Interactions Between Tryptase-Positive Mast Cells and Melanin-A+ Cells in the Microenvironment of Cutaneous Melanoma
Abstract
1. Introduction
2. Results
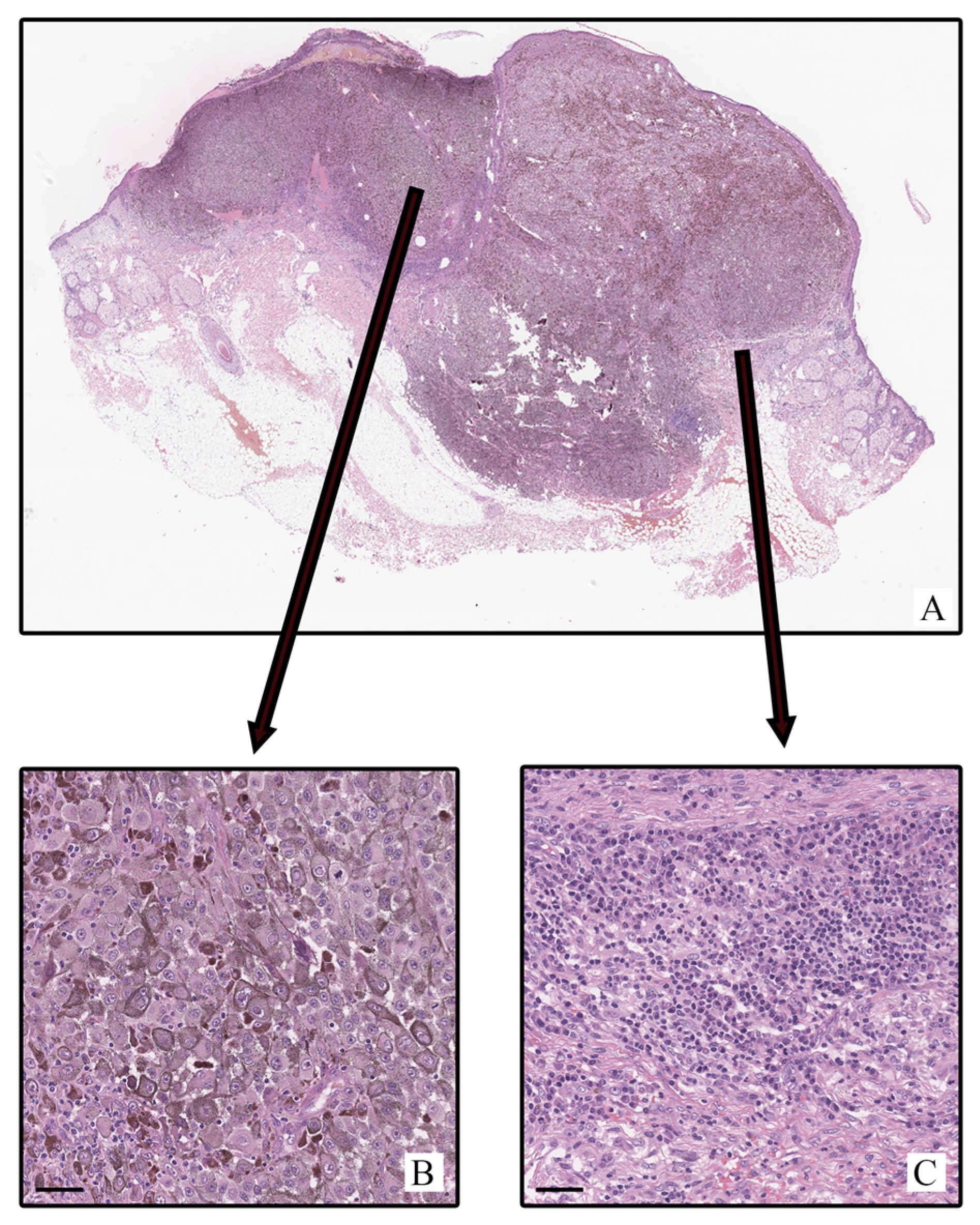
2.1. Histological Study
2.2. Histochemical Analysis
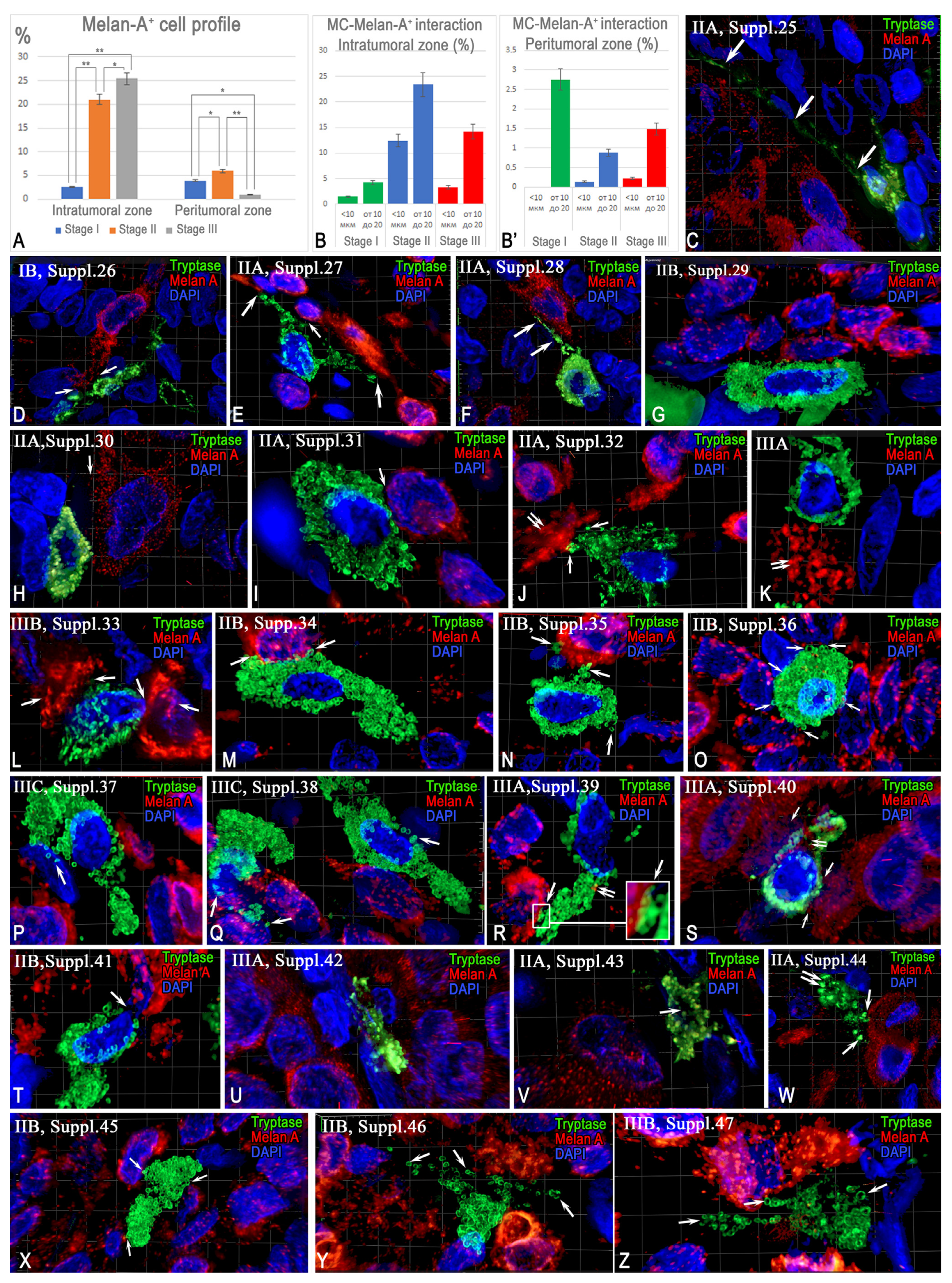
2.3. Immunohistochemical Analysis
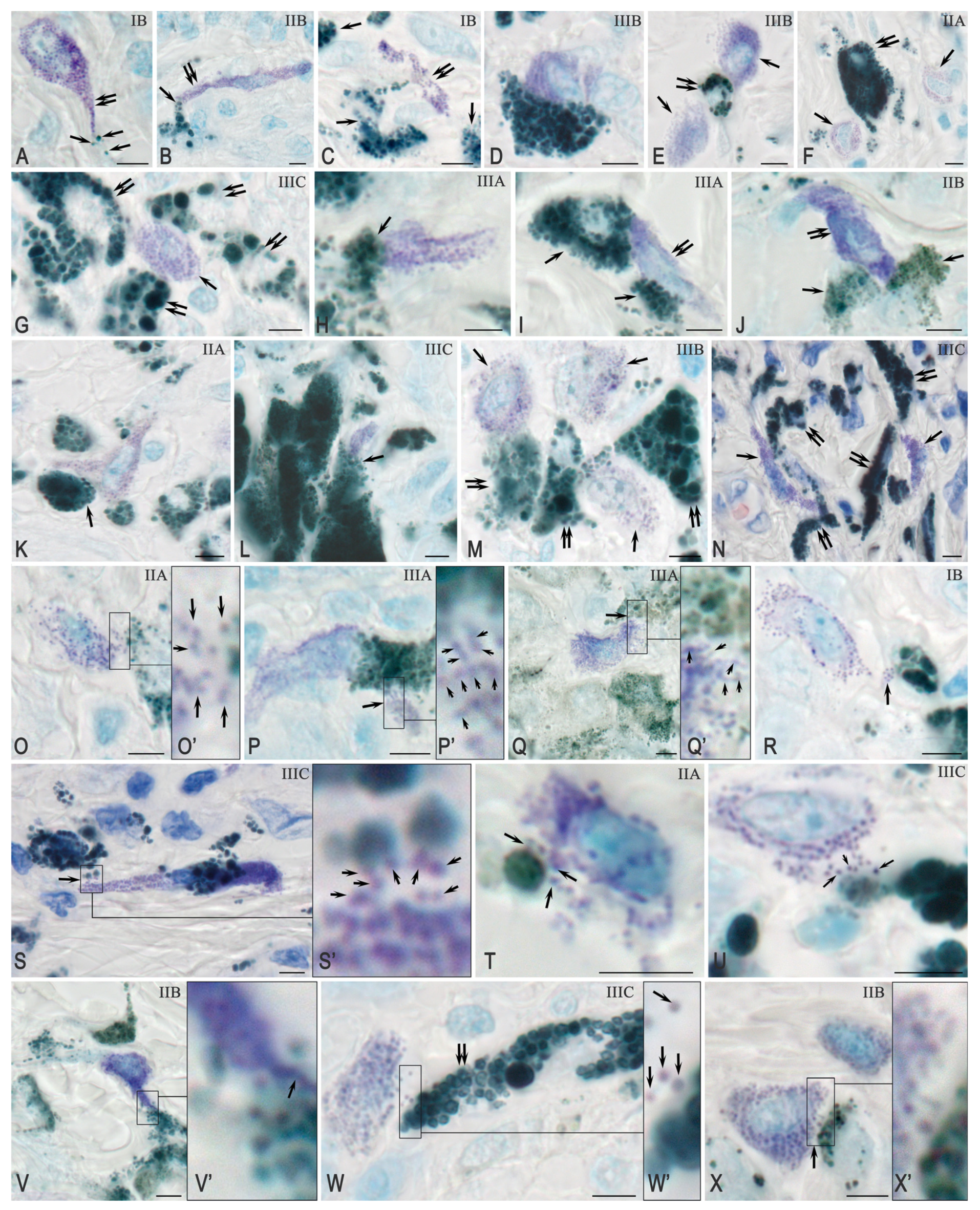
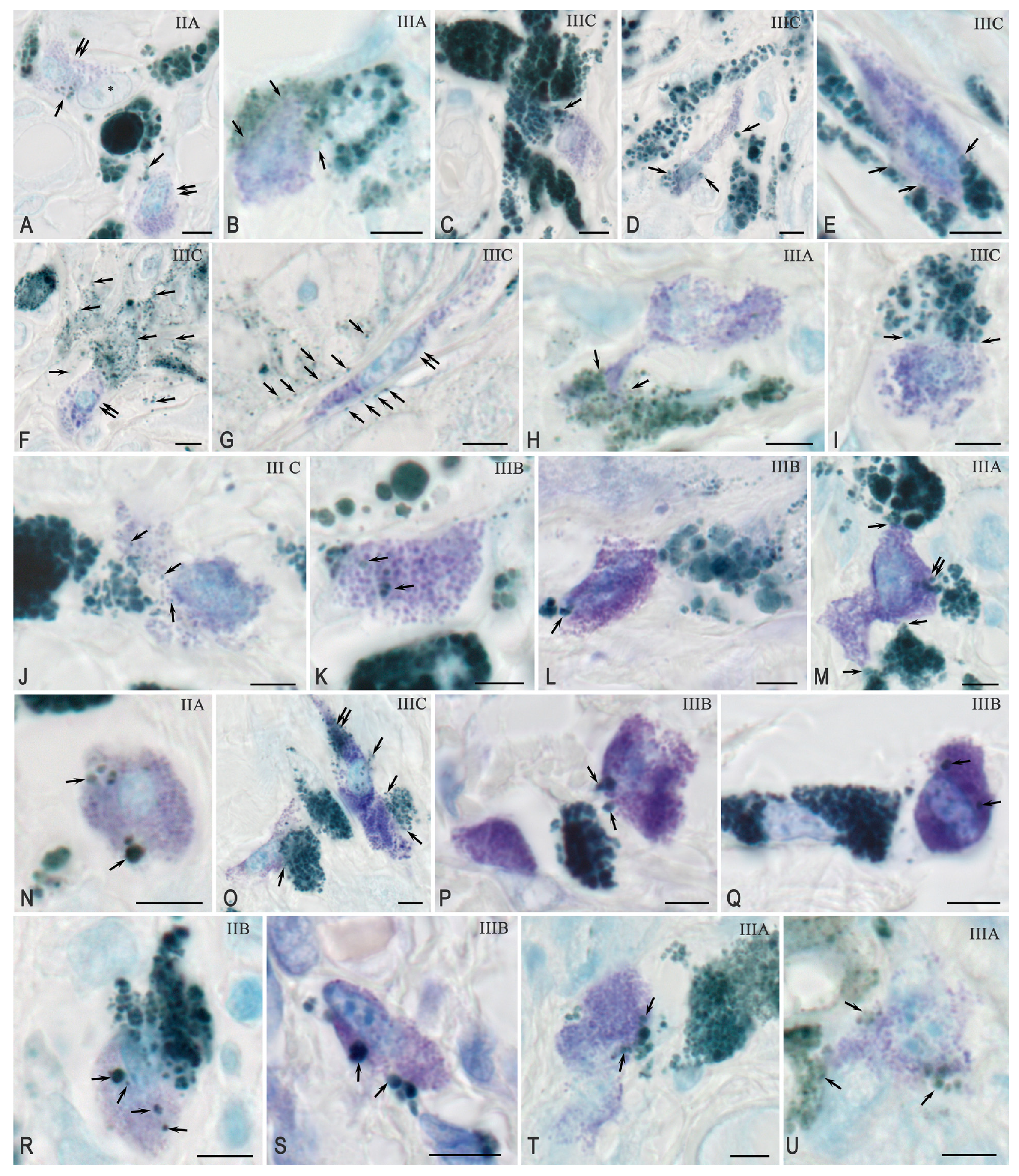
2.3.1. Quantitative and Cytological Features of Tryptase-Positive Mast Cells Associated with Cutaneous Melanoma
2.3.2. Interaction Between Tryptase-Positive Mast Cells and Atypical Melan-A+ Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. Tissue Probe Staining
4.2. Immunohistochemistry (IHC) and Histochemistry
4.3. Image Acquisition
4.4. Quantitative Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fruntelata, R.F.; Bakri, A.; Stoica, G.A.; Mogoanta, L.; Ionovici, N.; Popescu, G.; Pirscoveanu, D.F.V.; Raicea, A.; Ciurea, M.E. Assessment of tumoral and peritumoral inflammatory reaction in cutaneous malignant melanomas. Rom. J. Morphol. Embryol. 2023, 64, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Trocchia, M.; Ventrici, A.; Modestino, L.; Cristinziano, L.; Ferrara, A.L.; Palestra, F.; Loffredo, S.; Capone, M.; Madonna, G.; Romanelli, M.; et al. Innate Immune Cells in Melanoma: Implications for Immunotherapy. Int. J. Mol. Sci. 2024, 25, 8523. [Google Scholar] [CrossRef]
- Bida, M.; Miya, T.V.; Hull, R.; Dlamini, Z. Tumor-infiltrating lymphocytes in melanoma: From prognostic assessment to therapeutic applications. Front. Immunol. 2024, 15, 1497522. [Google Scholar] [CrossRef]
- Marzagalli, M.; Ebelt, N.D.; Manuel, E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin. Cancer Biol. 2019, 59, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Jalili, A. The emerging role of mast cells in skin cancers: Involved cellular and molecular mechanisms. Int. J. Dermatol. 2022, 61, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases—In Vivo Targets and Function. Front. Immunol. 2022, 13, 918305. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Shishkina, V.; Burtseva, A.; Buravleva, A.; Volodkin, A.; Elieh-Ali-Komi, D.; Buchwalow, I.; Tiemann, M. Space-Flight- and Microgravity-Dependent Alteration of Mast Cell Population and Protease Expression in Digestive Organs of Mongolian Gerbils. Int. J. Mol. Sci. 2023, 24, 13604. [Google Scholar] [CrossRef]
- Montero-Hernandez, J.E.; Zhang, K.; Blank, U.; Menasche, G. LRO biogenesis and function: What can we learn from mast cells? Front. Cell Dev. Biol. 2025, 13, 1613677. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Kostin, A.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Protease Profile of Tumor-Associated Mast Cells in Melanoma. Int. J. Mol. Sci. 2022, 23, 8930. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Kostin, A.; Trotsenko, I.; Samoilova, V.; Buchwalow, I.; Tiemann, M. Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells. Cells 2022, 11, 570. [Google Scholar] [CrossRef]
- Kohl, L.M.; Sumpter, T.L. Melanomas and mast cells: An ambiguous relationship. Melanoma Res. 2024, 34, 1–8. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Volodkin, A.; Nazarova, A.; Shishkina, V.; Esaulenko, D.; Buchwalow, I.; Tiemann, M.; Noda, M. Mast Cells as a Potential Target of Molecular Hydrogen in Regulating the Local Tissue Microenvironment. Pharmaceuticals 2023, 16, 817. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Shafaghat, F.; Alipoor, S.D.; Kazemi, T.; Atiakshin, D.; Pyatilova, P.; Maurer, M. Immunomodulatory Significance of Mast Cell Exosomes (MC-EXOs) in Immune Response Coordination. Clin. Rev. Allergy Immunol. 2025, 68, 20. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Asl, S.; Maurer, M.; Kiani, A.; Atiakshin, D.; Stahl Skov, P.; Elieh-Ali-Komi, D. Novel insights on the biology and immunologic effects of histamine: A road map for allergists and mast cell biologists. J. Allergy Clin. Immunol. 2024, 155, 1095–1114. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G. Mast Cells: Fascinating but Still Elusive after 140 Years from Their Discovery. Int. J. Mol. Sci. 2020, 21, 464. [Google Scholar] [CrossRef]
- Galvan-Morales, M.A.; Vizuet-de-Rueda, J.C.; Montero-Vargas, J.M.; Teran, L.M. Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies. Int. J. Mol. Sci. 2025, 26, 8895. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Pejler, G.; Ronnberg, E.; Waern, I.; Wernersson, S. Mast cell proteases: Multifaceted regulators of inflammatory disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef]
- Frenzel, L.; Hermine, O. Mast cells and inflammation. Jt. Bone Spine 2013, 80, 141–145. [Google Scholar] [CrossRef]
- Reber, L.L.; Frossard, N. Targeting mast cells in inflammatory diseases. Pharmacol. Ther. 2014, 142, 416–435. [Google Scholar] [CrossRef]
- Bonnekoh, H.; Scheffel, J.; Kambe, N.; Krause, K. The role of mast cells in autoinflammation. Immunol. Rev. 2018, 282, 265–275. [Google Scholar] [CrossRef]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Unsworth, L.D. Targeting active sites of inflammation using inherent properties of tissue-resident mast cells. Acta Biomater. 2023, 159, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Patsap, O.; Kostin, A.; Mikhalyova, L.; Buchwalow, I.; Tiemann, M. Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts. Int. J. Mol. Sci. 2023, 24, 6498. [Google Scholar] [CrossRef]
- Bacci, S. The evolution of mast cells across all vertebrate classes: The mystery continues. Histol. Histopathol. 2025, 18926. [Google Scholar] [CrossRef]
- Fukuishi, N.; Takahama, K.; Kurosaki, H.; Ono, S.; Asai, H. The Role of Endogenous Specialized Proresolving Mediators in Mast Cells and Their Involvement in Inflammation and Resolution. Int. J. Mol. Sci. 2025, 26, 1491. [Google Scholar] [CrossRef]
- Ligan, C.; Ma, X.H.; Zhao, S.L.; Zhao, W. The regulatory role and mechanism of mast cells in tumor microenvironment. Am. J. Cancer Res. 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Ribatti, D. New insights into the role of mast cells as a therapeutic target in cancer through the blockade of immune checkpoint inhibitors. Front. Med. 2024, 11, 1373230. [Google Scholar] [CrossRef] [PubMed]
- Kostin, A.; Lyundup, A.; Alekhnovich, A.; Prikhodko, A.; Patsap, O.; Gronskaia, S.; Belaya, Z.; Lesnyak, O.; Melnichenko, G.; Mokrysheva, N.; et al. Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23. Med. Sci. 2025, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Pearce, A.L.; Robertson, B.O.; Coventry, B.J.; Marshman, G.; Finlay-Jones, J.J.; Hart, P.H. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br. J. Dermatol. 2004, 150, 895–903. [Google Scholar] [CrossRef]
- Rajabi, P.; Bagheri, A.; Hani, M. Intratumoral and Peritumoral Mast Cells in Malignant Melanoma: An Immunohistochemical Study. Adv. Biomed. Res. 2017, 6, 39. [Google Scholar] [CrossRef]
- Grujic, M.; Hellman, L.; Gustafson, A.M.; Akula, S.; Melo, F.R.; Pejler, G. Protective role of mouse mast cell tryptase Mcpt6 in melanoma. Pigment. Cell Melanoma Res. 2020, 33, 579–590. [Google Scholar] [CrossRef]
- Bahri, R.; Kiss, O.; Prise, I.; Garcia-Rodriguez, K.M.; Atmoko, H.; Martinez-Gomez, J.M.; Levesque, M.P.; Dummer, R.; Smith, M.P.; Wellbrock, C.; et al. Human Melanoma-Associated Mast Cells Display a Distinct Transcriptional Signature Characterized by an Upregulation of the Complement Component 3 That Correlates With Poor Prognosis. Front. Immunol. 2022, 13, 861545. [Google Scholar] [CrossRef]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef]
- Li, J.; Peng, G.; Zhu, K.; Jie, X.; Xu, Y.; Rao, X.; Xu, Y.; Chen, Y.; Xing, B.; Wu, G.; et al. PD-1(+) mast cell enhanced by PD-1 blocking therapy associated with resistance to immunotherapy. Cancer Immunol. Immunother. 2023, 72, 633–645. [Google Scholar] [CrossRef]
- Siiskonen, H.; Poukka, M.; Bykachev, A.; Tyynela-Korhonen, K.; Sironen, R.; Pasonen-Seppanen, S.; Harvima, I.T. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. 2015, 25, 479–485. [Google Scholar] [CrossRef]
- Paolino, G.; Moliterni, E.; Didona, D.; Cardone, M.; Lopez, T.; Garelli, V.; Richetta, A.G.; Bottoni, U.; Cantisani, C.; Rossi, A.; et al. Serum tryptase levels in melanoma patients: Case-control study and review of the literature. G. Ital. Dermatol. Venereol. 2019, 154, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martin, A.; Nogues, L.; Hergueta-Redondo, M.; Castellano-Sanz, E.; Garvin, E.; Cioffi, M.; Sola-Castrillo, P.; Buehring, W.; Ximenez-Embun, P.; Munoz, J.; et al. Mast cells impair melanoma cell homing and metastasis by inhibiting HMGA1 secretion. Immunology 2023, 168, 362–373. [Google Scholar] [CrossRef]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Gruosso, T.; Gigoux, M.; Manem, V.S.K.; Bertos, N.; Zuo, D.; Perlitch, I.; Saleh, S.M.I.; Zhao, H.; Souleimanova, M.; Johnson, R.M.; et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Investig. 2019, 129, 1785–1800. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Siebenhaar, F.; Metz, M.; Maurer, M. Mast cells protect from skin tumor development and limit tumor growth during cutaneous de novo carcinogenesis in a Kit-dependent mouse model. Exp. Dermatol. 2014, 23, 159–164. [Google Scholar] [CrossRef]
- Rabelo Melo, F.; Nyman, L.; Menander, I.O.; Grujic, M.; Pejler, G. Melanoma cells suppress mast cell function via a melanin-dependent mechanism. FEBS J. 2025, 292, 6075–6099. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jung, H.; Kim, H.M.; Jeong, H.J. Surfactin exerts an anti-cancer effect through inducing allergic reactions in melanoma skin cancer. Int. Immunopharmacol. 2021, 99, 107934. [Google Scholar] [CrossRef] [PubMed]
- Chekmaryova, I.; Kalinin, D.; Kostin, A.; Buchwalow, I.; Tiemann, M.; Elieh-Ali-Komi, D.; Atiakshin, D. Ultrastructural features of tumor-associated mast cells in parasympathetic paragangliomas (chemodectomas) of the neck. Microsc. Res. Tech. 2024, 87, 1373–1383. [Google Scholar] [CrossRef]
- Rabelo Melo, F.; Santosh Martin, S.; Sommerhoff, C.P.; Pejler, G. Exosome-mediated uptake of mast cell tryptase into the nucleus of melanoma cells: A novel axis for regulating tumor cell proliferation and gene expression. Cell Death Dis. 2019, 10, 659. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I.T. Mast cell chymase is in contact with melanoma cells in vivo and it detaches melanoma cells from the substratum in vitro. Transl. Cancer Res. 2022, 11, 4315–4325. [Google Scholar] [CrossRef]
- Grujic, M.; Paivandy, A.; Gustafson, A.M.; Thomsen, A.R.; Ohrvik, H.; Pejler, G. The combined action of mast cell chymase, tryptase and carboxypeptidase A3 protects against melanoma colonization of the lung. Oncotarget 2017, 8, 25066–25079. [Google Scholar] [CrossRef] [PubMed]
- Massari, N.A.; Nicoud, M.B.; Sambuco, L.; Cricco, G.P.; Martinel Lamas, D.J.; Herrero Ducloux, M.V.; Blanco, H.; Rivera, E.S.; Medina, V.A. Histamine therapeutic efficacy in metastatic melanoma: Role of histamine H4 receptor agonists and opportunity for combination with radiation. Oncotarget 2017, 8, 26471–26491. [Google Scholar] [CrossRef] [PubMed]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol. Immunol. 2015, 63, 18–24. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Nilsson, G.; Harvima, I.T. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp. Dermatol. 2010, 19, 117–122. [Google Scholar] [CrossRef]
- Biswas, A.; Richards, J.E.; Massaro, J.; Mahalingam, M. Mast cells in cutaneous tumors: Innocent bystander or maestro conductor? Int. J. Dermatol. 2014, 53, 806–811. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-Convex Polygons. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2018, Granada, Spain, 16–20 September 2018; pp. 265–273. [Google Scholar]
- The R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006; Volume 1. [Google Scholar]

| Characteristic | Rate |
|---|---|
| Age | 29–88 years |
| Average age | 64.2 ± 6.8 years |
| Gender | |
| Male | 57 |
| Female | 73 |
| Histologic type | |
| Superficial spreading melanoma | 49 |
| Nodular melanoma | 69 |
| Desmoplastic melanoma | 12 |
| Breslow thickness | |
| <0.8–1.0 mm | 33 |
| >1.0–2.0 mm | 29 |
| >2.0–4.0 mm | 36 |
| >4.0 mm | 32 |
| Clark level of invasion | |
| I | 17 |
| II | 26 |
| III | 24 |
| IV | 49 |
| V | 12 |
| Localization of primary melanoma | |
| Head and neck | 19 |
| Trunk | 32 |
| Upper extremities | 29 |
| Lower extremities | 48 |
| pT | |
| pT1a | 24 |
| pT1b | 9 |
| pT2a | 21 |
| pT2b | 9 |
| pT3a | 21 |
| pT3b | 14 |
| pT4a | 17 |
| pT4b | 15 |
| AJCC stage | |
| IA | 23 |
| IB | 10 |
| IIA | 12 |
| IIB | 14 |
| IIC | 14 |
| IIIA | 17 |
| IIIB | 17 |
| IIIC | 17 |
| IIID | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiakshin, D.; Demyashkin, G.; Silakov, K.; Prikhodko, A.; Shchekin, V.; Alekhnovich, A.; Grivtsova, L.; Davydov, D.; Klabukov, I.; Baranovskii, D.; et al. Interactions Between Tryptase-Positive Mast Cells and Melanin-A+ Cells in the Microenvironment of Cutaneous Melanoma. Int. J. Mol. Sci. 2025, 26, 11313. https://doi.org/10.3390/ijms262311313
Atiakshin D, Demyashkin G, Silakov K, Prikhodko A, Shchekin V, Alekhnovich A, Grivtsova L, Davydov D, Klabukov I, Baranovskii D, et al. Interactions Between Tryptase-Positive Mast Cells and Melanin-A+ Cells in the Microenvironment of Cutaneous Melanoma. International Journal of Molecular Sciences. 2025; 26(23):11313. https://doi.org/10.3390/ijms262311313
Chicago/Turabian StyleAtiakshin, Dmitrii, Grigory Demyashkin, Kirill Silakov, Aleksandra Prikhodko, Vladimir Shchekin, Alexander Alekhnovich, Lyudmila Grivtsova, Demyan Davydov, Ilya Klabukov, Denis Baranovskii, and et al. 2025. "Interactions Between Tryptase-Positive Mast Cells and Melanin-A+ Cells in the Microenvironment of Cutaneous Melanoma" International Journal of Molecular Sciences 26, no. 23: 11313. https://doi.org/10.3390/ijms262311313
APA StyleAtiakshin, D., Demyashkin, G., Silakov, K., Prikhodko, A., Shchekin, V., Alekhnovich, A., Grivtsova, L., Davydov, D., Klabukov, I., Baranovskii, D., Ivanov, S., Elieh-Ali-Komi, D., Buchwalow, I., Tiemann, M., Kostin, A., Shegay, P., & Kaprin, A. (2025). Interactions Between Tryptase-Positive Mast Cells and Melanin-A+ Cells in the Microenvironment of Cutaneous Melanoma. International Journal of Molecular Sciences, 26(23), 11313. https://doi.org/10.3390/ijms262311313







