Epithelial–Mesenchymal Transition Increases the Susceptibility of Human A549 Cells to Nanosecond Pulsed Electric Fields
Abstract
1. Introduction
2. Results
2.1. Induction of EMT in A549 Cells by TGF-β1
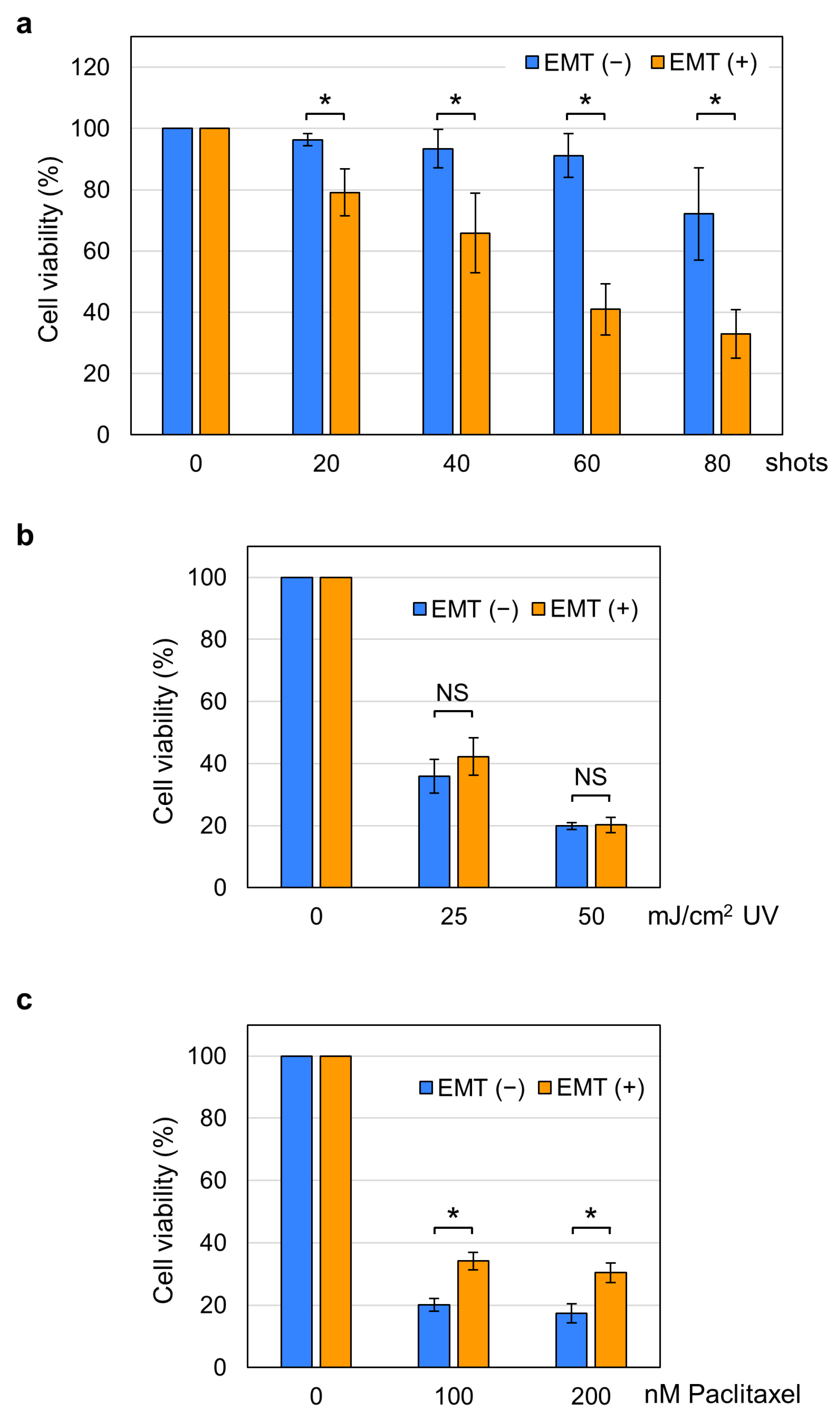
2.2. Increased Susceptibility of EMT Cells to nsPEFs
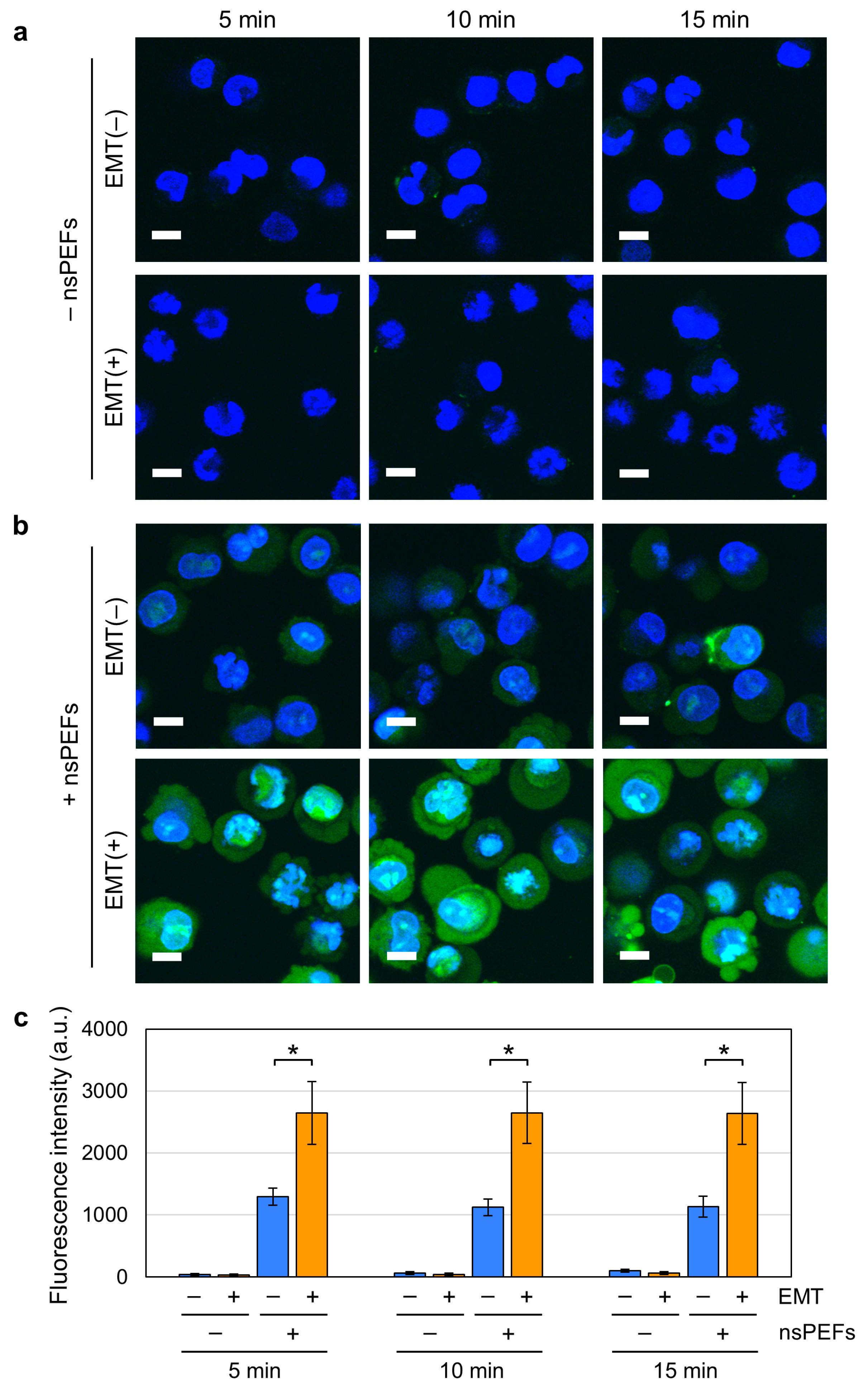
2.3. Increased Nanopore Formation by nsPEFs in EMT Cells
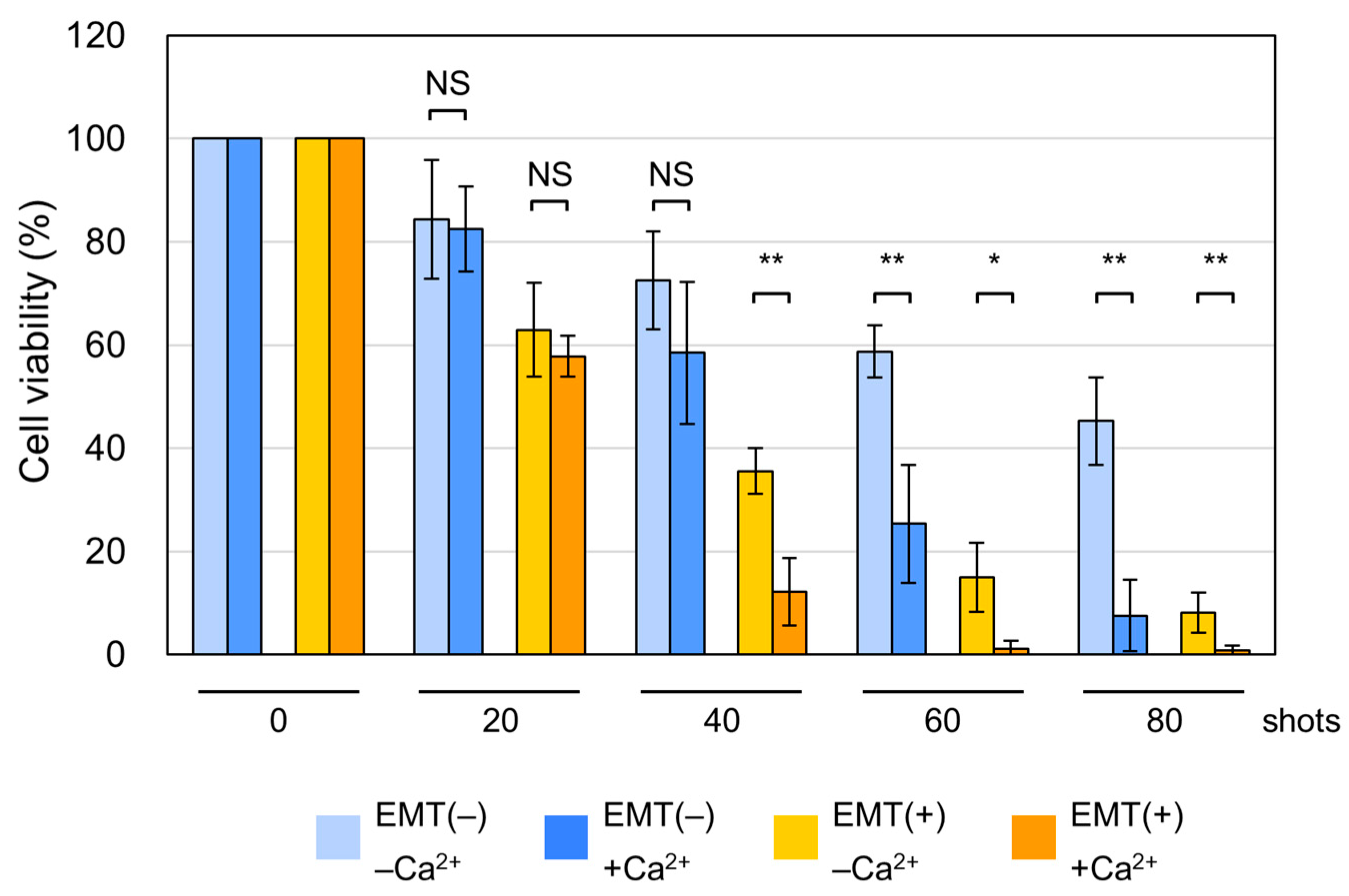
2.4. Induction of Non-Apoptotic Cell Death by nsPEFs in EMT Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and TGF-β1 Treatment
4.2. Generation of nsPEFs
4.3. Exposure of Cells to nsPEFs
4.4. Quantitative RT-PCR
4.5. Fluorescence Microscopy
4.6. Fluorescent Staining of Living Cells
4.7. Fluorescent Staining of Fixed Cells
4.8. YO-PRO-1 Staining
4.9. Cell Viability Assay
4.10. Western Blot Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| nsPEFs | Nanosecond pulsed electric fields |
| EMT | Epithelial–mesenchymal transition |
References
- Batista Napotnik, T.; Rebersek, M.; Vernier, P.T.; Mali, B.; Miklavcic, D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef]
- Ruiz-Fernandez, A.R.; Campos, L.; Gutierrez-Maldonado, S.E.; Nunez, G.; Villanelo, F.; Perez-Acle, T. Nanosecond Pulsed Electric Field (nsPEF): Opening the Biotechnological Pandora’s Box. Int. J. Mol. Sci. 2022, 23, 6158. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Dong, Y.; Alhaskawi, A.; Tu, T.; Hasan Abdullah Ezzi, S.; Goutham Kota, V.; Abdulla, M.H.A.H.; Li, P.; Wu, B.; et al. New advances in treatment of skin malignant tumors with nanosecond pulsed electric field: A literature review. Bioelectrochemistry 2023, 150, 108366. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Gundersen, M.A. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol. 2006, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Bowman, A.M.; Ibey, B.L.; Andre, F.M.; Pakhomova, O.N.; Schoenbach, K.H. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem. Biophys. Res. Commun. 2009, 385, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gianulis, E.; Vernier, P.T.; Semenov, I.; Xiao, S.; Pakhomova, O.N. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim. Biophys. Acta 2015, 1848, 958–966. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Salemi, S.; Craft, C.M.; Gundersen, M.A. Calcium bursts induced by nanosecond electric pulses. Biochem. Biophys. Res. Commun. 2003, 310, 286–295. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J. Biol. Chem. 2004, 279, 22964–22972. [Google Scholar] [CrossRef]
- Sozer, E.B.; Wu, Y.H.; Romeo, S.; Vernier, P.T. Nanometer-Scale Permeabilization and Osmotic Swelling Induced by 5-ns Pulsed Electric Fields. J. Membr. Biol. 2017, 250, 21–30. [Google Scholar] [CrossRef]
- Yang, Q.; Kajimoto, S.; Kobayashi, Y.; Hiramatsu, H.; Nakabayashi, T. Regulation of Cell Volume by Nanosecond Pulsed Electric Fields. J. Phys. Chem. B 2021, 125, 10692–10700. [Google Scholar] [CrossRef]
- Camera, F.; Colantoni, E.; Garcia-Sanchez, T.; Benassi, B.; Consales, C.; Muscat, A.; Vallet, L.; Mir, L.M.; Andre, F.; Merla, C. In Vitro Imaging and Molecular Characterization of Ca2+ Flux Modulation by Nanosecond Pulsed Electric Fields. Int. J. Mol. Sci. 2023, 24, 15616. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Wu, Y.H.; Gundersen, M.A.; Miklavcic, D.; Vernier, P.T. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics 2012, 33, 257–264. [Google Scholar] [CrossRef]
- Malakauskaite, P.; Zelvys, A.; Zinkeviciene, A.; Mickeviciute, E.; Radzeviciute-Valciuke, E.; Malysko-Ptasinske, V.; Lekesyte, B.; Novickij, J.; Kaseta, V.; Novickij, V. Mitochondrial depolarization and ATP loss during high frequency nanosecond and microsecond electroporation. Bioelectrochemistry 2024, 159, 108742. [Google Scholar] [CrossRef]
- Polajzer, T.; Peng, W.; Yao, C.; Miklavcic, D. Changes in Mitochondrial Membrane Potential in In Vitro Electroporation with Nano- and Microsecond Pulses. Bioelectricity 2024, 6, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K. Nanosecond pulsed electric fields activate MAPK pathways in human cells. Arch. Biochem. Biophys. 2011, 515, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Morotomi-Yano, K.; Oyadomari, S.; Akiyama, H.; Yano, K. Nanosecond pulsed electric fields act as a novel cellular stress that induces translational suppression accompanied by eIF2alpha phosphorylation and 4E-BP1 dephosphorylation. Exp. Cell Res. 2012, 318, 1733–1744. [Google Scholar] [CrossRef]
- Rossi, A.; Pakhomova, O.N.; Mollica, P.A.; Casciola, M.; Mangalanathan, U.; Pakhomov, A.G.; Muratori, C. Nanosecond Pulsed Electric Fields Induce Endoplasmic Reticulum Stress Accompanied by Immunogenic Cell Death in Murine Models of Lymphoma and Colorectal Cancer. Cancers 2019, 11, 2034. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.J.; Sain, N.M.; Ren, W. Induction of Cell Death Mechanisms and Apoptosis by Nanosecond Pulsed Electric Fields (nsPEFs). Cells 2013, 2, 136–162. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.W.; Semenov, I.; Pakhomov, A.G. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE 2013, 8, e70278. [Google Scholar] [CrossRef]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K. Nanosecond pulsed electric fields induce poly(ADP-ribose) formation and non-apoptotic cell death in HeLa S3 cells. Biochem. Biophys. Res. Commun. 2013, 438, 557–562. [Google Scholar] [CrossRef]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K. Different involvement of extracellular calcium in two modes of cell death induced by nanosecond pulsed electric fields. Arch. Biochem. Biophys. 2014, 555–556, 47–54. [Google Scholar] [CrossRef]
- Muratori, C.; Pakhomov, A.G.; Gianulis, E.C.; Jensen, S.D.; Pakhomova, O.N. The cytotoxic synergy of nanosecond electric pulses and low temperature leads to apoptosis. Sci. Rep. 2016, 6, 36835. [Google Scholar] [CrossRef]
- Nuccitelli, R.; McDaniel, A.; Anand, S.; Cha, J.; Mallon, Z.; Berridge, J.C.; Uecker, D. Nano-Pulse Stimulation is a physical modality that can trigger immunogenic tumor cell death. J. Immunother. Cancer 2017, 5, 32. [Google Scholar] [CrossRef]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640. [Google Scholar] [CrossRef]
- Mazzarda, F.; Chittams-Miles, A.E.; Pittaluga, J.; Sozer, E.B.; Vernier, P.T.; Muratori, C. Inflammasome Activation and IL-1beta Release Triggered by Nanosecond Pulsed Electric Fields in Murine Innate Immune Cells and Skin. J. Immunol. 2024, 212, 335–345. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Huynh, J.; Lui, K.; Wood, R.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanoelectroablation of human pancreatic carcinoma in a murine xenograft model without recurrence. Int. J. Cancer 2013, 132, 1933–1939. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Jiang, J.; Wu, L.; Yin, S.; Miao, X.; Swanson, R.J.; Zheng, S. Nano-pulse stimulation (NPS) ablate tumors and inhibit lung metastasis on both canine spontaneous osteosarcoma and murine transplanted hepatocellular carcinoma with high metastatic potential. Oncotarget 2017, 8, 44032–44039. [Google Scholar] [CrossRef]
- Ross, A.S.; Schlesinger, T.; Harmon, C.B.; Moy, R.L.; Rohrer, T.E.; Mehregan, D.R.; Nuccitelli, R.; Johnston, L.J.; Knape, W.A. Multicenter, prospective feasibility study of Nano-Pulse Stimulation technology for the treatment of both nodular and superficial low-risk basal cell carcinoma. Front. Oncol. 2022, 12, 1044694. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, W.; Xu, D.; Dong, G.; Ren, Z.; Aji, T.; Ji, J.; Zhao, Q.; Pan, J.; Chen, X.; et al. Nanosecond pulsed electric field ablation as first-line curative therapy for hepatocellular carcinoma in high-risk locations a prospective multicenter. Int. J. Surg. 2025, 111, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Ghafoor, S.; Garcia, E.; Jay, D.J.; Persad, S. Molecular Mechanisms Regulating Epithelial Mesenchymal Transition (EMT) to Promote Cancer Progression. Int. J. Mol. Sci. 2025, 26, 4364. [Google Scholar] [CrossRef] [PubMed]
- Skrypek, N.; Goossens, S.; De Smedt, E.; Vandamme, N.; Berx, G. Epithelial-to-Mesenchymal Transition: Epigenetic Reprogramming Driving Cellular Plasticity. Trends Genet. 2017, 33, 943–959. [Google Scholar] [CrossRef]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial-mesenchymal transition and its transcription factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.; Nabi, I.R. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PLoS ONE 2015, 10, e0119954. [Google Scholar] [CrossRef]
- Ilnitskaya, A.S.; Litovka, N.I.; Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. Actin Cytoskeleton Remodeling Accompanied by Redistribution of Adhesion Proteins Drives Migration of Cells in Different EMT States. Cells 2024, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, H.; Lee, S.; Youn, H.; Youn, B. Role of Metabolic Reprogramming in Epithelial(-)Mesenchymal Transition (EMT). Int. J. Mol. Sci. 2019, 20, 2042. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef]
- Amack, J.D. Cellular dynamics of EMT: Lessons from live in vivo imaging of embryonic development. Cell Commun. Signal. 2021, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Allen, J.T.; Mason, R.M.; Kamimura, T.; Zhang, Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir. Res. 2005, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, P.J.; Ebner, R.; Lopez, A.R.; Derynck, R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J. Cell Biol. 1994, 127, 2021–2036. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Yu, Y.; Xue, H.; Shen, R.; Zhang, Y.; Ding, J. Effects of cell shape and nucleus shape on epithelial-mesenchymal transition revealed using chimeric micropatterns. Biomaterials 2025, 317, 123013. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell. Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Germain, M.; Affar, E.B.; D’Amours, D.; Dixit, V.M.; Salvesen, G.S.; Poirier, G.G. Cleavage of automodified poly(ADP-ribose) polymerase during apoptosis. Evidence for involvement of caspase-7. J. Biol. Chem. 1999, 274, 28379–28384. [Google Scholar] [PubMed]
- Palchaudhuri, R.; Lambrecht, M.J.; Botham, R.C.; Partlow, K.C.; van Ham, T.J.; Putt, K.S.; Nguyen, L.T.; Kim, S.H.; Peterson, R.T.; Fan, T.M.; et al. A Small Molecule that Induces Intrinsic Pathway Apoptosis with Unparalleled Speed. Cell Rep. 2015, 13, 2027–2036. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.; Semenov, I.; Pakhomov, A.G. Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim. Biophys. Acta 2014, 1838, 2547–2554. [Google Scholar] [CrossRef]
- Janzen, C.; Sen, S.; Lei, M.Y.; Gagliardi de Assumpcao, M.; Challis, J.; Chaudhuri, G. The Role of Epithelial to Mesenchymal Transition in Human Amniotic Membrane Rupture. J. Clin. Endocrinol. Metab. 2017, 102, 1261–1269. [Google Scholar] [CrossRef]
- Gracia, M.; Theis, S.; Proag, A.; Gay, G.; Benassayag, C.; Suzanne, M. Mechanical impact of epithelial-mesenchymal transition on epithelial morphogenesis in Drosophila. Nat. Commun. 2019, 10, 2951. [Google Scholar] [CrossRef]
- Cantu, J.C.; Tarango, M.; Beier, H.T.; Ibey, B.L. The biological response of cells to nanosecond pulsed electric fields is dependent on plasma membrane cholesterol. Biochim. Biophys. Acta 2016, 1858, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Awasthi, K.; Chang, F.-L.; Wu, T.-E.; Hsu, H.-Y.; Ohta, N. Modulation of calcium signaling by nanosecond electric pulses and cell death through apoptosis in A549 lung cancerous cells. Sens. Actuators B Chem. 2022, 369, 132348. [Google Scholar] [CrossRef]
- Pellaud, J.; Schote, U.; Arvinte, T.; Seelig, J. Conformation and self-association of human recombinant transforming growth factor-beta3 in aqueous solutions. J. Biol. Chem. 1999, 274, 7699–7704. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsui, M.; Morotomi-Yano, K.; Yano, K.-i. Epithelial–Mesenchymal Transition Increases the Susceptibility of Human A549 Cells to Nanosecond Pulsed Electric Fields. Int. J. Mol. Sci. 2025, 26, 11360. https://doi.org/10.3390/ijms262311360
Mitsui M, Morotomi-Yano K, Yano K-i. Epithelial–Mesenchymal Transition Increases the Susceptibility of Human A549 Cells to Nanosecond Pulsed Electric Fields. International Journal of Molecular Sciences. 2025; 26(23):11360. https://doi.org/10.3390/ijms262311360
Chicago/Turabian StyleMitsui, Manato, Keiko Morotomi-Yano, and Ken-ichi Yano. 2025. "Epithelial–Mesenchymal Transition Increases the Susceptibility of Human A549 Cells to Nanosecond Pulsed Electric Fields" International Journal of Molecular Sciences 26, no. 23: 11360. https://doi.org/10.3390/ijms262311360
APA StyleMitsui, M., Morotomi-Yano, K., & Yano, K.-i. (2025). Epithelial–Mesenchymal Transition Increases the Susceptibility of Human A549 Cells to Nanosecond Pulsed Electric Fields. International Journal of Molecular Sciences, 26(23), 11360. https://doi.org/10.3390/ijms262311360




