Piperine and Tabersonine, but Not Lupinine, Inhibit S. proteamaculans Invasion of M-HeLa Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Alkaloids on Bacterial Growth and Biofilm Formation
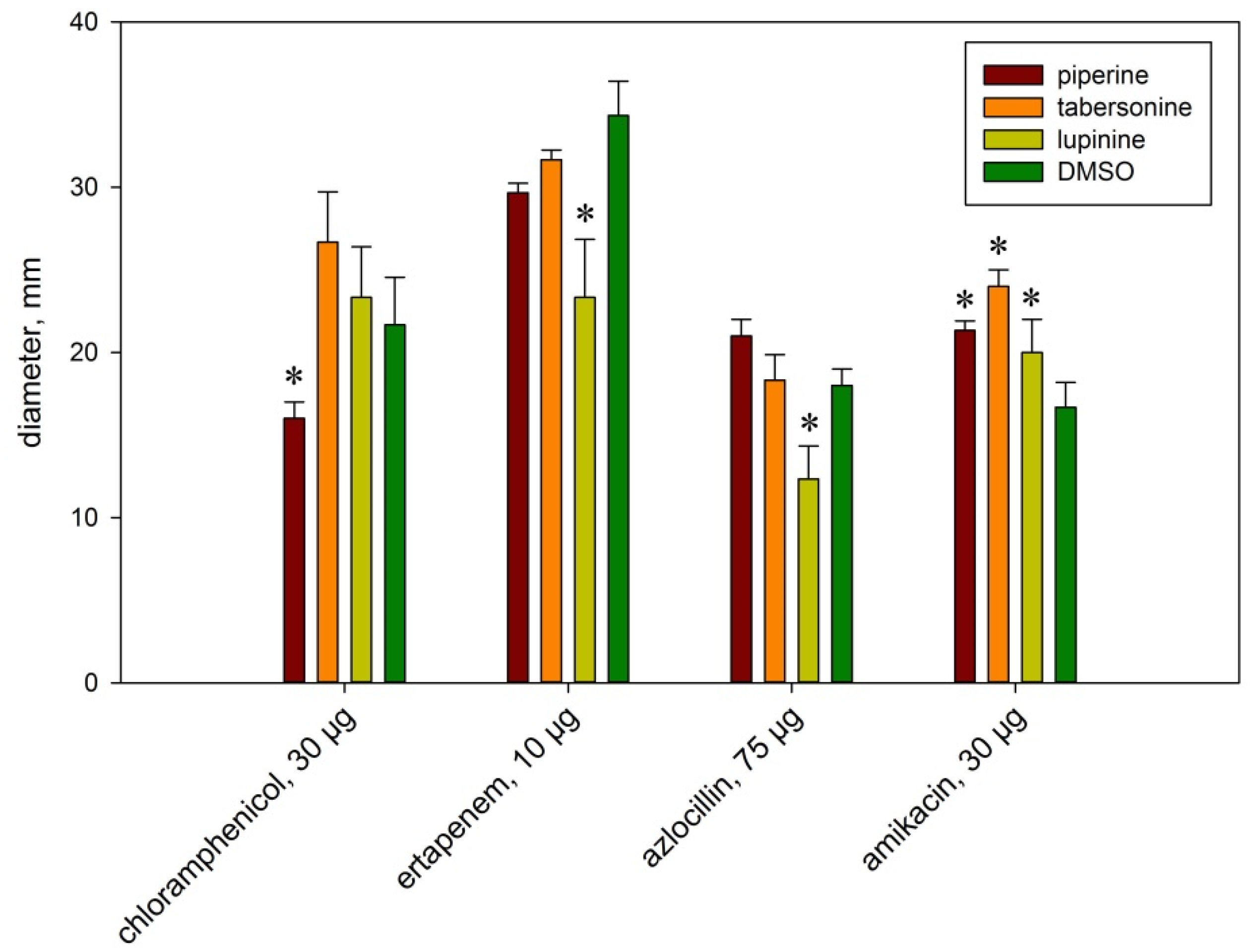
2.2. The Effect of Alkaloids on Bacterial Sensitivity to Antibiotics
2.3. Cytotoxic Effect of Alkaloids on Human Cells
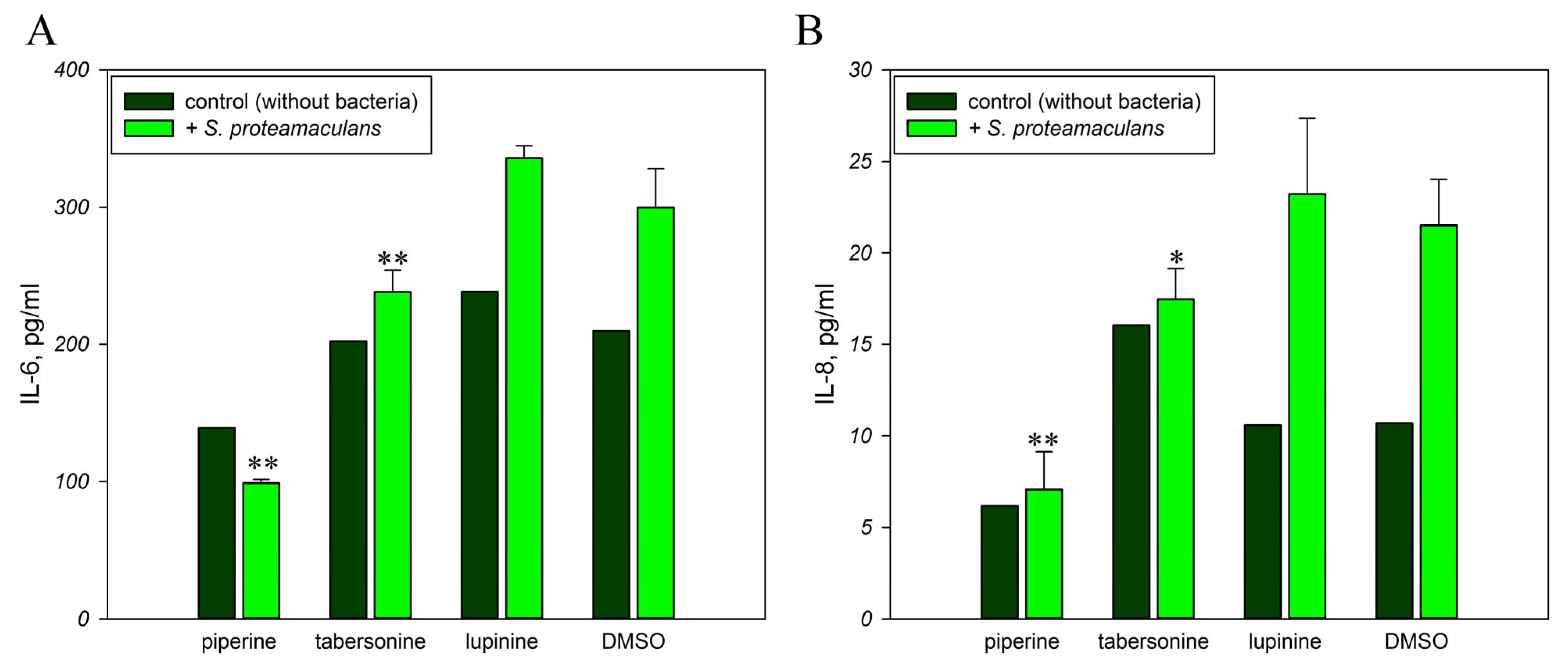
2.4. Inflammatory Response to Infection in the Presence of Alkaloids
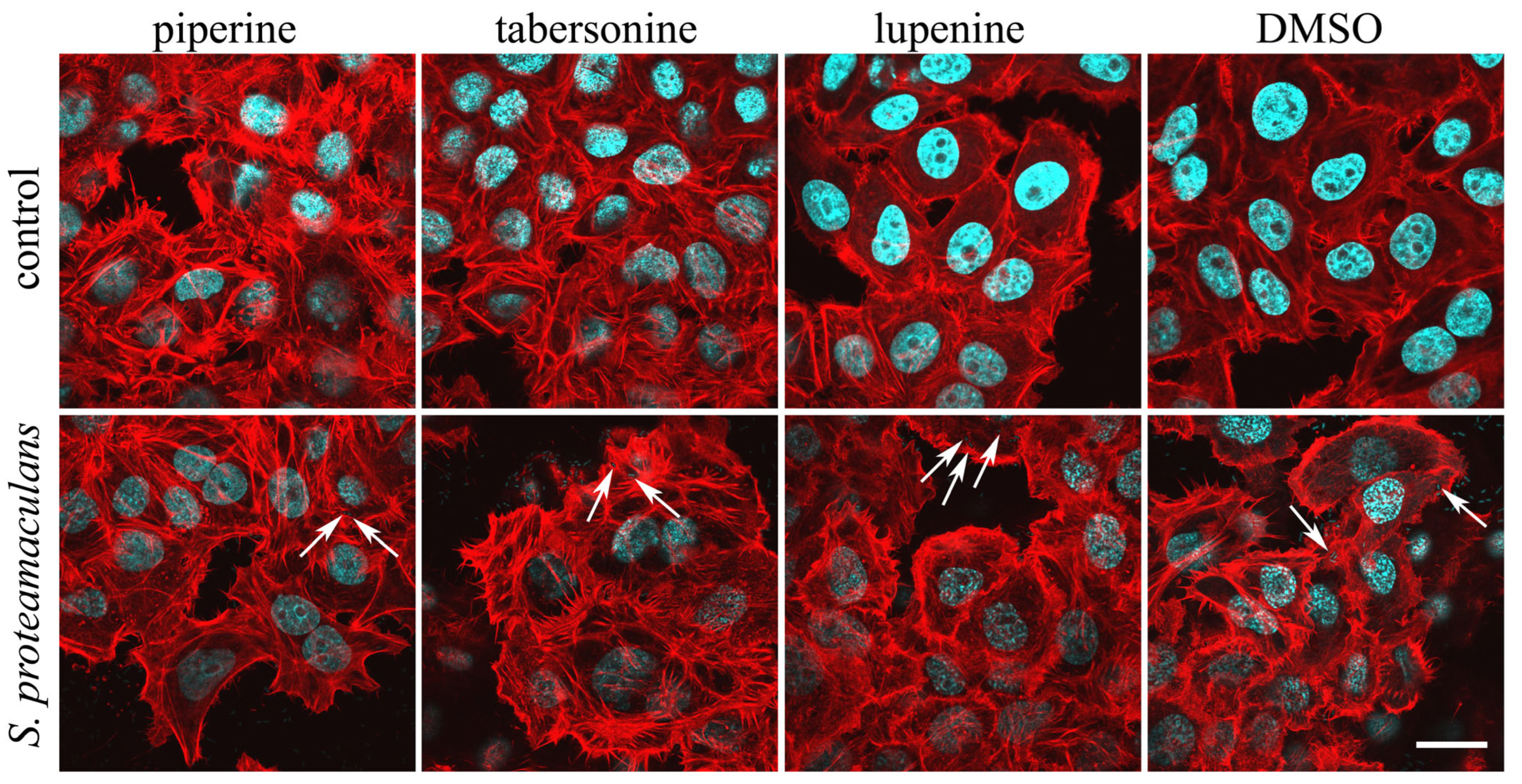
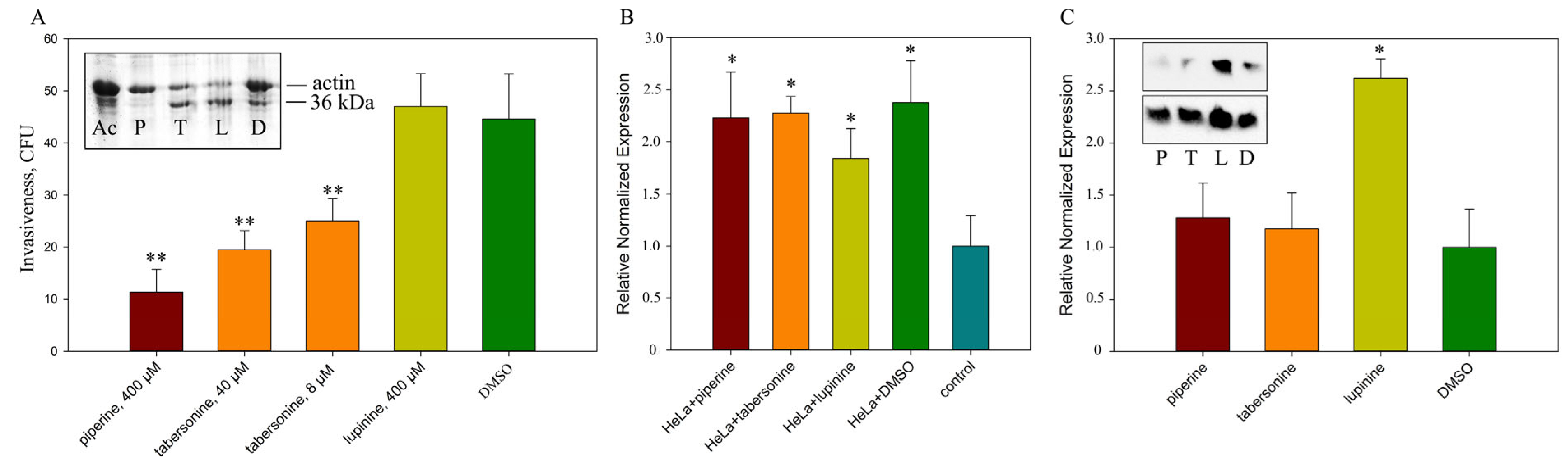
2.5. The Effect of Alkaloids on the Intensity of Invasion
2.6. Accumulation of Bacterial Virulence Factors in the Presence of Alkaloids
3. Materials and Methods
3.1. Cell Cultures, Bacterial Strains, and Growth Conditions
3.2. Biofilm Formation
3.3. Cell Viability Assessment Using MTT Assay
3.4. Antibiotic Resistance
3.5. Cytokine Enzyme-Linked Immunosorbent Assay
3.6. Fluorescence Microscopy
3.7. Quantitative Invasion Assay
3.8. Western Blot Analysis
3.9. Limited Proteolysis Assay
3.10. Real-Time RT-PCR
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomson, C.J.; Power, E.; Ruebsamen-Waigmann, H.; Labischinski, H. Antibacterial research and development in the 21(st) Century—An industry perspective of the challenges. Curr. Opin. Microbiol. 2004, 7, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Pizarro-Cerda, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Shekunov, E.V.; Efimova, S.S.; Yudintceva, N.M.; Muryleva, A.A.; Zarubaev, V.V.; Slita, A.V.; Ostroumova, O.S. Plant Alkaloids Inhibit Membrane Fusion Mediated by Calcium and Fragments of MERS-CoV and SARS-CoV/SARS-CoV-2 Fusion Peptides. Biomedicines 2021, 9, 1434. [Google Scholar] [CrossRef]
- Zivkovic Zaric, R.; Zaric, M.; Sekulic, M.; Zornic, N.; Nesic, J.; Rosic, V.; Vulovic, T.; Spasic, M.; Vuleta, M.; Jovanovic, J.; et al. Antimicrobial Treatment of Serratia marcescens Invasive Infections: Systematic Review. Antibiotics 2023, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Mahlen, S.D. Serratia infections: From military experiments to current practice. Clin. Microbiol. Rev. 2011, 24, 755–791. [Google Scholar] [CrossRef] [PubMed]
- Tsaplina, O.A.; Efremova, T.N.; Kever, L.V.; Komissarchik, Y.Y.; Demidyuk, I.V.; Kostrov, S.V.; Khaitlina, S.Y. Probing for actinase activity of protealysin. Biochemistry 2009, 74, 648–654. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Paul, P.; Das, S.; Chatterjee, S.; Shukla, A.; Chakraborty, P.; Sarkar, S.; Maiti, D.; Das, A.; Tribedi, P. 1,4-Naphthoquinone disintegrates the pre-existing biofilm of Staphylococcus aureus by accumulating reactive oxygen species. Arch. Microbiol. 2021, 203, 4981–4992. [Google Scholar] [CrossRef]
- Das, S.; Paul, P.; Chatterjee, S.; Chakraborty, P.; Sarker, R.K.; Das, A.; Maiti, D.; Tribedi, P. Piperine exhibits promising antibiofilm activity against Staphylococcus aureus by accumulating reactive oxygen species (ROS). Arch. Microbiol. 2021, 204, 59. [Google Scholar] [CrossRef]
- Das, S.; Paul, P.; Dastidar, D.G.; Chakraborty, P.; Chatterjee, S.; Sarkar, S.; Maiti, D.; Tribedi, P. Piperine Exhibits Potential Antibiofilm Activity Against Pseudomonas aeruginosa by Accumulating Reactive Oxygen Species, Affecting Cell Surface Hydrophobicity and Quorum Sensing. Appl. Biochem. Biotechnol. 2023, 195, 3229–3256. [Google Scholar] [CrossRef]
- Das, S.; Roy, R.; Paul, P.; Chakraborty, P.; Chatterjee, S.; Malik, M.; Sarkar, S.; Das Gupta, A.; Maiti, D.; Tribedi, P. Piperine, a Plant Alkaloid, Exhibits Efficient Disintegration of the Pre-existing Biofilm of Staphylococcus aureus: A Step Towards Effective Management of Biofilm Threats. Appl. Biochem. Biotechnol. 2024, 196, 1272–1291. [Google Scholar] [CrossRef]
- Khan, I.A.; Mirza, Z.M.; Kumar, A.; Verma, V.; Qazi, G.N. Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Ghandadi, M.; Ghoochi Atashbeyk, D.; Fazly Bazzaz, B.S.; Iranshahi, M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev. Ind. Pharm. 2015, 41, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Nargotra, A.; Sharma, S.; Koul, J.L.; Sangwan, P.L.; Khan, I.A.; Kumar, A.; Taneja, S.C.; Koul, S. Quantitative structure activity relationship (QSAR) of piperine analogs for bacterial NorA efflux pump inhibitors. Eur. J. Med. Chem. 2009, 44, 4128–4135. [Google Scholar] [CrossRef] [PubMed]
- Quijia, C.R.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Piperine: A Review. Crit. Rev. Anal. Chem. 2020, 50, 62–77. [Google Scholar] [CrossRef]
- Tsaplina, O.; Bozhokina, E.; Mardanova, A.; Khaitlina, S. Virulence factors contributing to invasive activities of Serratia grimesii and Serratia proteamaculans. Arch. Microbiol. 2015, 197, 481–488. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef]
- Chen, H.; Sheng, H.; Zhao, Y.; Zhu, G. Piperine Inhibits Cell Proliferation and Induces Apoptosis of Human Gastric Cancer Cells by Downregulating Phosphatidylinositol 3-Kinase (PI3K)/Akt Pathway. Med. Sci. Monit. 2020, 26, e928403. [Google Scholar] [CrossRef]
- Shaheer, K.; Somashekarappa, H.M.; Lakshmanan, M.D. Piperine sensitizes radiation-resistant cancer cells towards radiation and promotes intrinsic pathway of apoptosis. J. Food Sci. 2020, 85, 4070–4079. [Google Scholar] [CrossRef]
- Wu, C.; Qian, Y.; Jiang, J.; Li, D.; Feng, L. Piperine inhibits the proliferation of colorectal adenocarcinoma by regulating ARL3-mediated endoplasmic reticulum stress. Biomol. Biomed. 2025, 25, 391–405. [Google Scholar] [CrossRef]
- Chang, W.L.; Peng, J.Y.; Hong, C.L.; Li, P.C.; Chye, S.M.; Lu, F.J.; Lin, H.Y.; Chen, C.H. Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells. Antioxidants 2025, 14, 892. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Chen, L.; Deng, Y.; Zheng, Z.; Ming, Y. Tabersonine Induces the Apoptosis of Human Hepatocellular Carcinoma In vitro and In vivo. Anticancer. Agents Med. Chem. 2024, 24, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, T.; Shi, L.; Takasu, S.; Cho, Y.M.; Kiriyama, Y.; Nishikawa, A.; Ogawa, K.; Tatematsu, M.; Tsukamoto, T. Anti-Inflammatory Effects of Capsaicin and Piperine on Helicobacter pylori-Induced Chronic Gastritis in Mongolian Gerbils. Helicobacter 2016, 21, 131–142. [Google Scholar] [CrossRef]
- Xia, Y.; Khoi, P.N.; Yoon, H.J.; Lian, S.; Joo, Y.E.; Chay, K.O.; Kim, K.K.; Jung, Y.D. Piperine inhibits IL-1beta-induced IL-6 expression by suppressing p38 MAPK and STAT3 activation in gastric cancer cells. Mol. Cell Biochem. 2015, 398, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nguyen, T.T.; Ung, T.T.; Sah, D.K.; Park, S.Y.; Lakshmanan, V.K.; Jung, Y.D. Piperine Attenuates Lithocholic Acid-Stimulated Interleukin-8 by Suppressing Src/EGFR and Reactive Oxygen Species in Human Colorectal Cancer Cells. Antioxidants 2022, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, X.; Hu, Y.; Jiang, H.; Wu, Y.; Ding, Y.; Yu, K.; He, H.; Xu, J.; Sun, L.; et al. Tabersonine attenuates lipopolysaccharide-induced acute lung injury via suppressing TRAF6 ubiquitination. Biochem. Pharmacol. 2018, 154, 183–192. [Google Scholar] [CrossRef]
- de Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/beta-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef]
- Srivastava, S.; Dewangan, J.; Mishra, S.; Divakar, A.; Chaturvedi, S.; Wahajuddin, M.; Kumar, S.; Rath, S.K. Piperine and Celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/beta-catenin signaling pathway. Phytomedicine 2021, 84, 153484. [Google Scholar] [CrossRef]
- Tsaplina, O. Interaction of Serratia proteamaculans with Integrins Activates Invasion-Promoting Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 3955. [Google Scholar] [CrossRef]
- Tsaplina, O. The Balance between Protealysin and Its Substrate, the Outer Membrane Protein OmpX, Regulates Serratia proteamaculans Invasion. Int. J. Mol. Sci. 2024, 25, 6159. [Google Scholar] [CrossRef]
- Meng, X.; Liu, X.; Zhang, L.; Hou, B.; Li, B.; Tan, C.; Li, Z.; Zhou, R.; Li, S. Virulence characteristics of extraintestinal pathogenic Escherichia coli deletion of gene encoding the outer membrane protein X. J. Vet. Med. Sci. 2016, 78, 1261–1267. [Google Scholar] [CrossRef]
- Tsaplina, O.; Bozhokina, E. Bacterial Outer Membrane Protein OmpX Regulates beta1 Integrin and Epidermal Growth Factor Receptor (EGFR) Involved in Invasion of M-HeLa Cells by Serratia proteamaculans. Int. J. Mol. Sci. 2021, 22, 13246. [Google Scholar] [CrossRef] [PubMed]
- Demidyuk, I.V.; Kalashnikov, A.E.; Gromova, T.Y.; Gasanov, E.V.; Safina, D.R.; Zabolotskaya, M.V.; Rudenskaya, G.N.; Kostrov, S.V. Cloning, sequencing, expression, and characterization of protealysin, a novel neutral proteinase from Serratia proteamaculans representing a new group of thermolysin-like proteases with short N-terminal region of precursor. Protein Expr. Purif. 2006, 47, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tsaplina, O.; Lomert, E.; Berson, Y. Host-Cell-Dependent Roles of E-Cadherin in Serratia Invasion. Int. J. Mol. Sci. 2023, 24, 17075. [Google Scholar] [CrossRef]
- Spudich, J.A.; Watt, S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971, 246, 4866–4871. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.S., 3rd; Spencer, J.P. Aminoglycosides: A practical review. Am. Fam. Physician 1998, 58, 1811–1820. [Google Scholar]

| Target Gene | Primer Sequences |
|---|---|
| ompX | Forward 5′-GCAGTAGCAGCCTGTGTATTA-3′ |
| Reverse 5′-TTGGGCGTTGTCGATGTT-3′ | |
| protealysin | Forward 5′-GGTGAAGTCATCCGCGATATT-3′ |
| Reverse 5′-ATCAGCCAGTCGGCTTTATC-3′ | |
| S12 ribosomal protein | Forward 5′-CAGAAACGTGGCGTATGTACT-3′ |
| Reverse 5′-CGAGCTTGCTTACGGTCTTTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozhokina, E.; Berson, Y.; Tsaplina, O. Piperine and Tabersonine, but Not Lupinine, Inhibit S. proteamaculans Invasion of M-HeLa Cells. Int. J. Mol. Sci. 2025, 26, 11320. https://doi.org/10.3390/ijms262311320
Bozhokina E, Berson Y, Tsaplina O. Piperine and Tabersonine, but Not Lupinine, Inhibit S. proteamaculans Invasion of M-HeLa Cells. International Journal of Molecular Sciences. 2025; 26(23):11320. https://doi.org/10.3390/ijms262311320
Chicago/Turabian StyleBozhokina, Ekaterina, Yuliya Berson, and Olga Tsaplina. 2025. "Piperine and Tabersonine, but Not Lupinine, Inhibit S. proteamaculans Invasion of M-HeLa Cells" International Journal of Molecular Sciences 26, no. 23: 11320. https://doi.org/10.3390/ijms262311320
APA StyleBozhokina, E., Berson, Y., & Tsaplina, O. (2025). Piperine and Tabersonine, but Not Lupinine, Inhibit S. proteamaculans Invasion of M-HeLa Cells. International Journal of Molecular Sciences, 26(23), 11320. https://doi.org/10.3390/ijms262311320







