Assessment of the Antimicrobial Activity of Cistus salviifolius L. and Helichrysum stoechas (L.) DC Extracts and Their Synergistic Potential with Conventional Antibiotics Against Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
2.2. Activity of Cistus salviifolius and Helichrysum stoechas Extracts Against S. aureus Strains
2.3. Combined Effects of C. salviifolius and H. stoechas Extracts and Antibiotics, and Evaluation of Modulatory Activity of These Extracts
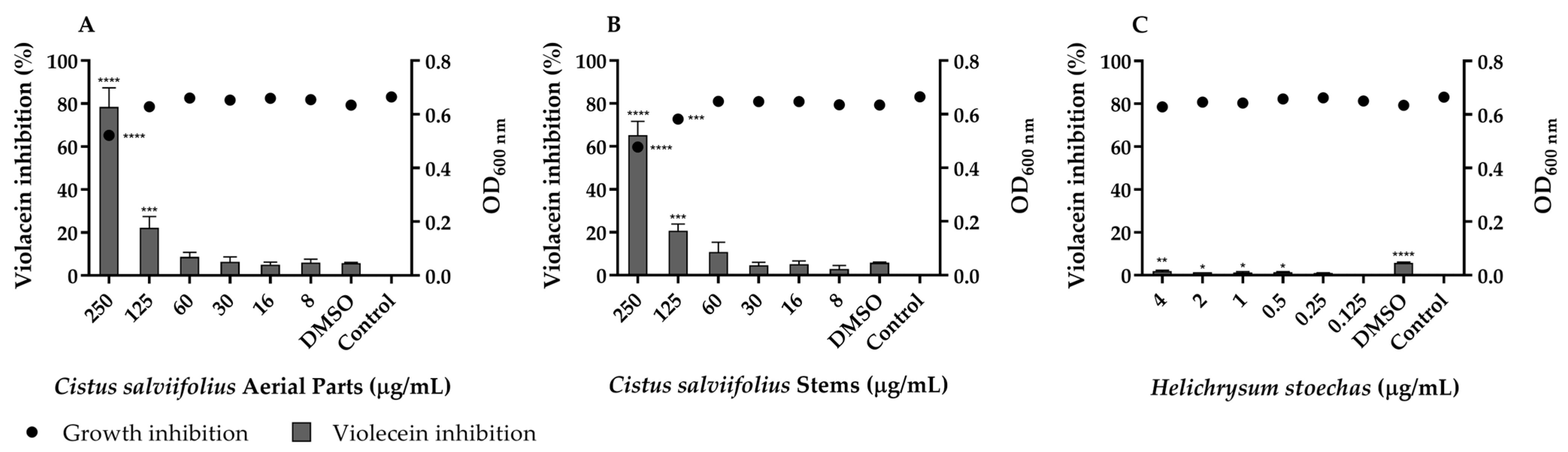
2.4. Inhibitory Effect of C. salviifolius and H. stoechas Extracts on Quorum Sensing
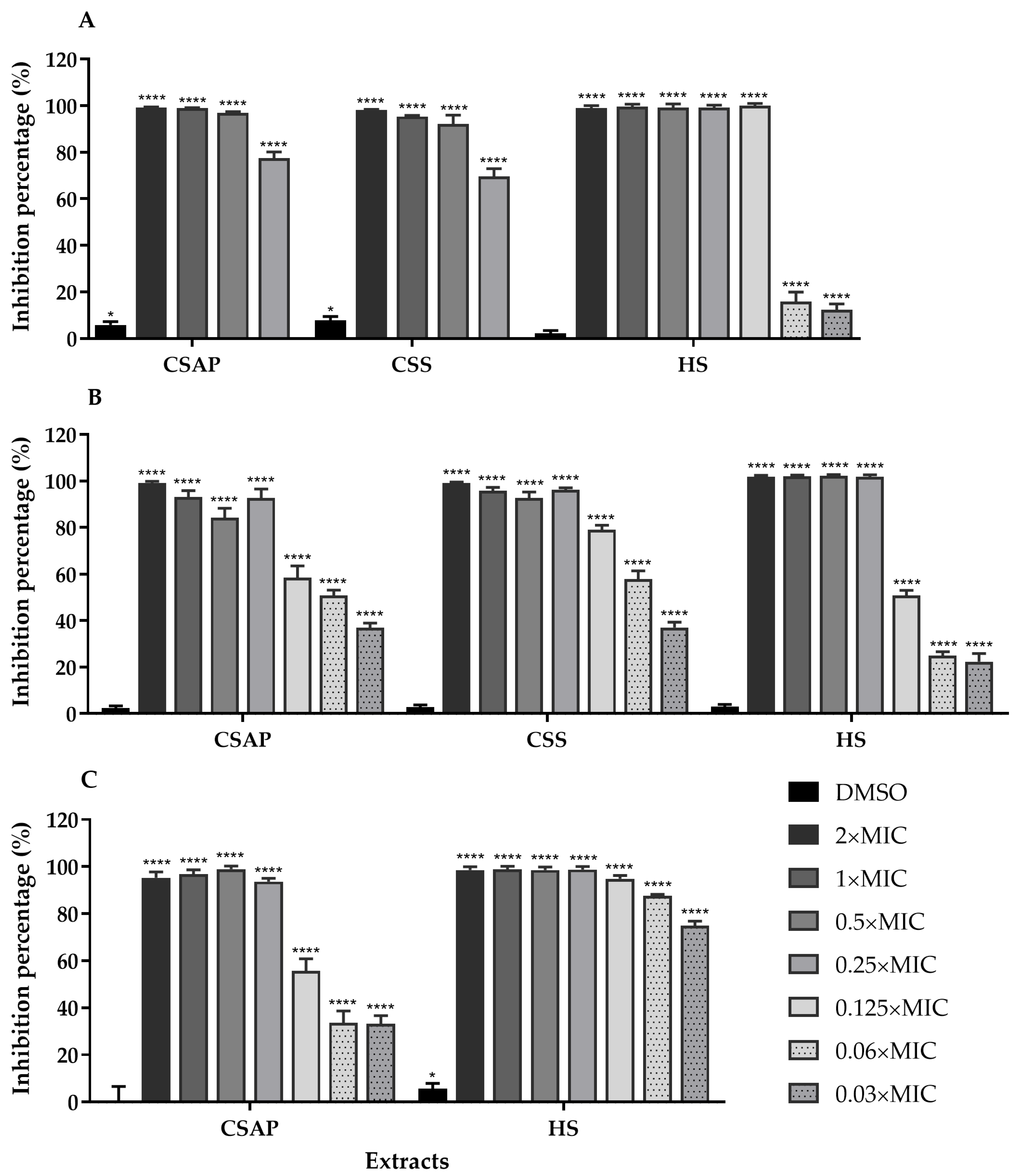
2.5. Inhibition of S. aureus Biofilm Formation by C. salviifolius and H. stoechas Extracts
3. Materials and Methods
3.1. Cistus salviifolius and Helichrysum stoechas Extracts
3.2. Plant Extracts, Microorganisms and Culture Media
3.3. Determination of the Minimum Inhibitory Concentration (MIC)
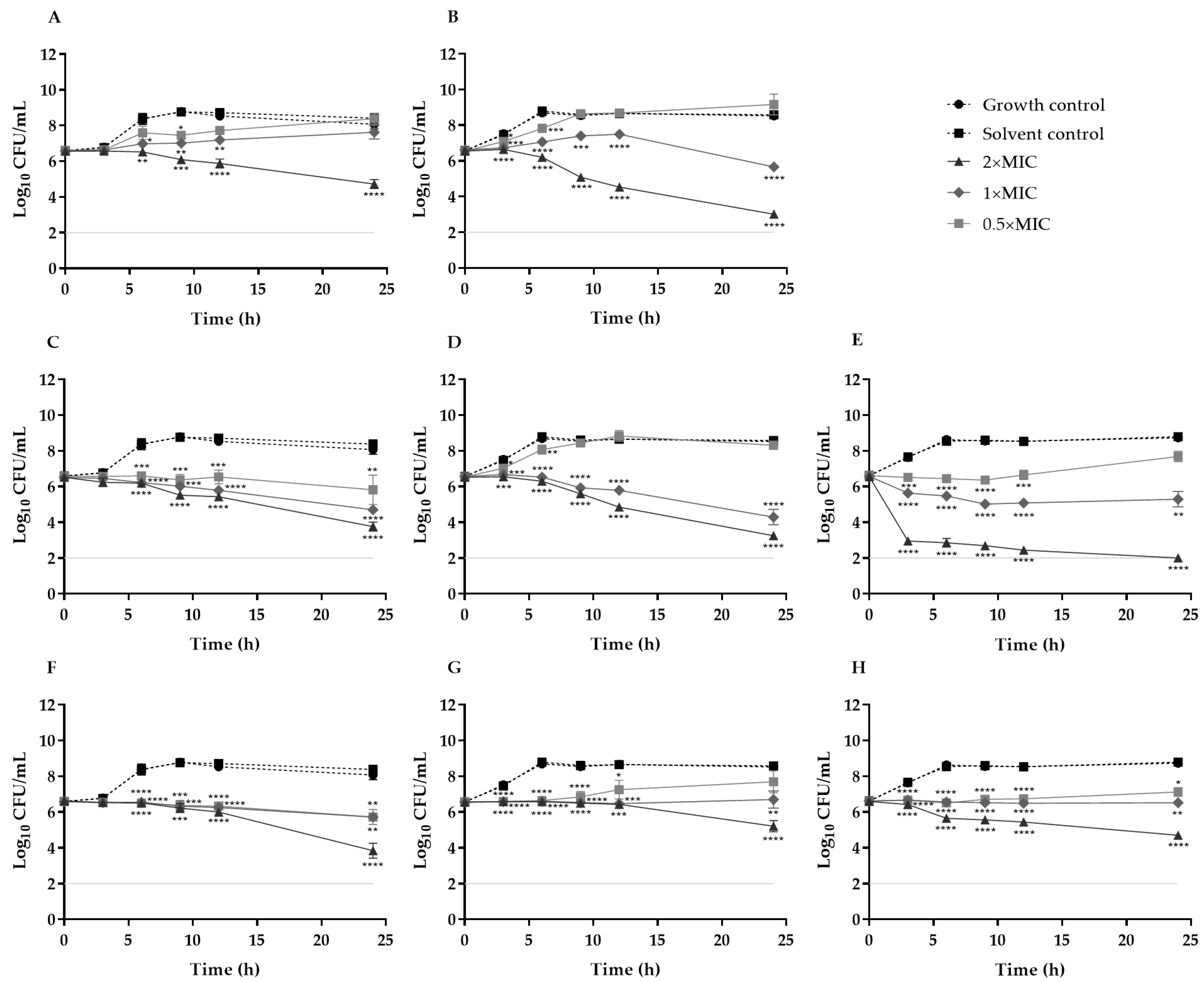
3.4. Time–Kill Curves
3.5. Checkerboard Assay
3.6. Evaluation of the Modulatory Effect of Extracts on Ethidium Bromide Activity
3.7. Inhibition of Quorum Sensing
3.8. Antibiofilm Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Lung, T.W.F.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.; Hu, G.; Liu, J.; Lian, S.; Pang, S.; Zhu, G.; Ding, X. Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures. Microorganisms 2025, 13, 1928. [Google Scholar] [CrossRef]
- Li, J.; Cheng, F.; Wei, X.; Bai, Y.; Wang, Q.; Li, B.; Zhou, Y.; Zhai, B.; Zhou, X.; Wang, W.; et al. Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics 2025, 14, 771. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm Exacerbates Antibiotic Resistance: Is This a Current Oversight in Antimicrobial Stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The Spread of Antibiotic Resistance to Humans and Potential Protection Strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic Action and Resistance: Updated Review of Mechanisms, Spread, Influencing Factors, and Alternative Approaches for Combating Resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Bao, M.; Zhang, L.; Liu, B.; Li, L.; Zhang, Y.; Zhao, H.; Ji, X.; Chen, Q.; Hu, M.; Bai, J.; et al. Synergistic Effects of Anti-MRSA Herbal Extracts Combined with Antibiotics. Future Microbiol. 2020, 15, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Rathore, D.; Janiyani, K.; Gupta, A.; Sulieman, A.M.E.; Tahir, H.E.; Mir, R.H.; Fatima, S.B.; Adnan, M.; Surti, M. A Comprehensive Review on Plant-Derived Bioactive Saponins as Promising Antimicrobial Agents: From Bioavailability Challenges, Molecular Mechanistic Insights to Therapeutic Applications. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gupta, N.; Dutta, A.; Bonomo, M.G.; Milella, L.; Sarker, S.D.; Nahar, L. Mitigating ROS Signalling Pathway-Mediated Defence Mechanism: A Novel Approach to Counteract Bacterial Resistance Using Natural Antioxidant-Based Antibiotics. Phytochem. Rev. 2025, 1–33. [Google Scholar] [CrossRef]
- Morguette, A.E.B.; Bartolomeu-Gonçalves, G.; Andriani, G.M.; Bertoncini, G.E.S.; de Castro, I.M.; Spoladori, L.F.d.A.; Bertão, A.M.S.; Tavares, E.R.; Yamauchi, L.M.; Yamada-Ogatta, S.F. The Antibacterial and Wound Healing Properties of Natural Products: A Review on Plant Species with Therapeutic Potential against Staphylococcus aureus Wound Infections. Plants 2023, 12, 2147. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Alamami, A.; Elshibani, F.; Alshalmani, S.; Sharkasi, M.A.; Elremali, N.; Daboub, A. High-Performance Liquid Chromatography Analysis and Antimicrobial Activities of Libyan Cistus salviifolius Extract. J. Pharm. Res. Int. 2021, 33, 32–40. [Google Scholar] [CrossRef]
- Bouabidi, M.; Salamone, F.L.; Gadhi, C.; Bouamama, H.; Speciale, A.; Ginestra, G.; Pulvirenti, L.; Siracusa, L.; Nostro, A.; Cristani, M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules 2023, 28, 2797. [Google Scholar] [CrossRef]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical Composition, Biological and Cytotoxic Activities of Cistus salviifolius Flower Buds and Leaves Extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- Christou, A.; Nikola, F.; Goulas, V. Optimization of the Ultrasound-Assisted Extraction of Phenolic Antioxidants from Cistus salvifolius L. Using Response Surface Methodology. Chem. Biodivers. 2025, 22, e202401337. [Google Scholar] [CrossRef]
- Hitl, M.; Bijelić, K.; Stilinović, N.; Božin, B.; Srđenović-Čonić, B.; Torović, L.; Kladar, N. Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae. Molecules 2022, 27, 8003. [Google Scholar] [CrossRef]
- Boy, F.R.; Casquete, R.; Martínez, A.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Benito, M.J. Antioxidant, Antihypertensive and Antimicrobial Properties of Phenolic Compounds Obtained from Native Plants by Different Extraction Methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Zalegh, I.; Bourhia, M.; Zerouali, K.; Katfy, K.; Nayme, K.; Khallouki, F.; Benzaarate, I.; Salamatullah, A.M.; Alzahrani, A.; Nafidi, H.A.; et al. Molecular Characterization of Gene-Mediated Resistance and Susceptibility of ESKAPE Clinical Isolates to Cistus monspeliensis L. and Cistus salviifolius L. Extracts. J. Evid.-Based Complement. Altern. Med. 2022, 2022, 7467279. [Google Scholar] [CrossRef]
- Carev, I.; Maravić, A.; Ilić, N.; Čulić, V.Č.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Boubekeur, S.; Messaoudi, M.; Awuchi, C.G.; Otekunrin, O.A.; Sawicka, B.; Idjeri-Mecherara, S.; Bouchareb, S.; Hassani, A.; Sharifi-Rad, M.; Begaa, S.; et al. Biological Properties and Polyphenols Content of Algerian Cistus salviifolius L. Aerial Parts. Eur. J. Biol. Res. 2022, 12, 163–180. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Ben Hamida, N.; Ben Nasri, M.; Ouerghi, Z.; Hosni, K. Comparison of Antioxidant and Antimicrobial activities of Two Cultivated Cistus Species from Tunisia. Biosci. J. 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef] [PubMed]
- Oyardi, Ö.; Hacioğlu, M.; Özdemir, E.; Erbay, M.Ş.; Kültür, Ş.; Bozkurt Güzel, Ç. Screening of Antimicrobial, Antibiofilm, and Cytotoxic Activities of Some Medicinal Plants from Balıkesir Province, Türkiye: Potential Effects of Allium paniculatum Flower. Turk. J. Pharm. Sci. 2024, 21, 252–258. [Google Scholar] [CrossRef]
- Onal, F.N.; Ozturk, I.; Kose, F.A.; Der, G.; Kilinc, E.; Baykan, S. Comparative Evaluation of Polyphenol Contents and Biological Activities of Five Cistus L. Species Native to Turkey. Chem. Biodivers. 2023, 20, e202200915. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Álvarez-Martínez, J.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Helichrysum stoechas (L.) Moench Inflorescence Extract for Tomato Disease Management. Molecules 2023, 28, 5861. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Cherif, J.K.; Ayadi, M.T. Total Phenolic Compounds and Antioxidant Potential of Rokrose (Cistus salviifolius) Leaves and Flowers Grown in Tunisia. Int. J. Pharm. Phytochem. Res. 2016, 8, 327–331. [Google Scholar]
- Kühn, C.; Arapogianni, N.E.; Halabalaki, M.; Hempel, J.; Hunger, N.; Wober, J.; Skaltsounis, A.L.; Vollmer, G. Constituents from Cistus salvifolius (Cistaceae) Activate Peroxisome Proliferator-Activated Receptor-γ but Not -δ And Stimulate Glucose Uptake by Adipocytes. Planta Med. 2011, 77, 346–353. [Google Scholar] [CrossRef]
- Coimbra, A.; Gallardo, E.; Luís, Â.; Gaspar, P.D.; Ferreira, S.; Duarte, A.P. Bioactive Potential of Wild Plants from Gardunha Mountain: Phytochemical Characterization and Biological Activities. Molecules 2025, 30, 3876. [Google Scholar] [CrossRef] [PubMed]
- Kutluk, I.; Aslan, M.; Orhan, I.E.; Özçelik, B. Antibacterial, Antifungal and Antiviral Bioactivities of Selected Helichrysum Species. S Afr. J. Bot. 2018, 119, 252–257. [Google Scholar] [CrossRef]
- Boubakeur, H.; Rebbas, K.; Belhattab, R. Activités Antioxydante et Antibactérienne Des Extraits d’Helichrysum stoechas (L.) Moench. Phytothérapie 2017, 16, 122–132. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Tešić, Ž.; Stupar, A.; Bulut, G.; Sinan, K.I.; Uysal, S.; Picot-Allain, M.C.N.; Mahomoodally, M.F. A Comparative Exploration of the Phytochemical Profiles and Bio-Pharmaceutical Potential of Helichrysum stoechas Subsp. barrelieri Extracts Obtained via Five Extraction Techniques. Process Biochem. 2020, 91, 113–125. [Google Scholar] [CrossRef]
- Kherbache, A.; Senator, A.; Laouicha, S.; Al-Zoubi, R.M.; Bouriche, H. Phytochemical Analysis, Antioxidant and Anti-Inflammatory Activities of Helichrysum stoechas (L.) Moench Extracts. Biocatal. Agric. Biotechnol. 2020, 29, 101826. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, Antioxidant and Antimicrobial Activities of Helichrysum (Asteraceae) Species Collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Bogdadi, H.A.A.; Kokoska, L.; Havlik, J.; Kloucek, P.; Rada, V.; Vorisek, K. In Vitro Antimicrobial Activity of Some Libyan Medicinal Plant Extracts. Pharm. Biol. 2007, 45, 386–391. [Google Scholar] [CrossRef]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting Flower (Helichrysum stoechas Moench) as a Potential Source of Bioactive Molecules with Antiproliferative, Antioxidant, Antidiabetic and Neuroprotective Properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Hussain, M.S.; Azam, F.; Eldarrat, H.A.; Haque, A.; Khalid, M.; Hassan, M.Z.; Ali, M.; Arif, M.; Ahmad, I.; Zaman, G.; et al. Structural, Functional, Molecular, and Biological Evaluation of Novel Triterpenoids Isolated from Helichrysum stoechas (L.) Moench. Collected from Mediterranean Sea Bank: Misurata- Libya. Arabian J. Chem. 2022, 15, 103818. [Google Scholar] [CrossRef]
- Hussain, M.S.; Azam, F.; Eldarrat, H.A.; Alkskas, I.; Mayoof, J.A.; Dammona, J.M.; Ismail, H.; Ali, M.; Arif, M.; Haque, A. Anti-Inflammatory, Analgesic and Molecular Docking Studies of Lanostanoic Acid 3-O-α-D-Glycopyranoside Isolated from Helichrysum stoechas. Arab. J. Chem. 2020, 13, 9196–9206. [Google Scholar] [CrossRef]
- Valero, M.S.; Nuñez, S.; Les, F.; Castro, M.; Gómez-Rincón, C.; Arruebo, M.P.; Plaza, M.Á.; Köhler, R.; López, V. The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants 2022, 11, 1092. [Google Scholar] [CrossRef]
- Silva, L.; Rodrigues, A.M.; Ciriani, M.; Falé, P.L.V.; Teixeira, V.; Madeira, P.; Machuqueiro, M.; Pacheco, R.; Florêncio, M.H.; Ascensão, L.; et al. Antiacetylcholinesterase Activity and Docking Studies with Chlorogenic Acid, Cynarin and Arzanol from Helichrysum stoechas (Asteraceae). Med. Chem. Res. 2017, 26, 2942–2950. [Google Scholar] [CrossRef]
- Abouzeed, Y.M.; Elfahem, A.; Zgheel, F.; Ahmed, M.O. Antibacterial in Vitro Activities of Selected Medicinal Plants against Methicillin Resistant Staphylococcus aureus from Libyan Environment. J. Environ. Anal. Toxicol. 2013, 3, 1000194. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-Resistant Bacteria: Prevalence in Food and Inactivation by Food-Compatible Compounds and Plant Extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Barrajón-Catalán, E.; Segura-Carretero, A.; Martí, N.; Saura, D.; Menéndez, J.A.; Joven, J.; Micol, V. The Promiscuous and Synergic Molecular Interaction of Polyphenols in Bactericidal Activity: An Opportunity to Improve the Performance of Antibiotics? Phytother. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Villar, A. Antimicrobial Activities of Helichrysum stoechas. Planta Med. 1990, 56, 646. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Villar, A.A. Isolation and Identification of the Antibacterial Compounds from Helichrysum stoechas. J. Ethnopharmacol. 1991, 33, 51–55. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, H.; Wang, Z.; Tian, R.; Li, S. Dimethyl Phthalate Damages Staphylococcus aureus by Changing the Cell Structure, Inducing Oxidative Stress and Inhibiting Energy Metabolism. J. Environ. Sci. 2021, 107, 171–183. [Google Scholar] [CrossRef]
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus Bacteriostatic Antibacterials: Clinical Significance, Differences and Synergistic Potential in Clinical Practice. J. Antimicrob. Chemother. 2025, 80, 1–17. [Google Scholar] [CrossRef]
- Nostro, A.; Bisignano, G.; Cannatelli, M.A.; Crisafi, G.; Germanò, M.P.; Alonzo, V. Effects of Helichrysum italicum Extract on Growth and Enzymatic Activity of Staphylococcus aureus. Int. J. Antimicrob. Agents 2001, 17, 517–520. [Google Scholar] [CrossRef]
- Sang, H.; Jin, H.; Song, P.; Xu, W.; Wang, F. Gallic Acid Exerts Antibiofilm Activity by Inhibiting Methicillin-Resistant Staphylococcus aureus Adhesion. Sci. Rep. 2024, 14, 17220. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Singh, K.; Coopoosamy, R.M.; Gumede, N.J.; Sabiu, S. Computational Insights and in Vitro Validation of Antibacterial Potential of Shikimate Pathway-Derived Phenolic Acids as NorA Efflux Pump Inhibitors. Molecules 2022, 27, 2601. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic Antimicrobial Activities of Epigallocatechin Gallate, Myricetin, Daidzein, Gallic Acid, Epicatechin, 3-Hydroxy-6-Methoxyflavone and Genistein Combined with Antibiotics against ESKAPE Pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic Activity of Sub-Inhibitory Concentrations of Curcumin with Ceftazidime and Ciprofloxacin against Pseudomonas aeruginosa Quorum Sensing Related Genes and Virulence Traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Zhao, W.-H.; Hara, Y.; Shimamura, T. Epigallocatechin Gallate Synergy with Ampicillin/Sulbactam against 28 Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin Gallate Synergistically Enhances the Activity of Carbapenems against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as Sources of New Antimicrobials and Resistance-Modifying Agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics against Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro and in Vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and Efflux Pump Inhibitory Activity of Caffeoylquinic Acids from Artemisia absinthium against Gram-Positive Pathogenic Bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Sharma, H.K.; Gupta, P.; Nagpal, D.; Mukherjee, M.; Parmar, V.S.; Lather, V. Virtual Screening and Antimicrobial Evaluation for Identification of Natural Compounds as the Prospective Inhibitors of Antibacterial Drug Resistance Targets in Staphylococcus aureus. Fitoterapia 2023, 168, 105554. [Google Scholar] [CrossRef]
- Gangwar, B.; Kumar, S.; Kumar, P.; Pal, A.; Darokar, M.P. A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus. Biomolecules 2025, 15, 750. [Google Scholar] [CrossRef]
- Martin, A.L.A.R.; Pereira, R.L.S.; Rocha, J.E.; Farias, P.A.M.; Freitas, T.S.; Caldas, F.R.d.L.; Figueredo, F.G.; Sampaio, N.F.L.; Oliveira-Tintino, C.D.d.M.; Tintino, S.R.; et al. Unlocking Bacterial Defense: Exploring the Potent Inhibition of NorA Efflux Pump by Coumarin Derivatives in Staphylococcus aureus. Microb. Pathog. 2024, 190, 106608. [Google Scholar] [CrossRef]
- Araújo-Neto, J.B.d.; Oliveira-Tintino, C.D.d.M.; de Araújo, G.A.; Alves, D.S.; Ribeiro, F.R.; Brancaglion, G.A.; Carvalho, D.T.; Lima, C.M.G.; Ali, H.S.H.M.; Rather, I.A.; et al. 3-Substituted Coumarins Inhibit NorA and MepA Efflux Pumps of Staphylococcus aureus. Antibiotics 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Rampacci, E.; Felicetti, T.; Cernicchi, G.; Stefanetti, V.; Sabatini, S.; Passamonti, F. Inhibition of Staphylococcus pseudintermedius Efflux Pumps by Using Staphylococcus aureus NorA Efflux Pump Inhibitors. Antibiotics 2023, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Santos, M.; Santos, R.; Soeiro, P.; Silvestre, S.; Ferreira, S. Resveratrol as an Inhibitor of the NorA Efflux Pump and Resistance Modulator in Staphylococcus aureus. Antibiotics 2023, 12, 1168. [Google Scholar] [CrossRef]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-Derived Secondary Metabolites as the Main Source of Efflux Pump Inhibitors and Methods for Identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef]
- Cernicchi, G.; Felicetti, T.; Sabatini, S. Microbial Efflux Pump Inhibitors: A Journey around Quinoline and Indole Derivatives. Molecules 2021, 26, 6996. [Google Scholar] [CrossRef]
- Shaheen, A.; Afridi, W.A.; Mahboob, S.; Sana, M.; Zeeshan, N.; Ismat, F.; Mirza, O.; Iqbal, M.; Rahman, M. Reserpine Is the New Addition into the Repertoire of AcrB Efflux Pump Inhibitors. Mol. Biol. 2019, 53, 596–605. [Google Scholar] [CrossRef]
- Randhawa, H.K.; Hundal, K.K.; Ahirrao, P.N.; Jachak, S.M.; Nandanwar, H.S. Efflux Pump Inhibitory Activity of Flavonoids Isolated from Alpinia calcarata against Methicillin-Resistant Staphylococcus aureus. Biologia 2016, 71, 484–493. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.E.; Petersen, B.; Mølgaard, P. Novel Inhibitory Activity of the Staphylococcus aureus NorA Efflux Pump by a Kaempferol Rhamnoside Isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Falcão-Silva, V.S.; Silva, D.A.; De Fátima, M.; Souza, V.; Siqueira-Junior, J.P. Modulation of Drug Resistance in Staphylococcus aureus by a Kaempferol Glycoside from Herissantia tiubae (Malvaceae). Phytother. Res. 2009, 23, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.N.; Schindler, B.D.; Kaatz, G.W.; Cech, N.B. A Mass Spectrometry-Based Assay for Improved Quantitative Measurements of Efflux Pump Inhibition. PLoS ONE 2015, 10, e0124814. [Google Scholar] [CrossRef]
- Dos Santos, J.F.S.; Tintino, S.R.; Da Silva, A.R.P.; Barbosa, C.R.d.S.; Scherf, J.R.; Silveira, Z.d.S.; de Freitas, T.S.; Neto, L.J.d.L.; Barros, L.M.; Menezes, I.R.d.A.; et al. Enhancement of the Antibiotic Activity by Quercetin against Staphylococcus aureus Efflux Pumps. J. Bioenerg. Biomembr. 2021, 53, 157–167. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.S. Molecular Determinants of β-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus (MRSA): An Updated Review. Antibiotics 2023, 12, 1362. [Google Scholar] [CrossRef]
- Ealand, C.S.; Machowski, E.E.; Kana, B.D. β-Lactam Resistance: The Role of Low Molecular Weight Penicillin Binding Proteins, β-Lactamases and LD-Transpeptidases in Bacteria Associated with Respiratory Tract Infections. IUBMB Life 2018, 70, 855–868. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of Antibacterial Resistance, Strategies and next-Generation Antimicrobials to Contain Antimicrobial Resistance: A Review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin Resistant Staphylococcus aureus Infections: A Review of Case Updating and Clinical Features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Savchenko, A. Molecular Mechanisms of Vancomycin Resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Kitpipit, W.; Scholfield, C.N.; Sangkanu, S.; Nissapatorn, V.; Pereira, M.L.; Paul, A.K.; Mitsuwan, W. Virulence Factors and Quorum Sensing as Targets of New Therapeutic Options by Plant-Derived Compounds against Bacterial Infections Caused by Human and Animal Pathogens. Vet. World 2023, 16, 1346–1355. [Google Scholar] [CrossRef]
- Díaz-Nuñez, J.L.; García-Contreras, R.; Castillo-Juárez, I. The New Antibacterial Properties of the Plants: Quo vadis Studies of Anti-Virulence Phytochemicals? Front. Microbiol. 2021, 12, 667126. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zuo, J.; Teng, J.; Yang, L.; Guo, J.; Liu, L.; Li, P. Antibiofilm Potential of Luteolin against Multidrug-Resistant Staphylococcus aureus Isolated from Dairy Goats and Farm Environments. Environ. Pollut. 2023, 335, 122274. [Google Scholar] [CrossRef]
- Neves, A.R.; Durães, F.; Freitas-Silva, J.; Szemerédi, N.; Martins-da-Costa, P.; Pinto, E.; Correia-da-Silva, M.; Spengler, G.; Sousa, E. Derivatives of Trimethoxybenzoic Acid and Gallic Acid as Potential Efflux Pump Inhibitors: In Silico and in Vitro Studies. Int. J. Mol. Sci. 2022, 23, 14468. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The Role of Biofilms as Environmental Reservoirs of Antibiotic Resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.W.; Thieman, C.; Gupta, S. Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-Resistant: Staphylococcus aureus (MRSA): Antibiotic-Resistance and the Biofilm Phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Bardhan, P.; Borah, M.; Sarkar, A.; Eldiehy, K.S.H.; Bhuyan, S.; Mandal, M. Microbial Biofilm: A Matter of Grave Concern for Human Health and Food Industry. J. Basic Microbiol. 2021, 61, 380–395. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm Formation and Persistence on Abiotic Surfaces in the Context of Food and Medical Environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing Phytochemicals as Anti-Virulent Agents to Attenuate Quorum Sensing-Regulated Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2022, 15, 1695–1718. [Google Scholar] [CrossRef]
- Kincses, A.; Ghazal, T.S.A.; Veres, K.; Spengler, G.; Hohmann, J. Phenolic Compounds from Origanum majorana with Biofilm-Inhibitory Activity against Methicillin-Resistant Staphylococcus aureus and Escherichia coli Strains. Pharm. Biol. 2025, 63, 402–410. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Zhou, Y.; Hu, T.; Zhang, Y.; Lv, X.; Li, J.; Zhou, Y. Discovery of Potential Anti-Microbial Molecules and Spectrum Correlation Effect of Ardisia crenata Sims via High-Performance Liquid Chromatography Fingerprints and Molecular Docking. Molecules 2024, 29, 1178. [Google Scholar] [CrossRef]
- Miyamoto, T.; Zhang, X.; Ueyama, Y.; Apisada, K.; Nakayama, M.; Suzuki, Y.; Ozawa, T.; Mitani, A.; Shigemune, N.; Shimatani, K.; et al. Development of Novel Monoclonal Antibodies Directed against Catechins for Investigation of Antibacterial Mechanism of Catechins. J. Microbiol. Methods 2017, 137, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm Activity of Flavonoids on Staphylococcal Biofilms through Targeting BAP Amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Xiao, K.; Zhang, J.-Q.; Zhong, K.; Grosu, E.; Gao, Z.; Wu, Y.-P.; Gao, H. Antibacterial and Antibiofilm Effects of Zanthoxylum bungeanum Leaves against Staphylococcus aureus. Nat. Prod. Commun. 2018, 13, 1001–1006. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin Protects Galleria mellonella against Staphylococcus aureus Infection and Inhibits Multiple Virulence Factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial Mechanism of Luteolin against Staphylococcus aureus and Listeria monocytogenes and Its Antibiofilm Properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Onmaz, N.E.; Yilmaz, D.D.; Imre, K.; Morar, A.; Gungor, C.; Yilmaz, S.; Gundog, D.A.; Dishan, A.; Herman, V.; Gungor, G. Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1466. [Google Scholar] [CrossRef]
- Bezek, K.; Kramberger, K.; Barlič-Maganja, D. Antioxidant and Antimicrobial Properties of Helichrysum italicum (Roth) G. Don Hydrosol. Antibiotics 2022, 11, 1017. [Google Scholar] [CrossRef]
- Soltanbeigi, E.; Isitez, N.; Erdogmus, S.F.; Ozliman, S. Helichrysum italicum (Roth) G. Don Essential Oil: Composition and Potential Anti-Tumour, Anti-Inflammatory, Antimicrobial, and Antibiofilm Effects. J. Biol. Act. Prod. Nat. 2025, 15, 353–368. [Google Scholar] [CrossRef]
- Willdigg, J.R.; Helmann, J.D. Mini Review: Bacterial Membrane Composition and Its Modulation in Response to Stress. Front. Mol. Biosci. 2021, 8, 634438. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, W.; Li, C.; Lin, L. Synergistic Effect between Helichrysum italicum Essential Oil and Cold Nitrogen Plasma against Staphylococcus aureus Biofilms on Different Food-Contact Surfaces. Int. J. Food Sci. Technol. 2016, 51, 2493–2501. [Google Scholar] [CrossRef]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.M.; Quiroga, P.R.; Al-Gburi, A.; Huang, Q.; Grosso, N.R. Rheological Behavior, Antimicrobial and Quorum Sensig Inhibition Study of an Argentinean Oregano Essential Oil Nanoemulsion. Front. Nutr. 2020, 7, 569913. [Google Scholar] [CrossRef]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm Formation by Salmonella spp. and Listeria monocytogenes on Plastic Surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]

| Species | MIC (MBC) | |||||
|---|---|---|---|---|---|---|
| Cistus salviifolius Aerial Parts | Cistus salviifolius Stems | Helichrysum stoechas | Ampicillin | Ciprofloxacin | Vancomycin | |
| Staphylococcus aureus ATCC 25923 | 500 (2000) | 500 (2000) | 7.8 (31.3) | 0.125 | 0.5 | 8 |
| Staphylococcus aureus MRSA 05/15 | 250 (2000) | 500 (>2000) | 7.8 (31.3) | 4 | 256 | 8 |
| Staphylococcus aureus MRSA 12/08 | 2000 (>2000) | >2000 (ND) | 62.5 (>250) | 32 | 32 | 64 |
| Antibiotics | S. aureus Strains | Extracts | ||
|---|---|---|---|---|
| Cistus salviifolius Aerial Parts | Cistus salviifolius Stems | Helichrysum stoechas | ||
| Ampicillin | ATCC 25923 | Additive (≤0.98) | Additive (≤0.98) | Additive (≤0.98) |
| MRSA 05/15 | No interaction (≤1.06) | No interaction (1.02) | No interaction (≤1.06) | |
| MRSA 12/08 | Synergistic (≤0.28) | Synergistic (≤0.38) | Synergistic (≤0.33) | |
| Ciprofloxacin | ATCC 25923 | Additive (≤1.00) | Synergistic (0.5) | Synergistic (0.37–0.5) |
| MRSA 05/15 | Additive (0.74) | Additive (0.75) | Additive (≤1.00) | |
| MRSA 12/08 | Synergistic (0.13) | Synergistic (≤0.27) | Synergistic (0.27–0.28) | |
| Vancomycin | ATCC 25923 | Additive (1.00) | No interaction (1.01) | Additive (1.00) |
| MRSA 05/15 | Additive (≤1.00) | No interaction (1.01) | Additive (≤1.00) | |
| MRSA 12/08 | Synergistic (0.50) | Additive (≤0.75) | Additive (0.57) | |
| Species | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| Ethidium Bromide | + Cistus salviifolius Aerial Parts | + Cistus salviifolius Stems | + Helichrysum stoechas | + Reserpine | |
| Staphylococcus aureus ATCC 25923 | 32 | 8 | 16 | 32 | 8 |
| Staphylococcus aureus MRSA 05/15 | 32 | 8 | 32 | 32 | 8 |
| Staphylococcus aureus MRSA 12/08 | 32 | 8 | ND | 8 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, A.; Luís, Â.; Gaspar, P.D.; Ferreira, S.; Duarte, A.P. Assessment of the Antimicrobial Activity of Cistus salviifolius L. and Helichrysum stoechas (L.) DC Extracts and Their Synergistic Potential with Conventional Antibiotics Against Staphylococcus aureus. Int. J. Mol. Sci. 2025, 26, 11331. https://doi.org/10.3390/ijms262311331
Coimbra A, Luís Â, Gaspar PD, Ferreira S, Duarte AP. Assessment of the Antimicrobial Activity of Cistus salviifolius L. and Helichrysum stoechas (L.) DC Extracts and Their Synergistic Potential with Conventional Antibiotics Against Staphylococcus aureus. International Journal of Molecular Sciences. 2025; 26(23):11331. https://doi.org/10.3390/ijms262311331
Chicago/Turabian StyleCoimbra, Alexandra, Ângelo Luís, Pedro Dinis Gaspar, Susana Ferreira, and Ana Paula Duarte. 2025. "Assessment of the Antimicrobial Activity of Cistus salviifolius L. and Helichrysum stoechas (L.) DC Extracts and Their Synergistic Potential with Conventional Antibiotics Against Staphylococcus aureus" International Journal of Molecular Sciences 26, no. 23: 11331. https://doi.org/10.3390/ijms262311331
APA StyleCoimbra, A., Luís, Â., Gaspar, P. D., Ferreira, S., & Duarte, A. P. (2025). Assessment of the Antimicrobial Activity of Cistus salviifolius L. and Helichrysum stoechas (L.) DC Extracts and Their Synergistic Potential with Conventional Antibiotics Against Staphylococcus aureus. International Journal of Molecular Sciences, 26(23), 11331. https://doi.org/10.3390/ijms262311331










