Isolation and Characterization of Extracellular Vesicles Derived from Mango Fruits
Abstract
1. Introduction
2. Results
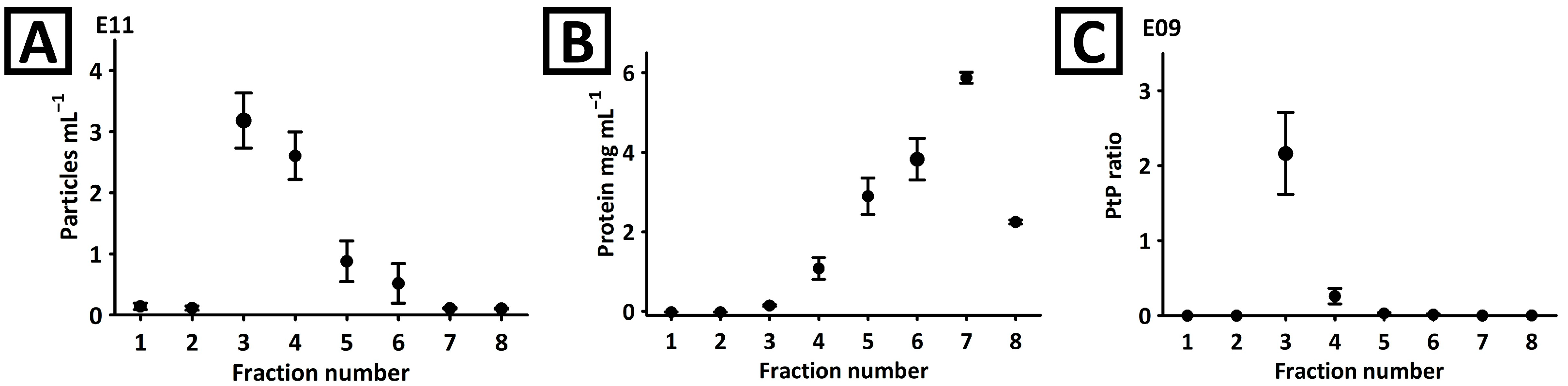
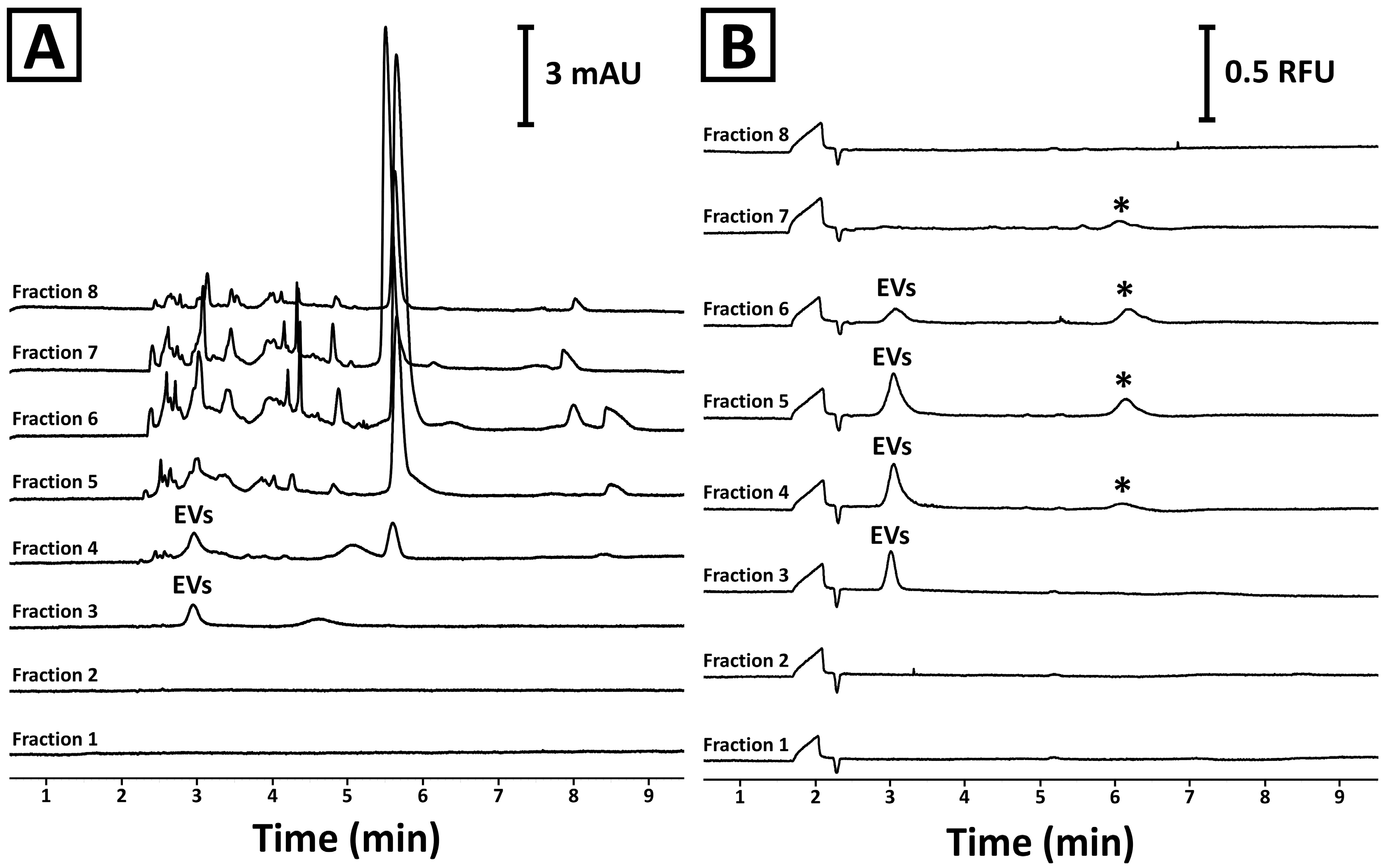
2.1. SEC Column Characterization
2.2. Physical Characterization of the Mango-Derived EVs
2.3. Proteomic Analysis of the Mango-Derived EVs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation of Plant EVs
4.3. Bicinchoninic Acid Assay (BCA)
4.4. Nanoparticles Tracking Analysis (NTA)
4.5. Capillary Electrophoresis (CE)
4.6. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
4.7. Electrophoretic Light Scattering (ELS)
4.8. Proteomic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic acid assay |
| BGE | Background electrolyte |
| BSA | Bovine serum albumin |
| CE | Capillary electrophoresis |
| Cryo-TEM | Cryogenic Transmission Electron Microscopy |
| ELS | Electrophoretic light scattering |
| EVs | Extracellular vesicles |
| LIF | Laser-induced fluorescence |
| NTA | Nanoparticles tracking analysis |
| PBS | Phosphate buffered saline |
| PES | Polyethersulfone |
| PtP | Particle-to-protein |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulfate |
| SEC | Size-exclusion chromatography |
| UF | Ultrafiltration |
References
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant Extracellular Vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles as Key Mediators of Plant–Microbe Interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Steć, A.; Targońska, M.; Jaikishan, S.; Chen, R.; Mucha, P.; Czyrski, G.; Jasiecki, J.; Płoska, A.; Heinz, A.; Wiedmer, S.K.; et al. Incorporation of Doxorubicin into Plant-Derived Nanovesicles: Process Monitoring and Activity Assessment. Drug Deliv. 2025, 32, 2439272. [Google Scholar] [CrossRef]
- Paolini, L.; Monguió-Tortajada, M.; Costa, M.; Antenucci, F.; Barilani, M.; Clos-Sansalvador, M.; Andrade, A.C.; Driedonks, T.A.P.; Giancaterino, S.; Kronstadt, S.M.; et al. Large-scale Production of Extracellular Vesicles: Report on the “MassivEVs” ISEV Workshop. J. Extracell. Biol. 2022, 1, e63. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus Limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of Exosome-Like Vesicles from Plants by Ultracentrifugation on Sucrose/Deuterium Oxide (D2O) Density Cushions. Methods Mol. Biol. 2016, 1459, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Raimondo, S.; Urzì, O.; Meraviglia, S.; Di Simone, M.; Corsale, A.M.; Rabienezhad Ganji, N.; Palumbo Piccionello, A.; Polito, G.; Lo Presti, E.; Dieli, F.; et al. Anti-Inflammatory Properties of Lemon-Derived Extracellular Vesicles Are Achieved through the Inhibition of ERK/NF-ΚB Signalling Pathways. J. Cell. Mol. Med. 2022, 26, 4195–4209. [Google Scholar] [CrossRef]
- Calzoni, E.; Bertoldi, A.; Cesaretti, A.; Alabed, H.B.R.; Cerrotti, G.; Pellegrino, R.M.; Buratta, S.; Urbanelli, L.; Emiliani, C. Aloe Extracellular Vesicles as Carriers of Photoinducible Metabolites Exhibiting Cellular Phototoxicity. Cells 2024, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe Vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Nanovesicles from Organic Agriculture-Derived Fruits and Vegetables: Characterization and Functional Antioxidant Content. Int. J. Mol. Sci. 2021, 22, 8170. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Wu, H.; Cai, Q.; Ramírez-Sánchez, O.; Abreu-Goodger, C.; Birch, P.R.J.; Jin, H. Plant MRNAs Move into a Fungal Pathogen via Extracellular Vesicles to Reduce Infection. Cell Host Microbe 2024, 32, 93–105.e6. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells In Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Cui, L.; Perini, G.; Minopoli, A.; Palmieri, V.; De Spirito, M.; Papi, M. Plant-Derived Extracellular Vesicles as a Natural Drug Delivery Platform for Glioblastoma Therapy: A Dual Role in Preserving Endothelial Integrity While Modulating the Tumor Microenvironment. Int. J. Pharm. X 2025, 10, 100349. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Masibo, M.; Qian, H. Major Mango Polyphenols and Their Potential Significance to Human Health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How Pure Are Your Vesicles? J. Extracell. Vesicles 2013, 2, 19861. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Tomé-Carneiro, J.; del Pozo-Acebo, L.; del Saz-Lara, A.; Chapado, L.A.; Balaguer, L.; Rojo, E.; Espín, J.C.; Crespo, C.; Moreno, D.A.; et al. Therapeutic Potential of Plant-Derived Extracellular Vesicles as Nanocarriers for Exogenous MiRNAs. Pharmacol. Res. 2023, 198, 106999. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Wang, S.; Fletcher, S.; Niu, D.; Mitter, N.; Birch, P.R.J.; Jin, H. Message in a Bubble: Shuttling Small RNAs and Proteins Between Cells and Interacting Organisms Using Extracellular Vesicles. Annu. Rev. Plant Biol. 2021, 72, 497–524. [Google Scholar] [CrossRef]
- Rodríguez de Lope, M.M.; Sánchez-Pajares, I.R.; Herranz, E.; López-Vázquez, C.M.; González-Moro, A.; Rivera-Tenorio, A.; González-Sanz, C.; Sacristán, S.; Chicano-Gálvez, E.; de la Cuesta, F. A Compendium of Bona Fide Reference Markers for Genuine Plant Extracellular Vesicles and Their Degree of Phylogenetic Conservation. J. Extracell. Vesicles 2025, 14, e70147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Wang, J.; Hillmer, S.; Miao, Y.; Lo, S.W.; Wang, X.; Robinson, D.G.; Jiang, L. EXPO, an Exocyst-Positive Organelle Distinct from Multivesicular Endosomes and Autophagosomes, Mediates Cytosol to Cell Wall Exocytosis in Arabidopsis and Tobacco Cells. Plant Cell 2010, 22, 4009–4030. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-Binding Proteins Contribute to Small RNA Loading in Plant Extracellular Vesicles. Nat. Plants 2021, 7, 342–352, Correction in Nat. Plants 2021, 7, 539. https://doi.org/10.1038/s41477-021-00901-5. [Google Scholar] [CrossRef]
- Steć, A.; Chodkowska, M.; Kasprzyk-Pochopień, J.; Mielczarek, P.; Piekoszewski, W.; Lewczuk, B.; Płoska, A.; Kalinowski, L.; Wielgomas, B.; Dziomba, S. Isolation of Citrus Lemon Extracellular Vesicles: Development and Process Control Using Capillary Electrophoresis. Food Chem. 2023, 424, 136333. [Google Scholar] [CrossRef]
- Hering, A.; Stefanowicz-Hajduk, J.; Dziomba, S.; Halasa, R.; Krzemieniecki, R.; Sappati, S.; Baginski, M.; Ochocka, J.R. Mangiferin Affects Melanin Synthesis by an Influence on Tyrosinase: Inhibition, Mechanism of Action and Molecular Docking Studies. Antioxidants 2023, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein Biocargo of Citrus Fruit-Derived Vesicles Reveals Heterogeneous Transport and Extracellular Vesicle Populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Kim, M.; Jang, H.; Kim, W.; Kim, D.; Park, J.H. Therapeutic Applications of Plant-Derived Extracellular Vesicles as Antioxidants for Oxidative Stress-Related Diseases. Antioxidants 2023, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Cafora, M.; Ganji, N.R.; Tinnirello, V.; Gasparro, R.; Raccosta, S.; Manno, M.; Corsale, A.M.; Conigliaro, A.; Pistocchi, A.; et al. Lemon-Derived Nanovesicles Achieve Antioxidant and Anti-Inflammatory Effects Activating the AhR/Nrf2 Signaling Pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, Z.; Zhao, L.; Yao, Y.; Zhou, Z. Evaluation of Antioxidant Activity and Drug Delivery Potential of Cell-Derived Extracellular Vesicles from Citrus Reticulata Blanco Cv. ‘Dahongpao’. Antioxidants 2023, 12, 1706. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Kumar, M.N.; Rajendran, S.; Bethi, C.M.S.; Ravilla, J.; Narayanan, J.; Sundaram, G.M. Natural Plant-Derived Nanovesicles for Effective Psoriasis Therapy via Dual Modulation of IL-17 and NRF2 Pathway. iScience 2025, 28, 112556. [Google Scholar] [CrossRef]
- Saito, R.F.; Machado, C.M.L.; Lomba, A.L.O.; Otake, A.H.; Rangel, M.C. Heat Shock Proteins Mediate Intercellular Communications within the Tumor Microenvironment through Extracellular Vesicles. Appl. Biosci. 2024, 3, 45–58. [Google Scholar] [CrossRef]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Berlin/Heidelberg, Germany, 2018; pp. 265–281. ISBN 9789811073717. [Google Scholar]
- Brakhage, A.A.; Zimmermann, A.K.; Rivieccio, F.; Visser, C.; Blango, M.G. Host-Derived Extracellular Vesicles for Antimicrobial Defense. MicroLife 2021, 2, uqab003. [Google Scholar] [CrossRef]
- Zhang, Y.; Belaid, M.; Luo, X.; Daci, A.; Limani, R.; Mantaj, J.; Zilbauer, M. Probing Milk Extracellular Vesicles for Intestinal Delivery of RNA Therapies. J. Nanobiotechnol. 2023, 21, 406. [Google Scholar] [CrossRef]
- Leng, Y.; Yang, L.; Zhu, H.; Li, D.; Pan, S. Stability of Blueberry Extracellular Vesicles and Their Gene Regulation Effects in Intestinal Caco-2 Cells. Biomolecules 2023, 13, 1412. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Ding, N.; Javorovic, J.; Yang, X.; Shenker, N.; Raimi-abraham, B.T.; Lynham, S. Mechanistic Insight into Human Milk Extracellular Vesicle-Intestinal Barrier Interactions. J. Extracell. Biol. 2025, 4, e70032. [Google Scholar] [CrossRef]
- Carmen, M.; De, L.; Lorena, H.; Maria, A.; Judit, S.H.; Zamorano, G.; Mantilla, D.C.; Diego, E.; Coronado, G.; Marín, F.; et al. Dietary Bovine Milk MiRNAs Transported in Extracellular Vesicles Are Partially Stable during GI Digestion, Are Bioavailable and Reach Target Tissues but Need a Minimum Dose to Impact on Gene Expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Akimoto, Y.; Ikemoto, M.; Goto, Y.; Ishikawa, A.; Ohta, S.; Takase, Y.; Kawakami, H.; Tsujimoto, M.; Yanoshita, R. Stability of Human Salivary Extracellular Vesicles Containing Dipeptidyl Peptidase IV under Simulated Gastrointestinal Tract Conditions. Biochem. Biophys. Rep. 2021, 27, 101034. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Mishra, V.S.N.; Santoro, J.; Mukhopadhya, A.; Buckley, F.; Driscoll, L.O.; Giblin, L.; Brodkorb, A. Effect of In Vitro Enzyme Digestion and Bile Treatment on Milk Extracellular Vesicles Stability. Mol. Nutr. Food Res. 2024, 68, 2300620. [Google Scholar] [CrossRef] [PubMed]
- Mazarío-Gárgoles, C.; del Saz-Lara, A.; Tomé-Carneiro, J.; Martel, R.; Ballesteros, L.; Burgos-Ramos, E.; Briand, O.; López-Aceituno, J.L.; Bernabè, G.; Ávila-Gálvez, M.Á.; et al. Impact of Thermal Culinary Processing and Gastrointestinal Digestion on the Stability, Count, and Biological Effects of Broccoli-Derived Extracellular Vesicles. Food Res. Int. 2025, 221, 117325. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steć, A.; Szaknis, G.; Skowrońska, A.; Mielczarek, P.; Czyrski, G.S.; Gade, L.; Heinz, A.; Płoska, A.; Kalinowski, L.; Wielgomas, B.; et al. Isolation and Characterization of Extracellular Vesicles Derived from Mango Fruits. Int. J. Mol. Sci. 2025, 26, 11375. https://doi.org/10.3390/ijms262311375
Steć A, Szaknis G, Skowrońska A, Mielczarek P, Czyrski GS, Gade L, Heinz A, Płoska A, Kalinowski L, Wielgomas B, et al. Isolation and Characterization of Extracellular Vesicles Derived from Mango Fruits. International Journal of Molecular Sciences. 2025; 26(23):11375. https://doi.org/10.3390/ijms262311375
Chicago/Turabian StyleSteć, Aleksandra, Grzegorz Szaknis, Anna Skowrońska, Przemysław Mielczarek, Grzegorz S. Czyrski, Luna Gade, Andrea Heinz, Agata Płoska, Leszek Kalinowski, Bartosz Wielgomas, and et al. 2025. "Isolation and Characterization of Extracellular Vesicles Derived from Mango Fruits" International Journal of Molecular Sciences 26, no. 23: 11375. https://doi.org/10.3390/ijms262311375
APA StyleSteć, A., Szaknis, G., Skowrońska, A., Mielczarek, P., Czyrski, G. S., Gade, L., Heinz, A., Płoska, A., Kalinowski, L., Wielgomas, B., & Dziomba, S. (2025). Isolation and Characterization of Extracellular Vesicles Derived from Mango Fruits. International Journal of Molecular Sciences, 26(23), 11375. https://doi.org/10.3390/ijms262311375






