Immune Checkpoint Signatures in Minimal Change Disease and Membranous Nephropathy: Divergent Pathways of a Shared Imbalance
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Patients Recruited into the Study
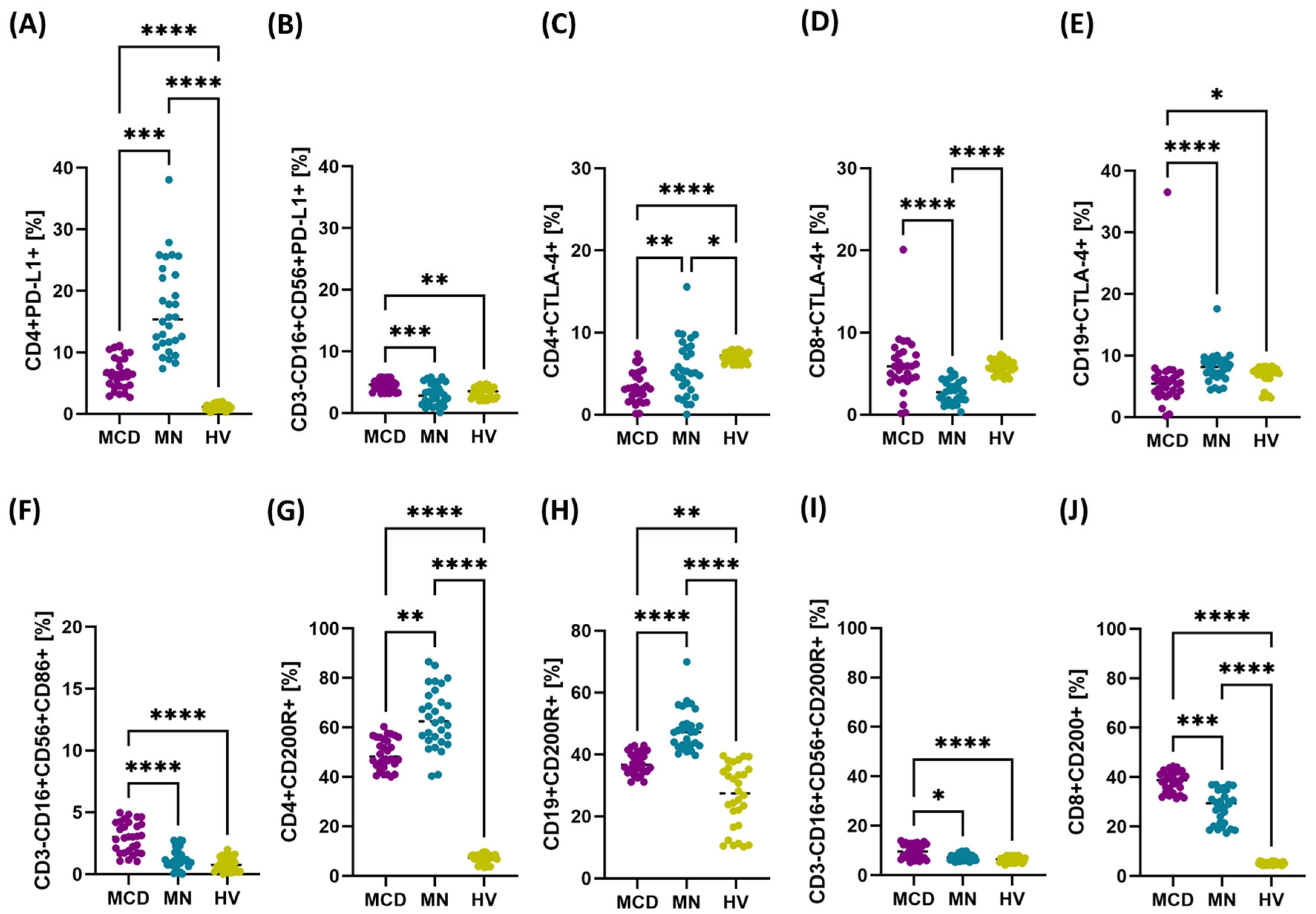
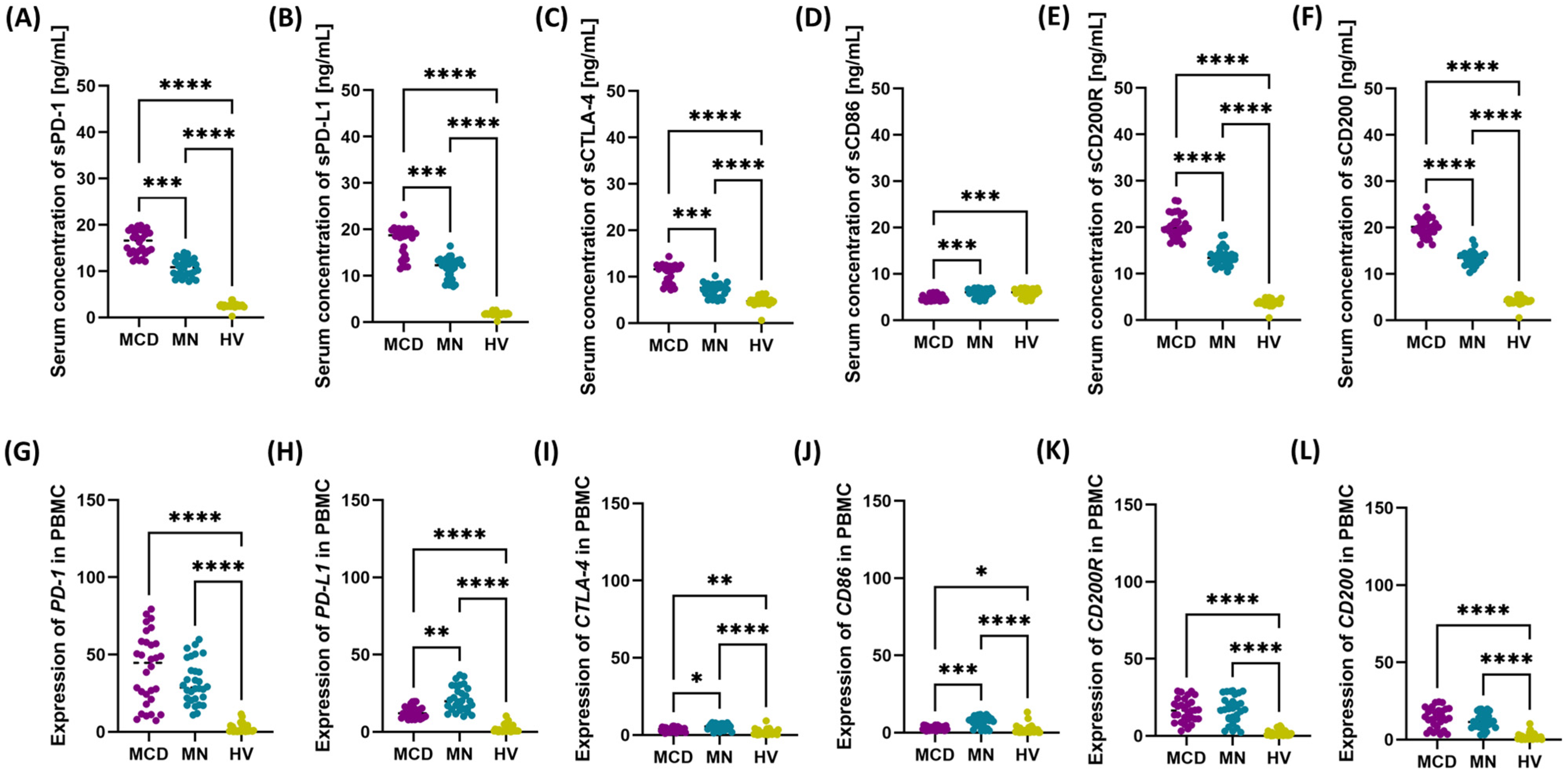
2.2. Upregulation of PD-1/PD-L1, CTLA-4/CD86, and CD200/CD200R Axes in MCD and MN Compared with Healthy Volunteers
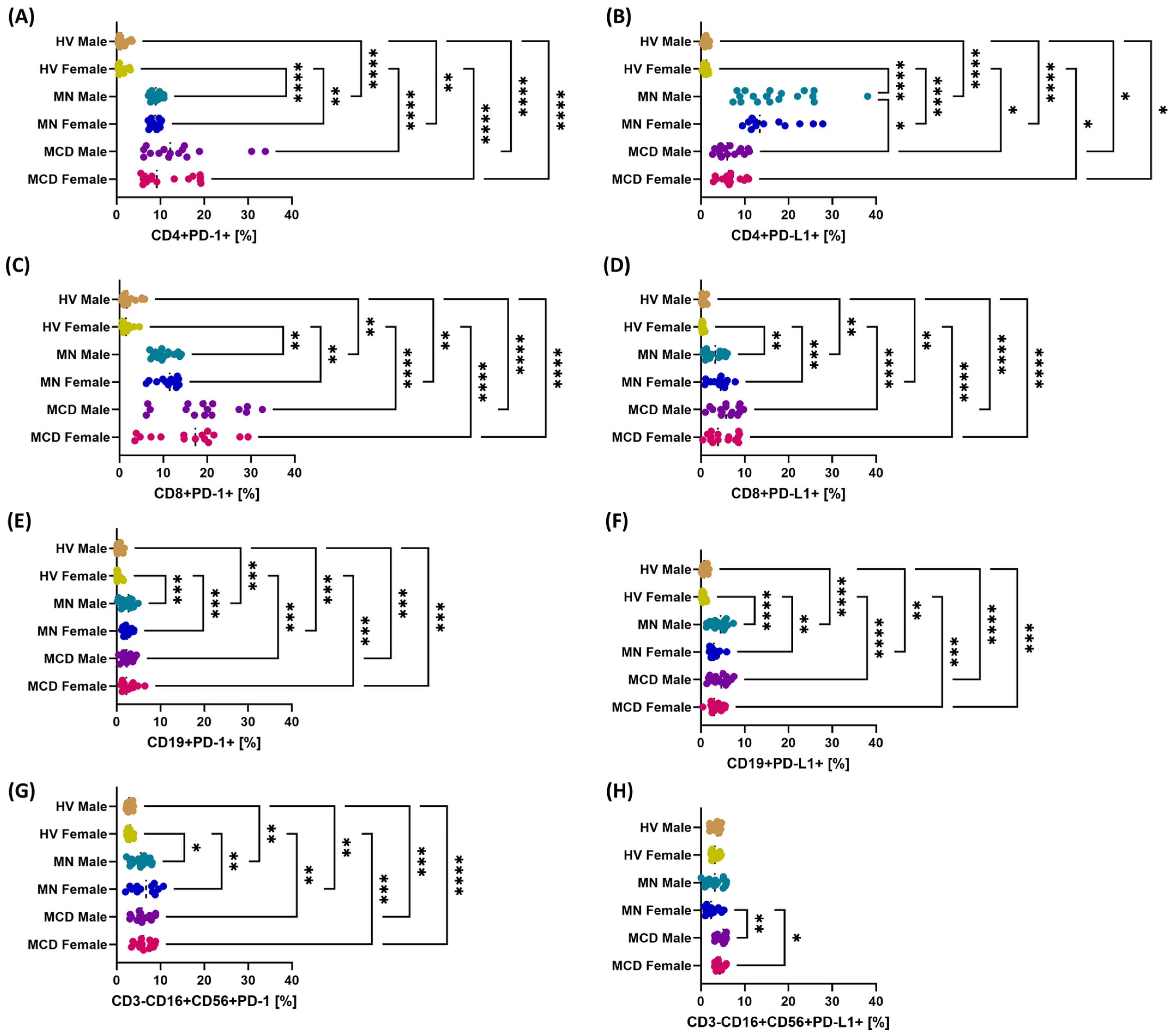
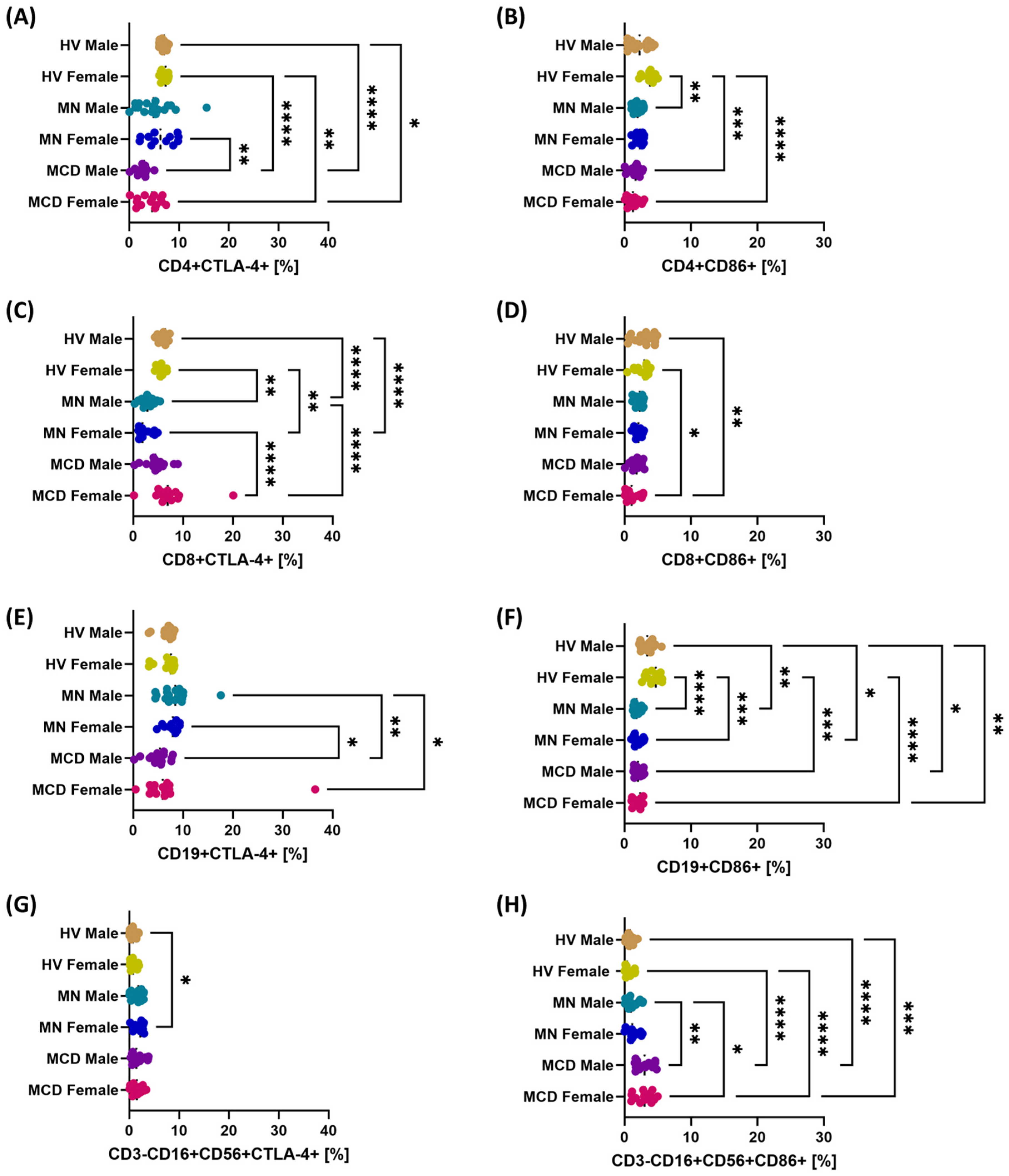
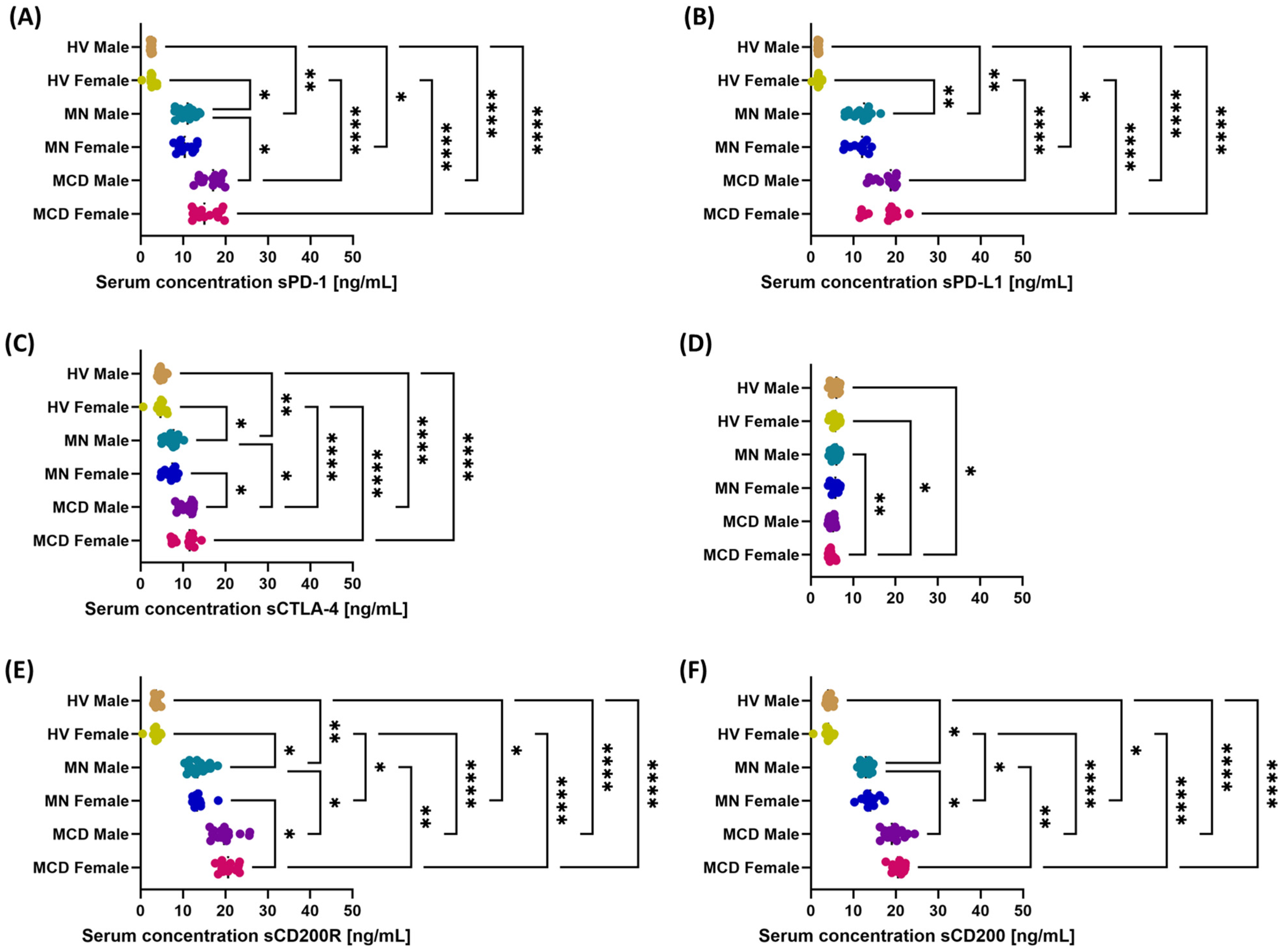
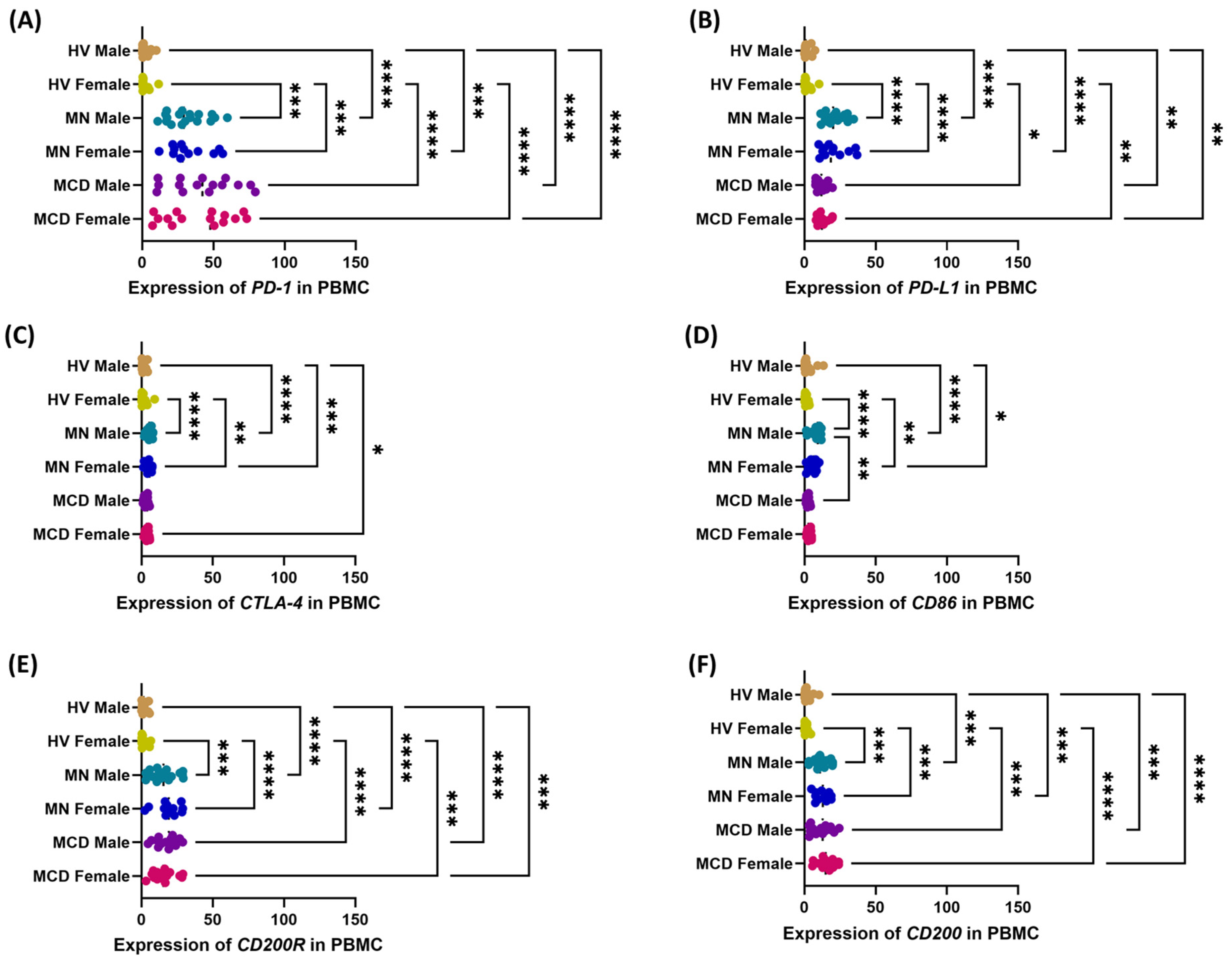
2.3. The Role of Gender in Modulating the Multilevel Profile of the PD-1/PD-L1, CTLA-4/CD86 and CD200/CD200R Axis—Cellular Expression, Soluble Forms and Transcripts in MCD and MN Compared to Healthy Volunteers
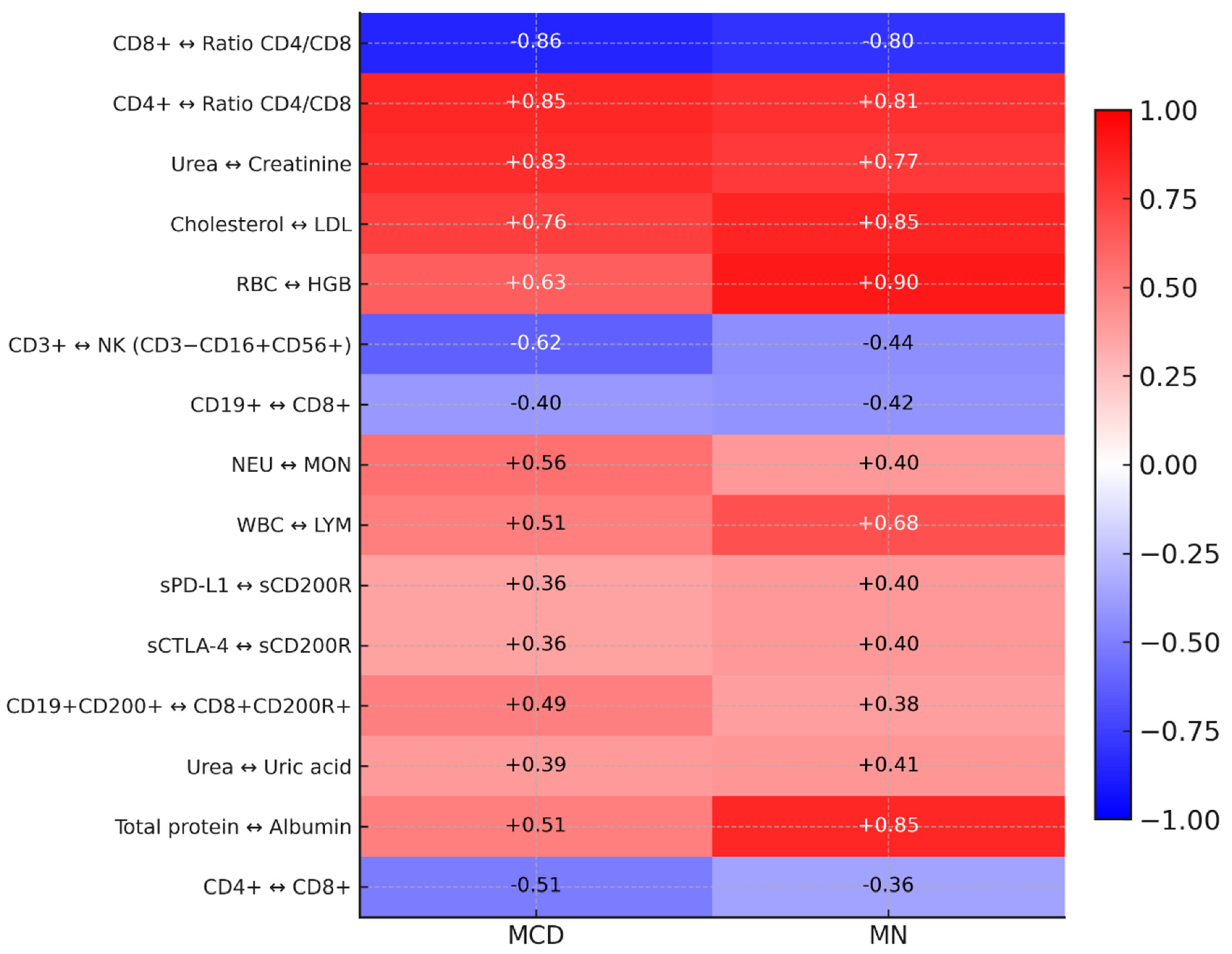
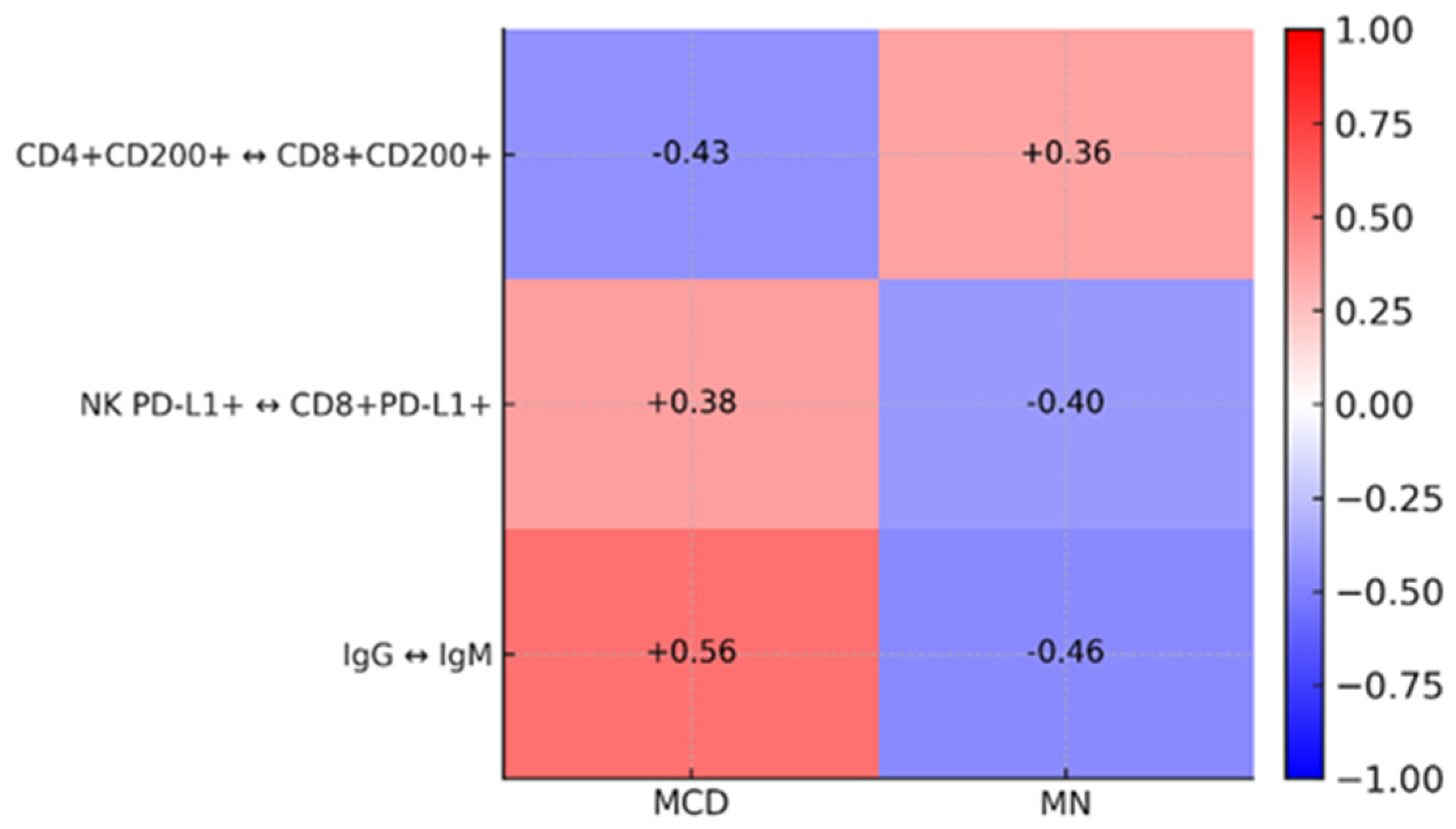
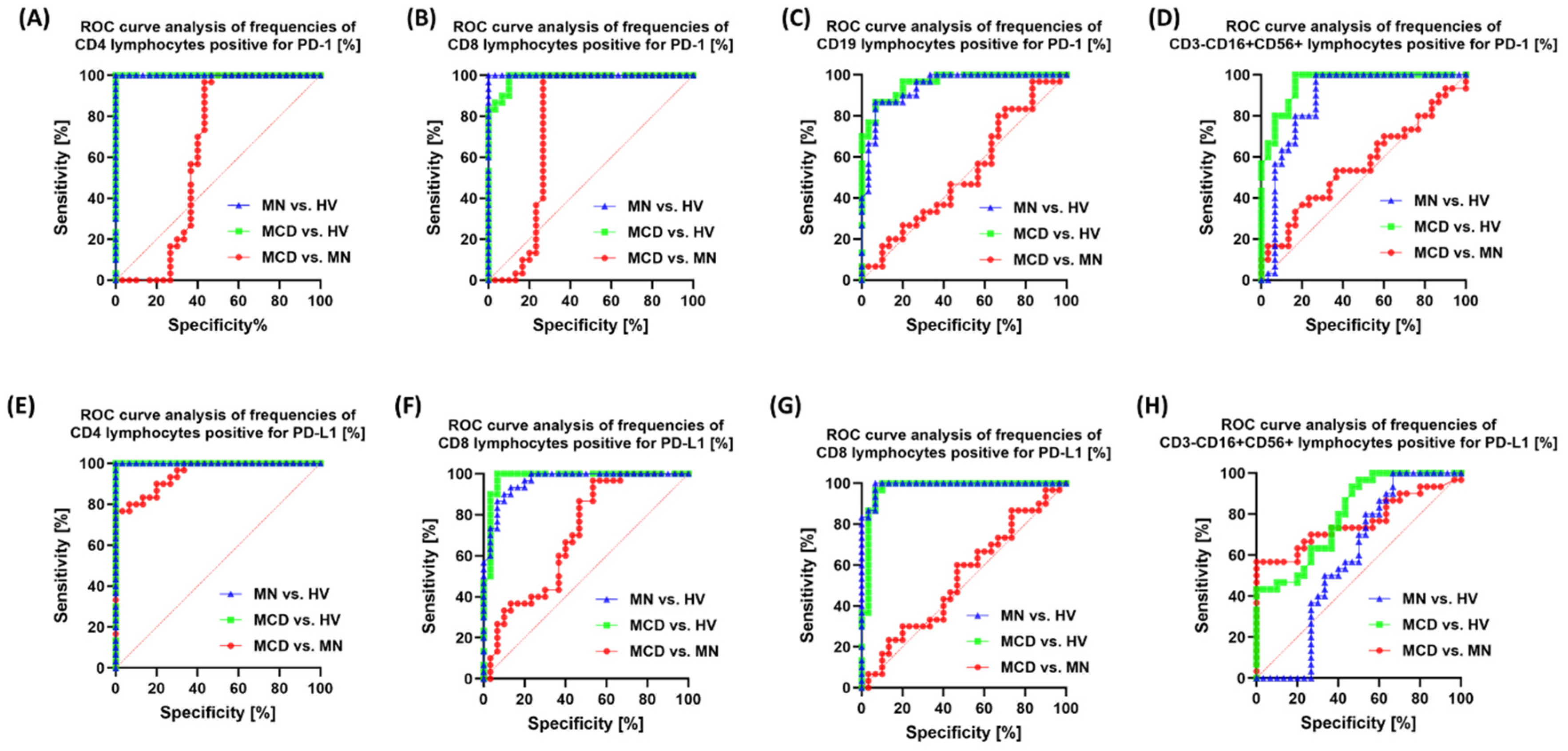
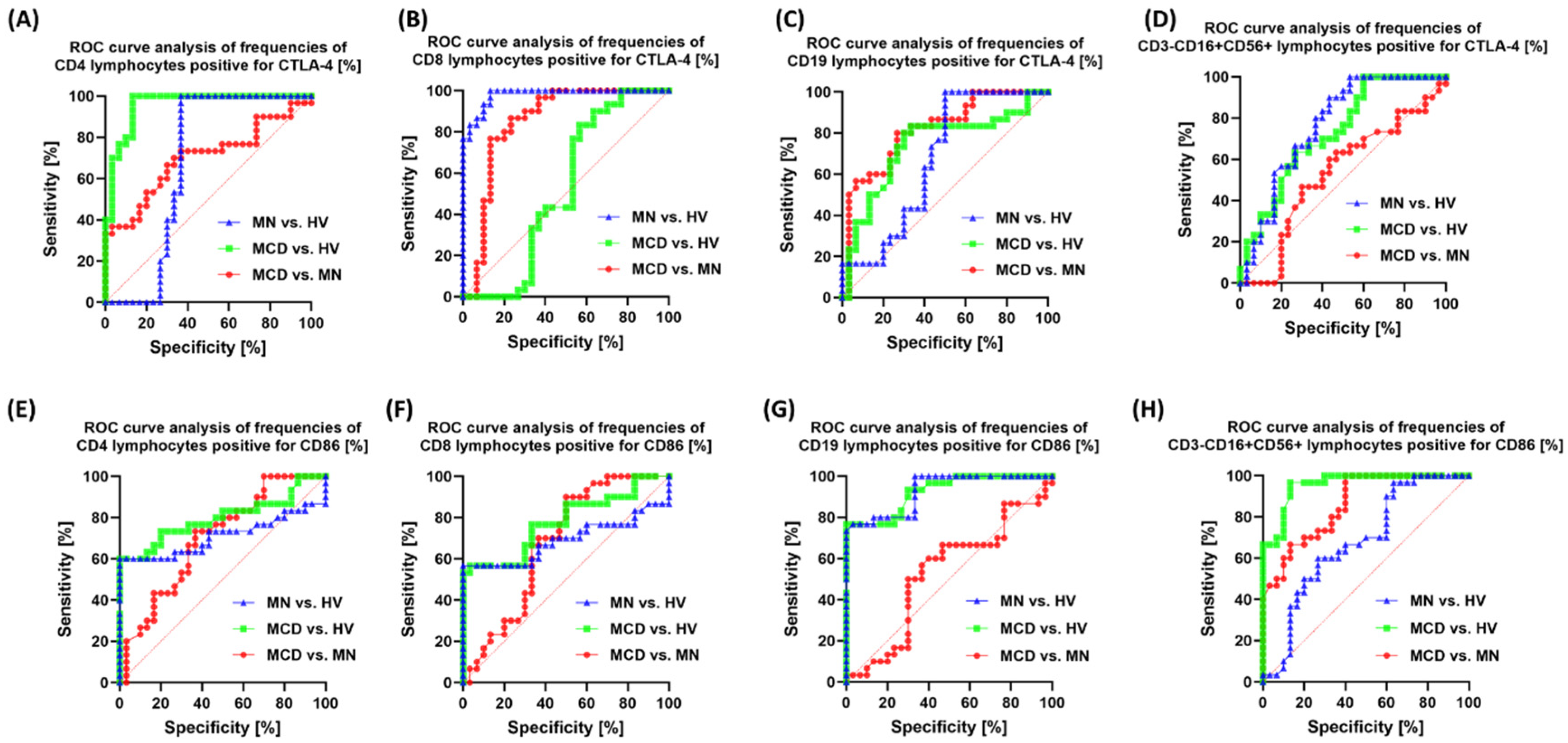
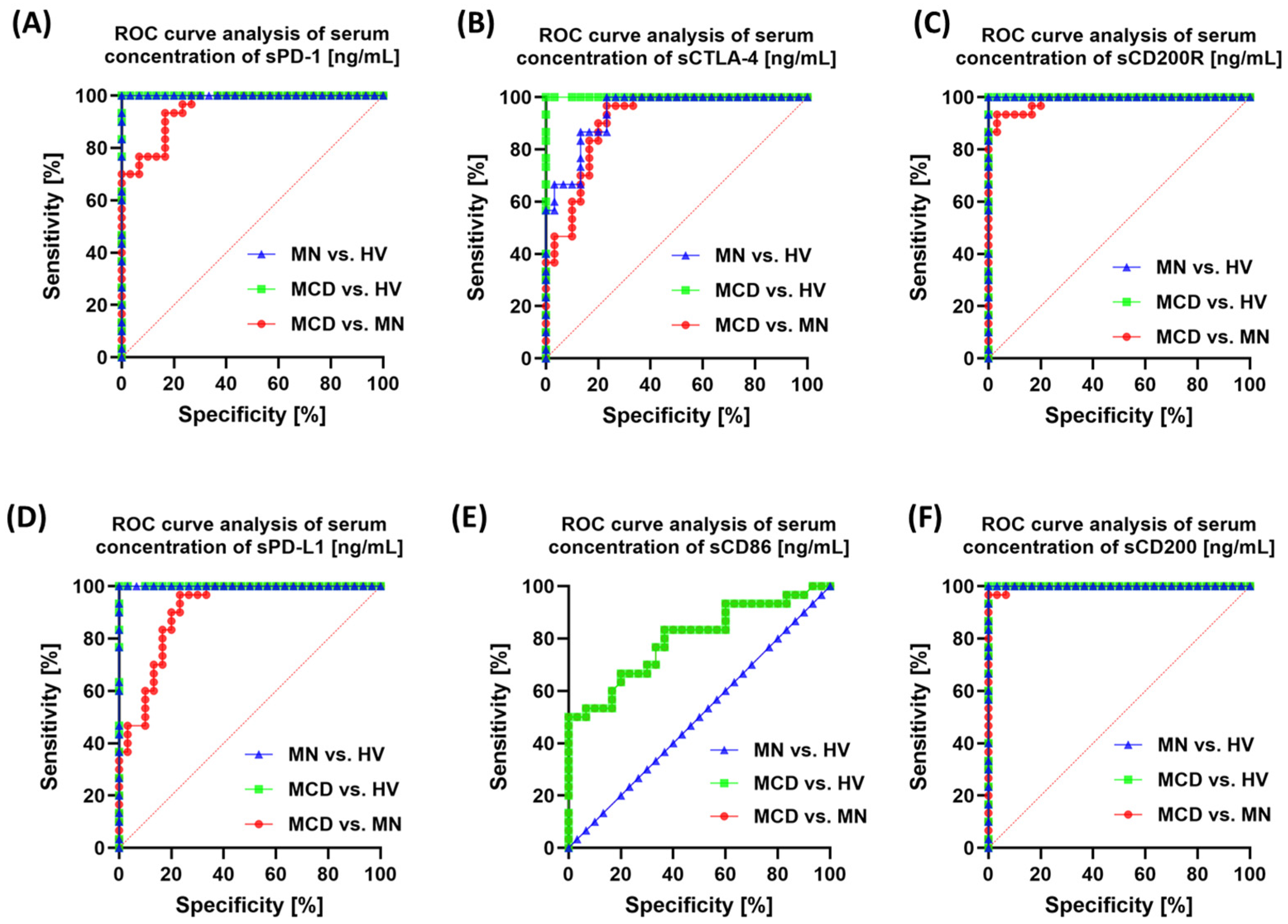
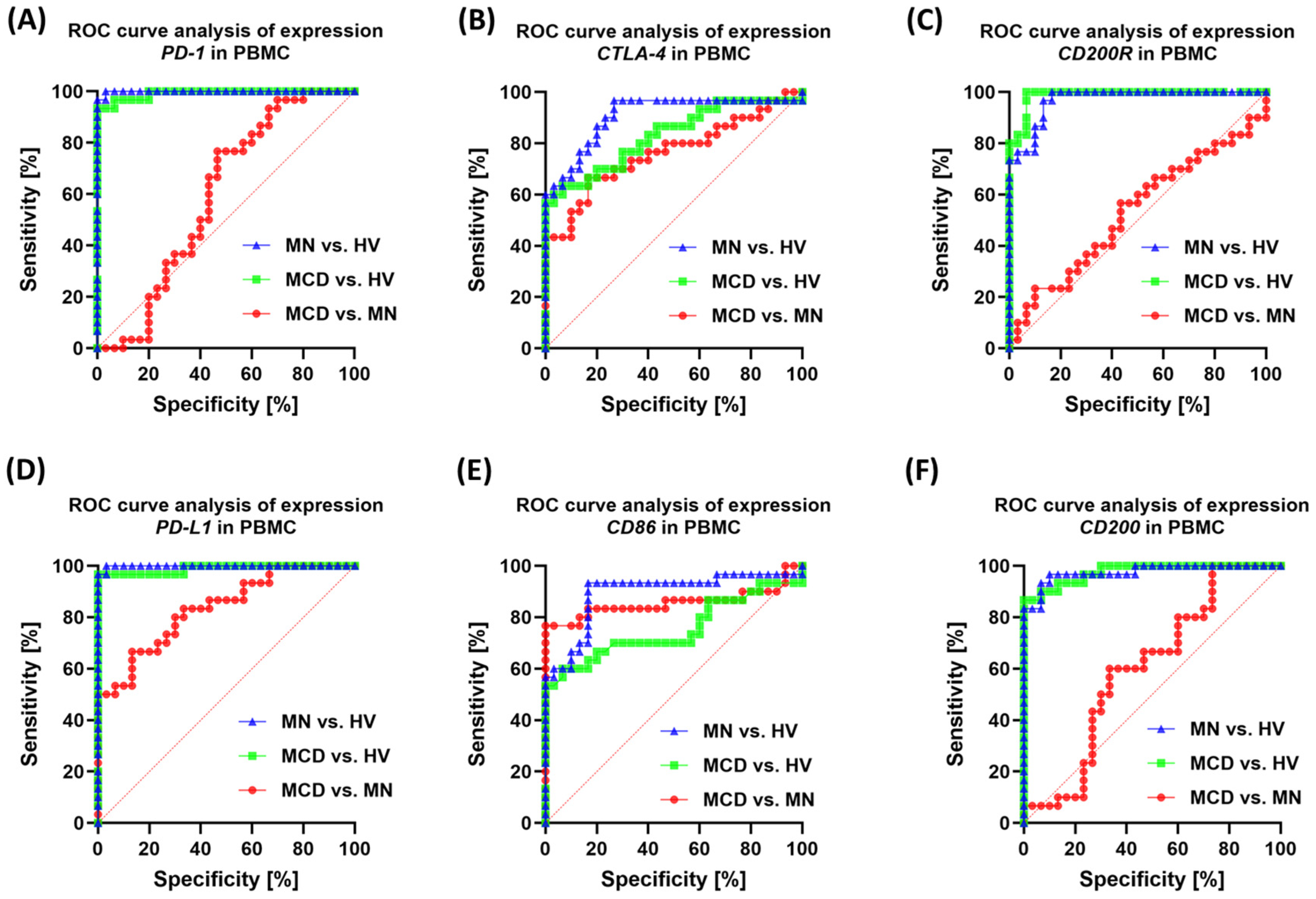
2.4. Correlation Analysis and ROC Curves
3. Discussion
3.1. Literature Analysis
3.2. Limitations of the Study
4. Materials and Methods
4.1. Participant Characteristics, Eligibility Criteria, and Study Material
4.2. Staining and Acquisition Parameters for Flow Cytometry
4.3. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
4.4. RNA Extraction from PBMCs, cDNA Synthesis, and Expression Analysis by qPCR
4.5. Serum Checkpoint Analysis (ELISA)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| MN | Membranous nephropathy |
| MCD | Minimal Change Disease |
| HV | Healthy volunteers |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CD86 | Cluster of differentiation 86 |
| CD200R | CD200 receptor |
| CD200 | Cluster of differentiation 200 |
| PBMC | Peripheral blood mononuclear cells |
| ROC | Receiver operating characteristic |
| sPD-1 | Soluble PD-1 |
| sPD-L1 | Soluble PD-L1 |
| sCTLA-4 | Soluble CTLA-4 |
| sCD86 | Soluble CD86 |
| sCD200 | Soluble CD200 |
| sCD200R | Soluble CD200R |
| GN | Glomerulonephritis |
| NK | Natural killer (cells) |
| ELISA | Enzyme-linked immunosorbent assay |
| CD4+/CD8+ T cells | T lymphocyte subsets expressing CD4 or CD8 |
| CD19+ B cells | B lymphocytes expressing CD19 |
References
- Roman, M.; Nowicki, M. Detailed Pathophysiology of Minimal Change Disease: Insights into Podocyte Dysfunction, Immune Dysregulation, and Genetic Susceptibility. Int. J. Mol. Sci. 2024, 25, 12174. [Google Scholar] [CrossRef]
- Qasim, H.; Abu Shugaer, M.; Dibian, S.; Ktaifan, M.; Khattab, K.; Leoni, M.L.G.; Varrassi, G. Patterns of Glomerular Injury: Histopathological Classification and Clinical Correlation. Cureus 2025, 17, e91728. [Google Scholar] [CrossRef] [PubMed]
- Wendt, R.; Sobhani, A.; Diefenhardt, P.; Trappe, M.; Völker, L.A. An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes. Biomedicines 2024, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Zamora, G.; Pearson-Shaver, A.L. Minimal Change Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S. Pathology of Podocytopathies Causing Nephrotic Syndrome in Children. Front. Pediatr. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Stangou, M.; Spartalis, Μ.; Daikidou, D.-V.; Kouloukourgiotou, T.; Sampani, E.; Lambropoulou, I.-T.; Pantzaki, A.; Papagianni, A.; Efstratiadis, G. Impact of Τh1 and Τh2 Cytokines in the Progression of Idiopathic Nephrotic Syndrome Due to Focal Segmental Glomerulosclerosis and Minimal Change Disease. J. Nephropathol. 2017, 6, 187–195. [Google Scholar] [CrossRef]
- Bertelli, R.; Bonanni, A.; Caridi, G.; Canepa, A.; Ghiggeri, G.M. Molecular and Cellular Mechanisms for Proteinuria in Minimal Change Disease. Front. Med. 2018, 5, 170. [Google Scholar] [CrossRef]
- Kaneko, K.; Tsuji, S.; Kimata, T.; Kitao, T.; Yamanouchi, S.; Kato, S. Pathogenesis of Childhood Idiopathic Nephrotic Syndrome: A Paradigm Shift from T-Cells to Podocytes. World J. Pediatr. 2015, 11, 21–28. [Google Scholar] [CrossRef]
- Maslyennikov, Y.; Bărar, A.A.; Rusu, C.C.; Potra, A.R.; Tirinescu, D.; Ticala, M.; Urs, A.; Pralea, I.E.; Iuga, C.A.; Moldovan, D.T.; et al. The Spectrum of Minimal Change Disease/Focal Segmental Glomerulosclerosis: From Pathogenesis to Proteomic Biomarker Research. Int. J. Mol. Sci. 2025, 26, 2450. [Google Scholar] [CrossRef]
- Tapia, C.; Bashir, K. Nephrotic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bogdanović, L.; Babić, I.; Prvanović, M.; Mijač, D.; Mladenović-Marković, A.; Popović, D.; Bogdanović, J. Uncommon Factors Leading to Nephrotic Syndrome. Biomedicines 2025, 13, 1907. [Google Scholar] [CrossRef]
- Koirala, A.; Jefferson, J.A. Steroid Minimization in Adults with Minimal Change Disease. Glomerular Dis. 2021, 1, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Zelikovich, A.S.; Salamone, I.M.; Fischer, J.A.; McNally, E.M. Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Xu, H.; Tang, D. Membranous Nephropathy Target Antigens Display Podocyte-Specific and Non-Specific Expression in Healthy Kidneys. Genes 2025, 16, 241. [Google Scholar] [CrossRef] [PubMed]
- Ronco, P.; Beck, L.; Debiec, H.; Fervenza, F.C.; Hou, F.F.; Jha, V.; Sethi, S.; Tong, A.; Vivarelli, M.; Wetzels, J. Membranous Nephropathy. Nat. Rev. Dis. Primers 2021, 7, 69. [Google Scholar] [CrossRef]
- Liu, W.; Huang, G.; Rui, H.; Geng, J.; Hu, H.; Huang, Y.; Huo, G.; Liu, B.; Xu, A. Course Monitoring of Membranous Nephropathy: Both Autoantibodies and Podocytes Require Multidimensional Attention. Autoimmun. Rev. 2022, 21, 102976. [Google Scholar] [CrossRef]
- Moroni, G.; Ponticelli, C. Secondary Membranous Nephropathy. A Narrative Review. Front. Med. 2020, 7, 611317. [Google Scholar] [CrossRef]
- Foster, M.H. Basement Membranes and Autoimmune Diseases. Matrix Biol. 2017, 57–58, 149–168. [Google Scholar] [CrossRef]
- Alok, A.; Yadav, A. Membranous Nephropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wais, M.; Warchoł, K.; Jasiura, A.; Wójcik, A.; Giżewska, K. Primary Membranous Nephropathy—What Do We Know Today? Qual. Sport 2023, 13, 81–87. [Google Scholar] [CrossRef]
- Smarz-Widelska, I.; Chojęta, D.; Kozioł, M.M. The Role of Anti-PLA2R and Anti-THSD7A Antibodies in the Pathogenesis and Diagnostics of Primary Membranous Nephropathy: A Review of Current Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 5301. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Z.; Lu, J.; Probst, C.; Zhang, Y.; Wang, X.; Qu, Z.; Wang, F.; Meng, L.; Cheng, X.; et al. Circulating Antibodies against Thrombospondin Type-I Domain-Containing 7A in Chinese Patients with Idiopathic Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 1642–1651. [Google Scholar] [CrossRef]
- Tesar, V.; Hruskova, Z. Autoantibodies in the Diagnosis, Monitoring, and Treatment of Membranous Nephropathy. Front. Immunol. 2021, 12, 593288. [Google Scholar] [CrossRef]
- Islam, S.; Janoudi, G.; Bryan, M.; Perras, C.; Jabr, M.; Haines, A.; Assasi, N.; Boucher, J.; Hafizi, D.; Lafleur, P.; et al. Rituximab for the Treatment of Primary Membranous Nephropathy: CADTH Health Technology Review; CADTH Health Technology Review; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2022. [Google Scholar]
- Shah, M.; DeLaat, A.; Cavanaugh, C. Treatment of Membranous Nephropathy: Perspectives on Current and Future Therapies. Front. Nephrol. 2023, 3, 1110355. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, L.; Peiti, S.; Pucci Bella, G.; Locatelli, F. SGLT2 Inhibitors in Glomerulonephritis: Beyond Nephroprotection? J. Clin. Med. 2025, 14, 3533. [Google Scholar] [CrossRef] [PubMed]
- Artan, A.S.; Mirioğlu, Ş.; Hocaoğlu, R.H.; Turgutalp, K.; Güllülü Boz, S.E.; Eren, N.; Dinçer, M.T.; Uzun, S.; Şahin, G.; Kutlay, S.; et al. Observational Study of Immunosuppressive Treatment Patterns and Outcomes in Primary Membranous Nephropathy: A Multicenter Retrospective Analysis. BMC Nephrol. 2024, 25, 327. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Kitching, A.R.; Leung, N.; Romagnani, P. Glomerulonephritis: Immunopathogenesis and Immunotherapy. Nat. Rev. Immunol. 2023, 23, 453–471. [Google Scholar] [CrossRef]
- Klaus, M.; Abinti, M.; Liébana, M.P.; Anders, H.-J. Update on Glomerulonephritis: How to Classify and How to Treat. J. Cell. Mol. Immunol. 2025, 4, 1–8. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, Z.; Zhuang, J.; Lu, C.; Qu, Y.; Xu, C.; Yang, S.; Tian, X. Understanding the Podocyte Immune Responses in Proteinuric Kidney Diseases: From Pathogenesis to Therapy. Front. Immunol. 2024, 14, 1335936. [Google Scholar] [CrossRef]
- Bruno, V.; Mühlig, A.K.; Oh, J.; Licht, C. New Insights into the Immune Functions of Podocytes: The Role of Complement. Mol. Cell. Pediatr. 2023, 10, 3. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Fang, X.; Lin, W.; Yang, Y. Membranous Nephropathy: Pathogenesis and Treatments. MedComm 2024, 5, e614. [Google Scholar] [CrossRef]
- Cantarelli, C.; Jarque, M.; Angeletti, A.; Manrique, J.; Hartzell, S.; O’Donnell, T.; Merritt, E.; Laserson, U.; Perin, L.; Donadei, C.; et al. A Comprehensive Phenotypic and Functional Immune Analysis Unravels Circulating Anti–Phospholipase A2 Receptor Antibody Secreting Cells in Membranous Nephropathy Patients. Kidney Int. Rep. 2020, 5, 1764–1776. [Google Scholar] [CrossRef]
- Strizzi, C.T.; Ambrogio, M.; Zanoni, F.; Bonerba, B.; Bracaccia, M.E.; Grandaliano, G.; Pesce, F. Epitope Spreading in Immune-Mediated Glomerulonephritis: The Expanding Target. Int. J. Mol. Sci. 2024, 25, 11096. [Google Scholar] [CrossRef]
- Pozdzik, A.; Brochériou, I.; David, C.; Touzani, F.; Goujon, J.M.; Wissing, K.M. Membranous Nephropathy and Anti-Podocytes Antibodies: Implications for the Diagnostic Workup and Disease Management. BioMed Res. Int. 2018, 2018, 6281054. [Google Scholar] [CrossRef]
- Mertowski, S.; Mertowska, P.; Czosnek, M.; Smarz-Widelska, I.; Załuska, W.; Grywalska, E. Checkpoint Imbalance in Primary Glomerulopathies: Comparative Insights into IgA Nephropathy and Membranoproliferative Glomerulonephritis. Cells 2025, 14, 1551. [Google Scholar] [CrossRef]
- Herrmann, S.M.; Abudayyeh, A.; Gupta, S.; Gudsoorkar, P.; Klomjit, N.; Motwani, S.S.; Karam, S.; Silva, V.T.C.E.; Khalid, S.B.; Anand, S.; et al. Diagnosis and Management of Immune Checkpoint Inhibitor–Associated Nephrotoxicity: A Position Statement from the American Society of Onco-Nephrology. Kidney Int. 2025, 107, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 Axis Converts Human Th1 Cells Into Regulatory T Cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, R.; Lazareth, H.; Flahault, A.; Brglez, V.; Seitz-Polski, B.; El Fil, S.; Mazières, J.; Ribes, D.; Huart, A.; Colombat, M.; et al. Membranous Nephropathy After Exposure to Immune Checkpoint Inhibitors. Kidney Int. Rep. 2023, 8, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Motavalli, R.; Hosseini, M.; Soltani-Zangbar, M.S.; Karimi, A.; Sadeghi, M.; Dolati, S.; Yousefi, M.; Etemadi, J. Evaluation of the Immune Checkpoint Factors in Idiopathic Membranous Nephropathy. Mol. Cell. Probes 2023, 69, 101914. [Google Scholar] [CrossRef]
- Grywalska, E.; Smarz-Widelska, I.; Krasowska-Zajac, E.; Korona-Glowniak, I.; Zaluska-Patel, K.; Mielnik, M.; Podgajna, M.; Malm, A.; Rolinski, J.; Zaluska, W. The PD-1/PD-L1 Inhibitory Pathway Is Altered in Primary Glomerulonephritides. Arch. Immunol. Ther. Exp. 2018, 66, 133–143. [Google Scholar] [CrossRef]
- Grywalska, E.; Smarz-Widelska, I.; Korona-Głowniak, I.; Mertowski, S.; Gosik, K.; Hymos, A.; Ludian, J.; Niedźwiedzka-Rystwej, P.; Roliński, J.; Załuska, W. PD-1 and PD-L1 Expression on Circulating Lymphocytes as a Marker of Epstein-Barr Virus Reactivation-Associated Proliferative Glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 8001. [Google Scholar] [CrossRef]
- Spink, C.; Stege, G.; Tenbrock, K.; Harendza, S. The CTLA-4 +49GG Genotype Is Associated with Susceptibility for Nephrotic Kidney Diseases. Nephrol. Dial. Transplant. 2013, 28, 2800–2805. [Google Scholar] [CrossRef]
- Chung, E.Y.M.; Wang, Y.M.; Shaw, K.; Ronning, E.; Wang, Y.; Yu Zhang, G.; Hu, M.; Keung, K.; McCarthy, H.J.; Harris, D.C.H.; et al. T Cell Costimulatory Blockade Ameliorates Induction of Experimental Membranous Nephropathy Potentially through T-Helper 17 Cell Suppression in the Kidney. Nephrol. Dial. Transplant. 2025, 40, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C. Membranous Nephropathy. J. Clin. Med. 2025, 14, 761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, G.; Wang, Q.; Wang, R.; Zhang, M. The CD200/CD200R Mechanism in Mesenchymal Stem Cells’ Regulation of Dendritic Cells. Am. J. Transl. Res. 2021, 13, 9607–9613. [Google Scholar]
- Gao, S.; Hao, B.; Yang, X.F.; Chen, W.Q. Decreased CD200R Expression on Monocyte-Derived Macrophages Correlates with Th17/Treg Imbalance and Disease Activity in Rheumatoid Arthritis Patients. Inflamm. Res. 2014, 63, 441–450. [Google Scholar] [CrossRef]
- Gorczynski, R.M. CD200:CD200R-Mediated Regulation of Immunity. Int. Sch. Res. Not. 2012, 2012, 682168. [Google Scholar] [CrossRef]
- Oweira, H.; Khajeh, E.; Mohammadi, S.; Ghamarnejad, O.; Daniel, V.; Schnitzler, P.; Golriz, M.; Mieth, M.; Morath, C.; Zeier, M.; et al. Pre-Transplant CD200 and CD200R1 Concentrations Are Associated with Post-Transplant Events in Kidney Transplant Recipients. Medicine 2019, 98, e17006. [Google Scholar] [CrossRef]
- Izzedine, H.; Perazella, M.A. Adverse Kidney Effects of Epidermal Growth Factor Receptor Inhibitors. Nephrol. Dial. Transplant. 2017, 32, 1089–1097. [Google Scholar] [CrossRef]
- Gallan, A.J.; Alexander, E.; Reid, P.; Kutuby, F.; Chang, A.; Henriksen, K.J. Renal Vasculitis and Pauci-Immune Glomerulonephritis Associated With Immune Checkpoint Inhibitors. Am. J. Kidney Dis. 2019, 74, 853–856. [Google Scholar] [CrossRef]
- He, X.; Liu, F.; Jin, Y.; Fu, H.; Mao, J. Glomerular Diseases after Immune Checkpoint Inhibitors Use: What Do We Know so Far? Ren. Fail. 2022, 44, 2046–2055. [Google Scholar] [CrossRef]
- Qu, L.; Jiao, B. The Interplay between Immune and Metabolic Pathways in Kidney Disease. Cells 2023, 12, 1584. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gallman, A.E.; Xie, C.; Shen, Q.; Ma, J.; Wolfreys, F.D.; Sandy, M.; Arsov, T.; Wu, X.; Qin, Y.; et al. P2RY8 Variants in Lupus Patients Uncover a Role for the Receptor in Immunological Tolerance. J. Exp. Med. 2022, 219, e20211004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, X.; Zhang, Y.; Li, J.; Qin, Y.; He, P.; Zhao, Y.; Su, B.; He, L. Immune Checkpoint Activity Exacerbate Renal Interstitial Fibrosis Progression by Enhancing PD-L1 Expression in Renal Tubular Epithelial Cells. Transl. Res. 2024, 271, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Fresquet, M.; Rhoden, S.J.; Jowitt, T.A.; McKenzie, E.A.; Roberts, I.; Lennon, R.; Brenchley, P.E. Autoantigens PLA2R and THSD7A in Membranous Nephropathy Share a Common Epitope Motif in the N-Terminal Domain. J. Autoimmun. 2020, 106, 102308. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.H. PLA2R and THSD7A: Disparate Paths to the Same Disease? J. Am. Soc. Nephrol. 2017, 28, 2579–2589. [Google Scholar] [CrossRef]
- van de Logt, A.-E.; Fresquet, M.; Wetzels, J.F.; Brenchley, P. The Anti-PLA2R Antibody in Membranous Nephropathy: What We Know and What Remains a Decade after Its Discovery. Kidney Int. 2019, 96, 1292–1302. [Google Scholar] [CrossRef]
- Shimada, M.; Araya, C.; Rivard, C.; Ishimoto, T.; Johnson, R.J.; Garin, E.H. Minimal Change Disease: A “Two-Hit” Podocyte Immune Disorder? Pediatr. Nephrol. 2011, 26, 645–649. [Google Scholar] [CrossRef]
- Yoo, T.-H.; Fornoni, A. Nonimmunologic Targets of Immunosuppressive Agents in Podocytes. Kidney Res. Clin. Pract. 2015, 34, 69–75. [Google Scholar] [CrossRef]
- Nair, R.; Somasundaram, V.; Kuriakose, A.; Krishn, S.R.; Raben, D.; Salazar, R.; Nair, P. Deciphering T-Cell Exhaustion in the Tumor Microenvironment: Paving the Way for Innovative Solid Tumor Therapies. Front. Immunol. 2025, 16, 1548234. [Google Scholar] [CrossRef]
- Zhao, Q.; Dai, H.; Liu, X.; Jiang, H.; Liu, W.; Feng, Z.; Zhang, N.; Gao, Y.; Dong, Z.; Zhou, X.; et al. Helper T Cells in Idiopathic Membranous Nephropathy. Front. Immunol. 2021, 12, 665629. [Google Scholar] [CrossRef]
- So, B.Y.F.; Yap, D.Y.H.; Chan, T.M. B Cells in Primary Membranous Nephropathy: Escape from Immune Tolerance and Implications for Patient Management. Int. J. Mol. Sci. 2021, 22, 13560. [Google Scholar] [CrossRef]
- Manich, G.; Recasens, M.; Valente, T.; Almolda, B.; González, B.; Castellano, B. Role of the CD200-CD200R Axis During Homeostasis and Neuroinflammation. Neuroscience 2019, 405, 118–136. [Google Scholar] [CrossRef]
- Zdravkova, I.; Tilkiyan, E.; Bozhkova, D.; Kuskunov, T.; Ronchev, Y.; Kirilov, B. Hypothetical Pathogenetic Model of Membranous Nephropathy. Int. J. Mol. Sci. 2025, 26, 2206. [Google Scholar] [CrossRef]
- Infantino, M. Editorial: Primary Membranous Nephropathy. Front. Immunol. 2022, 13, 1102036. [Google Scholar] [CrossRef]
- Courville, K.; Bustamante, N. Membranous Nephropathy: Genetics, Antigens and Antibodies. Genet. Clin. Genom. 2024, 2, 23–28. [Google Scholar] [CrossRef]


| Parameters | MCD Median (Q1–Q3) | MN Median (Q1–Q3) | HV Median (Q1–Q3) | p-Value | |||
|---|---|---|---|---|---|---|---|
| All | MCD vs. MN | MCD vs. HV | MN vs. HV | ||||
| Age | 49.50 (42.75–23.00) | 57.50 (49.00–34.00) | 44.50 (40.25–35.00) | 0.0034 | 0.0711 | 0.9077 | 0.003 |
| WBC [103/mm3] | 6.65 (5.83–4.33) | 6.50 (5.40–4.33) | 6.30 (5.91–4.20) | 0.5864 | >0.9999 | 0.9045 | >0.9999 |
| NEU [103/mm3] | 5.35 (5.01–3.90) | 4.17 (3.70–2.75) | 6.65 (4.82–3.06) | <0.0001 | 0.0007 | >0.9999 | <0.0001 |
| MON [103/mm3] | 0.48 (0.40–0.13) | 0.69 (0.56–0.26) | 0.57 (0.43–0.20) | 0.0003 | 0.0002 | 0.1313 | 0.1268 |
| LYM [103/mm3] | 2.08 (1.83–0.42) | 1.92 (1.37–0.27) | 1.95 (1.84–1.20) | 0.3432 | 0.4364 | >0.9999 | >0.9999 |
| EOS [103/mm3] | 0.20 (0.18–0.09) | 0.17 (0.14–0.00) | 0.17 (0.12–0.00) | 0.0059 | 0.0389 | 0.0083 | >0.9999 |
| BAS [103/mm3] | 0.03 (0.01–0.00) | 0.02 (0.00–0.00) | 0.02 (0.00–0.00) | 0.122 | 0.1888 | 0.2794 | >0.9999 |
| RBC [106/mm3] | 4.24 (3.87–3.20) | 4.11 (3.68–3.10) | 4.51 (4.24–3.67) | 0.0108 | >0.9999 | 0.0331 | 0.0231 |
| HGB [g/dL] | 12.24 (11.10–9.72) | 12.90 (11.14–9.20) | 14.76 (13.03–10.70) | 0.0003 | >0.9999 | 0.0005 | 0.0067 |
| PLT [103/mm3] | 238.46 (196.86–120.00) | 227.00 (193.50–126.42) | 247.00 (212.54–188.00) | 0.1037 | >0.9999 | 0.2368 | 0.1646 |
| Urea [mg/dL] | 56.22 (34.99–17.79) | 30.78 (23.32–15.48) | 21.00 (18.80–14.00) | <0.0001 | 0.0342 | <0.0001 | 0.003 |
| Creatine [mg/dL] | 1.04 (0.77–0.37) | 0.85 (0.67–0.38) | 0.70 (0.70–0.56) | 0.0119 | 0.5901 | 0.009 | 0.2799 |
| Uric acid [mg/dL] | 7.38 (6.03–3.80) | 5.80 (4.99–3.70) | 4.20 (3.60–3.29) | <0.0001 | 0.0288 | <0.0001 | <0.0001 |
| eGFR | 64.52 (61.26–30.30) | 87.32 (76.76–35.00) | 129.00 (126.00–117.50) | <0.0001 | 0.0249 | <0.0001 | <0.0001 |
| Cholesterol [mg/dL] | 279.25 (246.53–139.67) | 259.00 (209.83–118.68) | 149.20 (134.25–112.80) | <0.0001 | 0.6923 | <0.0001 | <0.0001 |
| Triglycerides [mg/dL] | 170.38 (150.28–51.63) | 124.32 (104.78–75.44) | 115.00 (69.75–55.46) | <0.0001 | 0.0913 | <0.0001 | 0.033 |
| HDL [mg/dL] | 61.17 (49.23–34.00) | 59.14 (48.74–25.67) | 60.00 (50.00–38.00) | 0.7384 | >0.9999 | >0.9999 | >0.9999 |
| LDL [mg/dL] | 199.86 (177.68–72.48) | 153.03 (110.71–60.95) | 102.00 (93.00–79.00) | <0.0001 | 0.0717 | <0.0001 | 0.0036 |
| IgG [g/L] | 4.20 (2.91–2.00) | 4.93 (4.25–1.91) | 5.20 (4.87–2.09) | 0.0025 | 0.0493 | 0.0023 | >0.9999 |
| IgM [g/L] | 1.06 (0.87–0.30) | 1.00 (0.54–0.34) | 1.80 (1.13–0.70) | 0.0002 | >0.9999 | 0.003 | 0.0003 |
| IgA [g/L] | 2.06 (1.73–0.82) | 2.21 (1.56–0.75) | 2.42 (1.82–0.95) | 0.3194 | >0.9999 | 0.3951 | >0.9999 |
| Total protein [g/dL] | 4.80 (4.03–2.83) | 4.48 (3.95–3.10) | 7.40 (7.09–3.60) | <0.0001 | >0.9999 | <0.0001 | <0.0001 |
| Albumin [g/L] | 1.97 (1.68–0.80) | 2.48 (1.95–1.20) | 4.28 (3.99–2.33) | <0.0001 | 0.5633 | <0.0001 | <0.0001 |
| Proteinuria [g/24 h] | 5.50 (4.13–2.49) | 5.87 (3.88–1.00) | 0.00 (0.00–0.00) | <0.0001 | >0.9999 | <0.0001 | <0.0001 |
| Parameters | MCD Median (Q1–Q3) | MN Median (Q1–Q3) | HV Median (Q1–Q3) | p-Value | |||
|---|---|---|---|---|---|---|---|
| ALL | MCD vs. MN | MCD vs. HV | MN vs. HV | ||||
| CD45+ [%] | 97.81 (97.17–99.11) | 97.92 (95.81–98.48) | 98.65 (97.09–99.33) | 0.3101 | >0.9999 | >0.9999 | 0.3782 |
| CD3+ [%] | 70.85 (66.88–76.30) | 72.75 (66.39–78.60) | 73.16 (70.47–78.95) | 0.2793 | >0.9999 | 0.3311 | >0.9999 |
| CD19+ [%] | 10.91 (8.40–16.42) | 11.12 (9.17–12.60) | 12.36 (7.97–14.74) | 0.9329 | >0.9999 | >0.9999 | >0.9999 |
| CD3-CD16+CD56+ [%] | 11.65 (6.18–18.71) | 8.60 (6.93–15.19) | 9.96 (8.63–14.06) | 0.3557 | 0.9688 | >0.9999 | 0.4858 |
| CD4+ [%] | 41.07 (33.27–48.50) | 42.11 (34.88–48.33) | 42.31 (36.01–49.01) | 0.8298 | >0.9999 | >0.9999 | >0.9999 |
| CD8+ [%] | 25.79 (22.35–29.73) | 25.75 (22.25–30.62) | 27.11 (22.17–29.98) | 0.6393 | >0.9999 | >0.9999 | >0.9999 |
| Ratio CD4/CD8 | 1.58 (1.02–2.14) | 1.51 (1.35–2.07) | 1.58 (0.97–2.27) | 0.9431 | >0.9999 | >0.9999 | >0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertowski, S.; Mertowska, P.; Czosnek, M.; Smarz-Widelska, I.; Załuska, W.; Grywalska, E. Immune Checkpoint Signatures in Minimal Change Disease and Membranous Nephropathy: Divergent Pathways of a Shared Imbalance. Int. J. Mol. Sci. 2025, 26, 11371. https://doi.org/10.3390/ijms262311371
Mertowski S, Mertowska P, Czosnek M, Smarz-Widelska I, Załuska W, Grywalska E. Immune Checkpoint Signatures in Minimal Change Disease and Membranous Nephropathy: Divergent Pathways of a Shared Imbalance. International Journal of Molecular Sciences. 2025; 26(23):11371. https://doi.org/10.3390/ijms262311371
Chicago/Turabian StyleMertowski, Sebastian, Paulina Mertowska, Milena Czosnek, Iwona Smarz-Widelska, Wojciech Załuska, and Ewelina Grywalska. 2025. "Immune Checkpoint Signatures in Minimal Change Disease and Membranous Nephropathy: Divergent Pathways of a Shared Imbalance" International Journal of Molecular Sciences 26, no. 23: 11371. https://doi.org/10.3390/ijms262311371
APA StyleMertowski, S., Mertowska, P., Czosnek, M., Smarz-Widelska, I., Załuska, W., & Grywalska, E. (2025). Immune Checkpoint Signatures in Minimal Change Disease and Membranous Nephropathy: Divergent Pathways of a Shared Imbalance. International Journal of Molecular Sciences, 26(23), 11371. https://doi.org/10.3390/ijms262311371





