Sublethal Doxorubicin Promotes Extracellular Vesicle Biogenesis in A375 Melanoma Cells: Implications for Vesicle-Loaded TGF-β-Mediated Cancer Progression and Cardiovascular Pathophysiology
Abstract
1. Introduction
2. Results
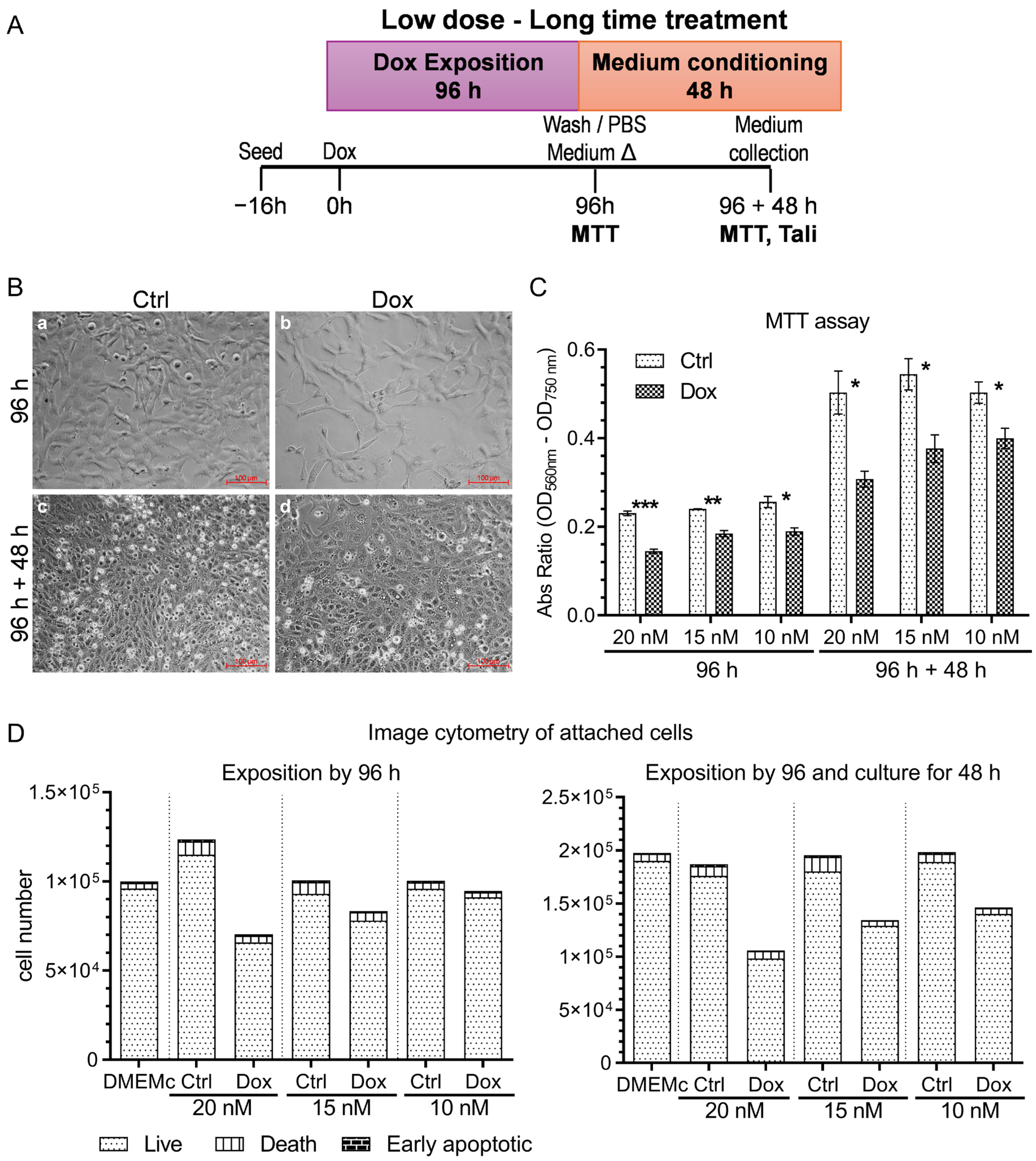
2.1. Doxorubicin Treatment Alters A375 Human Melanoma Cell Viability Without Inducing Apoptosis/Necrosis: Establishing an Optimal Sublethal Concentration for EV Studies
2.2. Doxorubicin at 10 nM Modulates Intracellular Vesicle Markers and Promotes Endosomal Activity in A375 Cells
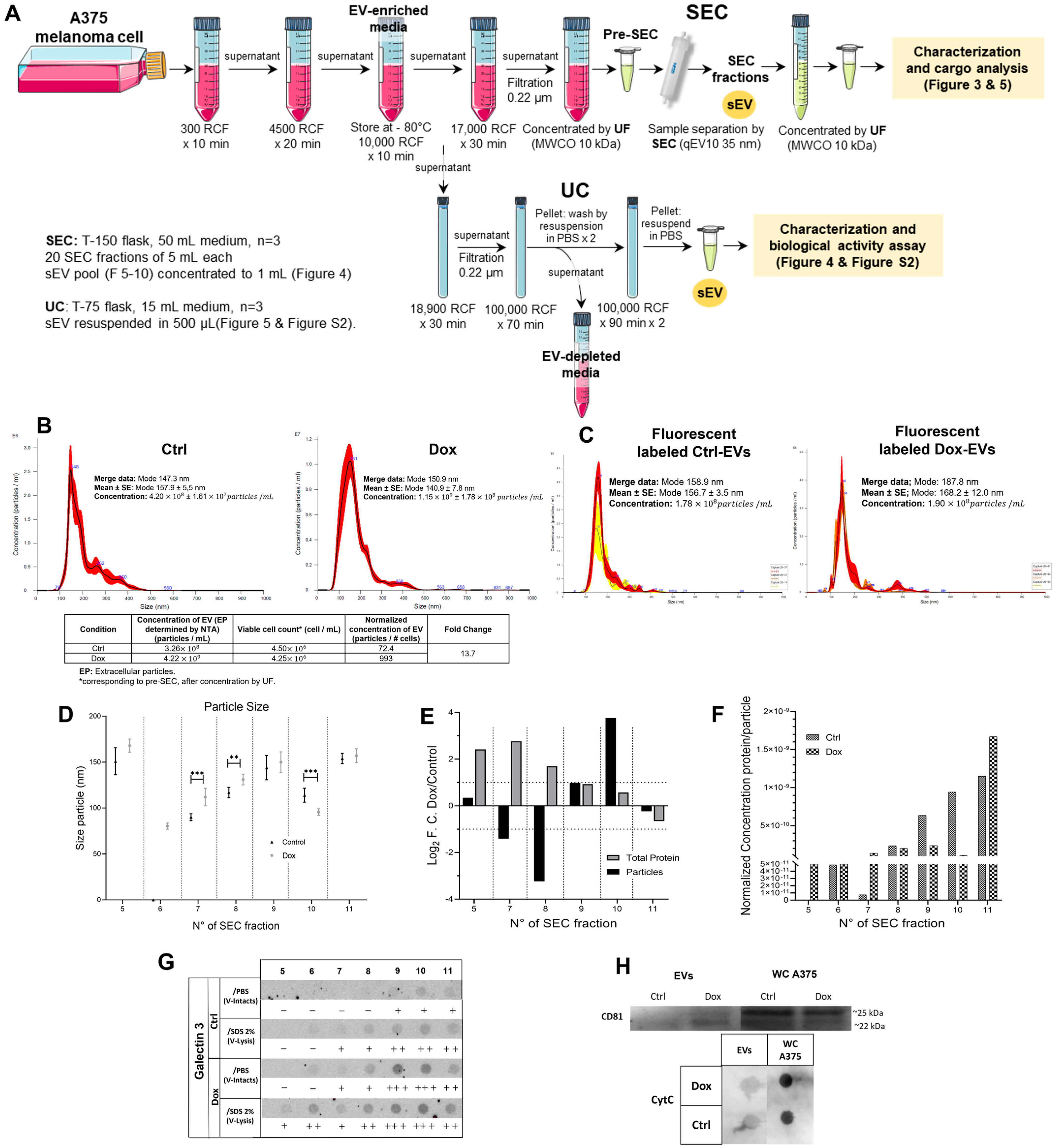
2.3. Isolation and Characterization of EVs from Doxorubicin-Treated A375 Cells
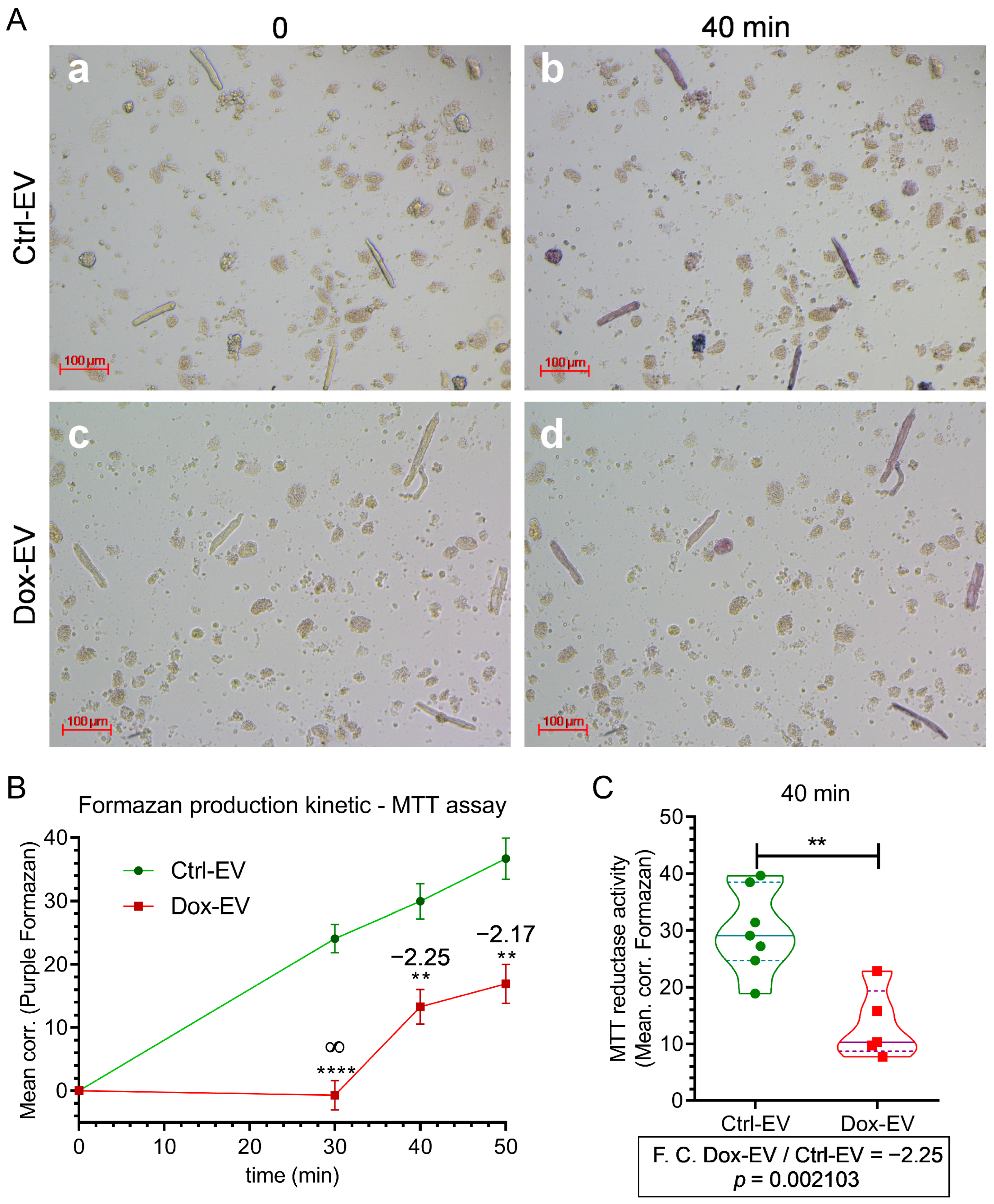
2.4. Doxorubicin-Induced EVs from A375 Cells Alter Metabolism in Primary Cardiomyocytes
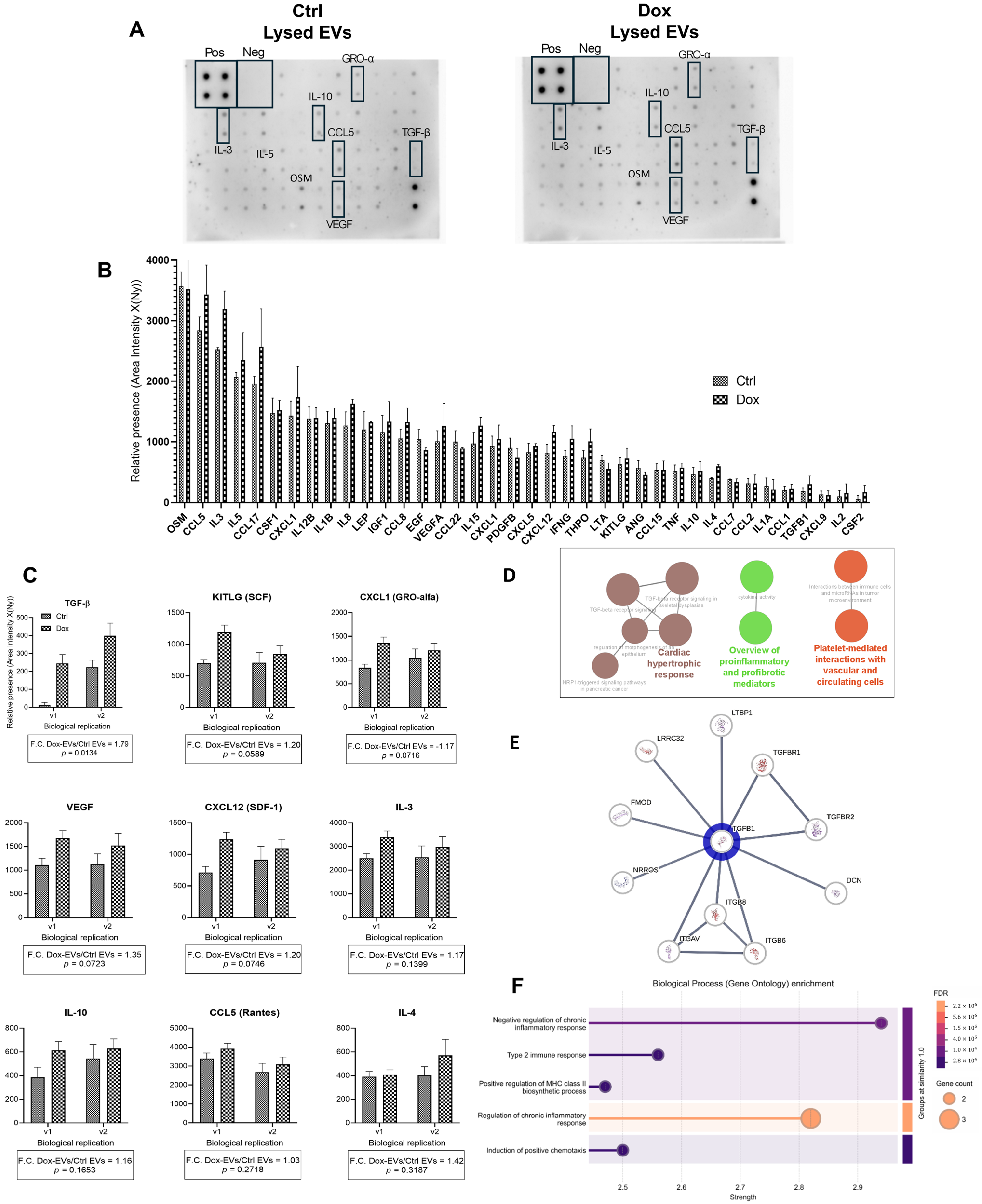
2.5. TGF-β Is Enriched in the Cargo of EVs Derived from Doxorubicin-Treated A375 Cells
3. Discussion
3.1. Sublethal Doxorubicin Induces Controlled Cellular Stress in A375 Melanoma Cells Without Triggering Cell Death
3.2. Doxorubicin Promotes Endosomal Trafficking and EVs Biogenesis in A375 Cells
3.3. Isolation and Characteristics of Small Extracellular Vesicles Derived from A375 Melanoma Cells Treated with Doxorubicin
3.4. Extracellular Vesicles from Doxo-Stressed Melanoma Cells Induce Metabolic Dysfunction in Cardiomyocytes
3.5. TGF-β-Enriched EVs from Doxorubicin-Treated Melanoma Cells: Functional and Bioinformatic Insights into Cytokines’ Roles in Cancer Progression and Cardiovascular Impact
3.6. Limitations
3.7. Future Directions
3.8. Potential Clinical Applications
4. Materials and Methods
4.1. Cell Culture and Doxorubicin Treatment
4.2. Cell Viability, Apoptosis, and Necrosis
4.2.1. MTT Assay
4.2.2. Apoptosis and Necrosis by Image Cytometry
4.3. Microscopy
4.3.1. Live Cell Imaging
4.3.2. Immunocytochemistry
4.4. Isolation and Characterization of Small Extracellular Vesicles
4.4.1. EVs Isolation and Purification
4.4.2. Scanning Electron Microscopy (SEM)
4.4.3. Nanoparticle Tracking Analysis (NTA)
4.4.4. Cytokine Profiling
4.5. Cardiomyocyte Isolation and Functional Assays
4.6. Bioinformatic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An Update on Anticancer Molecular Action, Toxicity and Novel Drug Delivery Systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, C.; Shi, H.; Zhang, B.; Zhang, L.; Zhang, H.; Qian, H.; Xu, W. Exosomal miRNA Derived from Chemoresistant Cells Contributes to the Chemoresistance of Sensitive Cells in Breast Cancer. Cancer Lett. 2021, 497, 100–111. [Google Scholar] [CrossRef]
- Vulpis, E.; Cuollo, L.; Borrelli, C.; Antonangeli, F.; Masuelli, L.; Cippitelli, M.; Fionda, C.; Caracciolo, G.; Petrucci, M.T.; Santoni, A.; et al. Doxorubicin-Mediated miR-433 Expression on Exosomes Promotes Bystander Senescence in Multiple Myeloma Cells in a DDR-Independent Manner. Int. J. Mol. Sci. 2023, 24, 6862. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Méndez, J.J.; Gómez-Grosso, L.A.; Montoya-Ortiz, G.; Novoa-Herrán, S.; Domínguez-Romero, Y. Small Extracellular Vesicles from Breast Cancer Cells Induce Cardiotoxicity. Int. J. Mol. Sci. 2025, 26, 945. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science. 2020, 367, 6977. [Google Scholar] [CrossRef] [PubMed]
- Ab Razak, N.S.; Ab Mutalib, N.S.; Mohtar, M.A.; Abu, N. Impact of Chemotherapy on Extracellular Vesicles: Understanding the Chemo-EVs. Front. Oncol. 2019, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Emam, S.E.; Ando, H.; Abu Lila, A.S.; Kobayashi, S.; Shimizu, T.; Okuhira, K.; Ishima, Y.; Ishida, T. Doxorubicin Expands In Vivo Secretion of Circulating Exosome in Mice. Biol. Pharm. Bull. 2018, 41, 1078–1083. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive Release of Extracellular Vesicles from Cancer Cells after Photodynamic Treatment or Chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New Insights into Extracellular Vesicle Biogenesis and Function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Yarana, C.; Carroll, D.; Chen, J.; Chaiswing, L.; Zhao, Y.; Noel, T.; Alstott, M.; Bae, Y.; Dressler, E.V.; Moscow, J.A.; et al. Extracellular Vesicles Released by Cardiomyocytes in a Doxorubicin-Induced Cardiac Injury Mouse Model Contain Protein Biomarkers of Early Cardiac Injury. Clin. Cancer Res. 2018, 24, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Badila, E.; Japie, C.; Vrabie, A.M.; Badila, A.; Georgescu, A. Cardiovascular Disease as a Consequence or a Cause of Cancer: Potential Role of Extracellular Vesicles. Biomolecules 2023, 13, 321. [Google Scholar] [CrossRef]
- Gray, W.D.; French, K.M.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Platt, M.O.; Searles, C.D.; Davis, M.E. Identification of Therapeutic Covariant MicroRNA Clusters in Hypoxia-Treated Cardiac Progenitor Cell Exosomes Using Systems Biology. Circ. Res. 2015, 116, 255–263. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma Exosomes Protect the Myocardium from Ischemia-Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Emanueli, C.; Shearn, A.I.U.; Angelini, G.D.; Sahoo, S. Exosomes and Exosomal miRNAs in Cardiovascular Protection and Repair. Vasc. Pharmacol. 2015, 71, 24–30. [Google Scholar] [CrossRef]
- Jadli, A.S.; Parasor, A.; Gomes, K.P.; Shandilya, R.; Patel, V.B. Exosomes in Cardiovascular Diseases: Pathological Potential of Nano-Messenger. Front. Cardiovasc. Med. 2021, 8, 767488. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming Growth Factor (TGF)-β Signaling in Cardiac Remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Andenæs, K.; Lunde, I.G.; Mohammadzadeh, N.; Dahl, C.P.; Aronsen, J.M.; Strand, M.E.; Palmero, S.; Sjaastad, I.; Christensen, G.; Engebretsen, K.V.; et al. The Extracellular Matrix Proteoglycan Fibromodulin Is Upregulated in Clinical and Experimental Heart Failure and Affects Cardiac Remodeling. PLoS ONE 2018, 13, e0201422. [Google Scholar] [CrossRef] [PubMed]
- Feola, M.; Garrone, O.; Occelli, M.; Francini, A.; Biggi, A.; Visconti, G.; Albrile, F.; Bobbio, M.; Merlano, M. Cardiotoxicity after Anthracycline Chemotherapy in Breast Carcinoma: Effects on Left Ventricular Ejection Fraction, Troponin I and Brain Natriuretic Peptide. Int. J. Cardiol. 2011, 148, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Asao, T.; Tobias, G.C.; Lucotti, S.; Jones, D.R.; Matei, I.; Lyden, D. Extracellular vesicles and particles as mediators of long-range communication in cancer: Connecting biological function to clinical applications. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 461–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Vanlandingham, P.A.; Ceresa, B.P. Rab7 Regulates Late Endocytic Trafficking Downstream of Multivesicular Body Biogenesis and Cargo Sequestration. J. Biol. Chem. 2009, 284, 12110–12124. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of microRNAs in Living Cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science. 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Jeong, H.; Koh, J.; Kim, S.; Song, S.G.; Lee, S.H.; Jeon, Y.; Lee, C.H.; Keam, B.; Lee, S.H.; Chung, D.H. Epithelial-mesenchymal transition induced by tumor cell-intrinsic PD-L1 signaling predicts a poor response to immune checkpoint inhibitors in PD-L1-high lung cancer. Br. J. Cancer 2024, 131, 23–36. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and Tumor-Mediated Immune Suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Castillo, R.L.; Gormaz, J.G.; Carrillo, M.; Thavendiranathan, P. Role of Oxidative Stress in the Mechanisms of Anthracycline-Induced Cardiotoxicity: Effects of Preventive Strategies. Oxid. Med. Cell. Longev. 2021, 2021, 8863789. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting Extracellular Vesicles Formation and Release: A Review of EV Inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.M.; Williams, J.K.; Zhang, C.; Saleh, A.H.; Liu, X.M.; Ma, L.; Schekman, R. Extracellular Vesicles and Cellular Homeostasis. Annu. Rev. Biochem. 2025, 94, 587–609. [Google Scholar] [CrossRef]
- Boudreault, J.; Wang, N.; Ghozlan, M.; Lebrun, J.-J. Transforming Growth Factor-β/Smad Signaling Inhibits Melanoma Cancer Stem Cell Self-Renewal, Tumor Formation and Metastasis. Cancers 2024, 16, 224. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-β Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 733123. [Google Scholar] [CrossRef]
- Benito-Martín, A.; Jasiulionis, M.G.; García-Silva, S. Extracellular Vesicles and Melanoma: New Perspectives on Tumor Microenvironment and Metastasis. Front. Cell Dev. Biol. 2023, 10, 1061982. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Yeung, V.; Kahale, F.; Parekh, M.; Cortinas, J.; Chen, L.; Ross, A.E.; Ciolino, J.B. Doxorubicin-Loaded Extracellular Vesicles Enhance Tumor Cell Death in Retinoblastoma. Bioengineering 2022, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, A.; Tsiapalis, D.; McNamee, N.; Talbot, B.; O’Driscoll, L. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics 2023, 15, 718. [Google Scholar] [CrossRef]
- Tian, C.; Yang, Y.; Bai, B.; Wang, S.; Liu, M.; Sun, R.C.; Yu, T.; Chu, X.M. Potential of Exosomes as Diagnostic Biomarkers and Therapeutic Carriers for Doxorubicin-Induced Cardiotoxicity. Int. J. Biol. Sci. 2021, 17, 1328–1340. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-Based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Gomez, L.A.; Alekseev, A.E.; Aleksandrova, L.A.; Brady, P.A.; Terzic, A. Use of the MTT Assay in Adult Ventricular Cardiomyocytes to Assess Viability: Effects of Adenosine and Potassium on Cellular Survival. J. Mol. Cell. Cardiol. 1997, 29, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules 2020, 25, 5585. [Google Scholar] [CrossRef]
- Elsner, C.; Ergün, S.; Wagner, N. Biogenesis and Release of Endothelial Extracellular Vesicles: Morphological Aspects. Ann. Anat. 2023, 245, 152006. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 3308. [Google Scholar] [CrossRef]
- Gomez-Grosso, L.A. Preacondicionamiento Isquémico en Cardiomiocitos Ventriculares Aislados. Identificación y Expresión de Algunos microRNAs Asociados. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 2013, 37, 433–447. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape Plugin: Pathway Insights Using Integrated Experimental and In Silico Data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8—A Global View on Proteins and Their Functional Interactions in 630 Organisms. Nucleic Acids Res. 2009, 37, D412–D416. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING Database in 2017: Quality-Controlled Protein–Protein Association Networks. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fonseca, L.F.; Novoa-Herrán, S.; Umaña-Pérez, A.; Gómez-Grosso, L.A. Sublethal Doxorubicin Promotes Extracellular Vesicle Biogenesis in A375 Melanoma Cells: Implications for Vesicle-Loaded TGF-β-Mediated Cancer Progression and Cardiovascular Pathophysiology. Int. J. Mol. Sci. 2025, 26, 8524. https://doi.org/10.3390/ijms26178524
Fernández-Fonseca LF, Novoa-Herrán S, Umaña-Pérez A, Gómez-Grosso LA. Sublethal Doxorubicin Promotes Extracellular Vesicle Biogenesis in A375 Melanoma Cells: Implications for Vesicle-Loaded TGF-β-Mediated Cancer Progression and Cardiovascular Pathophysiology. International Journal of Molecular Sciences. 2025; 26(17):8524. https://doi.org/10.3390/ijms26178524
Chicago/Turabian StyleFernández-Fonseca, Laura Fernanda, Susana Novoa-Herrán, Adriana Umaña-Pérez, and Luis Alberto Gómez-Grosso. 2025. "Sublethal Doxorubicin Promotes Extracellular Vesicle Biogenesis in A375 Melanoma Cells: Implications for Vesicle-Loaded TGF-β-Mediated Cancer Progression and Cardiovascular Pathophysiology" International Journal of Molecular Sciences 26, no. 17: 8524. https://doi.org/10.3390/ijms26178524
APA StyleFernández-Fonseca, L. F., Novoa-Herrán, S., Umaña-Pérez, A., & Gómez-Grosso, L. A. (2025). Sublethal Doxorubicin Promotes Extracellular Vesicle Biogenesis in A375 Melanoma Cells: Implications for Vesicle-Loaded TGF-β-Mediated Cancer Progression and Cardiovascular Pathophysiology. International Journal of Molecular Sciences, 26(17), 8524. https://doi.org/10.3390/ijms26178524







