Administration of Single or Repeated Doses of CDCs in a Swine Model of Reperfused Myocardial Infarction: Magnetic Resonance and Proteomics Evaluation
Abstract
1. Introduction
2. Results
2.1. Safety of Single or Repeated CDC Administration
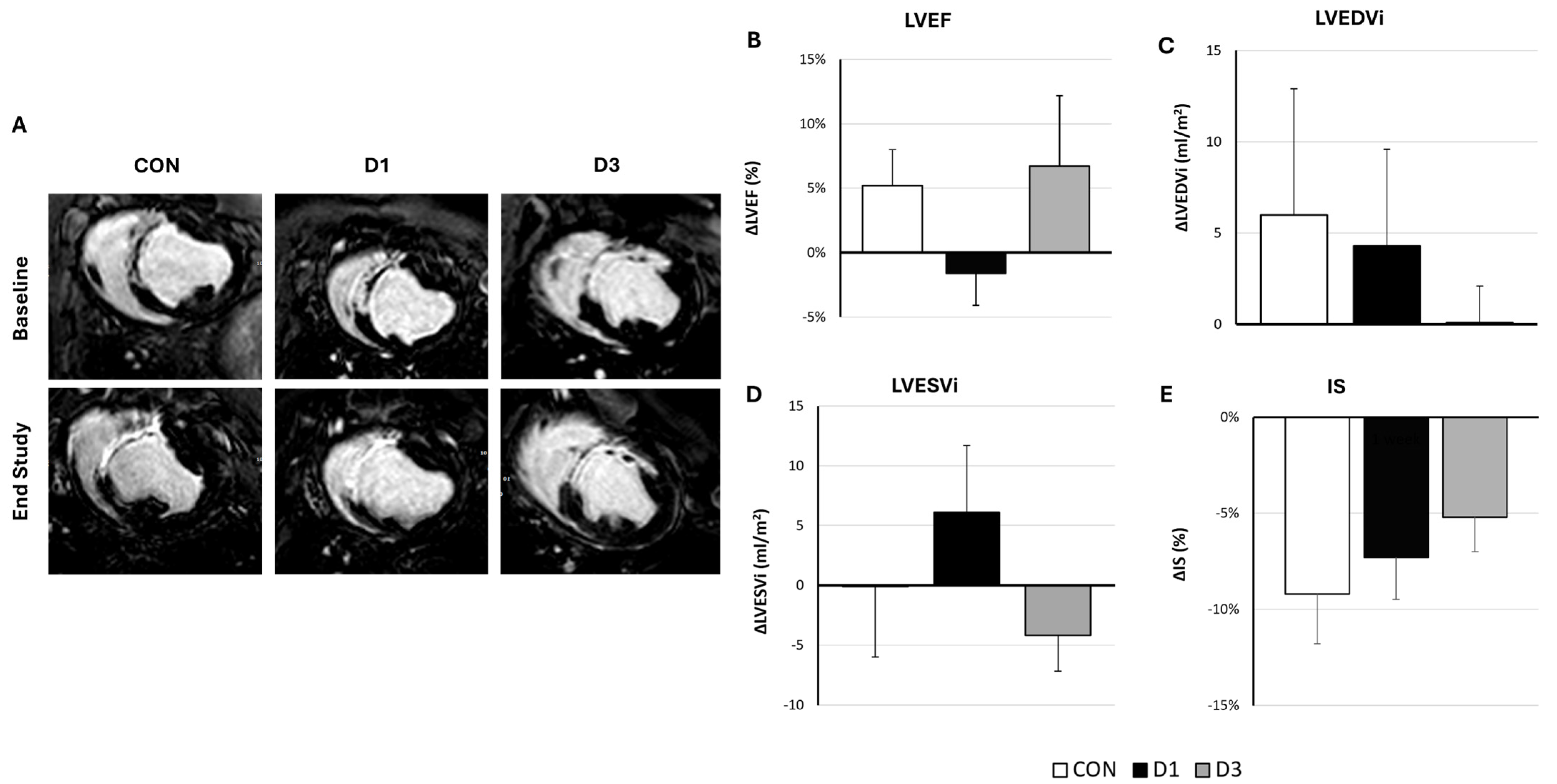
2.2. Efficacy of Single or Repeated CDC Administration
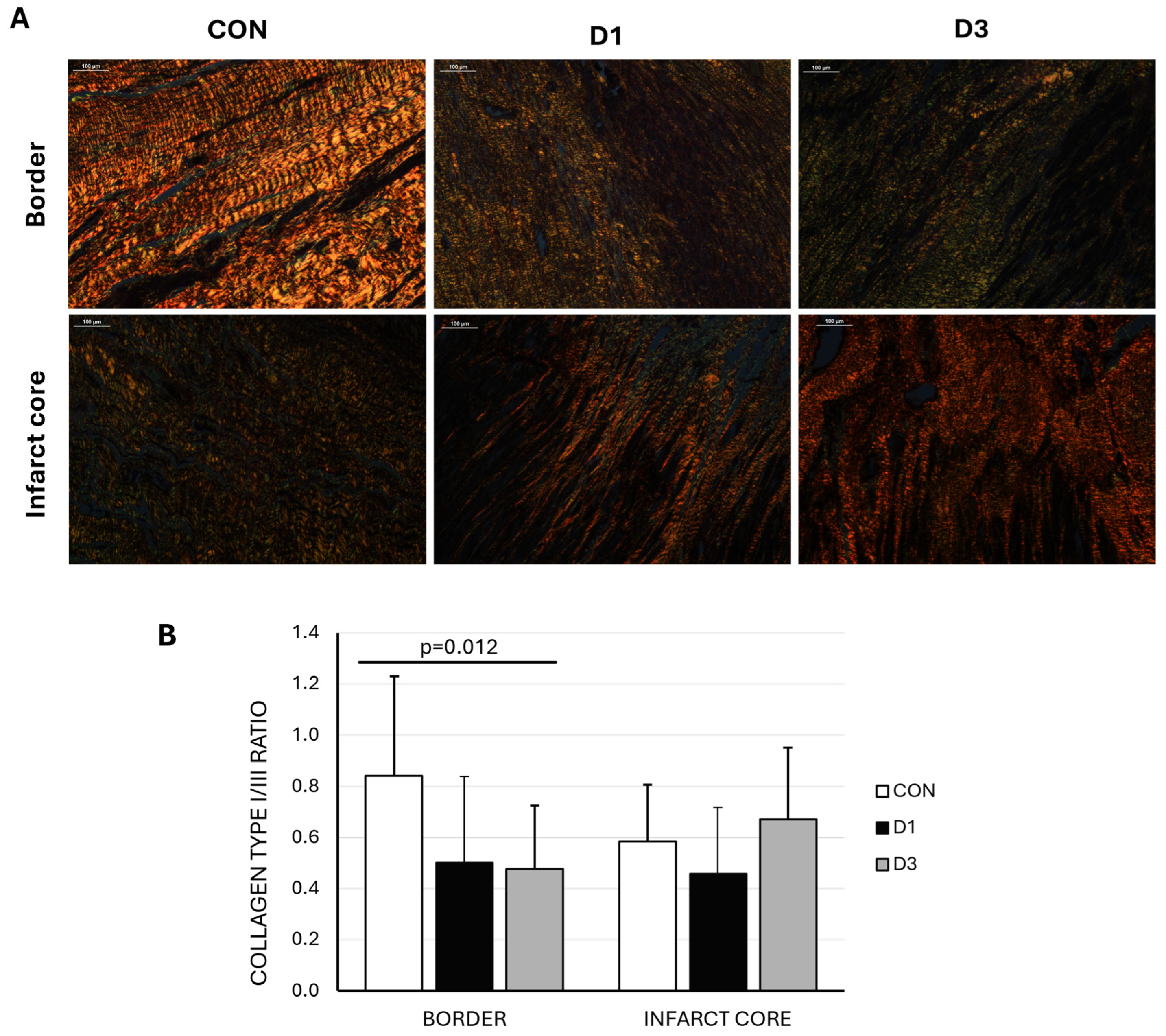
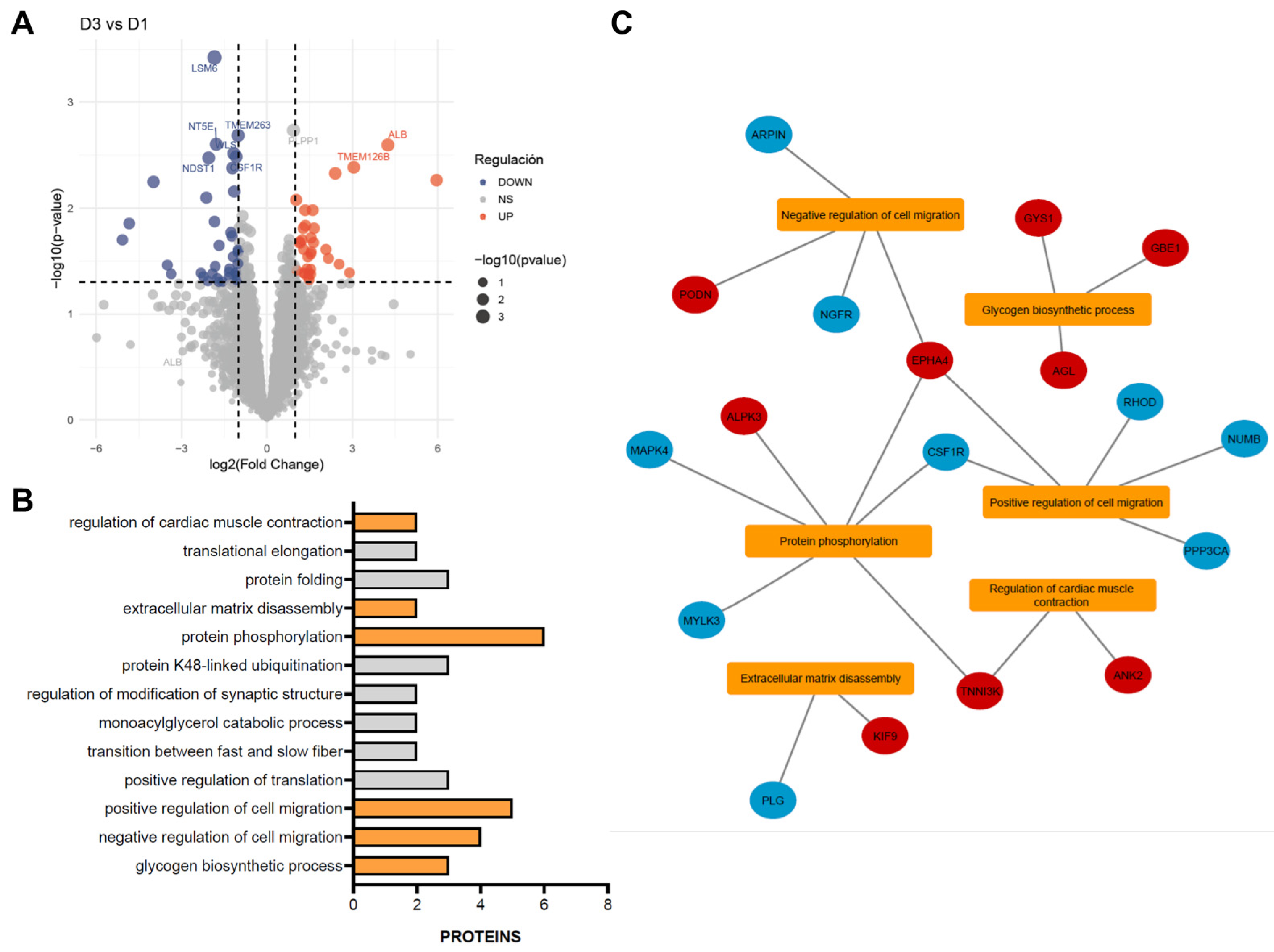
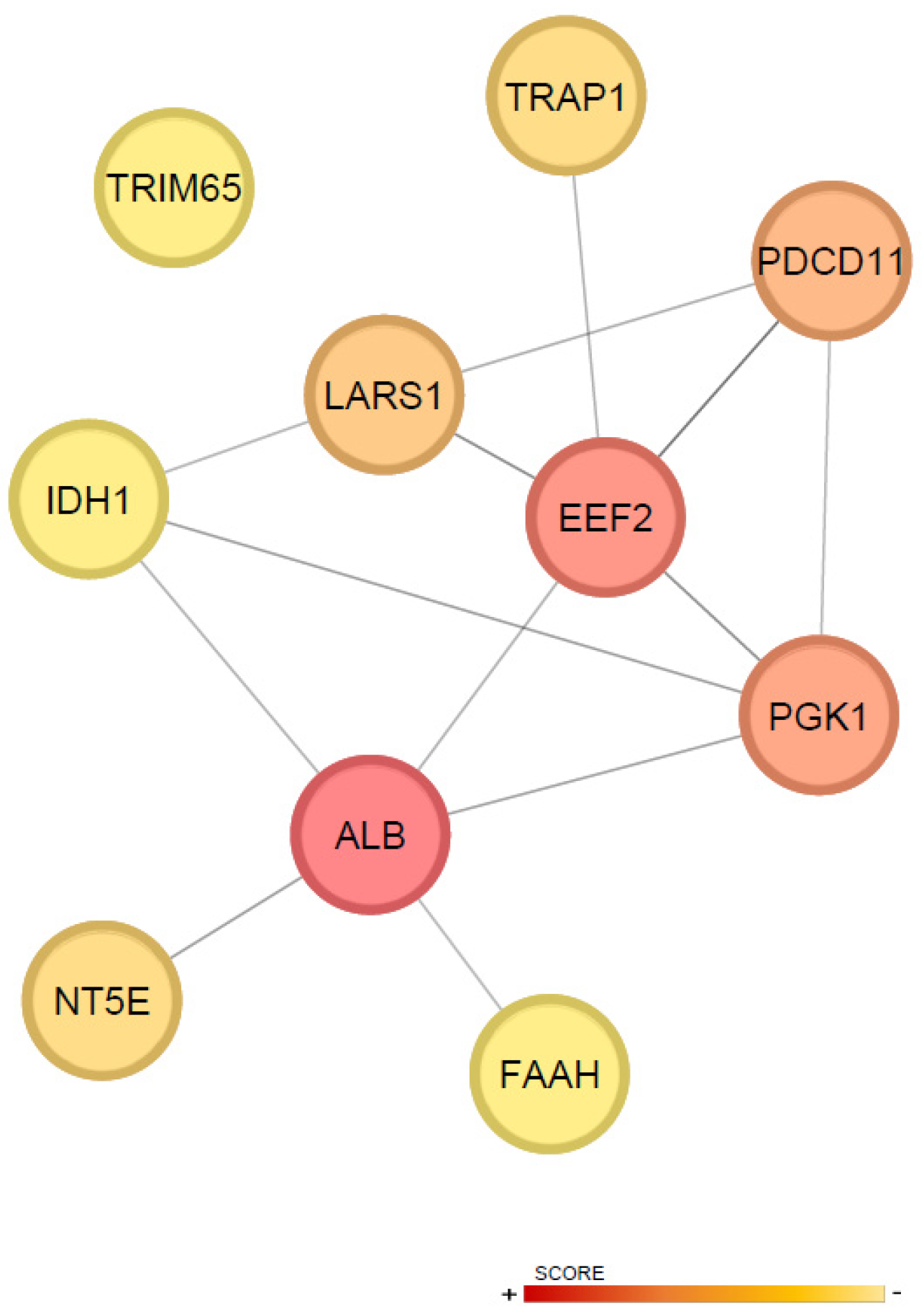
2.3. Proteomic Assessment of Cardiac Response to CDC Therapy
3. Discussion
4. Materials and Methods
4.1. Isolation of CDCs and Therapy Preparation
4.2. Swine Model of Reperfused Myocardial Infarction
4.3. Treatment Protocols
4.4. Therapy Administration
4.5. Cardiac Magnetic Resonance Follow-Up
4.6. End Study
4.7. Postmortem Studies
4.8. Proteomics
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MI | Myocardial infarction |
| CDCs | Cardiosphere-derived cells |
| CGM | Cardiomyocyte growth medium |
| LAD | Left anterior descending coronary artery |
| IR | Ischemia reperfusion |
| HF | Heart failure |
| CPCs | Cardiac progenitor cells |
| AMI | Acute myocardial infarction |
| CMR | Cardiac magnetic resonance |
| PES | Programmed electrical stimulation |
| LW | Large white |
| LVEF | Left ventricular ejection fraction |
| TnI | Troponin I |
| CRP | C-reactive protein |
| IS | Infarct size |
| LVEDVi | Left ventricular end-diastolic volume indexed to body surface area |
| LVESVi | End-systolic volume indexed to body surface area |
| HE | Hematoxylin eosin |
| MT | Masson’s trichrome |
| DAPs | Differentially abundant proteins |
| BP | Biological process |
| GO | Gene ontology |
| PPI | Protein–protein interaction |
| IC | Intracoronary |
| MSCs | Mesenchymal stromal cells |
| S-CDCs | Cardiosphere-derived cell secretomes |
| PBSC | Peripheral blood stem cell |
| BM-MNC | Bone marrow mononuclear cell |
| ECM | Extracellular matrix |
| ALB | Serum albumin |
| FAAH | Fatty acid amide hydrolase |
| BSA | Body surface area |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| MCC | Maximal clique centrality |
References
- Gyongyosi, M.; Haller, P.M.; Blake, D.J.; Martin Rendon, E. Meta-Analysis of Cell Therapy Studies in Heart Failure and Acute Myocardial Infarction. Circ. Res. 2018, 123, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chamuleau, S.A.J.; Van Der Naald, M.; Climent, A.M.; Kraaijeveld, A.O.; Wever, K.E.; Duncker, D.J.; Fernández-Avilés, F.; Bolli, R. Translational Research in Cardiovascular Repair. Circ. Res. 2018, 122, 310–318. [Google Scholar] [CrossRef]
- Bolli, R. Repeated Cell Therapy: A Paradigm Shift Whose Time Has Come. Circ. Res. 2017, 120, 1072–1074. [Google Scholar] [CrossRef]
- Tang, X.-L.; Wysoczynski, M.; Gumpert, A.M.; Solanki, M.; Li, Y.; Wu, W.-J.; Zheng, S.; Ruble, H.; Li, H.; Stowers, H.; et al. Intravenous infusions of mesenchymal stromal cells have cumulative beneficial effects in a porcine model of chronic ischaemic cardiomyopathy. Cardiovasc. Res. 2024, 120, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Nakamura, S.; Li, Q.; Wysoczynski, M.; Gumpert, A.M.; Wu, W.J.; Hunt, G.; Stowers, H.; Ou, Q.; Bolli, R. Repeated Administrations of Cardiac Progenitor Cells Are Superior to a Single Administration of an Equivalent Cumulative Dose. J. Am. Heart Assoc. 2018, 7, e007400. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Khan, A.; Bolli, R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ. Res. 2018, 123, 138–158. [Google Scholar] [CrossRef]
- Tokita, Y.; Tang, X.-L.; Li, Q.; Wysoczynski, M.; Hong, K.U.; Nakamura, S.; Wu, W.-J.; Xie, W.; Li, D.; Hunt, G.; et al. Repeated Administrations of Cardiac Progenitor Cells Are Markedly More Effective Than a Single Administration. Circ. Res. 2016, 119, 635–651. [Google Scholar] [CrossRef]
- Richardson, J.D.; Psaltis, P.J.; Frost, L.; Paton, S.; Carbone, A.; Bertaso, A.G.; Nelson, A.J.; Wong, D.T.; Worthley, M.I.; Gronthos, S.; et al. Incremental benefits of repeated mesenchymal stromal cell administration compared with solitary intervention after myocardial infarction. Cytotherapy 2014, 16, 460–470. [Google Scholar] [CrossRef]
- Guo, Y.; Wysoczynski, M.; Nong, Y.; Tomlin, A.; Zhu, X.; Gumpert, A.M.; Nasr, M.; Muthusamy, S.; Li, H.; Book, M.; et al. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res. Cardiol. 2017, 112, 18. [Google Scholar] [CrossRef] [PubMed]
- Poh, K.K.; Sperry, E.; Young, R.G.; Freyman, T.; Barringhaus, K.G.; Thompson, C.A. Repeated direct endomyocardial transplantation of allogeneic mesenchymal stem cells: Safety of a high dose, “off-the-shelf”, cellular cardiomyoplasty strategy. Int. J. Cardiol. 2007, 117, 360–364. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Trivedi, R.K.; Sharp, T.E.; Li, Z.; Goodchild, T.T.; Scarborough, A.; de Couto, G.; Marban, E.; Lefer, D.J. Repeated cell transplantation and adjunct renal denervation in ischemic heart failure: Exploring modalities for improving cell therapy efficacy. Basic Res. Cardiol. 2019, 114, 9. [Google Scholar] [CrossRef]
- Xu, J.; Xiong, Y.Y.; Li, Q.; Hu, M.J.; Huang, P.S.; Xu, J.Y.; Tian, X.Q.; Jin, C.; Liu, J.D.; Qian, L.; et al. Optimization of Timing and Times for Administration of Atorvastatin-Pretreated Mesenchymal Stem Cells in a Preclinical Model of Acute Myocardial Infarction. Stem Cells Transl. Med. 2019, 8, 1068–1083. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Nong, Y.; Tomlin, A.; Gumpert, A.; Zhu, X.; Hassan, S.A.; Bolli, R. Comparison of Repeated Doses of C-kit-Positive Cardiac Cells versus a Single Equivalent Combined Dose in a Murine Model of Chronic Ischemic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 3145. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.; Tseliou, E.; de Couto, G.; Angert, D.; Valle, J.; Kubota, Y.; Luthringer, D.; Mirocha, J.; Sun, B.; Smith, R.R.; et al. Repeated transplantation of allogeneic cardiosphere-derived cells boosts therapeutic benefits without immune sensitization in a rat model of myocardial infarction. J. Heart Lung Transplant. 2016, 35, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nong, Y.; Li, Q.; Tomlin, A.; Kahlon, A.; Gumpert, A.; Slezak, J.; Zhu, X.; Bolli, R. Comparison of One and Three Intraventricular Injections of Cardiac Progenitor Cells in a Murine Model of Chronic Ischemic Cardiomyopathy. Stem Cell Rev. Rep. 2021, 17, 604–615. [Google Scholar] [CrossRef]
- Attar, A.; Farjoud Kouhanjani, M.; Hessami, K.; Vosough, M.; Kojuri, J.; Ramzi, M.; Hosseini, S.A.; Faghih, M.; Monabati, A. Effect of once versus twice intracoronary injection of allogeneic-derived mesenchymal stromal cells after acute myocardial infarction: BOOSTER-TAHA7 randomized clinical trial. Stem Cell Res. Ther. 2023, 14, 264, Erratum in Stem Cell Res. Ther. 2024, 15, 40. [Google Scholar] [CrossRef]
- Vrtovec, B.; Bolli, R. Potential Strategies for Clinical Translation of Repeated Cell Therapy. Circ. Res. 2019, 124, 690–692. [Google Scholar] [CrossRef]
- Savko, C.; Sussman, M.A. Coming around again. Cardiovasc. Res. 2024, 120, 1830–1831. [Google Scholar] [CrossRef]
- Malliaras, K.; Smith, R.R.; Kanazawa, H.; Yee, K.; Seinfeld, J.; Tseliou, E.; Dawkins, J.F.; Kreke, M.; Cheng, K.; Luthringer, D.; et al. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 2013, 128, 2764–2775. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Arriola, C.G.; Badimon, L.; Crisostomo, V.; Giricz, Z.; Gyöngyösi, M.; Heusch, G.; Ibanez, B.; Kiss, A.; de Kleijn, D.P.V.; et al. The IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT): Multicenter pig study on the effect of ischemic preconditioning. Basic Res. Cardiol. 2024, 119, 893–909. [Google Scholar] [CrossRef]
- Sousonis, V.; Sfakianaki, T.; Ntalianis, A.; Nanas, I.; Kontogiannis, C.; Aravantinos, D.; Kapelios, C.; Katsaros, L.; Nana, M.; Sampaziotis, D.; et al. Intracoronary Administration of Allogeneic Cardiosphere-Derived Cells Immediately Prior to Reperfusion in Pigs with Acute Myocardial Infarction Reduces Infarct Size and Attenuates Adverse Cardiac Remodeling. J. Cardiovasc. Pharmacol. Ther. 2020, 26, 1074248420941672. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, V.; Baez-Diaz, C.; Blanco-Blazquez, V.; Alvarez, V.; Lopez-Nieto, E.; Maestre, J.; Bayes-Genis, A.; Galvez-Monton, C.; Casado, J.G.; Sanchez-Margallo, F.M. The epicardial delivery of cardiosphere derived cells or their extracellular vesicles is safe but of limited value in experimental infarction. Sci. Rep. 2021, 11, 22155. [Google Scholar] [CrossRef] [PubMed]
- Crisostomo, V.; Baez, C.; Abad, J.L.; Sanchez, B.; Alvarez, V.; Rosado, R.; Gomez-Mauricio, G.; Gheysens, O.; Blanco-Blazquez, V.; Blazquez, R.; et al. Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem Cell Res. Ther. 2019, 10, 152. [Google Scholar] [CrossRef]
- Gavira, J.J.; Nasarre, E.; Abizanda, G.; Pérez-Ilzarbe, M.; de Martino-Rodriguez, A.; García de Jalón, J.A.; Mazo, M.; Macias, A.; García-Bolao, I.; Pelacho, B.; et al. Repeated implantation of skeletal myoblast in a swine model of chronic myocardial infarction. Eur. Heart J. 2009, 31, 1013–1021. [Google Scholar] [CrossRef]
- Menasche, P.; Hagege, A.A.; Scorsin, M.; Pouzet, B.; Desnos, M.; Duboc, D.; Schwartz, K.; Vilquin, J.T.; Marolleau, J.P. Myoblast transplantation for heart failure. Lancet 2001, 357, 279–280. [Google Scholar] [CrossRef]
- Dede, M.; Putri, N.; Hari, H.; Muhammad Farhan Abdul, R. Safety and efficacy of stem cell therapy in acute myocardial infarction: A systematic review and meta-analysis of adverse events, infarct size and LV ejection fraction assessed by CMRI. Open Heart 2025, 12, e003301. [Google Scholar] [CrossRef]
- Grigorian Shamagian, L.; Madonna, R.; Taylor, D.; Climent, A.M.; Prosper, F.; Bras-Rosario, L.; Bayes-Genis, A.; Ferdinandy, P.; Fernández-Avilés, F.; Izpisua Belmonte, J.C.; et al. Perspectives on Directions and Priorities for Future Preclinical Studies in Regenerative Medicine. Circ. Res. 2019, 124, 938–951. [Google Scholar] [CrossRef]
- Macia, E.; Boyden, P.A. Stem Cell Therapy Is Proarrhythmic. Circulation 2009, 119, 1814–1823. [Google Scholar] [CrossRef]
- Báez-Díaz, C.; Torrescusa-Bermejo, A.; Sánchez-Margallo, F.M.; Vázquez-López, F.; Pulido, M.; López, E.; Arenal, Á.; Crisóstomo, V. Intracoronary-Cardiosphere-Derived Cell Secretome Therapy: Effects on Ventricular Tachycardia Inducibility and Cardiac Function in a Swine Model. Biomedicines 2025, 13, 1043. [Google Scholar] [CrossRef]
- Gu, X.; Xie, Y.; Gu, J.; Sun, L.; He, S.; Xu, R.; Duan, J.; Zhao, J.; Hang, F.; Xu, H.; et al. Repeated Intracoronary Infusion of Peripheral Blood Stem Cells With G-CSF in Patients with Refractory Ischemic Heart Failure; A Pilot Study. Circ. J. 2011, 75, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Huang, R.; Sun, A.; Qian, J.; Liu, X.; Ge, L.; Zhang, Y.; Zhang, S.; Niu, Y.; Wang, Q.; et al. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur. J. Heart Fail. 2009, 11, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A.C.P.; Møller, J.E.; Thayssen, P.; Junker, A.B.; Videbæk, L.; Sækmose, S.G.; Barington, T.; Kristiansen, M.; Kassem, M. Effect of repeated intracoronary injection of bone marrow cells in patients with ischaemic heart failure The Danish Stem Cell study—Congestive Heart Failure trial (DanCell-CHF). Eur. J. Heart Fail. 2008, 10, 661–667. [Google Scholar] [CrossRef]
- Mann, I.; Rodrigo, S.F.; van Ramshorst, J.; Beeres, S.L.; Dibbets-Schneider, P.; de Roos, A.; Wolterbeek, R.; Zwaginga, J.J.; Fibbe, W.E.; Bax, J.J.; et al. Repeated Intramyocardial Bone Marrow Cell Injection in Previously Responding Patients with Refractory Angina Again Improves Myocardial Perfusion, Anginal Complaints, and Quality of Life. Circ. Cardiovasc. Interv. 2015, 8, e002740. [Google Scholar] [CrossRef]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.V.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of Ejection Fraction on Cardiovascular Outcomes in a Broad Spectrum of Heart Failure Patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef]
- Dimmeler, S.; Burchfield, J.; Zeiher, A.M. Cell-Based Therapy of Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 208–216. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Eric Botker, H.; Dambrova, M.; et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J. Cell. Mol. Med. 2020, 24, 5937–5954, Erratum in J. Cell. Mol. Med. 2021, 25, 1325. [Google Scholar] [CrossRef]
- Chang, Z.; Li, H. KLF9 deficiency protects the heart from inflammatory injury triggered by myocardial infarction. Korean J. Physiol. Pharmacol. 2023, 27, 177–185. [Google Scholar] [CrossRef]
- Creemers, E.; Cleutjens, J.; Smits, J.; Heymans, S.; Moons, L.; Collen, D.; Daemen, M.; Carmeliet, P. Disruption of the Plasminogen Gene in Mice Abolishes Wound Healing after Myocardial Infarction. Am. J. Pathol. 2000, 156, 1865–1873. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Griffin, W.F.; Dries-Devlin, J.L.; Kent, S.D.; Chen, J.; Willis, M.S.; Virag, J.A.I. Ephrin–Eph signaling as a potential therapeutic target for the treatment of myocardial infarction. Med. Hypotheses 2013, 80, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, W.; Gao, X.; Chen, W.; Sun, C.; Li, J.; Gao, S.; Zhang, Y.; He, J.; Lu, D.; et al. EphA4 is highly expressed in the atria of heart and its deletion leads to atrial hypertrophy and electrocardiographic abnormalities in rats. Life Sci. 2021, 278, 119595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, L.; Chen, Q.; Zhang, F.; Wang, X.; Yao, F. Podocan unraveled: Understanding its role in tumor proliferation and smooth muscle regulation. Biomed. Pharmacother. 2024, 179, 117416. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, T.; Denise, M.; Joppich, M.; Fischer, M.; Garcia Rodriguez, C.; Kumaraswami, K.; Wimmler, V.; Ablinger, S.; Räuber, S.; Fang, J.; et al. Resident and recruited macrophages differentially contribute to cardiac healing after myocardial ischemia. eLife 2024, 12, RP89377. [Google Scholar] [CrossRef]
- Leinhos, L.; Robinson, P.; Poloni, G.; Broadway-Stringer, S.; Beglov, J.; Lokman, A.B.; Douglas, G.; Nuthay, S.; Fonseka, O.; Schmid, M.; et al. An ALPK3 truncation variant causing autosomal dominant hypertrophic cardiomyopathy is partially rescued by mavacamten. Sci. Rep. 2025, 15, 10090. [Google Scholar] [CrossRef]
- Agarwal, R.; Wakimoto, H.; Paulo, J.A.; Zhang, Q.; Reichart, D.; Toepfer, C.; Sharma, A.; Tai, A.C.; Lun, M.; Gorham, J.; et al. Pathogenesis of Cardiomyopathy Caused by Variants in ALPK3, an Essential Pseudokinase in the Cardiomyocyte Nucleus and Sarcomere. Circulation 2022, 146, 1674–1693. [Google Scholar] [CrossRef]
- Hitsumoto, T.; Tsukamoto, O.; Matsuoka, K.; Li, J.; Liu, L.; Kuramoto, Y.; Higo, S.; Ogawa, S.; Fujino, N.; Yoshida, S.; et al. Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin. Circulation 2023, 147, 1902–1918. [Google Scholar] [CrossRef]
- Li, J.; Kline, C.F.; Hund, T.J.; Anderson, M.E.; Mohler, P.J. Ankyrin-B Regulates Kir6.2 Membrane Expression and Function in Heart. J. Biol. Chem. 2010, 285, 28723–28730. [Google Scholar] [CrossRef]
- Koenig, S.N.; Mohler, P.J. The evolving role of ankyrin-B in cardiovascular disease. Heart Rhythm 2017, 14, 1884–1889. [Google Scholar] [CrossRef]
- Pham, C.; Muñoz-Martín, N.; Lodder, E.M. The Diverse Roles of TNNI3K in Cardiac Disease and Potential for Treatment. Int. J. Mol. Sci. 2021, 22, 6422. [Google Scholar] [CrossRef]
- Lal, H.; Ahmad, F.; Parikh, S.; Force, T. Troponin I-Interacting Protein Kinase– A Novel Cardiac-Specific Kinase, Emerging as a Molecular Target for the Treatment of Cardiac Disease. Circ. J. 2014, 78, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.-F.; Chen, Y.-Z.; Feng, L.-P.; Meng, X.-M.; Ding, J.-F.; Wang, L.-Y.; Ye, J.; Li, P.; Cheng, X.-S.; Kitamoto, Y.; et al. Overexpression of TNNI3K, a cardiac-specific MAP kinase, promotes P19CL6-derived cardiac myogenesis and prevents myocardial infarction-induced injury. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H708–H716. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, G.; Tanaka, A.; Nishihira, K.; Natsuaki, M.; Kawaguchi, A.; Watanabe, N.; Shibata, Y.; Node, K. Prognostic impact of follow-up serum albumin after acute myocardial infarction. ESC Heart Fail. 2021, 8, 5456–5465. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, Y.; Yu, D.; Zhang, D.; Hu, W. TRAP1 Provides Protection Against Myocardial Ischemia-Reperfusion Injury by Ameliorating Mitochondrial Dysfunction. Cell. Physiol. Biochem. 2015, 36, 2072–2082. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Zhang, C.; Li, J.; Guo, W.; Yan, D.; Yang, C.; Zhao, J.; Wu, X.; Shi, J. Phosphorylated eEF2 is SUMOylated and induces cardiomyocyte apoptosis during myocardial ischemia reperfusion. J. Cardiol. 2017, 69, 689–698. [Google Scholar] [CrossRef]
- Minor, M.; Alcedo, K.P.; Battaglia, R.A.; Snider, N.T. Cell type- and tissue-specific functions of ecto-5′-nucleotidase (CD73). Am. J. Physiol. Cell Physiol. 2019, 317, C1079–C1092. [Google Scholar] [CrossRef] [PubMed]
- Rajlic, S.; Surmann, L.; Zimmermann, P.; Weisheit, C.K.; Bindila, L.; Treede, H.; Velten, M.; Daiber, A.; Duerr, G.D. Fatty Acid Amide Hydrolase Deficiency Is Associated with Deleterious Cardiac Effects after Myocardial Ischemia and Reperfusion in Mice. Int. J. Mol. Sci. 2022, 23, 12690. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-H.; Chen, J.-H.; Tian, Z.; Quan, S.-B. Research and development prospects of TRIM65. J. Cancer Res. Clin. Oncol. 2025, 151, 232. [Google Scholar] [CrossRef]
- Álvarez, V.; Casado, J.G.; Blázquez, R.; Marinaro, F.; de Pedro, M.d.l.Á.; Pulido, M.; Nieto, E.L. Isolation, culture and, characterization of Cardiosphere-Derived Cells (CDCs). Protoc. Exch. 2022. [Google Scholar] [CrossRef]
- Galipeau, J.; Krampera, M.; Barrett, J.; Dazzi, F.; Deans, R.J.; DeBruijn, J.; Dominici, M.; Fibbe, W.E.; Gee, A.P.; Gimble, J.M.; et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016, 18, 151–159. [Google Scholar] [CrossRef]
- Binek, A.; Castans, C.; Jorge, I.; Bagwan, N.; Rodríguez, J.M.; Fernández-Jiménez, R.; Galán-Arriola, C.; Oliver, E.; Gómez, M.; Clemente-Moragón, A.; et al. Oxidative Post-translational Protein Modifications upon Ischemia/Reperfusion Injury. Antioxidants 2024, 13, 106. [Google Scholar] [CrossRef]
- Bonzon-Kulichenko, E.; Camafeita, E.; López, J.A.; Gómez-Serrano, M.; Jorge, I.; Calvo, E.; Núñez, E.; Trevisan-Herraz, M.; Bagwan, N.; Bárcena, J.A.; et al. Improved integrative analysis of the thiol redox proteome using filter-aided sample preparation. J. Proteom. 2020, 214, 103624. [Google Scholar] [CrossRef]
- Orsburn, B.C. Proteome Discoverer—A Community Enhanced Data Processing Suite for Protein Informatics. Proteomes 2021, 9, 15. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef]
- Wright, J.C.; Choudhary, J.S. DecoyPyrat: Fast Non-redundant Hybrid Decoy Sequence Generation for Large Scale Proteomics. J. Proteom. Bioinform. 2016, 9, 176–180. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical Statistical Model to Estimate the Accuracy of Peptide Identifications Made by MS/MS and Database Search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.; Vázquez, J. Protein Probability Model for High-Throughput Protein Identification by Mass Spectrometry-Based Proteomics. J. Proteome Res. 2020, 19, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Bonzon-Kulichenko, E.; Garcia-Marques, F.; Trevisan-Herraz, M.; Vázquez, J. Revisiting Peptide Identification by High-Accuracy Mass Spectrometry: Problems Associated with the Use of Narrow Mass Precursor Windows. J. Proteome Res. 2015, 14, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Trevisan-Herraz, M.; Bonzon-Kulichenko, E.; Núñez, E.; Martínez-Acedo, P.; Pérez-Hernández, D.; Jorge, I.; Mesa, R.; Calvo, E.; Carrascal, M.; et al. General Statistical Framework for Quantitative Proteomics by Stable Isotope Labeling. J. Proteome Res. 2014, 13, 1234–1247. [Google Scholar] [CrossRef]
- García-Marqués, F.; Trevisan-Herraz, M.; Martínez-Martínez, S.; Camafeita, E.; Jorge, I.; Lopez, J.A.; Méndez-Barbero, N.; Méndez-Ferrer, S.; del Pozo, M.A.; Ibáñez, B.; et al. A Novel Systems-Biology Algorithm for the Analysis of Coordinated Protein Responses Using Quantitative Proteomics. Mol. Cell. Proteom. 2016, 15, 1740–1760. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Jorge, I.; Martinez-Val, A.; Barrero-Rodríguez, R.; Magni, R.; Núñez, E.; Laguillo, A.; Devesa, C.A.; López, J.A.; Camafeita, E.; et al. iSanXoT: A standalone application for the integrative analysis of mass spectrometry-based quantitative proteomics data. Comput. Struct. Biotechnol. J. 2024, 23, 452–459. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
| CON (n = 8) | D1 (n = 8) | D3 (n = 8) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Dose | 2nd Dose | 3rd Dose | Final | ||||||||||
| PRE | POST | End-Study | PRE | POST | End-Study | PRE | POST | PRE | POST | PRE | POST | End-Study | |
| TnI (µg/L) | 0.42 (0.12) | 0.41 (0.01) | 0.01 (0.0) | 0.44 (0.11) | 0.43 (0.11) | 0.07 (0.07) | 0.19 (0.09) | 0.33 (0.15) | 0.05 (0.02) | 0.07 (0.02) | 0.03 (0.01) | 0.03 (0.01) | 0.02 (0.01) |
| CRP (mg/L) | 0.39 (0.12) | 0.44 (0.15) | 0.39 (0.13) | 0.93 (0.38) | 0.40 (0.20) | 0.55 (0.14) | 0.28 (0.10) | 0.26 (0.09) | 0.66 (0.15) | 0.44 (0.19) | 0.23 (0.12) | 0.14 (0.12) | 0.36 (0.15) |
| CON (n = 8) | D1 (n = 8) | D3 (n = 8) | p-Value (10 Weeks) | ||||
|---|---|---|---|---|---|---|---|
| 1 Week | 10 Weeks | 1 Week | 10 Weeks | 1 Week | 10 Weeks | ||
| IS (%) | 19.5 (2.5) | 10.3 (0.8) | 17.4 (1.3) | 10.1 (1.6) | 18.9 (2.4) | 12.7 (1.2) | 0.34 |
| LVEF (%) | 19.0 (1.9) | 24.2 (2.1) | 23 (2.0) | 21.4 (2.8) | 21.3 (1.1) | 27.5 (4.1) | 0.39 |
| LVEDVi (mL/m2) | 98.4 (5.6) | 104.4 (8.0) | 103 (5.3) | 107.3 (9.0) | 81.6 (5.6) | 81.6 (7.4) | 0.09 |
| LVESVi (mL/m2) | 79.4 (5.3) | 79.3 (7.0) | 79.5 (5.3) | 85.7 (10.1) | 64.1 (4.1) | 60.1 (7.9) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pedro, M.Á.; Báez-Díaz, C.; Jorge, I.; Vázquez-Lopez, F.; Torrescusa-Bermejo, A.; Martinez-Fernandez, B.; Pulido, M.; López, E.; Vázquez, J.; Sánchez-Margallo, F.M.; et al. Administration of Single or Repeated Doses of CDCs in a Swine Model of Reperfused Myocardial Infarction: Magnetic Resonance and Proteomics Evaluation. Int. J. Mol. Sci. 2025, 26, 11294. https://doi.org/10.3390/ijms262311294
de Pedro MÁ, Báez-Díaz C, Jorge I, Vázquez-Lopez F, Torrescusa-Bermejo A, Martinez-Fernandez B, Pulido M, López E, Vázquez J, Sánchez-Margallo FM, et al. Administration of Single or Repeated Doses of CDCs in a Swine Model of Reperfused Myocardial Infarction: Magnetic Resonance and Proteomics Evaluation. International Journal of Molecular Sciences. 2025; 26(23):11294. https://doi.org/10.3390/ijms262311294
Chicago/Turabian Stylede Pedro, María Ángeles, Claudia Báez-Díaz, Inmaculada Jorge, Fátima Vázquez-Lopez, Axiel Torrescusa-Bermejo, Beatriz Martinez-Fernandez, María Pulido, Esther López, Jesús Vázquez, Francisco M. Sánchez-Margallo, and et al. 2025. "Administration of Single or Repeated Doses of CDCs in a Swine Model of Reperfused Myocardial Infarction: Magnetic Resonance and Proteomics Evaluation" International Journal of Molecular Sciences 26, no. 23: 11294. https://doi.org/10.3390/ijms262311294
APA Stylede Pedro, M. Á., Báez-Díaz, C., Jorge, I., Vázquez-Lopez, F., Torrescusa-Bermejo, A., Martinez-Fernandez, B., Pulido, M., López, E., Vázquez, J., Sánchez-Margallo, F. M., & Crisostomo, V. (2025). Administration of Single or Repeated Doses of CDCs in a Swine Model of Reperfused Myocardial Infarction: Magnetic Resonance and Proteomics Evaluation. International Journal of Molecular Sciences, 26(23), 11294. https://doi.org/10.3390/ijms262311294









