Amyloid β Peptide Modifies Membrane Architecture and Surface Electrostatic Properties of Human Red Blood Cells
Abstract
1. Introduction
2. Results
2.1. RBC Aging Monitored by Laurdan GP
2.2. Interaction of Aβ42 Oligomers with RBC
2.3. Monitoring RBC Aging with and Without Aβ42 Using GP
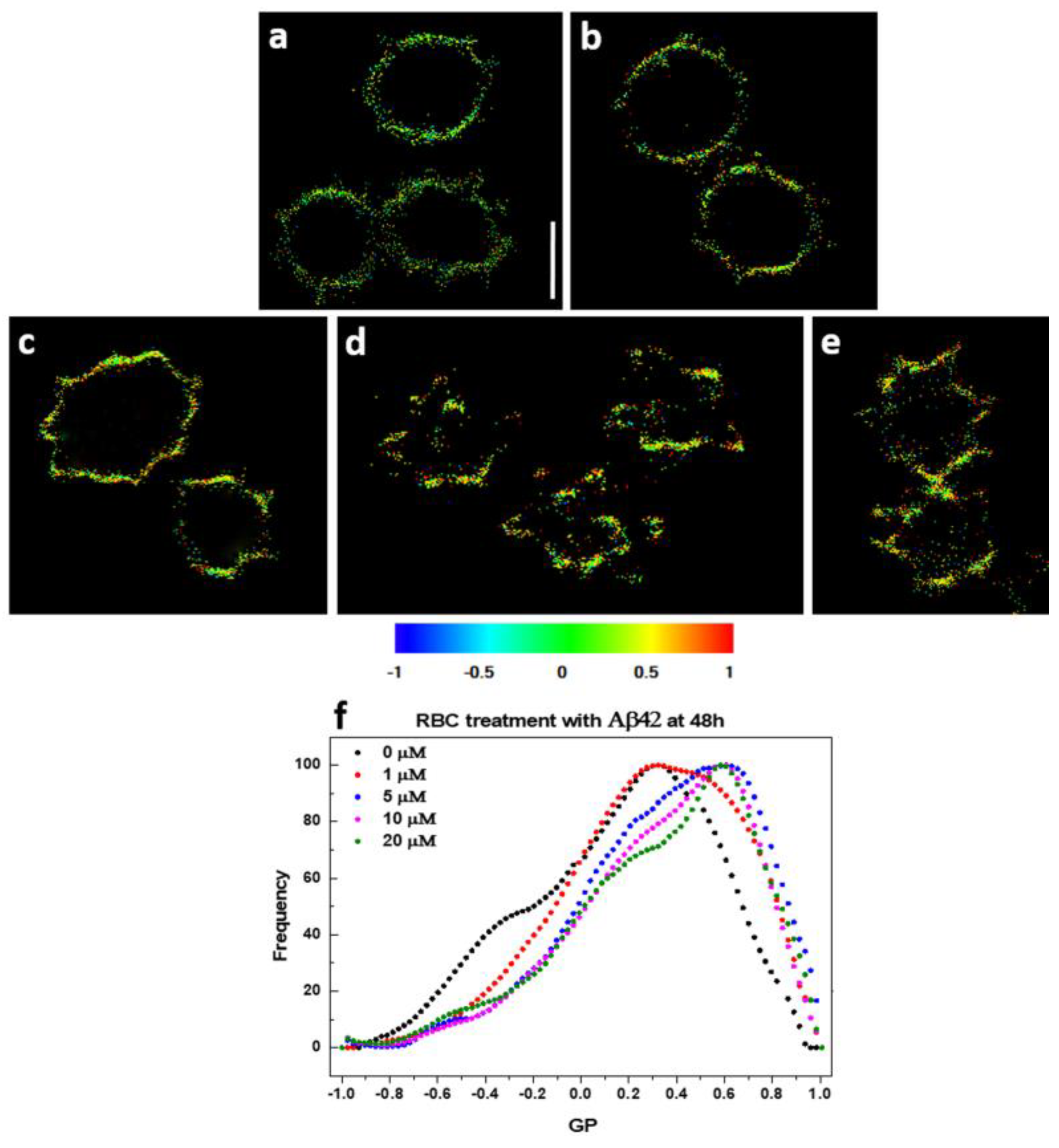
2.4. Visualization of Aβ42-RBCs Interaction
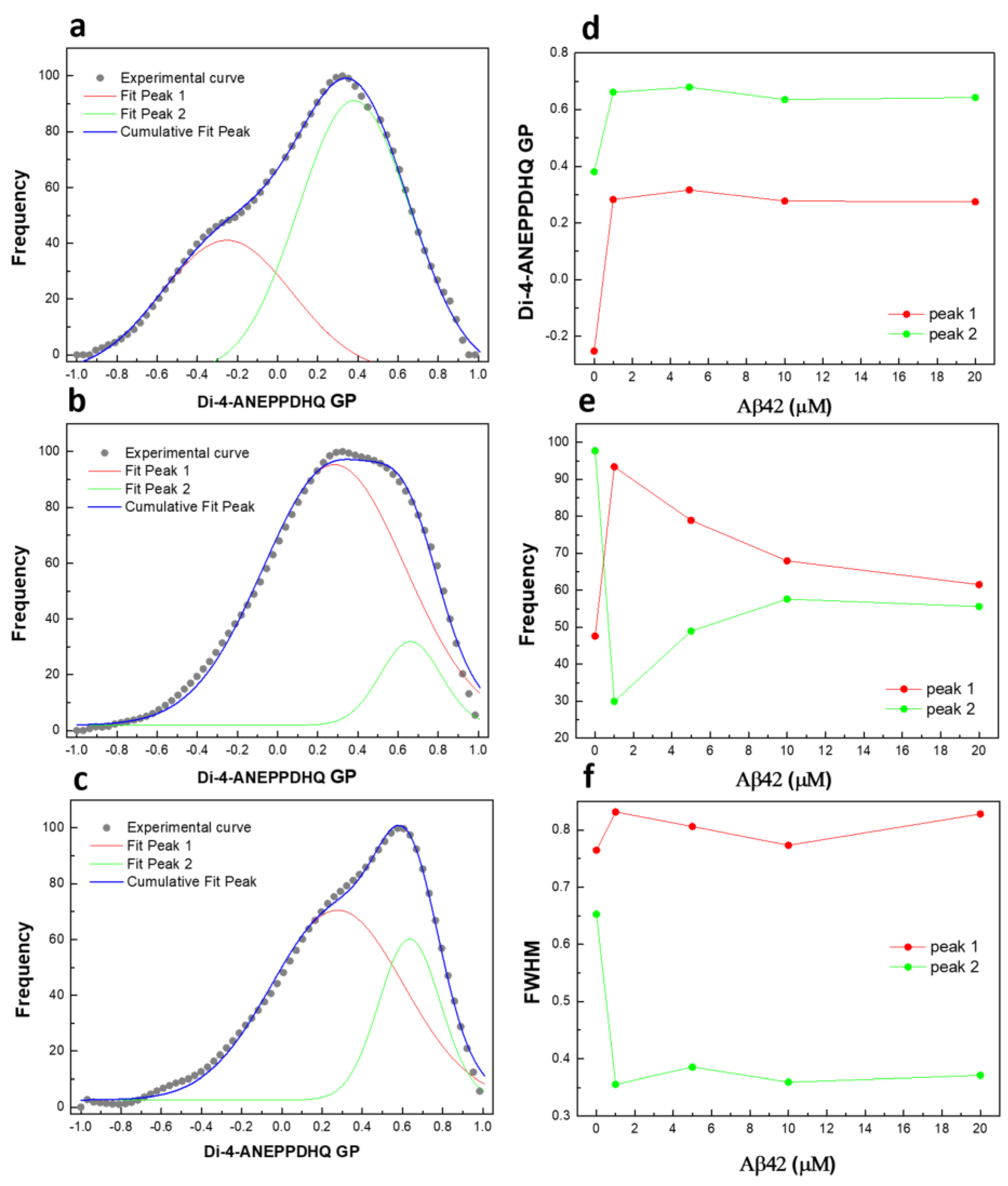
2.5. Plasma Membrane Lipid Order Visualization by Di-4-ANEPPDHQ Fluorescence Microscopy
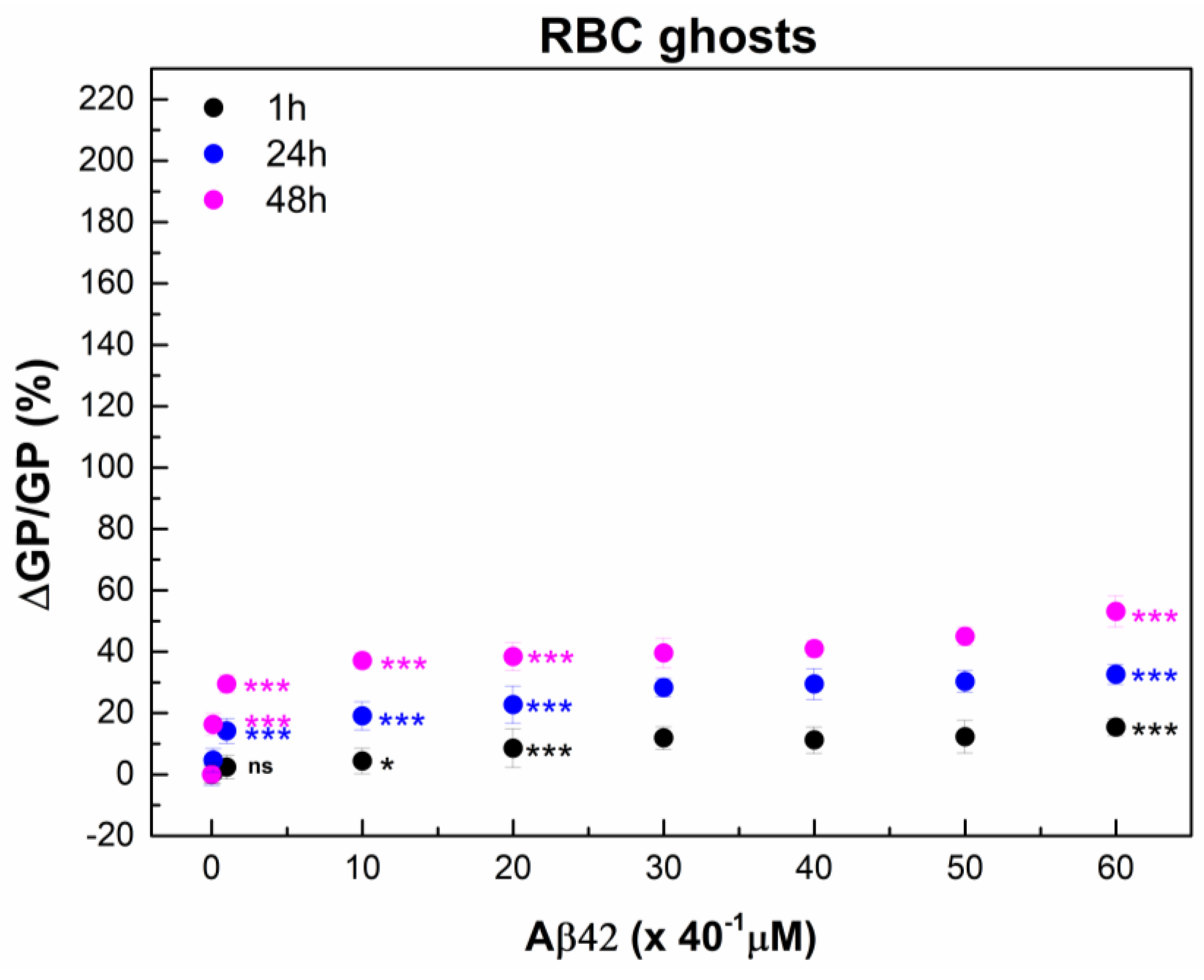
2.6. Interaction of Aβ42 Oligomers with RBC Ghosts
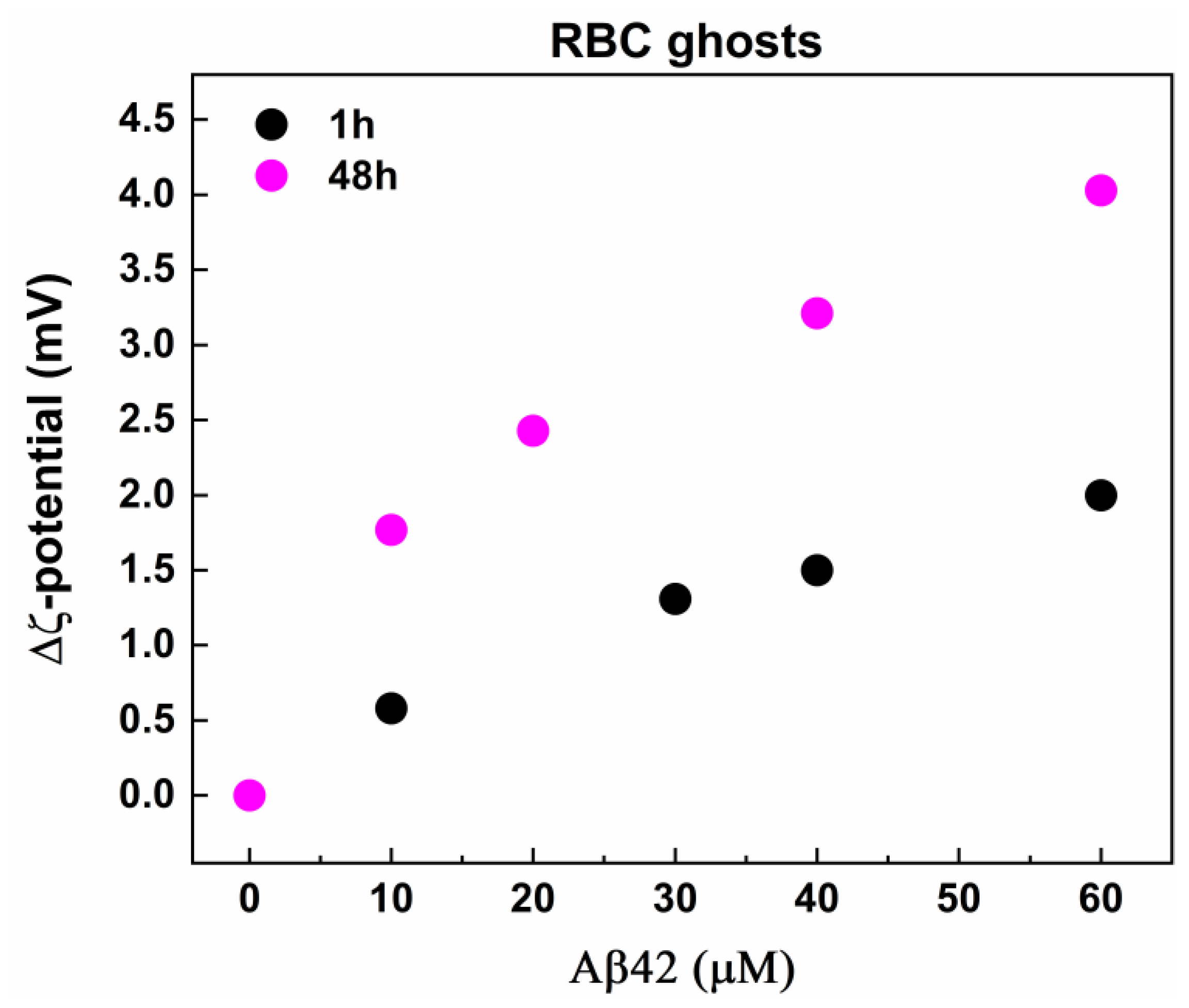
2.7. ζ-Potential of Human RBCs After Interaction with Aβ42 Oligomers
3. Discussion
4. Materials and Methods
4.1. Preparation of RBC and Erythrocyte Ghost Suspensions and Treatment with Aβ42 Oligomers
4.2. Preparation of Aβ42 Solutions
4.3. Laurdan Fluorescence Spectroscopy of RBCs and Ghosts
4.4. Di-4-ANEPPDHQ Microscopy Measurements
4.5. Electrokinetic Measurements of RBCs and Ghosts
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glenner, G.G.; Wong, C.W. Alzheimer’s Disease and Down’s Syndrome: Sharing of a Unique Cerebrovascular Amyloid Fibril Protein. Biochem. Biophys. Res. Commun. 1984, 122, 1131–1135. [Google Scholar] [CrossRef]
- Seubert, P.; Vigo-Pelfrey, C.; Esch, F.; Lee, M.; Dovey, H.; Davis, D.; Sinha, S.; Schiossmacher, M.; Whaley, J.; Swindlehurst, C.; et al. Isolation and Quantification of Soluble Alzheimer’s Β-Peptide from Biological Fluids. Nature 1992, 359, 325–327. [Google Scholar] [CrossRef]
- Selkoe, D.J.J. Normal and Abnormal Biology of the Beta-Amyloid Precursor Protein. Annu. Rev. Neurosci. 1994, 17, 489–517. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward Defining the Preclinical Stages of Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-Β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Ashe, K.H. The Biogenesis and Biology of Amyloid Β Oligomers in the Brain. Alzheimer’s Dement. 2020, 16, 1561–1567, Erratum in Alzheimer’s Dement. 2023, 2, 1104–1104. [Google Scholar] [CrossRef]
- Liu, N.; Haziyihan, A.; Zhao, W.; Chen, Y.; Chao, H. Trajectory of Brain-Derived Amyloid Beta in Alzheimer’s Disease: Where Is It Coming from and Where Is It Going? Transl. Neurodegener. 2024, 13, 42. [Google Scholar] [CrossRef]
- Johnson, J.T.; Awosiminiala, F.W.; Anumudu, C.K. Exploring Protein Misfolding and Aggregate Pathology in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Interventions. Appl. Sci. 2025, 15, 10285. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein Misfolding in Neurodegenerative Diseases: Implications and Strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk-Węgrzynowicz, M.; Adamiak, K.; Strużyńska, L. Targeting Protein Misfolding and Aggregation as a Therapeutic Perspective in Neurodegenerative Disorders. Int. J. Mol. Sci. 2024, 25, 12448. [Google Scholar] [CrossRef]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-Associated Protein Tau (Tau) Is a Major Antigenic Component of Paired Helical Filaments in Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Lee, V.M.-Y.; Trojanowski, J.Q. Tau-Mediated Neurodegeneration in Alzheimer’s Disease and Related Disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Hanger, D.P.; Lau, D.H.W.; Phillips, E.C.; Bondulich, M.K.; Guo, T.; Woodward, B.W.; Pooler, A.M.; Noble, W. Intracellular and Extracellular Roles for Tau in Neurodegenerative Disease. J. Alzheimer’s Dis. 2014, 40 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef]
- Wu, J.-R.; Zhou, B.; Huang, Y.-P.; Sun, Z.-D.; Hu, J.-X. Toxicities of Amyloid-Beta and Tau Protein Are Reciprocally Enhanced in the Drosophila Model. Neural Regen. Res. 2022, 17, 2286–2292. [Google Scholar] [CrossRef]
- Lacosta, A.M.; Insua, D.; Badi, H.; Pesini, P.; Sarasa, M. Neurofibrillary Tangles of Aβx-40 in Alzheimer’s Disease Brains. J. Alzheimer’s Dis. 2017, 58, 661–667. [Google Scholar] [CrossRef]
- Moda, F.; Ciullini, A.; Dellarole, I.; Lombardo, A.; Campanella, N.; Bufano, G.; Cazzaniga, F.; Giaccone, G. Secondary Protein Aggregates in Neurodegenerative Diseases: Almost the Rule Rather Than the Exception. FBL 2023, 28, 255. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Yang, Y.H.; Huang, L.C.; Hsieh, S.W.; Huang, L.J. Dynamic Blood Concentrations of Aβ1–40 and Aβ1–42 in Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 768. [Google Scholar] [CrossRef]
- Blömeke, L.; Rehn, F.; Pils, M.; Kraemer-Schulien, V.; Cousin, A.; Kutzsche, J.; Bujnicki, T.; Freiesleben, S.D.; Schneider, L.-S.; Preis, L.; et al. Blood-Based Quantification of Aβ Oligomers Indicates Impaired Clearance from Brain in Apoe Ε4 Positive Subjects. Commun. Med. 2024, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Ghiso, J.; Frangione, B.; Amari, M.; Tomidokoro, Y.; Ikeda, Y.; Harigaya, Y.; Okamoto, K.; Shoji, M. Lipoprotein-Free Amyloidogenic Peptides in Plasma Are Elevated in Patients with Sporadic Alzheimer’s Disease and Down’s Syndrome. Ann. Neurol. 1999, 45, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Chen, K.; Liu, X.; Bandy, D.; Yu, M.; Lee, W.; Ayutyanont, N.; Keppler, J.; Reeder, S.A.; Langbaum, J.B.S.; et al. Fibrillar Amyloid-Β Burden in Cognitively Normal People at 3 Levels of Genetic Risk for Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 6820–6825. [Google Scholar] [CrossRef] [PubMed]
- Blass, J.P.; Hanin, I.; Barclay, L.; Kopp, U.; Reding, M.J. Red Blood Cell Abnormalities in Alzheimer Disease. J. Am. Geriatr. Soc. 1985, 33, 401–405. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Satoh, A.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. Amyloid Β Levels in Human Red Blood Cells. PLoS ONE 2012, 7, e49620. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Whyte, S.; E Tanner, J.; Evin, G.; Beyreuther, K.; Masters, C.L. Secretion of Alzheimer’s Disease Abeta Amyloid Peptide by Activated Human Platelets. Lab Investig. 1998, 78, 461–469. [Google Scholar]
- E Gibson, G.; Huang, H.-M. Oxidative Processes in the Brain and Non-Neuronal Tissues as Biomarkers of Alzheimer’s Disease. Front. Biosci. A J. Virtual Libr. 2002, 7, d1007–d1015. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Kokjohn, T.A.; Kalback, W.; Luehrs, D.; Galasko, D.R.; Chevallier, N.; Koo, E.H.; Emmerling, M.R.; Roher, A.E. Amyloid-Β Peptides Interact with Plasma Proteins and Erythrocytes: Implications for Their Quantitation in Plasma. Biochem. Biophys. Res. Commun. 2000, 268, 750–756. [Google Scholar] [CrossRef]
- Zamolodchikov, D.; Berk-Rauch, H.E.; Oren, D.A.; Stor, D.S.; Singh, P.K.; Kawasaki, M.; Aso, K.; Strickland, S.; Ahn, H.J. Biochemical and Structural Analysis of the Interaction between Β-Amyloid and Fibrinogen. Blood 2016, 128, 1144–1151. [Google Scholar] [CrossRef]
- Ahn, H.J.; Chen, Z.L.; Zamolodchikov, D.; Norris, E.H.; Strickland, S. Interactions of Β-Amyloid Peptide with Fibrinogen and Coagulation Factor Xii May Contribute to Alzheimer’s Disease. Curr. Opin. Hematol. 2017, 24, 427–431. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Peripheral Blood Amyloid-Β Involved in the Pathogenesis of Alzheimer’s Disease Via Impacting on Peripheral Innate Immune Cells. J. Neuroinflam. 2024, 21, 5. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Higgins. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Strazdaite, S.; Roeters, S.J.; Sakalauskas, A.; Sneideris, T.; Kirschner, J.; Pedersen, K.B.; Schiøtt, B.; Jensen, F.; Weidner, T.; Smirnovas, V.; et al. Interaction of Amyloid-Β-(1–42) Peptide and Its Aggregates with Lipid/Water Interfaces Probed by Vibrational Sum-Frequency Generation Spectroscopy. J. Phys. Chem. B 2021, 125, 11208–11218. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhang, Z.; Zhao, W.; Yang, J. Interactions between Amyloid Β Peptide and Lipid Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 1663–1669. [Google Scholar] [CrossRef]
- Wiatrak, B.; Piasny, J.; Kuźniarski, A.; Gąsiorowski, K. Interactions of Amyloid-Β with Membrane Proteins. Int. J. Mol. Sci. 2021, 22, 6075. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Three Dimensions of the Amyloid Hypothesis: Time, Space and ‘Wingmen’. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Jamshidi, P.; Plascencia-Villa, G.; Perry, G. The Amyloid Cascade Hypothesis: A Conclusion in Search of Support. Am. J. Pathol. 2025, 195, 1988–1997. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Emmerling, M.R.; Vigo-Pelfrey, C.; Kasunic, T.C.; Kirkpatrick, J.B.; Murdoch, G.H.; Ball, M.J.; Roher, A.E. Water-Soluble Aβ(N-40, N-42) Oligomers in Normal and Alzheimer Disease Brains. J. Biol. Chem. 1996, 271, 4077–4081. [Google Scholar] [CrossRef]
- McLean, C.A.; Cherny, R.A.; Fraser, F.W.; Fuller, S.J.; Smith, M.J.; Vbeyreuther, K.; Bush, A.I.; Masters, C.L. Soluble Pool of Aβ Amyloid as a Determinant of Severity of Neurodegeneration in Alzheimer’s Disease. Ann. Neurol. 1999, 46, 860–866. [Google Scholar] [CrossRef]
- Tabaton, M.; Piccini, A. Role of Water-Soluble Amyloid-Β in the Pathogenesis of Alzheimer’s Disease. Int. J. Exp. Pathol. 2005, 86, 139–145. [Google Scholar] [CrossRef]
- Wirths, O.; Multhaup, G.; Bayer, T.A. A Modified Β-Amyloid Hypothesis: Intraneuronal Accumulation of the Β-Amyloid Peptide—The First Step of a Fatal Cascade. J. Neurochem. 2004, 91, 513–520. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-Β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimer’s Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Andrikopoulos, N.; Li, Y.; Ke, S.; Sun, Y.; Ding, F.; Ke, P.C. Emerging Biophysical Origins and Pathogenic Implications of Amyloid Oligomers. Nat. Commun. 2025, 16, 2937. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-Term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid Β-42 and A-Synuclein in Children and Young Adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Abrahamson, E.E.; Ciallella, J.R.; Paljug, W.R.; Wisniewski, S.R.; Clark, R.S.B.; Ikonomovic, M.D. Association of Increased Cortical Soluble Aβ42 Levels with Diffuse Plaques after Severe Brain Injury in Humans. Arch. Neurol. 2007, 64, 541–544. [Google Scholar] [CrossRef]
- de la Torre, J.C. Is Alzheimer’s Disease a Neurodegenerative or a Vascular Disorder? Data, Dogma, and Dialectics. Lancet Neurol. 2004, 3, 184–190. [Google Scholar] [CrossRef]
- Teller, J.K.; Russo, C.; Debusk, L.M.; Angelini, G.; Zaccheo, D.; Dagna-Bricarelli, F.; Scartezzini, P.; Bertolini, S.; Mann, D.M.; Tabaton, M.; et al. Presence of Soluble Amyloid Β–Peptide Precedes Amyloid Plaque Formation in Down’s Syndrome. Nat. Med. 1996, 2, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Lott, I.T. Down Syndrome and Beta-Amyloid Deposition. Curr. Opin. Neurol. 2004, 17, 95–100. [Google Scholar] [CrossRef]
- Head, E.; TLott, I.; MWilcock, D.; ALemere, C. Aging in Down Syndrome and the Development of Alzheimer’s Disease Neuropathology. Curr. Alzheimer Res. 2016, 13, 18–29. [Google Scholar] [CrossRef]
- Salehi, A.; Wesson Ashford, J.; Mufson, J.E. The Link between Alzheimer’s Disease and Down Syndrome. A Historical Perspective. Curr. Alzheimer Res. 2016, 13, 2–6. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic Brain Injury and Risk of Alzheimer’s Disease and Related Dementias in the Population. J. Alzheimer’s Dis. 2022, 88, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.A.; Jones, D.; South, P.W.; Snowden, J.S.; Neary, D. Deposition of Amyloid Β Protein in Non-Alzheimer Dementias: Evidence for a Neuronal Origin of Parenchymal Deposits of Β Protein in Neurodegenerative Disease. Acta Neuropathol. 1992, 83, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Massey, A.M.; Roher, A.E. Cerebral Amyloid Angiopathy: Accumulation of Aβ in Interstitial Fluid Drainage Pathways in Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2000, 903, 110–117. [Google Scholar] [CrossRef]
- Gallardo, G.; Holtzman, D.M. Amyloid-Β and Tau at the crossroads of Alzheimer’s Disease. In Tau Biology; Akihiko, T., Benjamin, W., Luc, B., Eds.; Springer: Singapore, 2019; pp. 187–203. [Google Scholar]
- Kosenko, E.; Tikhonova, L.; Alilova, G.; Urios, A.; Montoliu, C. The Erythrocytic Hypothesis of Brain Energy Crisis in Sporadic Alzheimer Disease: Possible Consequences and Supporting Evidence. J. Clin. Med. 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lauderback, C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid Β-Peptide-Associated Free Radical Oxidative Stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Aliev, G.; Kaminsky, Y.G. Relationship between Chronic Disturbance of 2,3-Diphosphoglycerate Metabolism in Erythrocytes and Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2016, 15, 113–123. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Tikhonova, L.A.; Montoliu, C.; Barreto, G.E.; Aliev, G.; Kaminsky, Y.G. Metabolic Abnormalities of Erythrocytes as a Risk Factor for Alzheimer’s Disease. Front. Neurosci. 2018, 11, 728. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: A Current Overview. J. Alzheimer’s Dis. 2023, 92, 751–768. [Google Scholar] [CrossRef]
- Mossmann, D.; Vögtle, F.N.; Taskin, A.A.; Teixeira, P.F.; Ring, J.; Burkhart, J.M.; Burger, N.; Pinho, C.M.; Tadic, J.; Loreth, D.; et al. Amyloid-β Peptide Induces Mitochondrial Dysfunction by Inhibition of Preprotein Maturation. Cell Metab. 2014, 20, 662–669. [Google Scholar] [CrossRef]
- Cenini, G.; Rüb, C.; Bruderek, M.; Voos, W. Amyloid Β-Peptides Interfere with Mitochondrial Preprotein Import Competence by a Coaggregation Process. Mol. Biol. Cell 2016, 27, 3257–3272. [Google Scholar] [CrossRef]
- Hemmerová, E.; Špringer, T.; Krištofiková, Z.; Homola, J. Ionic Environment Affects Biomolecular Interactions of Amyloid?: Spr Biosensor Study. Int. J. Mol. Sci. 2020, 21, 9727. [Google Scholar] [CrossRef]
- Banerjee, S.; Lyubchenko, Y.L. Interaction of Amyloidogenic Proteins with Membranes and Molecular Mechanism for the Development of Alzheimer’s Disease. Alzheimer’s Res. Ther. 2019, 2, 106. [Google Scholar]
- Chang, C.-C.; Edwald, E.; Veatch, S.; Steel, D.G.; Gafni, A. Interactions of Amyloid-Β Peptides on Lipid Bilayer Studied by Single Molecule Imaging and Tracking. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 1616–1624. [Google Scholar] [CrossRef]
- Meker, S.; Chin, H.; Sut, T.N.; Cho, N.J. Amyloid-Β Peptide Triggers Membrane Remodeling in Supported Lipid Bilayers Depending on Their Hydrophobic Thickness. Langmuir 2018, 34, 9548–9560. [Google Scholar] [CrossRef] [PubMed]
- Goñi, F.M. Rafts: A Nickname for Putative Transient Nanodomains. Chem. Phys. Lipids 2019, 218, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Staneva, G.; Puff, N.; Stanimirov, S.; Tochev, T.; Angelova, M.I.; Seigneuret, M. The Alzheimer’s Disease Amyloid-Β Peptide Affects Size-Dynamics of Raft-Mimicking Lo Domains in Gm1 Containing Lipid Bilayers. Soft Matter 2018, 14, 9609–9618. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Kusiak, J.W.; Chrest, F.J.; Demehin, A.A.; Murali, J.; Wersto, R.P.; Nagababu, E.; Ravi, L.; Rifkind, J.M. Red Cell Perturbations by Amyloid Β-Protein. Biochim. Biophys. Acta (BBA) Gen. Subj. 2003, 1622, 20–28. [Google Scholar] [CrossRef]
- Dinarelli, S.; Girasole, M.; Misiti, F. Amyloid Β Peptide Affects Erythrocyte Morphology: Role of Intracellular Signaling Pathways. Clin. Hemorheol. Microcirc. 2019, 71, 437–449. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; Schneider, T.; Felbecker, A. Spatial Organization of Protein Aggregates on Red Blood Cells as Physical Biomarkers of Alzheimer’s Disease Pathology. Sci. Adv. 2021, 7, eabj2137. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Giardina, B.; Misiti, F. Amyloid Beta Peptide (1–42)-Mediated Antioxidant Imbalance Is Associated with Activation of Protein Kinase C in Red Blood Cells. Cell Biochem. Funct. 2015, 33, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Stöckli, L.; Felbecker, A.; Nirmalraj, P.N. Protein Fibril Aggregation on Red Blood Cells: A Potential Biomarker to Distinguish Neurodegenerative Diseases from Healthy Aging. Brain Commun. 2024, 6, fcae180. [Google Scholar] [CrossRef]
- Banerjee, S.; Hashemi, M.; Zagorski, K.; Lyubchenko, Y.L. Interaction of Aβ42 with Membranes Triggers the Self-Assembly into Oligomers. Int. J. Mol. Sci. 2020, 21, 1129. [Google Scholar] [CrossRef]
- Stefani, M.; Rigacci, S. Protein Folding and Aggregation into Amyloid: The Interference by Natural Phenolic Compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef]
- Drabik, D.; Chodaczek, G.; Kraszewski, S. Effect of Amyloid-Beta Monomers on Lipid Membrane Mechanical Parameters–Potential Implications for Mechanically Driven Neurodegeneration in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 18. [Google Scholar] [CrossRef]
- Ahyayauch, H.; Raab, M.; Busto, J.V.; Andraka, N.; Arrondo, J.-L.R.; Masserini, M.; Tvaroska, I.; Goñi, F.M. Binding of Β-Amyloid (1–42) Peptide to Negatively Charged Phospholipid Membranes in the Liquid-Ordered State: Modeling and Experimental Studies. Biophys. J. 2012, 103, 453–463. [Google Scholar] [CrossRef]
- Ahyayauch, H.; De La Arada, I.; Masserini, M.E.; Arrondo, J.L.R.; Goñi, F.M.; Alonso, A. The Binding of Aβ42 Peptide Monomers to Sphingomyelin/Cholesterol/Ganglioside Bilayers Assayed by Density Gradient Ultracentrifugation. Int. J. Mol. Sci. 2020, 21, 1674. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.P.; Igbavboa, U.; Wang, L.; Wood, W.G.; Duff, K. Cholesterol Distribution, Not Total Levels, Correlate with Altered Amyloid Precursor Protein Processing in Statin-Treated Mice. NeuroMol. Med. 2006, 8, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, S.; Salamon, Z.; Lindblom, G.; Gröbner, G.; Tollin, G. Effects of Sphingomyelin, Cholesterol and Zinc Ions on the Binding, Insertion and Aggregation of the Amyloid Aβ1−40 Peptide in Solid-Supported Lipid Bilayers. FEBS J. 2006, 273, 1389–1402. [Google Scholar] [CrossRef]
- Watanabe, C.; Puff, N.; Staneva, G.; Seigneuret, M.; Angelova, M.I. Antagonism and Synergy of Single Chain Sphingolipids Sphingosine and Sphingosine-1-Phosphate toward Lipid Bilayer Properties. Consequences for Their Role as Cell Fate Regulators. Langmuir 2014, 30, 13956–13963. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Dinarelli, S.; Sampaolese, B.; Misiti, F.; Girasole, M. Morphological Changes Induced in Erythrocyte by Amyloid Beta Peptide and Glucose Depletion: A Combined Atomic Force Microscopy and Biochemical Study. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Strijkova-Kenderova, V.; Todinova, S.; Andreeva, T.; Bogdanova, D.; Langari, A.; Danailova, A.; Krumova, S.; Zlatareva, E.; Kalaydzhiev, N.; Milanov, I.; et al. Morphometry and Stiffness of Red Blood Cells—Signatures of Neurodegenerative Diseases and Aging. Int. J. Mol. Sci. 2022, 23, 227. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Shukla, H.D.; Williamson, J.D.; Launer, L.J.; Saxena, S.; Rifkind, J.M. Alterations in the Red Blood Cell Membrane Proteome in Alzheimer’s Subjects Reflect Disease-Related Changes and Provide Insight into Altered Cell Morphology. Proteome Sci. 2010, 8, 11. [Google Scholar] [CrossRef]
- Lue, L.-F.; Kuo, Y.-M.; Sabbagh, M. Advance in Plasma Ad Core Biomarker Development: Current Findings from Immunomagnetic Reduction-Based Squid Technology. Neurol. Ther. 2019, 8, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.V.; Forlenza, O.V.; Diniz, B.S. Plasma Biomarkers of Alzheimer’s Disease: A Review of Available Assays, Recent Developments, and Implications for Clinical Practice. J. Alzheimer’s Dis. Rep. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and Function in Native and Pathological Erythrocytes: A Quantitative View from the Nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Günther, G.; Herlax, V.; Lillo, M.P.; Sandoval-Altamirano, C.; Belmar, L.N.; Sánchez, S.A. Study of Rabbit Erythrocytes Membrane Solubilization by Sucrose Monomyristate Using Laurdan and Phasor Analysis. Colloids Surf. B Biointerfaces 2018, 161, 375–385. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Lee, C.-W.; Shih, Y.-H.; Yang, T.; Yu, L.; Kuo, Y.-M. Interactions between Amyloid-Β and Hemoglobin: Implications for Amyloid Plaque Formation in Alzheimer’s Disease. PLoS ONE 2012, 7, e33120. [Google Scholar] [CrossRef]
- Perry, R.T.; Gearhart, D.A.; Wiener, H.W.; Harrell, L.E.; Barton, J.C.; Kutlar, A.; Kutlar, F.; Ozcan, O.; Go, R.C.; Hill, W.D. Hemoglobin Binding to Aβ and Hbg2 Snp Association Suggest a Role in Alzheimer’s Disease. Neurobiol. Aging 2008, 29, 185–193. [Google Scholar] [CrossRef]
- Tokumasu, F.; A Nardone, G.; Ostera, G.R.; Fairhurst, R.M.; Beaudry, S.D.; Hayakawa, E.; A Dvorak, J. Altered Membrane Structure and Surface Potential in Homozygous Hemoglobin C Erythrocytes. PLoS ONE 2009, 4, e5828. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro, M.d.L. Electrical Properties of the Red Blood Cell Membrane and Immunohematological Investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef]
- Yagi-Utsumi, M.; Kato, K.; Nishimura, K. Membrane-Induced Dichotomous Conformation of Amyloid Β with the Disordered N-Terminal Segment Followed by the Stable C-Terminal Β Structure. PLoS ONE 2016, 11, e0146405. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Yahi, N. Molecular inside into Amyloid Regulation by Membrane Cholesterol and Sphingolipids: Common Mechanisms in Neurodegenerative Diseases. Expert Rev. Mol. Med. 2010, 12, e27. [Google Scholar] [CrossRef]
- Itoh, S.G.; Okumura, H. Promotion and Inhibition of Amyloid-Β Peptide Aggregation: Molecular Dynamics Studies. Int. J. Mol. Sci. 2021, 22, 1859. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Eckley, D.M.; Williamson, J.D.; Launer, L.J.; Rifkind, J.M. Do Red Blood Cell-Β-Amyloid Interactions Alter Oxygen Delivery in Alzheimer’s Disease? Adv. Exp. Med. Biol. 2008, 614, 29–35. [Google Scholar]
- Villaflores, O.B.; Chen, Y.-J.; Chen, C.-P.; Yeh, J.-M.; Wu, T.-Y. Curcuminoids and Resveratrol as Anti-Alzheimer Agents. Taiwan. J. Obstet. Gynecol. 2012, 51, 515–525. [Google Scholar] [CrossRef]
- Azouz, M.; Cullin, C.; Lecomte, S.; Lafleur, M. Membrane Domain Modulation of Aβ1–42 Oligomer Interactions with Supported Lipid Bilayers: An Atomic Force Microscopy Investigation. Nanoscale 2019, 11, 20857–20867. [Google Scholar] [CrossRef]
- Bokvist, M.; Lindström, F.; Watts, A.; Gröbner, G. Two Types of Alzheimer’s Β-Amyloid (1–40) Peptide Membrane Interactions: Aggregation Preventing Transmembrane Anchoring Versus Accelerated Surface Fibril Formation. J. Mol. Biol. 2004, 335, 1039–1049. [Google Scholar] [CrossRef]
- Lai, A.Y.; McLaurin, J. Mechanisms of Amyloid-Beta Peptide Uptake by Neurons: The Role of Lipid Rafts and Lipid Raft-Associated Proteins. Int. J. Alzheimer’s Dis. 2011, 2011, 548380. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.; Conrard, L.; Guthmann, M.; Pollet, H.; Carquin, M.; Vermylen, C.; Gailly, P.; Van Der Smissen, P.; Mingeot-Leclercq, M.P.; Tyteca, D. Contribution of Plasma Membrane Lipid Domains to Red Blood Cell (Re)Shaping. Sci. Rep. 2017, 7, 4264. [Google Scholar] [CrossRef] [PubMed]
- Carquin, M.; Pollet, H.; Veiga-Da-Cunha, M.; Cominelli, A.; Van Der Smissen, P.; N’KUli, F.; Emonard, H.; Henriet, P.; Mizuno, H.; Courtoy, P.J.; et al. Endogenous Sphingomyelin Segregates into Submicrometric Domains in the Living Erythrocyte Membrane [S]. J. Lipid Res. 2014, 55, 1331–1342. [Google Scholar] [CrossRef]
- Conrard, L.; Stommen, A.; Cloos, A.-S.; Steinkühler, J.; Dimova, R.; Pollet, H.; Tyteca, D. Spatial Relationship and Functional Relevance of Three Lipid Domain Populations at the Erythrocyte Surface. Cell. Physiol. Biochem. 2018, 51, 1544–1565. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.; Pollet, H.; Vermylen, C.; Gov, N.; Tyteca, D.; Mingeot-Leclercq, M.-P. Tuning of Differential Lipid Order between Submicrometric Domains and Surrounding Membrane Upon Erythrocyte Reshaping. Cell. Physiol. Biochem. 2018, 48, 2563–2582. [Google Scholar] [CrossRef]
- Li, H.; Lykotrafitis, G. Erythrocyte Membrane Model with Explicit Description of the Lipid Bilayer and the Spectrin Network. Biophys. J. 2014, 107, 642–653. [Google Scholar] [CrossRef]
- Carquin, M.; Conrard, L.; Pollet, H.; Van Der Smissen, P.; Cominelli, A.; Veiga-Da-Cunha, M.; Courtoy, P.J.; Tyteca, D. Cholesterol Segregates into Submicrometric Domains at the Living Erythrocyte Membrane: Evidence and Regulation. Cell. Mol. Life Sci. 2015, 72, 4633–4651. [Google Scholar] [CrossRef]
- Himbert, S.; Alsop, R.J.; Rose, M.; Hertz, L.; Dhaliwal, A.; Moran-Mirabal, J.M.; Verschoor, C.P.; Bowdish, D.M.E.; Kaestner, L.; Wagner, C.; et al. The Molecular Structure of Human Red Blood Cell Membranes from Highly Oriented, Solid Supported Multi-Lamellar Membranes. Sci. Rep. 2017, 7, 39661. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T. A Comprehensive Review of Our Current Understanding of Red Blood Cell (Rbc) Glycoproteins. Membranes 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.R.; Santa-Maria, A.R.; Mészáros, M.; Veszelka, S.; Dér, A.; Deli, M.A. Surface Charge, Glycocalyx, and Blood-Brain Barrier Function. Tissue Barriers 2021, 9, 1904773. [Google Scholar] [CrossRef]
- Yagi-Utsumi, M.; Itoh, S.G.; Okumura, H.; Yanagisawa, K.; Kato, K.; Nishimura, K. The Double-Layered Structure of Amyloid-Β Assemblage on Gm1-Containing Membranes Catalytically Promotes Fibrillization. ACS Chem. Neurosci. 2023, 14, 2648–2657. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Huang, Y.-X.; Liu, W.-J.; Yuan, Z.-J. Membrane Surface Charge and Morphological and Mechanical Properties of Young and Old Erythrocytes. Curr. Appl. Phys. 2007, 7, e94–e96. [Google Scholar] [CrossRef]
- Peters, D.G.; Connor, J.R.; Meadowcroft, M.D. The Relationship between Iron Dyshomeostasis and Amyloidogenesis in Alzheimer’s Disease: Two Sides of the Same Coin. Neurobiol. Dis. 2015, 81, 49–65. [Google Scholar] [CrossRef]
- Bukara, K.; Jovanić, S.; Drvenica, I.T.; Stančić, A.; Ilić, V.; Rabasović, M.D.; Pantelić, D.; Jelenković, B.; Bugarski, B.; Krmpot, A.J. Mapping of Hemoglobin in Erythrocytes and Erythrocyte Ghosts Using Two Photon Excitation Fluorescence Microscopy. J. Biomed. Opt. 2017, 22, 026003. [Google Scholar] [CrossRef]
- Flores, V.G.; Martínez-Martínez, A.; Pérez, J.A.R.; Bezada, J.A.; Aguirre-Tostado, F.S.; Casillas, P.E.G. Biointeraction of Erythrocyte Ghost Membranes with Gold Nanoparticles Fluorescents. Materials 2021, 14, 6390. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Gill, D. A Micro-Biuret Method for Estimating Proteins. Anal. Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef]
- Watanabe, C.; Seigneuret, M.; Staneva, G.; Puff, N.; Angelova, M.I. On the Possible Structural Role of Single Chain Sphingolipids Sphingosine and Sphingosine 1-Phosphate in the Amyloid-Β Peptide Interactions with Membranes. Consequences for Alzheimer’s Disease Development. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 317–327. [Google Scholar] [CrossRef]
- Staneva, G.; Watanabe, C.; Puff, N.; Yordanova, V.; Seigneuret, M.; Angelova, M.I. Amyloid-Β Interactions with Lipid Rafts in Biomimetic Systems: A Review of Laboratory Methods. In Lipid Rafts: Methods and Protocols; Erhard, B., Ed.; Springer: New York, NY, USA, 2021; pp. 47–86. [Google Scholar]
- Numaguchi, Y.; Tsukakoshi, K.; Takeuchi, N.; Suzuki, Y.; Ikebukuro, K.; Kawano, R. Real-Time Monitoring of the Amyloid Β1–42 Monomer-to-Oligomer Channel Transition Using a Lipid Bilayer System. PNAS Nexus 2024, 3, pgad437. [Google Scholar] [CrossRef] [PubMed]
- Sant, V.; Som, M.; Karkisaval, A.G.; Carnahan, P.; Lal, R. Scavenging Amyloid Oligomers from Neurons with Silica Nanobowls: Implications for Amyloid Diseases. Biophys. J. 2021, 120, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Nirmalraj, P.N.; List, J.; Battacharya, S.; Howe, G.; Xu, L.; Thompson, D.; Mayer, M. Complete Aggregation Pathway of Amyloid Β (1-40) and (1-42) Resolved on an Atomically Clean Interface. Sci. Adv. 2020, 6, eaaz6014. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Luo, J.-C.; Liu, T.-I.; Lu, I.-L.; Shen, M.-Y.; Chuang, C.-Y.; Chern, C.-S.; Chiu, H.-C. Capturing Amyloid-Β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells. Nanomaterials 2020, 10, 1284. [Google Scholar] [CrossRef]
- Watson, D.; Castaño, E.; Kokjohn, T.A.; Kuo, Y.M.; Lyubchenko, Y.; Pinsky, D.; Connolly, E.S.; Esh, C.; Luehrs, D.C.; Stine, W.B.; et al. Physicochemical Characteristics of Soluble Oligomeric Aβ and Their Pathologic Role in Alzheimer’s Disease. Neurol. Res. 2005, 27, 869–881. [Google Scholar] [CrossRef]
- Sehlin, D.; Stina, S.; Bénédicte, B.; Henryk, B.; Gérard, N.B.; Delphine, B.; Hennig, B.; Karl, P.B.; Per, B.; Donna, C.; et al. Engineered Antibodies: New Possibilities for Brain Pet? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative Imaging of Membrane Lipid Order in Cells and Organisms. Nat. Protoc. 2012, 7, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Danovski, G.; Dyankova-Danovska, T.; Stamatov, R.; Aleksandrov, R.; Kanev, P.-B.; Stoynov, S. CellTool: An Open-Source Software Combining Bio-Image Analysis and Mathematical Modeling for the Study of DNA Repair Dynamics. Int. J. Mol. Sci. 2023, 24, 16784. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Castanho, M.A.R.B.; Santos, N.C. Rbpi21 Promotes Lipopolysaccharide Aggregation and Exerts Its Antimicrobial Effects by (Hemi)Fusion of Pg-Containing Membranes. PLoS ONE 2009, 4, e8385. [Google Scholar] [CrossRef]
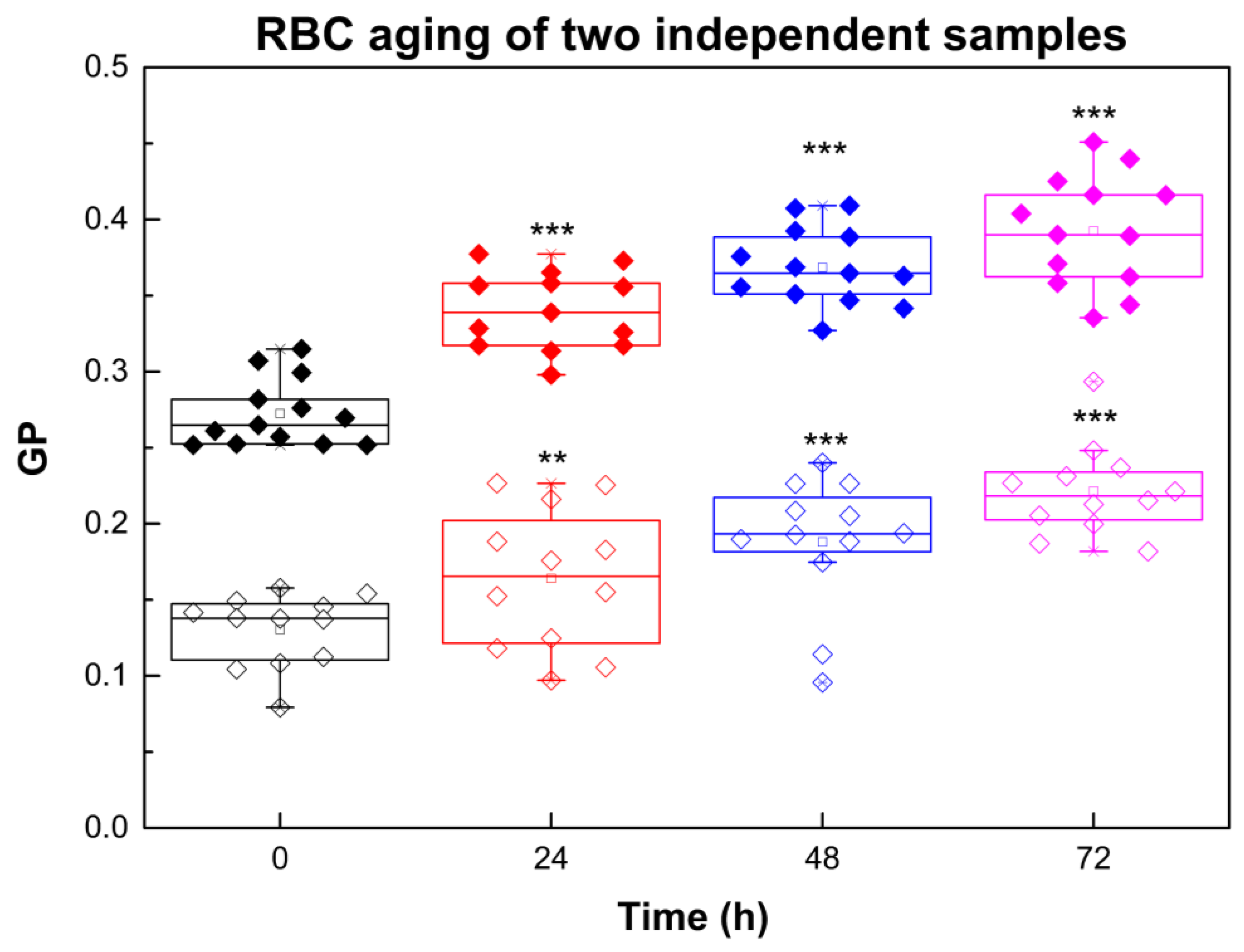
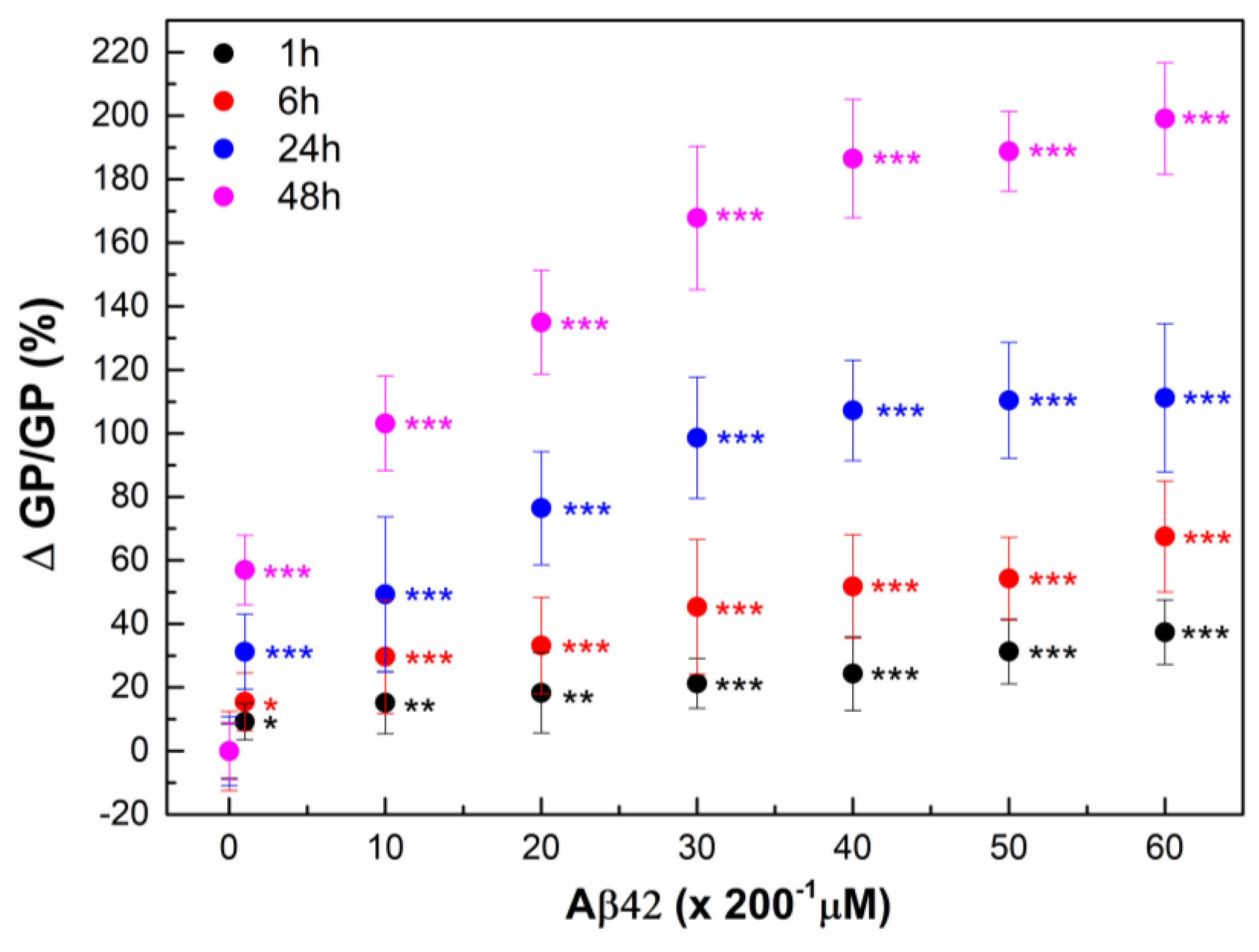
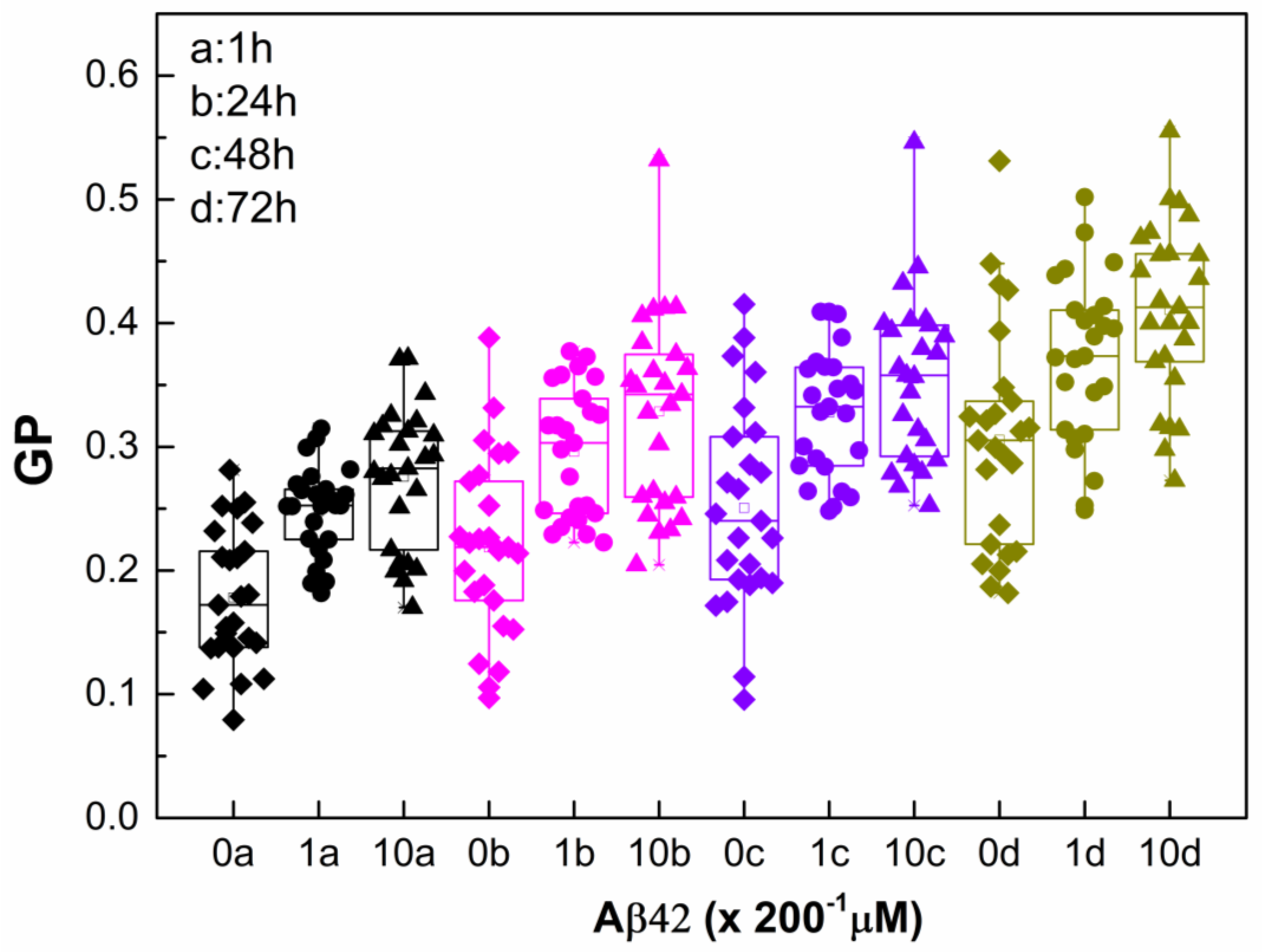
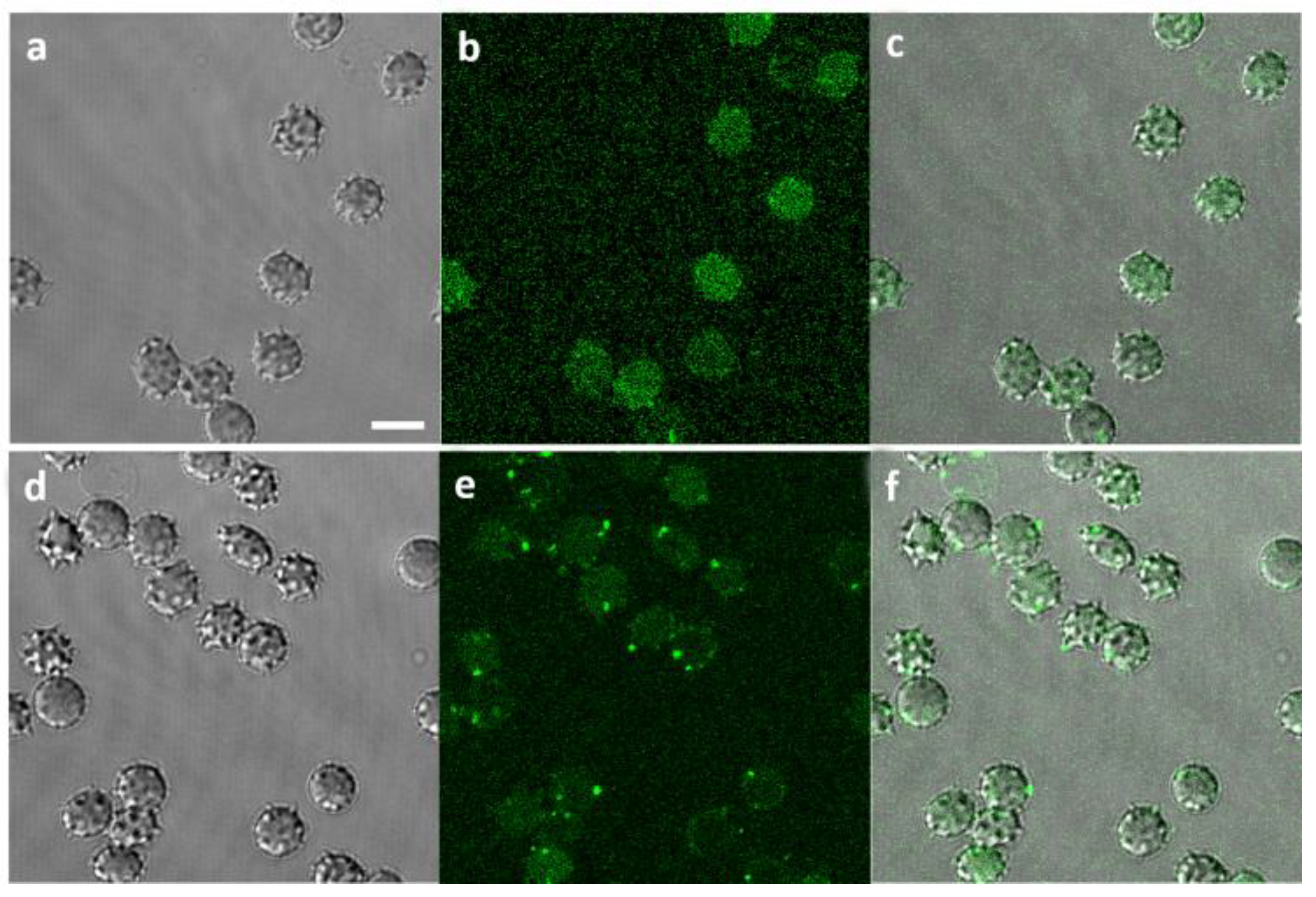

| Sample | ΔGP at 24 h | ΔGP/GP at 24 h (%) | ΔGP at 48 h | ΔGP/GP at 48 h (%) | ΔGP at 72 h | ΔGP/GP at 72 h (%) |
|---|---|---|---|---|---|---|
| Sample 1 | 0.07 ± 0.03 | 25 | 0.09 ± 0.03 | 35 | 0.12 ± 0.03 | 44 |
| Sample 2 | 0.03 ± 0.02 | 26 | 0.06 ± 0.05 | 44 | 0.09 ± 0.03 | 69 |
| Time (h) | Aβ42 (µM) | GP | ΔGP | ΔGP/GP (%) |
|---|---|---|---|---|
| 1 | 0 | 0.1780 | ||
| 1 | 0.2479 | 0.0699 | 39 | |
| 10 | 0.2756 | 0.0976 | 55 | |
| 24 | 0 | 0.2187 | ||
| 1 | 0.2961 | 0.0774 | 35 | |
| 10 | 0.3285 | 0.1098 | 50 | |
| 48 | 0 | 0.2505 | ||
| 1 | 0.3276 | 0.0771 | 31 | |
| 10 | 0.3552 | 0.1047 | 42 | |
| 72 | 0 | 0.3057 | ||
| 1 | 0.3714 | 0.0657 | 21 | |
| 10 | 0.4105 | 0.1048 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staneva, G.; Yordanova, V.; Danailova, A.; Marinovska, A.-M.; Dér, A.; Taneva, S.G. Amyloid β Peptide Modifies Membrane Architecture and Surface Electrostatic Properties of Human Red Blood Cells. Int. J. Mol. Sci. 2025, 26, 11361. https://doi.org/10.3390/ijms262311361
Staneva G, Yordanova V, Danailova A, Marinovska A-M, Dér A, Taneva SG. Amyloid β Peptide Modifies Membrane Architecture and Surface Electrostatic Properties of Human Red Blood Cells. International Journal of Molecular Sciences. 2025; 26(23):11361. https://doi.org/10.3390/ijms262311361
Chicago/Turabian StyleStaneva, Galya, Vesela Yordanova, Avgustina Danailova, Ana-Maria Marinovska, András Dér, and Stefka G. Taneva. 2025. "Amyloid β Peptide Modifies Membrane Architecture and Surface Electrostatic Properties of Human Red Blood Cells" International Journal of Molecular Sciences 26, no. 23: 11361. https://doi.org/10.3390/ijms262311361
APA StyleStaneva, G., Yordanova, V., Danailova, A., Marinovska, A.-M., Dér, A., & Taneva, S. G. (2025). Amyloid β Peptide Modifies Membrane Architecture and Surface Electrostatic Properties of Human Red Blood Cells. International Journal of Molecular Sciences, 26(23), 11361. https://doi.org/10.3390/ijms262311361








