Cladribine Preserves Normal Central Nervous System Cellular Activity and Promotes Neuroprotection to Oxidative Stress Damage
Abstract
1. Introduction
2. Results
2.1. Effect of Cladribine on Human Microglial Cells and Primary Astrocytes
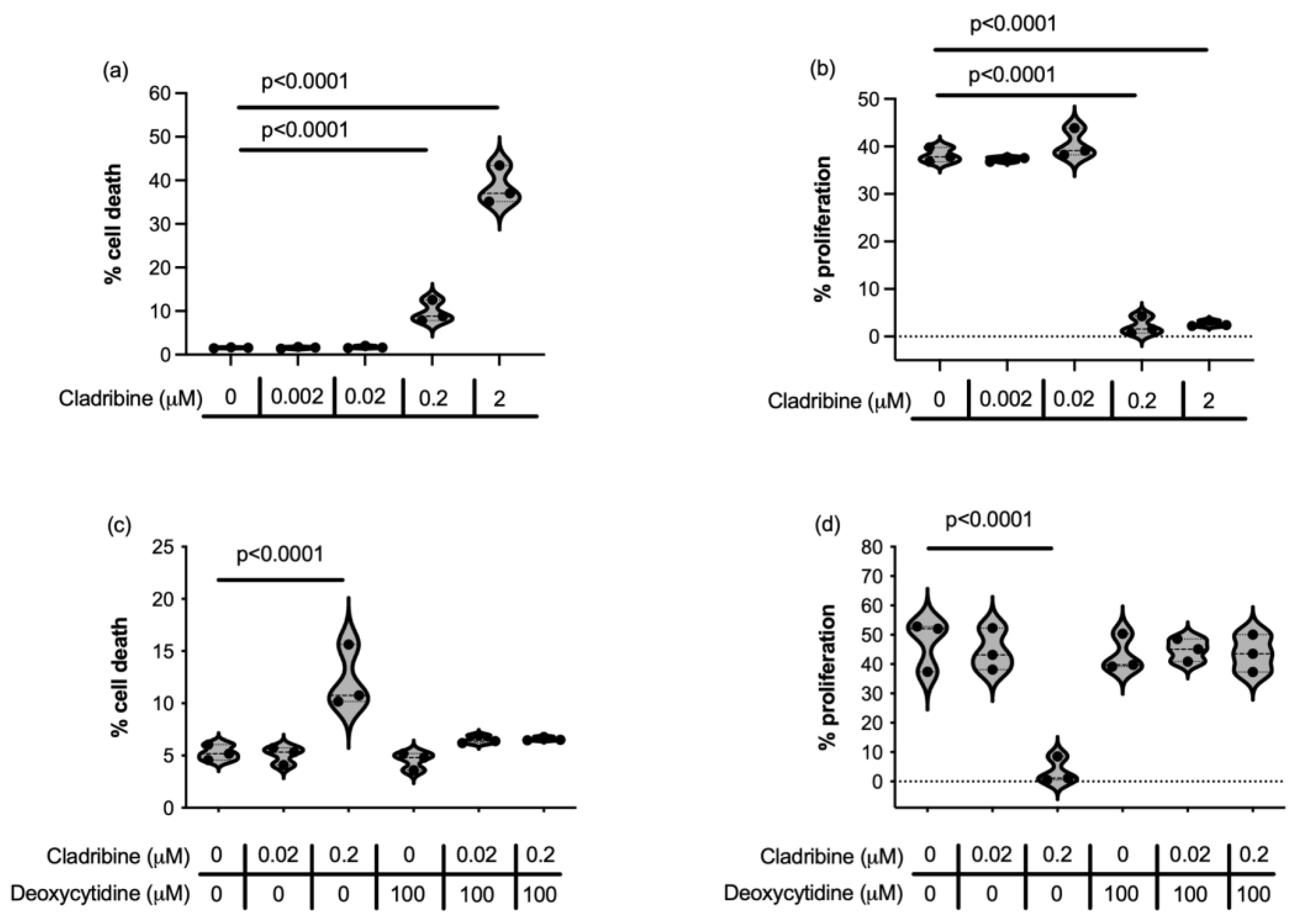
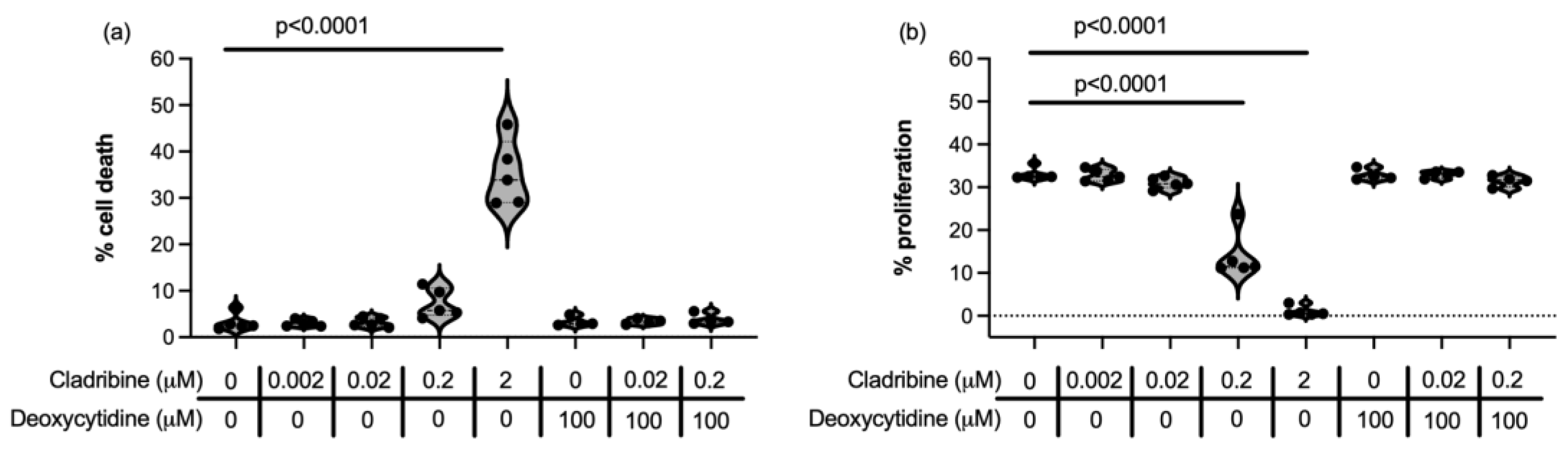
2.1.1. Steady-State Cladribine Concentration Does Not Affect Cell Survival or Proliferation of HMC3 Human Microglia Cell Line and Human Primary Astrocytes
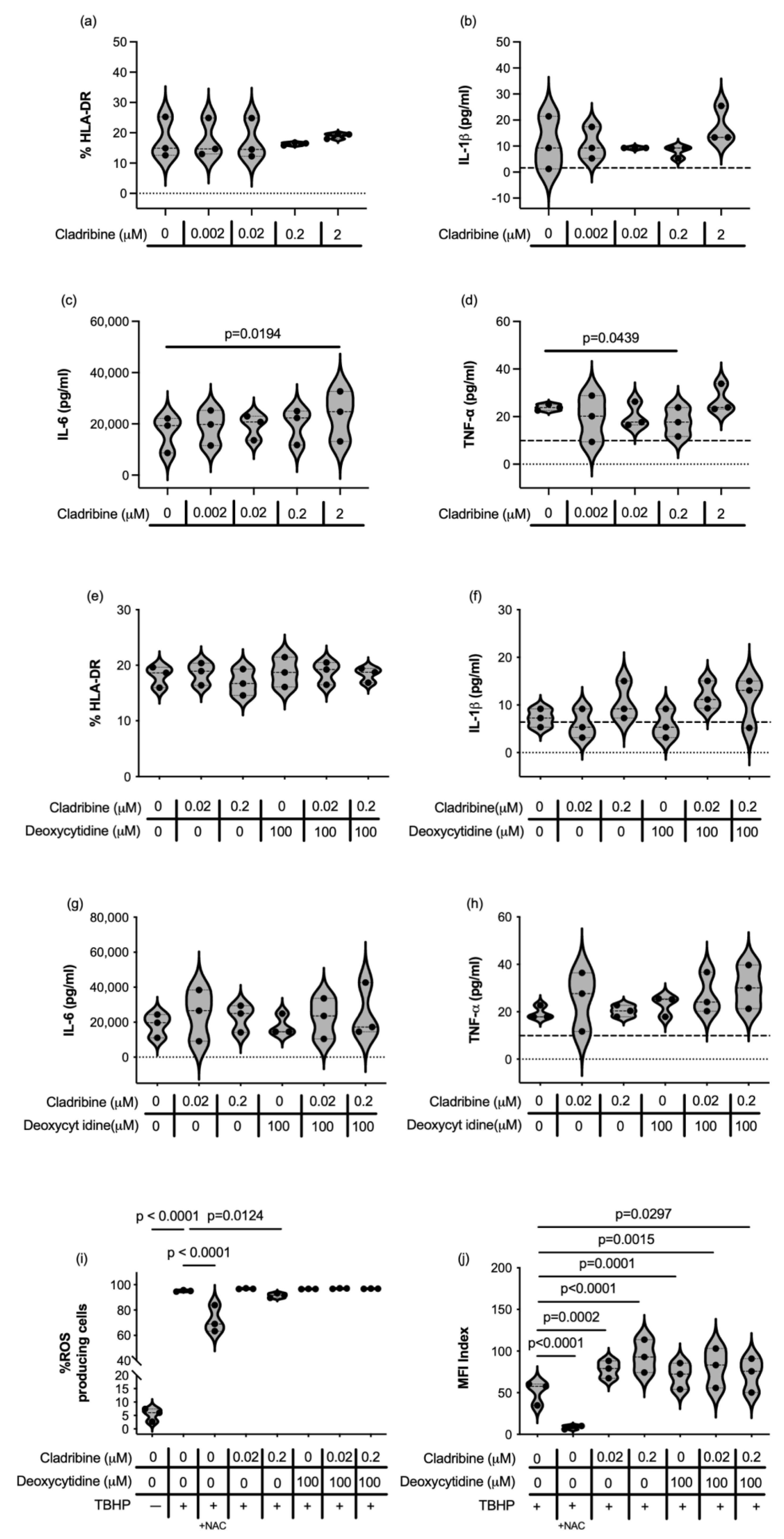
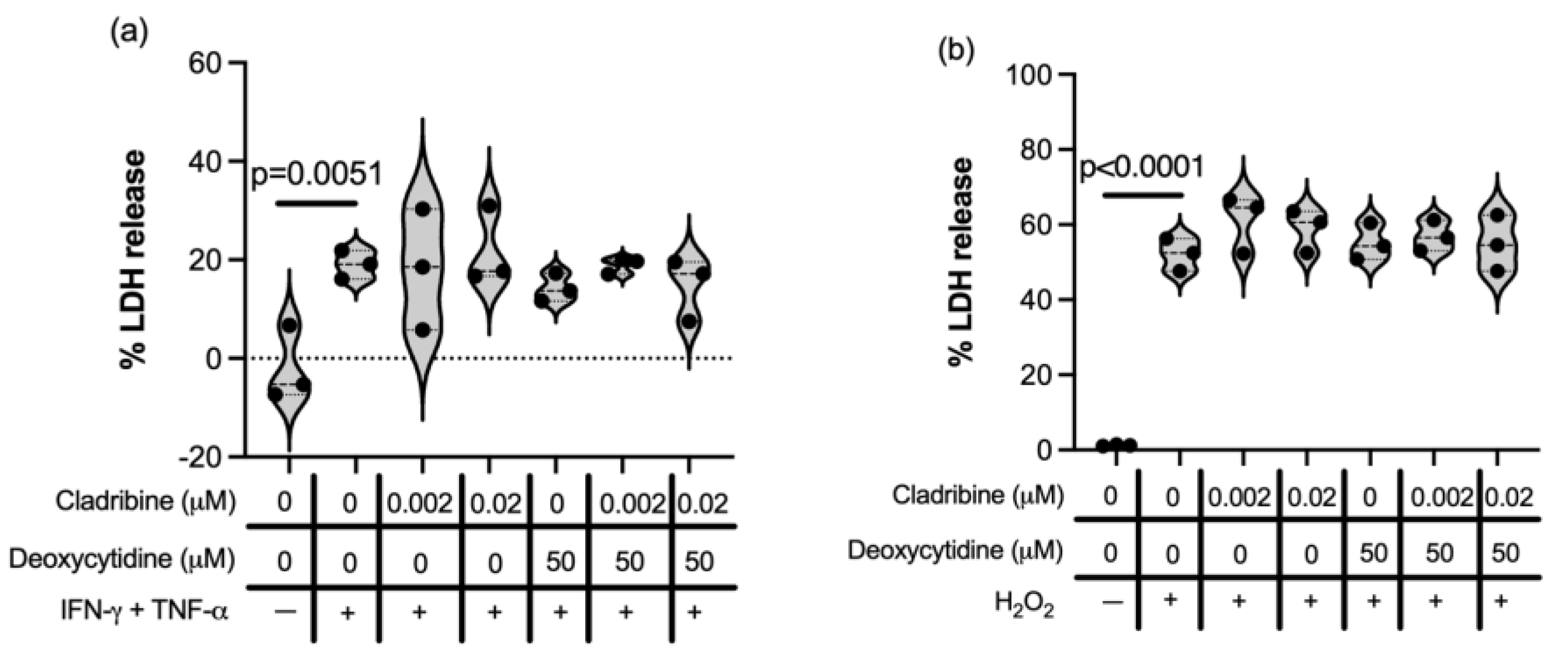
2.1.2. Immune Function of HMC3 Human Microglia Cell Line and Human Primary Astrocytes Exposed to Cladribine
2.2. Effect of Cladribine on Neural and Oligodendroglial Cells
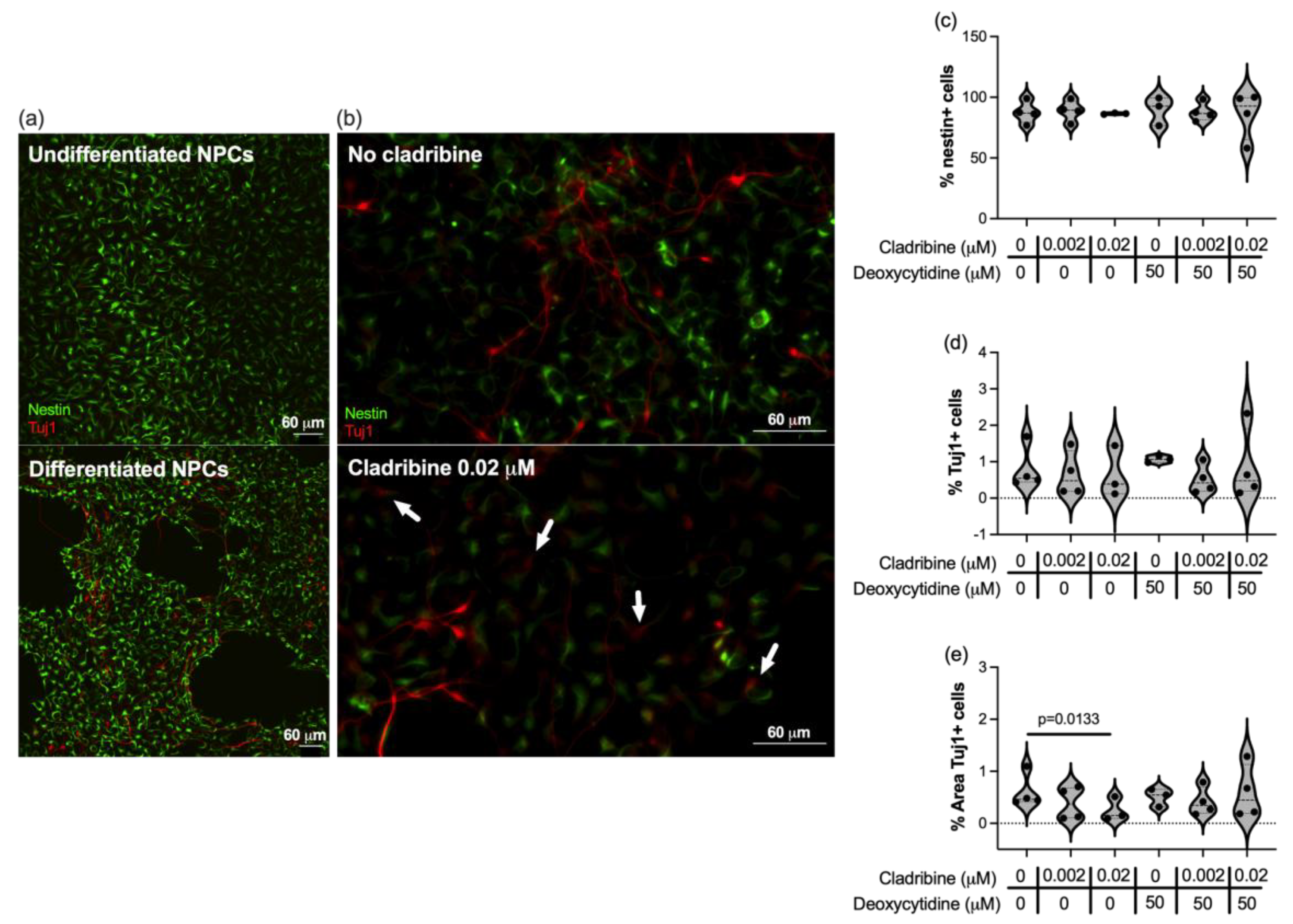
2.2.1. Cladribine Does Not Impair the Differentiation of Neuronal Progenitors into Neurons
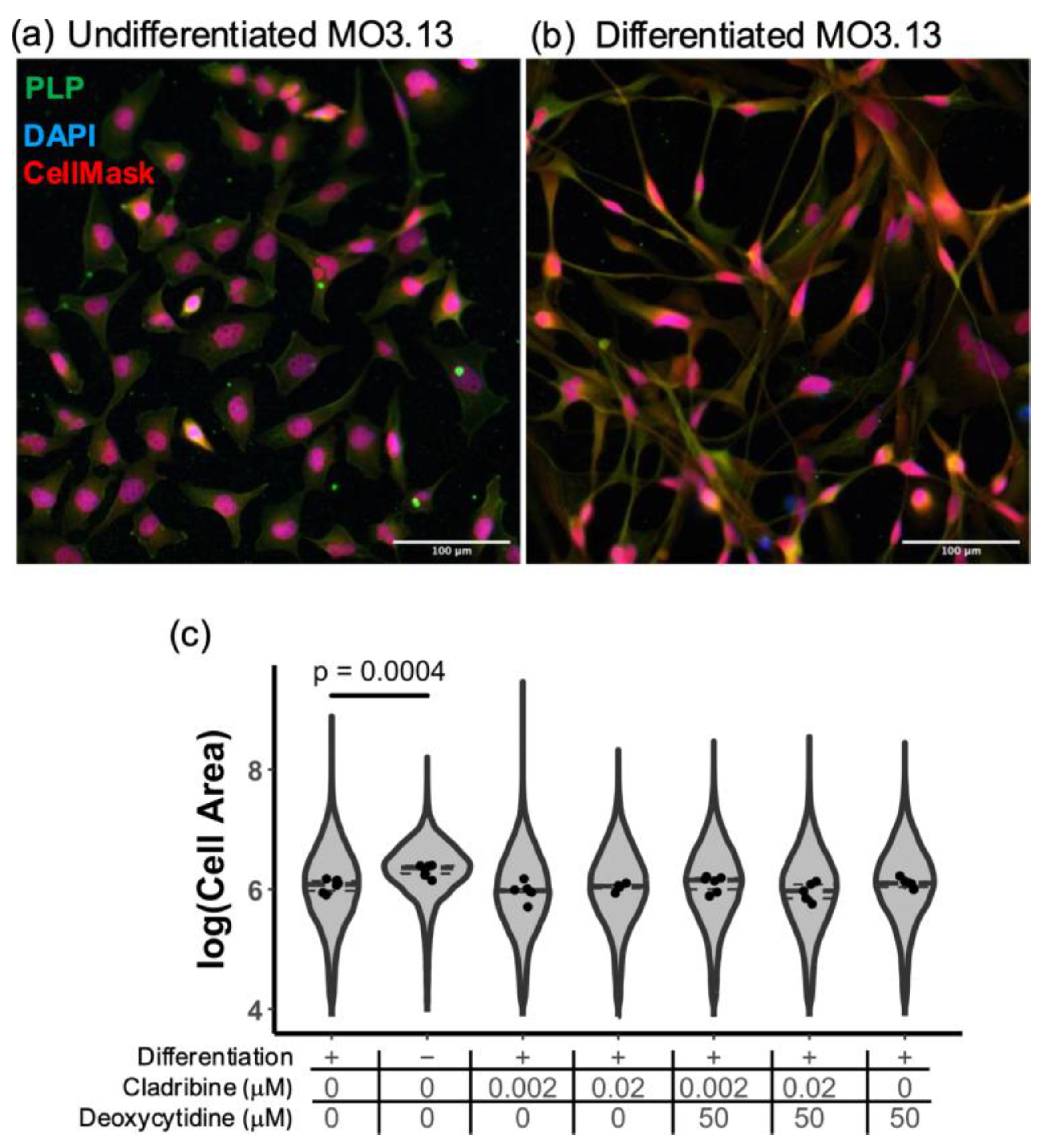
2.2.2. Cladribine Does Not Affect the Differentiation of MO3.13 Human Oligodendroglia Cell Line
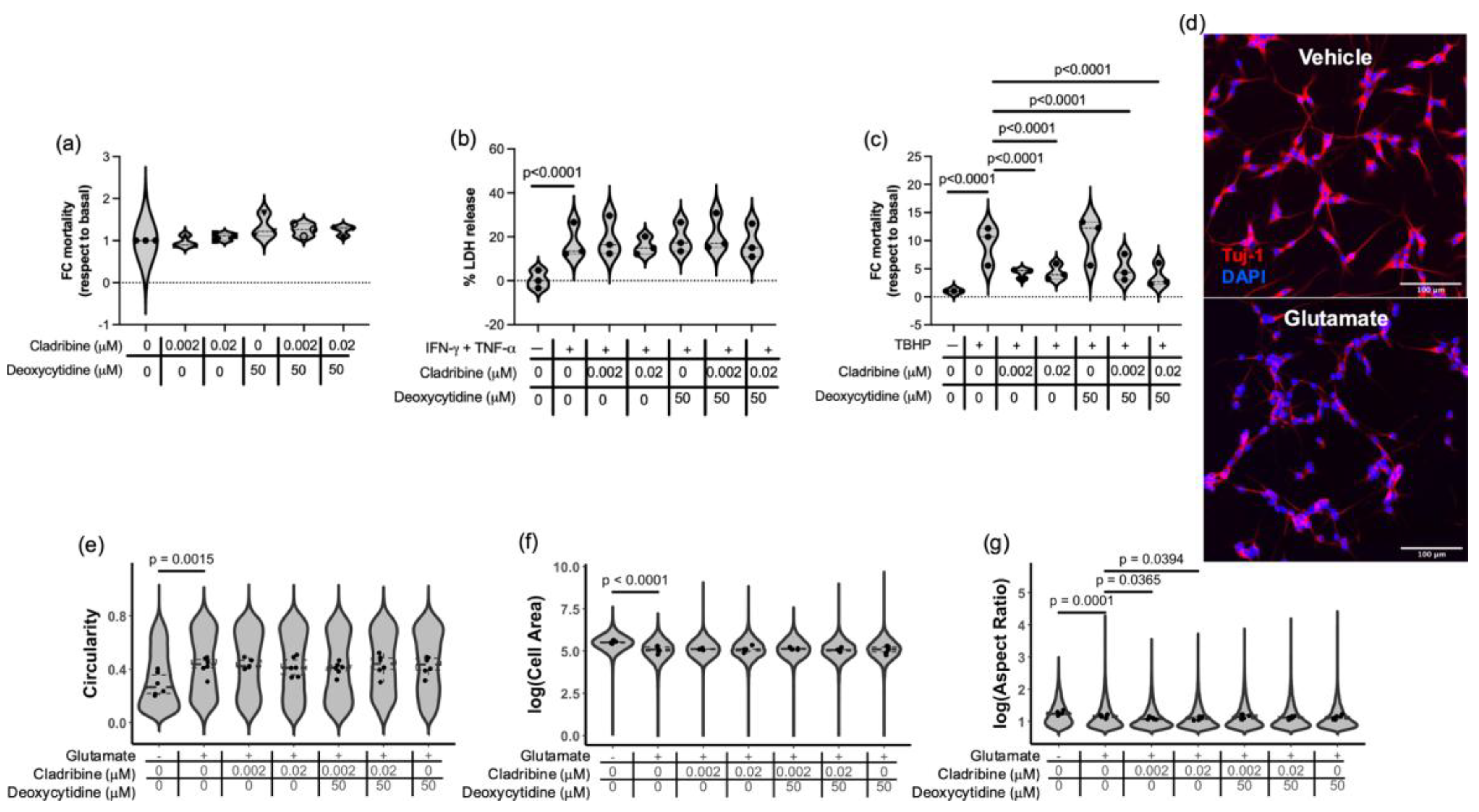
2.2.3. Effect of Cladribine at Estimated Brain Concentrations in Neural- and Oligodendroglial-like Cells Exposed to Inflammatory, Oxidative and Excitotoxic Stress
3. Discussion
4. Materials and Methods
4.1. Human Primary Cells and Cell Lines, Media and Differentiation Protocols
4.2. Cladribine and Deoxycytidine
4.3. Cytokine Stimulation
4.4. Determination of Cell Death
4.5. Proliferation Assay
4.6. Cytokine Release Quantification
4.7. Determination of Oxidative Stress
4.8. Cytotoxicity Detection by LDH Release
4.9. Excitotoxic Insult in Differentiated SH-SY5Y Cells
4.10. Immunocytochemistry
4.11. Image Acquisition and Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Franz, J.; Nessler, S. Recent developments in multiple sclerosis neuropathology. Curr. Opin. Neurol. 2025, 38, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, T.; Thompson, A.J. Atlas of MS 2020: Informing global policy change. Mult. Scler. 2020, 26, 1807–1808. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2; Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Baxter, A.G. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 2007, 7, 904–912. [Google Scholar] [CrossRef]
- Bielekova, B.; Sung, M.H.; Kadom, N.; Simon, R.; McFarland, H.; Martin, R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 2004, 172, 3893–3904. [Google Scholar] [CrossRef]
- Wang, J.; Jelcic, I.; Muhlenbruch, L.; Haunerdinger, V.; Toussaint, N.C.; Zhao, Y.; Cruciani, C.; Faigle, W.; Naghavian, R.; Foege, M.; et al. HLA-DR15 Molecules Jointly Shape an Autoreactive T Cell Repertoire in Multiple Sclerosis. Cell 2020, 183, 1264–1281.e20. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Semin. Neurol. 2016, 36, 115–127. [Google Scholar] [CrossRef]
- Heming, M.; Wiendl, H. Learning multiple sclerosis immunopathogenesis from anti-CD20 therapy. Proc. Natl. Acad. Sci. USA 2023, 120, e2221544120. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.; Alvarez, E. Advances in Multiple Sclerosis Neurotherapeutics, Neuroprotection, and Risk Mitigation Strategies. Neurol. Clin. 2024, 42, 115–135. [Google Scholar] [CrossRef]
- Brown, J.W.L.; Coles, A.; Horakova, D.; Havrdova, E.; Izquierdo, G.; Prat, A.; Girard, M.; Duquette, P.; Trojano, M.; Lugaresi, A.; et al. Association of Initial Disease-Modifying Therapy with Later Conversion to Secondary Progressive Multiple Sclerosis. JAMA 2019, 321, 175–187, Erratum in JAMA 2020, 323, 1318. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Soelberg Sorensen, P.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sorensen, P.S.; Vermersch, P.; Hamlett, A.; Viglietta, V.; Greenberg, S.; CLARITY Study Group. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: A post-hoc and subgroup analysis. Lancet Neurol. 2011, 10, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.W.; Rieckmann, P.; Comi, G.; Dangond, F.; Hicking, C.; Vermersch, P. Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study. Mult. Scler. 2019, 25, 819–827. [Google Scholar] [CrossRef]
- Leist, T.P.; Comi, G.; Cree, B.A.; Coyle, P.K.; Freedman, M.S.; Hartung, H.P.; Vermersch, P.; Casset-Semanaz, F.; Scaramozza, M.; Oral Cladribine for Early MS (ORACLE MS) Study Group. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): A phase 3 randomised trial. Lancet Neurol. 2014, 13, 257–267. [Google Scholar] [CrossRef]
- Beutler, E. Cladribine (2-chlorodeoxyadenosine). Lancet 1992, 340, 952–956. [Google Scholar] [CrossRef]
- Hermann, R.; Karlsson, M.O.; Novakovic, A.M.; Terranova, N.; Fluck, M.; Munafo, A. The Clinical Pharmacology of Cladribine Tablets for the Treatment of Relapsing Multiple Sclerosis. Clin. Pharmacokinet. 2019, 58, 283–297, Erratum in Clin. Pharmacokinet. 2019, 58, 401. [Google Scholar] [CrossRef]
- Wiendl, H.; Barkhof, F.; Montalban, X.; Achiron, A.; Derfuss, T.; Chan, A.; Hodgkinson, S.; Prat, A.; Leocani, L.; Schmierer, K.; et al. Blood biomarker dynamics in people with relapsing multiple sclerosis treated with cladribine tablets: Results of the 2-year MAGNIFY-MS study. Front. Immunol. 2025, 16, 1512189, Erratum in Front. Immunol. 2025, 16, 1571978. [Google Scholar] [CrossRef]
- Schmierer, K.; Wiendl, H.; Barkhof, F.; Montalban, X.; Achiron, A.; Derfuss, T.; Chan, A.; Hodgkinson, S.; Prat, A.; Leocani, L.; et al. Clinical and mechanistic effects of cladribine in relapsing multiple sclerosis: 2-year results from the MAGNIFY-MS Study. Ther. Adv. Neurol. Disord. 2025, 18, 17562864251351760. [Google Scholar] [CrossRef]
- Jensen, K.; Johnson, L.A.; Jacobson, P.A.; Kachler, S.; Kirstein, M.N.; Lamba, J.; Klotz, K.N. Cytotoxic purine nucleoside analogues bind to A1, A2A, and A3 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 519–525. [Google Scholar] [CrossRef]
- Canals, M.; Angulo, E.; Casado, V.; Canela, E.I.; Mallol, J.; Vinals, F.; Staines, W.; Tinner, B.; Hillion, J.; Agnati, L.; et al. Molecular mechanisms involved in the adenosine A and A receptor-induced neuronal differentiation in neuroblastoma cells and striatal primary cultures. J. Neurochem. 2005, 92, 337–348. [Google Scholar] [CrossRef]
- Zarrinmayeh, H.; Territo, P.R. Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and Their Applications. Mol. Imaging 2020, 19, 1536012120927609. [Google Scholar] [CrossRef]
- Riff, R.; Naamani, O.; Mazar, J.; Haviv, Y.S.; Chaimovitz, C.; Douvdevani, A. A(1) and A(2A) adenosine receptors play a protective role to reduce prevalence of autoimmunity following tissue damage. Clin. Exp. Immunol. 2021, 205, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Schnermann, J.; Noorbakhsh, F.; Henry, S.; Yong, V.W.; Winston, B.W.; Warren, K.; Power, C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004, 24, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Liliemark, J. The clinical pharmacokinetics of cladribine. Clin. Pharmacokinet. 1997, 32, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Janabi, N.; Peudenier, S.; Heron, B.; Ng, K.H.; Tardieu, M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci. Lett. 1995, 195, 105–108. [Google Scholar] [CrossRef]
- Etemad, S.; Zamin, R.M.; Ruitenberg, M.J.; Filgueira, L. A novel in vitro human microglia model: Characterization of human monocyte-derived microglia. J. Neurosci. Methods 2012, 209, 79–89. [Google Scholar] [CrossRef]
- Boscia, F.; D’Avanzo, C.; Pannaccione, A.; Secondo, A.; Casamassa, A.; Formisano, L.; Guida, N.; Sokolow, S.; Herchuelz, A.; Annunziato, L. Silencing or knocking out the Na+/Ca2+ exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012, 19, 562–572, Erratum in Cell Death Differ. 2013, 20, 184. [Google Scholar] [CrossRef]
- Jorgensen, L.O.; Hyrlov, K.H.; Elkjaer, M.L.; Weber, A.B.; Pedersen, A.E.; Svenningsen, A.F.; Illes, Z. Cladribine modifies functional properties of microglia. Clin. Exp. Immunol. 2020, 201, 328–340. [Google Scholar] [CrossRef]
- Singh, V.; Voss, E.V.; Benardais, K.; Stangel, M. Effects of 2-chlorodeoxyadenosine (Cladribine) on primary rat microglia. J. Neuroimmune Pharmacol. 2012, 7, 939–950. [Google Scholar] [CrossRef]
- Kraus, S.H.; Luessi, F.; Trinschek, B.; Lerch, S.; Hubo, M.; Poisa-Beiro, L.; Paterka, M.; Jonuleit, H.; Zipp, F.; Jolivel, V. Cladribine exerts an immunomodulatory effect on human and murine dendritic cells. Int. Immunopharmacol. 2014, 18, 347–357. [Google Scholar] [CrossRef]
- Rojo, A.I.; McBean, G.; Cindric, M.; Egea, J.; Lopez, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Sign. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Gutierrez, C.; Bonora, N.; Bobo-Jimenez, V.; Jimenez-Blasco, D.; Lopez-Fabuel, I.; Fernandez, E.; Josephine, C.; Bonvento, G.; Enriquez, J.A.; Almeida, A.; et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metab. 2019, 1, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, M.; Gargano, C.D.; Ferretta, A.; Manni, A.; Capacchione, A.; Frigeri, A.; Iaffaldano, P.; Trojano, M.; Paolicelli, D. Effect of Cladribine on Neuronal Apoptosis: New Insight of In Vitro Study in Multiple Sclerosis Therapy. Brain Sci. 2020, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, D.; Ruggieri, M.; Manni, A.; Gargano, C.D.; Carleo, G.; Palazzo, C.; Iaffaldano, A.; Bollo, L.; Guerra, T.; Saracino, A.; et al. Real-Life Experience of the Effects of Cladribine Tablets on Lymphocyte Subsets and Serum Neurofilament Light Chain Levels in Relapsing Multiple Sclerosis Patients. Brain Sci. 2022, 12, 1595. [Google Scholar] [CrossRef]
- Schroeter, C.B.; Rolfes, L.; Gothan, K.S.S.; Gruchot, J.; Herrmann, A.M.; Bock, S.; Fazio, L.; Henes, A.; Narayanan, V.; Pfeuffer, S.; et al. Cladribine treatment improves cortical network functionality in a mouse model of autoimmune encephalomyelitis. J. Neuroinflamm. 2022, 19, 270. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Manna, P.; Sil, P.C. Protective role of a coumarin-derived schiff base scaffold against tertiary butyl hydroperoxide (TBHP)-induced oxidative impairment and cell death via MAPKs, NF-kappaB and mitochondria-dependent pathways. Free Radic. Res. 2011, 45, 620–637. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, H.; Gong, W.; Cao, F.; Wu, T.; Hu, F. Plumbagin protects H9c2 cardiomyocytes against TBHP-induced cytotoxicity by alleviating ROS-induced apoptosis and modulating autophagy. Exp. Ther. Med. 2022, 24, 501. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology 2011, 18, 221–234. [Google Scholar] [CrossRef]
- Jamroz-Wisniewska, A.; Beltowski, J.; Wojcicka, G.; Bartosik-Psujek, H.; Rejdak, K. Cladribine Treatment Improved Homocysteine Metabolism and Increased Total Serum Antioxidant Activity in Secondary Progressive Multiple Sclerosis Patients. Oxid. Med. Cell Longev. 2020, 2020, 1654754. [Google Scholar] [CrossRef]
- Stone, T.W.; Ceruti, S.; Abbracchio, M.P. Adenosine receptors and neurological disease: Neuroprotection and neurodegeneration. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 535–587. [Google Scholar]
- Petcharat, K.; Singh, M.; Ingkaninan, K.; Attarat, J.; Yasothornsrikul, S. Bacopa monnieri protects SH-SY5Y cells against tert-Butyl hydroperoxide-induced cell death via the ERK and PI3K pathways. Siriraj Med. J. 2015, 67, 20–26. [Google Scholar]
- Laugel, B.; Borlat, F.; Galibert, L.; Vicari, A.; Weissert, R.; Chvatchko, Y.; Bruniquel, D. Cladribine inhibits cytokine secretion by T cells independently of deoxycytidine kinase activity. J. Neuroimmunol. 2011, 240–241, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eixarch, H.; Calvo-Barreiro, L.; Fissolo, N.; Boschert, U.; Hervera, A.; Comabella, M.; Montalban, X.; Espejo, C. Cladribine Preserves Normal Central Nervous System Cellular Activity and Promotes Neuroprotection to Oxidative Stress Damage. Int. J. Mol. Sci. 2025, 26, 11311. https://doi.org/10.3390/ijms262311311
Eixarch H, Calvo-Barreiro L, Fissolo N, Boschert U, Hervera A, Comabella M, Montalban X, Espejo C. Cladribine Preserves Normal Central Nervous System Cellular Activity and Promotes Neuroprotection to Oxidative Stress Damage. International Journal of Molecular Sciences. 2025; 26(23):11311. https://doi.org/10.3390/ijms262311311
Chicago/Turabian StyleEixarch, Herena, Laura Calvo-Barreiro, Nicolás Fissolo, Ursula Boschert, Arnau Hervera, Manuel Comabella, Xavier Montalban, and Carmen Espejo. 2025. "Cladribine Preserves Normal Central Nervous System Cellular Activity and Promotes Neuroprotection to Oxidative Stress Damage" International Journal of Molecular Sciences 26, no. 23: 11311. https://doi.org/10.3390/ijms262311311
APA StyleEixarch, H., Calvo-Barreiro, L., Fissolo, N., Boschert, U., Hervera, A., Comabella, M., Montalban, X., & Espejo, C. (2025). Cladribine Preserves Normal Central Nervous System Cellular Activity and Promotes Neuroprotection to Oxidative Stress Damage. International Journal of Molecular Sciences, 26(23), 11311. https://doi.org/10.3390/ijms262311311







