Redox on the Clock: Sex-Dependent Dynamics of Xanthine Oxidoreductase Isoforms and Melatonin
Abstract
1. Introduction
- Characterize circadian variations in xanthine oxidoreductase isoforms and their metabolic products.
- Evaluate relationships between melatonin concentration, oxidative stress markers, and the balance of XOR isoforms.
2. Results
2.1. Analysis of Biochemical, Morphological, and Mineral Parameters
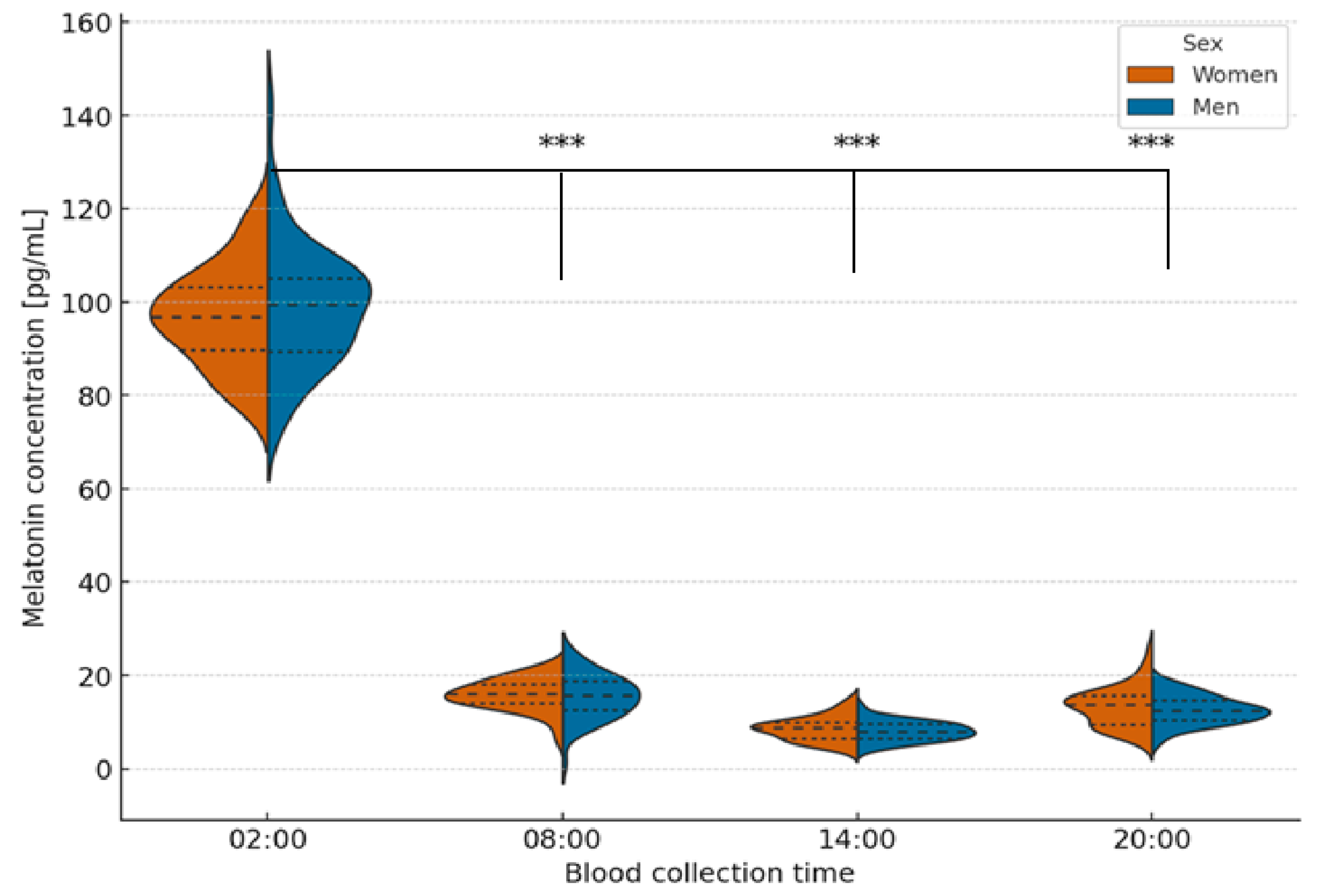
2.2. Analysis of Melatonin Concentration at Different Times of the Day
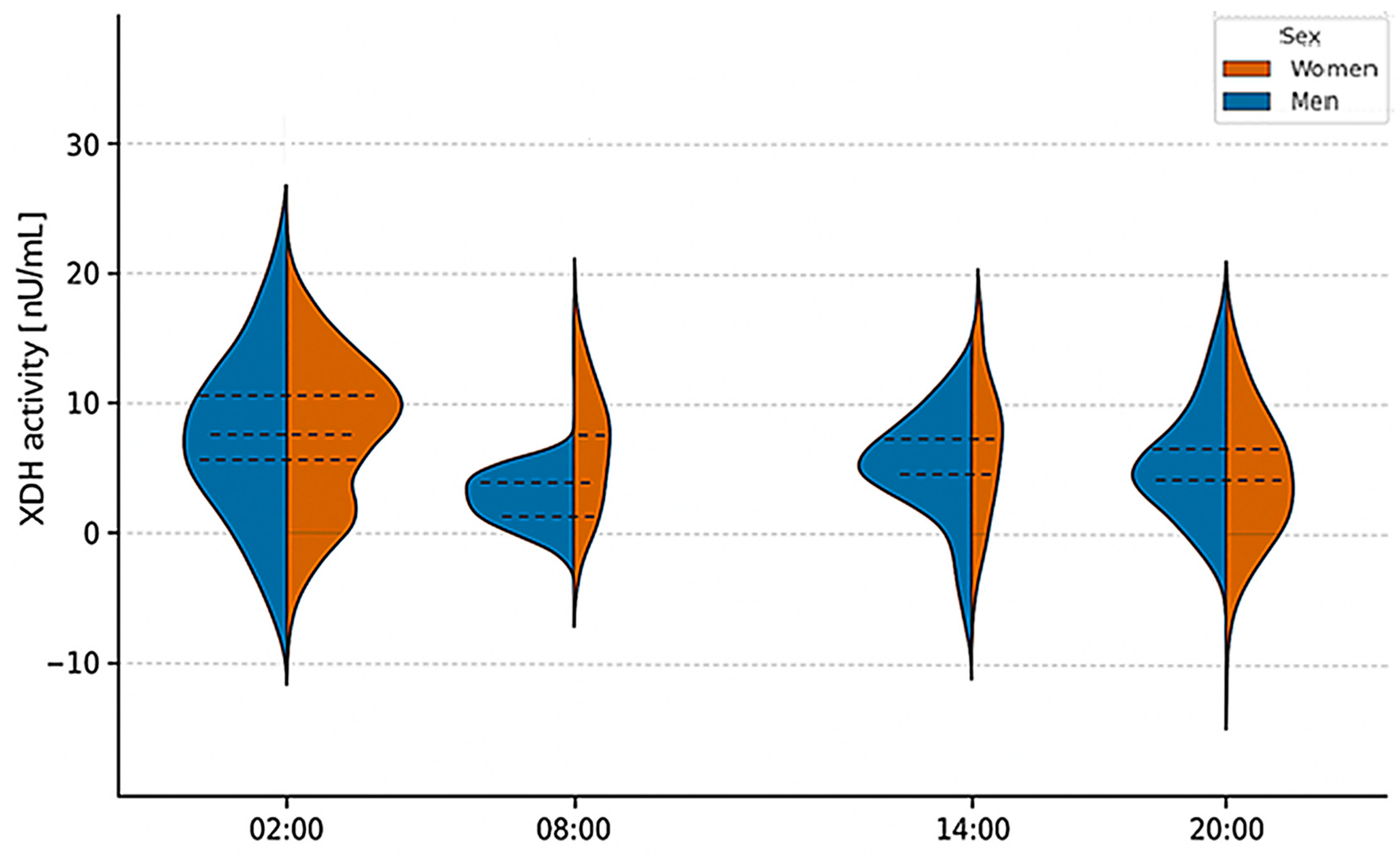
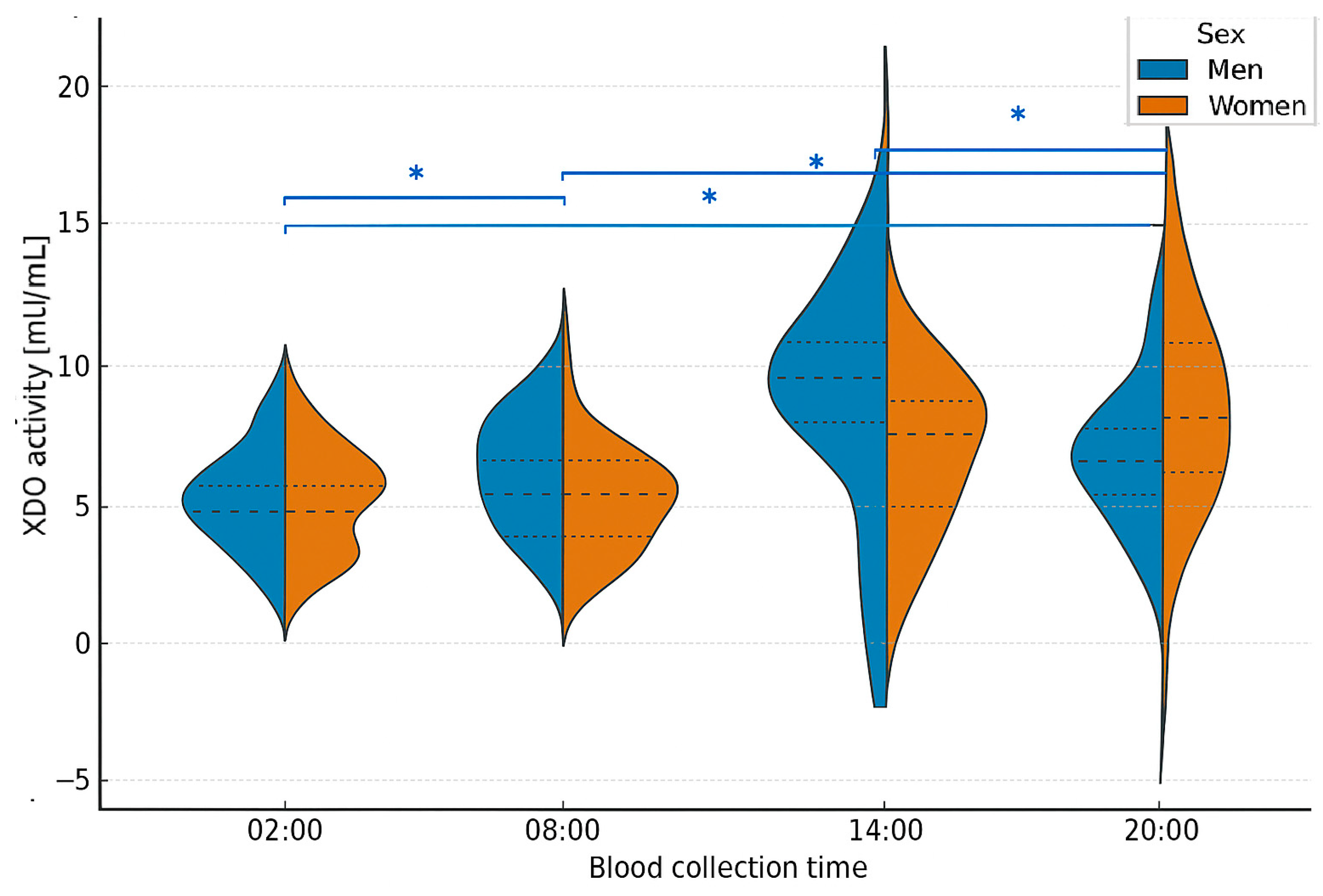
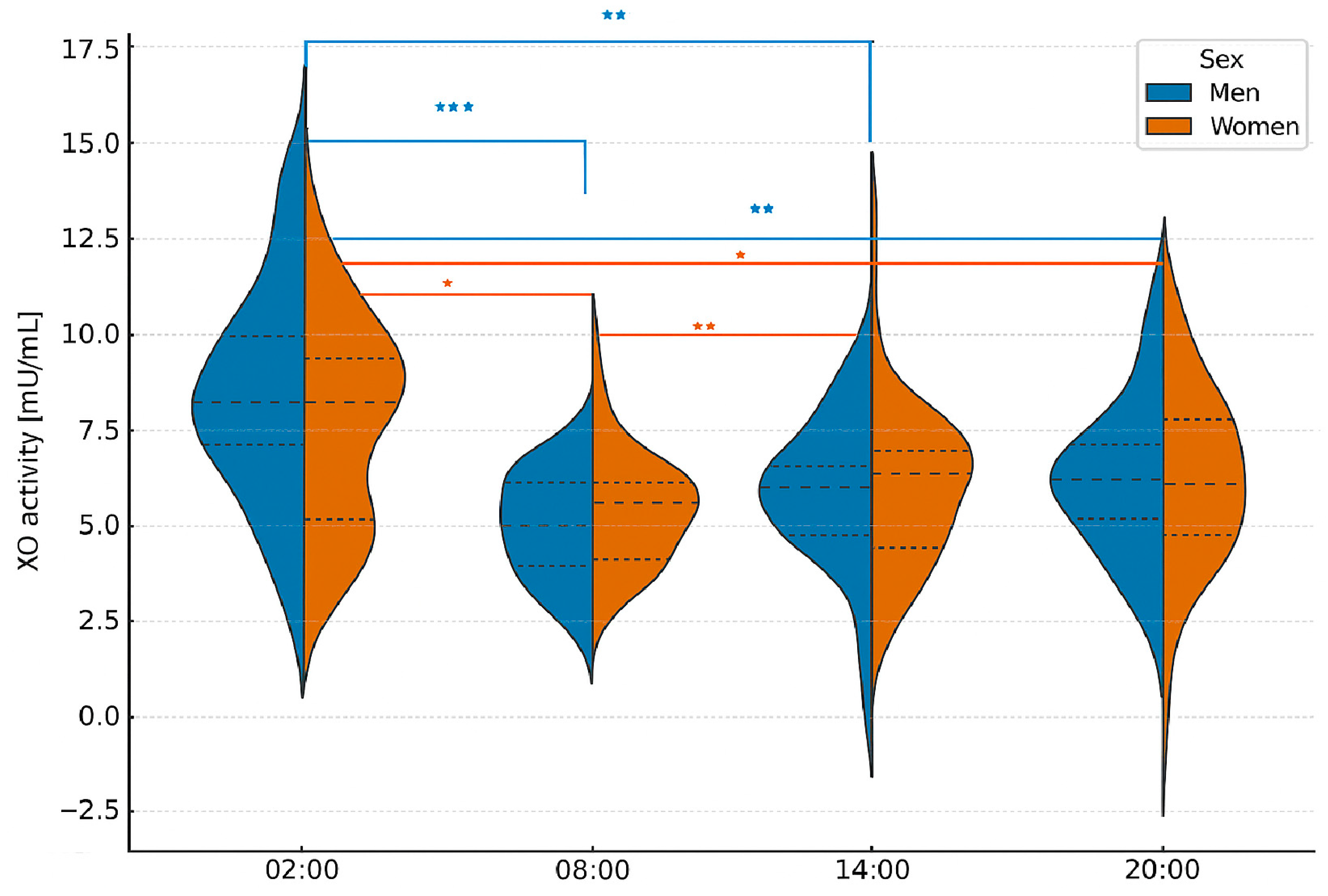
2.3. Analysis of XOR Isoform Concentrations at Different Times of Day
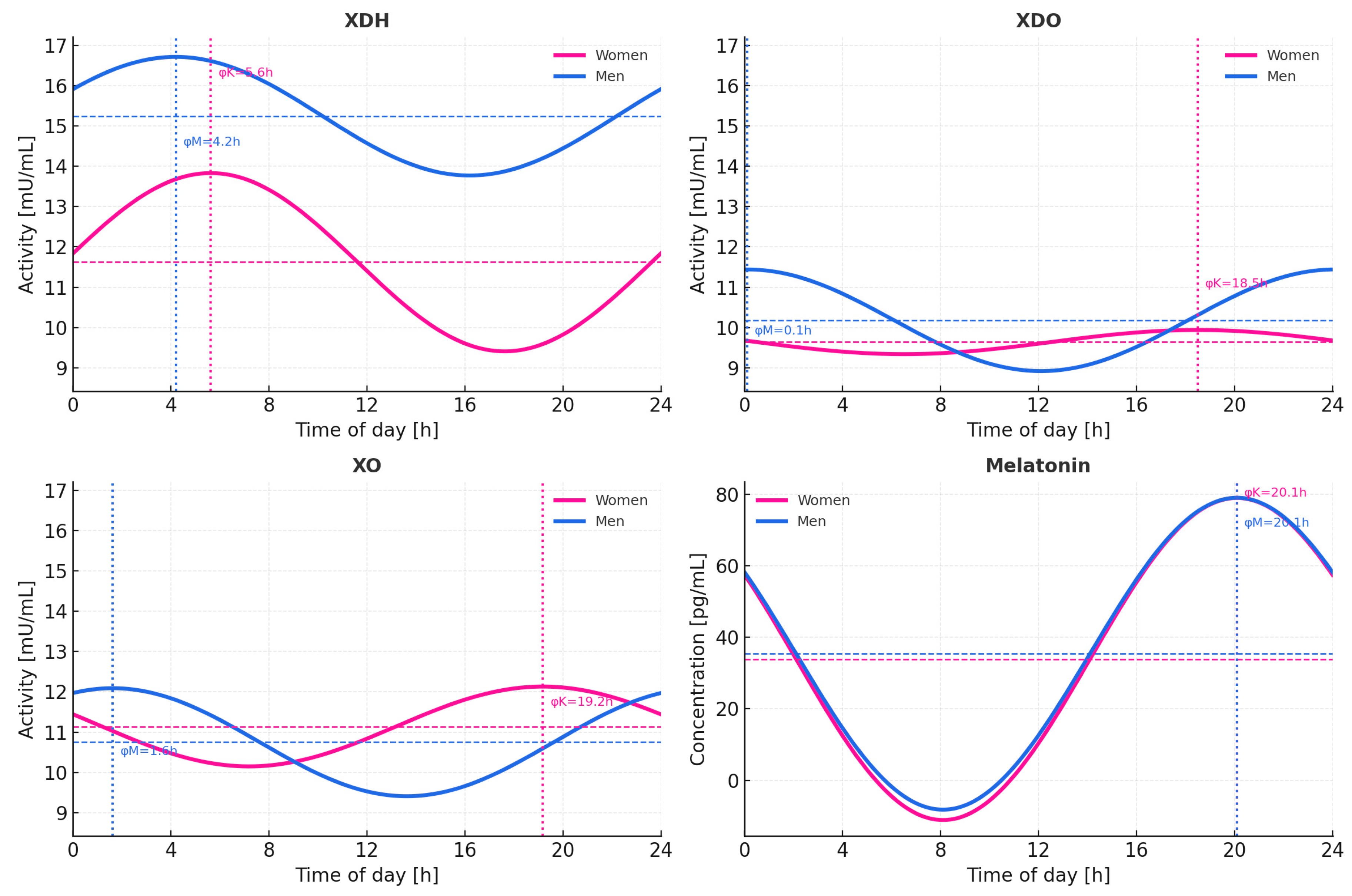
2.4. Circadian Profiles of XOR Isoforms and Melatonin-Cosinor Analysis
3. Discussion
4. Materials and Methods
4.1. Characteristics of Study Groups
- -
- Healthy adult women aged 24 to 38 years and men aged 24 to 44 years.
- -
- No chronic diseases and no long-term pharmacotherapy.
- -
- No antibiotic treatment within the recent period.
- -
- No use of painkillers in the two weeks before sample collection.
- -
- No hormone replacement therapy.
- -
- For women: not using oral contraceptives and not pregnant.
- -
- Normal results for laboratory analyses, including complete blood count, glucose, albumin, total protein, creatinine, uric acid, lipid profile (total cholesterol, HDL, LDL, TAG), and mineral parameters (total calcium, inorganic phosphorus, magnesium).
- -
- Regular daily rhythm (no night or shift work).
- -
- Written informed consent for participation.
- -
- Smoking
- -
- Night-shift or rotating-shift work that disrupts circadian rhythm.
- -
- Ongoing pharmacotherapy for chronic diseases.
- -
- Current or recent (within two weeks) antibiotic therapy.
- -
- Use of analgesics in the two weeks preceding sample collection.
- -
- Hormone replacement therapy or use of oral contraceptives.
- -
- Pregnancy (for women participants).
- -
- Abnormal laboratory findings indicating metabolic disturbances or chronic illness.
- -
- Withdrawal of consent at any point during the study.
4.2. Study Material
- K2EDTA tubes for hematological analysis and plasma preparation.
- Clot activator tubes for serum biochemical and hormonal assays.
4.3. Melatonin Assay
4.4. Determination of Xanthine Oxidoreductase Activity and Its Isoforms
- (1)
- buffer + NAD+ + xanthine (without plasma),
- (2)
- buffer + NAD+ + plasma (without xanthine), and
- (3)
- buffer + xanthine + plasma (without NAD+).
4.4.1. Dehydrogenase (XDH) Activity Assay
4.4.2. Dehydrogenase–Oxidase (XDO) Activity Assay
4.4.3. Oxidase (XO) Activity Assay
4.5. Statistical Analysis
- In men at 02:00, XO activity was inversely correlated with melatonin concentration (ρ = –0.52, p = 0.006, N = 33), with post hoc power ≈ 0.88.
- In women at 14:00, XDO activity correlated significantly with melatonin (ρ = –0.48, p = 0.01, N = 33), yielding post hoc power ≈ 0.82.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. Int. J. Mol. Sci. 2023, 24, 12143. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Manucha, W.; Rosales-Corral, S.; Chuffa, L.G.A.; Loh, D.; Luchetti, F.; Balduini, W.; Govitrapong, P. Dysfunctional mitochondria in age-related neurodegeneration: Utility of melatonin as an antioxidant treatment. Ageing Res. Rev. 2024, 101, 102480. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Nachvak, S.M.; Fazelian, S.; Moradi, F.; Persad, E.; Heshmati, J. Effect of melatonin supplementation on oxidative stress parameters: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 1483–1494. [Google Scholar]
- Cecerska-Heryć, E.; Jesionowska, A.; Klaudyna, S.; Katarzyna, S.; Dominika, M.; Dominika, P.; Marta, U.; Dołęgowska, B. Xanthine oxidoreductase reference values in platelet-poor plasma and platelets in healthy volunteers. Oxid. Med. Cell Longev. 2015, 2015, 341926. [Google Scholar] [PubMed]
- Nishino, T. XDH and XO Research and Drug Discovery—Personal Perspectives. Molecules 2023, 28, 4440. [Google Scholar]
- Shimizu, M.; Naito, R.; Sato, A.; Ishiwata, S.; Yatsu, S.; Shitara, J.; Matsumoto, H.; Murata, A.; Kato, T.; Suda, S.; et al. Diurnal Variations in Serum Uric Acid. Xanthine, and Xanthine Oxidoreductase Activity in Male Patients with Coronary Artery Disease. Nutrients 2023, 15, 4480. [Google Scholar] [CrossRef]
- Kanemitsu, T.; Tsurudome, Y.; Kusunose, N.; Oda, M.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Periodic variation in bile acids controls circadian changes in uric acid via regulation of xanthine oxidase by the orphan nuclear receptor PPARα. J. Biol. Chem. 2017, 292, 21397–21406. [Google Scholar] [CrossRef]
- Budkowska, M.; Cecerska-Heryć, E.; Marcinowska, Z.; Siennicka, A.; Dołęgowska, B. The Influence of Circadian Rhythm on the Activity of Oxidative Stress Enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Heryć, R.; Dutkiewicz, G.; Michalczyk, A.; Grygorcewicz, B.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Xanthine oxidoreductase activity in platelet-poor and rich plasma as a oxidative stress indicator in patients required renal replacement therapy. BMC Nephrol. 2022, 23, 35. [Google Scholar] [CrossRef]
- Cedernaes, J.; Waldeck, N.; Bass, J. Neurogenetic basis for circadian regulation of metabolism and health. J. Clin. Investig. 2019, 129, 3666–3673. [Google Scholar]
- Zhao, R.; Wang, H.; Xu, L. Circadian rhythm and its disruption in human health. Front. Physiol. 2021, 12, 848374. [Google Scholar]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication, and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef]
- Abbas, A.B.; Aldomaini, A.; Al-Qadri, A.A.; Algorbani, Z.; Aljamali, S.; Alsiri, S.; Alghorbani, K.; Abo Osba’a, S. Determine Complete Blood Count Reference Values and Associated Factors for Healthy Adults. Med. Sci. Monit. 2024, 30, e928471. [Google Scholar]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Connor Westfall, J.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 725–735. [Google Scholar] [CrossRef]
- Ohlander, S.J.; Varghese, B.; Pastuszak, A.W. Erythrocytosis Following Testosterone Therapy. Sex. Med. Rev. 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Bachman, E.; Feng, R.; Travison, T.; Li, M.; Olbina, G.; Ostland, V.; Ulloor, J.; Zhang, A.; Basaria, S.; Ganz, T.; et al. Testosterone suppresses hepcidin in men: Evidence for a novel erythropoietic mechanism for testosterone. J. Clin. Endocrinol. Metab. 2010, 95, 4743–4747. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Effects of testosterone treatment on hemoglobin, hematocrit, and red cell indices in hypogonadal men. JAMA Netw. Open 2023, 6, e2338893. [Google Scholar]
- Yu, W.; Zhou, G.; Fan, B.; Gao, C.; Li, C.; Wei, M.; Lv, J.; He, L.; Feng, G.; Zhang, T. Temporal Sequence of Blood Lipids and Insulin Resistance in Mid-life Women. Diabet. Res. Clin. Pract. 2022, 182, 109068. [Google Scholar]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not So Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef]
- Ávila, M.; Mora Sánchez, M.G.; Bernal Amador, A.S.; Paniagua, R. The metabolism of creatinine: Implications for assessing renal function. Biomolecules 2025, 15, 41. [Google Scholar]
- Kuhns, V.L.H.; Fullerton, D.; McCormick, K.; Cai, X.; Woodward, O.M. Sex Differences in Urate Handling: A Mechanistic Review. Front. Med. 2020, 7, 601. [Google Scholar]
- Cruz-Sanabria, F.; Carmassi, C.; Bruno, S.; Bazzani, A.; Carli, M.; Scarselli, M.; Faraguna, U. Melatonin as a Chronobiotic with Sleep-promoting Properties. Curr. Neuropharmacol. 2023, 21, 951–987. [Google Scholar] [CrossRef]
- Chen, I.Y.; Radom-Aizik, S.; Stehli, A.; Palmer, J.R.; Lui, K.K.; Dave, A.; Chappel-Farley, M.G.; Vinces, K.G.; Gealer, D.; Lim, A. Cardiorespiratory fitness and circadian rhythms in adolescents: A pilot study. J. Appl. Physiol. (1985) 2024, 136, 372–384. [Google Scholar] [CrossRef]
- Folkard, S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol. Int. 2008, 25, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kandel, R.; Sawant, N.; Singh, K.P. Estrogen-induced reactive oxygen species, through epigenetic reprogramming, causes increased growth in breast cancer cells. Mol. Cell Endocrinol. 2024, 579, 112092. [Google Scholar] [CrossRef]
- Lucas-Herald, A.K.; Touyz, R.M. Androgens and androgen receptors as determinants of vascular sex differences across the lifespan. Can. J. Cardiol. 2022, 38, 1854–1864. [Google Scholar] [CrossRef]
- Alonso-Alvarez, C.; Bertrand, S.; Faivre, B.; Chastel, O.; Sorci, G. Testosterone and oxidative stress: The oxidation handicap hypothesis. Proc. Biol. Sci. 2007, 274, 819–825. [Google Scholar] [CrossRef]
- Patel, S.A.; Velingkaar, N.S.; Kondratov, R.V. Transcriptional control of antioxidant defense by the circadian clock. Antioxid. Redox Signal. 2014, 20, 2997–3006. [Google Scholar] [CrossRef]
- Doi, R.; Oishi, K.; Ishida, N. CLOCK regulates circadian rhythms of hepatic glycogen synthesis through transcriptional activation of Gys2. J. Biol. Chem. 2010, 285, 22114–22121. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.-X.; Jou, M.-J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One molecule, multiple actions. J. Pineal Res. 2017, 63, e12401. [Google Scholar]
- Teixeira, A.; Morfim, M.P.; de Cordova, C.A.S.; Charão, C.C.T.; de Lima, V.R.; Creczynski-Pasa, T.B. Melatonin protects against pro-oxidant enzymes and reduces oxidative stress: Inhibition of xanthine oxidase activity. J. Pineal Res. 2003, 35, 115–118. [Google Scholar]
- Zang, L.-Y.; Cosma, G.; Gardner, H.; Vallyathan, V. Scavenging of reactive oxygen species by melatonin. Biochim. Biophys. Acta 1998, 1425, 469–477. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Mohammadpour Fard, R.; Rashno, M.; Bahreiny, S.S. Effects of melatonin supplementation on markers of inflammation and oxidative stress in patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2024, 63, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Matsumoto, M.; Tanaka, M.; Moniwa, N.; Murase, T.; Nakamura, T.; Ohnishi, H.; Saitoh, S.; Shimamoto, K.; Miura, T. P lasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ. J. 2018, 82, 1892–1899. [Google Scholar] [CrossRef]
- Kotozaki, Y.; Satoh, M.; Tanno, K.; Ohmomo, H.; Otomo, R.; Tanaka, F.; Nasu, T.; Taguchi, S.; Kikuchi, H.; Kobayashi, T. Plasma xanthine oxidoreductase activity is associated with a high risk of cardiovascular disease in a general Japanese population. Int. J. Environ. Res. Public Health 2021, 18, 1894. [Google Scholar] [CrossRef]
- Yoshida, S.; Kurajoh, M.; Fukumoto, S.; Murase, T.; Nakamura, T.; Yoshida, H.; Nagata, Y.; Nakatani, S.; Tsuda, A.; Yamada, S. Association of plasma xanthine oxidoreductase activity with blood pressure affected by oxidative stress level: MedCity21 health examination registry. Sci. Rep. 2020, 10, 4437. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Higashiura, Y.; Koyama, M.; Tanaka, M.; Murase, T.; Nakamura, T.; Akari, S.; Sakai, A.; Mori, K.; Ohnishi, H.; et al. Independent association of plasma xanthine oxidoreductase activity with hypertension in nondiabetic subjects not using medication. Hypertens. Res. 2021, 44, 1213–1220. [Google Scholar] [CrossRef]
- Ali, N.; Taher, A.; Islam, N.; Zaman, S.; Islam, F.; Rahman, M.M.; Hossain, M.G.; Ahmed, S.; Akhter, S.; Miah, R. Evaluation of the relationship between xanthine oxidase activity and metabolic syndrome in a population group in Bangladesh. Sci. Rep. 2024, 14, 20380. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Kinoshita, D.; Yokoyama, M.; Takahashi, T.; Toshima, T.; Suzuki, S.; Saito, Y.; Nakazawa, Y.; Sagara, K. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol. 2017, 228, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Watanabe, T.; Otaki, Y.; Shishido, T.; Murase, T.; Nakamura, T.; Tsuda, A.; Fujimoto, S.; Okazaki, H.; Shibata, Y. Impact of plasma xanthine oxidoreductase activity in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2020, 7, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Nasu, T.; Satoh, M.; Kotozaki, Y.; Tanno, K.; Tanaka, F.; Murase, T.; Nakamura, T.; Kikuchi, H.; Kobayashi, T. Association between plasma xanthine oxidoreductase activity and the renal function in a general Japanese population: The Tohoku Medical Megabank Community-Based Cohort Study. Kidney Blood Press. Res. 2022, 47, 722–728. [Google Scholar] [CrossRef]
- Tiberi, J.; Cesarini, V.; Stefanelli, R.; La Rosa, P. Sex differences in antioxidant defense and the regulation of redox homeostasis in physiology and pathology. Mech. Ageing Dev. 2023, 211, 111802. [Google Scholar] [CrossRef]
- Walton, J.C.; Bumgarner, J.R.; Nelson, R.J. Sex Differences in Circadian Rhythms. Cold Spring Harb. Perspect. Biol. 2022, 14, a039107. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex differences in sleep, circadian rhythms, and metabolism: Implications for precision medicine. Sleep Med. Rev. 2024, 75, 101926. [Google Scholar] [CrossRef]
- Okamoto, K.; Matsumoto, K.; Hille, R.; Eger, B.T.; Pai, E.F.; Nishino, T. The crystal structure of xanthine oxidoreductase during catalysis: Implications for reaction mechanism and enzyme inhibition. Proc. Natl. Acad. Sci. USA 2004, 101, 7931–7936. [Google Scholar] [CrossRef]
- Meneshian, A.; Bulkley, G.B. The physiology of endothelial xanthine oxidase: From urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation 2002, 9, 161–175. [Google Scholar] [CrossRef]
- Dołęgowska, B.; Błogowski, W.; Domański, L. Clinical evidence of the association between serum perioperative changes in xanthine metabolizing enzymes activity and early post-transplant kidney allograft function. J. Am. Coll. Surg. 2010, 211, 587–595. [Google Scholar] [CrossRef]
- Hille, R.; Nishino, T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Vorbach, C.; Harrison, R.; Capecchi, M.R. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003, 24, 512–517. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2020, 307, 29–31. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bolognesi, A.; Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multifaceted enzyme. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166045. [Google Scholar] [CrossRef]
- Nishino, T.; Okamoto, K. Mechanistic insights into xanthine oxidoreductase from development studies of candidate drugs to treat hyperuricemia and gout. Molecules 2021, 26, 2085. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Nishino, T.; Okamoto, K.; Hille, R.; Nishino, T. The mechanism and significance of the conversion of xanthine dehydrogenase to xanthine oxidase in mammalian secretory gland cells. Redox Biol. 2023, 59, 102573. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro-e-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- El Solh, A.A.; Saliba, R.; Bosinski, T.; Grant, B.J.B.; Berbary, E.; Miller, N. Allopurinol improves endothelial function in sleep apnoea: A randomized placebo-controlled crossover trial. Eur. Respir. J. 2006, 27, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E827–E834. [Google Scholar] [CrossRef]
- Matsushita, M.; Tamura, S.; Okazaki, H.; Yoshida, S.; Murase, T.; Nakamura, T.; Ito, K.; Sato, A.; Fujimoto, S.; Tsuda, A. Plasma xanthine oxidoreductase (XOR) activity in outpatients with cardiovascular disease and its association with metabolic parameters. Circ. J. 2020, 84, 1234–1242. [Google Scholar]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef] [PubMed]
- Kotozaki, Y.; Satoh, M.; Tanno, K.; Ohmomo, H.; Otomo, R.; Tanaka, F. Human plasma xanthine oxidoreductase activity in cardiovascular disease: Evidence from a population-based study. Biomedicines 2023, 11, 754. [Google Scholar] [CrossRef]
- McClean, C.; Davison, G.W. Circadian clocks, redox homeostasis, and exercise: Time to connect the dots? Antioxidants 2022, 11, 256. [Google Scholar] [CrossRef]
- O’Siorain, J.R.; Piggins, H.D.; Garvey, J.F.; Ray, D.W.; Loudon, A.S.I. Circadian control of redox reactions in the macrophage: Implications for immunity and disease. Antioxid. Redox Signal. 2022, 37, 592–610. [Google Scholar]
- Maury, E. Off the clock: From circadian disruption to metabolic disease. Endocr. Rev. 2019, 40, 1597. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, 15524–15533. [Google Scholar] [CrossRef]
- Grosjean, E.; Simonneaux, V.; Challet, E. Reciprocal interactions between circadian clocks, food intake, and energy metabolism. Biology 2023, 12, 539. [Google Scholar] [CrossRef]
- Belloir, J.; Makarem, N.; Shechter, A.; Florea, E.; Ghisletta, P.; Vetter, C.; Hébert, M.; Dumitrescu, L.; Zimmet, P.; Florez, J. Sleep and circadian disturbance in cardiovascular risk. Curr. Cardiol. Rep. 2022, 24, 1833–1848. [Google Scholar] [CrossRef]
- Young, M.E.; Bray, M.S.B.; Shaw, R.M.; Durgan, D.J.; Gamble, K.L.; Wu, Y.; Tsai, J.Y.; Jeong, W.-J.; Bugger, H.; Chatham, J.C. The cardiac circadian clock: Implications for cardiovascular disease and chronotherapy. JACC Basic Transl. Sci. 2023, 8, 665–684. [Google Scholar]
- Lecacheur, M.; Kervadec, A.; Davy, A.; Bernard, C.; Chen, Y.; Smith, J.; Tremblay, J.; Durant, M.; Roy, P.; Lambert, G. Circadian rhythms in cardiovascular (dys)function. Nat. Rev. Cardiol. 2024, 21, 21. [Google Scholar]
- Hermida, R.C.; Crespo, J.J.; Domínguez-Sardiña, M.; Otero, A.; Moyá, A.; Ríos, M.T.; Sineiro, E.; Castiñeira, M.C.; Callejas, P.A.; Pousa, L. Chronotherapy with conventional blood pressure medications improves management and reduces cardiovascular risk. Hypertens. Res. 2016, 39, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L.; Gil-Lozano, M. Metabolic homeostasis: It’s all in the timing. Physiol. Rev. 2021, 101, 1171–1201. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Men (n = 33) | Women (n = 33) | p |
|---|---|---|---|
| RBC [1012/L] | 5.3 ± 0.5 | 4.5 ± 0.4 | <0.0001 |
| HGB [mM/L] | 9.2 ± 0.6 | 7.9 ± 0.6 | <0.0001 |
| HCT [%] | 46 ± 3 | 40 ± 3 | <0.0001 |
| WBC [109/L] | 5.8 ± 1.1 | 6.0 ± 1.4 | 0.9929 |
| PLT [103/µL] | 253 ± 40 | 252 ± 41 | 0.9715 |
| Glucose [mg/dL] | 92 ± 7 | 90 ± 8 | 0.1311 |
| Phosphorus [mM/L] | 1.5 ± 0.1 | 1.5 ± 0.2 | 0.9359 |
| Magnesium [mM/L] | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.6487 |
| Calcium [mM/L] | 2.4 ± 0.1 | 2.4 ± 0.2 | 0.7141 |
| Ch–C [mg/dL] | 187 ± 22 | 179 ± 23 | 0.1555 |
| Triglycerides [mg/dL] | 111 ± 35 | 92 ± 19 | 0.0207 |
| LDL [mg/dL] | 92 ± 25 | 89 ± 23 | 0.7141 |
| HDL [mg/dL] | 72 ± 14 | 70 ± 11 | 0.3669 |
| Total protein [g/dL] | 6.6 ± 0.5 | 6.5 ± 0.4 | 0.2491 |
| Albumin [g/dL] | 4.0 ± 0.3 | 3.9 ± 0.3 | 0.0525 |
| Creatynine [mg/dL] | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.0012 |
| Uric acid [mg/dL] | 5.3 ± 1.0 | 4.5 ± 0.7 | 0.0005 |
| Outcome | Zero amp. p (Women) | Zero amp. p (Men) | MESOR (Women) | MESOR (Men) | Amplitude (Women) | Acrophase [h] (Women) | Amplitude (Men) | Acrophase [h] (Men) | Δ Phase (Men−Women) [h] |
|---|---|---|---|---|---|---|---|---|---|
| XDH | 1.32 × 10−1 | 3.71 × 10−1 | 11.62 | 15.24 | 2.21 | 5.62 | 1.47 | 4.19 | −1.43 |
| XDO | 6.88 × 10−1 | 1.17 × 10−1 | 9.64 | 10.18 | 0.30 | 18.53 | 1.26 | 0.09 | −18.44 |
| XO | 4.84 × 10−1 | 2.00 × 10−1 | 11.14 | 10.75 | 0.99 | 19.18 | 1.34 | 1.62 | −17.56 |
| Melatonin | <1 × 10−5 | <1 × 10−5 | 33.95 | 35.42 | 45.08 | 3.87 | 43.65 | 3.84 | −0.03 |
| Enzymes | MESOR | Amplitude (Global) | Acrophase (Rad) | R2 | p-Value |
|---|---|---|---|---|---|
| XDH | 13.43 | 1.85 | 1.29 | 0.012 | 0.094 |
| XDO | 9.90 | 0.62 | 0.28 | 0.005 | 0.265 |
| XO | 10.94 | 0.78 | 1.11 | 0.004 | 0.375 |
| Melatonin | 34.66 | 44.38 | 3.85 | 0.66 | <1 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecerska-Heryć, E.; Zoń, M.; Budkowska, M.; Serwin, N.; Michalczyk, A.; Goszka, M.; Polikowska, A.; Wojciuk, B.; Dołęgowska, B. Redox on the Clock: Sex-Dependent Dynamics of Xanthine Oxidoreductase Isoforms and Melatonin. Int. J. Mol. Sci. 2025, 26, 11272. https://doi.org/10.3390/ijms262311272
Cecerska-Heryć E, Zoń M, Budkowska M, Serwin N, Michalczyk A, Goszka M, Polikowska A, Wojciuk B, Dołęgowska B. Redox on the Clock: Sex-Dependent Dynamics of Xanthine Oxidoreductase Isoforms and Melatonin. International Journal of Molecular Sciences. 2025; 26(23):11272. https://doi.org/10.3390/ijms262311272
Chicago/Turabian StyleCecerska-Heryć, Elżbieta, Martyn Zoń, Marta Budkowska, Natalia Serwin, Anna Michalczyk, Małgorzata Goszka, Aleksandra Polikowska, Bartosz Wojciuk, and Barbara Dołęgowska. 2025. "Redox on the Clock: Sex-Dependent Dynamics of Xanthine Oxidoreductase Isoforms and Melatonin" International Journal of Molecular Sciences 26, no. 23: 11272. https://doi.org/10.3390/ijms262311272
APA StyleCecerska-Heryć, E., Zoń, M., Budkowska, M., Serwin, N., Michalczyk, A., Goszka, M., Polikowska, A., Wojciuk, B., & Dołęgowska, B. (2025). Redox on the Clock: Sex-Dependent Dynamics of Xanthine Oxidoreductase Isoforms and Melatonin. International Journal of Molecular Sciences, 26(23), 11272. https://doi.org/10.3390/ijms262311272








