History of Gap Junction Architecture and Potential Role of Calmodulin in Channel Arrays
Abstract
1. Introduction
2. Early Evidence for Direct Cell-to-Cell Communication
3. Early Ultrastructural Images of Gap Junctions in Excitable Cells of Invertebrates
4. Gap Junctions in Vertebrates—Early Images
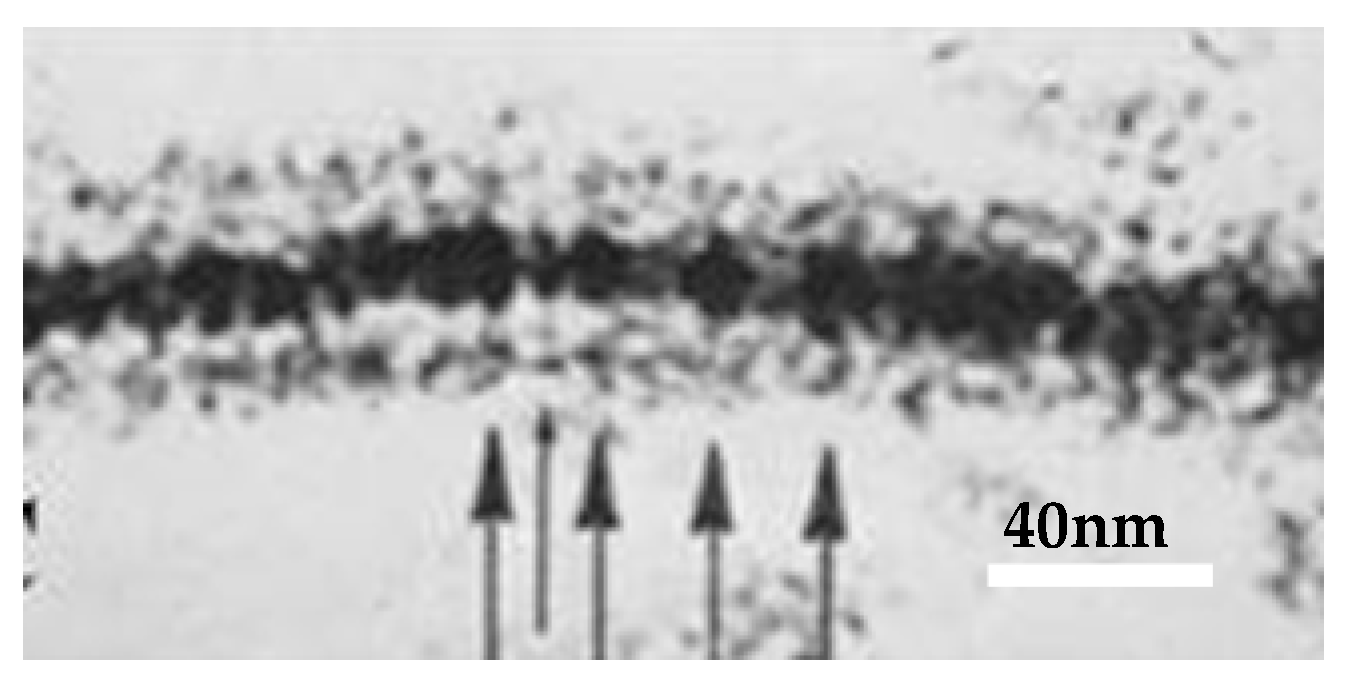
4.1. Pentalaminar Junction Profile
4.2. Septilaminar Junctional Profiles
4.3. The Puzzle of the 1960s
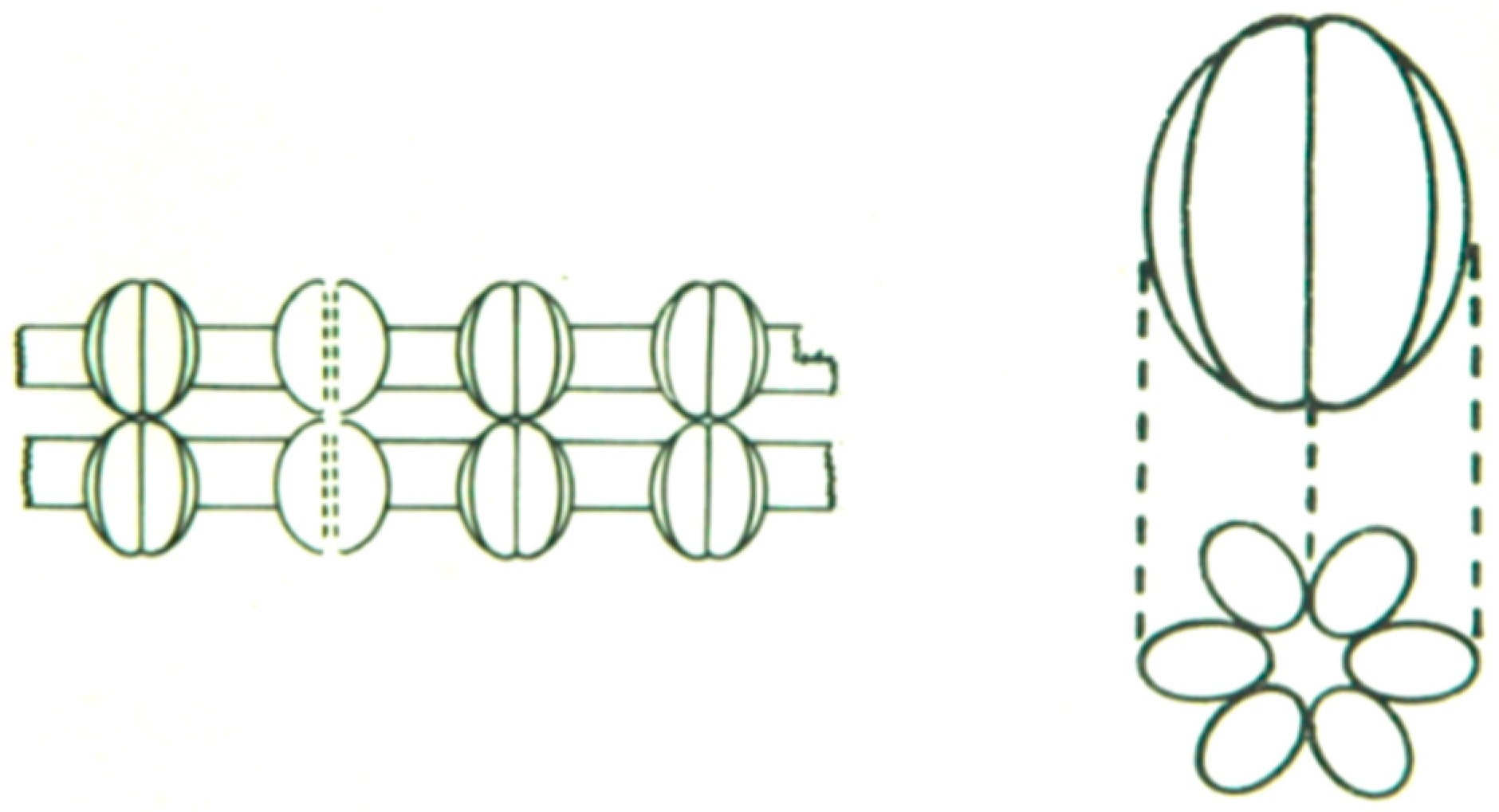
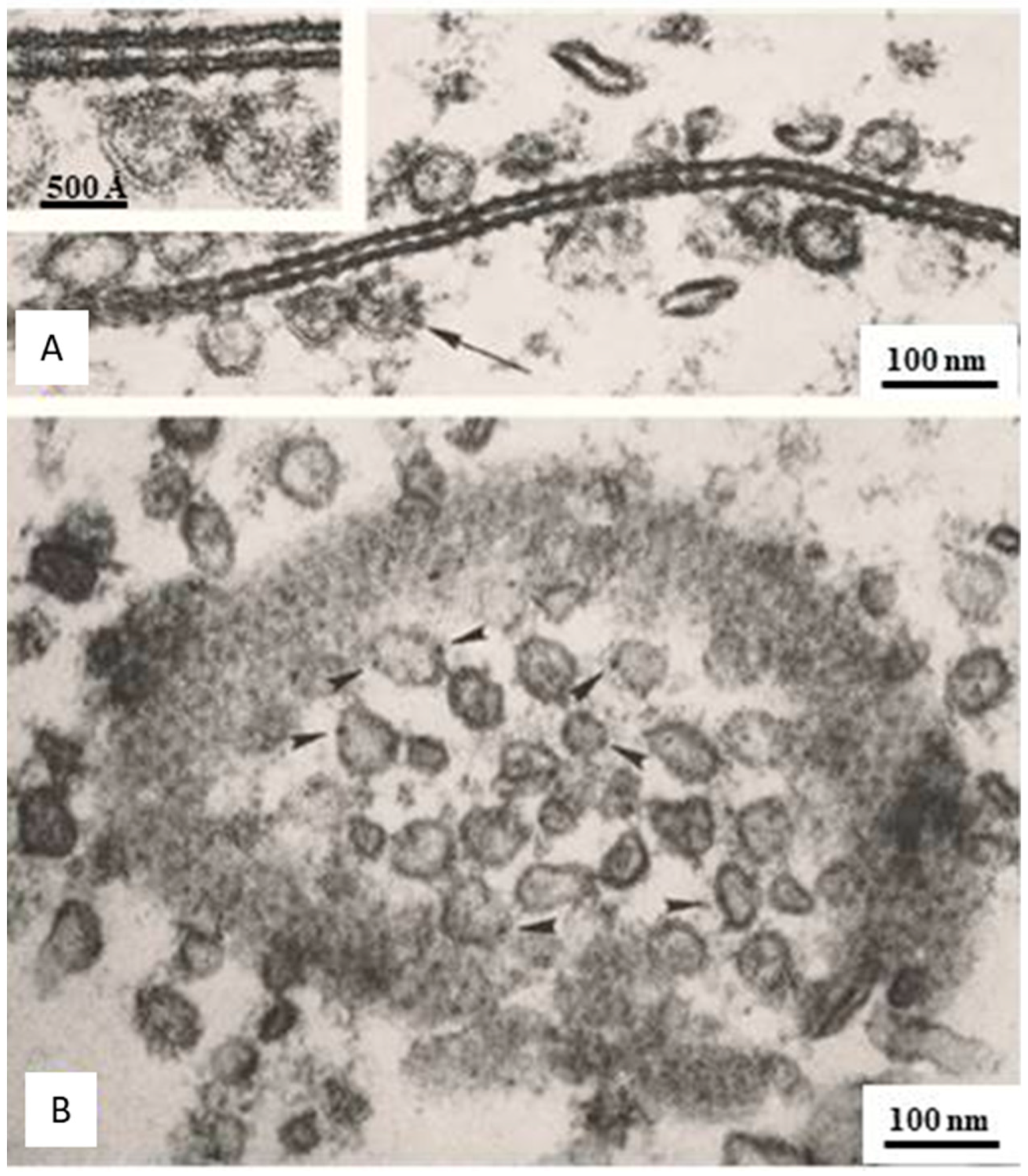
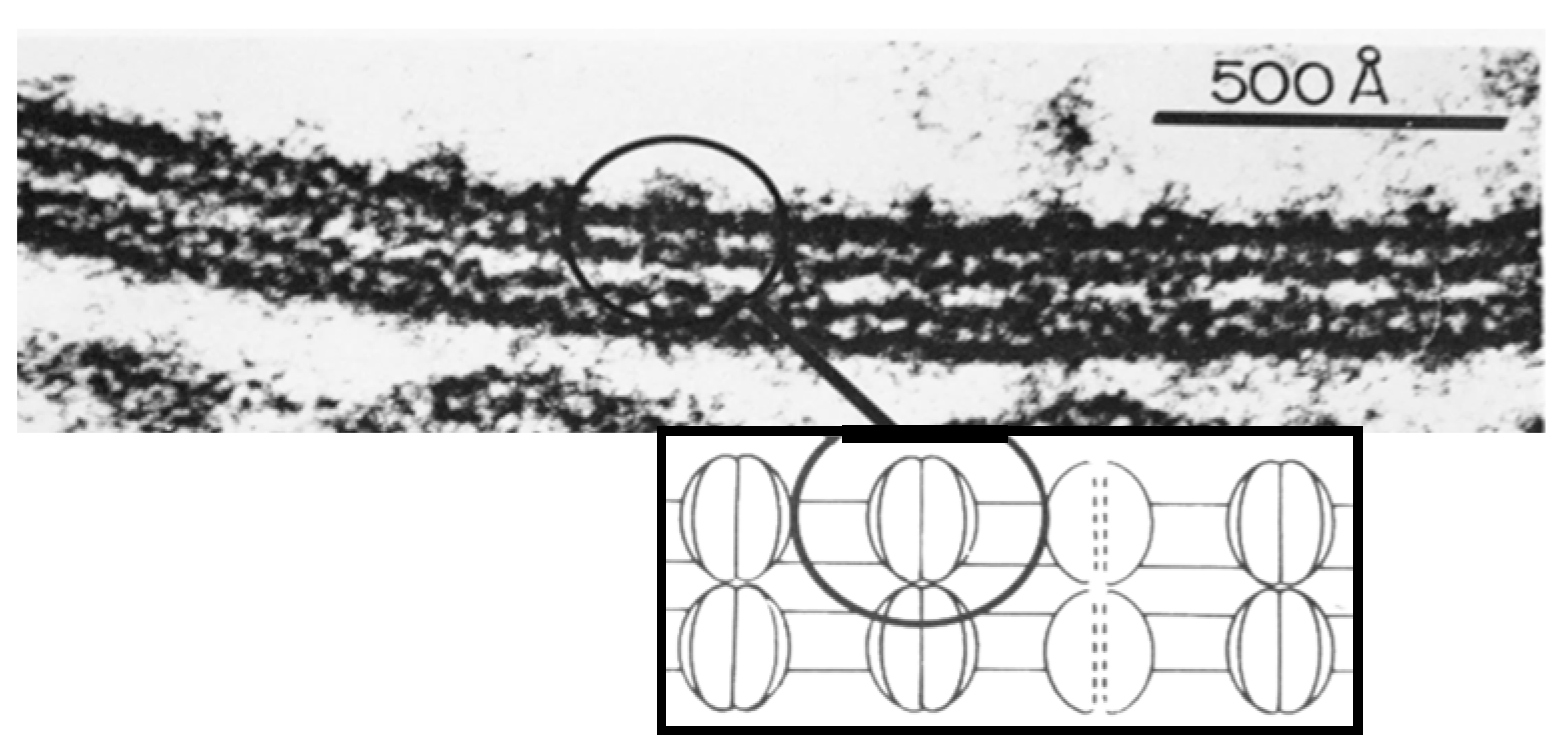
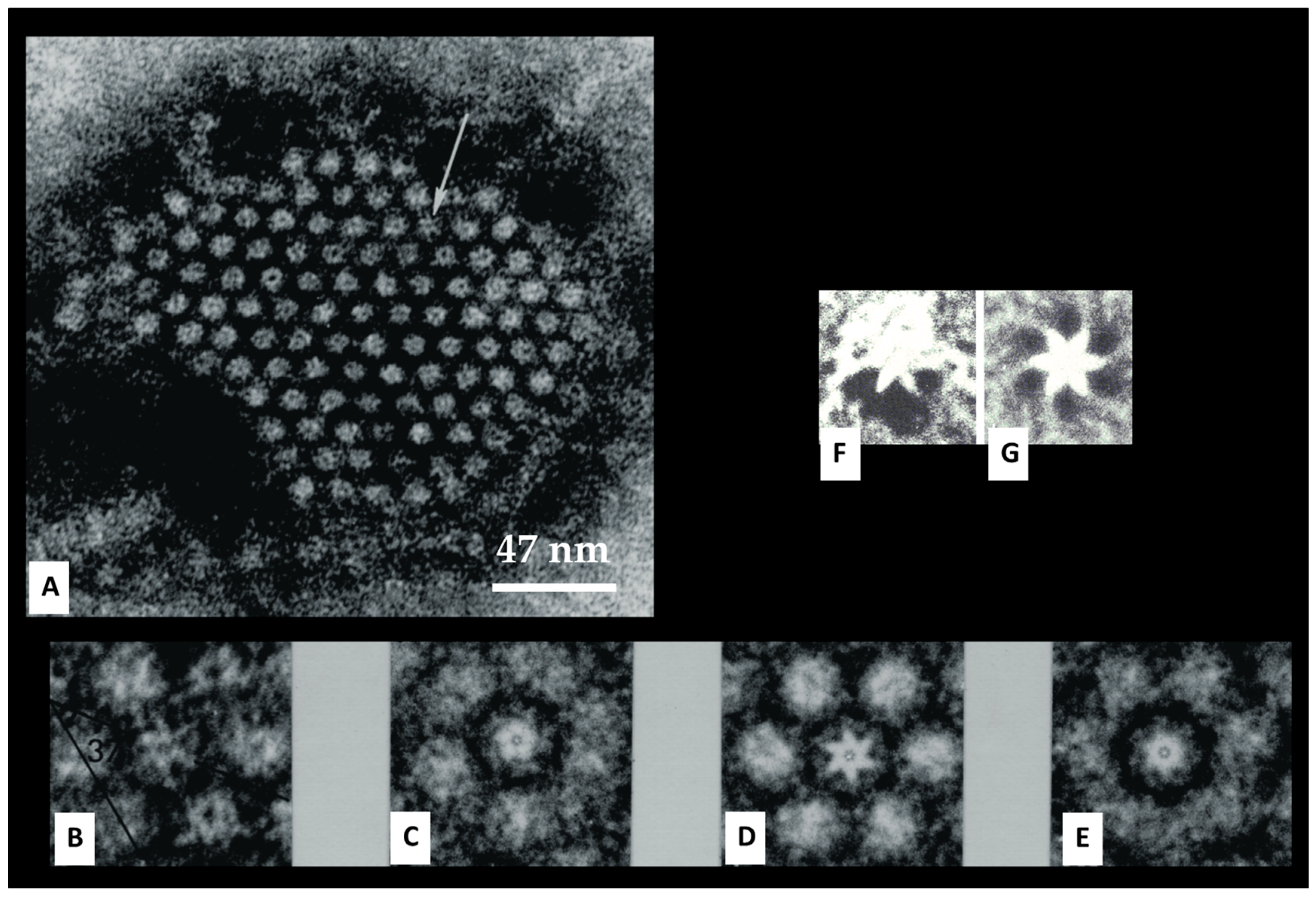
4.4. Hexagonal Arrays in Gap Junctions
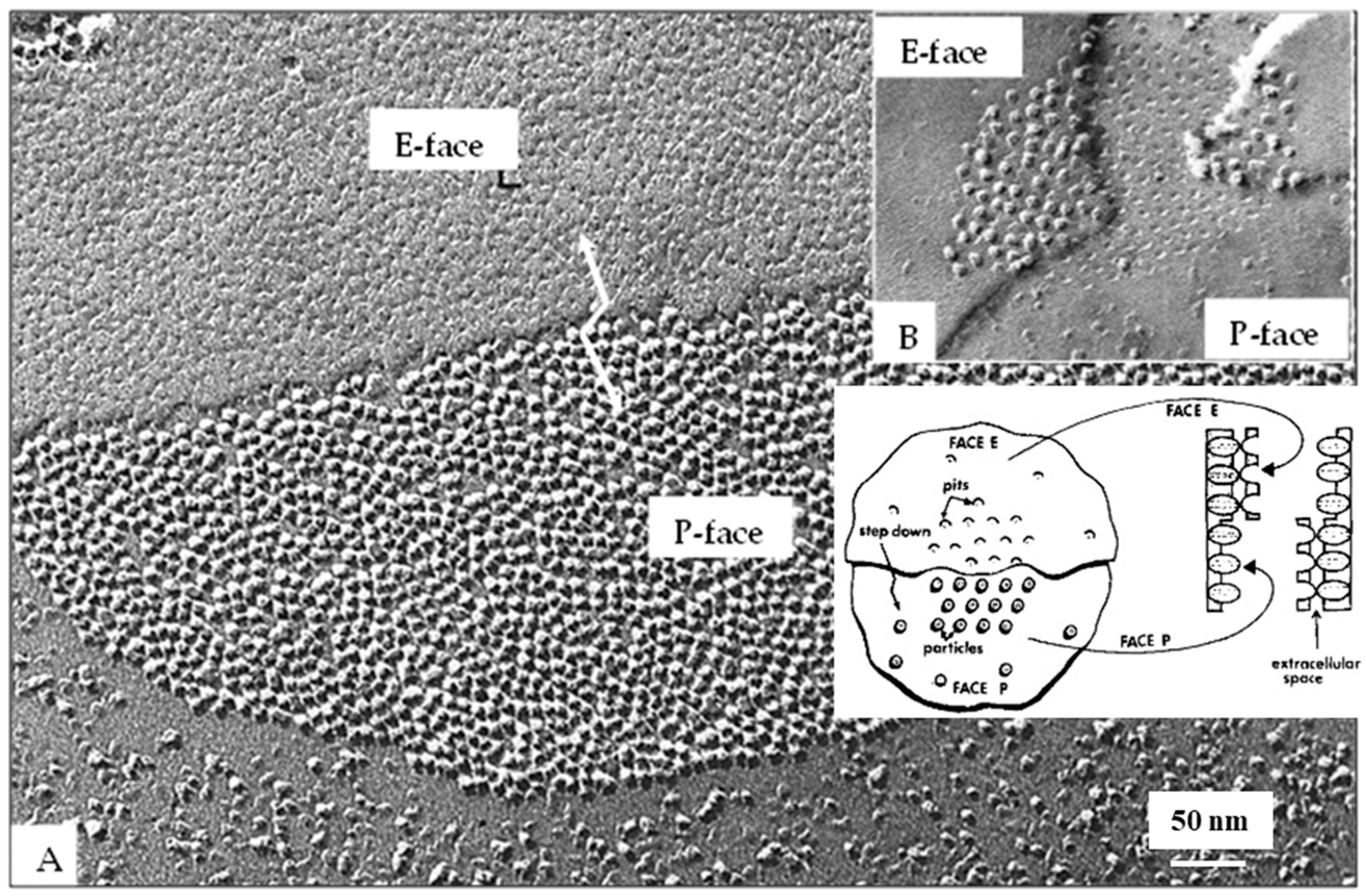
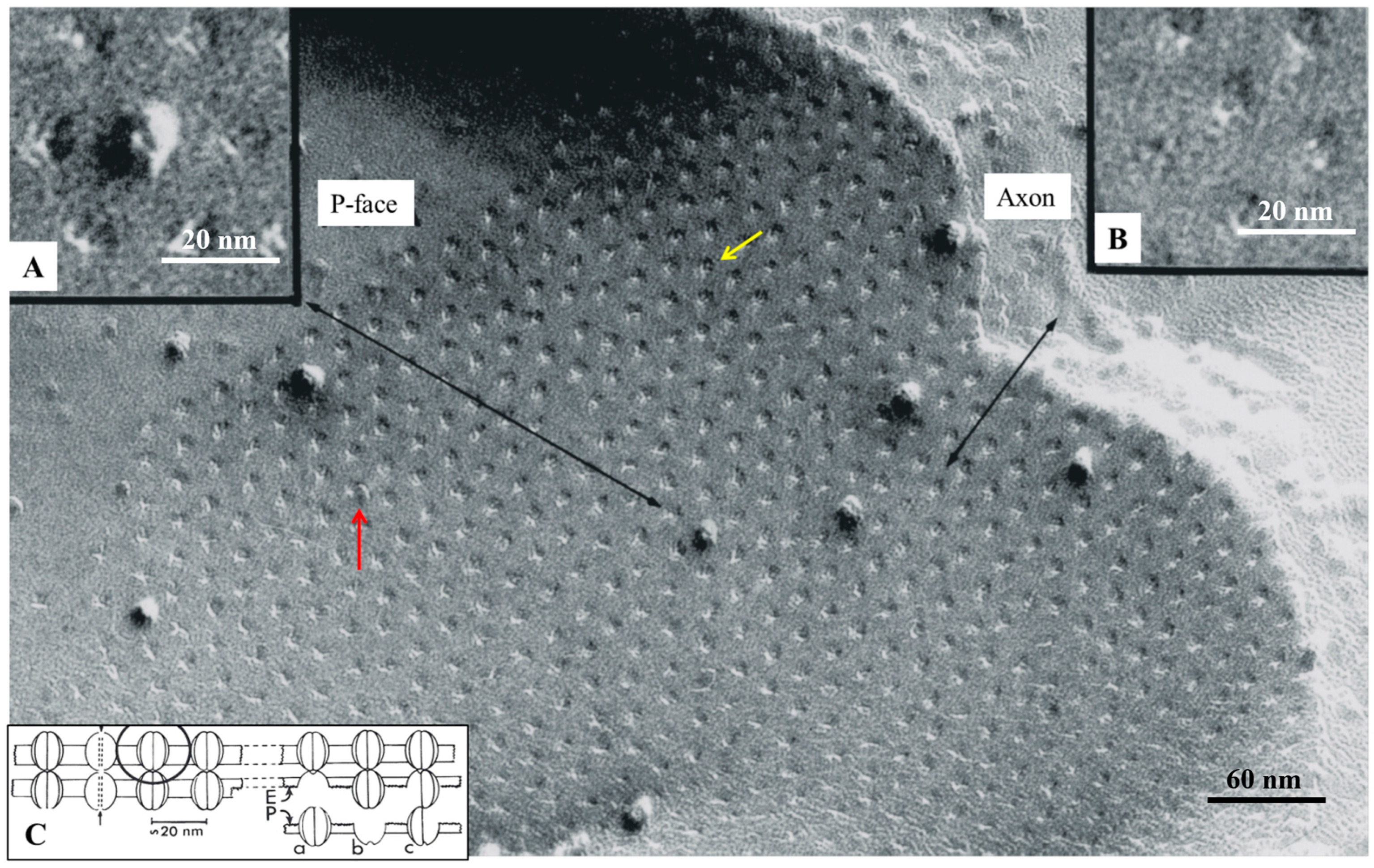
4.5. Freeze-Fracture of Gap Junctions
5. Early Models of Gap Junction Architecture
5.1. Model of Chalcroft and Bullivant
5.2. Model of Sommer and Steer
5.3. Model of Spycher
5.4. Model of McNutt–Weinstein
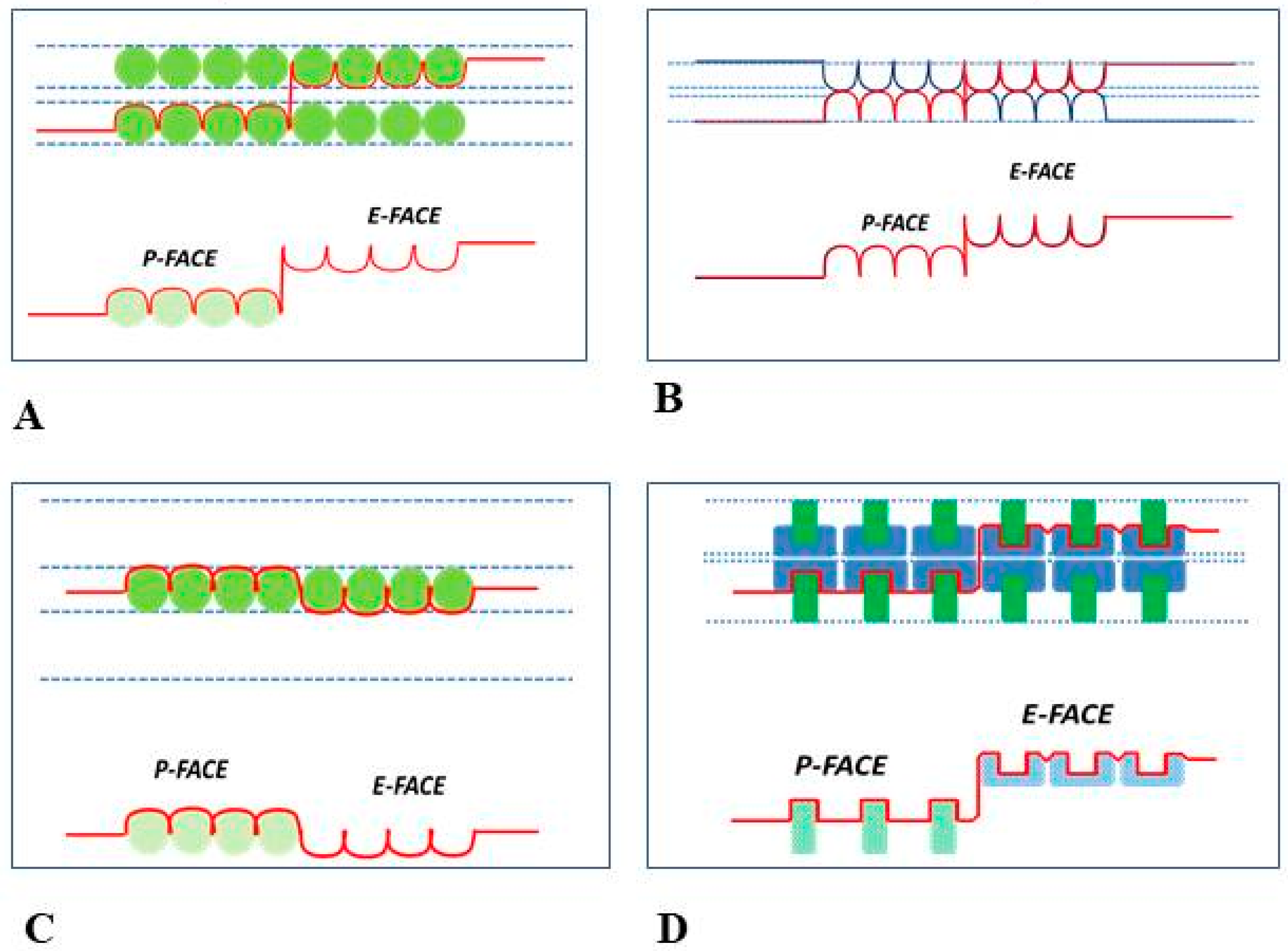
5.5. The Correct Model of Gap Junction Architecture
6. The Gap Junction Channels Span the Thickness of the Membrane
7. The Channels Project from Both Surfaces of the Membrane
8. The Channels Are Made of Six Radially Arranged Subunits (Hexamers)
9. The Channels Display Dimples on the Cytoplasmic and Extracellular Ends, Representing the Cytoplasmic and Extracellular Channel Opening, Respectively
Freeze-Fracture Images of Central Pits on the Cytoplasmic and Extracellular Ends Match the Electron-Dense Spots Seen in Sections Negatively Stained as Well as in Junctions Isolated, Demonstrating the Location of the Channel
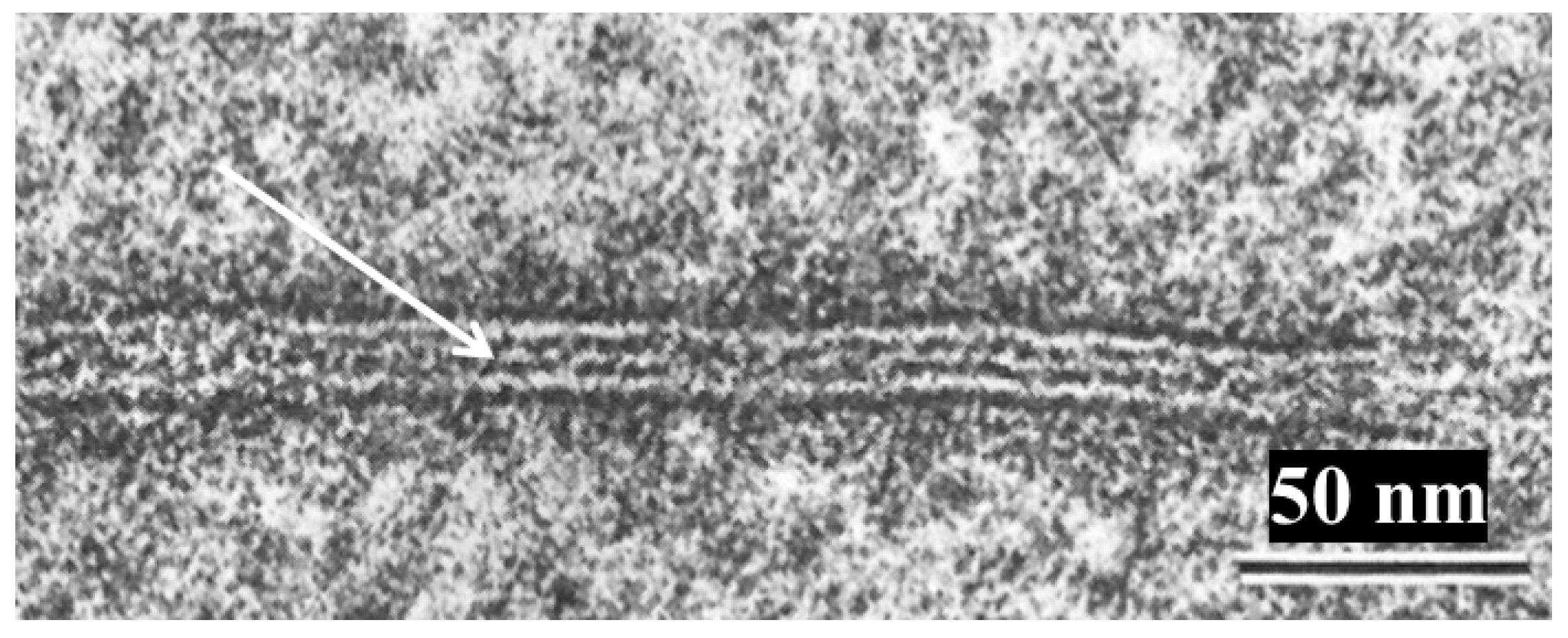
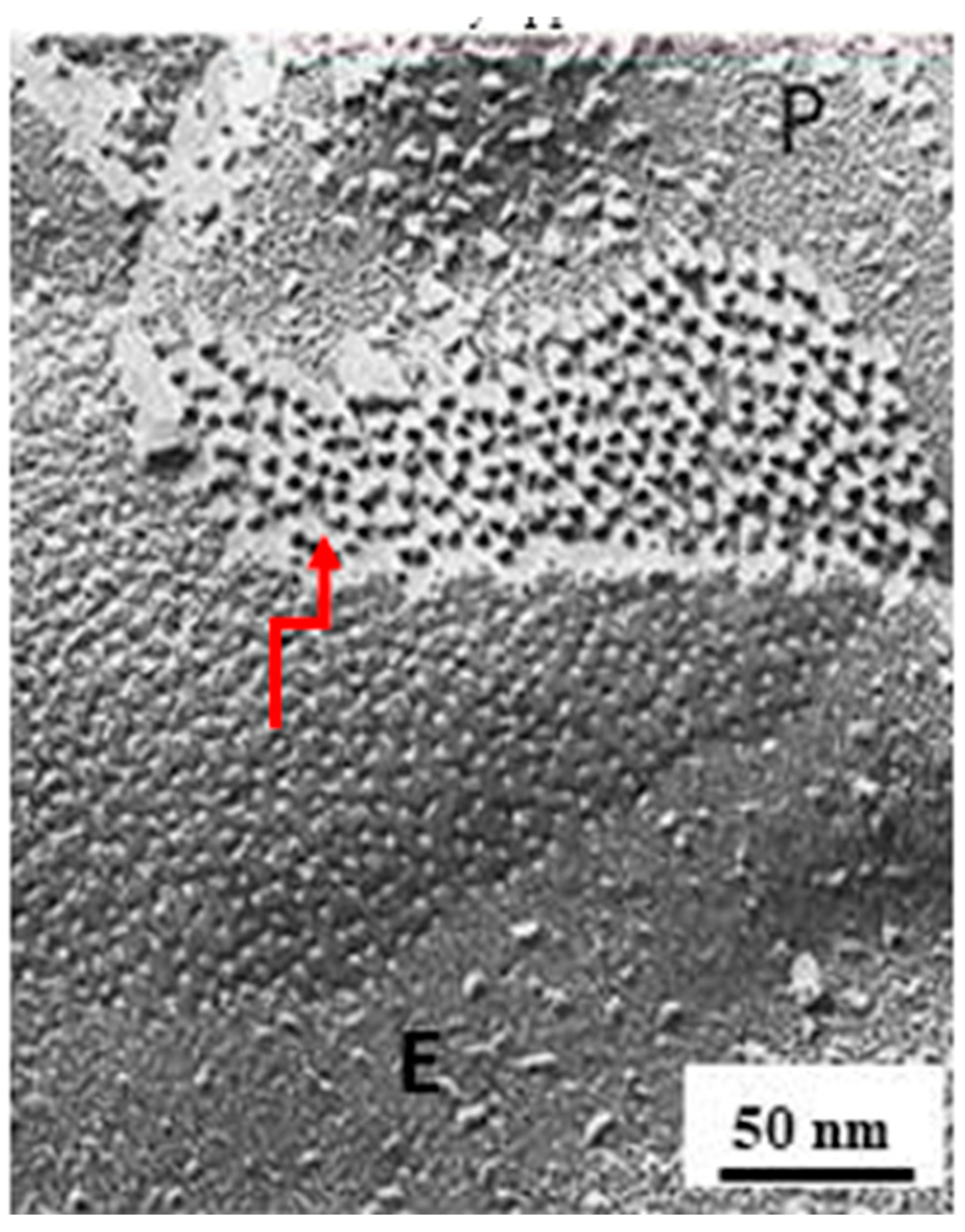
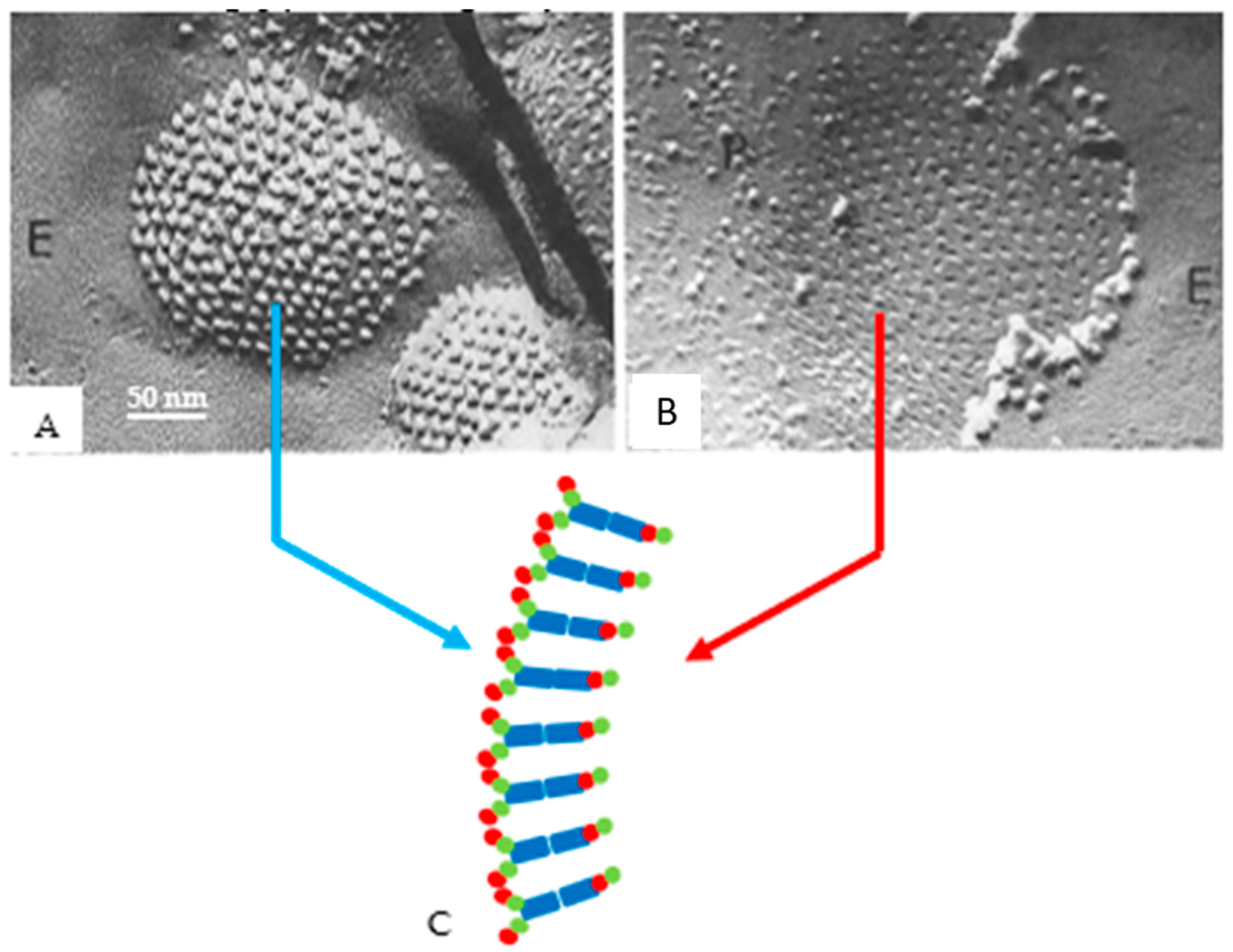
10. The Channels Are in Register and Are Linked to Each Other Across the Gap
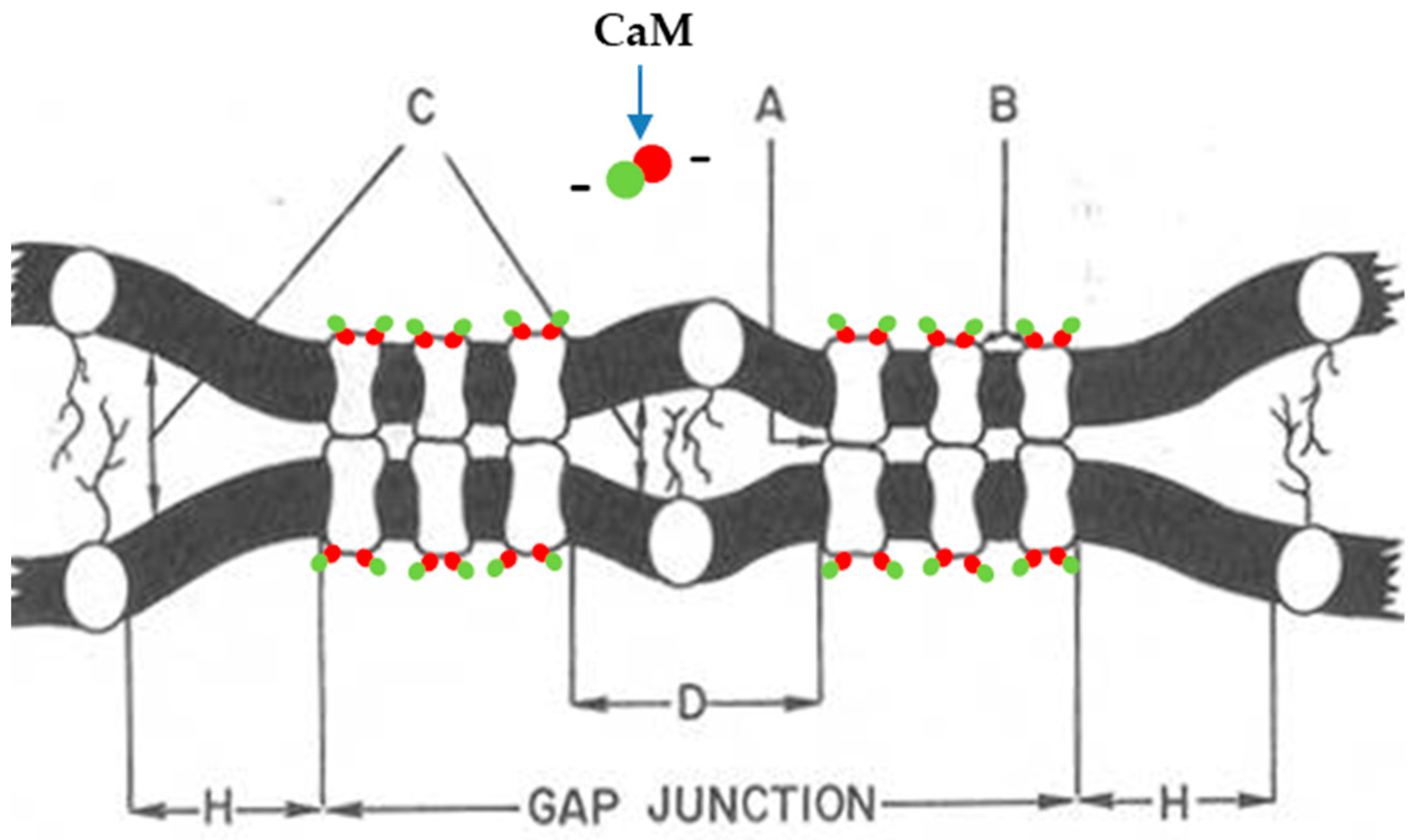
11. Calmodulin (CaM) Is an Accessory Protein of Connexin/Innexin Channels
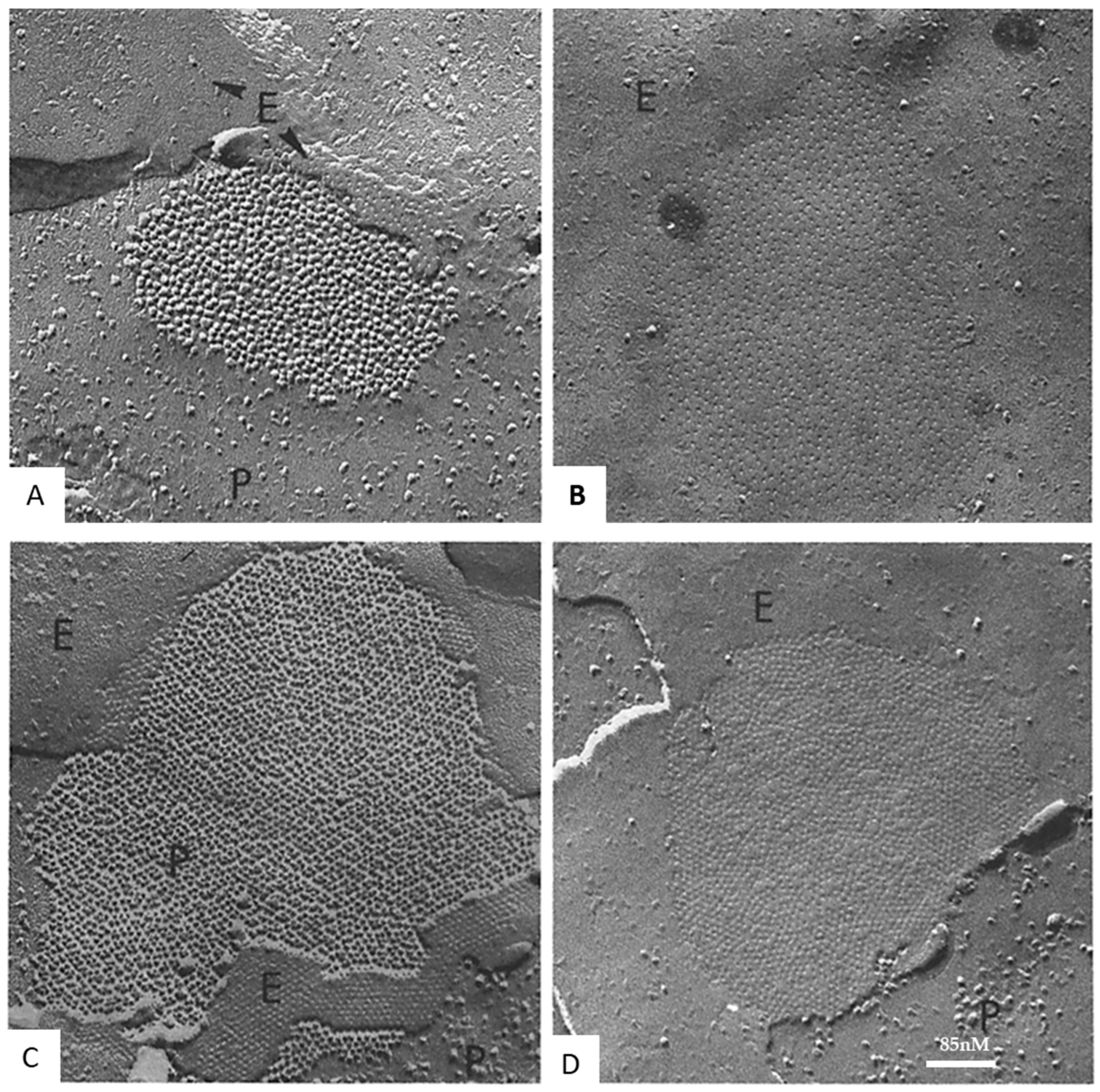
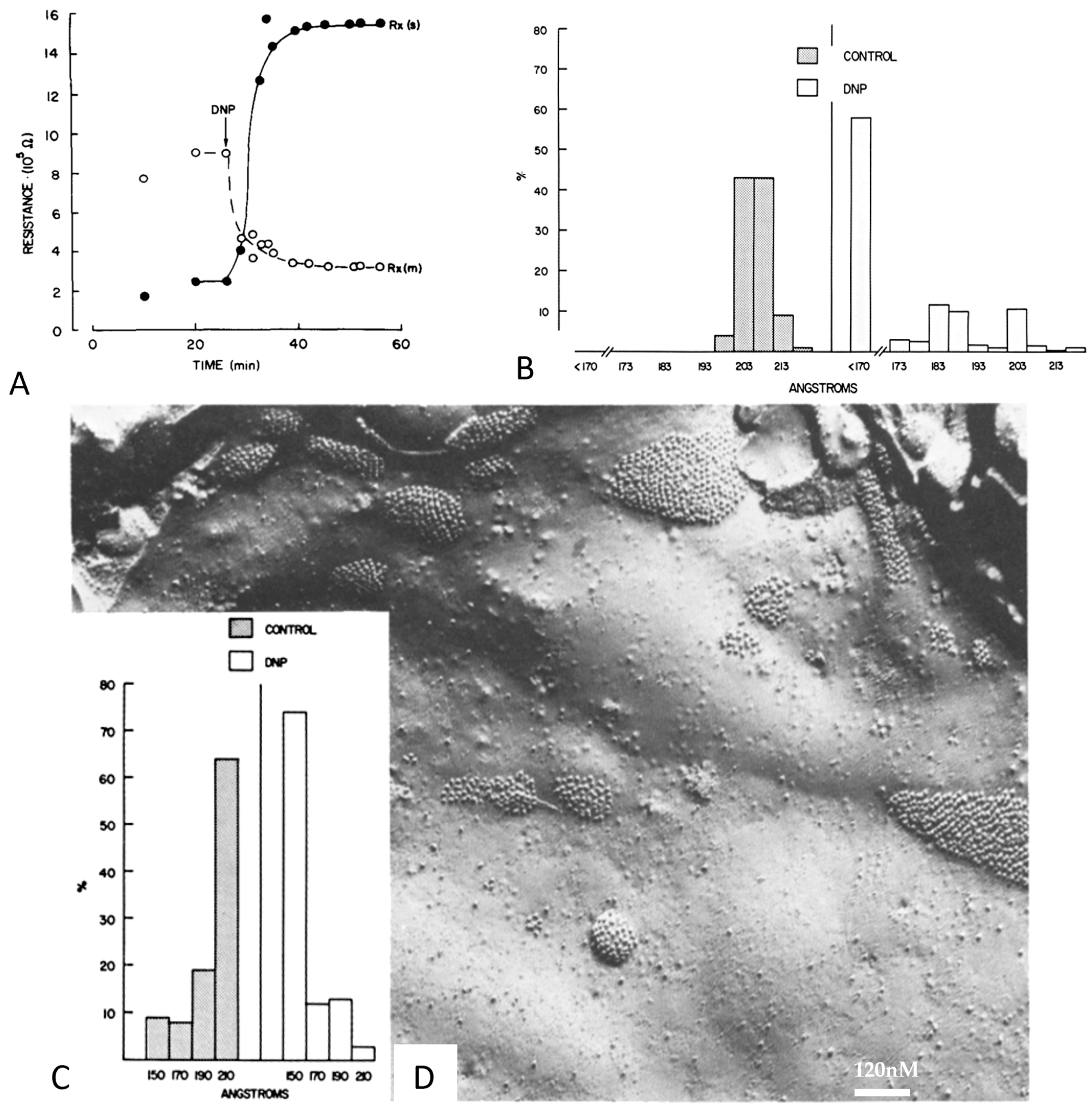
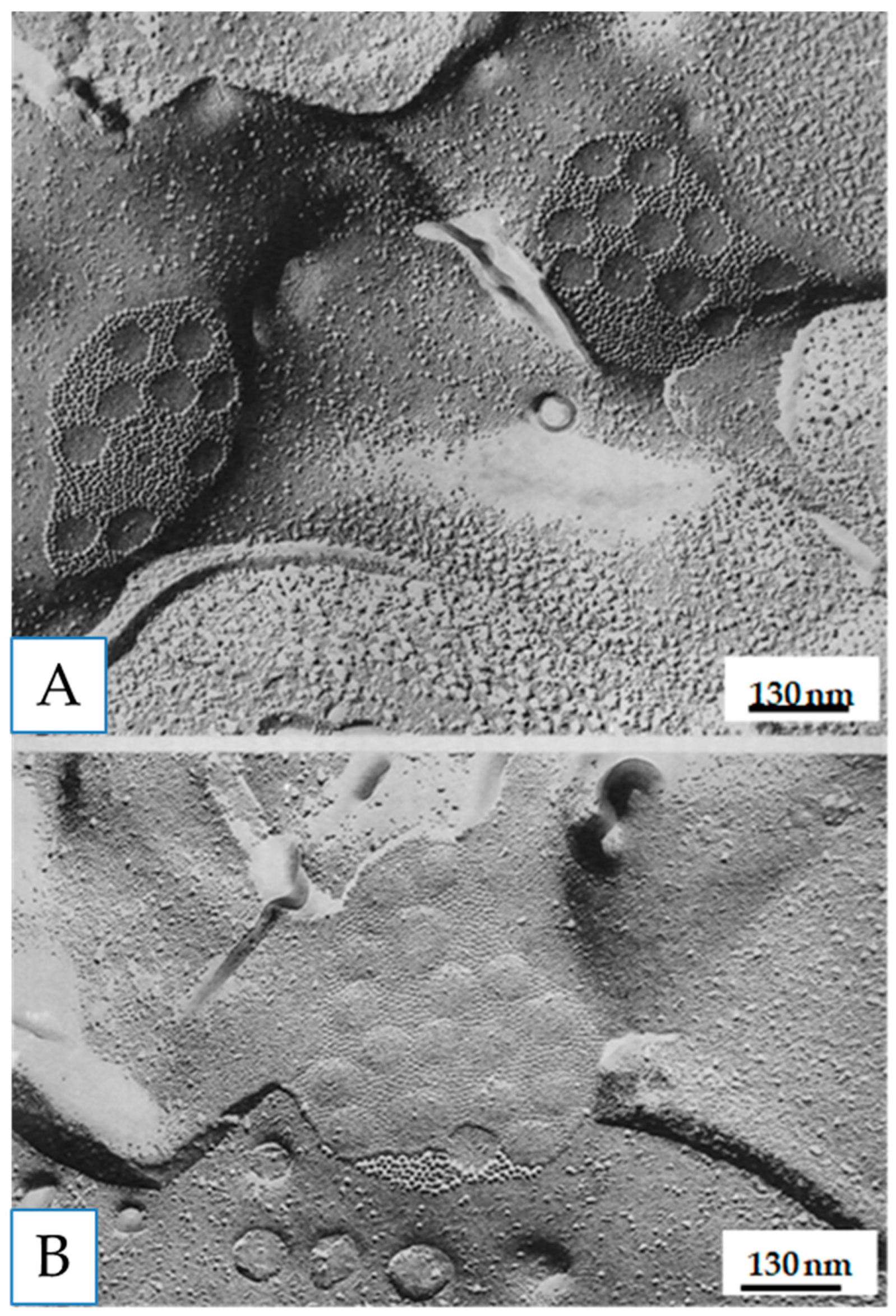
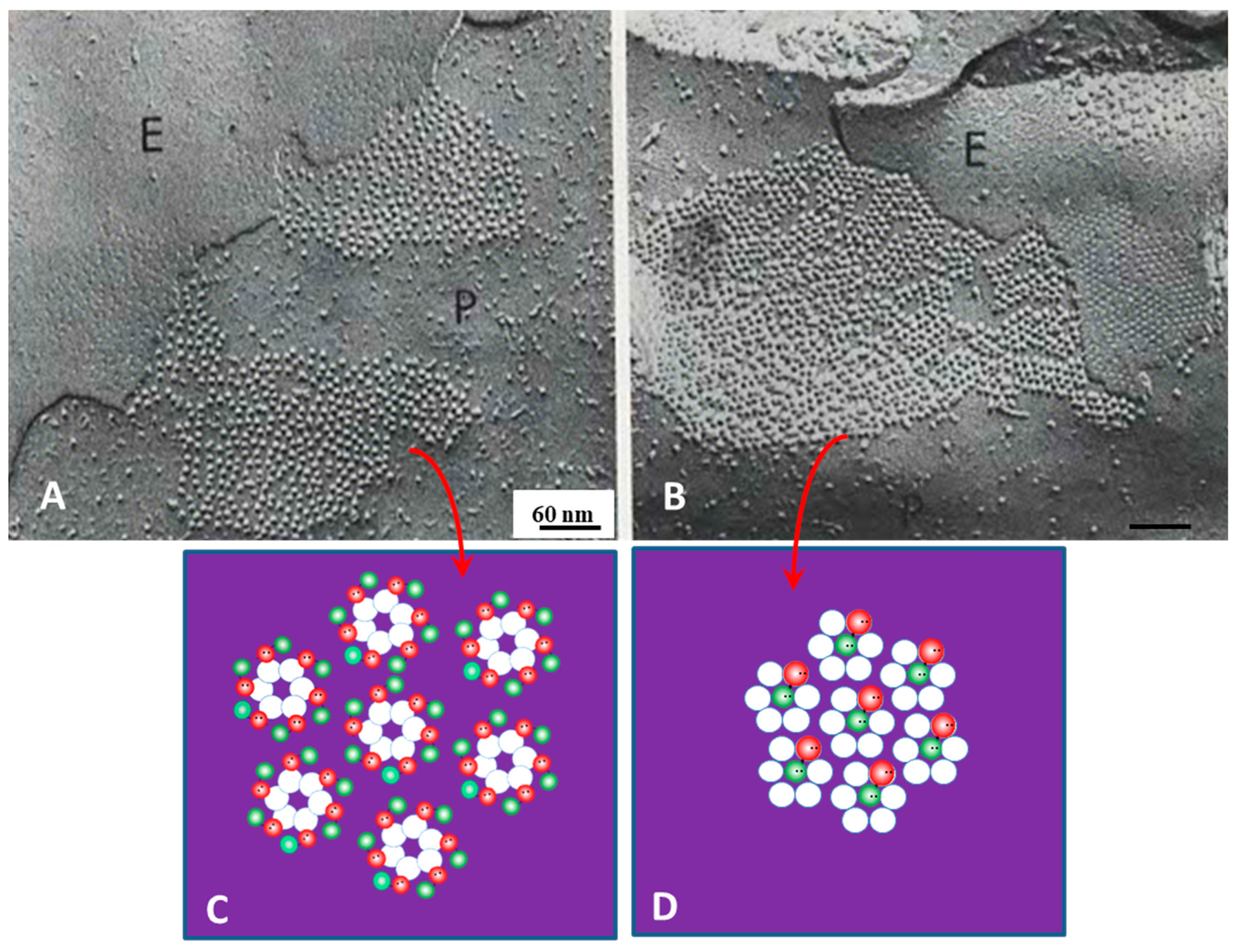
12. Channel Arrays in Coupled and Uncoupled Gap Junctions
13. Calmodulin Role in Gap Junction Channel Gating and Aggregation
14. What Causes Different Channel Aggregations in Gap Junctions?
15. Dome-Shaped Gap Junctions
16. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagielnicki, M.; Kucharska, I.; Bennett, B.C.; Harris, A.L.; Yeager, M. Connexin Gap Junction Channels and Hemichannels: Insights from High-Resolution Structures. Biology 2024, 13, 298. [Google Scholar] [CrossRef]
- Khan, A.K.; Jagielnicki, M.; Bennett, B.C.; Purdy, M.D.; Yeager, M. Cryo-EM structure of an open conformation of a gap junction hemichannel in lipid bilayer nanodiscs. Structure 2021, 29, 1040–1047.e3. [Google Scholar] [CrossRef]
- Stough, H.B. Giant nerve fibers of the earthworn. J. Comp. Neurol. 1926, 40, 409–463. [Google Scholar] [CrossRef]
- Stough, H.B. Polarization of the giant nerve fibers of the earthworm. J. Comp. Neurol. 1930, 50, 217–229. [Google Scholar] [CrossRef]
- Boule, L. Recherches sur le système nerveux central normal du Lombric. Névraxe 1908, 10, 16–58. [Google Scholar]
- Johnson, G.E. Giant nerve fibers in crustaceans, with special reference to Cambarus and Palaemonetes. J. Comp. Neurol. 1924, 36, 323–375. [Google Scholar] [CrossRef]
- Smallwood, W.M.; Holmes, M.T. The neurofibrillar structure of the giant fibers in Lumbricus terrestris and Eisenia foetida. J. Comp. Neurol. 1927, 43, 327–345. [Google Scholar] [CrossRef]
- Kanno, Y.; Loewenstein, W.R. Low-resistance coupling between gland cells. Some observations on intercellular contact membranes and intercellular space. Nature 1964, 201, 194–195. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Kanno, Y. Studies on an epithelial (gland) cell junction: I. modifications of surface membrane permeability. J. Cell Biol. 1964, 22, 565–586. [Google Scholar] [CrossRef]
- Loewenstein, W.R.; Socolar, S.J.; Higashino, S.; Kanno, Y.; Davidson, N. Intercellular Communication: Renal, Urinary Bladder, Sensory, and Salivary Gland Cells. Science 1965, 149, 295–298. [Google Scholar] [CrossRef]
- Kuffler, S.W.; Potter, D.D. Glia in the leech central nervous system: Physiological properties and neuron-glia relationship. J. Neurophysiol. 1964, 27, 290–320. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D. Ultrastructure of two invertebrate synapses. Proc. Soc. Exp. Biol. Med. 1953, 82, 219–223. [Google Scholar] [CrossRef]
- Robertson, J.D. Recent electron microscope observations on the ultrastructure of the crayfish median-to-motor giant synapse. Exp. Cell Res. 1955, 8, 226–229. [Google Scholar] [CrossRef]
- Issidorides, M. Ultrastructure of the synapse in the giant axons of the earthworm. Exp. Cell Res. 1956, 11, 423–436. [Google Scholar] [CrossRef]
- Robertson, J.D. New observations on the ultrastructure of the membranes of frog peripheral nerve fibers. J. Biophys. Biochem. Cytol. 1957, 3, 1043–1048. [Google Scholar] [CrossRef]
- Robertson, J.D. The molecular structure and contact relationships of cell membranes. Prog. Biophys. Mol. Biol. 1960, 10, 343–418. [Google Scholar] [CrossRef]
- De Lorenzo, A.J. Electron microscopy of the electrical synapses in the crayfish. Biol. Bull. 1960, 119, 325. [Google Scholar]
- Hama, K. Some observations on the fine structure of the giant fibers of the crayfishes (Cambarus virilus and Cambarus clarkii) with special reference to the submicroscopic organization of the synapses. Anat. Rec. 1961, 141, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Hama, K. Some observations on the fine structure of the giant nerve fibers of the earthworm, Eisenia foetida. J. Biophys. Biochem. Cytol. 1959, 6, 61–66. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, E.; Franchi, C.M. Electron microscope observations on synaptic vesicles in synapses of the retinal rods and cones. J. Biophys. Biochem. Cytol. 1956, 2, 307–318. [Google Scholar] [CrossRef]
- Peracchia, C. Low resistance junctions in crayfish: I. Two arrays of globules in junctional membranes. J. Cell Biol. 1973, 57, 66–76. [Google Scholar] [CrossRef]
- Sjostrand, F.S.; Andersson-Cedergren, E.; Dewey, M.M. The ultrastructure of the intercalated discs of frog, mouse and guinea pig cardiac muscle. J. Ultrastruct. Res. 1958, 1, 271–287. [Google Scholar] [CrossRef]
- Karrer, H.E. Cell interconnections in normal human cervical epithelium. J. Biophys. Biochem. Cytol. 1960, 7, 181–184. [Google Scholar] [CrossRef]
- Karrer, H.E. The striated musculature of blood vessels: II. Cell interconnections and cell surface. J. Biophys. Biochem. Cytol. 1960, 8, 135–150. [Google Scholar] [CrossRef]
- Dewey, M.M.; Barr, L. Intercellular Connection between Smooth Muscle Cells: The Nexus. Science 1962, 137, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.R.; Peters, A. Quintuple-layered membrane junctions at terminal bars between endothelial cells. J. Cell Biol. 1962, 12, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Cell junctions in amphibian skin. J. Cell Biol. 1965, 26, 263–291. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, E.; Ryter, A.; Sechaud, J. Electron microscope study of DNA-containing plasms: II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J. Biophys. Biochem. Cytol. 1958, 4, 671–678. [Google Scholar] [CrossRef]
- Revel, J.P.; Karnovsky, M.J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 1967, 33, C7–C12. [Google Scholar] [CrossRef]
- Revel, J.P. Studies on the Fine Structure of Intercellular Junctions; Proceedings Electron Microscopy Society of America; Claitor’s Publishing Division: Baton Rouge, LA, USA, 1968; p. 40. [Google Scholar]
- Peracchia, C. Gap Junction Stucture and Chemical Regulation: Direct Calmodulin Role in Cell-to-Cell Channel Gating; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019. [Google Scholar]
- Doggenweiler, C.F.; Frenk, S. Staining properties of lanthanum on cell membranes. Proc. Natl. Acad. Sci. USA 1965, 53, 425–430. [Google Scholar] [CrossRef]
- Friend, D.S.; Gilula, N.B. Variations in tight and gap junctions in mammalian tissues. J. Cell Biol. 1972, 53, 758–776. [Google Scholar] [CrossRef]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Revel, J.P. The permeability of isolated and in situ mouse hepatic gap junctions studied with enzymatic tracers. J. Cell Biol. 1971, 50, 81–91. [Google Scholar] [CrossRef]
- Peters, A. Plasma membrane contacts in the central nervous system. J. Anat. 1962, 96, 237–248. [Google Scholar]
- Robertson, J.D. The occurrence of a subunit pattern in the unit membranes of club endings in mauthner cell synapses in goldfish brains. J. Cell Biol. 1963, 19, 201–221. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Propagation of electrical signals along giant nerve fibers. Proc. R. Soc. Lond B Biol. Sci. 1952, 140, 177–183. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 1952, 116, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.L.; Emmelot, P. Electron microscopic observations on negatively stained plasma membranes isolated from rat liver. J. Cell Biol. 1965, 26, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Payton, B.W.; Bennett, M.V.; Pappas, G.D. Permeability and structure of junctional membranes at an electrotonic synapse. Science 1969, 166, 1641–1643. [Google Scholar] [CrossRef] [PubMed]
- McNutt, N.S.; Weinstein, R.S. The ultrastructure of the nexus: A correlated thin-section and freeze-cleave study. J. Cell Biol. 1970, 47, 666–688. [Google Scholar] [CrossRef]
- Rose, B. Intercellular communication and some structural aspects of membrane junctions in a simple cell system. J. Membr. Biol. 1971, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J.; Revel, J.P. Coexistence of gap and septate junctions in an invertebrate epithelium. J. Cell Biol. 1971, 50, 92–101. [Google Scholar] [CrossRef]
- Hand, A.R.; Gobel, S. The structural organization of the septate and gap junctions of Hydra. J. Cell Biol. 1972, 52, 397–408. [Google Scholar] [CrossRef]
- Peracchia, C. Low resistance junctions in crayfish: II. Structural details and further evidence for intercellular channels by freeze-fracture and negative staining. J. Cell Biol. 1973, 57, 54–65. [Google Scholar] [CrossRef]
- Robertson, J.D.; Bodenheimer, T.S.; Stage, D.E. The ultrastructure of Mauthner cell synapses and nodes in goldfish brains. J. Cell Biol. 1963, 19, 159–199. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, E.L.; Emmelot, P. Hexagonal array of subunits in tight junctions separated from isolated rat liver plasma membranes. J. Cell Biol. 1968, 38, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Steere, R.L. Electron microscopy of structural detail in frozen biological specimens. J. Biophys. Biochem. Cytol. 1957, 3, 45–60. [Google Scholar] [CrossRef]
- Moor, H.; Muhlethaler, K.; Waldner, H.; Frey-Wyssling, A. A new freezing-ultramicrotome. J. Biophys. Biochem. Cytol. 1961, 10, 1–13. [Google Scholar] [CrossRef]
- Branton, D. Fracture faces of frozen membranes. Proc. Natl. Acad. Sci. USA 1966, 55, 1048–1056. [Google Scholar] [CrossRef]
- Deamer, D.W.; Branton, D. Fracture planes in an ice-bilayer model membrane system. Science 1967, 158, 655–657. [Google Scholar] [CrossRef]
- Spycher, M.A. Intercellular adhesions: An electron microscope study on freeze-etched rat hepatocytes. Z. Zellforsch. Mikrosk. Anat. 1970, 111, 64–74. [Google Scholar] [CrossRef]
- McNutt, N.S.; Weinstein, R.S. Interlocking Subunit Arrays Forming Nexus Membranes; Caitor’s Publishing Division: Carlstadt, NJ, USA, 1969; pp. 330–331. [Google Scholar]
- Sommer, J.R.; Steere, R.L. The nexus freeze-etched. J. Cell Biol. 1969, 43, 136a. [Google Scholar]
- Chalcroft, J.P.; Bullivant, S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J. Cell Biol. 1970, 47, 49–60. [Google Scholar] [CrossRef]
- Kreutziger, G.O. Freeze-Etching of Intercellular Junctions of Mouse Liver; Caitor’s Publishing Division: Carlstadt, NJ, USA, 1968; pp. 234–235. [Google Scholar]
- Bullivant, S. Freeze-fracturing of biological materials. Micron 1969, 1, 46–51. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Revel, J.P. A fine structural analysis of intercellular junctions in the mouse liver. J. Cell Biol. 1970, 45, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M. Cardiac gap junction configuration after an uncoupling treatment as a function of time. J. Cell Biol. 1979, 82, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Steere, R.L.; Sommer, J.R. Stereo ultrastructure of nexus faces exposed by freeze-fracturing. J. Microsc. 1972, 15, 205–218. [Google Scholar]
- Pinto da Silva, P.; Branton, D. Membrane splitting in freeze-ethching: Covalently bound ferritin as a membrane marker. J. Cell Biol. 1970, 45, 598–605. [Google Scholar]
- Tillack, T.W.; Marchesi, V.T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J. Cell Biol. 1970, 45, 649–653. [Google Scholar] [CrossRef]
- Peracchia, C.; Peracchia, L.L. Gap junction dynamics: Reversible effects of divalent cations. J. Cell Biol. 1980, 87 Pt 1, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M. The fine structure of healing over in mammalian cardiac muscle. J. Mol. Cell Cardiol. 1977, 9, 959–966. [Google Scholar] [CrossRef]
- Barr, L.; Dewey, M.M.; Brrger, W. Propagation of action potentials and the structure of the nexus in cardiac muscle. J. Gen. Physiol. 1965, 48, 797–823. [Google Scholar] [CrossRef]
- Peracchia, C.; Leverone Peracchia, L.M. Calmodulin-Connexin Partnership in Gap Junction Channel Regulation-Calmodulin-Cork Gating Model. Int. J. Mol. Sci. 2021, 22, 13055. [Google Scholar] [CrossRef]
- Peracchia, C.; Dulhunty, A.F. Low resistance junctions in crayfish. Structural changes with functional uncoupling. J. Cell Biol. 1976, 70 Pt 1, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Gap junctions. Structural changes after uncoupling procedures. J. Cell Biol. 1977, 72, 628–641. [Google Scholar] [CrossRef]
- Peracchia, C. Calcium effects on gap junction structure and cell coupling. Nature 1978, 271, 669–671. [Google Scholar] [CrossRef]
- Peracchia, C. Structural correlates of gap junction permeation. Int. Rev. Cytol. 1980, 66, 81–146. [Google Scholar] [PubMed]
- Asada, Y.; Bennett, M.V. Experimental alteration of coupling resistance at an electrotonic synapse. J. Cell Biol. 1971, 49, 159–172. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Gilula, N.B. The splitting of hepatocyte gap junctions and zonulae occludentes with hypertonic disaccharides. J. Cell Biol. 1974, 61, 575–590. [Google Scholar] [CrossRef]
- Peracchia, C.; Bernardini, G. Gap junction structure and cell-to-cell coupling regulation: Is there a calmodulin involvement? Fed. Proc. 1984, 43, 2681–2691. [Google Scholar]
- Noma, A.; Tsuboi, N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J. Physiol. 1987, 382, 193–211. [Google Scholar] [CrossRef]
- Noma, A.; Tsuboi, N. Direct measurement of the gap junctional conductance under the influence of Ca2+ in dissociated paired myocytes of guinea-pig. Jpn. Heart J. 1986, 27 (Suppl. S1), 161–166. [Google Scholar]
- Dekker, L.R.; Fiolet, J.W.; VanBavel, E.; Coronel, R.; Opthof, T.; Spaan, J.A.; Janse, M.J. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle: Effects of preconditioning and metabolic blockade. Circ. Res. 1996, 79, 237–246. [Google Scholar] [CrossRef]
- Peracchia, C. Effects of caffeine and ryanodine on low pHi-induced changes in gap junction conductance and calcium concentration in crayfish septate axons. J. Membr. Biol. 1990, 117, 79–89. [Google Scholar] [CrossRef]
- Peracchia, C. Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J. Membr. Biol. 1990, 113, 75–92. [Google Scholar] [CrossRef]
- Peracchia, C.; Wang, X.; Li, L.; Peracchia, L.L. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. Pflügers. Arch. 1996, 431, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Neyton, J.; Trautmann, A. Single-channel currents of an intercellular junction. Nature 1985, 317, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Peracchia, C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys. J. 1993, 65, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Peres, A.; Giovannardi, S.; Peracchia, C. Ca-mediated and independent effects of arachidonic acid on gap junctions and Ca-independent effects of oleic acid and halothane. Biophys. J. 1994, 67, 1052–1059. [Google Scholar] [CrossRef]
- Enkvist, M.O.; McCarthy, K.D. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J. Neurochem. 1994, 62, 489–495. [Google Scholar] [CrossRef]
- Giaume, C.; Venance, L. Characterization and regulation of gap junction channels in cultured astrocytes. In Gap Junctions in the Nervous System; Spray, D.C., Dermietzel, R., Eds.; R.G Landes Medical Pub.: Austin, TX, USA, 1996; pp. 135–157. [Google Scholar]
- Cotrina, M.L.; Kang, J.; Lin, J.H.; Bueno, E.; Hansen, T.W.; He, L.; Liu, Y.; Nedergaard, M. Astrocytic gap junctions remain open during ischemic conditions. J. Neurosci. 1998, 18, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.M.; Atkinson, M.M.; Johnson, R.G. Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3332–3341. [Google Scholar]
- Dakin, K.; Zhao, Y.; Li, W.H. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat. Methods 2005, 2, 55–62. [Google Scholar] [CrossRef]
- Xu, Q.; Kopp, R.F.; Chen, Y.; Yang, J.J.; Roe, M.W.; Veenstra, R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012, 302, C1548–C1556. [Google Scholar] [CrossRef]
- Matthews, E.K.; Petersen, O.H. Pancreatic acinar cells: Ionic dependence of the membrane potential and acetycholine-induced depolarization. J. Physiol. 1973, 231, 283–295. [Google Scholar] [CrossRef]
- Scheele, G.A.; Palade, G.E. Studies on the guinea pig pancreas. Parallel discharge of exocrine enzyme activities. J. Biol. Chem. 1975, 250, 2660–2670. [Google Scholar] [CrossRef]
- Iwatsuki, N.; Petersen, O.H. Membrane potential, resistance, and intercellular communication in the lacrimal gland: Effects of acetylcholine and adrenaline. J. Physiol. 1978, 275, 507–520. [Google Scholar] [CrossRef]
- Iwatsuki, N.; Petersen, O.H. Pancreatic acinar cells: Acetylcholine-evoked electrical uncoupling and its ionic dependency. J. Physiol. 1978, 274, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, N.; Petersen, O.H. Electrical coupling and uncoupling of exocrine acinar cells. J. Cell Biol. 1978, 79 Pt 1, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Mears, D.; Sheppard, N.F., Jr.; Atwater, I.; Rojas, E. Magnitude and modulation of pancreatic beta-cell gap junction electrical conductance in situ. J. Membr. Biol. 1995, 146, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Nakas, M.; Socolar, S.J. Junctional membrane uncoupling: Permeability transformations at a cell membrane junction. J. Gen. Physiol. 1967, 50, 1865–1891. [Google Scholar] [CrossRef]
- Rose, B.; Loewenstein, W.R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature 1975, 254, 250–252. [Google Scholar] [CrossRef]
- Rose, B.; Loewenstein, W.R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: A study with aequorin. J. Membr. Biol. 1976, 28, 87–119. [Google Scholar] [CrossRef]
- Kretsinger, R.H. Hipothesis: Calcium modulated proteins contain EF-hands. In Calcium Transport in Contraction and Secretion; Carafoli, E., Clementi, F., Drabinowski, W., Margreth, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1975; pp. 469–478. [Google Scholar]
- Kretsinger, R.H.; Barry, C.D. The predicted structure of the calcium-binding component of troponin. Biochim. Biophys. Acta 1975, 405, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Persechini, A.; Moncrief, N.D.; Kretsinger, R.H. The EF-hand family of calcium-modulated proteins. Trends. Neurosci. 1989, 12, 462–467. [Google Scholar] [CrossRef]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef]
- Zou, J.; Salarian, M.; Chen, Y.; Veenstra, R.; Louis, C.F.; Yang, J.J. Gap junction regulation by calmodulin. FEBS Lett. 2014, 588, 1430–1438. [Google Scholar] [CrossRef]
- Lurtz, M.M.; Louis, C.F. Calmodulin and protein kinase C regulate gap junctional coupling in lens epithelial cells. Am. J. Physiol. Cell Physiol. 2003, 285, C1475–C1482. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Y. Role of intramolecular interaction in connexin50: Mediating the Ca2+-dependent binding of calmodulin to gap junction. Arch. Biochem. Biophys. 2005, 440, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Liu, Y.; Qi, Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol. Chem. 2006, 387, 595–601. [Google Scholar] [CrossRef]
- Siu, R.C.; Smirnova, E.; Brown, C.A.; Zoidl, C.; Spray, D.C.; Donaldson, L.W.; Zoidl, G. Structural and Functional Consequences of Connexin 36 (Cx36) Interaction with Calmodulin. Front. Mol. Neurosci. 2016, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Salarian, M.; Chen, Y.; Zhuo, Y.; Brown, N.E.; Hepler, J.R.; Yang, J. Direct Visualization of Interaction between Calmodulin and Connexin45. Biochem. J. 2017, 474, 4035–4051. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.; Peracchia, C.; Stolady, D.; Török, K. Calmodulin association with connexin32-derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J. Biol. Chem. 2008, 283, 26911–26920. [Google Scholar] [CrossRef]
- Stauch, K.; Kieken, F.; Sorgen, P. Characterization of the structure and intermolecular interactions between the connexin 32 carboxyl-terminal domain and the protein partners synapse-associated protein 97 and calmodulin. J. Biol. Chem. 2012, 287, 27771–27788. [Google Scholar] [CrossRef]
- Burr, G.S.; Mitchell, C.K.; Keflemariam, Y.J.; Heidelberger, R.; O’Brien, J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem. Biophys. Res. Commun. 2005, 335, 1191–1198. [Google Scholar] [CrossRef]
- Hu, Z.; Riquelme, M.A.; Gu, S.; Jiang, J.X. Regulation of Connexin Gap Junctions and Hemichannels by Calcium and Calcium Binding Protein Calmodulin. Int. J. Mol. Sci. 2020, 21, 8194. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Riquelme, M.A.; Wang, B.; Bugay, V.; Brenner, R.; Gu, S.; Jiang, J.X. Cataract-associated Connexin 46 Mutation Alters Its Interaction with Calmodulin and Function of Hemichannels. J. Biol. Chem. 2018, 293, 2573–2585. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Lin, X.; Wong, H.C.; Xu, Q.; Jiang, J.; Wang, S.; Lurtz, M.M.; Louis, C.F.; Veenstra, R.D.; et al. Molecular interaction and functional regulation of connexin50 gap junctions by calmodulin. Biochem. J. 2011, 435, 711–722. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Chen, Y.; Jiang, J.; Huang, Y.; Louis, C.F.; Yang, J.J. Calmodulin mediates the Ca2+-dependent regulation of Cx44 gap junctions. Biophys. J. 2009, 96, 2832–2848. [Google Scholar] [CrossRef]
- Myllykoski, M.; Kuczera, K.; Kursula, P. Complex formation between calmodulin and a peptide from the intracellular loop of the gap junction protein connexin43: Molecular conformation and energetics of binding. Biophys. Chem. 2009, 144, 130–135. [Google Scholar] [CrossRef]
- Török, K.; Stauffer, K.; Evans, W.H. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem. J. 1997, 326 Pt 2, 479–483. [Google Scholar] [CrossRef]
- Tran, O.; Kerruth, S.; Coates, C.; Kaur, H.; Peracchia, C.; Carter, T.; Török, K. Ca2+-dependent and -independent calmodulin binding to the cytoplasmic loop of gap junction connexins. Int. J. Mol. Sci. 2023, 24, 4153. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Bernardini, G.; Peracchia, L.L. Is calmodulin involved in the regulation of gap junction permeability? Pflügers Arch. 1983, 399, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.A.; Duncan, G.; Tomlinson, J.; Maraini, G. Mammalian lens inter-fiber resistance is modulated by calcium and calmodulin. Curr. Eye Res. 1990, 9, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Sotkis, A.; Wang, X.G.; Peracchia, L.L.; Persechini, A. Calmodulin directly gates gap junction channels. J. Biol. Chem. 2000, 275, 26220–26224. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Huang, X.; Bai, X.; Wang, Z.; Yang, R.; Deng, Q.; Gao, H. Disrupting the interaction between connexin 43 and calmodulin restores gap junction function and mitigates reperfusion arrhythmias. Sci. Rep. 2025, 15, 34716. [Google Scholar] [CrossRef]
- Wang, X.G.; Peracchia, C. Connexin 32/38 chimeras suggest a role for the second half of inner loop in gap junction gating by low pH. Am. J. Physiol. 1996, 271 Pt 1, C1743–C1749. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Ye, Y.; Huang, Y.; Lee, H.W.; Chen, Y.; Louis, C.F.; Yang, J.J. Identification of the calmodulin binding domain of connexin 43. J. Biol. Chem. 2007, 282, 35005–35017. [Google Scholar] [CrossRef]
- Wei, S.; Cassara, C.; Lin, X.; Veenstra, R.D. Calcium-calmodulin gating of a pH-insensitive isoform of connexin43 gap junctions. Biochem. J. 2019, 476, 1137–1148. [Google Scholar] [CrossRef]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: A revision. BMC Cell Biol. 2016, 17 (Suppl. S1), 11. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Calmodulin-Mediated Regulation of Gap Junction Channels. Int. J. Mol. Sci. 2020, 21, 485. [Google Scholar] [CrossRef]
- Black, D.J.; Tran, Q.K.; Persechini, A. Monitoring the total available calmodulin concentration in intact cells over the physiological range in free Ca2+. Cell Calcium 2004, 35, 415–425. [Google Scholar] [CrossRef]
- Wu, X.; Bers, D.M. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium 2007, 41, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Caspar, D.L.; Goodenough, D.A.; Phillips, W.C. Gap Junction Structures: III. The Effect of Variations in the Isolation Procedure. Biophys J. 1982, 37, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Caspar, D.L.; Phillips, W.C.; Goodenough, D.A. Gap junction structures: V. Structural chemistry inferred from X-ray diffraction measurements on sucrose accessibility and trypsin susceptibility. J. Mol. Biol. 1984, 174, 449–481. [Google Scholar] [CrossRef]
- Makowski, L. Structural domains in gap junctions: Imnplications for the control of intercellular communication. In Gap Junctions; Bennett, M.V.L.S., David, C., Eds.; Cold Spring Harbor Laboratories: New York, NY, USA, 1985; pp. 5–12. [Google Scholar]
- Oshima, A.; Tani, K.; Hiroaki, Y.; Fujiyoshi, Y.; Sosinsky, G.E. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc. Natl. Acad. Sci. USA 2007, 104, 10034–10039. [Google Scholar] [CrossRef]
- Bennett, B.C.; Purdy, M.D.; Baker, K.A.; Acharya, C.; McIntire, W.E.; Stevens, R.C.; Zhang, Q.; Harris, A.L.; Abagyan, R.; Yeager, M. An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat. Commun. 2016, 7, 8770. [Google Scholar] [CrossRef]
- Raviola, E.; Goodenough, D.A.; Raviola, G. Structure of rapidly frozen gap junctions. J. Cell Biol. 1980, 87, 273–279. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracchia, C. History of Gap Junction Architecture and Potential Role of Calmodulin in Channel Arrays. Int. J. Mol. Sci. 2025, 26, 11337. https://doi.org/10.3390/ijms262311337
Peracchia C. History of Gap Junction Architecture and Potential Role of Calmodulin in Channel Arrays. International Journal of Molecular Sciences. 2025; 26(23):11337. https://doi.org/10.3390/ijms262311337
Chicago/Turabian StylePeracchia, Camillo. 2025. "History of Gap Junction Architecture and Potential Role of Calmodulin in Channel Arrays" International Journal of Molecular Sciences 26, no. 23: 11337. https://doi.org/10.3390/ijms262311337
APA StylePeracchia, C. (2025). History of Gap Junction Architecture and Potential Role of Calmodulin in Channel Arrays. International Journal of Molecular Sciences, 26(23), 11337. https://doi.org/10.3390/ijms262311337





