
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- Gupta, A.; Liu, B.; Chen, Q.-J.; Yang, B. High-efficiency prime editing enables new strategies for broad-spectrum resistance to bacterial blight of rice. Plant Biotechnol. J. 2023, 21, 1454–1464. [Google Scholar] [CrossRef]
- Juhas, M.; Rodekohr, B.; Bauer-Panskus, A.; Then, C. Combining AI and new genomic techniques to ‘fine-tune’ plants: Challenges in risk assessment. Front. Plant Sci. 2025, 16, 1677066. [Google Scholar] [CrossRef]
- Koller, F.; Schulz, M.; Juhas, M.; Bauer-Panskus, A.; Then, C. The need for assessment of risks arising from interactions between NGT organisms from an EU perspective. Environ. Sci. Eur. 2023, 35, 27. [Google Scholar] [CrossRef]
- Kawall, K.; Cotter, J.; Then, C. Broadening the GMO risk assessment in the EU for genome editing technologies in agriculture. Env. Sci. Eur. 2020, 32, 106. [Google Scholar] [CrossRef]
- Ledford, H. Fixing the tomato: CRISPR edits correct plant-breeding snafu. Nature 2017, 545, 394–395. [Google Scholar] [CrossRef]
- Klee, H.J. Loss of Function of JOINTLESS2 (J2) due to CRISPR-Cas9 Induced Mutation. 2019. Available online: https://www.aphis.usda.gov/sites/default/files/19-282-01-a3-air-inquiry-cbidel.pdf (accessed on 22 November 2025).
- Roldan, M.V.G.; Périlleux, C.; Morin, H.; Huerga-Fernandez, S.; Latrasse, D.; Benhamed, M.; Bendahmane, A. Natural and induced loss of function mutations in SlMBP21 MADS-box gene led to jointless-2 phenotype in tomato. Sci. Rep. 2017, 7, 4402. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J. Genetic Control of Floral Architecture: Insights into Improving Crop Yield. Cell 2017, 169, 983–984. [Google Scholar] [CrossRef]
- Gasparini, K.; Moreira, J.d.R.; Peres, L.E.P.; Zsögön, A. De novo domestication of wild species to create crops with increased resilience and nutritional value. Curr. Opin. Plant Biol. 2021, 60, 102006. [Google Scholar] [CrossRef] [PubMed]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X.; et al. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014, 46, 1220–1226. [Google Scholar] [CrossRef]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-mediated metabolic engineering of γ-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef]
- Yang, T.; Ali, M.; Lin, L.; Li, P.; He, H.; Zhu, Q.; Sun, C.; Wu, N.; Zhang, X.; Huang, T.; et al. Recoloring tomato fruit by CRISPR/Cas9-mediated multiplex gene editing. Hortic. Res. 2023, 10, uhac214. [Google Scholar] [CrossRef]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogué, F.; Faure, J.-D. Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol. J. 2017, 15, 729–739. [Google Scholar] [CrossRef]
- Kawall, K. genome-edited Camelina sativa with a unique fatty acid content and its potential impact on ecosystems. Environ. Sci. Eur. 2021, 33, 38. [Google Scholar] [CrossRef]
- Bellec, Y.; Guyon-Debast, A.; François, T.; Gissot, L.; Biot, E.; Nogué, F.; Faure, J.-D.; Tepfer, M. New Flowering and Architecture Traits Mediated by Multiplex CRISPR-Cas9 Gene Editing in Hexaploid Camelina sativa. Agronomy 2022, 12, 1873. [Google Scholar] [CrossRef]
- Yan, D.; Tajima, H.; Cline, L.C.; Fong, R.Y.; Ottaviani, J.I.; Shapiro, H.-Y.; Blumwald, E. Genetic modification of flavone biosynthesis in rice enhances biofilm formation of soil diazotrophic bacteria and biological nitrogen fixation. Plant Biotechnol. J. 2022, 20, 2135–2148. [Google Scholar] [CrossRef]
- Chen, M.; Presting, G.; Barbazuk, W.B.; Goicoechea, J.L.; Blackmon, B.; Fang, G.; Kim, H.; Frisch, D.; Yu, Y.; Sun, S.; et al. An Integrated Physical and Genetic Map of the Rice Genome. Plant Cell 2002, 14, 537–545. [Google Scholar] [CrossRef]
- Sasaki, T. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef]
- Yan, H.; Talbert, P.B.; Lee, H.-R.; Jett, J.; Henikoff, S.; Chen, F.; Jiang, J. Intergenic Locations of Rice Centromeric Chromatin. PLoS Biol. 2008, 6, e286. [Google Scholar] [CrossRef]
- Flowers, J.M.; Molina, J.; Rubinstein, S.; Huang, P.; Schaal, B.A.; Purugganan, M.D. Natural Selection in Gene-Dense Regions Shapes the Genomic Pattern of Polymorphism in Wild and Domesticated Rice. Mol. Biol. Evol. 2012, 29, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Fayos, I.; Mieulet, D.; Petit, J.; Meunier, A.C.; Périn, C.; Nicolas, A.; Guiderdoni, E. Engineering meiotic recombination pathways in rice. Plant Biotechnol. J. 2019, 17, 2062–2077. [Google Scholar] [CrossRef]
- Song, J.-M.; Xie, W.-Z.; Wang, S.; Guo, Y.-X.; Koo, D.-H.; Kudrna, D.; Gong, C.; Huang, Y.; Feng, J.-W.; Zhang, W.; et al. Two gap-free reference genomes and a global view of the centromere architecture in rice. Mol. Plant 2021, 14, 1757–1767. [Google Scholar] [CrossRef]
- Peñuela, M.; Riccio-Rengifo, C.; Finke, J.; Rocha, C.; Gkanogiannis, A.; Wing, R.A.; Lorieux, M. Prediction of crossover recombination using parental genomes. PLoS ONE 2023, 18, e0281804. [Google Scholar] [CrossRef]
- Shang, L.; He, W.; Wang, T.; Yang, Y.; Xu, Q.; Zhao, X.; Yang, L.; Zhang, H.; Li, X.; Lv, Y.; et al. A complete assembly of the rice Nipponbare reference genome. Mol. Plant 2023, 16, 1232–1236. [Google Scholar] [CrossRef]
- Zou, M.; Shabala, S.; Zhao, C.; Zhou, M. Molecular mechanisms and regulation of recombination frequency and distribution in plants. Theor. Appl. Genet. 2024, 137, 86. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yunusbaev, U.; Fritz, A.; Tilley, M.; Akhunova, A.; Trick, H.; Akhunov, E. CRISPR-based editing of the ω- and γ-gliadin gene clusters reduces wheat immunoreactivity without affecting grain protein quality. Plant Biotechnol. J. 2023, 22, 892–903. [Google Scholar] [CrossRef]
- Sánchez-León, S.; Marín-Sanz, M.; Guzmán-López, M.H.; Gavilán-Camacho, M.; Simón, E.; Barro, F. CRISPR/Cas9-mediated multiplex gene editing of gamma and omega gliadins: Paving the way for gliadin-free wheat. J. Exp. Bot. 2024, 75, 7079–7095. [Google Scholar] [CrossRef] [PubMed]
- Raffan, S.; Sparks, C.; Huttly, A.; Hyde, L.; Martignago, D.; Mead, A.; Hanley, S.J.; Wilkinson, P.A.; Barker, G.; Edwards, K.J.; et al. Wheat with greatly reduced accumulation of free asparagine in the grain, produced by CRISPR/Cas9 editing of asparagine synthetase gene TaASN2. Plant Biotechnol. J. 2021, 19, 1602–1613. [Google Scholar] [CrossRef]
- Wakasa, Y.; Kawakatsu, T.; Ishimaru, K.; Ozawa, K. Generation of major glutelin-deficient (GluA, GluB, and GluC) semi-dwarf Koshihikari rice line. Plant Cell Rep. 2024, 43, 51. [Google Scholar] [CrossRef]
- Kannan, B.; Jung, J.H.; Moxley, G.W.; Lee, S.-M.; Altpeter, F. TALEN-mediated targeted mutagenesis of more than 100 copies/alleles in highly polyploid sugarcane improves saccharification efficiency without compromising biomass yield. Plant Biotechnol. J. 2018, 16, 856–866. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Hua, X.; Wang, Y.; Wang, B.; Qi, Y.; Huang, Y.; Yu, Z.; Gao, R.; Zhang, Y.; et al. The highly allo-autopolyploid modern sugarcane genome and very recent allopolyploidization in Saccharum. Nat. Genet. 2025, 57, 242–253. [Google Scholar] [CrossRef]
- Eid, A.; Mohan, C.; Sanchez, S.; Wang, D.; Altpeter, F. Multiallelic, Targeted Mutagenesis of Magnesium Chelatase With CRISPR/Cas9 Provides a Rapidly Scorable Phenotype in Highly Polyploid Sugarcane. Front. Genome Ed. 2021, 3, 654996. [Google Scholar] [CrossRef]
- Oz, M.T.; Altpeter, A.; Karan, R.; Merotto, A.; Altpeter, F. CRISPR/Cas9-Mediated Multi-Allelic Gene Targeting in Sugarcane Confers Herbicide Tolerance. Front. Genome Ed. 2021, 3, 673566. [Google Scholar] [CrossRef]
- Laksana, C.; Sophiphun, O.; Chanprame, S. Lignin reduction in sugarcane by performing CRISPR/Cas9 site-direct mutation of SoLIM transcription factor. Plant Sci. 2024, 340, 111987. [Google Scholar] [CrossRef]
- Liu, Y.; Merrick, P.; Zhang, Z.; Ji, C.; Yang, B.; Fei, S. Targeted mutagenesis in tetraploid switchgrass (Panicum virgatum L.) using CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Lipka, A.E.; Glaubitz, J.; Elshire, R.; Cherney, J.H.; Casler, M.D.; Buckler, E.S.; Costich, D.E. Switchgrass genomic diversity, ploidy, and evolution: Novel insights from a network-based SNP discovery protocol. PLoS Genet. 2013, 9, e1003215. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Donohoe, B.S.; Ahuja, N.; Garrity, D.M.; Qu, R.; Tucker, M.P.; Himmel, M.E.; Wei, H. Evaluation of parameters affecting switchgrass tissue culture: Toward a consolidated procedure for Agrobacterium-mediated transformation of switchgrass (Panicum virgatum). Plant Methods 2017, 13, 113. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, R.; Liu, M.; Liu, Y.; Yuan, X.; Chen, L.; Zhao, S.; Qin, X.; Zhou, C.; Fu, C.; et al. CRISPR/Cas9-mediated knockout of PvTCP19/22 enhances tiller number and biomass yield in switchgrass. Ind. Crops Prod. 2025, 226, 120689. [Google Scholar] [CrossRef]
- Biselli, C.; Vietto, L.; Rosso, L.; Cattivelli, L.; Nervo, G.; Fricano, A. Advanced Breeding for Biotic Stress Resistance in Poplar. Plants 2022, 11, 2032. [Google Scholar] [CrossRef]
- Mohamed, R.; Wang, C.-T.; Ma, C.; Shevchenko, O.; Dye, S.J.; Puzey, J.R.; Etherington, E.; Sheng, X.; Meilan, R.; Strauss, S.H.; et al. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. Plant J. 2010, 62, 674–688. [Google Scholar] [CrossRef]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 2019, 565, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vernet, A.; Meynard, D.; Lian, Q.; Mieulet, D.; Gibert, O.; Bissah, M.; Rivallan, R.; Autran, D.; Leblanc, O.; Meunier, A.C.; et al. High-frequency synthetic apomixis in hybrid rice. Nat. Commun. 2022, 13, 7963. [Google Scholar] [CrossRef]
- Qi, X.; Gao, H.; Lv, R.; Mao, W.; Zhu, J.; Liu, C.; Mao, L.; Li, X.; Xie, C. CRISPR/dCas-mediated gene activation toolkit development and its application for parthenogenesis induction in maize. Plant Commun. 2023, 4, 100449. [Google Scholar] [CrossRef] [PubMed]
- Karlson, D.; Mojica, J.P.; Poorten, T.J.; Lawit, S.J.; Jali, S.; Chauhan, R.D.; Pham, G.M.; Marri, P.; Guffy, S.L.; Fear, J.M.; et al. Targeted Mutagenesis of the Multicopy Myrosinase Gene Family in Allotetraploid Brassica juncea Reduces Pungency in Fresh Leaves across Environments. Plants 2022, 11, 2494. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Zhang, C.; Li, N.; Zhao, J. The application of CRISPR/Cas technologies to Brassica crops: Current progress and future perspectives. aBIOTECH 2022, 3, 146–161. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2016, 15, 207–216. [Google Scholar] [CrossRef]
- Xue, C.; Qiu, F.; Wang, Y.; Li, B.; Zhao, K.T.; Chen, K.; Gao, C. Tuning plant phenotypes by precise, graded downregulation of gene expression. Nat. Biotechnol. 2023, 41, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Zhang, H.; Wang, Y.; Chen, K.; Gao, C. Manipulating gene translation in plants by CRISPR–Cas9-mediated genome editing of upstream open reading frames. Nat. Protoc. 2020, 15, 338–363. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480.e8. [Google Scholar] [CrossRef]
- EFSA P on GMO (GMO). Applicability of the EFSA Opinion on site-directed nucleases type 3 for the safety assessment of plants developed using site-directed nucleases type 1 and 2 and oligonucleotide-directed mutagenesis. EFSA J. 2020, 18, e06299. [Google Scholar] [CrossRef]
- EFSA P on GM. Criteria for risk assessment of plants produced by targeted mutagenesis, cisgenesis and intragenesis. EFSA J. 2022, 20, e07618. [Google Scholar] [CrossRef]
- EFSA P on GM. In vivo and in vitro random mutagenesis techniques in plants. EFSA J. 2021, 19, e06611. [Google Scholar] [CrossRef]
- EFSA. Literature horizon scan for new scientific data on plants and their products obtained by new genomic techniques (January 2022 to May 2025). EFSA J. 2025, 23, e9619. [Google Scholar] [CrossRef]
- Schnell, J.; Steele, M.; Bean, J.; Neuspiel, M.; Girard, C.; Dormann, N.; Pearson, C.; Savoie, A.; Bourbonnière, L.; Macdonald, P. A comparative analysis of insertional effects in genetically engineered plants: Considerations for pre-market assessments. Transgenic Res. 2015, 24, 1–17. [Google Scholar] [CrossRef]
- Holme, I.B.; Gregersen, P.L.; Brinch-Pedersen, H. Induced Genetic Variation in Crop Plants by Random or Targeted Mutagenesis: Convergence and Differences. Front. Plant Sci. 2019, 10, 1468. [Google Scholar] [CrossRef]
- Modrzejewski, D.; Hartung, F.; Lehnert, H.; Sprink, T.; Kohl, C.; Keilwagen, J.; Wilhelm, R. Which Factors Affect the Occurrence of Off-Target Effects Caused by the Use of CRISPR/Cas: A Systematic Review in Plants. Front. Plant Sci. 2020, 11, 574959. [Google Scholar] [CrossRef]
- Li, T.; Xu, H.; Teng, S.; Suo, M.; Bahitwa, R.; Xu, M.; Qian, Y.; Ramstein, G.P.; Song, B.; Buckler, E.S.; et al. Modeling 0.6 million genes for the rational design of functional cis-regulatory variants and de novo design of cis-regulatory sequences. Proc. Natl. Acad. Sci. USA 2024, 121, e2319811121. [Google Scholar] [CrossRef] [PubMed]
- Sikora, P.; Chawade, A.; Larsson, M.; Olsson, J.; Olsson, O. Mutagenesis as a Tool in Plant Genetics, Functional Genomics, and Breeding. Int. J. Plant Genom. 2011, 2011, 314829. [Google Scholar] [CrossRef] [PubMed]
- Szurman-Zubrzycka, M.; Kurowska, M.; Till, B.J.; Szarejko, I. Is it the end of TILLING era in plant science? Front. Plant Sci. 2023, 14, 1160695. [Google Scholar] [CrossRef]
- Carrère, S.; Routaboul, J.-M.; Savourat, P.; Bellenot, C.; López, H.; Sahoo, A.; Quiroz Monnens, T.; Ricou, A.; Camilleri, C.; Declerck, N.; et al. A fully sequenced collection of homozygous EMS mutants for forward and reverse genetic screens in Arabidopsis thaliana. Plant J. 2024, 119, 3015–3026. [Google Scholar] [CrossRef]
- Fanelli, V.; Ngo, K.J.; Thompson, V.L.; Silva, B.R.; Tsai, H.; Sabetta, W.; Montemurro, C.; Comai, L.; Harmer, S.L. A TILLING by sequencing approach to identify induced mutations in sunflower genes. Sci. Rep. 2021, 11, 9885. [Google Scholar] [CrossRef] [PubMed]
- Ndreca, B.; Huttly, A.; Bibi, S.; Bayon, C.; Lund, G.; Ham, J.; Alarcón-Reverte, R.; Addy, J.; Tarkowská, D.; Pearce, S.; et al. Stacked mutations in wheat homologues of rice SEMI-DWARF1 confer a novel semi-dwarf phenotype. BMC Plant Biol. 2024, 24, 384. [Google Scholar] [CrossRef] [PubMed]
- Romera-Branchat, M.; Severing, E.; Pocard, C.; Ohr, H.; Vincent, C.; Née, G.; Martinez-Gallegos, R.; Jang, S.; Andrés, F.; Madrigal, P.; et al. Functional Divergence of the Arabidopsis Florigen-Interacting bZIP Transcription Factors FD and FDP. Cell Rep. 2020, 31, 107717. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Pacher, M.; Puchta, H. Efficient induction of heritable inversions in plant genomes using the CRISPR/Cas system. Plant J. 2019, 98, 577–589. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation on New Genomic Techniques (NGT)—Technical Paper on the Rationale for the Equivalence Criteria in Annex I. 2023. Available online: https://eur-lex.europa.eu/legal-content/DE/TXT/?uri=CONSIL:ST_14204_2023_INIT (accessed on 22 November 2025).
- European Commission. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on Plants Obtained by Certain New Genomic Techniques and Their Food and Feed, and Amending Regulation (EU) 2017/625. 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52023PC0411 (accessed on 22 November 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, F. The Potential of NGTs to Overcome Constraints in Plant Breeding and Their Regulatory Implications. Int. J. Mol. Sci. 2025, 26, 11391. https://doi.org/10.3390/ijms262311391
Koller F. The Potential of NGTs to Overcome Constraints in Plant Breeding and Their Regulatory Implications. International Journal of Molecular Sciences. 2025; 26(23):11391. https://doi.org/10.3390/ijms262311391
Chicago/Turabian StyleKoller, Franziska. 2025. "The Potential of NGTs to Overcome Constraints in Plant Breeding and Their Regulatory Implications" International Journal of Molecular Sciences 26, no. 23: 11391. https://doi.org/10.3390/ijms262311391
APA StyleKoller, F. (2025). The Potential of NGTs to Overcome Constraints in Plant Breeding and Their Regulatory Implications. International Journal of Molecular Sciences, 26(23), 11391. https://doi.org/10.3390/ijms262311391






