CCN2/CTGF-Driven Myocardial Fibrosis and NT-proBNP Synergy as Predictors of Mortality in Maintenance Hemodialysis
Abstract
1. Introduction
2. Results
2.1. Primary Analyses: Evaluation of the Clinical Candidate Predictors
2.2. Univariate Cox Regression Analyses of Mortality Predictors
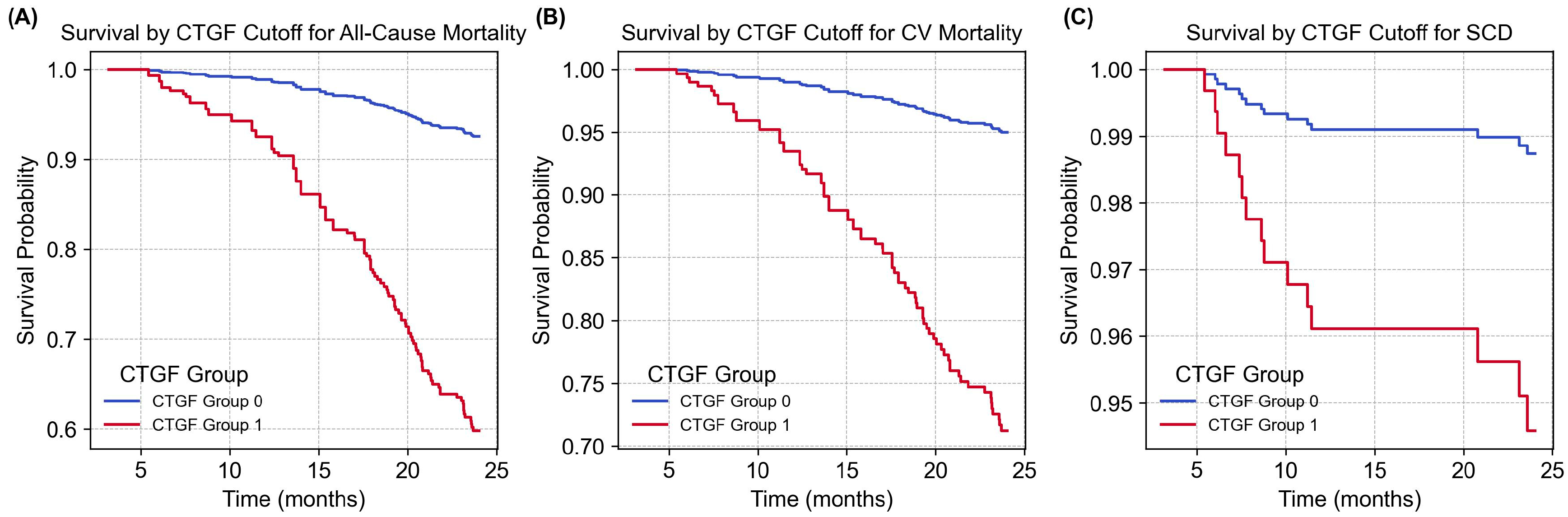
2.3. Multivariate Cox Regression and Survival Analysis
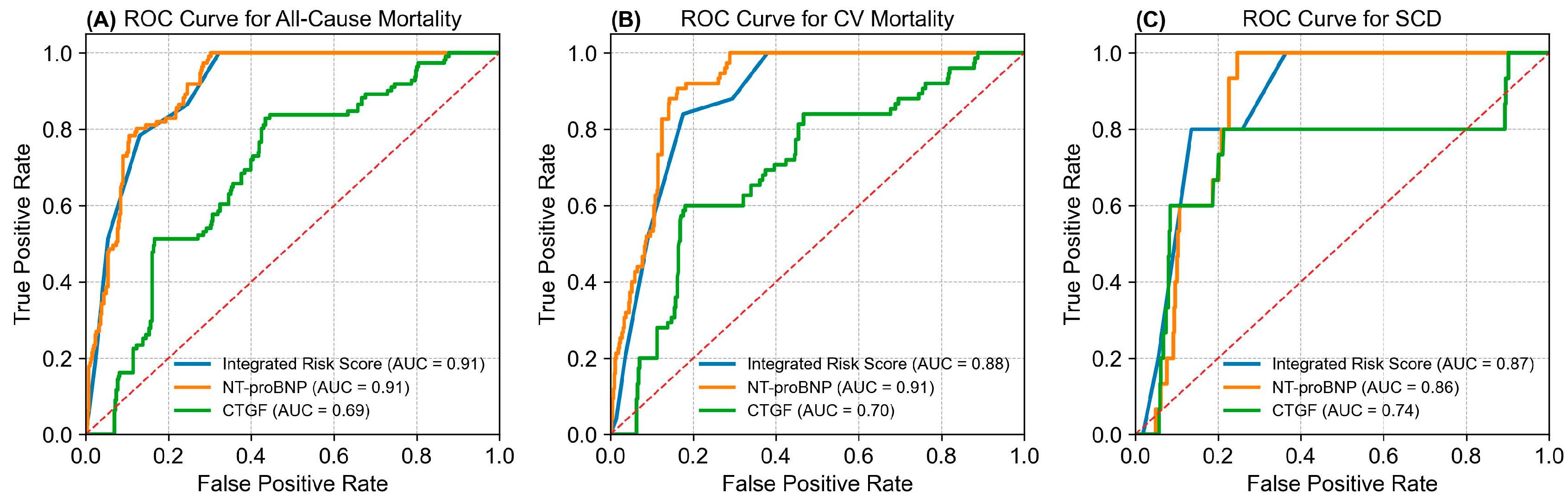
2.4. Integrated Risk Score Model Summary
3. Discussion
4. Materials and Methods
4.1. Participants in the Cohort
4.2. Assessment of Exposures
4.3. Assessment of Covariates
4.4. Ascertainment of Outcomes
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Regidor, D.L.; Kovesdy, C.P.; Van Wyck, D.; Bunnapradist, S.; Horwich, T.B.; Fonarow, G.C. Fluid Retention Is Associated with Cardiovascular Mortality in Patients Undergoing Long-Term Hemodialysis. Circulation 2009, 119, 671–679. [Google Scholar] [CrossRef]
- Hijazi, Z.; Oldgren, J.; Lindbäck, J.; Alexander, J.H.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Held, C.; Hylek, E.M.; Lopes, R.D.; et al. A biomarker-based risk score to predict death in patients with atrial fibrillation: The ABC (age, biomarkers, clinical history) death risk score. Eur. Hear. J. 2017, 39, 477–485. [Google Scholar] [CrossRef]
- Lopez, T.; Banerjee, D. Management of Fluid Overload in Hemodialysis Patients. Kidney Int. 2021, 100, 1170–1173. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Kamal, O.M.M.; Hassan, M.S.; Khalifa, M.M.M. Interdialytic Weight Gain and Its Relation to Outcome among Patients on Maintenance Hemodialysis. QJM Int. J. Med. 2020, 113, pi123. [Google Scholar] [CrossRef]
- Chang, J.F.; Wu, C.C.; Hsieh, C.Y.; Li, Y.Y.; Wang, T.M.; Liou, J.C. A Joint Evaluation of Impaired Cardiac Sympathetic Responses and Malnutrition-Inflammation Cachexia for Mortality Risks in Hemodialysis Patients. Front. Med. 2020, 27, 7. [Google Scholar] [CrossRef]
- Mitsas, A.C.; Elzawawi, M.; Mavrogeni, S.; Boekels, M.; Khan, A.; Eldawy, M.; Stamatakis, I.; Kouris, D.; Daboul, B.; Gunkel, O.; et al. Heart Failure and Cardiorenal Syndrome: A Narrative Review on Pathophysiology, Diagnostic and Therapeutic Regimens—From a Cardiologist’s View. J. Clin. Med. 2022, 11, 7041. [Google Scholar] [CrossRef]
- Chava, R.; Rosemarijn, B.; Evans, M.; Olof, H.; Snead, C.M.; Caskey, F.; Torino, C.; Porto, G.; Szymczak, M.; Krajewska, M.; et al. Predicting Kidney Failure, Cardiovascular Disease and Death in Advanced CKD Patients. Kidney Int. Rep. 2022, 7, 2230–2241. [Google Scholar]
- Hunt, K.J.; Jaffa, M.A.; Garrett, S.M.; Luttrell, D.K.; Lipson, K.E.; Lopes-Virella, M.F.; Luttrell, L.M.; Jaffa, A.A.; VADT Investigators. Plasma Connective Tissue Growth Factor (CTGF/CCN2) Levels Predict Myocardial Infarction in the Veterans Affairs Diabetes Trial (VADT) Cohort. Diabetes Care 2018, 41, 840–846. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Fu, Y.-C.; Wu, M.-J. NT-ProBNP Predicts Total Mortality, Emergency Department Visits, Hospitalization, Intensive-Care Unit Admission, and Cardiovascular Events in Hemodialysis Patients. J. Clin. Med. 2019, 8, 238. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.-K.; Zhang, G.; Zhang, B.-T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef]
- Ren, M.; Yao, S.; Chen, T.; Luo, H.; Tao, X.; Jiang, H.; Yang, X.; Zhang, H.; Yu, S.; Wang, Y.; et al. Connective Tissue Growth Factor: Regulation, Diseases, and Drug Discovery. Int. J. Mol. Sci. 2024, 25, 4692. [Google Scholar] [CrossRef] [PubMed]
- Vainio, L.E.; Szabó, Z.; Lin, R.; Ulvila, J.; Yrjölä, R.; Alakoski, T.; Piuhola, J.; Koch, W.J.; Ruskoaho, H.; Fouse, S.D.; et al. Connective Tissue Growth Factor Inhibition Enhances Cardiac Repair and Limits Fibrosis After Myocardial Infarction. JACC Basic Transl. Sci. 2019, 4, 83–94. [Google Scholar] [CrossRef]
- Watanabe, K.; Sato, E.; Mishima, E.; Miyazaki, M.; Tanaka, T. What’s New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances. Int. J. Mol. Sci. 2023, 24, 570. [Google Scholar] [CrossRef] [PubMed]
- Behnes, M.; Brueckmann, M.; Lang, S.; Weiß, C.; Ahmad-Nejad, P.; Neumaier, M.; Borggrefe, M.; Hoffmann, U. Connective tissue growth factor (CTGF/CCN2): Diagnostic and prognostic value in acute heart failure. Clin. Res. Cardiol. 2013, 103, 107–116. [Google Scholar] [CrossRef]
- Hoedt, C.H.D.; van Gelder, M.K.; Grooteman, M.P.; Nubé, M.J.; Blankestijn, P.J.; Goldschmeding, R.; Kok, R.J.; Bots, M.L.; Dorpel, M.A.v.D.; Gerritsen, K.G.F. Connective Tissue Growth Factor Is Related to All-cause Mortality in Hemodialysis Patients and Is Lowered by On-line Hemodiafiltration: Results from the Convective Transport Study. Toxins 2019, 11, 268. [Google Scholar] [CrossRef]
- Gallo, G.; Lanza, O.; Savoia, C. New Insight in Cardiorenal Syndrome: From Biomarkers to Therapy. Int. J. Mol. Sci. 2023, 24, 5089. [Google Scholar] [CrossRef]
- Goffredo, G.; Barone, R.; Di Terlizzi, V.; Correale, M.; Brunetti, N.D.; Iacoviello, M. Biomarkers in cardiorenal syndrome. J. Clin. Med. 2021, 10, 3433. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Liu, P.; Sawhney, S.; Heide-Jørgensen, U.; Quinn, R.R.; Jensen, S.K.; Mclean, A.; Christiansen, C.F.; Gerds, T.A.; Ravani, P. Predicting the risks of kidney failure and death in adults with moderate to severe chronic kidney disease: Multinational, longitudinal, population based, cohort study. BMJ 2024, 385, e078063. [Google Scholar] [CrossRef]
- Chang, J.F.; Chen, P.C.; Hsieh, C.Y.; Liou, J.C. A Growth Differentiation Factor 15-Based Risk Score Model to Predict Mortality in Hemodialysis Patients. Diagnostics 2021, 11, 286. [Google Scholar] [CrossRef]
- Ravizza, S.; Huschto, T.; Adamov, A.; Böhm, L.; Büsser, A.; Flöther, F.F.; Hinzmann, R.; König, H.; McAhren, S.M.; Robertson, D.H.; et al. Predicting the early risk of chronic kidney disease in patients with diabetes using real-world data. Nat. Med. 2019, 25, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Leask, A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010, 106, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β and fibrosis. World J. Gastroenterol. 2007, 13, 3056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Abraham, D.J.; Shi-Wen, X.; Pearson, J.D.; Black, C.M.; Lyons, K.M.; Leask, A. CCN2 (Connective Tissue Growth Factor) Promotes Fibroblast Adhesion to Fibronectin. Mol. Biol. Cell 2004, 15, 5635–5646. [Google Scholar] [CrossRef]
- Wu, S.; Platteau, A.; Chen, S.; McNamara, G.; Whitsett, J.A.; Bancalari, E. Conditional Overexpression of Connective Tissue Growth Factor Disrupts Postnatal Lung Development. Am. J. Respir. Cell Mol. Biol. 2010, 42, 552–563. [Google Scholar] [CrossRef]
- Liang, F.; Gardner, D.G. Mechanical strain activates BNP gene transcription through a p38/NF-κB–dependent mechanism. J. Clin. Investig. 1999, 104, 1603–1612. [Google Scholar] [CrossRef]
- Takahashi, T.; Allen, P.D.; Izumo, S. Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles. Correlation with expression of the Ca(2+)-ATPase gene. Circ. Res. 1992, 71, 9–17. [Google Scholar] [CrossRef]
- Takimoto, E.; Kass, D.A. Role of Oxidative Stress in Cardiac Hypertrophy and Remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef]
- Khan, S.Q.; Ng, K.; Dhillon, O.; Kelly, D.; Quinn, P.; Squire, I.B.; Davies, J.E.; Ng, L.L. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur. Heart J. 2009, 30, 1057–1065. [Google Scholar] [CrossRef]
- Pollmann, D.L.; Knott, J.D.; Carvalho, P.E.P.; Apple, F.S.; Jaffe, A.S.; Sandoval, Y. Cardiac Biomarkers for the Prediction, Discrimination, and Risk-Stratification of Type 2 Myocardial Infarction: A Critical Appraisal. Am. J. Med. 2025, 138, 1338–1345. [Google Scholar] [CrossRef]
- Yoon, S.J.; Kwon, W.; Lee, O.J.; Jung, J.H.; Shin, Y.C.; Lim, C.S.; Kim, H.; Jang, J.Y.; Shin, S.H.; Heo, J.S.; et al. External validation of risk prediction platforms for pancreatic fistula after pancreatoduodenectomy using nomograms and artificial intelligence. Ann. Surg. Treat. Res. 2022, 102, 147. [Google Scholar] [CrossRef] [PubMed]
- Loutati, R.; Ben-Yehuda, A.; Rosenberg, S.; Rottenberg, Y. Multimodal Machine Learning for Prediction of 30-Day Readmission Risk in Elderly Population. Am. J. Med. 2024, 137, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, J.; Han, C.; Ai, Z. The Application of Machine Learning in Predicting Mortality Risk in Patients with Severe Femoral Neck Fractures: Prediction Model Development Study. JMIR Bioinform. Biotechnol. 2022, 3, e38226. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Jang, S.Y.; Adler, E.; Ahmad, F.; Campagnari, C.; Yagil, A.; Greenberg, B. A machine learning-derived risk score predicts mortality in East Asian patients with acute heart failure. Eur. J. Heart Fail. 2023, 25, 2331–2333. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF—β signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Liu, X.; Gai, Y.; Liu, F.; Gao, W.; Zhang, Y.; Xu, M.; Li, Z. Trimetazidine inhibits pressure overload-induced cardiac fibrosis through NADPH oxidase–ROS–CTGF pathway. Cardiovasc. Res. 2010, 88, 150–158. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Ko, W.-C.; Choy, C.-S.; Lin, W.-N.; Chang, S.-W.; Liou, J.-C.; Tung, T.-H.; Hsieh, C.-Y.; Chang, J.-F. Galectin-3 Interacts with Vascular Cell Adhesion Molecule-1 to Increase Cardiovascular Mortality in Hemodialysis Patients. J. Clin. Med. 2018, 7, 300. [Google Scholar] [CrossRef]
- Dai, X.; Gil, G.F.; Reitsma, M.B.; Ahmad, N.S.; Anderson, J.A.; Bisignano, C.; Carr, S.; Feldman, R.; Hay, S.I.; He, J.; et al. Health effects associated with smoking: A Burden of Proof study. Nat. Med. 2022, 28, 2045–2055. [Google Scholar] [CrossRef]
- Sweeney, C.; Pharithi, R.B.; Kerr, B.; Ryan, C.; Ryan, F.; Collins, L.; Halley, C.; Barrett, M.; Watson, C.J.; McDonald, K.; et al. NT-proBNP/BNP ratio for prognostication in European Caucasian patients enrolled in a heart failure prevention programme. ESC Heart Fail. 2021, 8, 5081–5091. [Google Scholar] [CrossRef]
| Variables | CTGF ≤ 30.2 ng/mL | CTGF > 30.2 ng/mL | p-Value |
|---|---|---|---|
| Diabetes Mellitus (n, %) | 29 (35.8) | 47 (54.0) | 0.020 |
| Cardiovascular Diseases (n, %) | 28 (34.6) | 49 (56.3) | 0.005 |
| Hypertension (n, %) | 37 (45.7) | 53 (60.9) | 0.063 |
| Smoking (n, %) | 14 (17.3) | 19 (21.8) | 0.561 |
| All-cause Mortality (n, %) | 6 (7.4) | 31 (35.6) | <0.001 |
| Cardiovascular Death (n, %) | 4 (4.9) | 21 (24.1) | <0.001 |
| Age (years ± std) | 64.2 ± 6.9 | 73.6 ± 8.6 | <0.001 |
| Hemodialysis Vintage (months ± std) | 66.6 ± 55.7 | 77.6 ± 42.4 | 0.150 |
| Systolic Blood Pressure (mmHg ± std) | 135.7 ± 21.1 | 138.8 ± 23.3 | 0.376 |
| Diastolic Blood Pressure (mmHg ± std) | 77.6 ± 10.0 | 78.4 ± 13.1 | 0.640 |
| NT-ProBNP (pg/mL ± std) | 475.7 ± 270.9 | 828.9 ± 370.9 | <0.001 |
| Albumin (g/dL ± std) | 3.9 ± 0.4 | 3.8 ± 0.5 | 0.086 |
| Alanine Aminotransferase (IU/L ± std) | 12.6 ± 8.3 | 17.0 ± 14.3 | 0.016 |
| Total Cholesterol (mg/dL ± std) | 191.5 ± 50.2 | 190.1 ± 47.9 | 0.853 |
| Triglycerides (mg/dL ± std) | 243.4 ± 209.2 | 180.6 ± 156.4 | 0.028 |
| Blood Urea Nitrogen (mg/dL ± std) | 55.0 ± 17.0 | 63.0 ± 19.3 | 0.005 |
| Creatinine (mg/dL ± std) | 9.6 ± 2.1 | 10.1 ± 1.6 | 0.103 |
| Blood Glucose (mg/dL ± std) | 126.7 ± 43.5 | 141.2 ± 72.2 | 0.120 |
| Uric Acid (mg/dL ± std) | 7.3 ± 1.4 | 7.3 ± 1.1 | 0.813 |
| Potassium (mmol/L ± std) | 4.6 ± 0.9 | 4.4 ± 0.8 | 0.172 |
| Calcium (mg/dL ± std) | 9.2 ± 0.7 | 9.2 ± 0.7 | 0.811 |
| Phosphate (mg/dL ± std) | 4.4 ± 1.6 | 4.9 ± 1.5 | 0.062 |
| Intact Parathyroid Hormone (pg/mL ± std) | 229.3 ± 264.4 | 217.6 ± 220.3 | 0.755 |
| Hematocrit (%) ± std | 31.6 ± 3.9 | 31.9 ± 3.4 | 0.624 |
| Platelet Count (k/μL ± std) | 199.1 ± 69.6 | 197.6 ± 61.7 | 0.886 |
| high-sensitivity C-Reactive Protein (mg/L ± std) | 1.0 ± 0.4 | 1.7 ± 0.8 | <0.001 |
| All-Cause Mortality HR (95% CI) | p-Value | Cardiovascular Mortality HR (95% CI) | p-Value | Sudden Cardiac Death HR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Male | 0.836 (0.438–1.597) | p = 0.588 | 1.070 (0.488–2.345) | p = 0.866 | 0.676 (0.113–4.044) | p = 0.667 |
| Diabetes Mellitus | 2.653 (1.350–5.214) | p = 0.005 | 3.007 (1.296–6.973) | p = 0.010 | 5.177 (0.577–46.432) | p = 0.142 |
| Cardiovascular Diseases | 1.616 (0.846–3.086) | p = 0.146 | 2.900 (1.251–6.723) | p = 0.013 | 5.059 (0.565–45.301) | p = 0.147 |
| Hypertension | 1.082 (0.567–2.067) | p = 0.810 | 1.630 (0.720–3.690) | p = 0.241 | 1.322 (0.221–7.918) | p = 0.760 |
| Smoking | 2.143 (1.057–4.347) | p = 0.035 | 3.302 (1.479–7.370) | p = 0.004 | 6.656 (1.106–40.053) | p = 0.038 |
| CTGF (ng/mL) | 1.014 (1.005–1.022) | p = 0.002 | 1.015 (1.006–1.025) | p = 0.002 | 1.020 (1.001–1.040) | p = 0.042 |
| Age (years) | 1.065 (1.026–1.105) | p = 0.001 | 1.071 (1.023–1.121) | p = 0.003 | 1.273 (1.051–1.541) | p = 0.014 |
| Hemodialysis Vintage (months) | 1.007 (1.001–1.012) | p = 0.014 | 1.004 (0.997–1.011) | p = 0.232 | 0.998 (0.981–1.017) | p = 0.869 |
| Systolic Blood Pressure (mmHg) | 1.011 (0.997–1.026) | p = 0.135 | 1.023 (1.004–1.041) | p = 0.014 | 1.021 (0.980–1.063) | p = 0.322 |
| Diastolic Blood Pressure (mmHg) | 0.975 (0.948–1.004) | p = 0.086 | 0.979 (0.946–1.014) | p = 0.240 | 0.971 (0.900–1.048) | p = 0.452 |
| NT-ProBNP (pg/mL) | 1.003 (1.002–1.004) | p < 0.001 | 1.004 (1.003–1.005) | p < 0.001 | 1.003 (1.001–1.005) | p = 0.007 |
| Albumin (g/dL) | 0.191 (0.090–0.408) | p < 0.001 | 0.390 (0.153–0.997) | p = 0.049 | 0.586 (0.079–4.348) | p = 0.601 |
| Alanine Aminotransferase (IU/L) | 1.015 (0.993–1.038) | p = 0.181 | 1.021 (0.996–1.046) | p = 0.100 | 1.028 (0.980–1.078) | p = 0.258 |
| Total Cholesterol (mg/dL) | 0.996 (0.990–1.003) | p = 0.278 | 0.998 (0.990–1.006) | p = 0.662 | 0.995 (0.976–1.014) | p = 0.590 |
| Triglycerides (mg/dL) | 0.998 (0.995–1.000) | p = 0.108 | 0.997 (0.993–1.001) | p = 0.106 | 0.990 (0.975–1.005) | p = 0.208 |
| Blood Urea Nitrogen (mg/dL) | 1.009 (0.993–1.026) | p = 0.270 | 1.011 (0.991–1.031) | p = 0.300 | 0.982 (0.934–1.033) | p = 0.486 |
| Creatinine (mg/dL) | 1.027 (0.866–1.216) | p = 0.762 | 1.119 (0.909–1.379) | p = 0.289 | 0.678 (0.439–1.049) | p = 0.081 |
| Blood Glucose (mg/dL) | 1.002 (0.997–1.007) | p = 0.436 | 1.004 (0.998–1.009) | p = 0.168 | 0.999 (0.985–1.014) | p = 0.936 |
| Uric Acid (mg/dL) | 1.085 (0.848–1.388) | p = 0.516 | 1.091 (0.809–1.472) | p = 0.568 | 0.754 (0.355–1.605) | p = 0.464 |
| Potassium (mmol/L) | 0.740 (0.504–1.088) | p = 0.125 | 0.689 (0.430–1.105) | p = 0.122 | 0.728 (0.253–2.095) | p = 0.556 |
| Calcium (mg/dL) | 0.869 (0.548–1.377) | p = 0.549 | 0.672 (0.372–1.212) | p = 0.186 | 0.429 (0.098–1.867) | p = 0.259 |
| Phosphate (mg/dL) | 1.100 (0.910–1.330) | p = 0.325 | 1.050 (0.823–1.341) | p = 0.693 | 0.442 (0.193–1.013) | p = 0.054 |
| Intact Parathyroid Hormone (pg/mL) | 1.001 (1.000–1.002) | p = 0.101 | 1.000 (0.999–1.002) | p = 0.748 | 0.997 (0.990–1.004) | p = 0.409 |
| Hematocrit (%) | 1.032 (0.933–1.141) | p = 0.538 | 1.025 (0.906–1.159) | p = 0.700 | 1.254 (0.943–1.667) | p = 0.119 |
| Platelet Count (k/uL) | 1.004 (0.998–1.009) | p = 0.173 | 1.007 (1.001–1.013) | p = 0.029 | 1.002 (0.989–1.015) | p = 0.746 |
| high-sensitivity C-Reactive Protein (mg/L) | 3.888 (2.755–5.486) | p < 0.001 | 3.617 (2.366–5.530) | p < 0.001 | 3.405 (1.296–8.948) | p = 0.013 |
| All-Cause Mortality aHR (95% CI and p-Value) | Cardiovascular Mortality aHR (95% CI and p-Value) | |||
|---|---|---|---|---|
| Model 1 | ||||
| CTGF (ng/mL) | 1.012 (1.000–1.023) | p = 0.045 | 1.018 (1.004–1.032) | p = 0.010 |
| NT-ProBNP (pg/mL) | 1.003 (1.002–1.004) | p < 0.001 | 1.004 (1.003–1.005) | p < 0.001 |
| Albumin (g/dL) | 0.286 (0.133–0.616) | p = 0.001 | 0.608 (0.233–1.584) | p = 0.309 |
| Model 2 | ||||
| CTGF (ng/mL) | 1.014 (1.003–1.026) | p = 0.014 | 1.019 (1.006–1.033) | p = 0.006 |
| NT-ProBNP (pg/mL) | 1.003 (1.002–1.004) | p < 0.001 | 1.004 (1.003–1.005) | p < 0.001 |
| Diabetes Mellitus | 1.751 (0.875–3.505) | p = 0.113 | 1.889 (0.796–4.484) | p = 0.149 |
| Model 3 | ||||
| CTGF (ng/mL) | 1.014 (1.003–1.026) | p = 0.016 | 1.018 (1.005–1.032) | p = 0.008 |
| NT-ProBNP (pg/mL) | 1.003 (1.002–1.004) | p < 0.001 | 1.004 (1.003–1.005) | p < 0.001 |
| Hemodialysis Vintage (months) | 1.004 (0.997–1.011) | p = 0.236 | 1.000 (0.992–1.009) | p = 0.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, W.-C.; Chen, C.-S.; Chang, Y.-P.; Wu, C.-S.; Yang, H.-C.; Chang, J.-F. CCN2/CTGF-Driven Myocardial Fibrosis and NT-proBNP Synergy as Predictors of Mortality in Maintenance Hemodialysis. Int. J. Mol. Sci. 2025, 26, 11350. https://doi.org/10.3390/ijms262311350
Ko W-C, Chen C-S, Chang Y-P, Wu C-S, Yang H-C, Chang J-F. CCN2/CTGF-Driven Myocardial Fibrosis and NT-proBNP Synergy as Predictors of Mortality in Maintenance Hemodialysis. International Journal of Molecular Sciences. 2025; 26(23):11350. https://doi.org/10.3390/ijms262311350
Chicago/Turabian StyleKo, Wen-Chin, Che-Shao Chen, Yi-Ping Chang, Chi-Sheng Wu, Hung-Chi Yang, and Jia-Feng Chang. 2025. "CCN2/CTGF-Driven Myocardial Fibrosis and NT-proBNP Synergy as Predictors of Mortality in Maintenance Hemodialysis" International Journal of Molecular Sciences 26, no. 23: 11350. https://doi.org/10.3390/ijms262311350
APA StyleKo, W.-C., Chen, C.-S., Chang, Y.-P., Wu, C.-S., Yang, H.-C., & Chang, J.-F. (2025). CCN2/CTGF-Driven Myocardial Fibrosis and NT-proBNP Synergy as Predictors of Mortality in Maintenance Hemodialysis. International Journal of Molecular Sciences, 26(23), 11350. https://doi.org/10.3390/ijms262311350







