Unveiling the Skin Anti-Aging Potential of the Novel Spirulina platensis Extract Elixspir®
Abstract
1. Introduction
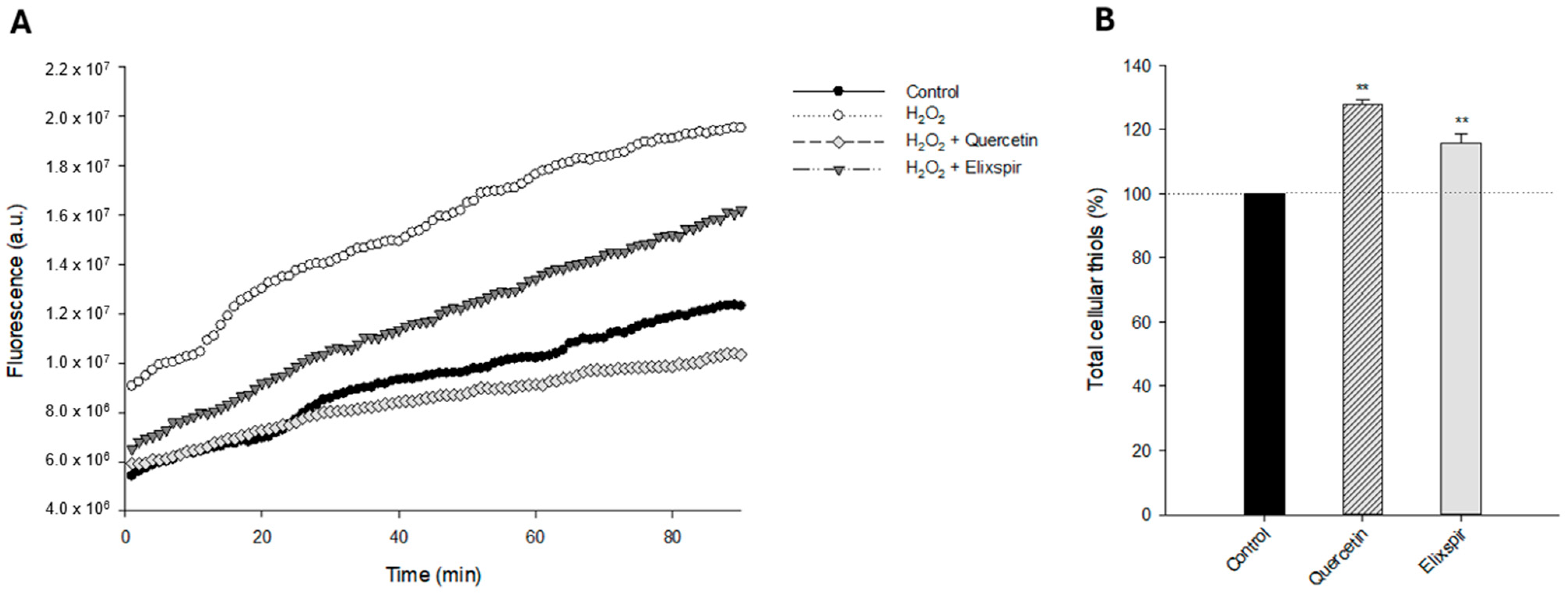
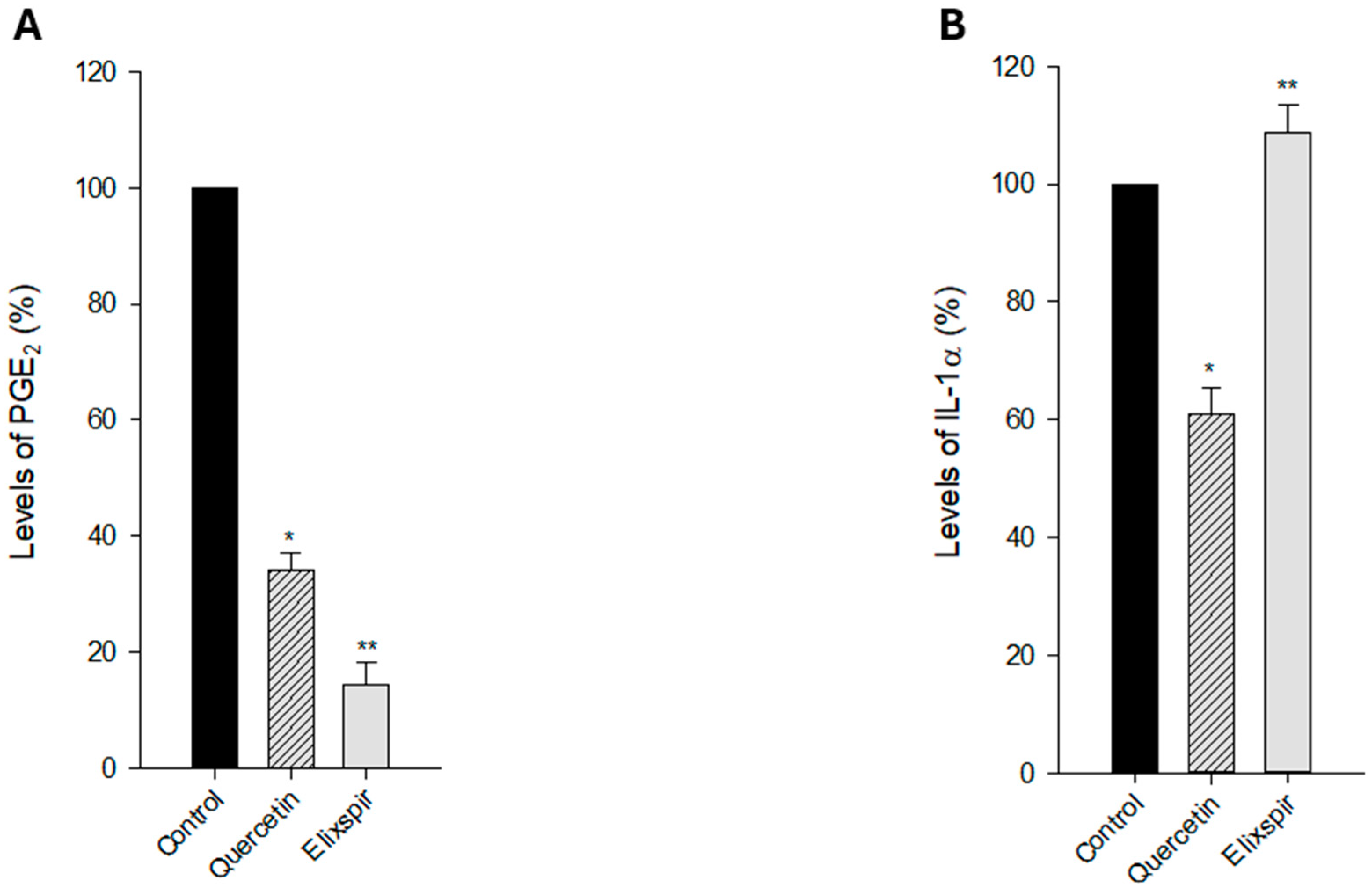
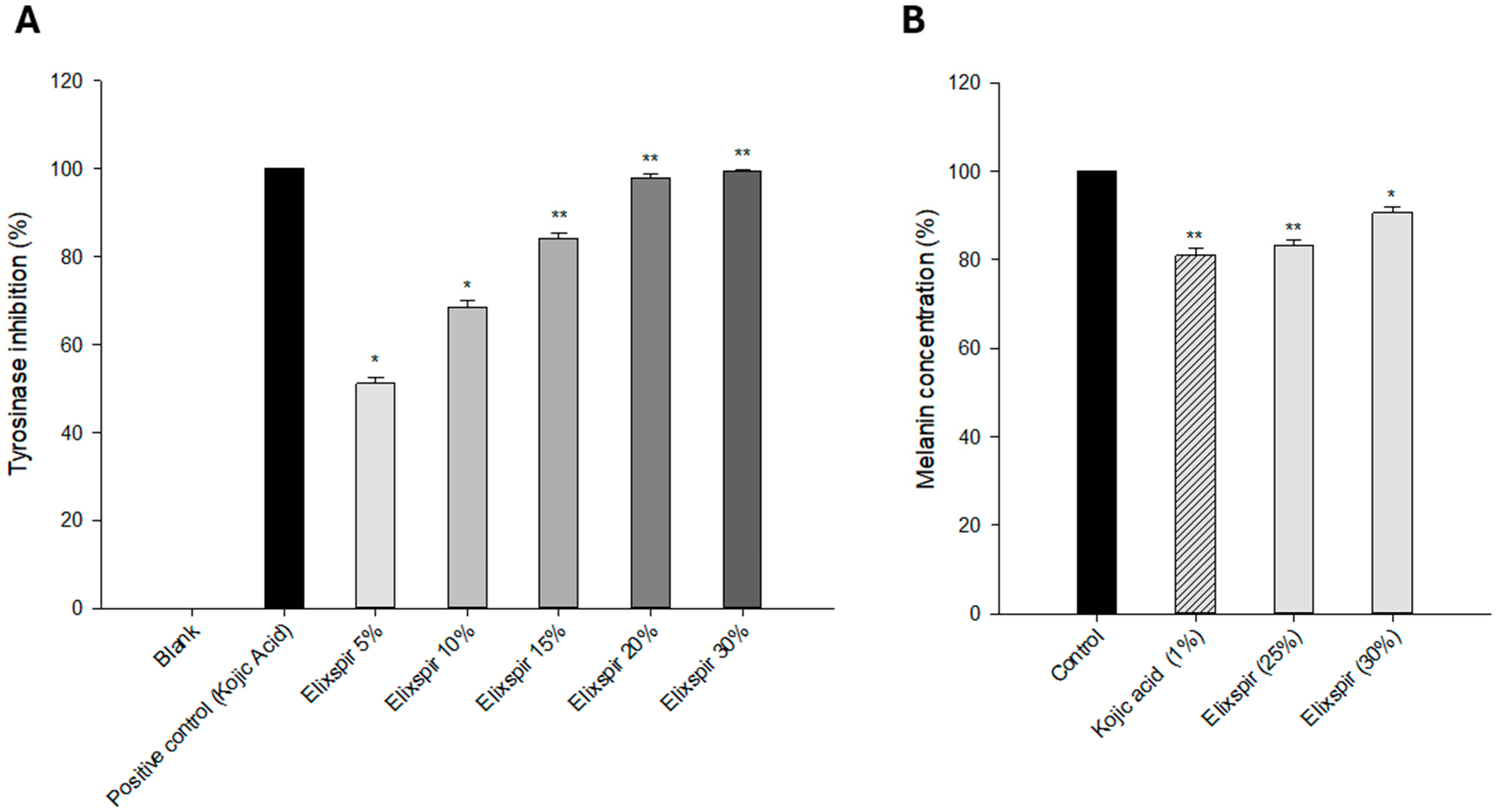
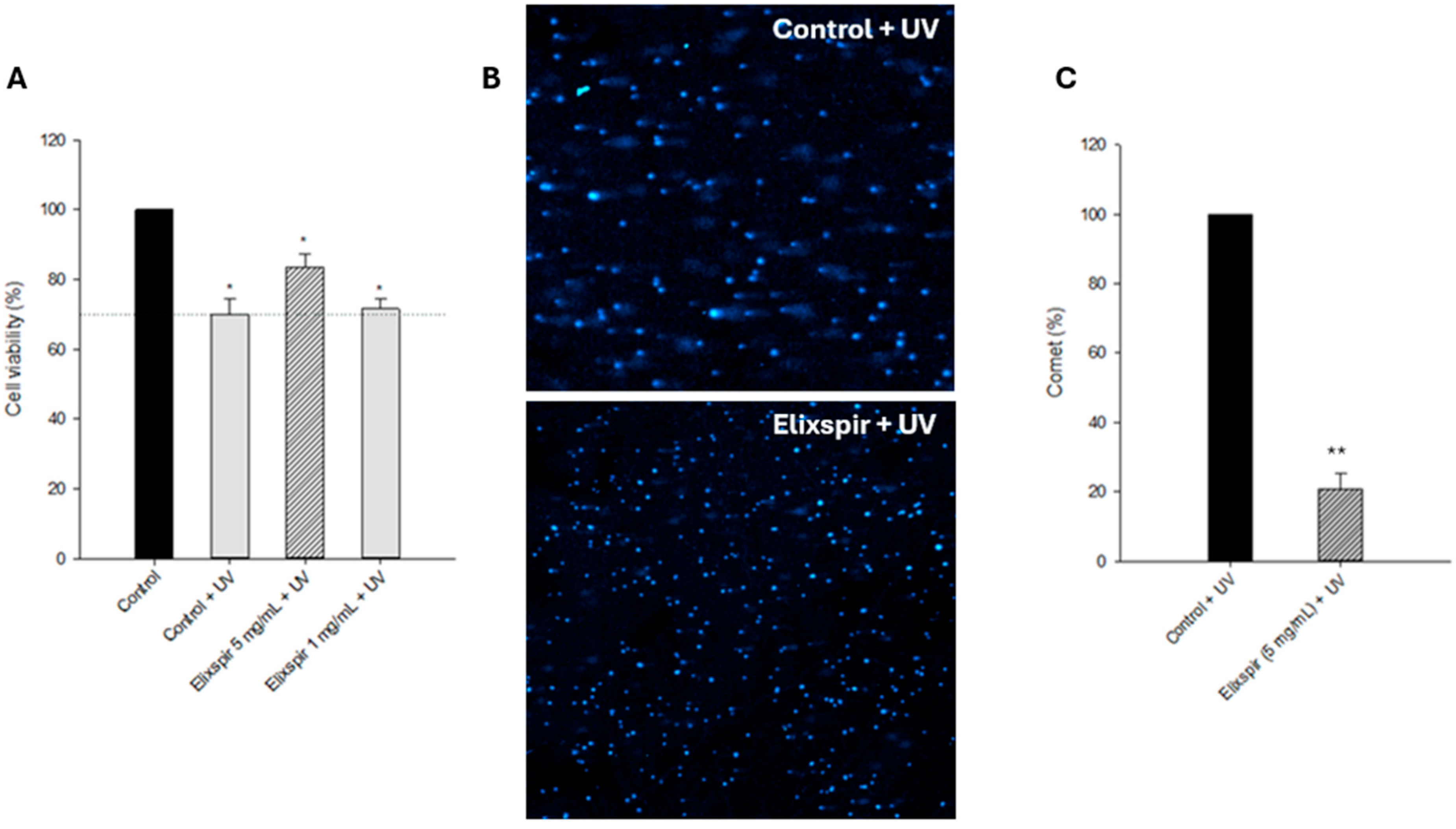
2. Results
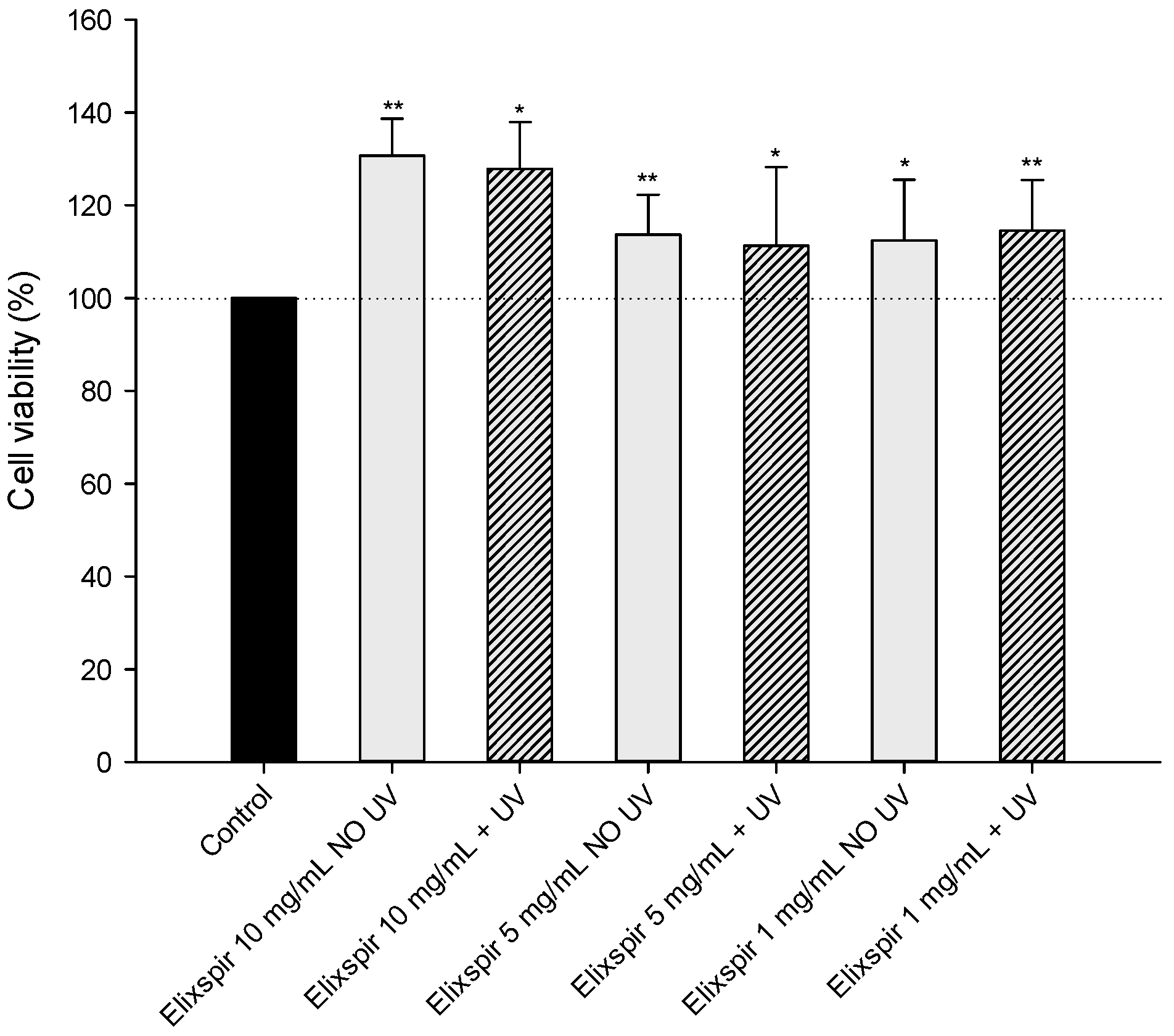
2.1. Evaluation of Elixspir® Safety
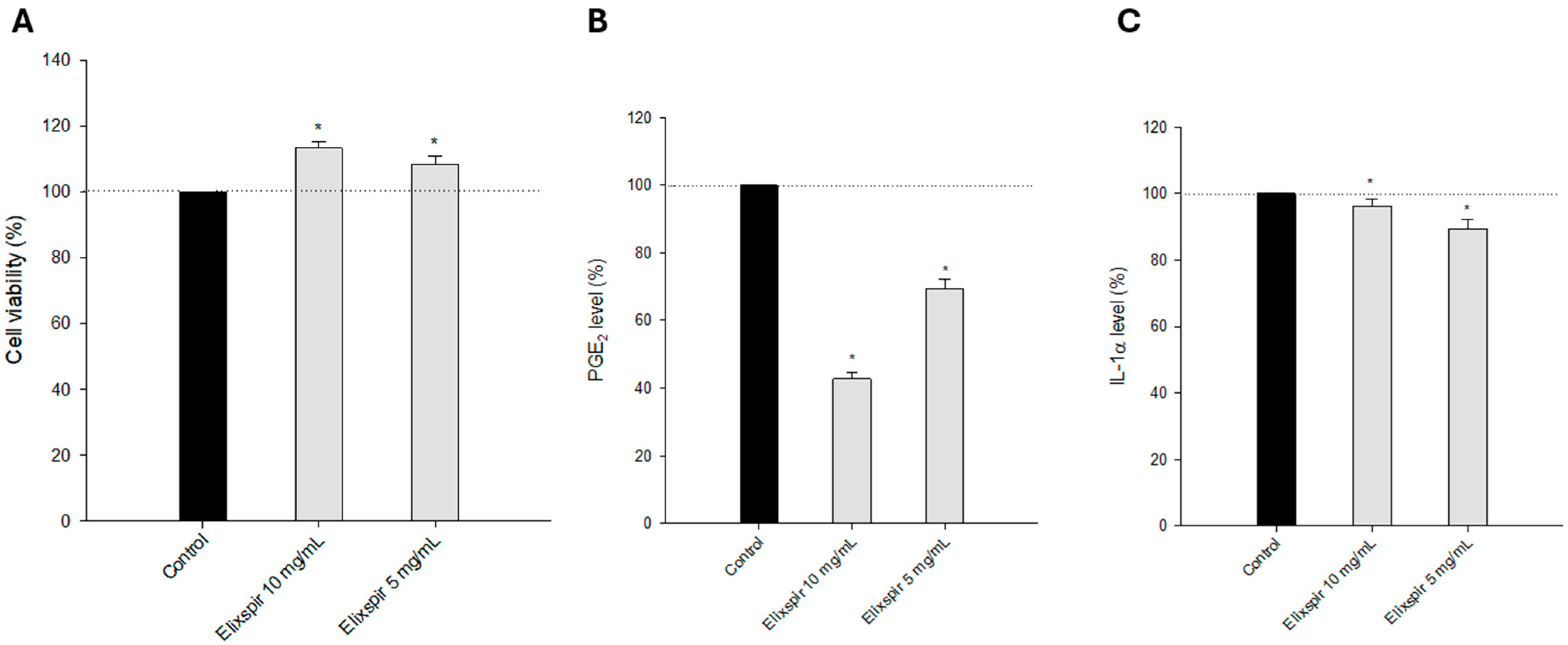
2.2. Evaluation of Elixspir Antioxidant, Anti-Inflammatory, Anti-Tyrosinase and Photoprotective Properties
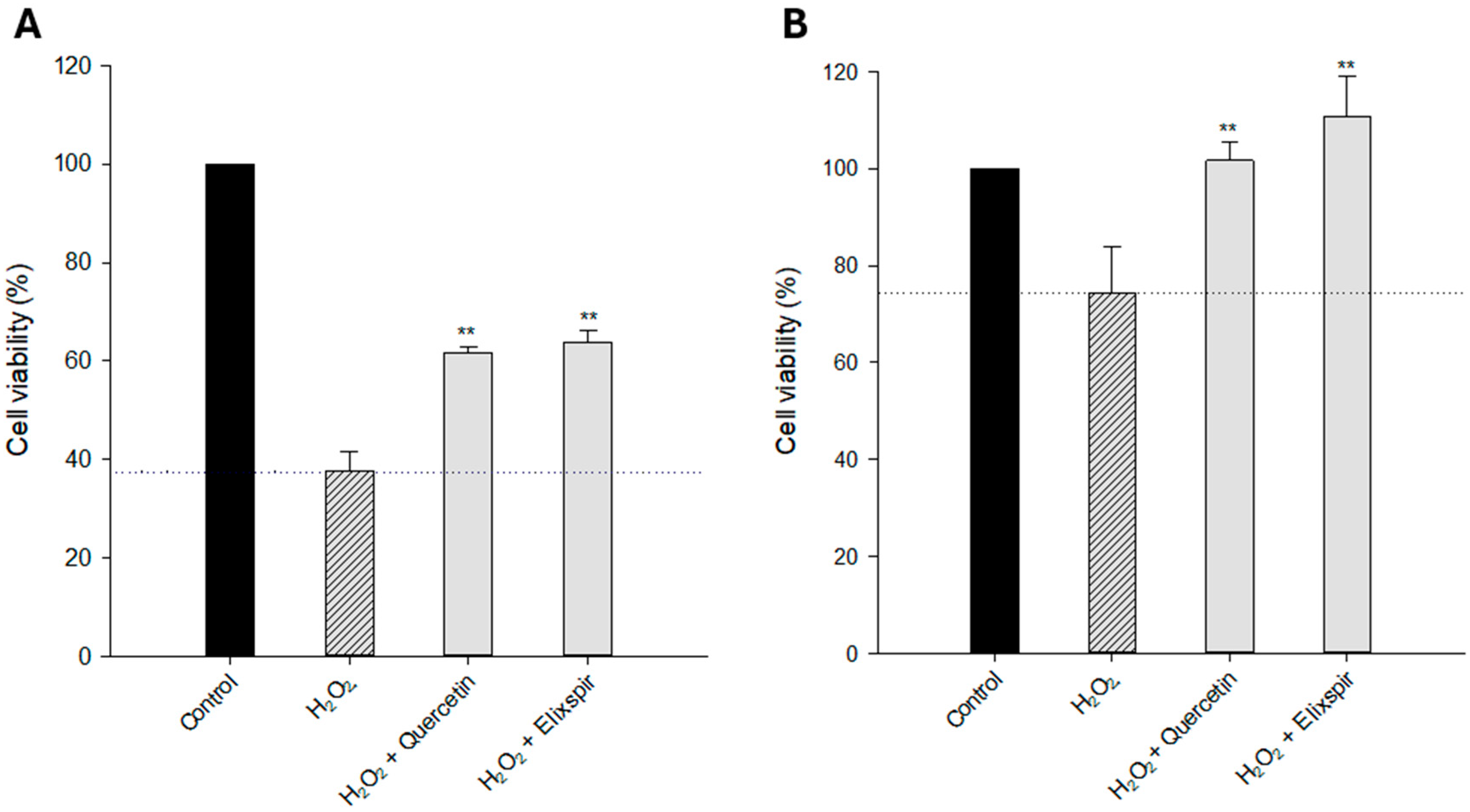
2.3. Photoprotective Effect
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Cultures
4.3. Biocompatibility Studies
4.3.1. MTT Assay
4.3.2. Spheroid Cultures and Acid Phosphatase (APH) Assay
4.4. Evaluation of the Photoprotective Effect
4.4.1. Cell Viability
4.4.2. Comet Assay
4.5. Skin Irritation Assay
4.6. Antioxidant Activity
4.6.1. DPPH Scavenging Activity
4.6.2. Evaluation of Cytoprotective Effect Against Oxidative Stress in 2D Cellular Models
4.6.3. Evaluation of Cytoprotective Effects Against Oxidative Stress in 3D Cellular Models
4.6.4. Evaluation of Cytoprotective Effects and Reduction in ROS Production in Response to Oxidative Stress
4.6.5. Determination of Redox State of Thiols
4.7. Anti-Inflammatory Activity
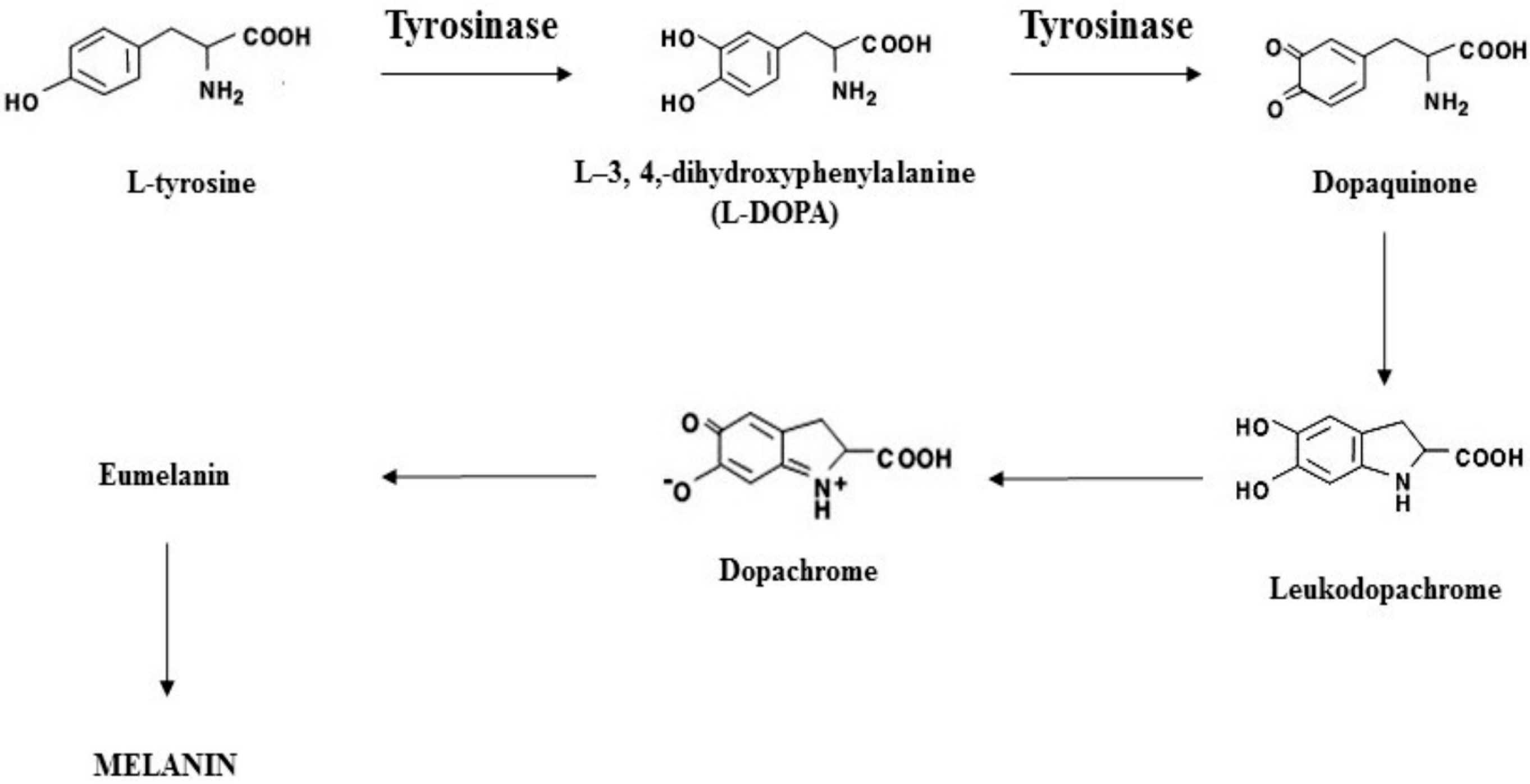
4.8. Anti-Tyrosinase Activity
4.8.1. Determination of the Tyrosinase Inhibition Activity of Elixspir
4.8.2. Depigmentation Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Quan, N.V.; Thien, D.D.; Khanh, T.D.; Tran, H.-D.; Xuan, T.D. Momilactones A, B, and Tricin in Rice Grain and By-Products are Potential Skin Aging Inhibitors. Foods 2019, 8, 602. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Derm.-Endocrinol. 2012, 4, 227–231. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics-a review. Ind. Crops Prod. 2016, 90, 38. [Google Scholar] [CrossRef]
- Naser, W. The cosmetic effects of various natural biofunctional ingredients against skin aging: A Review. Int. J. Appl. Pharm. 2021, 13, 10–18. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, R.; Xiong, B.; Zhu, J.; Sang, J.; Li, H.; Chen, C.; Xu, Z.; Zhang, W.; Chen, Y.; et al. Clinical Investigation of Tyrosinase Inhibitors: Past, Present, and Future. Drug Dev. Res. 2025, 86, e70113. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Safety Assessment of Kojic Acid as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 244S–273S. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Plants and Natural Products for the Treatment of Skin Hyperpigmentation—A Review. Planta Medica 2018, 84, 988. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Topical treatment of melasma. Indian J. Dermatol. 2009, 54, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Zubair, R.; Lyons, A.B.; Vellaichamy, G.; Peacock, A.; Hamzavi, I. What’s New in Pigmentary Disorders. Dermatol. Clin. 2019, 37, 175–181. [Google Scholar] [CrossRef]
- Clarys, P.; Barel, O.A. New trends in antiaging cosmetic ingredients and treatments: An overview. In Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Rathee, P.; Kumar, S.; Kumar, D.; Kumari, B.; Yadav, S.S. Skin hyperpigmentation and its treatment with herbs: An alternative method. Future J. Pharm. Sci. 2021, 7, 132. [Google Scholar] [CrossRef]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Stelmach, J.; Zajdel, K.; Kucharska, E.; Zajdel, R. Plants as Modulators of Melanogenesis: Role of Extracts, Pure Compounds and Patented Compositions in Therapy of Pigmentation Disorders. Int. J. Mol. Sci. 2022, 23, 14787. [Google Scholar] [CrossRef]
- Fuyuno, I. Spotlight turns on cosmetics for Asian skin. Nature 2004, 432, 938. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Khan, S.; Mobashar, M.; Mahsood, F.K.; Javaid, S.; Abdel-Wareth, A.A.; Ammanullah, H.; Mahmood, A. Spirulina inclusion levels in a broiler ration: Evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020, 52, 3233–3240. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, J.Y.; Im, A.-R.; Chae, S. Phycocyanin Protects Against UVB-induced Apoptosis Through the PKC α/βII-Nrf-2/HO-1 Dependent Pathway in Human Primary Skin Cells. Molecules 2018, 23, 478. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.-S.; Kang, C.-M.; Ferruzzi, M.G.; Ahn, M.-J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Desai, K.; Sivakami, S. Purification and biochemical characterization of a superoxide dismutase from the soluble fraction of the cyanobacterium, Spirulina platensis. World J. Microbiol. Biotechnol. 2007, 23, 1661–1666. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Fusco, L.; Garrido, M.; Martín, C.; Sosa, S.; Ponti, C.; Centeno, A.; Alonso, B.; Zurutuza, A.; Vázquez, E.; Tubaro, A.; et al. Skin irritation potential of graphene-based materials using a non-animal test. Nanoscale 2020, 12, 610–622. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Yusof, N.Z.; Abd Gani, S.S.; Azizul Hasan, Z.A.; Idris, Z. Skin and Eye Irritation Assessment of Oil Palm (Elaeis guineensis) Leaf Extract for Topical Application. Int. J. Toxicol. 2018, 37, 335–343. [Google Scholar] [CrossRef]
- Park, Y.D.; Lee, J.-R.; Park, K.-H.; Hahn, H.-S.; Hahn, M.-J.; Yang, J.-M. A New Continuous Spectrophotometric Assay Method for DOPA Oxidase Activity of Tyrosinase. J. Protein Chem. 2003, 22, 473–480. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Pelle, E.; Khan, N.; Syed, D.N.; Matsui, M.S.; Mukhtar, H. Aloe vera protects the skin against ultraviolet radiation-induced suppression of contact hypersensitivity, oxidative stress, and DNA damage. Toxicol. Appl. Pharmacol. 2005, 207, 159–167. [Google Scholar]
- Hashim, P.; Sidek, H.; Helan, M.H.M.; Sabery, A.; Palanisamy, U.D.; Ilham, M. Triterpene composition and bioactivities of Centella asiatica. Molecules 2011, 16, 1310–1322. [Google Scholar] [CrossRef]
- Xie, Q.W.; Leung, M.; Fuortes, M.; Sassa, S.; Nathan, C. Complementation analysis of mutants of nitric oxide synthase reveals that the active site requires two hemes. Proc. Natl. Acad. Sci. USA 1996, 93, 4891–4896. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Margulets, V.; Segev, H.; Shariki, K.; Laevsky, I.; Coleman, R.; Itskovitz-Eldor, J. Human feeder layers for human embryonic stem cells. Biol. Reprod. 2003, 68, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Dzarlieva-Petrusevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.F.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, F.D.; Shenk, T.; Ornelles, D.A. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J. Virol. 1996, 70, 6323–6335. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Santini, C.; Bagnarelli, L.; Caviglia, M.; Sgarbossa, P.; De Franco, M.; Zancato, M.; Marzano, C.; Gandin, V. Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management. Int. J. Mol. Sci. 2023, 24, 4091. [Google Scholar] [CrossRef]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; Montalbano, A. Insight on pyrimido[5,4-g]indolizine and pyrimido[4,5-c]pyrrolo[1,2-a]azepine systems as promising photosensitizers on malignant cells. Eur. J. Med. Chem. 2022, 237, 114399. [Google Scholar] [CrossRef]
- Helbing, T.; Carraro, C.; Francke, A.; Sosic, A.; De Franco, M.; Gandin, V.; Göttlich, R.; Gatto, B. Aromatic Linkers Unleash the Antiproliferative Potential of 3-Chloropiperidines Against Pancreatic Cancer Cells. ChemMedChem 2020, 15, 2040–2051. [Google Scholar] [CrossRef]
- Hermosilha, D.; Trigo, G.; Coelho, M.; Lehmann, I.; Melosini, M.; Serro, A.P.; Reis, C.P.; Gaspar, M.M.; Santos, S. Valorization of thyme combined with phytocannabinoids as anti-inflammatory agents for skin diseases. Pharmaceutics 2025, 17, 1291. [Google Scholar] [CrossRef]
- Bruić, M.; Pirković, A.; Vilotić, A.; Jovanović-Krivokuća, M.; Spremo-Potparević, B. Cytoprotective and genoprotective effects of taxifolin against oxidative damage in HTR-8/SVneo human trophoblast cells. Mutagenesis 2023, 38, 64–70. [Google Scholar] [CrossRef]
- Rigobello, M.P.; Gandin, V.; Folda, A.; Rundlöf, A.-K.; Fernandes, A.P.; Bindoli, A.; Marzano, C.; Björnstedt, M. Treatment of human cancer cells with selenite or tellurite in combination with auranofin enhances cell death due to redox shift. Free Radic. Biol. Med. 2009, 47, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Jaehyeok, Y.; Jong-Eun, K. Luteolin targets MKK4 to attenuate particulate matter-induced MMP-1 and inflammation in human keratinocytes. Sci. Rep. 2025, 15, 16848. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.; Calcaterra, E.; Scuderi, S.A.; Caffo, M.; De Luca, F.; Catalfamo, A.; Casili, G.; Paterniti, I. Troxerutin Potentiated Temozolomide Induced Antitumor Effect in 2D and 3D Glioblastoma Models. J. Cell. Mol. Med. 2025, 29, e70862. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, L.; Song, L.; Sommerfeld, M.; Hu, Q. An improved phenol-sulfuric acid method for the quantitative measurement of total carbohydrates in algal biomass. Algal Res. 2023, 70, 102986. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Boussiba, S.; Richmond, A.E. Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
| Cell-Line | IC50 (mg/mL) |
|---|---|
| HEK-293 (ATCC CRL-1573) | >10 |
| V-79 (ATCC CCL-93) | >10 |
| HFF-1 (ATCC SCRC-1041) | >10 |
| HaCaT | >10 |
| Cell-Line | IC50 (mg/mL) |
|---|---|
| HEK-293 (ATCC CRL-1573) | >0.2 |
| V-79 (ATCC CCL-93) | >0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, C.; Nardone, G.N.; Mason, V.; Di Gregorio, E.; Ragusa, I.; Amadio, E.; Zampieri, E.; Bassetto, R.; Gandin, V.; Zanatta, S. Unveiling the Skin Anti-Aging Potential of the Novel Spirulina platensis Extract Elixspir®. Int. J. Mol. Sci. 2025, 26, 11372. https://doi.org/10.3390/ijms262311372
Donati C, Nardone GN, Mason V, Di Gregorio E, Ragusa I, Amadio E, Zampieri E, Bassetto R, Gandin V, Zanatta S. Unveiling the Skin Anti-Aging Potential of the Novel Spirulina platensis Extract Elixspir®. International Journal of Molecular Sciences. 2025; 26(23):11372. https://doi.org/10.3390/ijms262311372
Chicago/Turabian StyleDonati, Chiara, Giulia Nerina Nardone, Vera Mason, Emanuela Di Gregorio, Irene Ragusa, Emanuele Amadio, Eleonora Zampieri, Rebecca Bassetto, Valentina Gandin, and Samuele Zanatta. 2025. "Unveiling the Skin Anti-Aging Potential of the Novel Spirulina platensis Extract Elixspir®" International Journal of Molecular Sciences 26, no. 23: 11372. https://doi.org/10.3390/ijms262311372
APA StyleDonati, C., Nardone, G. N., Mason, V., Di Gregorio, E., Ragusa, I., Amadio, E., Zampieri, E., Bassetto, R., Gandin, V., & Zanatta, S. (2025). Unveiling the Skin Anti-Aging Potential of the Novel Spirulina platensis Extract Elixspir®. International Journal of Molecular Sciences, 26(23), 11372. https://doi.org/10.3390/ijms262311372









