Emerging Roles of Bile Acids in Neuroinflammation
Abstract
1. Introduction
2. Bile Acids: Multifunctional Molecules in Digestion and Metabolic Regulation
3. Bile Acids as Neuroimmune Signaling Molecules
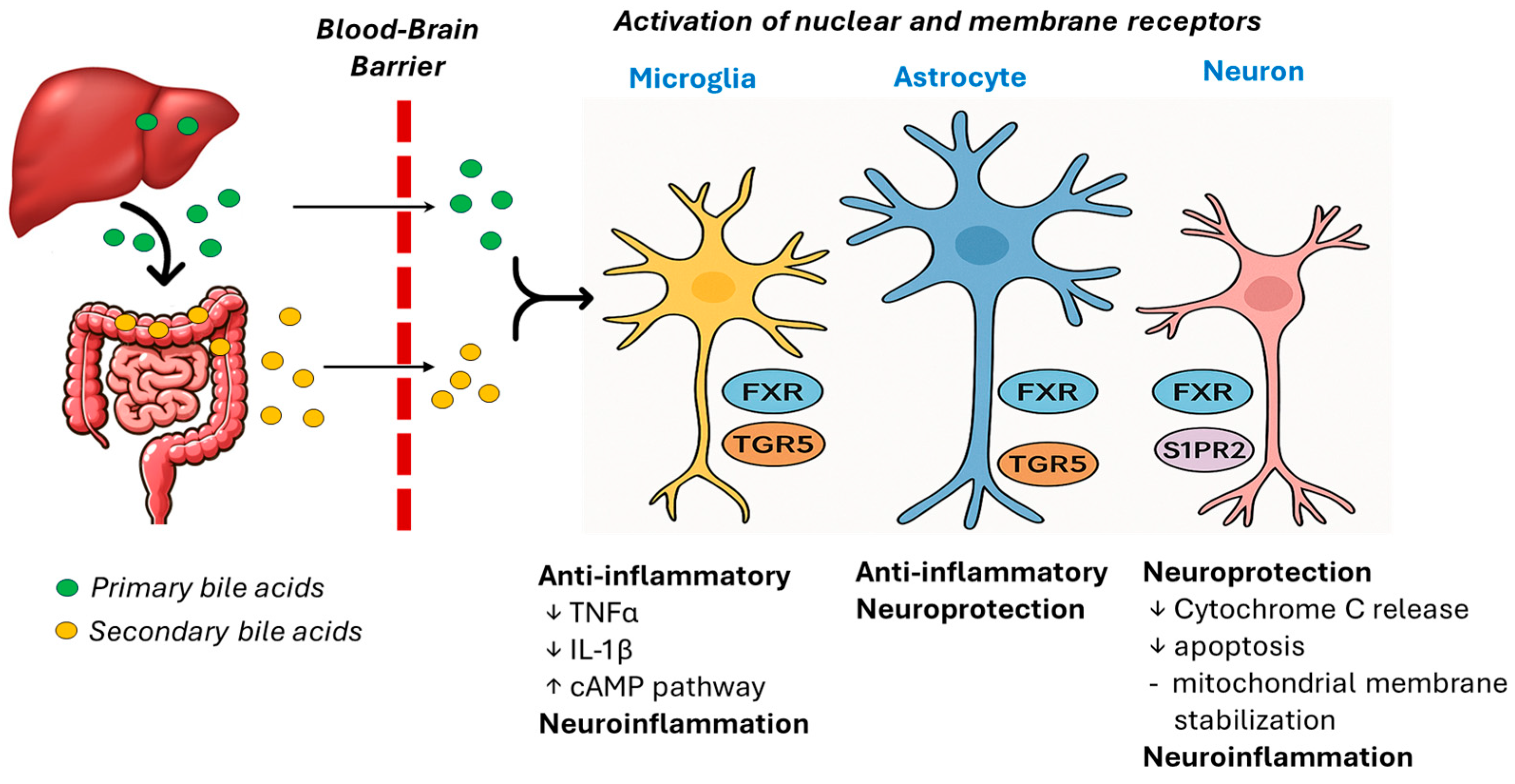
4. Bile Acids and the Blood-Brain-Barrier
5. Mechanistic Links Between Bile Acids and Neuroinflammation
6. Gut–Microbiome–Bile Acid–Brain Axis and Neuroinflammation
7. Bile Acids in Neurological and Neuroimmune Disorders
7.1. Alzheimer’s and Parkinson’s Disease
7.2. Multiple Sclerosis
7.3. Hepatic Encephalopathy
7.4. Aging and Cognitive Decline
7.5. Amyotrophic Lateral Sclerosis
7.6. Huntington’s Disease
8. Therapeutic and Translational Potential
9. Knowledge Gaps and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| A2M | Alpha-2 macroglobulin |
| AHSG | Alpha-2 HS glycoprotein |
| ALB | Albumin |
| ALS | Amyotrophic lateral sclerosis |
| APOA1 | Apolipoprotein A1 |
| APOH | Apolipoprotein H |
| AKT/NFκB | Ak strain transforming/nuclear factor kappa B (signaling pathway) |
| BBB | Blood–brain barrier |
| BSH | Bile salt hydrolases |
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| CDCA | Chenodeoxycholic acid |
| CA | Cholic acid |
| cAMP | Cyclic adenosine monophosphate |
| DCA | Deoxycholic acid |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| EAE | Experimental autoimmune encephalomyelitis |
| FXR | Farnesoid x receptor |
| GPBAR1 | G-protein coupled bile acid receptor 1 (GPBAR1 aka TGR5) |
| GPCR | G-protein coupled receptor |
| GCA | Glycocholic acid |
| GDCA | Glycodeoxycholic acid |
| GUDCA | Glucoursodeoxycholic acid |
| GCDCA | Glycochenodeoxycholic acid |
| HD | Huntington’s disease |
| HE | Hepatic encephalopathy |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| LCA | Lithocholic acid |
| MS | Multiple sclerosis |
| MG6 | Microglial cells |
| OATP | Organic anion transport polypeptide |
| OCA | Obeticholic acid |
| PBC | Primary biliary cirrhosis |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acid |
| SPP2 | Secreted phosphoprotein 2 |
| S1PR2 | Sphingosine-1-phosphate receptor 2 |
| TCA | Taurocholic acid |
| TDCA | Taurodeoxycholic acid |
| TUDCA | Tauroursodeoxycholic acid |
| TβMCA | Tauro-beta-muricholic acid |
| TGR5 | Takeda G-protein coupled receptor 5 |
| TNFα | Tumor necrosis factor alpha |
| UDCA | Ursodeoxycholic acid |
| 6-ECDCA | 6alpha-ethyl-chenodeoxycholic acid |
References
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, Y.; Cheung, K.C.P.; Zheng, X. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis. Sci. China Life Sci. 2024, 67, 865–878. [Google Scholar] [CrossRef]
- Liu, X.; Fang, W.; Pang, S.; Song, G.; Wang, Y.; Qi, W. Total dietary fiber of tartary buckwheat alleviates T2DM through the IRS-1/PI3K/AKT pathway and gut microbiota-bile acids-TGR5/FXR axis in db/db mice. Int. J. Biol. Macromol. 2025, 308, 142145. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Xie, Y.; Yi, L.; Cheng, W.; Jia, H.; Shi, W.; Liu, Q.; Fang, L.; Xue, S.; Liu, D.; et al. Bile acids affect intestinal barrier function through FXR and TGR5. Front. Med. 2025, 12, 1607899. [Google Scholar] [CrossRef]
- Lin, X.; Xia, L.; Zhou, Y.; Xie, J.; Tuo, Q.; Lin, L.; Liao, D. Crosstalk Between Bile Acids and Intestinal Epithelium: Multidimensional Roles of Farnesoid X Receptor and Takeda G Protein Receptor 5. Int. J. Mol. Sci. 2025, 26, 4240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; He, Z.; Xia, Z.; Liu, H.; Wu, Y.; Chen, S.; Wu, B.; Li, H. Salidroside attenuates NASH through regulating bile acid-FXR/TGR5 signaling pathway via targeting gut microbiota. Int. J. Biol. Macromol. 2025, 307, 142276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mu, X.; Hu, H.; Zhao, S.; Hu, N.; Yang, M.; Jiang, J. DHLCA Alleviates Diabetic Kidney Disease via TGR5/FXR Activation and Gut Microbiota Remodeling. Drug Des. Dev. Ther. 2025, 19, 6469–6485. [Google Scholar] [CrossRef]
- Ay, Ü.; Leníček, M.; Haider, R.S.; Classen, A.; van Eijk, H.; Koelfat, K.V.K.; van der Kroft, G.; Neumann, U.P.; Hoffmann, C.; Bolm, C.; et al. Microbially conjugated bile salts found in human bile activate the bile salt receptors TGR5 and FXR. Hepatol. Commun. 2024, 8, e0383. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Zhao, Y.; Zhou, Q.; Yang, X.; Gao, Y.; Li, Q.; Bai, M.; Liu, J.; Liang, Y.; et al. Study on the mechanism of modified Gegen Qinlian decoction in regulating the intestinal flora-bile acid-TGR5 axis for the treatment of type 2 diabetes mellitus based on macro genome sequencing and targeted metabonomics integration. Phytomedicine 2024, 132, 155329. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Jagadeesan, A.; Paulraj, R.S.; Sundaram, U.; Arthur, S. Novel Expression of Apical Bile Acid Transport (ASBT) More Proximally than Distal Ileum Contributing to Enhanced Intestinal Bile Acid Absorption in Obesity. Int. J. Mol. Sci. 2024, 25, 11452. [Google Scholar] [CrossRef]
- Wang, J.; Zang, J.; Yu, Y.; Liu, Y.; Cao, H.; Guo, R.; Zhang, L.; Liu, M.; Zhang, Z.; Li, X.; et al. Lingguizhugan oral solution alleviates MASLD by regulating bile acids metabolism and the gut microbiota through activating FXR/TGR5 signaling pathways. Front. Pharmacol. 2024, 15, 1426049. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, L.; Zou, T.; Lian, S.; Luo, J.; Lu, Y.; Hao, H.; Xu, Y.; Xiang, Y.; Zhang, X.; et al. Ileitis promotes MASLD progression via bile acid modulation and enhanced TGR5 signaling in ileal CD8(+) T cells. J. Hepatol. 2024, 80, 764–777. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Zhang, B.; Xie, S.; Wang, M. Bile Acid Injection Regulated Blood Glucose in T2DM Rats via the TGR5/GLP-1 Rather than FXR/FGF15 Pathway. Altern. Ther. Health Med. 2024, 30, 480–485. [Google Scholar] [PubMed]
- Ackerman, H.D.; Gerhard, G.S. Bile Acids in Neurodegenerative Disorders. Front. Aging Neurosci. 2016, 8, 263. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Watanabe, S.; Tomaru, K.; Yamazaki, W.; Yoshizawa, K.; Ogawa, S.; Nagao, H.; Minato, K.; Maekawa, M.; Mano, N. Unconjugated bile acids in rat brain: Analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids 2017, 125, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.M.; Chiang, J.Y.L. Bile acid receptors and signaling crosstalk in the liver, gut and brain. Liver Res. 2021, 5, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shao, Q.; Chen, J.; Lv, X.; Ji, J.; Liu, Y.; Song, Y. Bile acid signalling and its role in anxiety disorders. Front. Endocrinol. 2023, 14, 1268865. [Google Scholar] [CrossRef]
- Xing, C.; Huang, X.; Wang, D.; Yu, D.; Hou, S.; Cui, H.; Song, L. Roles of bile acids signaling in neuromodulation under physiological and pathological conditions. Cell Biosci. 2023, 13, 106, Correction in Cell Biosci. 2023, 13, 125. [Google Scholar] [CrossRef]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—An emerging role for gut microbiome. Alzheimers Dement. 2019, 15, 76–92, Erratum in Alzheimers Dement. 2019, 15, 604. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, M.; Li, M.; Guo, Q.; Ren, Z.; Zheng, X.; Chen, T. The clinical and mechanistic roles of bile acids in depression, Alzheimer’s disease, and stroke. Proteomics 2022, 22, e2100324. [Google Scholar] [CrossRef]
- Khalaf, K.; Tornese, P.; Cocco, A.; Albanese, A. Tauroursodeoxycholic acid: A potential therapeutic tool in neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 33. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.; Xie, G.; Kristal, B.S.; Zheng, X.; Sun, T.; Arnold, M.; Louie, G.; Li, M.; Wu, L.; et al. Serum Bile Acids Improve Prediction of Alzheimer’s Progression in a Sex-Dependent Manner. Adv. Sci. 2024, 11, e2306576. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Bai, X.; Zhao, Y.; Du, Z.; Liu, F.; Wang, Y.-D.; Chen, W.-D. Farnesoid X receptor activation alleviates hepatic encephalopathy by improving hepatic ammonia metabolism in murine models. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167750. [Google Scholar] [CrossRef]
- Ren, C.; Cha, L.; Huang, S.Y.; Bai, G.H.; Li, J.H.; Xiong, X.; Feng, Y.X.; Feng, D.P.; Gao, L.; Li, J.Y. Dysregulation of bile acid signal transduction causes neurological dysfunction in cirrhosis rats. World J. Hepatol. 2025, 17, 101340. [Google Scholar] [CrossRef]
- Payne, T.; Appleby, M.; Buckley, E.; van Gelder, L.M.A.; Mullish, B.H.; Sassani, M.; Dunning, M.J.; Hernandez, D.; Scholz, S.W.; McNeill, A.; et al. A Double-Blind, Randomized, Placebo-Controlled Trial of Ursodeoxycholic Acid (UDCA) in Parkinson’s Disease. Mov. Disord. 2023, 38, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lai, Y.; Mo, C.; Zhang, Y.; Ai, P.; Xu, S.; Qian, Y.; Xiao, Q.; Yang, X. Association between Fecal Bile Acids and Levodopa Response in Patients with Parkinson’s Disease. Microorganisms 2024, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Fleishman, J.S.; Kumar, S. Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Stellaard, F.; Lütjohann, D. Dynamics of the enterohepatic circulation of bile acids in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G55–G66. [Google Scholar] [CrossRef]
- van de Peppel, I.P.; Verkade, H.J.; Jonker, J.W. Metabolic consequences of ileal interruption of the enterohepatic circulation of bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G619–G625. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Carino, A.; Zampella, A.; Biagioli, M. Bile acids and their receptors in metabolic disorders. Prog. Lipid Res. 2021, 82, 101094. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Xia, Y.; Jia, X.; Chen, Y.; Liu, Y.; Zhang, L.; Chai, H.; Sun, L. Review on chronic metabolic diseases surrounding bile acids and gut microbiota: What we have explored so far. Life Sci. 2024, 336, 122304, Correction in Life Sci. 2024, 338, 122384. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Apte, U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv. Pharmacol. 2015, 74, 263–302. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Hillotte, G.L.; Ahumada, N.; Jaureguizahar, F.; Medeot, A.C.; Roma, M.G. UDCA for Drug-Induced Liver Disease: Clinical and Pathophysiological Basis. Semin. Liver Dis. 2024, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Wang, X.; Wang, Z.; Gu, L.; Yue, D.; Wang, Z.; Li, X.; Yang, L.; Huang, W.; Ding, L. Biological functions and pharmacological behaviors of bile acids in metabolic diseases. J. Adv. Res. 2025, 75, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, H.; Jia, Y.; Xiao, Y.; Luo, S.; Zhang, D.; Han, L.; Dai, L.; Xiao, C.; Feng, L.; et al. Ganoderic acid A exerted antidepressant-like action through FXR modulated NLRP3 inflammasome and synaptic activity. Biochem. Pharmacol. 2021, 188, 114561. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.M.; Zang, M.; Zhang, Q.; Shi, R.B.; Shi, X.J.; Mamtilahun, M.; Liu, C.; Luo, L.L.; Tian, X.; Zhang, Z.; et al. Farnesoid X receptor knockout protects brain against ischemic injury through reducing neuronal apoptosis in mice. J. Neuroinflam. 2020, 17, 164. [Google Scholar] [CrossRef]
- Ho, P.P.; Steinman, L. Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2016, 113, 1600–1605. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramírez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J. Cell Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef]
- Perino, A.; Velázquez-Villegas, L.A.; Bresciani, N.; Sun, Y.; Huang, Q.; Fénelon, V.S.; Castellanos-Jankiewicz, A.; Zizzari, P.; Bruschetta, G.; Jin, S.; et al. Central anorexigenic actions of bile acids are mediated by TGR5. Nat. Metab. 2021, 3, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Deng, S.; Tian, M.; Lenahan, C.; Wei, P.; Wang, Y.; Tan, J.; Wen, H.; Zhao, F.; Gao, Y.; et al. INT-777 prevents cognitive impairment by activating Takeda G protein-coupled receptor 5 (TGR5) and attenuating neuroinflammation via cAMP/ PKA/ CREB signaling axis in a rat model of sepsis. Exp. Neurol. 2021, 335, 113504. [Google Scholar] [CrossRef]
- Hu, X.; Yan, J.; Huang, L.; Araujo, C.; Peng, J.; Gao, L.; Liu, S.; Tang, J.; Zuo, G.; Zhang, J.H. INT-777 attenuates NLRP3-ASC inflammasome-mediated neuroinflammation via TGR5/cAMP/PKA signaling pathway after subarachnoid hemorrhage in rats. Brain Behav. Immun. 2021, 91, 587–600, Correction in Brain Behav. Immun. 2024, 119, 1021–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Deng, Y.; Wang, H.; Fu, J.; Wu, G.; Duan, Z.; Zhang, X.; Cai, Y.; Zhou, H.; Yin, J.; et al. Gut microbiota-mediated ursodeoxycholic acids regulate the inflammation of microglia through TGR5 signaling after MCAO. Brain Behav. Immun. 2024, 115, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramírez, L.; Mey, J. Emerging Roles of Bile Acids and TGR5 in the Central Nervous System: Molecular Functions and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 9279. [Google Scholar] [CrossRef]
- Xu, N.; Bai, Y.; Han, X.; Yuan, J.; Wang, L.; He, Y.; Yang, L.; Wu, H.; Shi, H.; Wu, X. Taurochenodeoxycholic acid reduces astrocytic neuroinflammation and alleviates experimental autoimmune encephalomyelitis in mice. Immunobiology 2023, 228, 152388. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Tobin, R.; Dusio, G.; Smith, J.; Shin, H.; Newell-Rogers, K.; Grant, S.; DeMorrow, S. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J. Neurochem. 2015, 135, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.G.; Yao, Y.; Liang, Y.J.; Lei, J.; Feng, S.Y.; Zhang, Z.X.; Tian, Y.; Cai, J.; Xing, G.G.; Fu, K.Y. Activation of TGR5 in the injured nerve site according to a prevention protocol mitigates partial sciatic nerve ligation-induced neuropathic pain by alleviating neuroinflammation. Pain 2025, 166, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Grant, S.; Khan, S.; Diocares, J.; Petrescu, A.; Wyatt, A.; Kain, J.; Jefferson, B.; DeMorrow, S. Bile Acid-Mediated Sphingosine-1-Phosphate Receptor 2 Signaling Promotes Neuroinflammation During Hepatic Encephalopathy in Mice. Front. Cell. Neurosci. 2017, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Hong, Y.H.; Sung, J.J.; Kim, S.M.; Lee, J.B.; Lee, K.W. Oral solubilized ursodeoxycholic acid therapy in amyotrophic lateral sclerosis: A randomized cross-over trial. J. Korean Med. Sci. 2012, 27, 200–206. [Google Scholar] [CrossRef]
- Parry, G.J.; Rodrigues, C.M.; Aranha, M.M.; Hilbert, S.J.; Davey, C.; Kelkar, P.; Low, W.C.; Steer, C.J. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 2010, 33, 17–21. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Chen, L.; Du, Y.F.; Hu, M.; Reed, M.N.; Long, Y.; Suppiramaniam, V.; Hong, H.; Tang, S.S. Protective effects of tauroursodeoxycholic acid on lipopolysaccharide-induced cognitive impairment and neurotoxicity in mice. Int. Immunopharmacol. 2019, 72, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Yeo, X.Y.; Tan, L.Y.; Chae, W.R.; Lee, D.-Y.; Lee, Y.-A.; Wuestefeld, T.; Jung, S. Liver’s influence on the brain through the action of bile acids. Front. Neurosci. 2023, 17, 1123967. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Vazquez-Juarez, E.; Muñoz, A.; Gerenu, G.; Gómez-Galán, M.; Lindskog, M.; DeFelipe, J.; Cedazo-Minguez, A.; Merino-Serrais, P. High levels of 27-hydroxycholesterol results in synaptic plasticity alterations in the hippocampus. Sci. Rep. 2021, 11, 3736. [Google Scholar] [CrossRef]
- Hurley, M.J.; Bates, R.; Macnaughtan, J.; Schapira, A.H.V. Bile acids and neurological disease. Pharmacol. Ther. 2022, 240, 108311. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.R.; Cunha, C.; Gomes, C.; Schmucki, N.; Barbosa, M.; Brites, D. Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 2015, 51, 864–877. [Google Scholar] [CrossRef]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Investig. 2020, 130, 3467–3482. [Google Scholar] [CrossRef]
- Shtilbans, A.; Reintsch, W.E.; Piscopo, V.E.C.; Krahn, A.I.; Durcan, T.M. Combination of tauroursodeoxycholic acid, co-enzyme Q10 and creatine demonstrates additive neuroprotective effects in in-vitro models of Parkinson’s disease. Front. Neurosci. 2024, 18, 1492028. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, K.; Guo, J.; Xu, L. Bile acid-mediated gut-liver axis crosstalk: The role of nuclear receptor signaling in dynamic regulation of inflammatory networks. Front. Immunol. 2025, 16, 1595486. [Google Scholar] [CrossRef] [PubMed]
- Luxenburger, A.; Harris, L.D.; Ure, E.M.; Jiao, W.; Woolhouse, A.D.; Cameron, S.A.; Weymouth-Wilson, A.; Furneaux, R.H.; Pitman, J.L.; Hinkley, S.F.R. The discovery of 12β-methyl-17-epi-18-nor-bile acids as potent and selective TGR5 agonists. Eur. J. Med. Chem. 2023, 250, 115143. [Google Scholar] [CrossRef]
- Pan, L.; Xie, L.; Yang, W.; Feng, S.; Mao, W.; Ye, L.; Cheng, H.; Wu, X.; Mao, X. The role of brain–liver–gut Axis in neurological disorders. Burn. Trauma 2025, 13, tkaf011. [Google Scholar] [CrossRef] [PubMed]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Mahmoudiandehkordi, S.; Louie, G.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.; Xie, W.; Chen, E.; Chen, Y.; Li, H.; Can, D.; Lei, A.; Wang, Y.; Zhang, J. TGR5 deficiency in excitatory neurons ameliorates Alzheimer’s pathology by regulating APP processing. Sci. Adv. 2024, 10, eado1855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, L.; Zhang, Y.; Zhang, Z.; Li, H.; Fan, F.; He, J.; Kang, J.; Zuo, L. Integrated untargeted and targeted metabolomics to reveal therapeutic effect and mechanism of Alpiniae oxyphyllae fructus on Alzheimer’s disease in APP/PS1 mice. Front. Pharmacol. 2023, 13, 1104954. [Google Scholar] [CrossRef]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2021, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Ladakis, D.C.; Harrison, K.L.; Smith, M.D.; Solem, K.; Gadani, S.; Jank, L.; Hwang, S.; Farhadi, F.; Dewey, B.E.; Fitzgerald, K.C.; et al. Bile acid metabolites predict multiple sclerosis progression and supplementation is safe in progressive disease. Med 2025, 6, 100522. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Dodge, J.C.; Yu, J.; Sardi, S.P.; Shihabuddin, L.S. Sterol auto-oxidation adversely affects human motor neuron viability and is a neuropathological feature of amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 803. [Google Scholar] [CrossRef]
- Chiang, P.I.; Chang, K.H.; Tang, H.Y.; Wu, Y.R.; Cheng, M.L.; Chen, C.M. Diagnostic Potential of Alternations of Bile Acid Profiles in the Plasma of Patients with Huntington’s Disease. Metabolites 2024, 14, 394. [Google Scholar] [CrossRef]
- Jia, W.; Rajani, C.; Kaddurah-Daouk, R.; Li, H. Expert insights: The potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer’s disease and hepatic encephalopathy. Med. Res. Rev. 2020, 40, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Cardoso, V.F.; Corlianò, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromolecular Med. 2021, 23, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Zhou, Z.; Yang, D.; Yan, W. Gut Microbiota-Bile Acid-Brain Axis and TGR5-ERK1/2 Signaling Mediate ADT-Induced Cognitive Impairment. CNS Neurosci. Ther. 2025, 31, e70608. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, M.; Bao, Y.; Huang, W.; He, X.; Hong, Y.; Wei, W.; Liu, Z.; Gao, X.; Yang, Y.; et al. Gut microbiota-brain bile acid axis orchestrates aging-related neuroinflammation and behavior impairment in mice. Pharmacol. Res. 2024, 208, 107361. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Valizadeh, P.; Fallahi, M.S.; Alzheimer’s disease Neuroimaging, I. Bile acid profile associated with CSF and PET biomarkers in Alzheimer’s disease. Aging Clin. Exp. Res. 2024, 36, 62. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Li, Y.; Zhang, J.; Gao, Y.; Qiu, Y.; Gan, R.; Zhang, Y.; Wang, L. Distinct Bile Acid Signature in Parkinson’s Disease With Mild Cognitive Impairment. Front. Neurol. 2022, 13, 897867. [Google Scholar] [CrossRef]
- Hou, Y.; Luan, J.; Huang, T.; Deng, T.; Li, X.; Xiao, Z.; Zhan, J.; Luo, D.; Hou, Y.; Xu, L.; et al. Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J. Neuroinflammation 2021, 18, 216. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Solon, C.; Bronczek, G.A.; Engel, D.F.; Kurauti, M.A.; Soares, G.M.; Rodrigues, K.S.; Velloso, L.A.; Boschero, A.C.; et al. The bile acid TUDCA improves glucose metabolism in streptozotocin-induced Alzheimer’s disease mice model. Mol. Cell. Endocrinol. 2021, 521, 111116. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shen, D.; Jiang, C.; Wang, H.; Chang, M. Ursodeoxycholic acid protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis in MPTP/MPP(+)-induced Parkinson’s disease. Neurosci. Lett. 2021, 741, 135493. [Google Scholar] [CrossRef]
- Huang, F. Ursodeoxycholic acid as a potential alternative therapeutic approach for neurodegenerative disorders: Effects on cell apoptosis, oxidative stress and inflammation in the brain. Brain Behav. Immun. Health 2021, 18, 100348. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Steer, C.J. The therapeutic effects of ursodeoxycholic acid as an anti-apoptotic agent. Expert. Opin. Investig. Drugs 2001, 10, 1243–1253. [Google Scholar] [CrossRef]
- Huang, F.; Pariante, C.M.; Borsini, A. From dried bear bile to molecular investigation: A systematic review of the effect of bile acids on cell apoptosis, oxidative stress and inflammation in the brain, across pre-clinical models of neurological, neurodegenerative and neuropsychiatric disorders. Brain Behav. Immun. 2022, 99, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shen, D.; Li, K.; Wang, H.; Sang, W.; Qi, H. Protective Effects of Ursodeoxycholic Acid Against Oxidative Stress and Neuroinflammation Through Mitogen-Activated Protein Kinases Pathway in MPTP-Induced Parkinson Disease. Clin. Neuropharmacol. 2022, 45, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered Bile Acid Profile in Mild Cognitive Impairment and Alzheimer’s Disease: Relationship to Neuroimaging and CSF Biomarkers. Alzheimer’s Dement. 2019, 15, 232–244. [Google Scholar] [CrossRef]
- Li, P.; Killinger, B.A.; Ensink, E.; Beddows, I.; Yilmaz, A.; Lubben, N.; Lamp, J.; Schilthuis, M.; Vega, I.E.; Woltjer, R.; et al. Gut Microbiota Dysbiosis Is Associated with Elevated Bile Acids in Parkinson’s Disease. Metabolites 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Erngren, I.; Vaivade, A.; Carlsson, H.; Al-Grety, A.; Åkerfeldt, T.; Kockum, I.; Hedström, A.K.; Alfredsson, L.; Olsson, T.; Burman, J.; et al. Bile acid metabolism in multiple sclerosis is perturbed and associated with the risk of confirmed disability worsening. BMC Med. 2025, 23, 212. [Google Scholar] [CrossRef]
- Crick, P.J.; Griffiths, W.J.; Zhang, J.; Beibel, M.; Abdel-Khalik, J.; Kuhle, J.; Sailer, A.W.; Wang, Y. Reduced Plasma Levels of 25-Hydroxycholesterol and Increased Cerebrospinal Fluid Levels of Bile Acid Precursors in Multiple Sclerosis Patients. Mol. Neurobiol. 2017, 54, 8009–8020. [Google Scholar] [CrossRef]
- Sonoda, Y.; Aizawa, F.; Tomochika, N.; Miyauchi, K.; Nishibashi, A.; Takahashi, S.; Kosako, H.; Tanida, S.; Yagi, K.; Niimura, T.; et al. Ursodeoxycholic acid alleviates multiple sclerosis via TGR5-dependent microglial regulation in mice. Eur. J. Pharmacol. 2025, 1003, 177941. [Google Scholar] [CrossRef]
- Lewis, N.D.; Patnaude, L.A.; Pelletier, J.; Souza, D.J.; Lukas, S.M.; King, F.J.; Hill, J.D.; Stefanopoulos, D.E.; Ryan, K.; Desai, S.; et al. A GPBAR1 (TGR5) small molecule agonist shows specific inhibitory effects on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS ONE 2014, 9, e100883. [Google Scholar] [CrossRef]
- Verma, S.; Goswami, S.; Palanimuthu, D.; Ghosh, T.S. Gut Microbiota-Brain Axis in Healthy Ageing. In Brain and Mental Health in Ageing; Kaur, G., Rattan, S.I.S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 143–164. [Google Scholar]
- Elia, A.E.; Lalli, S.; Monsurrò, M.R.; Sagnelli, A.; Taiello, A.C.; Reggiori, B.; La Bella, V.; Tedeschi, G.; Albanese, A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016, 23, 45–52, Correction in Eur. J. Neurol. 2017, 24, 659. [Google Scholar] [CrossRef]
- Thams, S.; Lowry, E.R.; Larraufie, M.H.; Spiller, K.J.; Li, H.; Williams, D.J.; Hoang, P.; Jiang, E.; Williams, L.A.; Sandoe, J.; et al. A Stem Cell-Based Screening Platform Identifies Compounds that Desensitize Motor Neurons to Endoplasmic Reticulum Stress. Mol. Ther. 2019, 27, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Nandakumar, R.; Haeusler, R.A. Alteration of serum bile acids in amyotrophic lateral sclerosis. Lipids 2024, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.L.; Spila Alegiani, S.; Mayer, F.; Cipriani, M.; Lo Giudice, M.; Ludolph, A.C.; McDermott, C.J.; Corcia, P.; Van Damme, P.; Van den Berg, L.H.; et al. A randomized double-blind clinical trial on safety and efficacy of tauroursodeoxycholic acid (TUDCA) as add-on treatment in patients affected by amyotrophic lateral sclerosis (ALS): The statistical analysis plan of TUDCA-ALS trial. Trials 2023, 24, 792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Li, Z.; Liang, N.; Zhou, X.; Nie, X.; Zhang, T.; Qi, W. Amyotrophic Lateral Sclerosis and Primary Biliary Cirrhosis Overlap Syndrome: Two Cases Report. Front. Neurol. 2019, 10, 890. [Google Scholar] [CrossRef]
- Warby, S.C.; Montpetit, A.; Hayden, A.R.; Carroll, J.B.; Butland, S.L.; Visscher, H.; Collins, J.A.; Semaka, A.; Hudson, T.J.; Hayden, M.R. CAG expansion in the Huntington disease gene is associated with a specific and targetable predisposing haplogroup. Am. J. Hum. Genet. 2009, 84, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.D.; Rodrigues, C.M.P.; Eich, T.; Linehan-Stieers, C.; Abt, A.; Kren, B.T.; Steer, C.J.; Low, W.C. A Bile Acid Protects against Motor and Cognitive Deficits and Reduces Striatal Degeneration in the 3-Nitropropionic Acid Model of Huntington’s Disease. Exp. Neurol. 2001, 171, 351–360. [Google Scholar] [CrossRef]
- Keene, C.D.; Rodrigues, C.M.; Eich, T.; Chhabra, M.S.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, R.M.; Viana, R.J.; Low, W.C.; Steer, C.J.; Rodrigues, C.M. Bile acids and apoptosis modulation: An emerging role in experimental Alzheimer’s disease. Trends Mol. Med. 2008, 14, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Esmaealzadeh, N.; Ataei, M.; Afshari, N.; Saleh, M.; Amini, Y.; Hasrati, S.; Ghazizadeh Hashemi, F.; Mortazavi, A.; Mohaghegh Shalmani, L.; et al. The effects of ursodeoxycholic acid on Parkinson’s disease, a mechanistic review of the recent evidence. Metab. Brain Dis. 2025, 40, 115. [Google Scholar] [CrossRef] [PubMed]
- Vang, S.; Longley, K.; Steer, C.J.; Low, W.C. The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases. Glob. Adv. Health Med. 2014, 3, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Burks, S.; Raymick, J.; Robinson, B.; Gómez-Crisóstomo, N.P.; Escudero-Lourdes, C.; Lopez, A.G.G.; Chigurupati, S.; Hanig, J.; Ferguson, S.A.; et al. Tauroursodeoxycholic acid (TUDCA) is neuroprotective in a chronic mouse model of Parkinson’s disease. Nutr. Neurosci. 2022, 25, 1374–1391. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.I.; Fonseca, I.; Nunes, M.J.; Moreira, S.; Rodrigues, E.; Carvalho, A.N.; Rodrigues, C.M.P.; Gama, M.J.; Castro-Caldas, M. Novel insights into the antioxidant role of tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yang, S.; Tang, C.; Li, D.; Kan, Y.; Yao, L. New insights into microbial bile salt hydrolases: From physiological roles to potential applications. Front. Microbiol. 2025, 16, 1513541. [Google Scholar] [CrossRef]
- Otto, C.; Kalantzis, R.; Kübler-Weller, D.; Kühn, A.A.; Böld, T.; Regler, A.; Strathmeyer, S.; Wittmann, J.; Ruprecht, K.; Heelemann, S. Comprehensive analysis of the cerebrospinal fluid and serum metabolome in neurological diseases. J. Neuroinflam. 2024, 21, 234. [Google Scholar] [CrossRef]
- Vidicevic, S.; Tasic, J.; Stanojevic, Z.; Ciric, D.; Martinovic, T.; Paunovic, V.; Petricevic, S.; Tomonjic, N.; Isakovic, A.; Trajkovic, V. Endoplasmic reticulum stress response in immune cells contributes to experimental autoimmune encephalomyelitis pathogenesis in rats. Immunol. Lett. 2024, 267, 106855. [Google Scholar] [CrossRef]
- Albanese, A.; Ludolph, A.C.; McDermott, C.J.; Corcia, P.; Van Damme, P.; Van den Berg, L.H.; Hardiman, O.; Rinaldi, G.; Vanacore, N.; Dickie, B. Tauroursodeoxycholic acid in patients with amyotrophic lateral sclerosis: The TUDCA-ALS trial protocol. Front. Neurol. 2022, 13, 1009113. [Google Scholar] [CrossRef]
- Joo, S.S.; Won, T.J.; Lee, D.I. Potential role of ursodeoxycholic acid in suppression of nuclear factor kappa B in microglial cell line (BV-2). Arch. Pharm. Res. 2004, 27, 954–960. [Google Scholar] [CrossRef]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar]
- Amaral, J.D.; Viana, R.J.S.; Ramalho, R.M.; Steer, C.J.; Rodrigues, C.M.P. Bile acids: Regulation of apoptosis by ursodeoxycholic acid. J. Lipid Res. 2009, 50, 1721–1734. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Barnes, K.; Clemmens, H.; Al-Rafiah, A.R.; Al-Ofi, E.A.; Leech, V.; Bandmann, O.; Shaw, P.J.; Blackburn, D.J.; Ferraiuolo, L.; et al. Ursodeoxycholic Acid Improves Mitochondrial Function and Redistributes Drp1 in Fibroblasts from Patients with Either Sporadic or Familial Alzheimer’s Disease. J. Mol. Biol. 2018, 430, 3942–3953. [Google Scholar] [CrossRef]
- Fathima, A.; Jamma, T. UDCA ameliorates inflammation driven EMT by inducing TGR5 dependent SOCS1 expression in mouse macrophages. Sci. Rep. 2024, 14, 24285, Correction in Sci. Rep. 2024, 14, 29359. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Hu, X.; Zhang, S.; Yu, Q.; Kuang, G.; Liu, L.; Yu, D.; Huang, J.; Xia, Y.; et al. NR1H4 ameliorates Parkinson’s disease via inhibiting astrocyte activation and neuroinflammation in a CEBPβ/NF-κB dependent manner. Int. Immunopharmacol. 2024, 142, 113087. [Google Scholar] [CrossRef]
- Liang, H.; Matei, N.; McBride, D.W.; Xu, Y.; Zhou, Z.; Tang, J.; Luo, B.; Zhang, J.H. TGR5 activation attenuates neuroinflammation via Pellino3 inhibition of caspase-8/NLRP3 after middle cerebral artery occlusion in rats. J. Neuroinflam. 2021, 18, 40. [Google Scholar] [CrossRef]
- Huang, R.; Gao, Y.; Chen, J.; Duan, Q.; He, P.; Zhang, J.; Huang, H.; Zhang, Q.; Ma, G.; Zhang, Y.; et al. TGR5 Agonist INT-777 Alleviates Inflammatory Neurodegeneration in Parkinson’s Disease Mouse Model by Modulating Mitochondrial Dynamics in Microglia. Neuroscience 2022, 490, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.B.; Flynn, J.F.; Palacios, A.M. Capitalizing on Hope: Questionable Marketing Approval and Pricing of a New ALS Drug. Int. J. Soc. Determ. Health Health Serv. 2024, 54, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Alqallaf, A.; Cates, D.W.; Render, K.P.; Patel, K.A. Sodium Phenylbutyrate and Taurursodiol: A New Therapeutic Option for the Treatment of Amyotrophic Lateral Sclerosis. Ann. Pharmacother. 2024, 58, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.K.; Setayesh, T.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Wan, Y.Y. Intestinal Microbiota Remodeling Protects Mice from Western Diet-Induced Brain Inflammation and Cognitive Decline. Cells 2022, 11, 504. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; Yin, H.; Li, H.; Du, J.; Bao, H. Ganoderic Acid A Attenuates LPS-Induced Neuroinflammation in BV2 Microglia by Activating Farnesoid X Receptor. Neurochem. Res. 2021, 46, 1725–1736. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Delbridge, A.R.D.; Huh, D.; Brickelmaier, M.; Burns, J.C.; Roberts, C.; Challa, R.; Raymond, N.; Cullen, P.; Carlile, T.M.; Ennis, K.A.; et al. Organotypic Brain Slice Culture Microglia Exhibit Molecular Similarity to Acutely-Isolated Adult Microglia and Provide a Platform to Study Neuroinflammation. Front. Cell. Neurosci. 2020, 14, 592005. [Google Scholar] [CrossRef]
- Musto, M.; Rauti, R.; Rodrigues, A.F.; Bonechi, E.; Ballerini, C.; Kostarelos, K.; Ballerini, L. 3D Organotypic Spinal Cultures: Exploring Neuron and Neuroglia Responses Upon Prolonged Exposure to Graphene Oxide. Front. Syst. Neurosci. 2019, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Suh, M.; Choi, H.; Choi, Y.; Hwang, D.W.; Bae, S.; Lee, D.S. Spatial transcriptomic brain imaging reveals the effects of immunomodulation therapy on specific regional brain cells in a mouse dementia model. BMC Genom. 2024, 25, 516. [Google Scholar] [CrossRef] [PubMed]
- Chen-Plotkin, A.S. Unbiased Approaches to Biomarker Discovery in Neurodegenerative Diseases. Neuron 2014, 84, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, T.; Zanella, D.; Bhatt, M.; Di Iacovo, A.; Galli, A.; Bossi, E. Bile acid interactions with neurotransmitter transporters. Front. Cell. Neurosci. 2023, 17, 1161930. [Google Scholar] [CrossRef] [PubMed]

| Disorder | Key Features | Altered Bile Acids | Receptors Involved | Pathogenic Mechanisms | References |
|---|---|---|---|---|---|
| Alzheimer’s Disease | Amyloid-beta deposition, tau pathology | ↑ DCA, TDCA, GDCA; ↓ CDCA, TUDCA | FXR, TGR5 | Neuroinflammation, oxidative stress, gut–brain signaling | [66,67,68] |
| Parkinson’s Disease | Cognitive decline, motor symptoms | ↓ CDCA, CA, UDCA, TUDCA; ↑ DCA, LCA | FXR, TGR5 | Mitochondrial dysfunction, inflammation, appendix bile acid shifts | [49,69] |
| Multiple Sclerosis | Demyelination, immune dysregulation | Altered serum/CSF bile acids | FXR, TGR5 | Microglial modulation, AKT/NFκB signaling, immune cell trafficking | [61,70] |
| Hepatic Encephalopathy | Cognitive impairment, motor disturbances | ↑ TCA | S1PR2, TGR5 | Microglial activation, systemic bile acid toxicity | [51,53] |
| Aging and Cognitive Decline | Synaptic dysfunction, memory loss | ↑ TβMCA | FXR | Microbial dysbiosis, neuroimmune signaling disruption | [71] |
| Amyotrophic lateral sclerosis | Motor neuron degeneration, muscle wasting | ↑ LCA, CDCA; ↓ UDCA | TGR5, FXR | Neuroinflammation, mitochondrial dysfunction, bile acid neurotoxicity | [55,72] |
| Huntington’s Disease | Chorea, psychiatric symptoms, cognitive decline | ↓ CDCA, CA, UDCA; ↑ DCA | FXR, TGR5 | Impaired bile acid metabolism, neuroinflammation, gut–brain axis disruption | [73] |
| Compound | Target Disorders | Mechanisms of Action | Preclinical Insights | Clinical Insights |
|---|---|---|---|---|
| TUDCA | Alzheimer’s, Parkinson’s, MS, ALS | Anti-inflammatory, anti-apoptotic, antioxidant; neuroprotective | Anti-apoptotic and mitochondrial protection, reduction in ER stress; improved neuronal survival; reduced Experimental Autoimmune Encephalomyelitis (EAE) scores [61]; decreased Aβ deposition in Alzheimer’s Disease models [24,110]; attenuated autophagy in Parkinson’s disease [105]. | Safe in ALS Phase II trials; Phase III showed no significant benefit but good tolerability [111]; changes in circulating T cells and the gut microbiota in MS patients [70]. |
| UDCA (Ursodiol) | Alzheimer’s, Parkinson’s, MS, ALS, HD | Anti-inflammatory | attenuation of the production of pro-inflammatory cytokines and nitric oxide via inactivation of NF-kappaB in Alzheimer’s disease [112]; Neuroprotective effects in vitro and in vivo [82,113]; modulates bile acid receptors and apoptosis [114]; Mitochondrial stabilization [115]; microglial modulation via TGR5 [91,116]. | Safe and well-tolerated in Parkinson’s Phase II trial (UP Study); improved mitochondrial function and gait (ClinicalTrials.gov ID NCT03840005); Ursodiol in Huntington’s Disease (NCT00514774). |
| GUDCA | ALS | Anti-apoptotic and anti-inflammatory | reduces matrix metalloproteinase-9 and caspase-9 activation [60]. | - |
| Obeticholic acid/ 6-ECDCA (FXR agonist) | Parkinson’s Disease, Hepatic Encephalo-pathy | Anti-inflammatory and neuroprotective | inhibited astrocyte activation and neuroinflammation in a CEBPβ/NF-κB dependent manner [117]; suppresses pro-inflammatory cytokines and reduces C cell populations [43]. | - |
| GPBAR1/ TGR5 agonists (TUDCA, INT-777) | Hepatic Encephalo-pathy, Parkinson’s Disease | Anti-inflammatory and neuroprotective | Alleviates neuroinflammation by altering neuron and microglia paracrine signaling [51]; reduces neuroinflammation and microglial cell activation [46]; mitigates neuropathic pain by reducing neuroinflammation [52]; alleviates neuroinflammation via Pellino3 inhibition of caspase-8/NLRP3 [118], modulating Mitochondrial Dynamics in Microglia [119], reducing microglia activation [44]; modulates NF-κB signaling [56]. | - |
| AMX0035 (TUDCA combined with phenylbutyrate) | ALS | Neuroprotective | TUDCA administration delayed muscle denervation and reduced ER stress [95]. | Reduced disease progression and longer survivability [120] (clinical trials NCT04987671, NCT03127514, NCT03488524, NCT05286372 and NCT04516096), marketed as a new ALS drug [121]. |
| Cholestyramine | Hepatic Encephalopathy and cognitive decline | Anti-inflammatory; Bile acid chelation; cytokine modulation | Reduced IL-1β/IL-6; increased IL-10 and TGR5 expression in animal models [27]; diminishes brain inflammatory signaling [122]; reduces pro-inflammatory cytokine expression in the cortex [53]. | Used clinically for hepatic encephalopathy and NAFLD; combination trials with elobixibat show safety and efficacy. |
| Microbiota Modulation | Aging and Cognitive Decline | Anti-inflammatory; alters bile acid profiles; reduces TβMCA | Preserves cognition, reduces neuroinflammation, improves SCFA production [77]. | Emerging clinical interest; microbiome-targeted therapies under development for mild cognitive impairment and dementia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butcher, E.L.; Arthur, S. Emerging Roles of Bile Acids in Neuroinflammation. Int. J. Mol. Sci. 2025, 26, 11301. https://doi.org/10.3390/ijms262311301
Butcher EL, Arthur S. Emerging Roles of Bile Acids in Neuroinflammation. International Journal of Molecular Sciences. 2025; 26(23):11301. https://doi.org/10.3390/ijms262311301
Chicago/Turabian StyleButcher, Erika L., and Subha Arthur. 2025. "Emerging Roles of Bile Acids in Neuroinflammation" International Journal of Molecular Sciences 26, no. 23: 11301. https://doi.org/10.3390/ijms262311301
APA StyleButcher, E. L., & Arthur, S. (2025). Emerging Roles of Bile Acids in Neuroinflammation. International Journal of Molecular Sciences, 26(23), 11301. https://doi.org/10.3390/ijms262311301



_Kim.png)



