1. Introduction
Norrin (NDP, Norrie Disease Protein) is an atypical Wnt-protein that is essential for growth of the retinal vasculature and maintenance of the endothelial blood–retinal barrier [
1]. Norrin’s presence as an endogenous retinal growth factor and its ability to improve neovascular regrowth in the mouse OIR model highlights Norrin itself as a candidate protein therapeutic for the repair and regeneration of a compromised BRB in retinal vascular diseases. This led us to explore the development of a bacterially produced version of human Norrin
K86P and report here that it displays Norrin activity in both binding to FZD4 and as an activator of Norrin target gene expression in primary human retinal microvascular endothelial cells.
Norrin is produced by Müller glial cells in the retina, and it binds to the Wnt receptor Frizzled-4 (FZD4) on endothelial cells [
2]. Norrin functions as an angiogenic factor and as a neuroprotective growth factor [
3], mediating angiogenesis partly through the induction of insulin-like growth factor-1 [
4]. Frizzled-4 (FZD4) is the central receptor for Norrin. Early conditional knockout of Fzd4 in mouse endothelial cells impairs retinal vascular development while conditional knockout in adulthood causes conversion of endothelial cells from a PLVAP
(−) to PLVAP
(+) phenotype [
5]. Unlike fenestrated endothelium, Plasma-Lemma Vesicle-Associated Protein (PLVAP) concentrations are very low in high-barrier endothelia of the CNS, including the neural retina. The essential role of Norrin to human retinal vasculature development was revealed by
NDP gene variants that result in Norrie Disease or FEVR (Familial Exudative Vitreoretinopathy) [
6,
7,
8,
9]. Pathologic variants of Norrin are associated with several related vascular retinopathies, including persistent fetal vasculature syndrome (PFVS), retinopathy of prematurity (ROP), and Coats disease.
Norrin–Wnt signaling is essential for the formation of the three microvascular beds of the neural retina and for maintaining the high-barrier nature of the mature neural retina endothelium [
1,
10]. Norrin is secreted by Müller glial cells and binds to FZD4, triggering canonical Wnt signaling that regulates angiogenesis in the retina and the inner ear [
11,
12]. In the neural retina, Norrin stimulates the proliferation of the superficial vascular plexus and is important for the recruitment of Mural cells [
13]. TSPAN-12 and LRP-5 are essential co-receptors that enhance this binding, and expression of this triple-receptor complex is a specific marker of neuroretinal endothelial cells. Retinal vascular defects that arise from the disruption of Norrin/FZD4 signaling can be mitigated by stabilizing beta-catenin. Conversely, inhibiting beta-catenin-dependent transcription results in vascular defects comparable to those observed with the inactivation of Norrin or its receptor components [
14,
15].
Transgenic expression of Norrin in NDP-deficient mice, ectopically from the lens, restores vascular development in the neural retina [
16]. NDP
y/− transgenic mice with ectopic expression of Norrin from the retinal pigment epithelium also display accelerated vascular regrowth in a mouse model of oxygen induced retinopathy (OIR) [
17]. We have demonstrated that a single injection of recombinant human Norrin protein can also accelerate vascular regrowth in the mouse OIR model [
18]. More recently a tetravalent antibody to FZD4 and LRP5 was used to restore retinal angiogenesis and barrier function in Tspan12
−/− mice [
19]. These results suggest that activation of the Norrin–Wnt signaling pathway is a therapeutic target for the repair of a compromised neuroretinal vasculature.
Norrin is a small, disulfide-rich protein (11 cysteines), with essential disulfide bonds for the tertiary structure of the monomer and for intermolecular stability of the biologically active dimer [
12]. Norrin has been difficult to purify at a higher concentration from mammalian sources [
20] or insect cells [
21]. Expression of Norrin in
E. coli results in inclusion body formation, and X-ray crystallography analysis of Norrin required the bacterial production of Norrin fused to the C-terminus of MBP (Maltose-Binding Protein) for solubility and isolation [
12]. MBP-Norrin also required complete refolding to obtain active Norrin dimer formation. With this history in mind, seeking strategies to produce Norrin protein using bacterial production led us to investigate several options. While developed around Norrin protein, the results described here provide an example of strategies that can be applied to the production of other disulfide-rich mammalian proteins.
About one-third of mammalian proteins are synthesized in the endoplasmic reticulum (ER) for proper folding into their tertiary structures [
22]. The ER compartment is more oxidizing than the cytoplasm, permitting the shuffling of disulfide bonds until native disulfide bonds are formed. Key signaling proteins, such as insulin and human growth hormones, need structural disulfide bonds for normal activity. Producing polypeptides in bacteria may not yield soluble active protein, but it generates large amounts of recombinant protein for purification. Recombinantly expressed proteins can be unfolded and refolded, if necessary, but refolding must avoid trapping the protein with the wrong disulfide bonds. This requires conditions that permit disulfide bonds to form reversibly and prevent aggregation of misfolded protein until the desired active protein is formed [
23].
The first strategy we explored was the production of an MPB–Norrin fusion protein, the strategy used for previous X-ray structural studies [
12], with an HRV3C protease-cleavable site between MBP and Norrin. We used SHuffle strains of
E. coli, which have deletions of the genes for glutaredoxin reductase (Δgor) and thioredoxin reductase (ΔtrxB), permitting the formation of disulfide bonds in the cytoplasm. These strains also express a cytoplasmic version of the DsbC protein to facilitate shuffling of disulfide bonds [
24]. DsbC exists as a dimer, has disulfide isomerase activity, and has a V-shaped cleft with protein chaperone activity. DsbC can bind misfolded proteins and catalyze the exchange of disulfide bonds until soluble folded structures are formed [
25]. There is potential to produce mammalian proteins folded with their desired native disulfide bonds, although the chances of obtaining the desired native configuration may be reduced with increasing numbers of disulfides. Norrin is a small protein with seven essential disulfide bonds to form its active dimer.
We found that this soluble MPB–Norrin fusion protein was not protease-cleavable. A second version was then engineered, adding rigid alpha-helical spacers and a flexible linker [
26] around the protease cut site, which was efficiently cleavable by the HRV3C protease. Both MBP–Norrin
K86P and Norrin
K86P were soluble but not active until refolded while supporting disulfide shuffling in vitro. This led to a final strategy to make tag-free Norrin
K86P proteins as insoluble inclusion bodies. Denaturing solvation and refolding with disulfide reshuffling in vitro resulted in protein with the biological activity of Norrin based on FZD4 binding and effects on primary human retinal microvascular endothelial cells (HRMECs).
3. Discussion
Initial development of bacterial recombinant Norrin protein expression first utilized MBP (Maltose-Binding Protein) fused to the N-terminal of the mature human Norrin amino acid sequence. This was explored because it is known that MBP–Norrin can be produced in
E. coli, and in fact, this strategy was utilized by Ke Et Al. for X-ray crystallographic analysis of Norrin dimers bound to a Frizzled-4 and LRP5/6 complex [
12]. As illustrated in our results (
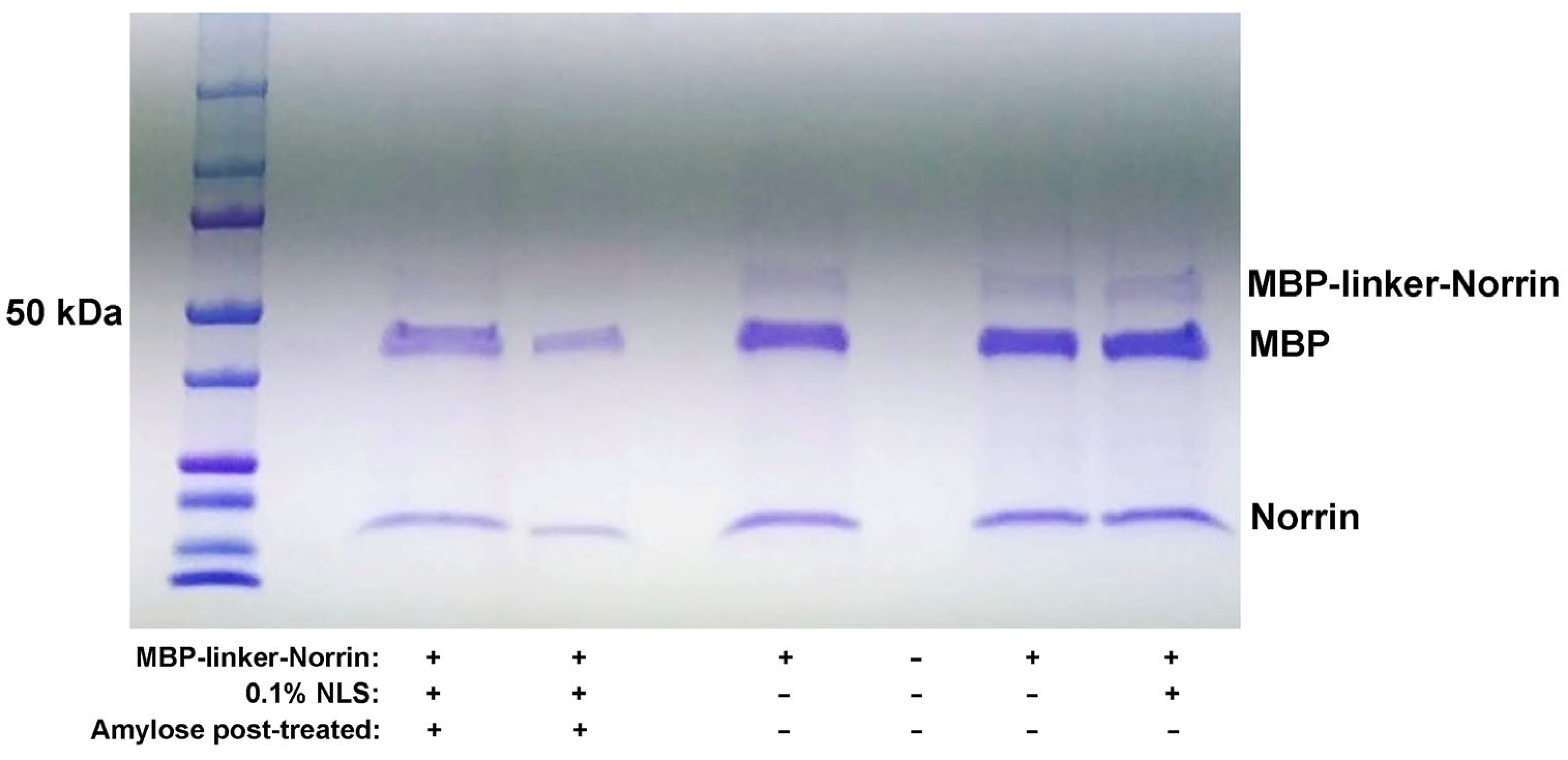
Figure 1), we found that purified MBP–Norrin could not be cleaved by the HRV3C viral protease, even though a cleavage site was provided between the MBP and Norrin moieties. While potentially useful for NDP dimerization studies, we desired to produce a recombinant Norrin protein without the large MBP tag.
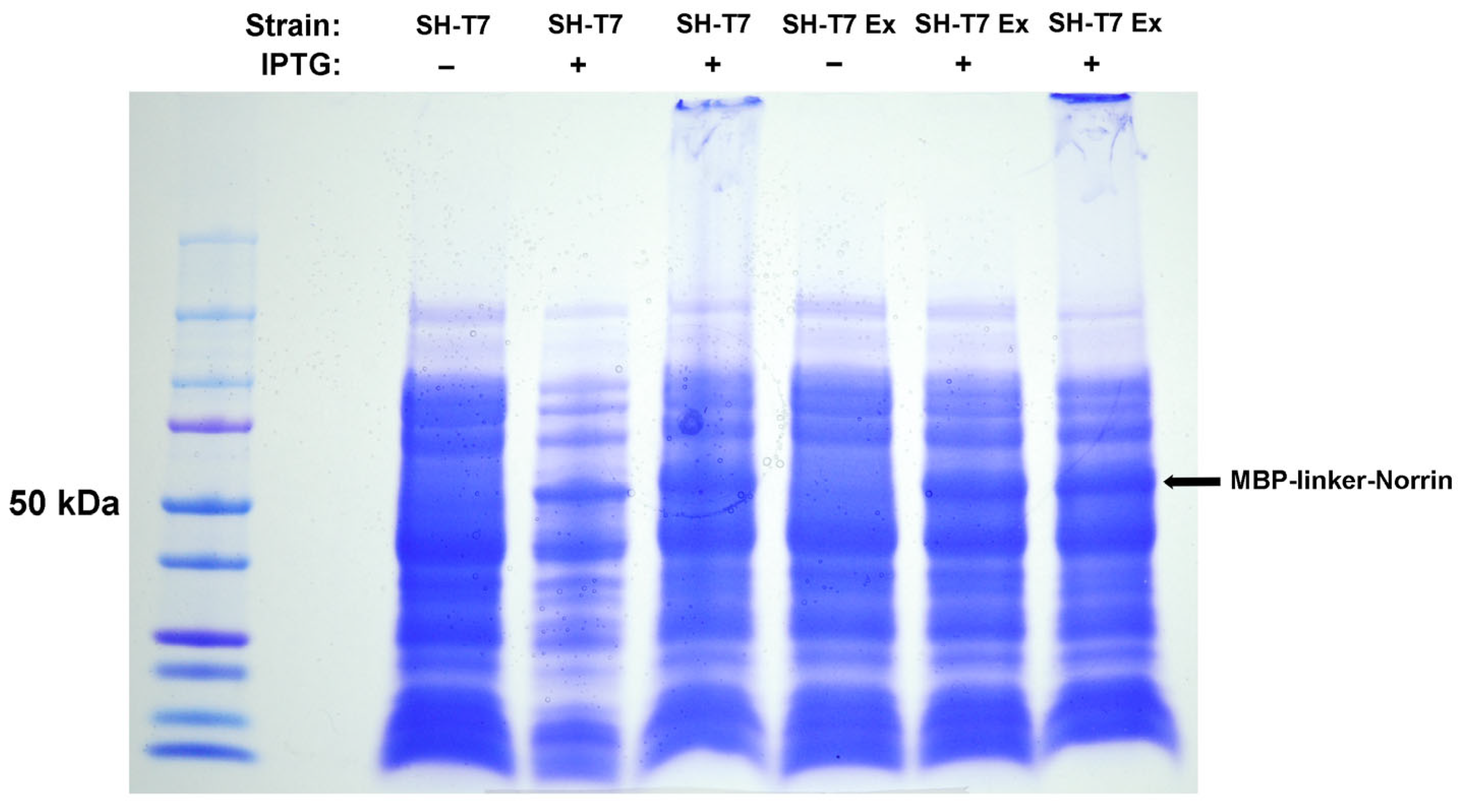
Based on our hypothesis that the MBP and Norrin structures were too large to allow access of the HRV3C protease to its cleavage site (LEVLFQGP), we tested a second construct, engineered to add rigid helical linker domains and additional flexible linkers surrounding the protease cleavage site. The intent was to increase the separation and flexibility between the MBP and Norrin components to improve protease access. Indeed, this strategy worked, and the MBP-linker-Norrin was a very efficient substrate for the HRV3C protease.
Aside from our particular interest in Norrin, this result suggests a useful strategy that could be employed when faced with fusion protein constructs that suffer a similar lack of physical access to an intermediate protease cleavage site. The amino acid sequence (EAAAK) has been used previously to add rigid helical linkers, while the glycine-rich sequence (GGGGS) provides the addition of flexible linkers [
26]. Engineering the addition of these linkers provided a viable solution to allow for protease cleavage of MBP from Norrin.
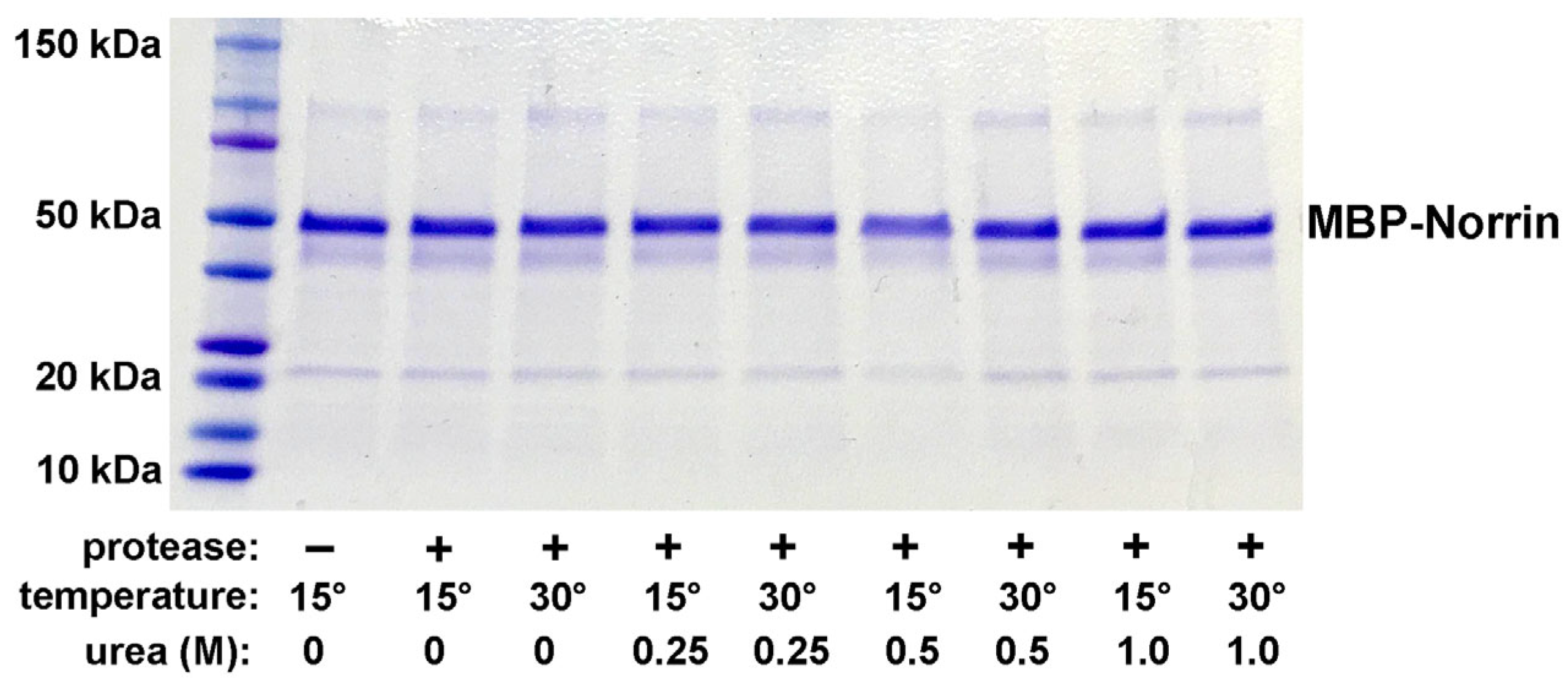
After solving a way to cleave recombinant Norrin from MBP, it was discovered that the resulting Norrin, while soluble, did not display the expected activity for the binding to Frizzled-4 in plate-binding assays. Expression in T7-Shuffle E. coli strains to permit disulfide bond formation resulted in Norrin that was soluble but lacked the required binding activity. We hypothesized that this initial Norrin was soluble, but only a small percentage of the Norrin was correctly folded with the required disulfide bonds. Thus, only a small percentage of this Norrin was active for Frizzled-4 binding. This directed us to a strategy of disulfide shuffling to refold Norrin into its native conformation while preventing the protein from becoming trapped in permanent but incorrect disulfide bonds.
Norrin is a disulfide-rich protein with a high density of cysteines, creating opportunity for incorrect disulfide bond pairings. We next employed complete denaturation and a refolding process that included disulfide reshuffling. This resulted in the recovery of Frizzled-4-binding activity. Refolding of either intact or cleaved MBP-linker-Norrin resulted in an increase in Norrin-like Frizzed-4-binding activity. This result established the requirement to denature and refold Norrin while permitting disulfide reshuffling. Based upon this conclusion, it was decided to switch to a third and final strategy in which tag-less Norrin was expressed in E. coli as inclusion bodies, which would be mostly recombinant Norrin.
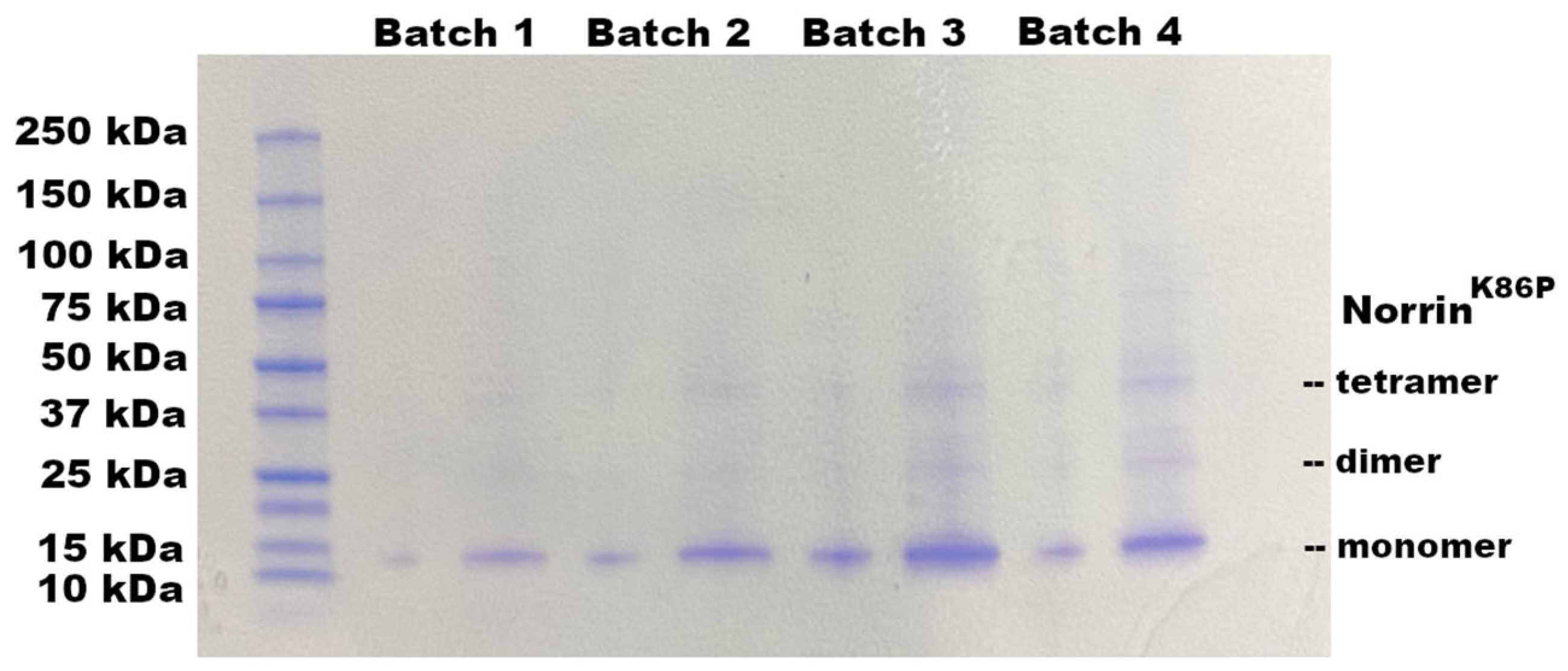
Expression of Norrin
K86P devoid of additional amino acid tags resulted in the expected production of a substantial amount of Norrin protein in the insoluble inclusion body fraction from bacterial cultures. While inclusion bodies of recombinant proteins are crystalline, insoluble, and generally comprise misfolded protein, they provide a substantial source of recombinant protein that is 95–99% recombinant protein. Inclusion body proteins can be utilized by first solvating them in protein denaturing conditions and then refolding the proteins [
23]. In the case of Norrin, refolding had to occur in conditions that permitted disulfide reshuffling. In that process, disulfide bonds are allowed to reversibly form, so the protein does not become trapped in a non-functional conformation by the wrong disulfide bond pairings. This requires a denaturing agent, such as 6M guanidine–HCl, to first solvate the IB protein. Refolding then involved a gradual reduction in the guanidine–HCl concentration by dialysis, in the presence of a mixture of both reduced (GSH) and oxidized glutathione (GSSH). In addition, a high concentration of arginine was used to prevent aggregation of intermediate misfolded protein.
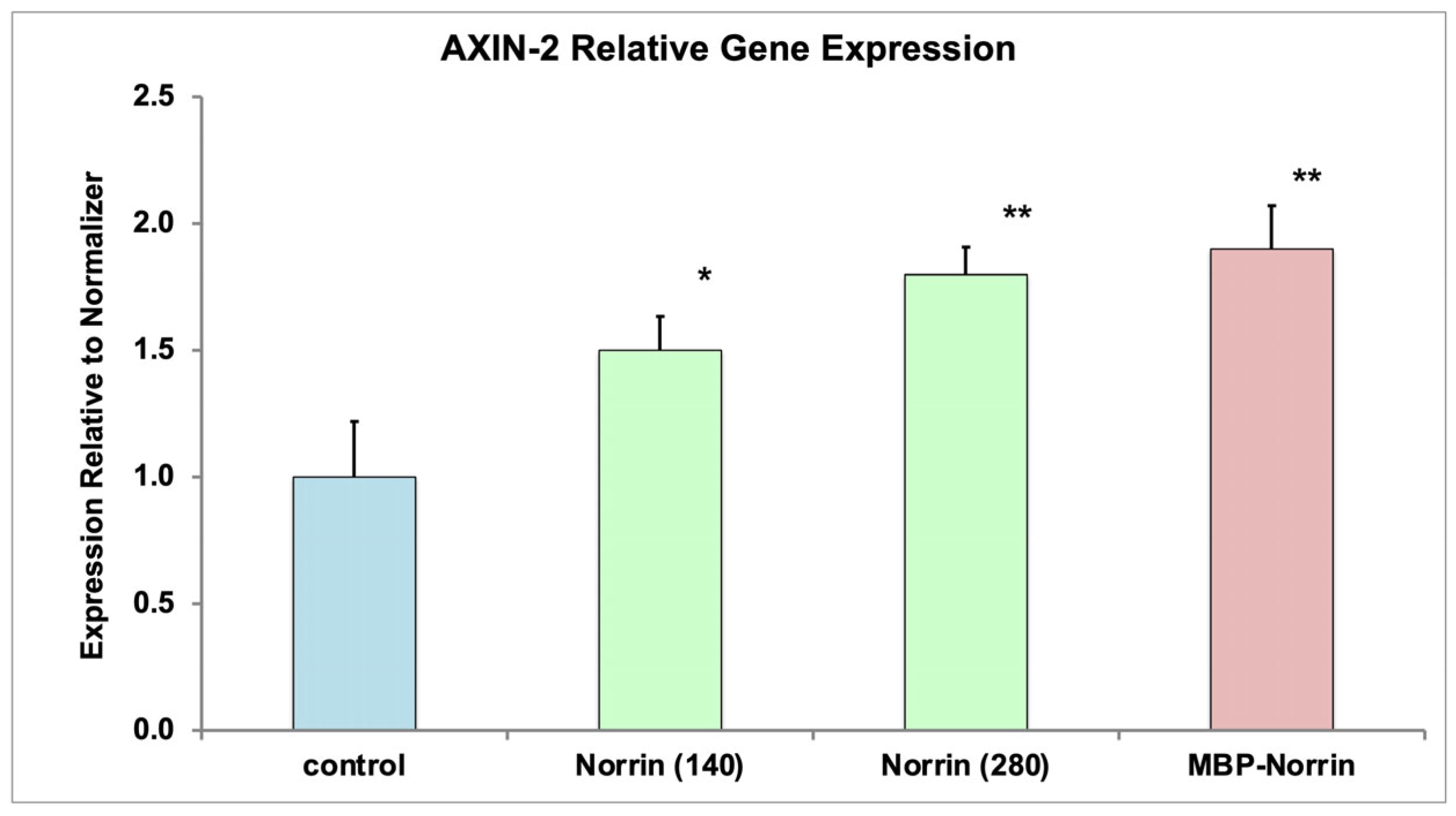
The refolding process of inclusion body protein was successful for generating recombinant NorrinK86P that was biologically active. Norrin biological activity was evaluated in two ways. First, using in vitro assays of Frizzled-4 binding, refolded NorrinK86P preparations displayed Frizzled-4-binding activity, compared to a control Norrin preparation. Second, refolded protein increased expression of the AXIN-2 gene in primary HRMECs, a known effect of Norrin.
Preparation of proteins like Norrin in bacteria requires sufficient purification from bacterial cell wall glycoproteins to allow introduction of the recombinant protein into the ocular environment. For the purposes of basic laboratory process development, we employed the strategy of denaturing size exclusion chromatography. That is, after denaturing and solvating inclusion body protein, the denatured recombinant Norrin was separated further from bacterial proteins under denaturing conditions (guanidine–HCl) prior to refolding the protein with disulfide reshuffling. This kind of preparation was used for testing biological activity but also tested with intraocular injection into rat vitreous to test ocular tolerance.
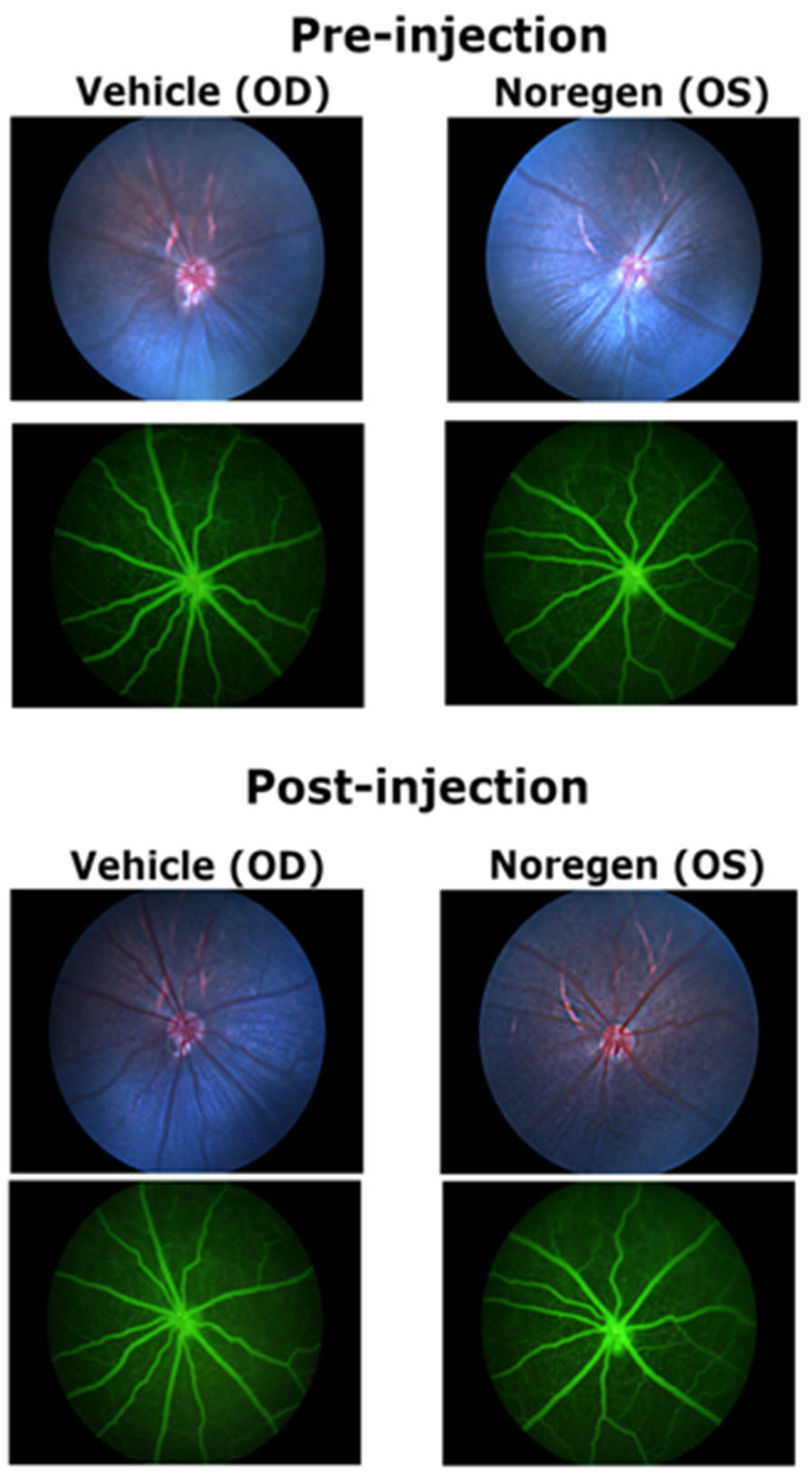
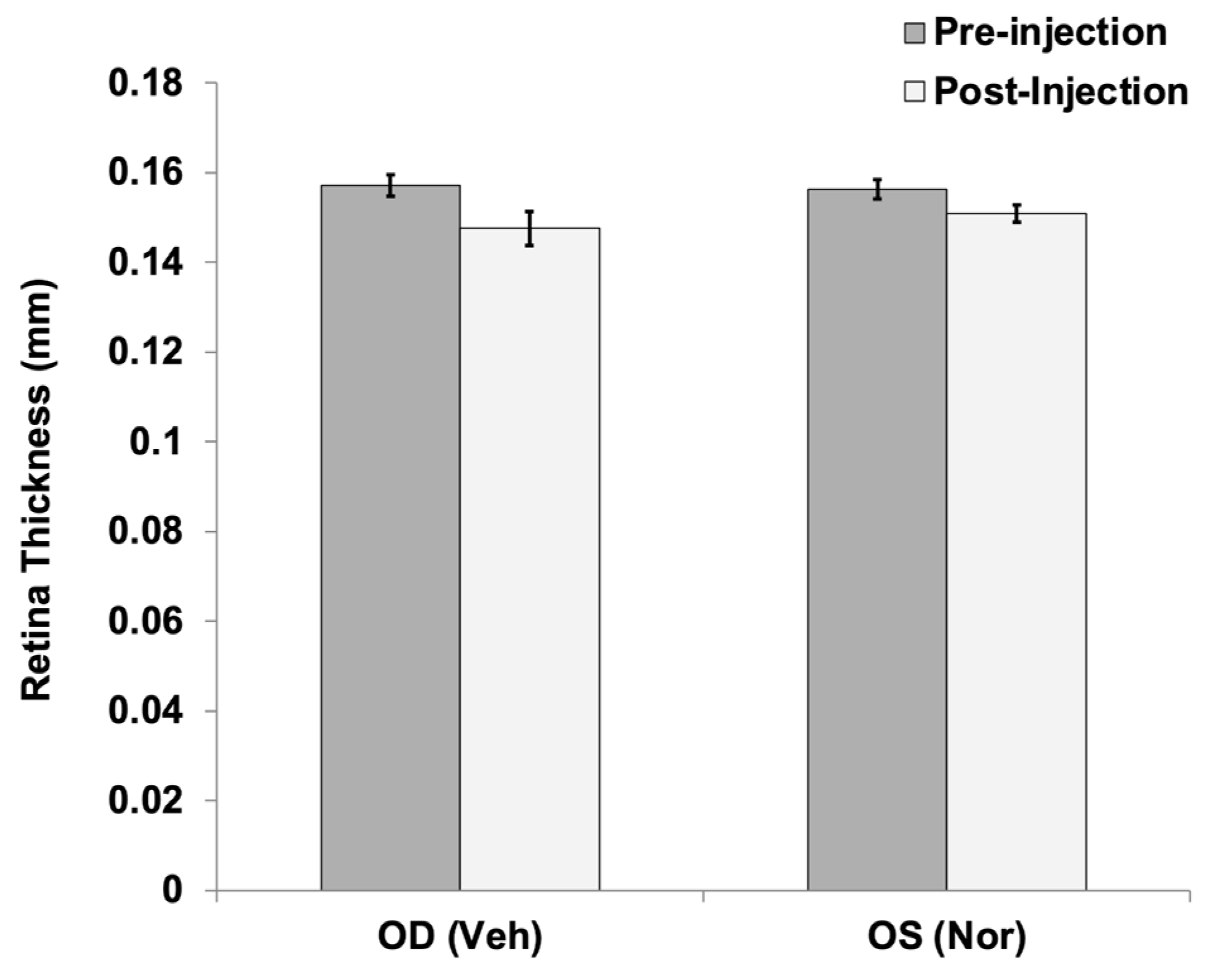
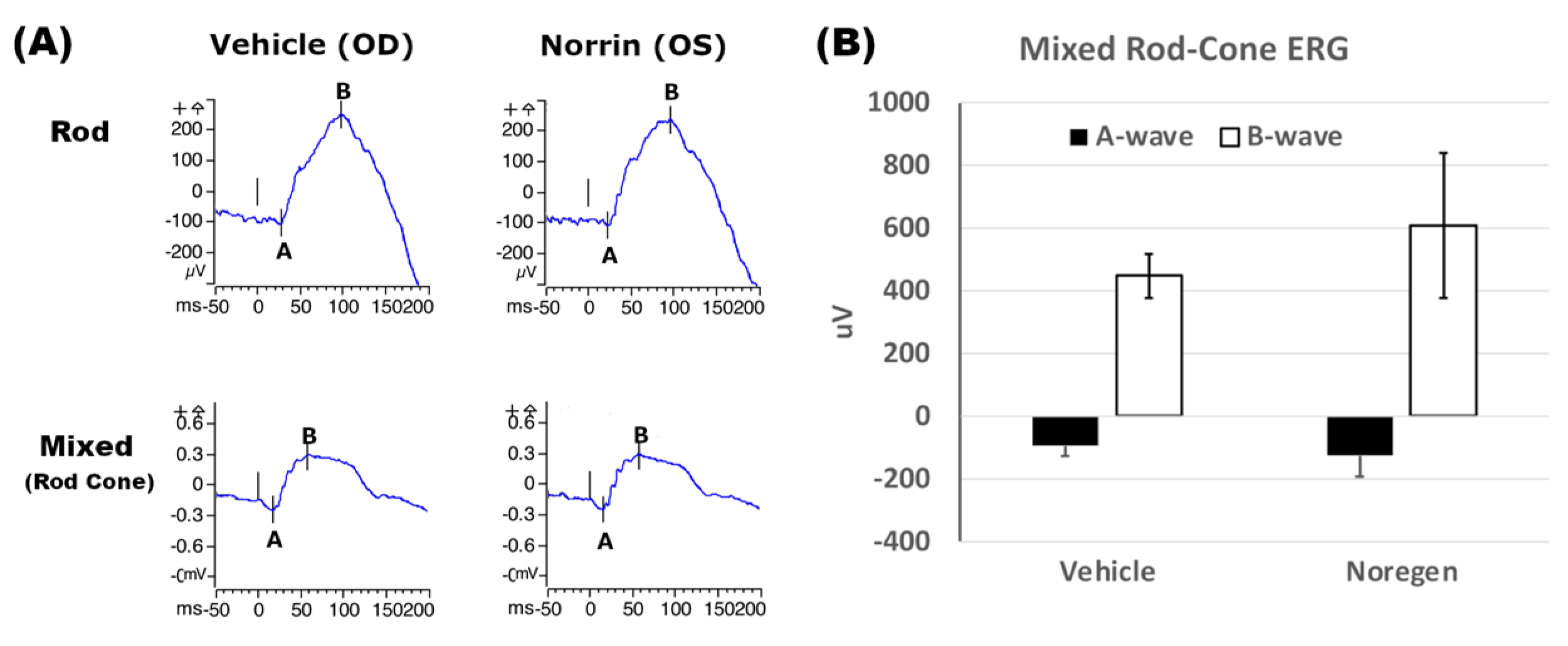
Analysis of rat retinas after injection of a large dose of NorrinK86P (250 ng, into vitreous) were completed using fluorescein angiography and SD-OCT analysis of retinal thickness. No changes to the retinal vasculature were detected when comparing fluorescein angiography images of vehicle-injected eyes and contralateral Norrin-injected eyes, before and after Norrin injection. Likewise, SD-OCT measurements of neural retina thickness did not find any significant differences when comparing vehicle- and Norrin-injected eyes. Finally, ERG analysis of retinal function did not reveal any ill effects of Norrin injection on either the Rod-only or mixed Rod–Cone ERG response. We concluded that this modest purification process of recombinant Norrin resulted in sufficiently low concentrations of bacterial protein to permit introduction into the living rodent eye.
Summary
To demonstrate feasibility, a process was established to produce a recombinant protein based on the human Norrin protein in bacterial culture. Recombinant Norrin protein has the potential as a future therapeutic for the repair of a damaged retinal vasculature and blood–retinal barrier due to Norrin’s biological function. Norrin is active as a dimer, is disulfide-rich, and has proven to be difficult to produce recombinantly. Our initial explorations of MBP-tagged strategies resulted in demonstrating the use of protein linker additions to solve the previous challenge of steric access for HRV3C protease cleavage. The strategy described here may be useful for similar situations with different fusion protein constructs. However, the need to completely refold recombinant protein to obtain Norrin’s biological activity led to a final production strategy based on expressing untagged NorrinK86P protein as bacterial inclusion bodies (IBs). The best final process was found to be denaturation solvation of IB protein followed by denaturing SEC and refolding of the protein using disulfide reshuffling conditions. We concluded that it was feasible to produce biologically active recombinant NorrinK86P in substantial quantity using bacterial fermentation and that the preparations were well tolerated by the eye after injection into the vitreous of Long Evans rats.
4. Materials and Methods
4.1. Animal Care and Use
All animal care and tissue collections performed in this study were carried out with the approval of Oakland University’s Institutional Animal Care and Use Committee, and research was carried out in compliance with the requirements contained in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research (Association for Research in Vision and Ophthalmology,
www.ARVO.org), IACUC approval number 2021–1130. Adult Long Evans rats (155–190 g body weight, female) were obtained from Charles River Laboratories (Wilmington, MA, USA) and were housed at Oakland University in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
4.2. Bacterial Protein Expression Plasmids
Plasmids for bacterial protein expression were designed, synthesized, and inserted into the pD454-MBP or pD454 plasmids (Atum,
https://www.atum.bio/, Newark, CA, USA). The pD454-MBP plasmid provided for inducible expression, in
E. coli, of an N-terminal fusion of mature Norrin
K86P with the Maltose-Binding Protein (MBP). An HRV3C protease target site was present between the Norrin and MBP domains (See
Figure 9). The pD454 plasmid provided for the inducible expression of mature human Norrin
K86P without the MPB fusion or any additional tags. The protein encoding sequences were configured to use bacteria-prefered codons in
E. coli. The plasmid construct Nor-2 was a direct fusion of MBP with human Norrin. The plasmid construct Nor-w-Linker was similar but added extra polypeptide linker sections, positioned on either side of the HRV3C protease cleavage site (
Figure 9). The plasmid construct pD454-Nor encoded only mature human Norrin
K86P without N- or C-terminal tags.
4.3. Bacterial Strains for Recombinant Protein Expression
SHuffle T7 (SHT7), SHuffle T7 Express (SHT7-Express), and BL21(DE3) competent E. coli cells (New England BioLabs, Ipswich, MA, USA) were used for bacterial protein expression. BL21(DE3) was used for the expression of untagged Norrin as bacterial inclusion body protein.
4.4. Transformation of Bacterial Strains for NorrinK86P Protein Expression
Plasmid DNAs were diluted in TE pH 8.0 to a working concentration of 0.1 ng/µL for transformation. In total, 2 µL of plasmid DNA was mixed into a freshly thawed aliquot of competent SHT7 or 1 µL of DNA with competent SHT7-Express cells or 3 µL for BL21(DE3) cells. Tubes were flicked 5 times to mix and incubated on ice for 30 min. DNA bacterial mixes were then subjected to 42 °C heat shock for 30 s for the SHT7 strains or 10 s for BL21(DE3), in a water bath, and then incubated on ice for 5 min. SOC media (950 µL) was added to each transformation mix; these were incubated for 60 min at 250 RPM and 30 °C. Transformation mixtures were centrifuged for 2 min 5000× g to pellet bacteria and the supernatant was discarded. Cells were resuspended in SOC media (100 µL). To select transformed clones, by ampicillin resistance, 5 µL and 95 µL of the cells were spread onto prewarmed LB/AMP plates (100 µg/mL ampicillin) and incubated for approximately 10 min to dry. Plates were then incubated overnight at 30 °C to allow individual colonies to form. Several clonal isolates of each transformation were used for the preparation of glycerol stocks, combining 850 µL of the overnight culture and 150 µL of glycerol (15%), followed by incubation on ice for 10 min, and then transfer to −70 °C.
4.5. Extraction of Inclusion Body Protein from E. coli
Extraction of inclusion body protein from E. coli was possible using common treatment with lysozyme and sonication, but more efficient extraction of inclusion body (IB) protein was afforded using a mixture of two recombinant enzymes, Benzonase and rLysosyme (BugBuster MasterMix (BBMM), Sigma Aldrich, St. Louis, MO, USA). This process degraded the bacterial cell wall, DNA and RNA, to allow more efficient washing of IB protein. Using the manufacturer’s instructions, 0.5 g of frozen bacterial cells were resuspended in 2.5 mL of BBMM solution, supplemented with 0.5 mM PMSF (phenylmethylsulfonyl fluoride, serine protease inhibitor) and incubated at room temperature for 15 min. An equal volume of BBMM was then added for an additional 10 min of incubation. After dilution with 9 volumes of deionized water (to 10% BBMM), inclusion body protein was collected by centrifuging (3200× g 25 min, 5 °C). IB material was resuspended and washed two times with 5 mL of 0.1× BBMM, and then collected by the centrifuge (3200× g 20 min, 5 °C). A final third wash in 1 mL of 0.1× BBMM was completed, with the final IB protein pellet collected in a microcentrifuge (20,000× g 15 min, 5 °C). The IB material was weighed and frozen.
4.6. Denatured SEC for Inclusion Body Protein
IB protein was solvated using denaturation conditions and then fractionated using size exclusion chromatography under denaturing conditions (Denaturing SEC). The IB protein was gently suspended and dissolved in unfolding buffer (10 mg/mL IB, 49 °C, 30 min): 50 mM Tris–HCl pH 8.5, 5 mM EDTA, 7 M guanidine–HCl, and 5 mM DTT. Solvated IB protein was centrifuged (1 min 20,000× g) to remove any insoluble material prior to column loading. The SEC column comprised Bio-Gel P100 Polyacrylamide Gel packed into a 100 mL bed volume Econo-column column per the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA), equilibrated with a column running buffer (50 mM Tris–HCl pH 8.5, 4 M guanidine–HCl). The column was run using top shelf gravity feed pressure and 1 mL fractions collected using a Pharmacia Frac-100 fraction collector. Eluted protein was monitored by A280 analysis of each fraction. A total of 50 µL of selected fractions were ethanol precipitated, separated by DISC-PAGE, and processed with Imperial Protein Stain (Thermo Fisher, Waltham, MA, USA) to identify Norrin fractions.
4.7. Refolding of Recombinant NorrinK86P with Disulfide Reshuffling
Refolding of NorrinK86P required conditions to remove guanidine–HCl gradually while preventing aggregation of incompletely folded protein during the transition from the denatured state to secondary and tertiary structures. Another essential requirement was to provide conditions that permitted the dynamic formation of disulfide bonds, without trapping NorrinK86P in an inactive tertiary structure. This was accomplished by dialysis of the denatured NorrinK86P protein fraction in refolding buffer: 20 mM Tris pH 8.5, 1 mM EDTA, 1 M L-Arginine, 500 mM NaCl, 1 mM GSH, and 1 mM GSSG. Dialysis used 10 kDa cutoff dialysis cassettes (Thermo Fisher).
4.8. Anesthesia for Intraocular Injections, Retinal Imaging, or ERG Testing
Pupils were dilated with tropicamide and phenylephrine eye drops (Covetrus, Portland, ME, USA) prior to anesthesia. Rats were anesthetized with an intraperitoneal injection of a Ketamine HCL (50 mg/kg) and Xylazine (7 mg/kg) (Covetrus).
4.9. NorrinK86P Intravitreal Injection (Rat)
Pupils were dilated with tropicamide and phenylephrine drops prior to anesthesia. Rats were anesthetized with an intraperitoneal injection of a Ketamine HCL (50 mg/kg) and Xylazine (7 mg/kg). Rat retinas were imaged before and 3 weeks after injections of 250 ng NorrinK86P, using fluorescein angiography and SD-OCT imaging to record the status of their normal retinal vasculature. Recombinant NorrinK86P was diluted with phosphate-buffered saline solution (1× PBS, no calcium, no magnesium) to a concentration of 100 ng/µL for intravitreal injections of a 2.5 μL volume (dose 250 ng/eye). Injections were given with a NanoFil syringe with 35-gauge beveled NanoFil needles (WPI Inc., Sarasota, FL, USA). The contralateral eye served as a control and received the same injection procedure using vehicle alone (1× PBS).
4.10. Fluorescein Angiography (FA)
After sedation and pupil dilation, rats were injected (intraperitoneal) with 50 µL of 10% sodium fluorescein in 1× PBS. Corneal surfaces were protected with GenTeal lubricant eye gel (Novartis, CVS Pharmacy) to maintain corneal hydration and to provide optical transmission with the camera lens. Retinal blood vessels were imaged using a MICRON-3 camera system (Phoenix Micron, Bend, OR, USA), using normal white-light illumination fundus images and a dial-in fluorescein filter set for capturing FA images (blue illumination, green emission).
4.11. Analysis of Rat Retinal Thickness In Vivo
Spectral Domain Optical Coherence Tomography (SD-OCT) was employed to measure retina thickness in vivo, comparing before and 3 weeks after injection of 250 ng Norrin
K86P. To maintain corneal transparency, artificial tear lubricant eye drop solution was applied often to both corneal surfaces. Anesthetized rats were secured in a three-axis positioning support cradle (Bioptigen, Durham, NC, USA). SD-OCT scans were taken using an Envisu R2200 model SD-OCT system (Bioptigen), equipped with a lens for the rat eye axial length. A rectangular scan pattern of rat sizes 2.6 mm × 2.6 mm was used (1000 A-scans by 100 B-scans). For measurement of retinal thickness, retinal layers were marked and measured using processed OCT images with InVivoVue Diver 2.0 software (Bioptigen), as previously described [
27]. A fixed 5 × 5 grid was first centered on the optic disk. Boundaries of all retinal layers were marked at measurement grid locations. The central grid position was not used, as it marks the optic disk center. The remaining 24 grid positions were then used to generate an average thickness between the inner limiting membrane (ILM) and the outer limiting membrane (OLM) for each eye, both pre and post injection. Retinal thickness was measured between the ILM and the OLM.
4.12. Electroretinography (ERG)
ERG analysis was used to compare the Rod and mixed Rod–Cone responses of Long Evans rat retinas before and 6 weeks after intraocular injection of 250 ng NorrinK86P in vivo. A Diagnosys LLC Espion-III ERG system with ColorDome was used to obtain the full-field ERG responses in response to white light flash stimulation. Rats were dark-adapted for 2 h and then handled under red light room lighting in a rodent ERG imaging suite. Pupils were dilated with two applications of tropicamide and phenylephrine–HCl eye drops to ensure maximum pupil size prior to anesthesia. Once anesthetized, rats were positioned on a custom support platform with an integrated warming pad to maintain normal body temperature. Gold loop electrodes were used for electrical coupling to corneas bathed in a small application of corneal protectant (GenTeal lubricant gel). One platinum microneedle was inserted into the hind flank skin (ground electrode), and a second platinum microneedle (reference electrode) was inserted into the skull cap skin just forward of the ears to minimize the detection of cardiac activity (EKG) in the ERG trace. A custom program was used to reproducibly automate the ERG testing. For the Rod-only response, dark-adapted rats were first stimulated with white light (6500K) flashes using a dimmer illumination intensity of 0.01 cd-s/m2. Immediately after the Rod test sequence, the mixed Rod–Cone ERG sequence was recorded using a brighter flash intensity of 3 cd-s/m2 to activate both Rod and Cone photoreceptors. Five traces were time-averaged to generate all ERG traces.
4.13. Receptor Binding ELISA
An In Vitro ELISA was created to assess NorrinK86P potency by its ability to bind to its receptor, Frizzled-4 (FZD4). Briefly, a rhFrizzled-4/FC Chimera (R&D Systems, Minneapolis, MN, USA; 5847-FZ-050) was coated onto the surface of a 96-well plate overnight. The next day, the plate was washed with buffered surfactant (R&D Systems # WA126). The surfaces were then blocked with BSA (1% in PBS) or Casein (1% in PBS) and a serial dilution of the test NorrinK86P (0.8–500 ng/mL) was made in the blocking agent. Each of the 7–9 levels of NorrinK86P were added to duplicate or triplicate wells of the plate and allowed to incubate for 2 h. After washing away unbound NorrinK86P, a biotinylated Norrin antibody (R&D Systems; BAF3014) was added to the wells and allowed to incubate for 2 h at room temperature. Following incubation with the antibody, the wells were washed and incubated with Streptavidin–HRP (R&D Systems; DY998), followed by more washing. TMB substrate (R&D Systems, DY999) was added to the wells, the plate was stored in the dark, and color development was stopped by the addition of 2N Sulfuric Acid after 20 min. The absorbance at 450 nm was read on a BioTek Cytation 3 or Epoch 2 Plate Reader. The data was analyzed by four-parameter logistic (4-PL) curve fitting to determine the half maximal effective concentration (EC50).
4.14. Culturing of Primary Human Microvascular Retinal Endothelial Cells (HMREC)
Primary HMRECs were obtained from Cell Systems (Kirkland, WA, USA). They were grown to confluence in six well plates that had been pre-coated with Cell Attachment Factor (Cell Systems, Kirkland, WA, USA) and grown to confluence in fully supplemented EndoGRO-MV media (Millipore, Burlington, MA, USA), a low serum media that does not contain VEGF. Supplements included EndoGRO-LS supplement (0.2%), rh EGF (5 ng/mL), L-Glutamine (10 mM), Heparin Sulfate (0.75 U/mL), Ascorbic Acid, (50 µg/mL), FBS (5%), and Hydrocortisone Hemisuccinate (1 µg/mL). The cells were weaned to media containing no Hydrocortisone Hemisuccinate prior to stimulation with NorrinK86P or rhNorrin (R&D Systems, Minneapolis, MN, USA; 3014-NR). Concentrations ranged from 2 to 2000 ng/mL, and after a 24 h duration, the cells were trypsinized and collected for RNA isolation.
4.15. Quantitative PCR Analysis of HMREC Gene Expression
Total RNA was isolated using the Monarch Total RNA Miniprep kit (NEB, Ipswich, MA, USA; T2010) with the optional On-Column DNase I treatment to remove residual DNA, according to the kit instructions. First-strand cDNA was synthesized by reverse transcribing 1 µg of total RNA per sample using the LunaScript RT Super Mix Kit (NEB, Ipswich, MA, USA; E3010L). The reaction conditions were according to the manufacturer’s instructions: 25 °C for 2 min, 55 °C for 10 min, and 95 °C for 1 min. All compared samples were processed using the same reagent set. Stock first-strand cDNA preparations were stored at −70 °C and were not used for analysis after a maximum of three freeze-thaws. qPCR was performed using a duplex reaction format with FAM-labeled probe/primer pairs for the gene of interest and VIC-labeled probe/primer-limited pairs for TBP (Tata-Binding Protein) as the normalizer gene. For real-time PCR reactions, sample first-strand cDNA was diluted 5-fold with deionized water, and 2 µL was added to 18 µL of master mix for 20 µL PCR reactions. Triplicate reactions were used for each sample using the Luna Universal Probe qPCR 2× Master Mix with Rox reference dye (NEB, Ipswich, MA, USA; M3004). Reactions were run on an AriaMx Real-time PCR System (Agilent, Santa Clara, CA, USA) and the comparative method used to determine the relative gene expression. Gene expression assays were evaluated for high PCR efficiency using a dilution series of HMREC cDNA to ensure validity using the delta–delta Ct method for comparing relative gene expression. Each replicate reaction was internally normalized relative to endogenous
TBP gene expression. The specific assay probe sets used for gene expression analysis are listed in
Table 2.