Retinal Organoid-Derived Exosomes Reduce CNV Lesion and Restore RPE Integrity in Mouse Laser-Induced Choroidal Neovascularization (CNV) Model
Abstract
1. Introduction
2. Results and Discussion
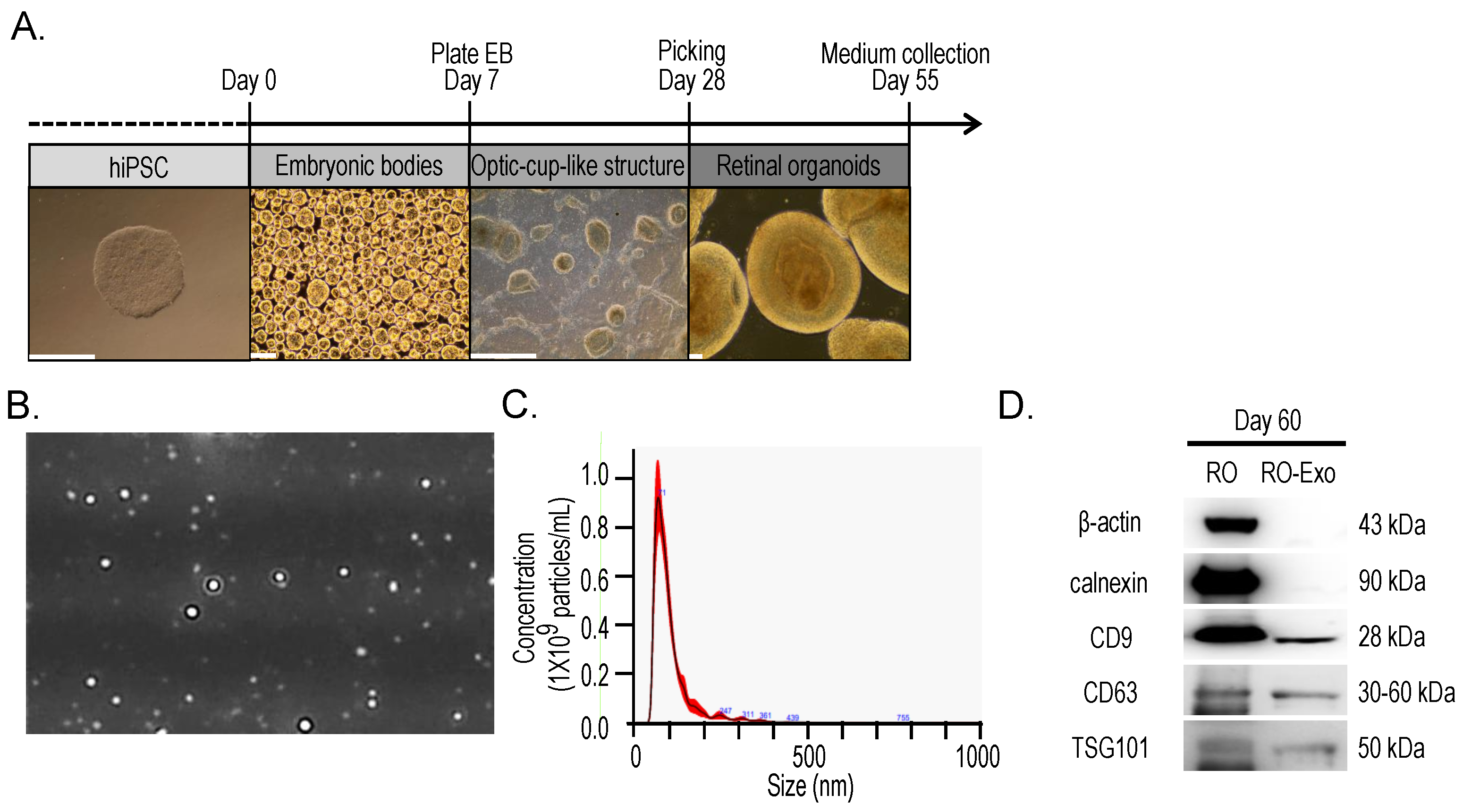
2.1. Retinal Organoids Differentiation and Exosomes Isolation
2.2. Retinal and RPE Distribution of Calcein-Labeled RO-Exo in Mice
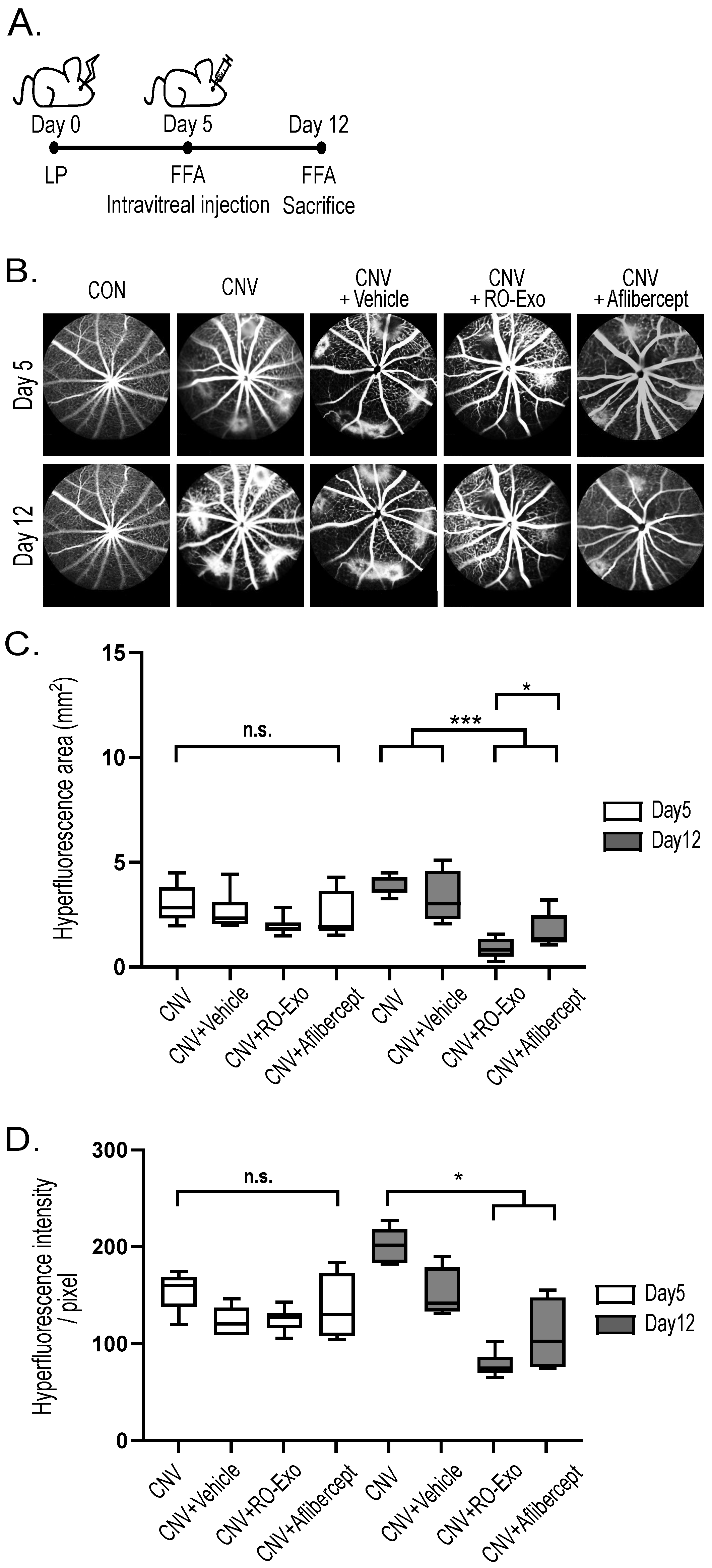
2.3. Vascular Leakage Suppression by RO-Exo
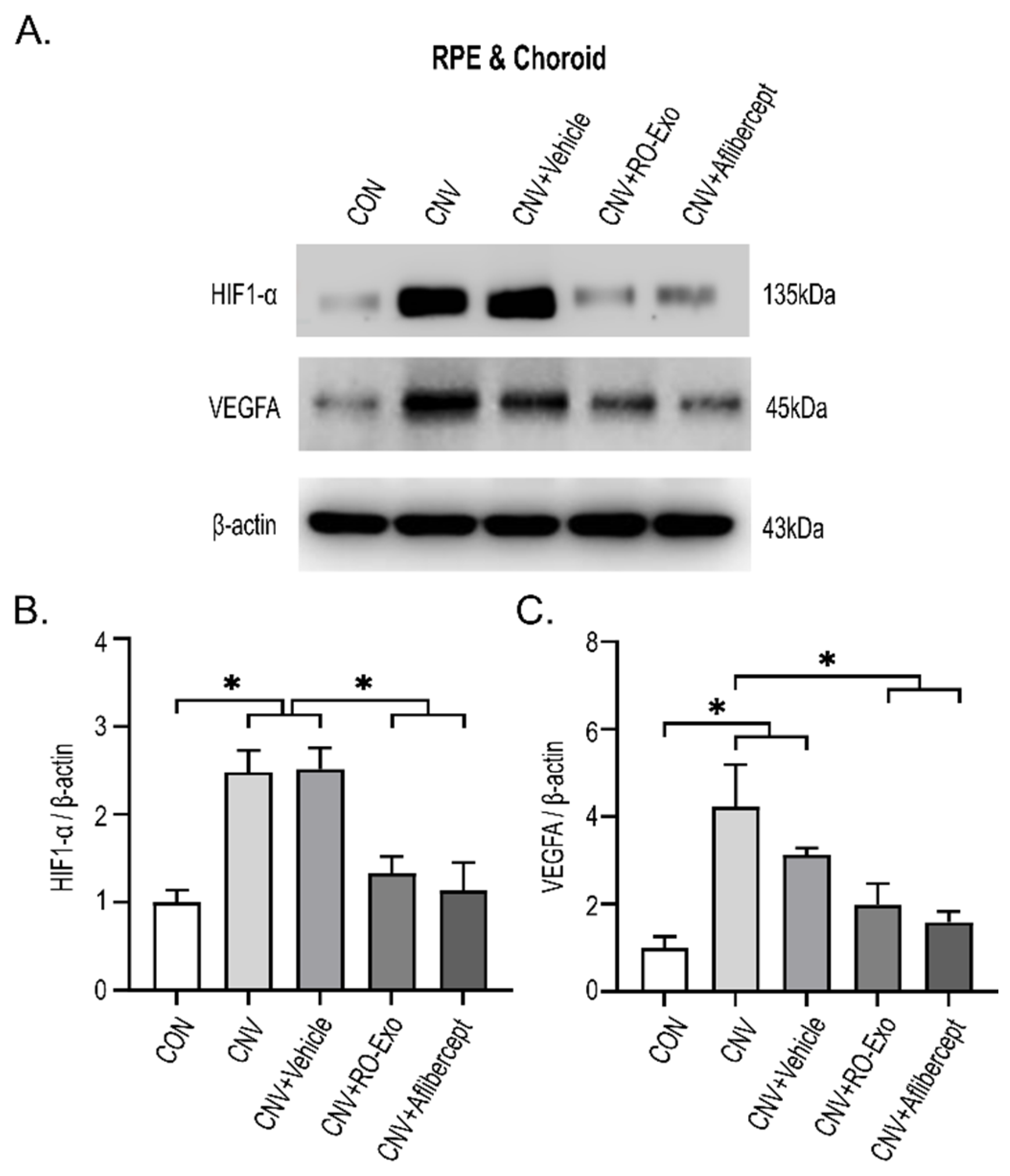
2.4. Reduction in HIF-1α and VEGFA Expression by RO-Exo
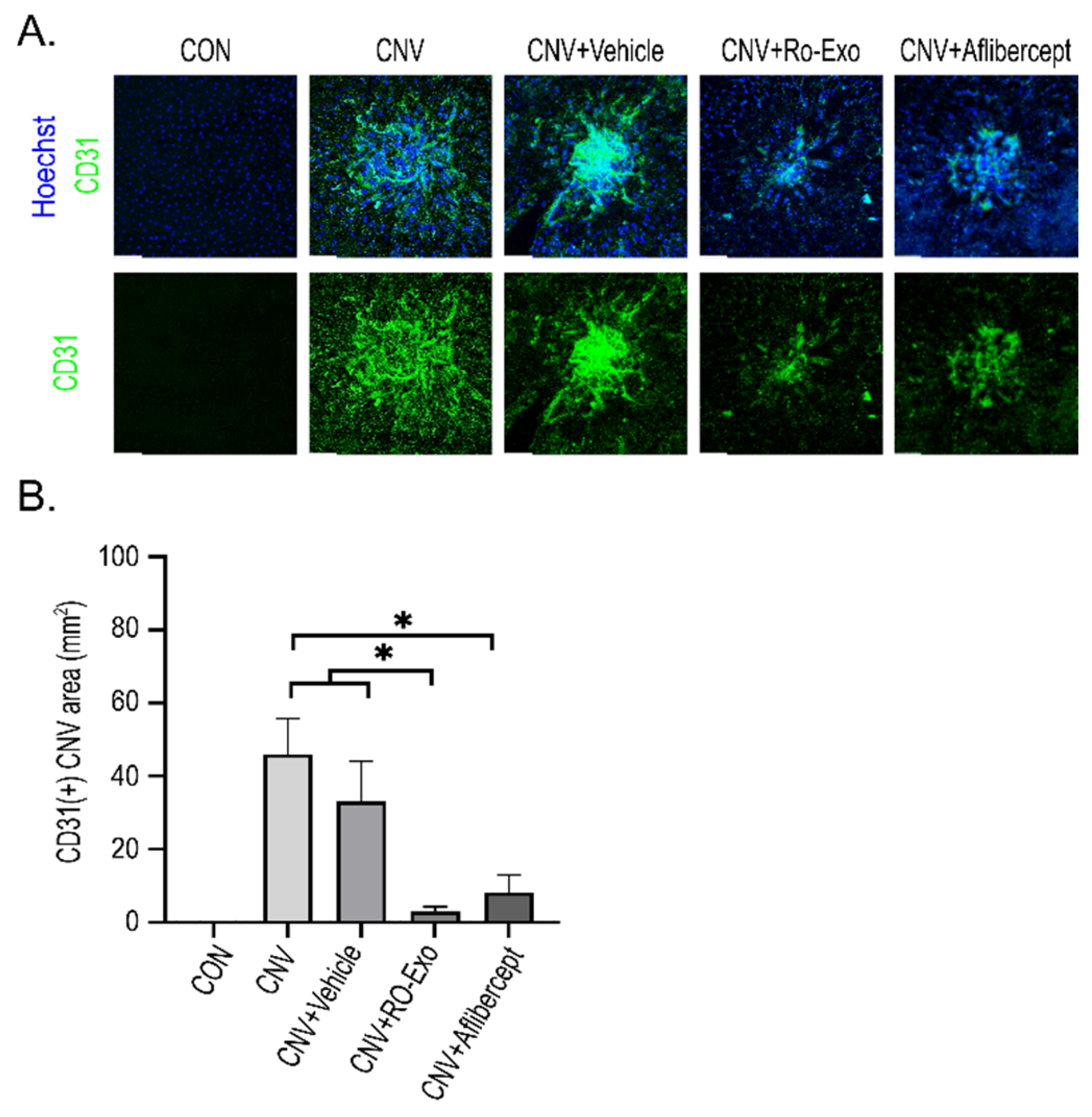
2.5. Suppression of Pathologic Neovascularization by RO-Exo
2.6. Restoration of RPE Monolayer Structure and Morphology by RO-Exo
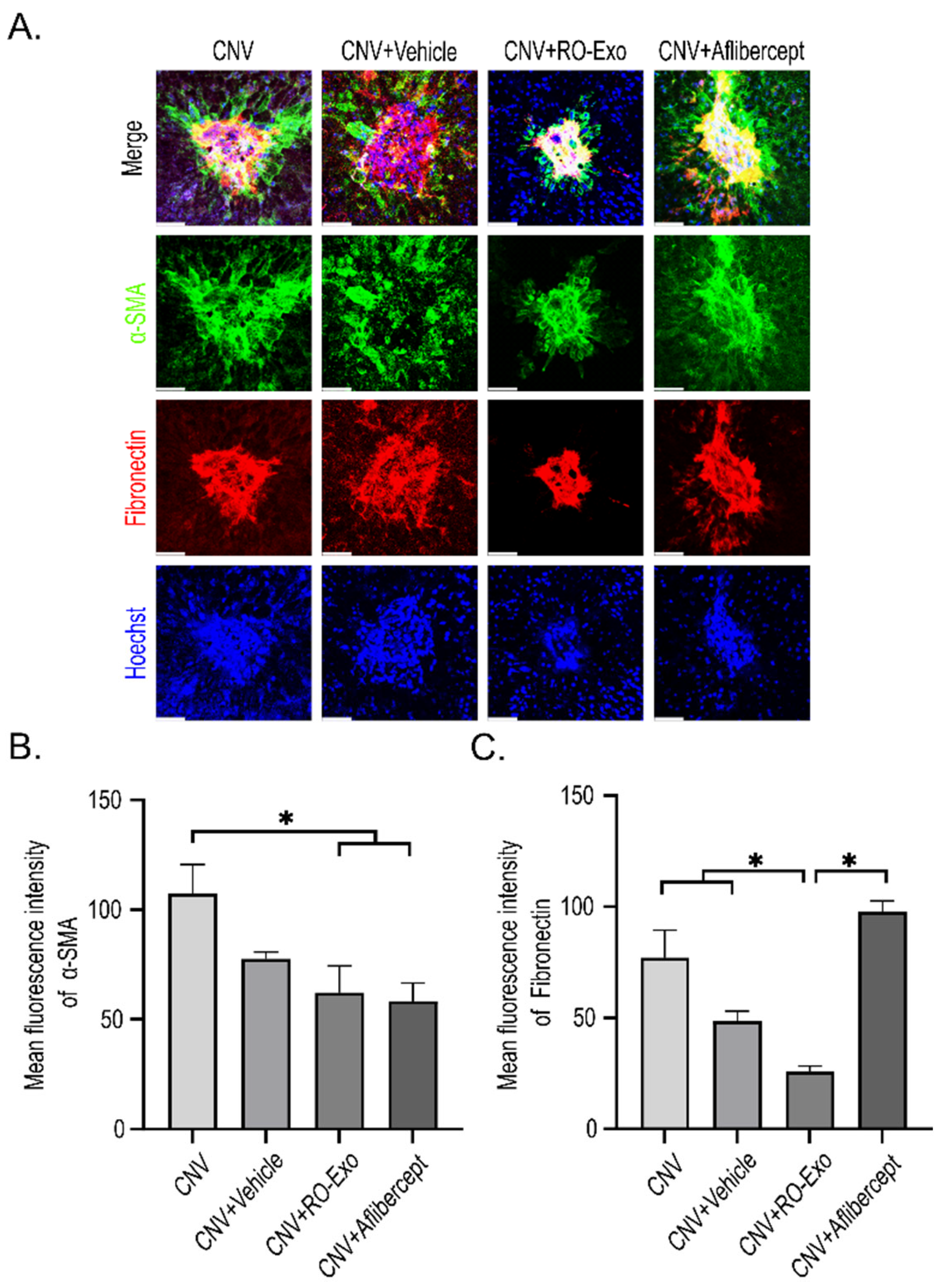
2.7. Suppression of Epithelial–Mesenchymal Transition in RPE Cells by RO-Exo
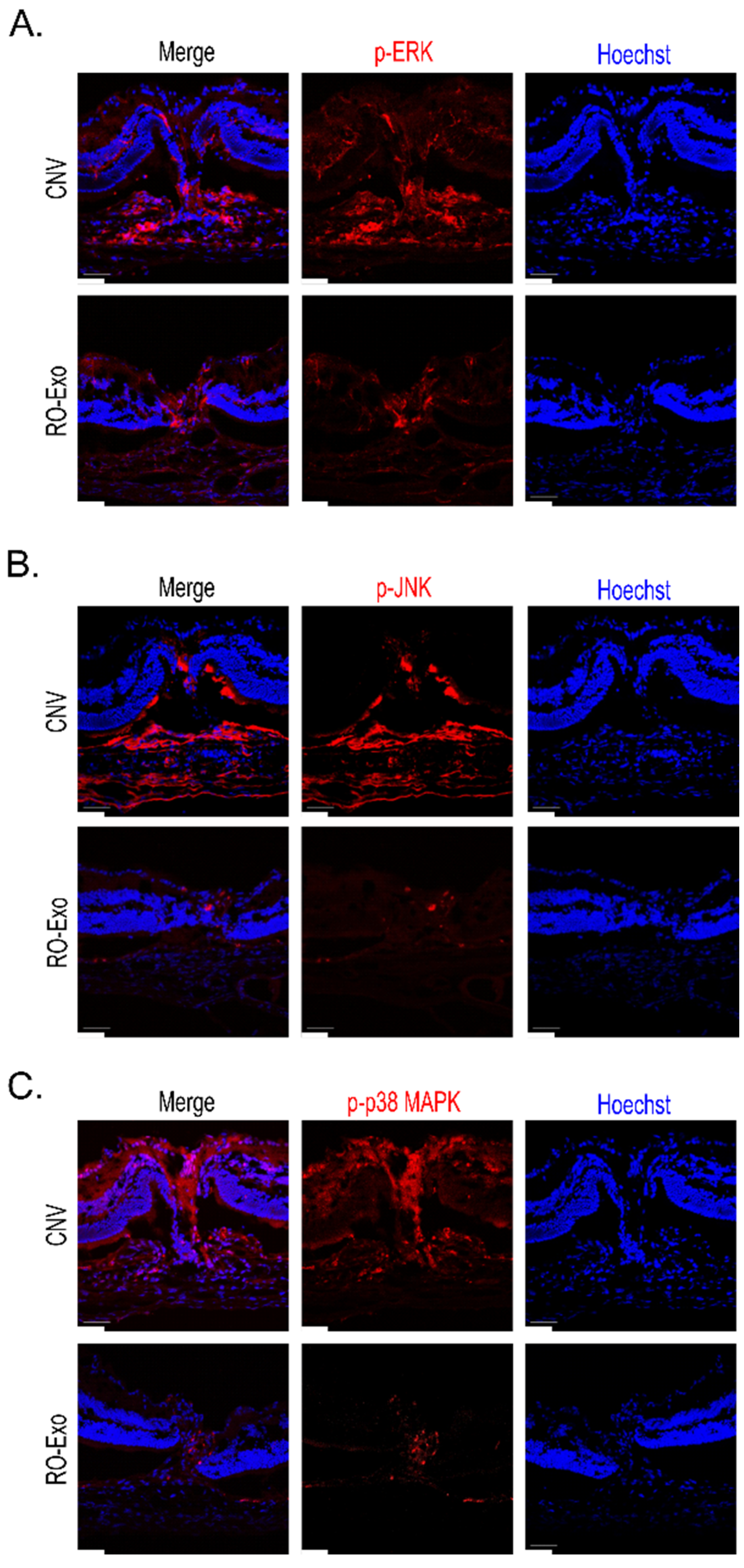
2.8. Inhibition of MAPK Pathway RO-Exo
3. Materials and Methods
3.1. Retinal Organoid and Exosome Preparation
3.1.1. Human Induced Pluripotent Stem Cells Culture and Retinal Organoid Differentiation
3.1.2. Exosome Isolation
3.1.3. Nanoparticle Tracking Analysis and Western Blotting
3.1.4. Intravitreal Delivery of Labeled Exosomes
3.2. Experimental Design and Treatment for In Vivo Study
3.2.1. Animals
3.2.2. Laser Photocoagulation and Intravitreal Injection
3.3. Fundus Fluorescein Angiography
3.4. Tissue Analysis by Immunofluorescence and Western Blotting
3.4.1. Immunofluorescence
3.4.2. RPE Hexagonality
3.5. Western Blot Analysis of RPE–Choroid Complex
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2020, 2, e106–e116. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, 339–349. [Google Scholar] [CrossRef]
- Chappelow, A.V.; Kaiser, P.K. Neovascular age-related macular degeneration: Potential therapies. Drugs 2008, 68, 1029–1036. [Google Scholar] [CrossRef]
- Ambati, J.; Ambati, B.K.; Yoo, S.H.; Ianchulev, S.; Adamis, A.P. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 2003, 48, 257–293. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular macular degeneration: A review of etiology, risk factors, and recent advances in research and therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Toma, H.S.; Barnett, J.M.; Penn, J.S.; Kim, S.J. Improved assessment of laser-induced choroidal neovascularization. Microvasc. Res. 2010, 80, 295–302. [Google Scholar] [CrossRef]
- Parmeggiani, F.; Campa, C.; Costagliola, C.; Incorvaia, C.; Sheridan, C.; Semeraro, F.; De Nadai, K.; Sebastiani, A. Inflammatory mediators and angiogenic factors in choroidal neovascularization: Pathogenetic interactions and therapeutic implications. Mediat. Inflamm. 2010, 2010, 546826. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Xu, M.; Fan, R.; Fan, X.; Shao, Y.; Li, X. Progress and Challenges of Anti-VEGF Agents and Their Sustained-Release Strategies for Retinal Angiogenesis. Drug Des. Dev. Ther. 2022, 16, 3241–3262. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Z.; Webster, K.A.; Paulus, Y.M. Treatment Strategies for Anti-VEGF Resistance in Neovascular Age-Related Macular Degeneration by Targeting Arteriolar Choroidal Neovascularization. Biomolecules 2024, 14, 252. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Maharaj, A.S.R.; Walshe, T.E.; Tucker, B.A.; Sekiyama, E.; Kurihara, T.; Darland, D.C.; Young, M.J.; D’Amore, P.A. Endogenous VEGF is required for visual function: Evidence for a survival role on Müller cells and photoreceptors. PLoS ONE 2008, 3, e3554. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhao, J.; Yang, Y.; Cai, X.; Xu, J.; Cao, P. Exosomes: A novel strategy for treatment and prevention of diseases. Front. Pharmacol. 2017, 8, 300. [Google Scholar] [CrossRef]
- Zhang, Z.; Mugisha, A.; Fransisca, S.; Liu, Q.; Xie, P.; Hu, Z. Emerging Role of Exosomes in Retinal Diseases. Front. Cell Dev. Biol. 2021, 9, 643680. [Google Scholar] [CrossRef]
- Lee, S.; Han, J.; Yang, J.; Lyu, J.; Park, H.; Bang, J.; Kim, Y.; Chang, H.; Park, T. Exosomes from Human iPSC-Derived Retinal Organoids Enhance Corneal Epithelial Wound Healing. Int. J. Mol. Sci. 2024, 25, 8925. [Google Scholar] [CrossRef]
- Neves, K.B.; Rios, F.J.; Sevilla-Montero, J.; Montezano, A.C.; Touyz, R.M. Exosomes and the cardiovascular system: Role in cardiovascular health and disease. J. Physiol. 2023, 601, 4923–4936. [Google Scholar] [CrossRef] [PubMed]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H.J.; Zhang, H.G.; Shao, H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Investig. Ophthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Y.; Zhang, X.; Wang, J.; Wang, X.; Gu, Q.; Xu, C.; Fan, Y.; Li, X.; Xie, P.; et al. Lens epithelial cell-derived exosome inhibits angiogenesis in ocular pathological neovascularization through its delivery of miR-146a-5p. FASEB J. 2023, 37, e23192. [Google Scholar] [CrossRef] [PubMed]
- Capowski, E.E.; Samimi, K.; Mayerl, S.J.; Phillips, M.J.; Pinilla, I.; Howden, S.E.; Saha, J.; Jansen, A.D.; Edwards, K.L.; Jager, L.D.; et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 2019, 146, dev171686. [Google Scholar] [CrossRef]
- Berber, P.; Bondarenko, S.; Michaelis, L.; Weber, B.H.F. Transient Retention of Photoreceptor Outer Segments in Matrigel-Embedded Retinal Organoids. Int. J. Mol. Sci. 2022, 23, 14893. [Google Scholar] [CrossRef]
- Zhou, J.; Flores-Bellver, M.; Pan, J.; Benito-Martin, A.; Shi, C.; Onwumere, O.; Mighty, J.; Qian, J.; Zhong, X.; Hogue, T.; et al. Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells. Sci. Rep. 2021, 11, 21128. [Google Scholar] [CrossRef]
- Arthur, P.; Kandoi, S.; Sun, L.; Kalvala, A.; Kutlehria, S.; Bhattacharya, S.; Kulkarni, T.; Nimma, R.; Li, Y.; Lamba, D.A.; et al. Biophysical, Molecular and Proteomic Profiling of Human Retinal Organoid-Derived Exosomes. Pharm. Res. 2023, 40, 801–816. [Google Scholar] [CrossRef]
- Han, J.W.; Chang, H.S.; Yang, J.Y.; Choi, H.S.; Park, H.S.; Jun, H.O.; Choi, J.H.; Paik, S.S.; Chung, K.H.; Shin, H.J.; et al. Intravitreal Administration of Retinal Organoids-Derived Exosomes Alleviates Photoreceptor Degeneration in Royal College of Surgeons Rats by Targeting the Mitogen-Activated Protein Kinase Pathway. Int. J. Mol. Sci. 2023, 24, 12068. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Henry, J.; Zhe, J.; Hu, J.; Wang, X.; Paulus, Y.M. Multimodal imaging of laser-induced choroidal neovascularization in pigmented rabbits. Sci. Rep. 2023, 13, 8396. [Google Scholar] [CrossRef]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular agerelated macular degeneration. Cochrane Database Syst. Rev. 2019, 2019, CD005139. [Google Scholar] [CrossRef]
- Alizadeh, E.; Mammadzada, P.; André, H. The different facades of retinal and choroidal endothelial cells in response to hypoxia. Int. J. Mol. Sci. 2018, 19, 3846. [Google Scholar] [CrossRef]
- Barben, M.; Samardzija, M.; Grimm, C. The role of hypoxia, hypoxia-inducible factor (HIF), and VEGF in retinal angiomatous proliferation. Adv. Exp. Med. Biol. 2018, 1074, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhang, Q.Y.; Liu, Q.; Feng, S.G.; Ma, Y.; Wang, F.S.; Zhu, Y.; Yao, J.; Yan, B. Exosome-loading miR-205: A two-pronged approach to ocular neovascularization therapy. J. Nanobiotechnol. 2025, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Geathers, J.S.; Grillo, S.L.; Weber, S.R.; Wang, W.; Zhao, Y.; Sundstrom, J.M. Role of Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium Dysfunction. Front. Cell Dev. Biol. 2020, 8, 501. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A. Retinal pigment epithelial cells as a therapeutic tool and target against retinopathies. Drug Discov. Today 2018, 23, 1672–1679. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Park, H.S.; Bang, J.H.; Jung, W.H.; Yang, J.Y.; Shin, H.J.; Son, J.H.; Han, J.W.; Lee, S.H.; Chung, K.H.; Kim, K.; et al. Development of Non-Invasive miRNA Markers for Assessing the Quality of Human Induced Pluripotent Stem Cell-Derived Retinal Organoids. Int. J. Mol. Sci. 2024, 25, 8011. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Gwon, Y.; Khan, S.A.; Jang, K.J.; Kim, J. Engineering considerations of iPSC-based personalized medicine. Biomater. Res. 2023, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.L. Human induced pluripotent stem cell-derived exosomes as a new therapeutic strategy for various diseases. Int. J. Mol. Sci. 2021, 22, 1769. [Google Scholar] [CrossRef]
- Jakobsen, T.S.; Fabian-Jessing, B.K.; Hansen, S.; Bek, T.; Askou, A.L.; Corydon, T.J. Porcine models of choroidal neovascularization: A systematic review. Exp. Eye Res. 2023, 234, 109590. [Google Scholar] [CrossRef]
- Salas, A.; Badia, A.; Fontrodona, L.; Zapata, M.; Duarri, A. Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model. Biomedicines 2023, 11, 2445. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, J.W.; Yang, J.Y.; Jun, H.O.; Bang, J.H.; Shin, H.; Choi, J.H.; Lee, J.; Madrakhimov, S.B.; Chung, K.H.; et al. Role of mTORC1 activity during early retinal development and lamination in human-induced pluripotent stem cell-derived retinal organoids. Cell Death Discov. 2022, 8, 56. [Google Scholar] [CrossRef]
- Rangel-Fonseca, P.; Gómez-Vieyra, A.; Malacara-Hernández, D.; Wilson, M.C.; Williams, D.R.; Rossi, E.A. Automated segmentation of retinal pigment epithelium cells in fluorescence adaptive optics images. J. Opt. Soc. Am. A 2013, 30, 2595. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.Y.; Kim, Y.; An, S.; Han, J.W.; Choi, J.-S.; Park, T.K. Retinal Organoid-Derived Exosomes Reduce CNV Lesion and Restore RPE Integrity in Mouse Laser-Induced Choroidal Neovascularization (CNV) Model. Int. J. Mol. Sci. 2025, 26, 11327. https://doi.org/10.3390/ijms262311327
Yang JY, Kim Y, An S, Han JW, Choi J-S, Park TK. Retinal Organoid-Derived Exosomes Reduce CNV Lesion and Restore RPE Integrity in Mouse Laser-Induced Choroidal Neovascularization (CNV) Model. International Journal of Molecular Sciences. 2025; 26(23):11327. https://doi.org/10.3390/ijms262311327
Chicago/Turabian StyleYang, Jin Young, Yeji Kim, Sumin An, Jung Woo Han, Jun-Sub Choi, and Tae Kwann Park. 2025. "Retinal Organoid-Derived Exosomes Reduce CNV Lesion and Restore RPE Integrity in Mouse Laser-Induced Choroidal Neovascularization (CNV) Model" International Journal of Molecular Sciences 26, no. 23: 11327. https://doi.org/10.3390/ijms262311327
APA StyleYang, J. Y., Kim, Y., An, S., Han, J. W., Choi, J.-S., & Park, T. K. (2025). Retinal Organoid-Derived Exosomes Reduce CNV Lesion and Restore RPE Integrity in Mouse Laser-Induced Choroidal Neovascularization (CNV) Model. International Journal of Molecular Sciences, 26(23), 11327. https://doi.org/10.3390/ijms262311327





