A Novel Chimeric Molecule of Heparanase and Ig-Fc Enables Histochemical and Cytochemical Detection of O-sulfated Heparan Sulfate
Abstract
1. Introduction
2. Results
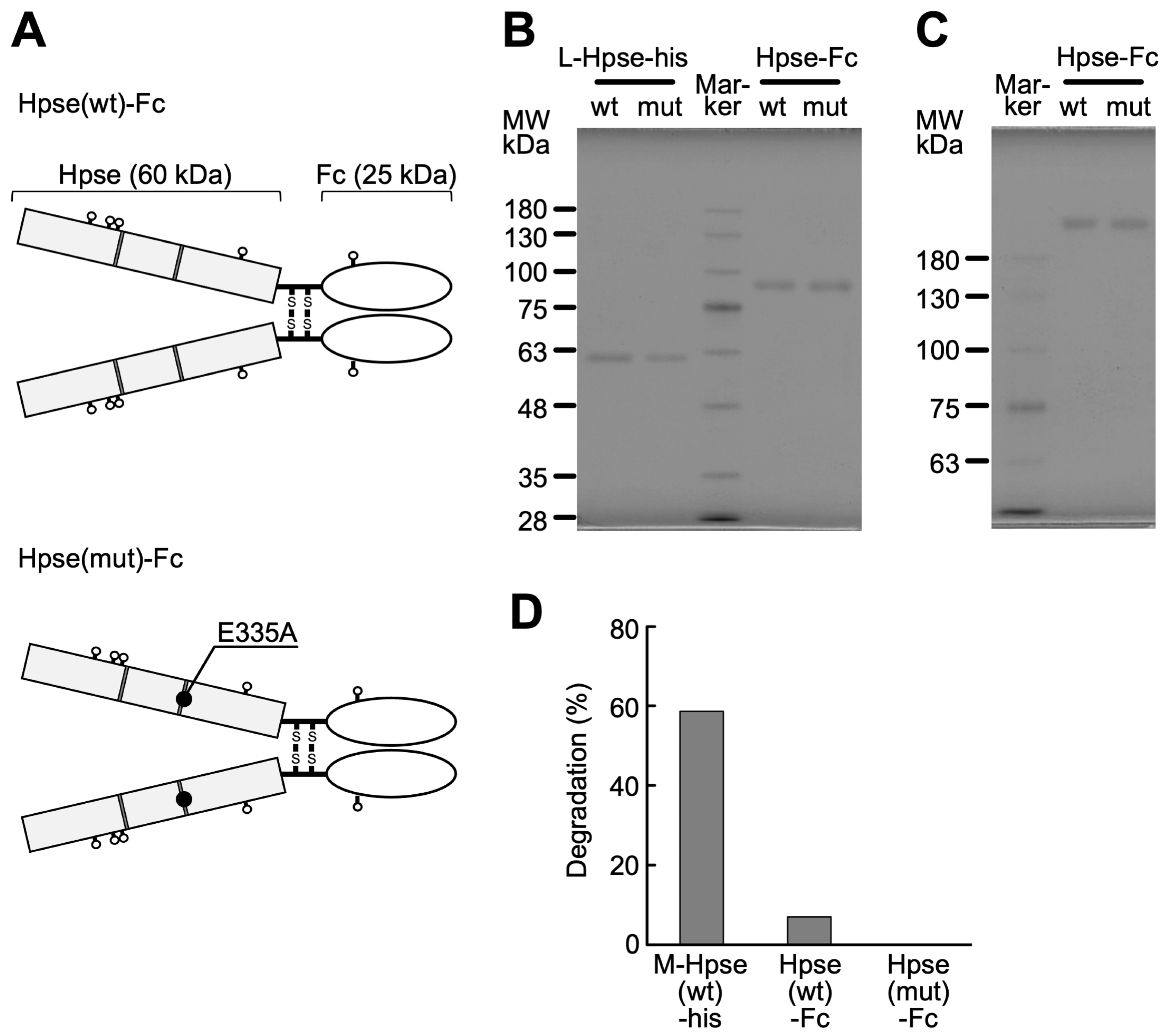
2.1. Biochemical Characterization of Purified Hpse-Fc Chimeric Proteins
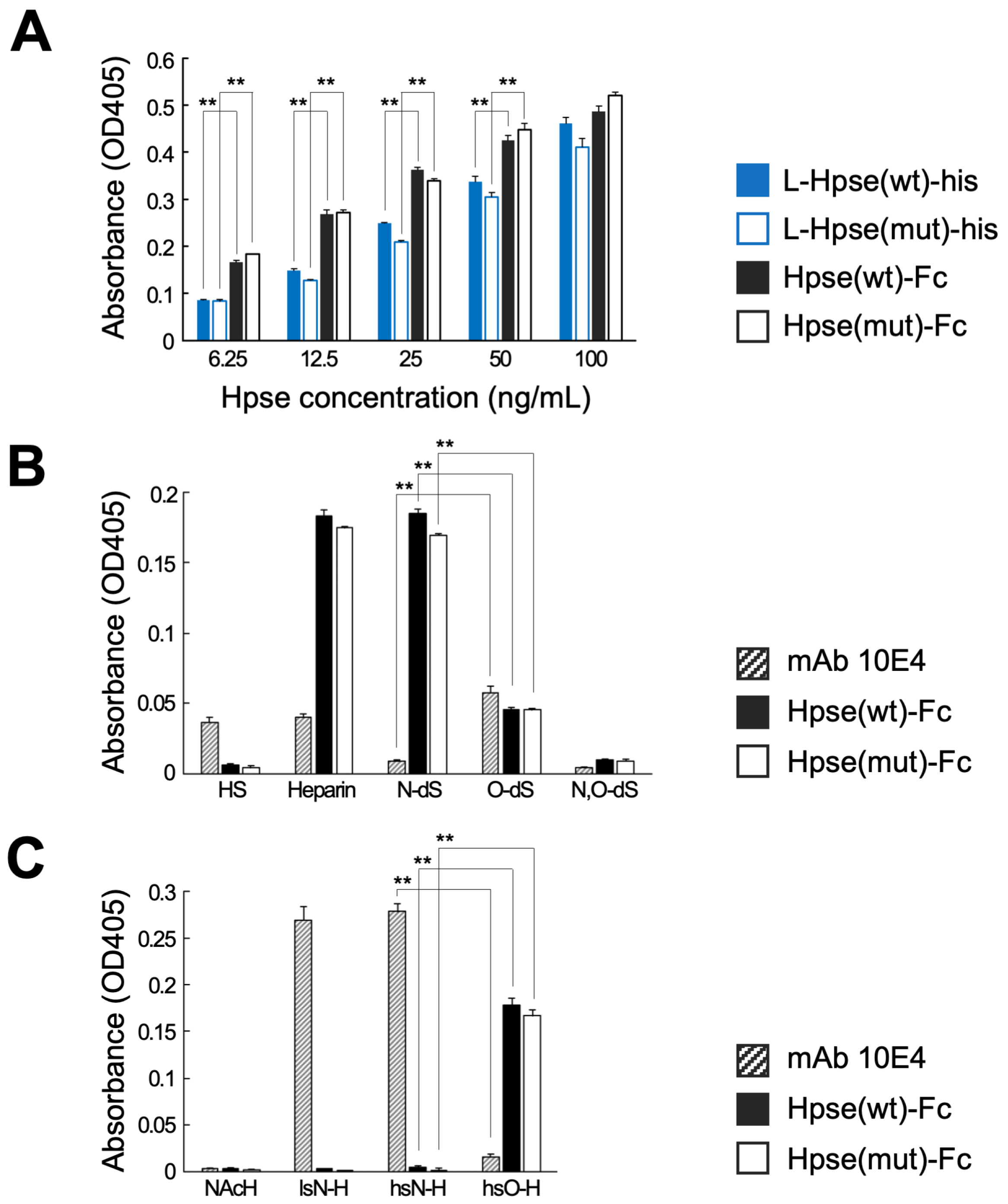
2.2. Hpse-Fc Chimeric Proteins Preferentially Bound to O-Sulfated GAGs
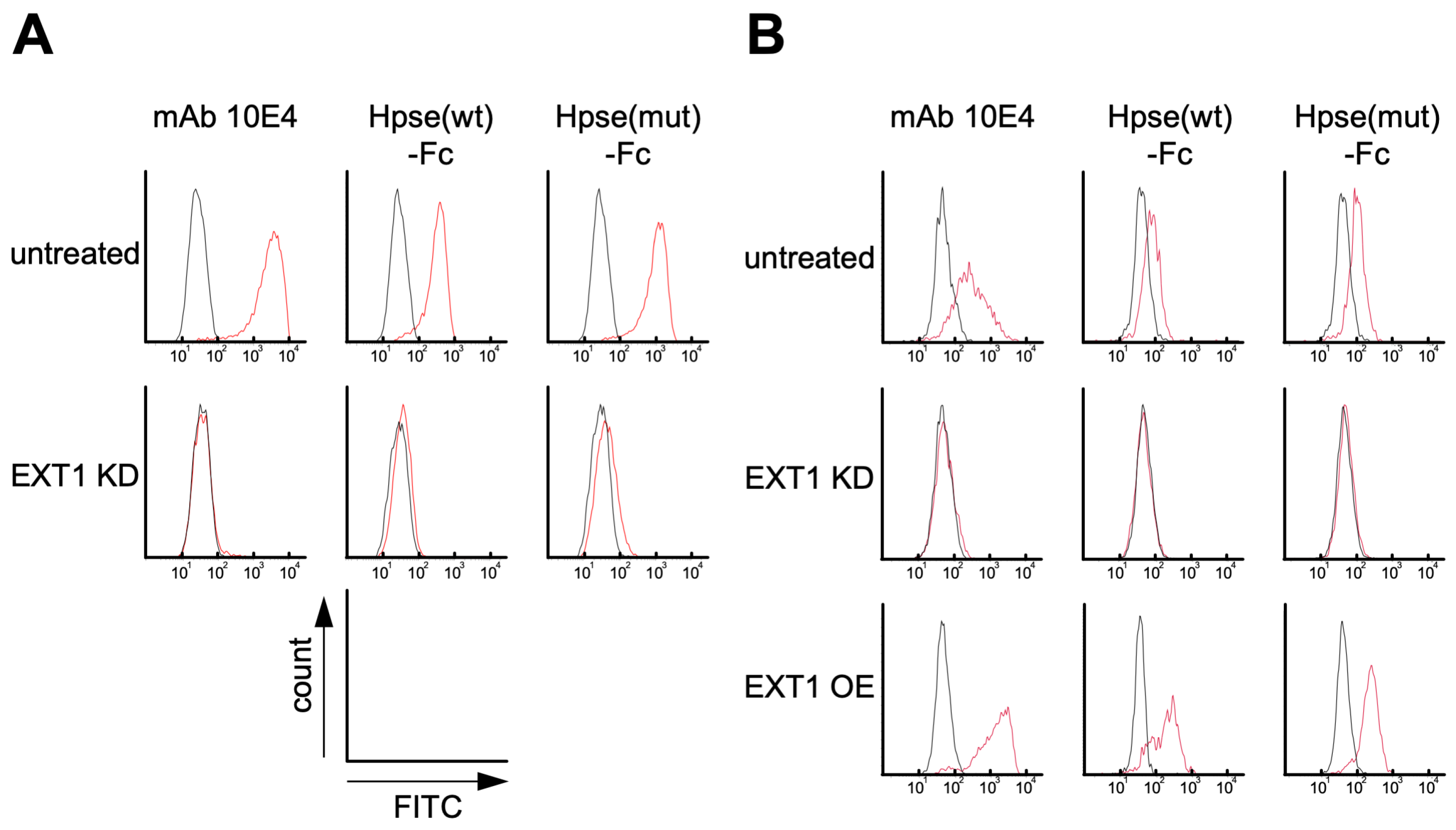
2.3. Hpse-Fc Chimeric Proteins Bound to Carcinoma Cell Lines via Cell Surface HS
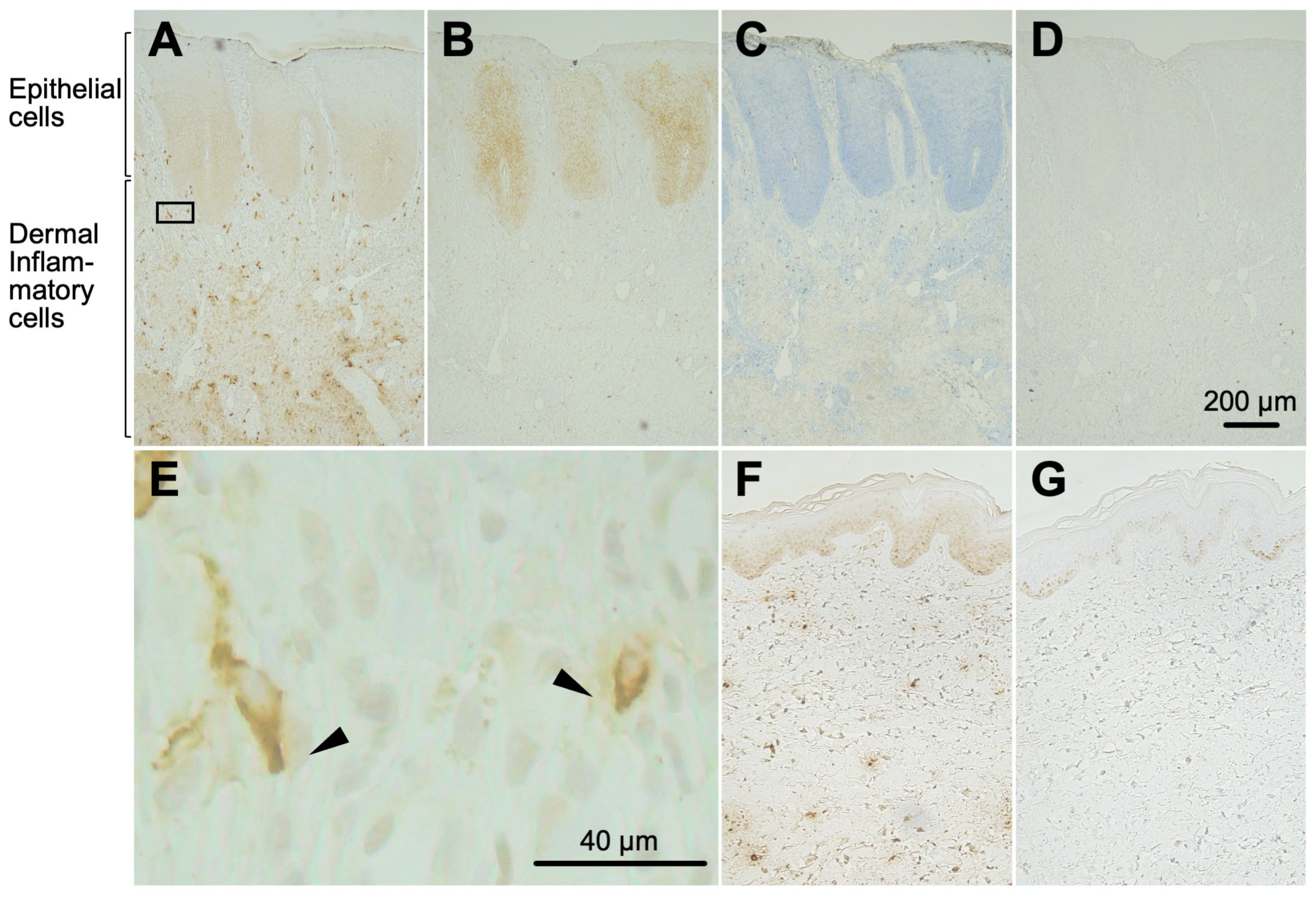
2.4. Hpse-Fc Chimeric Proteins Bound to Epidermal Cells and Dermal Inflammatory Cells in Atopic Dermatitis Skin
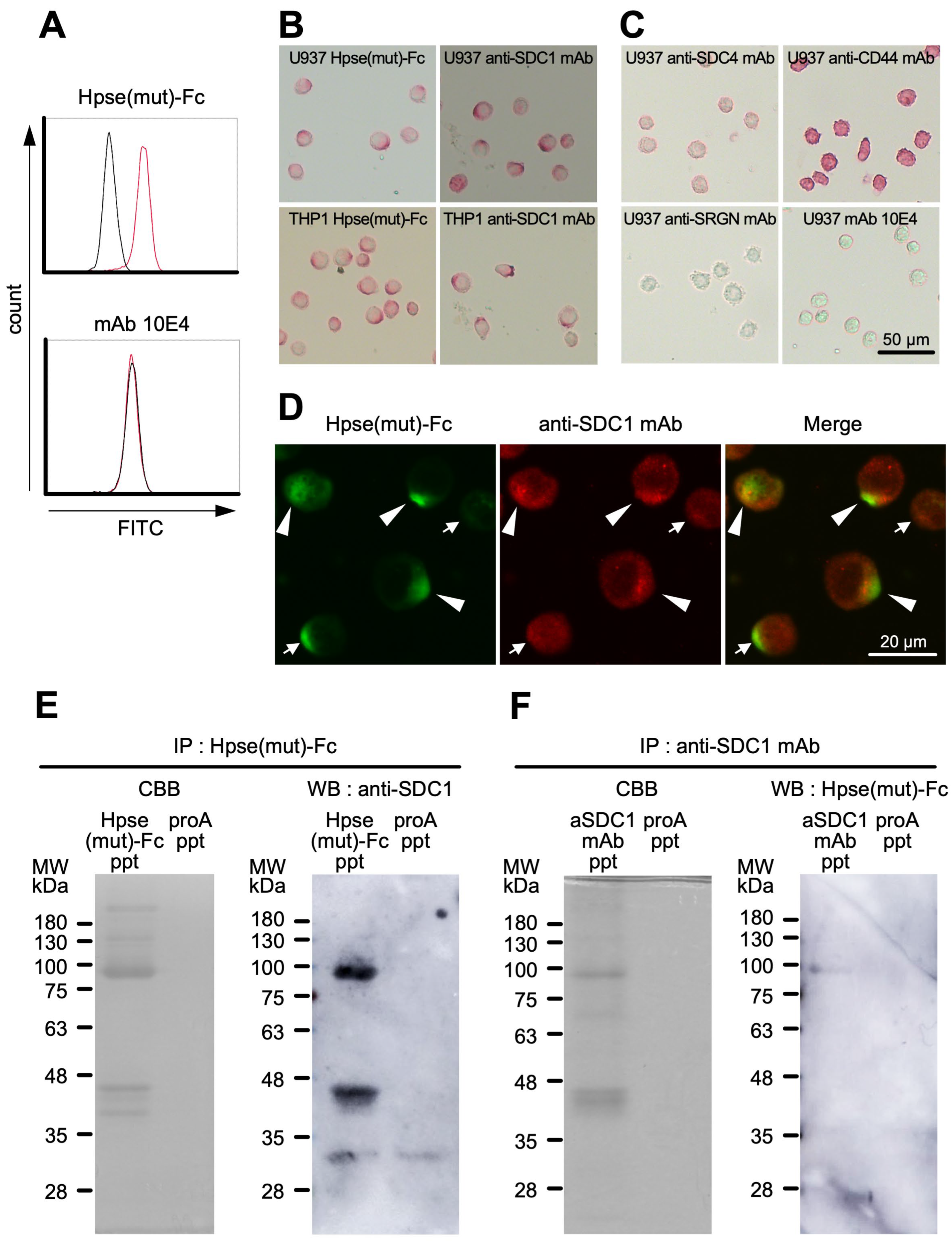
2.5. Hpse-Fc Chimeric Proteins Bound to Cell Surface Syndecan-1 in Monocytic U937 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Cells
4.2. Design and Preparation of Chimeric Proteins
4.3. Purification of Chimeric Proteins
4.4. Heparan Sulfate Degradation Activity
4.5. Binding Experiments to Immobilized GAGs
4.6. Detection of Cell Surface Antigens Using Flow Cytometry
4.7. Histochemical Detection of Hpse(mut)-Fc Binding Sites in Human Skin
4.8. Cytochemical Detection of Hpse(mut)-Fc Binding Molecules in Monocytic U937 and THP1 Cells
4.9. Precipitation of Hpse(mut)-Fc Binding Molecules from Monocytic U937 Cells
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBB | Coomassie Brilliant Blue |
| CS | chondroitin sulfate |
| ELISA | enzyme-linked immunosorbent assay |
| GAG | glycosaminoglycan |
| HA | hyaluronic acid |
| HBD | heparin binding domain |
| Hpse | heparanase |
| L-Hpse | recombinant mouse Hpse protein mimicking latent-form Hpse |
| M-Hpse | recombinant mouse Hpse protein mimicking mature-form Hpse |
| Hpse-Fc | chimeric molecule of mouse heparanase and Fc region of mouse IgG1 |
| HRP | horseradish peroxidase |
| HS | heparan sulfate |
| mAb | monoclonal antibody |
| MFI | median fluorescence intensity |
| mut | mutated (with mutation at E335A) |
| PMA | phorbol myristate acetate |
| SDC | syndecan |
| wt | wild type |
References
- Habuchi, H.; Habuchi, O.; Kimata, K. Sulfation pattern in glycosaminoglycan: Does it have a code? Glycoconj. J. 2004, 21, 47–52. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef] [PubMed]
- Petitou, M.; Casu, B.; Lindahl, U. heparin sequence, FGF sequence. 1976–1983, a critical period in the history of heparin: The discovery of the antithrombin binding site. Biochimie 2003, 85, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Maccarana, M.; Casu, B.; Lindahl, U. Minimal sequence in heparin/heparan sulfate required for binding of basic fibroblast growth factor. J. Biol. Chem. 1993, 268, 23898–23905. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shriver, Z.; Pope, R.M.; Thorp, S.C.; Duncan, M.B.; Copeland, R.J.; Raska, C.S.; Yoshida, K.; Eisenberg, R.J.; Cohen, G.; et al. Characterization of a heparan sulfate octasaccharide that binds to herpes simplex virus type 1 glycoprotein D. J. Biol. Chem. 2002, 277, 33456–33467. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Bai, X.M.; Van der Schueren, B.; Cassiman, J.J.; Van den Berghe, H. Developmental changes in heparan sulfate expression: In situ detection with mAbs. J. Cell Biol. 1992, 119, 961–975. [Google Scholar] [CrossRef]
- van den Born, J.; van den Heuvel, L.P.; Bakker, M.A.; Veerkamp, J.H.; Assmann, K.J.; Berden, J.H. Monoclonal antibodies against the protein core and glycosaminoglycan side chain of glomerular basement membrane heparan sulfate proteoglycan: Characterization and immunohistological application in human tissues. J. Histochem. Cytochem. 1994, 42, 89–102. [Google Scholar] [CrossRef]
- Huhle, G.; Harenberg, J.; Malsch, R.; Heene, D.L. Monoclonal antibodies against heparin and heparinoids. Semin. Thromb. Hemost. 1997, 23, 17–22. [Google Scholar] [CrossRef]
- van den Born, J.; van den Heuvel, L.P.; Bakker, M.A.; Veerkamp, J.H.; Assmann, K.J.; Berden, J.H. Production and characterization of a monoclonal antibody against human glomerular heparan sulfate. Lab. Investig. 1991, 65, 287–297. [Google Scholar]
- van den Born, J.; van den Heuvel, L.P.; Bakker, M.A.; Veerkamp, J.H.; Assmann, K.J.; Berden, J.H. A monoclonal antibody against GBM heparan sulfate induces an acute selective proteinuria in rats. Kidney Int. 1992, 41, 115–123. [Google Scholar] [CrossRef]
- ten Dam, G.B.; Kurup, S.; van de Westerlo, E.M.A.; Versteeg, E.M.M.; Lindahl, U.; Spillmann, D.; van Kuppevelt, T.H. 3-O-Sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J. Biol. Chem. 2006, 281, 4654–4662. [Google Scholar] [CrossRef]
- Watanabe, I.; Hikita, T.; Mizuno, H.; Sekita, R.; Minami, A.; Ishii, A.; Minamisawa, Y.; Suzuki, K.; Maeda, H.; Hidari, K.I.; et al. Isolation and characterization of monoclonal antibodies specific for chondroitin sulfate E. Glycobiology 2015, 25, 953–962. [Google Scholar] [CrossRef]
- Wittrup, A.; Zhang, S.H.; ten Dam, G.B.; van Kuppevelt, T.H.; Bengtson, P.; Johansson, M.; Welch, J.; Mörgelin, M.; Belting, M. ScFv antibody-induced translocation of cell-surface heparan sulfate proteoglycan to endocytic vesicles: Evidence for heparan sulfate epitope specificity and role of both syndecan and glypican. J. Biol. Chem. 2009, 284, 32959–32967. [Google Scholar] [CrossRef]
- van den Born, J.; Gunnarsson, K.; Bakker, M.A.; Kjellén, L.; Kusche-Gullberg, M.; Maccarana, M.; Berden, J.H.; Lindahl, U. Presence of N-unsubstituted glucosamine units in native heparan sulfate revealed by a monoclonal antibody. J. Biol. Chem. 1995, 270, 31303–31309. [Google Scholar] [CrossRef] [PubMed]
- Dennissen, M.A.; Jenniskens, G.J.; Pieffers, M.; Versteeg, E.M.; Petitou, M.; Veerkamp, J.H.; van Kuppevelt, T.H. Large, tissue-regulated domain diversity of heparan sulfates demonstrated by phage display antibodies. J. Biol. Chem. 2002, 277, 10982–10986. [Google Scholar] [CrossRef] [PubMed]
- Leteux, C.; Chai, W.; Nagai, K.; Herbert, C.G.; Lawson, A.M.; Feizi, T. 10E4 antigen of Scrapie lesions contains an unusual nonsulfated heparan motif. J. Biol. Chem. 2001, 276, 12539–12545. [Google Scholar] [CrossRef] [PubMed]
- Mani, K.; Cheng, F.; Sandgren, S.; van den Born, J.; Havsmark, B.; Ding, K.; Fransson, L.A. The heparan sulfate-specific epitope 10E4 is NO-sensitive and partly inaccessible in glypican-1. Glycobiology 2004, 14, 599–607. [Google Scholar] [CrossRef] [PubMed]
- van den Born, J.; Salmivirta, K.; Henttinen, T.; Ostman, N.; Ishimaru, T.; Miyaura, S.; Yoshida, K.; Salmivirta, M. Novel heparan sulfate structures revealed by monoclonal antibodies. J. Biol. Chem. 2005, 280, 20516–20523. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.; Chang, Z.; Tierney, A.; Rapraeger, A.C. Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparan sulfate: Comparison of normal and abnormal human tissues. Am. J. Pathol. 1997, 150, 1443–1455. [Google Scholar]
- Murakami, K.; Tamura, R.; Ikehara, S.; Ota, H.; Ichimiya, T.; Matsumoto, N.; Matsubara, H.; Nishihara, S.; Ikehara, Y.; Yamamoto, K. Construction of mouse cochlin mutants with different GAG-binding specificities and their use for immunohistochemistry. Biochem. J. 2023, 480, 41–56. [Google Scholar] [CrossRef]
- Brunetti, J.; Depau, L.; Falciani, C.; Gentile, M.; Mandarini, E.; Riolo, G.; Lupetti, P.; Pini, A.; Bracci, L. Insights into the role of sulfated glycans in cancer cell adhesion and migration through use of branched peptide probe. Sci. Rep. 2016, 6, 27174. [Google Scholar] [CrossRef]
- Bray, B.A.; Hsu, W.; Turino, G.M. Lung hyaluronan as assayed with a biotinylated hyaluronan-binding protein. Exp. Lung Res. 1994, 20, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Irimura, T.; Di Ferrante, N.; Nicolson, G.L. Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J. Biol. Chem. 1984, 259, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Irimura, T.; Nicolson, G.L. Heparanases and tumor metastasis. J. Cell. Biochem. 1988, 36, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Nakajima, M. Human heparanase. Purification, characterization, cloning, and expression. J. Biol. Chem. 1999, 274, 24153–24160. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Beckhove, P.; Lerner, I.; Pisano, C.; Meirovitz, A.; Ilan, N.; Elkin, M. Significance of heparanase in cancer and inflammation. Cancer Microenviron. 2012, 5, 115–132. [Google Scholar] [CrossRef]
- Miao, H.Q.; Navarro, E.; Patel, S.; Sargent, D.; Koo, H.; Wan, H.; Plata, A.; Zhou, Q.; Ludwig, D.; Bohlen, P.; et al. Cloning, expression, and purification of mouse heparanase. Protein Expr. Purif. 2002, 26, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hulett, M.D.; Hornby, J.R.; Ohms, S.J.; Zuegg, J.; Freeman, C.; Gready, J.E.; Parish, C.R. Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry 2000, 39, 15659–15667. [Google Scholar] [CrossRef] [PubMed]
- Elli, S.; Guerrini, M. Molecular Aspects of Heparanase Interaction with Heparan Sulfate, Heparin and Glycol Split Heparin. Adv. Exp. Med. Biol. 2020, 1221, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Pikas, D.S.; Li, J.P.; Vlodavsky, I.; Lindahl, U. Substrate specificity of heparanases from human hepatoma and platelets. J. Biol. Chem. 1998, 273, 18770–18777. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Yamada, S.; Toyoshima, M.; Dong, J.; Nakajima, M.; Sugahara, K. Structural recognition by recombinant human heparanase that plays critical roles in tumor metastasis. Hierarchical sulfate groups with different effects and the essential target disulfated trisaccharide sequence. J. Biol. Chem. 2002, 277, 42488–42495. [Google Scholar] [CrossRef]
- Peterson, S.; Liu, J. Deciphering mode of action of heparanase using structurally defined oligosaccharides. J. Biol. Chem. 2012, 287, 34836–34843. [Google Scholar] [CrossRef]
- Bame, K.J.; Venkatesan, I.; Stelling, H.D.; Tumova, S. The spacing of S-domains on HS glycosaminoglycans determines whether the chain is a substrate for intracellular heparanases. Glycobiology 2000, 10, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Huang, Y.; Buczek-Thomas, J.A.; Ethen, C.M.; Nugent, M.A.; Wu, Z.L.; Zaia, J. A liquid chromatography-mass spectrometry-based approach to characterize the substrate specificity of mammalian heparanase. J. Biol. Chem. 2014, 289, 34141–34151. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Jemth, P.; Escobar Galvis, M.L.; Vlodavsky, I.; Horner, A.; Lindahl, U.; Li, J.P. Processing of macromolecular heparin by heparanase. J. Biol. Chem. 2003, 278, 35152–35158. [Google Scholar] [CrossRef]
- Nasser, N.J.; Sarig, G.; Brenner, B.; Nevo, E.; Goldshmidt, O.; Zcharia, E.; Li, J.P.; Vlodavsky, I. Heparanase neutralizes the anticoagulation properties of heparin and low-molecular-weight heparin. J. Thromb. Haemost. 2006, 4, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Levy-Adam, F.; Abboud-Jarrous, G.; Guerrini, M.; Beccati, D.; Vlodavsky, I.; Ilan, N. Identification and characterization of heparin/heparan sulfate binding domains of the endoglycosidase heparanase. J. Biol. Chem. 2005, 280, 20457–20466. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Ilan, N.; Nadir, Y.; Brenner, B.; Katz, B.Z.; Naggi, A.; Torri, G.; Casu, B.; Sasisekharan, R. Heparanase, heparin and the coagulation system in cancer progression. Thromb. Res. 2007, 120 (Suppl. S2), S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Freeman, C.; Parish, C.R.; Mancera, R.L. Computational analyses of the catalytic and heparin-binding sites and their interactions with glycosaminoglycans in glycoside hydrolase family 79 endo-beta-D-glucuronidase (heparanase). Glycobiology 2012, 22, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Viola, C.M.; Brzozowski, A.M.; Davies, G.J. Structural characterization of human heparanase reveals insights into substrate recognition. Nat. Struct. Mol. Biol. 2015, 22, 1016–1022, Erratum in Nat. Struct. Mol. Biol. 2016, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Gingis-Velitski, S.; Zetser, A.; Kaplan, V.; Ben-Zaken, O.; Cohen, E.; Levy-Adam, F.; Bashenko, Y.; Flugelman, M.Y.; Vlodavsky, I.; Ilan, N. Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J. Biol. Chem. 2004, 279, 44084–44092. [Google Scholar] [CrossRef]
- Ben-Zaken, O.; Shafat, I.; Gingis-Velitski, S.; Bangio, H.; Kelson, I.K.; Alergand, T.; Amor, Y.; Maya, R.B.; Vlodavsky, I.; Ilan, N. Low and high affinity receptors mediate cellular uptake of heparanase. Int. J. Biochem. Cell Biol. 2008, 40, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, N.; Higashi, N.; Kogane, Y.; Waki, M.; Shida, H.; Nishimura, Y.; Adachi, H.; Nakajima, M.; Irimura, T. Heparanase augments inflammatory chemokine production from colorectal carcinoma cell lines. Biochem. Biophys. Res. Commun. 2016, 469, 878–883. [Google Scholar] [CrossRef]
- Higashi, N.; Waki, M.; Sudo, Y.; Suzuki, S.; Oku, T.; Tsuiji, M.; Tsuji, T.; Miyagishi, M.; Takahashi, K.; Nakajima, M.; et al. Incorporation, intracellular trafficking and processing of extracellular heparanase by mast cells: Involvement of syndecan-4-dependent pathway. Biochem. Biophys. Res. Commun. 2018, 503, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Onuki, Y.; Kawanami, F.; Miyagawa, N.; Iwasaki, F.; Tsuda, H.; Takahashi, K.; Oku, T.; Suzuki, M.; Higashi, K.; et al. The Uptake of Heparanase into Mast Cells Is Regulated by Its Enzymatic Activity to Degrade Heparan Sulfate. Int. J. Mol. Sci. 2024, 25, 6281. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Higashi, N.; Taka, T.; Nakajima, M.; Irimura, T. Cell surface localization of heparanase on macrophages regulates degradation of extracellular matrix heparan sulfate. J. Immunol. 2004, 172, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Philo, J.S.; Tsumoto, K.; Yumioka, R.; Ejima, D. Elution of antibodies from a Protein-A column by aqueous arginine solutions. Protein Expr. Purif. 2004, 36, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Do, A.T.; Lozynska, O.; Kusche-Gullberg, M.; Lindahl, U.; Emerson, C.P., Jr. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 2003, 162, 341–351. [Google Scholar] [CrossRef]
- Safaiyan, F.; Kolset, S.O.; Prydz, K.; Gottfridsson, E.; Lindahl, U.; Salmivirta, M. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J. Biol. Chem. 1999, 274, 36267–36273. [Google Scholar] [CrossRef]
- Higashi, N.; Maeda, R.; Sesoko, N.; Isono, M.; Ishikawa, S.; Tani, Y.; Takahashi, K.; Oku, T.; Higashi, K.; Onishi, S.; et al. Chondroitin sulfate E blocks enzymatic action of heparanase and heparanase-induced cellular responses. Biochem. Biophys. Res. Commun. 2019, 520, 152–158. [Google Scholar] [CrossRef]
- Shi, J.; Kanoya, R.; Tani, Y.; Ishikawa, S.; Maeda, R.; Suzuki, S.; Kawanami, F.; Miyagawa, N.; Takahashi, K.; Oku, T.; et al. Sulfated Hyaluronan Binds to Heparanase and Blocks Its Enzymatic and Cellular Actions in Carcinoma Cells. Int. J. Mol. Sci. 2022, 23, 5055. [Google Scholar] [CrossRef] [PubMed]
- Staples, G.O.; Shi, X.; Zaia, J. Extended N-sulfated domains reside at the nonreducing end of heparan sulfate chains. J. Biol. Chem. 2010, 285, 18336–18343. [Google Scholar] [CrossRef]
- Nakao, M.; Sugaya, M.; Takahashi, N.; Otobe, S.; Nakajima, R.; Oka, T.; Kabasawa, M.; Suga, H.; Morimura, S.; Miyagaki, T.; et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch. Dermatol. Res. 2016, 308, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Priestley, M.J.; Hains, A.K.; Mulholland, I.Z.; Spijkers-Shaw, S.; Müller, J.C.; Howell, G.; Ridley, A.J.L.; Davies-Strickleton, H.; Miller, R.L.; Nobis, M.; et al. Leukocytes have a heparan sulfate glycocalyx that regulates recruitment during psoriasis-like skin inflammation. Sci. Signal. 2025, 18, eadr0011. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Macleod, V.; Miao, H.Q.; Theus, A.; Zhan, F.; Shaughnessy, J.D., Jr.; Sawyer, J.; Li, J.P.; Zcharia, E.; Vlodavsky, I.; et al. Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. J. Biol. Chem. 2007, 282, 13326–13333. [Google Scholar] [CrossRef]
- Irimura, T.; Nakajima, M.; Nicolson, G.L. Chemically modified heparins as inhibitors of heparan sulfate specific endo-beta-glucuronidase (heparanase) of metastatic melanoma cells. Biochemistry 1986, 25, 5322–5328. [Google Scholar] [CrossRef]
- Lind, T.; Tufaro, F.; McCormick, C.; Lindahl, U.; Lidholt, K. The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J. Biol. Chem. 1998, 273, 26265–26268. [Google Scholar] [CrossRef]
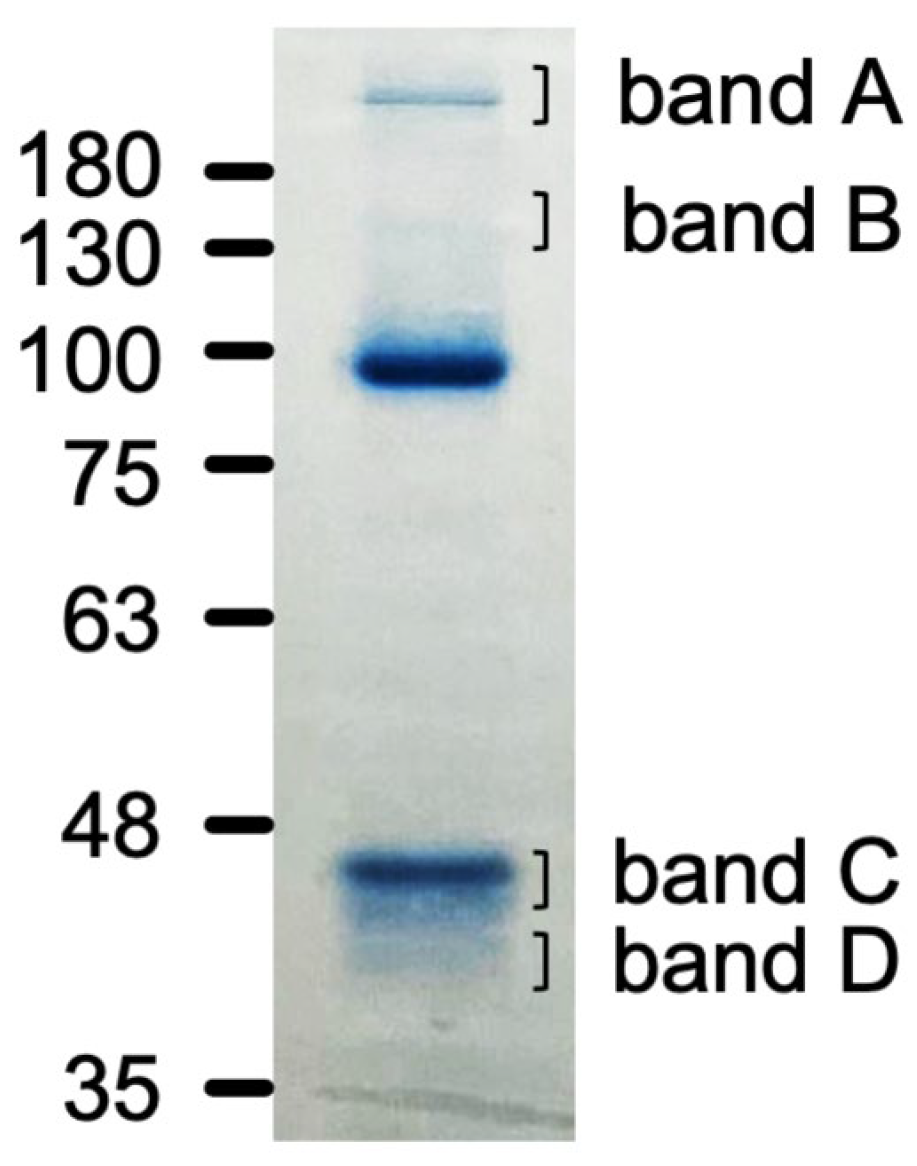
| (a) | |
|---|---|
| Proteins | Total Spectrum Count |
| Spectrin beta chain, non-erythrocytic 1 | 2574 |
| Spectrin alpha chain, non-erythrocytic 1 | 1971 |
| Alpha-actinin-4 | 739 |
| Filamin A | 376 |
| Myosin-9 | 356 |
| Ras GTPase-activating-like protein IQGAP1 | 149 |
| (b) | |
| Proteins | Total Spectrum Count |
| Alpha-actinin-4 | 1494 |
| Spectrin alpha chain, non-erythrocytic 1 | 1254 |
| Spectrin beta chain, non-erythrocytic 1 | 302 |
| Actin, cytoplasmic 1 | 135 |
| Ras GTPase-activating-like protein IQGAP1 | 61 |
| (c) | |
| Proteins | Total Spectrum Count |
| Actin, cytoplasmic 1 | 1745 |
| Alpha-actinin-4 | 95 |
| Eukaryotic initiation factor 4A-III | 29 |
| Tubulin alpha-1B chain | 27 |
| Eukaryotic initiation factor 4A-I | 19 |
| (d) | |
| Proteins | Total Spectrum Count |
| Actin, cytoplasmic 1 | 831 |
| Alpha-actinin-4 | 74 |
| Lymphocyte-specific protein 1 | 19 |
| Tubulin beta chain | 12 |
| Twinfilin-2 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Nakamura, M.; Baba, R.; Arakawa, S.; Yamaguchi, A.; Hariya, T.; Suzuki, R.; Inazuki, Y.; Takahashi, K.; Tsuiji, M.; et al. A Novel Chimeric Molecule of Heparanase and Ig-Fc Enables Histochemical and Cytochemical Detection of O-sulfated Heparan Sulfate. Int. J. Mol. Sci. 2025, 26, 11293. https://doi.org/10.3390/ijms262311293
Shi J, Nakamura M, Baba R, Arakawa S, Yamaguchi A, Hariya T, Suzuki R, Inazuki Y, Takahashi K, Tsuiji M, et al. A Novel Chimeric Molecule of Heparanase and Ig-Fc Enables Histochemical and Cytochemical Detection of O-sulfated Heparan Sulfate. International Journal of Molecular Sciences. 2025; 26(23):11293. https://doi.org/10.3390/ijms262311293
Chicago/Turabian StyleShi, Jia, Momoko Nakamura, Ryoya Baba, Sojiro Arakawa, Arisa Yamaguchi, Tomonori Hariya, Rin Suzuki, Yu Inazuki, Katsuhiko Takahashi, Makoto Tsuiji, and et al. 2025. "A Novel Chimeric Molecule of Heparanase and Ig-Fc Enables Histochemical and Cytochemical Detection of O-sulfated Heparan Sulfate" International Journal of Molecular Sciences 26, no. 23: 11293. https://doi.org/10.3390/ijms262311293
APA StyleShi, J., Nakamura, M., Baba, R., Arakawa, S., Yamaguchi, A., Hariya, T., Suzuki, R., Inazuki, Y., Takahashi, K., Tsuiji, M., Oku, T., Komine, M., Shimekake, M., Higashi, K., Nakamura, M., Sasaki, K., Nakajima, M., Irimura, T., & Higashi, N. (2025). A Novel Chimeric Molecule of Heparanase and Ig-Fc Enables Histochemical and Cytochemical Detection of O-sulfated Heparan Sulfate. International Journal of Molecular Sciences, 26(23), 11293. https://doi.org/10.3390/ijms262311293





