Potential of Essential Oils from Different Mint Species Against Multidrug-Resistant Escherichia coli Strains Isolated from Clinical Cases in Poultry
Abstract
1. Introduction
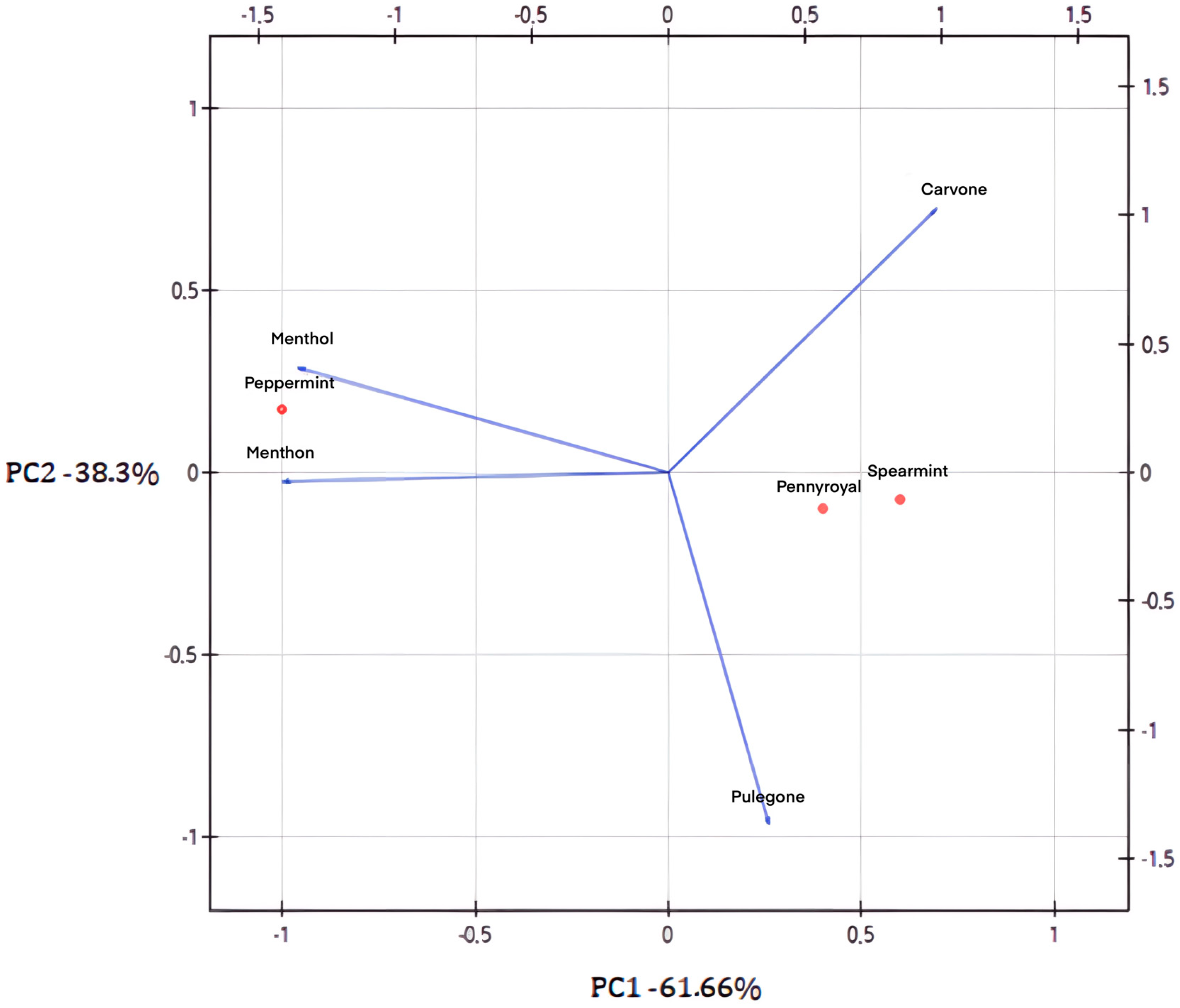
2. Results
3. Discussion
4. Materials and Methods
4.1. Essential Oils and Their Main Chemical Constituents
4.2. Analysis of EO Chemical Composition
4.3. Bacterial Strains
4.4. Antibacterial Activity Assessment
4.5. MIC Readings
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APEC | Avian Pathogenic Escherichia coli |
| EO | Essential oil |
| EOs | Essential oils |
| MIC | Minimum inhibitory concentration |
References
- Sfaxi, A.; Tavaszi-Sárosi, S.; Flórián, K.; Patonay, K.; Radácsi, P.; Juhász, Á. Comparative Evaluation of Different Mint Species Based on Their In Vitro Antioxidant and Antibacterial Effect. Plants 2025, 14, 105. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Hutsol, T.; Priss, O.; Kiurcheva, L.; Serdiuk, M.; Panasiewicz, K.; Jakubus, M.; Barabasz, W.; Furyk-Grabowska, K.; Kukharets, M. Mint Plants (Mentha) as a Promising Source of Biologically Active Substances to Combat Hidden Hunger. Sustainability 2023, 15, 11648. [Google Scholar] [CrossRef]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the Potential Use of Medicinal Plants from Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Telcï, I.; Özek, T.; Özek, G.; Devrïm, S.; Eryiğit, S. Assessing chemical diversity in essential oil compositions of Mint (Mentha spp.) cultivars and clones using Multivariate analysis. Biochem. Syst. Ecol. 2025, 120, 104972. [Google Scholar] [CrossRef]
- Majkowska-Gadomska, J.; Kaliniewicz, Z.; Mikulewicz, E.; Francke, A.; Jadwisieńczak, K.K.; Marks, M.; Choszcz, D.J.; Kozłowski, W. Effect of Different Sustainable Cultivation Methods on the Biometric Parameters and Yield of Mint. Sustainability 2024, 16, 7126. [Google Scholar] [CrossRef]
- Kadoglidou, K.I.; Chatzopoulou, P. Approaches and Applications of Mentha Species in Sustainable Agriculture. Sustainability 2023, 15, 5245. [Google Scholar] [CrossRef]
- Lawrence, B.M. Oil composition of other Mentha species and hybrids. W: Mint: The Genus Mentha. In Medicinal and Aromatic Plants-Industrial Profiles; Lawrence, B.M., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 1–547. [Google Scholar]
- Tafrihi, M.; Imran, M.; Tufail, T.; Gondal, T.A.; Caruso, G.; Sharma, S.; Sharma, R.; Atanassova, M.; Atanassov, L.; Valere Tsouh Fokou, P.; et al. The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules 2021, 26, 1118. [Google Scholar] [CrossRef]
- van Vuuren, S.; Suliman, S.; Viljoen, A. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef]
- Yap, P.S.; Yiap, B.C.; Ping, H.C.; Lim, S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef]
- Talei, G.R.; Mohmmadi, M.; Bahmani, M.; Kopaei, M.R. Synergistic effect of Carum copticum and Mentha piperita essential oils with ciprofloxacin, vancomycin, and gentamicin on Gram-negative and Gram-positive bacteria. Int. J. Pharm. Investig. 2017, 2, 82–87. [Google Scholar] [CrossRef]
- Shalayel, M.H.F.; Asaad, A.M.; Qureshi, M.A.; Elhussein, A.B. Anti-bacterial activity of peppermint (Mentha piperita) extracts against some emerging multi-drug resistant human bacterial pathogens. J. Herb. Med. 2017, 7, 27–30. [Google Scholar] [CrossRef]
- Fokas, R.; Anastopoulou, Z.; Vantarakis, A. Antimicrobial Activity of Greek Native Essential Oils Against Escherichia coli O157:H7 and Antibiotic Resistance Strains Harboring pNorm Plasmid, mecA, mcr-1 and blaOXA Genes. Antibiotics 2025, 14, 741. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. Use of essential oils in broiler chicken production—A review. Ann. Anim. Sci. 2017, 2, 317–335. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, 4, 43–167. Available online: http://data.europa.eu/eli/reg/2019/6/oj (accessed on 1 October 2025).
- Wilczyński, J.; Stępień-Pyśniak, D.; Wystalska, D.; Wernicki, A. Molecular and Serological Characteristics of Avian Pathogenic Escherichia coli Isolated from Various Clinical Cases of Poultry Colibacillosis in Poland. Animals 2022, 12, 1090. [Google Scholar] [CrossRef]
- Singh, S.; Kriti, M.; KS, A.; Sharma, P.; Pal, N.; Sarma, K.D.; Tiwari, R.; Kumar, R. A one health approach addressing poultry-associated antimicrobial resistance: Human, animal and environmental perspectives. Microbe 2025, 7, 100309. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D.; Zych, S. The Use of Lavender (Lavandula angustifolia) Essential Oil as an Additive to Drinking Water for Broiler Chickens and Its In Vitro Reaction with Enrofloxacin. Animals 2021, 11, 1535. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, S.; Madsen, J.S. Cooperative resistance varies among β-lactamases in E. coli, with some enabling cross-protection and sustained extracellular activity. Commun. Biol. 2025, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Brătfelan, D.O.; Tabaran, A.; Colobatiu, L.; Mihaiu, R.; Mihaiu, M. Prevalence and Antimicrobial Resistance of Escherichia coli Isolates from Chicken Meat in Romania. Animals 2023, 13, 3488. [Google Scholar] [CrossRef] [PubMed]
- Menck-Costa, M.F.; Baptista, A.A.S.; Sanches, M.S.; Santos, B.Q.d.; Cicero, C.E.; Kitagawa, H.Y.; Justino, L.; Medeiros, L.P.; Souza, M.d.; Rocha, S.P.D.; et al. Resistance and Virulence Surveillance in Escherichia coli Isolated from Commercial Meat Samples: A One Health Approach. Microorganisms 2023, 11, 2712. [Google Scholar] [CrossRef]
- Xedzro, C.; Kimura, T.; Shimamoto, T.; Ahmed, A.M.; Shimamoto, T. Comparative molecular profiling of antimicrobial resistance and phylogenetic characterization of multidrug-resistant Escherichia coli isolated from meat sources in 2009 and 2021 in Japan. Int. J. Food Microbiol. 2023, 391–393, 110146. [Google Scholar] [CrossRef]
- Chodkowska, K.A.; Iwiński, H.; Wódz, K.; Nowak, T.; Różański, H. In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens. Antibiotics 2022, 11, 1818. [Google Scholar] [CrossRef]
- Zych, S.; Adaszyńska-Skwirzyńska, M.; Szewczuk, M.A.; Szczerbińska, D. Interaction between Enrofloxacin and Three Essential Oils (Cinnamon Bark, Clove Bud and Lavender Flower)—A Study on Multidrug-Resistant Escherichia coli Strains Isolated from 1-Day-Old Broiler Chickens. Int. J. Mol. Sci. 2024, 25, 5220. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Y.; Chen, C.; Wang, N.; Yang, F. Solutions to the Dilemma of Antibiotics Use in Livestock and Poultry Farming: Regulation Policy and Alternatives. Toxics 2025, 13, 348. [Google Scholar] [CrossRef]
- Dec, M.; Wernicki, A.; Urban-Chmiel, R. Efficacy of experimental phage therapies in livestock. Anim. Health Res. Rev. 2020, 21, 69–83. [Google Scholar] [CrossRef]
- Zamojska, D.; Nowak, A.; Nowak, I.; Macierzyńska-Piotrowska, E. Probiotics and Postbiotics as Substitutes of Antibiotics in Farm Animals: A Review. Animals 2021, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Pieri, A.; Aschbacher, R.; Fasani, G.; Mariella, J.; Brusetti, L.; Pagani, E.; Sartelli, M.; Pagani, L. Country Income Is Only One of the Tiles: The Global Journey of Antimicrobial Resistance among Humans, Animals, and Environment. Antibiotics 2020, 9, 473. [Google Scholar] [CrossRef]
- Paramitadevi, Y.V.; Priadi, C.R.; Rahmatika, I.; Rukmana, A.; Moersidik, S.S. Integration of Water, Sanitation, and Hygiene Program with Biosecurity: A One Health Approach to Reduce the Prevalence and Exposure of Antibiotic-Resistant Bacteria in the Livestock Community. Int. J. One Health 2023, 9, 181–193. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential oils: Chemical constituents, potential neuropharmacological effects and aromatherapy—A review. Pharmacol. Res.-Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Mikaili, P.; Mojaverrostami, S.; Moloudizargari, M.; Aghajanshakeri, S. Pharmacological and Therapeutic Effects of Mentha Longifolia L. and Its Main Constituent, Menthol. Anc. Sci. Life 2013, 33, 131. [Google Scholar] [CrossRef]
- Gandova, V.; Muntean, E.; Dranca, F.; Diaconeasa, Z.; Pintea, A.; Vlase, L. Comparative Analysis of Antibacterial Activity and Physicochemical Properties of Peppermint and Cornmint Essential Oils and Their Main Compound Menthol. J. Chem. Technol. Metall. 2023, 58, 664–671. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Saba, I.; Ahmed, M.; Khan, A.; Muneer, M.; Rehman, Z.U.; Haq, I.U.; Haider, S. Spearmint (Mentha spicata L.) Leaves Essential Oil: Comparative Compositional and Biological Attributes as a Function of Different Agroclimatic Regions. Biocatal. Agric. Biotechnol. 2024, 56, 102984. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; De Feo, V. Mentha spicata Essential Oil: Chemical Composition, Antioxidant and Antibacterial Activities against Planktonic and Biofilm Cultures of Vibrio spp. Strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef] [PubMed]
- Rached, S.; Habsaoui, A.; Mzioud, K.; Er-rajy, M.; Abujaber, F.; Imtara, H.; Oubihi, A.; Haida, S.; El-guourrami, O.; Noman, O.M.; et al. Chemical profiling, safety assessment, bioactive properties, and molecular interactions of the essential oil derived from Mentha pulegium L. Front. Sustain. Food Syst. 2025, 9, 1511848. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical Composition, Antifungal and Antibiofilm Activities of the Essential Oil of Mentha piperita L. ISRN Pharm. 2012, 2012, 718645. [Google Scholar] [CrossRef] [PubMed]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Mentha piperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Comparative chemical composition of the essential oil of Mentha × piperita L. from various geographical sources. Proc. Est. Acad. Sci. Chem. 2004, 53, 174–181. [Google Scholar] [CrossRef]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A.; et al. Effect of Extraction Methods on Polyphenols, Flavonoids, Mineral Elements, and Biological Activities of Essential Oil and Extracts of Mentha pulegium L. Molecules 2022, 27, 11. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid. Based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F. Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil. Antibiotics 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- El-Ghorab, A.H. The Chemical Composition of the Mentha pulegium L. Essential Oil from Egypt and its Antioxidant Activity. J. Essent. Oil Bear. Plants 2013, 9, 183–195. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Elsevier, Churchill Livingstone: London, UK, 2014. [Google Scholar]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- EFSA Plain Language Summary of the European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2022–2023. EFSA J. 2025, 23, ep230301. [CrossRef]
- Monroy, I.; Catala-Gregori, P.; Sevilla-Navarro, S. Assessment of antibiotic resistance and virulence in Escherichia coli strains from poultry in Spain. Poult. Sci. 2025, 104, 104838. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Tadevosyan, S.; Sahakyan, N. Influence of menthol on membrane-associated properties of tetracycline-resistant Escherichia coli. AIMS Biophys. 2024, 11, 329–339. [Google Scholar] [CrossRef]
- Aperce, C.C.; Amachawadi, R.; Van Bibber-Krueger, C.L.; Nagaraja, T.G.; Scott, H.M.; Vinasco-Torre, J.; Drouillard, J.S. Effects of Menthol Supplementation in Feedlot Cattle Diets on the Fecal Prevalence of Antimicrobial-Resistant Escherichia coli. PLoS ONE 2016, 11, e0168983. [Google Scholar] [CrossRef]
- Nogueira, J.E.; Campolina, A.G.; Batista, L.R.; Alves, E.; Silva Caetano, A.R.; Magalhães Brandão, R.; Nelson, D.L.; das Graças Cardoso, M. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021, 368, fnab052. [Google Scholar] [CrossRef]
- Moro, J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; de Sousa, D.P.; Santos, A.G. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz. J. Pharm. Sci. 2017, 53, e00076. [Google Scholar] [CrossRef]
- Gordon, W.P.; Forte, A.J.; McMurtry, R.J.; Gal, J.; Nelson, S.D. Hepatotoxicity and Pulmonary Toxicity of Pennyroyal Oil and Its Constituent Terpenes in the Mouse. Toxicol. Appl. Pharmacol. 1982, 65, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.B. Pennyroyal Toxicity: Measurement of Toxic Metabolite Levels in Two Cases and Review of the Literature. Ann. Intern. Med. 1996, 124, 726. [Google Scholar] [CrossRef] [PubMed]
- Fadli, M.; Saad, A.; Sayadi, S.; Chevalier, J.; Mezrioui, N.E.; Pagès, J.M.; Hassani, L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection—Bacteria and their synergistic potential with antibiotics. Phytomedicine 2012, 15, 464–471. [Google Scholar] [CrossRef]
- Noui Mehidi, I.; Ait Ouazzou, A.; Tachoua, W.; Hosni, K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules 2024, 29, 4119. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef]
- Broniatowski, M.; Mastalerz, P.; Flasiński, M. Studies of the interactions of ursane-type bioactive terpenes with the model of Escherichia coli inner membrane—Langmuir monolayer approach. Biochim. Biophys. Acta 2015, 1848, 469–476. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Wang, Z. Menthol and Its Derivatives: Exploring the Medical Application Potential. Eng. Life Sci. 2025, 25, e70039. [Google Scholar] [CrossRef]
- Azmi, N.; Elgharbawy, A.S.; Adeeb, H.H. Evaluation of the antimicrobial performance of menthol and menthol-based deep eutectic solvents as potential future antibiotic. E3S Web Conf. 2021, 287, 02010. [Google Scholar] [CrossRef]
- Turcheniuk, V.; Raks, V.; Issa, R.; Cooper, I.R.; Cragg, P.J.; Jijie, R.; Dumitrascu, N.; Mikhalovska, L.I.; Barras, A.; Zaitsev, V.; et al. Antimicrobial activity of menthol modified nanodiamond particles. Diam. Relat. Mater. 2015, 57, 2–8. [Google Scholar] [CrossRef]
- Landau, E.; Shapira, R. Effects of subinhibitory concentrations of menthol on adaptation, morphological, and gene expression changes in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 2012, 15, 5361–5367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, C.; Zhang, N.; Peng, Y.; Ma, Y.; Gu, K.; Liu, X.; Liu, Y.; Li, S.; Zhao, L. Menthone Exerts its Antimicrobial Activity Against Methicillin Resistant Staphylococcus aureus by Affecting Cell Membrane Properties and Lipid Profile. Drug Des. Dev. Ther. 2023, 17, 219–236. [Google Scholar] [CrossRef]
- Gong, H.; He, L.; Zhao, Z.; Mao, X.; Zhang, C. The specific effect of (R)-(+)-pulegone on growth and biofilm formation in multi-drug resistant Escherichia coli and molecular mechanisms underlying the expression of pgaABCD genes. Biomed. Pharmacother. 2021, 134, 111149. [Google Scholar] [CrossRef]
- Farhanghi, A.; Aliakbarlu, J.; Tajik, H.; Mortazavi, N.; Manafi, L.; Jalilzadeh-Amin, G. Antibacterial interactions of pulegone and 1,8-cineole with monolaurin ornisin against Staphylococcus aureus. Food Sci. Nutr. 2025, 10, 2659–2666. [Google Scholar] [CrossRef]
- Pl’uchtova, M.; Gervasi, T.; Benameur, Q.; Pellizzeri, V.; Grul’ova, D.; Campone, L.; Sedlak, V.; Cicero, N. Antibacterial Activity of two Mentha Species Essential Oil and its Dependence on Different Origin and Chemical Diversity. Nat. Prod. Commun. 2018, 13, 1051. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; document VET01Ed5; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Development of Quality Control Ranges, Breakpoints, and Interpretive Categories for Antimicrobial Agents Used in Veterinary Medicine, 4th ed.; document VET02Ed4; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
| No. | Compound | RT 1 (min) | LRI 2 | LRIRef 3 | Mint Variety | ||
|---|---|---|---|---|---|---|---|
| M. piperita (Peppermint) | M. spicata (Spearmint) | M. pulegium (Pennyroyal) | |||||
| Relative Content ± SD 4 (%) | |||||||
| 1 | α-Pinene | 5.64 | 932 | 936 | 0.89 ± 0.06 | 0.80 ± 0.06 | 0.66 ± 0.02 |
| 2 | Sabinene | 6.66 | 950 | 969 | 0.59 ± 0.05 | 0.60 ± 0.02 | 0.15 ± 0.01 |
| 3 | β-Pinene | 6.74 | 975 | 978 | 1.17 ± 0.09 | 1.05 ± 0.02 | 0.50 ± 0.01 |
| 4 | β-Myrcene | 7.12 | 990 | 989 | 0.22 ± 0.02 | 1.26 ± 0.04 | 0.10 ± 0.00 |
| 5 | 3-Octanole | 7.28 | 996 | 993 | 0.19 ± 0.01 | 0.65 ± 0.01 | 1.02 ± 0.03 |
| 6 | Limonene | 8.23 | 1027 | 1030 | 7.95 ± 0.30 | 1.93 ± 0.06 | 1.65 ± 0.02 |
| 7 | 1,8-Cineole | 8.36 | 1031 | 1032 | 0.36 ± 0.04 | 18.73 ± 0.73 | 0.19 ± 0.01 |
| 8 | trans-β-Ocimene | 8.53 | 1037 | 1048 | 0.18 ± 0.02 | nd | nd |
| 9 | γ-Terpinene | 9.16 | 1057 | 1060 | 0.39 ± 0.02 | 0.55 ± 0.01 | nd |
| 10 | p-Mentha-3,8-diene | 9.51 | 1068 | 1068 | nd | nd | 0.71 ± 0.01 |
| 11 | trans-Menthone | 12.33 | 1155 | 1150 | 23.11 ± 0.28 | 0.40 ± 0.01 | 6.60 ± 0.10 |
| 12 | Menthofuran | 12.72 | 1167 | 1159 | 4.87 ± 0.20 | nd | nd |
| 13 | cis-Menthone | 12.73 | 1167 | 1159 | nd | nd | 1.64 ± 0.05 |
| 14 | neo-Menthol | 12.80 | 1169 | 1167 | 6.80 ± 0.23 | nd | nd |
| 15 | Menthol | 13.02 | 1176 | 1177 | 35.14 ± 0.06 | 1.83 ± 0.07 | nd |
| 16 | trans-Isopulegone | 13.05 | 1177 | 1177 | nd | nd | 2.98 ± 0.11 |
| 17 | Terpinen-4-ol | 13.08 | 1178 | 1177 | nd | 1.27 ± 0.05 | nd |
| 18 | Isomenthol | 13.47 | 1189 | 1179 | 0.60 ± 0.10 | nd | nd |
| 19 | α-Terpineol | 13.68 | 1196 | 1190 | 0.57 ± 0.04 | nd | nd |
| 20 | cis-Dihydrocarvone | 13.75 | 1198 | 1191 | nd | 3.09 ± 0.02 | nd |
| 21 | trans-Dihydrocarvone | 13.96 | 1204 | 1201 | nd | 1.03 ± 0.09 | nd |
| 22 | Pulegone | 15.42 | 1248 | 1234 | 1.58 ± 0.01 | nd | 76.54 ± 0.65 |
| 23 | Carvone | 15.57 | 1253 | 1242 | nd | 58.61 ± 0.51 | nd |
| 24 | Piperitone | 15.95 | 1264 | 1254 | 0.57 ± 0.01 | 0.70 ± 0.20 | nd |
| 25 | Neomenthyl acetate | 16.33 | 1276 | 1271 | 0.45 ± 0.01 | nd | nd |
| 25 | Menthyl acetate | 17.05 | 1298 | 1296 | 6.96 ± 0.12 | 0.48 ± 0.02 | nd |
| 27 | cis-Dihydrocarvyl acetate | 18.10 | 1330 | 1326 | nd | 0.50 ± 0.01 | nd |
| 28 | Piperitenone | 18.50 | 1343 | 1341 | nd | nd | 1.98 ± 0.16 |
| 29 | Carvyl acetate | 19.18 | 1364 | 1362 | nd | 0.34 ± 0.01 | nd |
| 30 | β-Bourbonene | 19.85 | 1385 | 1384 | 0.23 ± 0.00 | 2.18 ± 0.06 | nd |
| 31 | β-Elemene | 20.09 | 1393 | 1390 | nd | 0.26 ± 0.02 | nd |
| 32 | β-Caryophyllene | 20.95 | 1420 | 1420 | 3.55 ± 0.23 | 1.74 ± 0.05 | 1.44 ± 0.05 |
| 33 | α-Humulene | 21.95 | 1453 | 1453 | 0.17 ± 0.01 | 0.35 ± 0.02 | 1.91 ± 0.09 |
| 34 | β-Farnesene | 22.09 | 1457 | 1457 | 0.27 ± 0.01 | nd | nd |
| 35 | Germacrene D | 22.81 | 1481 | 1481 | 1.25 ± 0.02 | nd | 0.15 ± 0.01 |
| 36 | Bicyclogermacrene | 23.27 | 1496 | 1494 | 0.27 ± 0.01 | nd | nd |
| Total | 98.43 | 98.38 | 98.24 | ||||
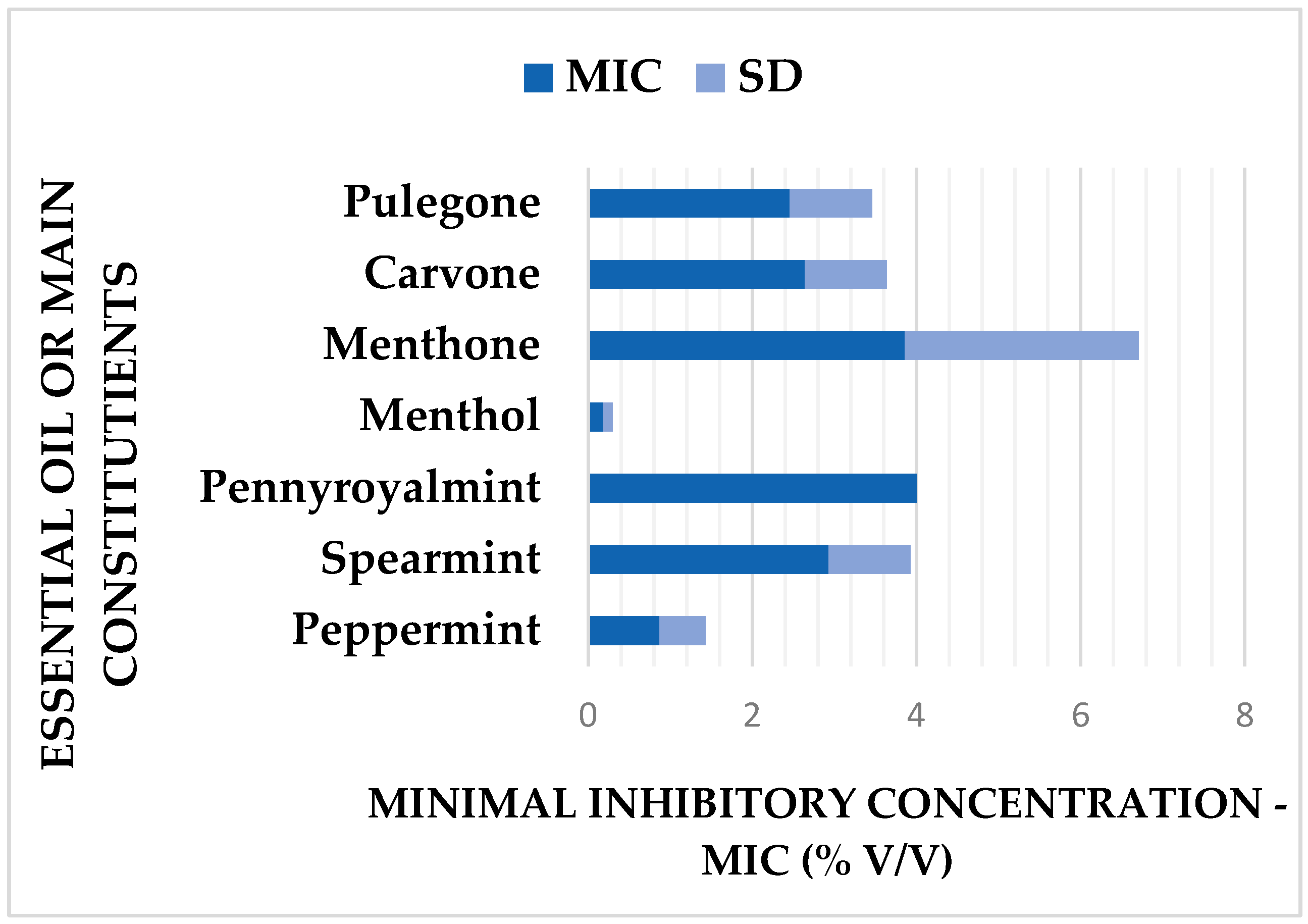
| Escherichia coli Strain | MIC 1 of Main Constituents or Essential Oil (% v/v) | |||||||
|---|---|---|---|---|---|---|---|---|
| Menthol | Menthone | PM EO 2 | Carvone | SM EO 3 | Pulegone | PR EO 4 | ||
| Multidrug-resistant strains | ||||||||
| 1 | 0.125 | 2 | 1 | 2 | 2 | 2 | 4 | |
| 2 | 0.25 | 8 | 1 | 4 | 4 | 4 | 4 | |
| 3 | 0.25 | 8 | 2 | 4 | 4 | 4 | 4 | |
| 4 | 0.25 | 8 | 1 | 4 | 4 | 4 | 4 | |
| 5 | 0.125 | 4 | 0.5 | 2 | 2 | 1 | 4 | |
| 6 | 0.25 | 4 | 1 | 2 | 4 | 2 | 4 | |
| 7 | 0.25 | 8 | 1 | 2 | 2 | 2 | 4 | |
| 8 | 0.5 | 8 | 2 | 4 | 4 | 4 | 4 | |
| 9 | 0.5 | 8 | 2 | 4 | 4 | 2 | 4 | |
| 10 | 0.125 | 8 | 1 | 4 | 4 | 2 | 4 | |
| Susceptible strains | ||||||||
| 11 | 0.125 | 4 | 0.5 | 2 | 4 | 2 | 4 | |
| 12 | 0.125 | 2 | 0.5 | 2 | 2 | 2 | 4 | |
| 13 | 0.125 | 2 | 0.5 | 2 | 2 | 2 | 4 | |
| 14 | 0.125 | 2 | 0.5 | 2 | 2 | 2 | 4 | |
| 15 | 0.25 | 8 | 1 | 4 | 4 | 4 | 4 | |
| 16 | 0.25 | 2 | 0.5 | 2 | 2 | 2 | 4 | |
| 17 | 0.125 | 2 | 1 | 2 | 4 | 4 | 4 | |
| 18 | 0.125 | 1 | 0.5 | 2 | 2 | 2 | 4 | |
| 19 | 0.125 | 4 | 2 | 4 | 4 | 4 | 4 | |
| ATCC 25922 | 0.125 | 2 | 0.5 | 2 | 2 | 2 | 4 | |
| Summary | ||||||||
| Mean MIC | 0.18 | 3.86 | 0.87 | 2.64 | 2.93 | 2.46 | 4 | |
| SD 5 | 0.12 | 2.85 | 0.56 | 1 | 1 | 1 | 0 | |
| CV 6 % | 66.7 | 73.8 | 64.4 | 37.9 | 34.1 | 40.7 | 0 | |
| most frequent MICs | % v/v | 0.125 | 8 | 0.5/1 | 2 | 4 | 2 | 4 |
| % of cases | 55 | 40 | 40/40 | 60 | 55 | 60 | 100 | |
| Group | Total | Multidrug-Resistant Strains | Susceptible Strains | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PM EO 2 | SM EO 3 | PM EO 4 | PM EO 2 | SM EO 3 | PR EO 4 | PM EO 2 | SM EO 3 | PR EO 4 | ||
| (1) | (2) | (3) | (1) | (2) | (3) | (1) | (2) | (3) | ||
| Number of strains | 20 | 20 | 20 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Arithmetic mean | 1 | 3.1 | 4 | 1.25 | 3.4 | 4 | 0.75 | 2.8 | 4 | |
| Standard deviation | 0.56 | 1.02 | 0 | 0.5401 | 0.9661 | 0 | 0.4859 | 1.0328 | 0 | |
| Friedman test | T1 | 37.1304 | 18.7273 | 18.6667 | ||||||
| p | <0.0001 | 0.0001 | 0.0001 | |||||||
| Post hoc 1 | (1) | <0.0001 | 0.0002 | 0.0007 | 0.0076 | 0.0002 | 0.0219 | |||
| (2) | 0.0002 | 0.4642 | 0.0076 | 1 | 0.0219 | 0.5391 | ||||
| (3) | <0.0001 | 0.4642 | 0.0007 | 1 | 0.0002 | 0.5391 | ||||
| Homogeneous groups | a | b | b | a | b | b | a | b | b | |
| Test page for trend | Z | 4.0319 | 3.0187 | 2.6833 | ||||||
| p | 0.0001 | 0.0025 | 0.0073 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adaszyńska-Skwirzyńska, M.; Zych, S.; Dzięcioł, M.; Konieczka, P.; Kowalik, B.; Witkowska, D.; Bucław, M. Potential of Essential Oils from Different Mint Species Against Multidrug-Resistant Escherichia coli Strains Isolated from Clinical Cases in Poultry. Int. J. Mol. Sci. 2025, 26, 11263. https://doi.org/10.3390/ijms262311263
Adaszyńska-Skwirzyńska M, Zych S, Dzięcioł M, Konieczka P, Kowalik B, Witkowska D, Bucław M. Potential of Essential Oils from Different Mint Species Against Multidrug-Resistant Escherichia coli Strains Isolated from Clinical Cases in Poultry. International Journal of Molecular Sciences. 2025; 26(23):11263. https://doi.org/10.3390/ijms262311263
Chicago/Turabian StyleAdaszyńska-Skwirzyńska, Michalina, Sławomir Zych, Małgorzata Dzięcioł, Paweł Konieczka, Barbara Kowalik, Dorota Witkowska, and Mateusz Bucław. 2025. "Potential of Essential Oils from Different Mint Species Against Multidrug-Resistant Escherichia coli Strains Isolated from Clinical Cases in Poultry" International Journal of Molecular Sciences 26, no. 23: 11263. https://doi.org/10.3390/ijms262311263
APA StyleAdaszyńska-Skwirzyńska, M., Zych, S., Dzięcioł, M., Konieczka, P., Kowalik, B., Witkowska, D., & Bucław, M. (2025). Potential of Essential Oils from Different Mint Species Against Multidrug-Resistant Escherichia coli Strains Isolated from Clinical Cases in Poultry. International Journal of Molecular Sciences, 26(23), 11263. https://doi.org/10.3390/ijms262311263






