The Gut Microbial Metabolite Indole-3-Acetic Acid Reprograms Systemic Homeostasis and Ameliorates IBD-Associated Cachexia Independent of Food Intake
Abstract
1. Introduction
2. Results
2.1. IAA Reduces the Severity of Clinical Colitis
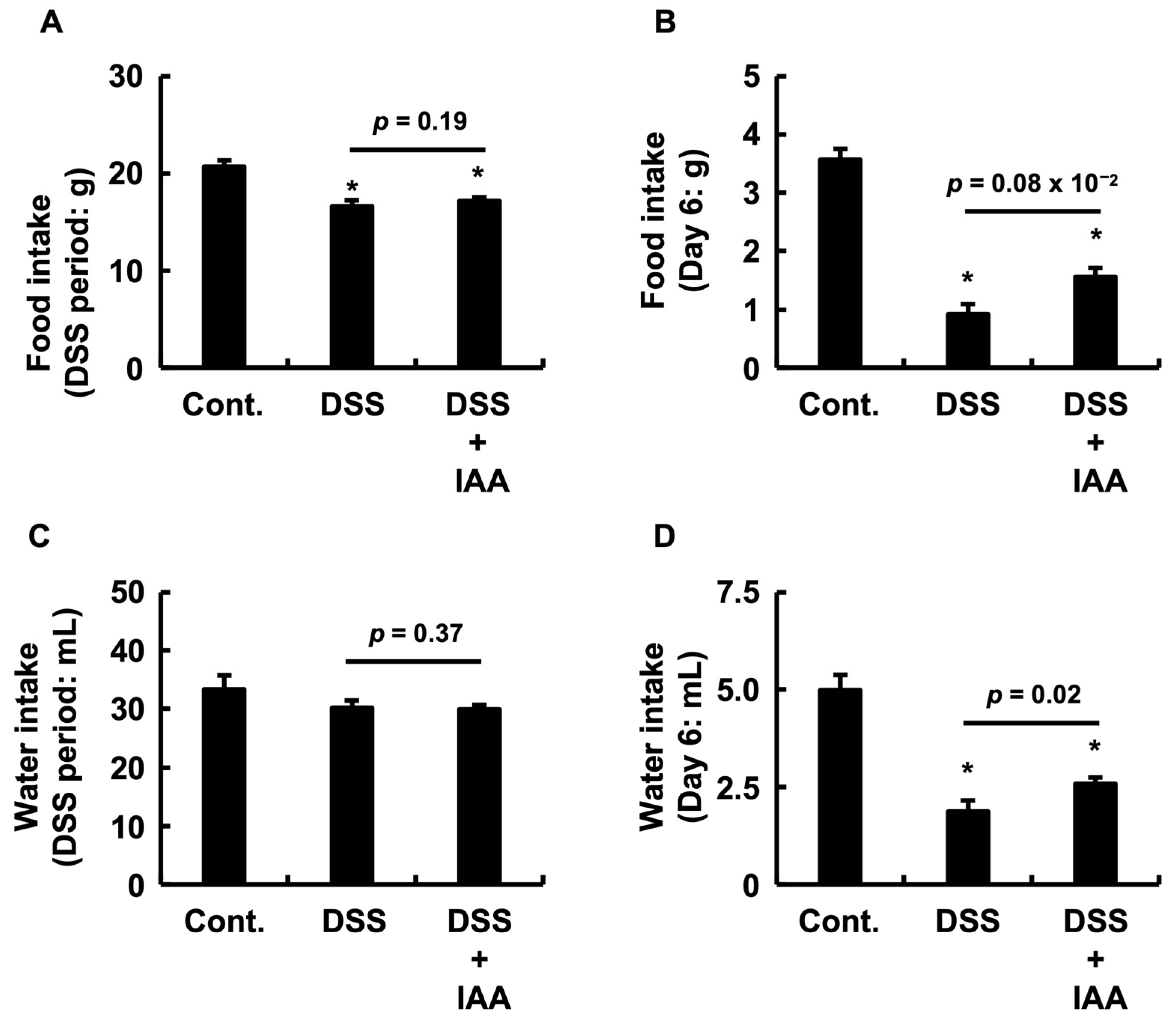
2.2. IAA Ameliorates Anorexia and Dehydration During Acute Colitis
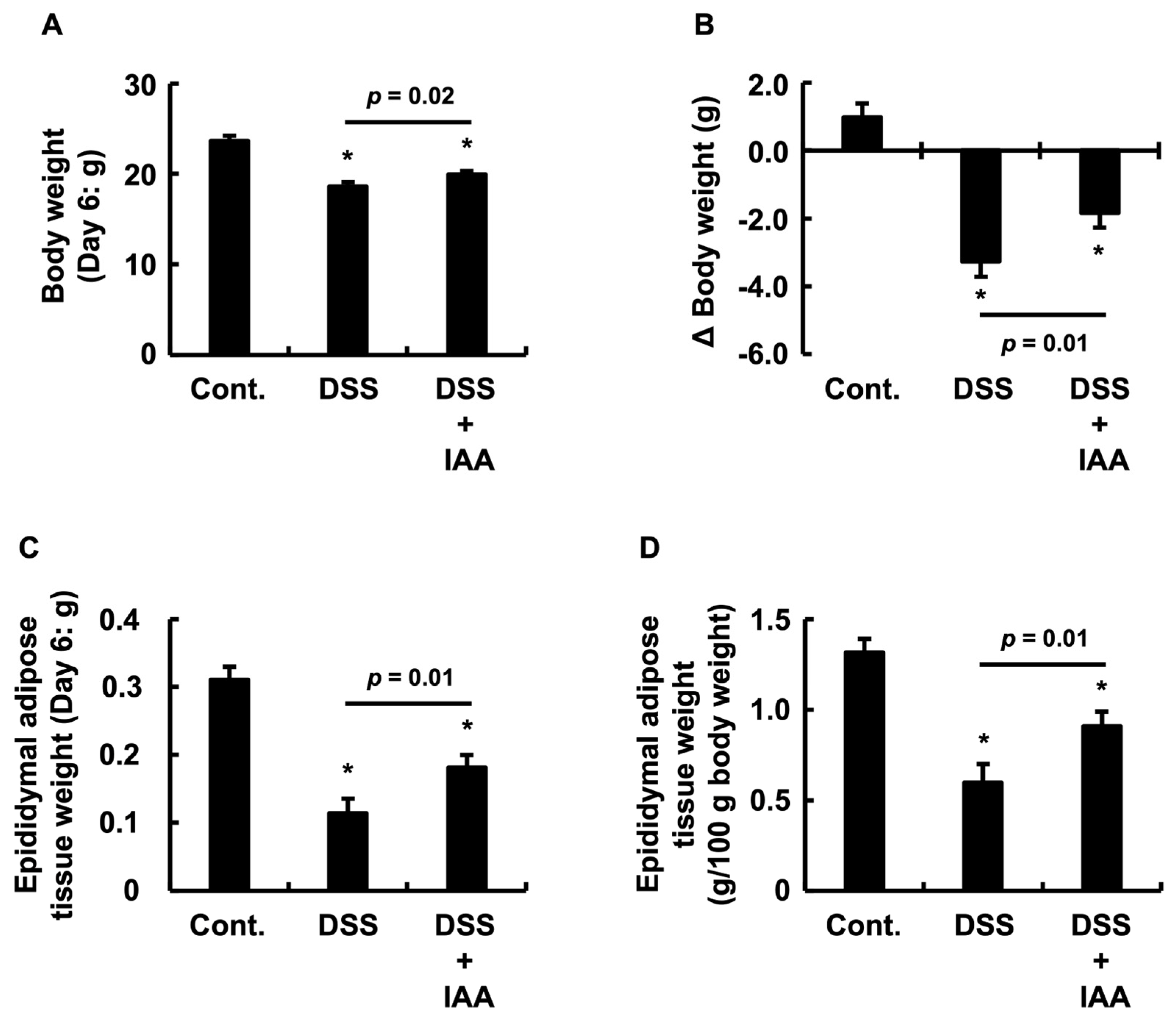
2.3. IAA Attenuates Weight Loss and Adipose Tissue Depletion in DSS-Induced Colitis
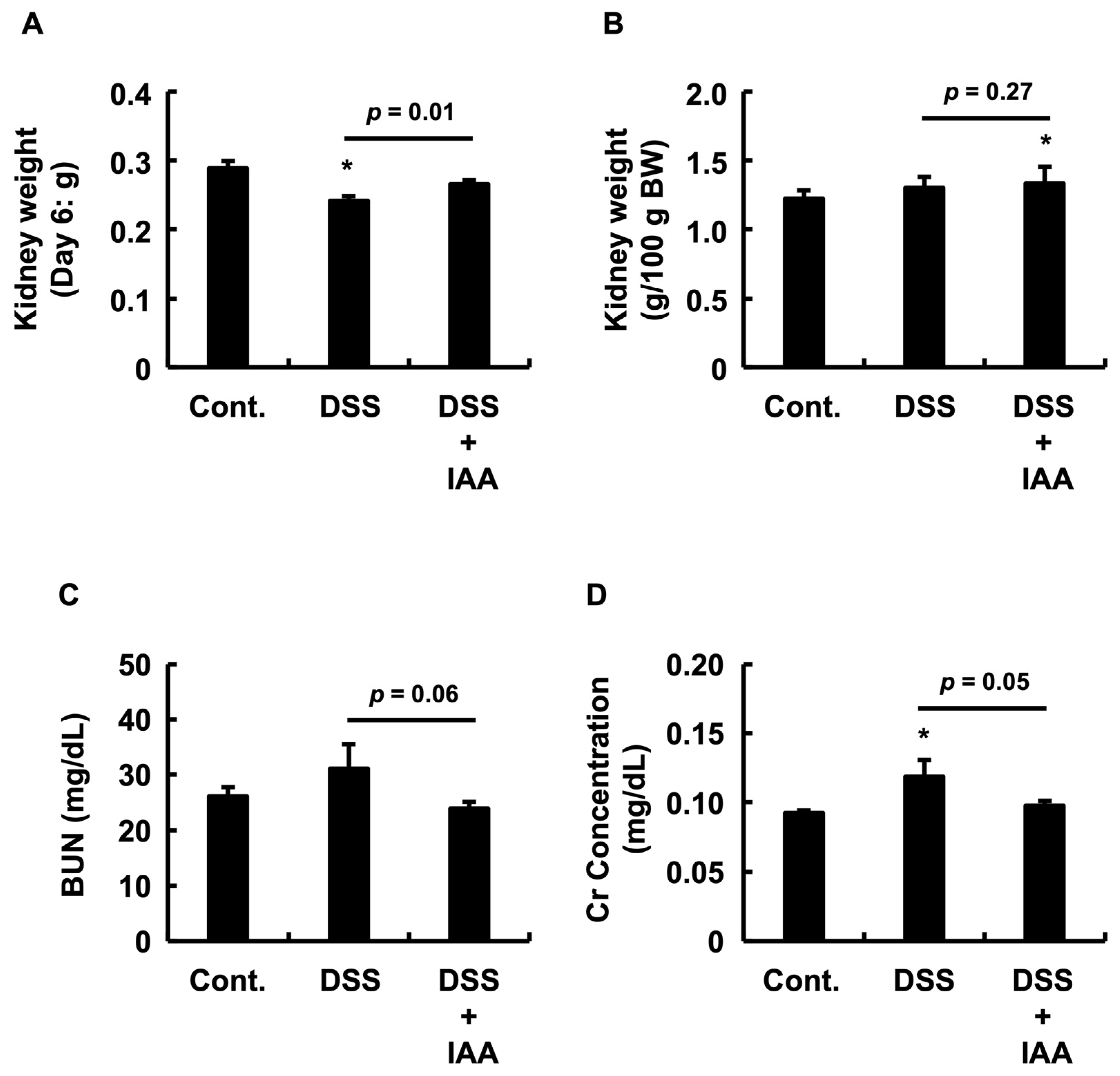
2.4. IAA Mitigates Incipient Renal Dysfunction in DSS-Induced Colitis
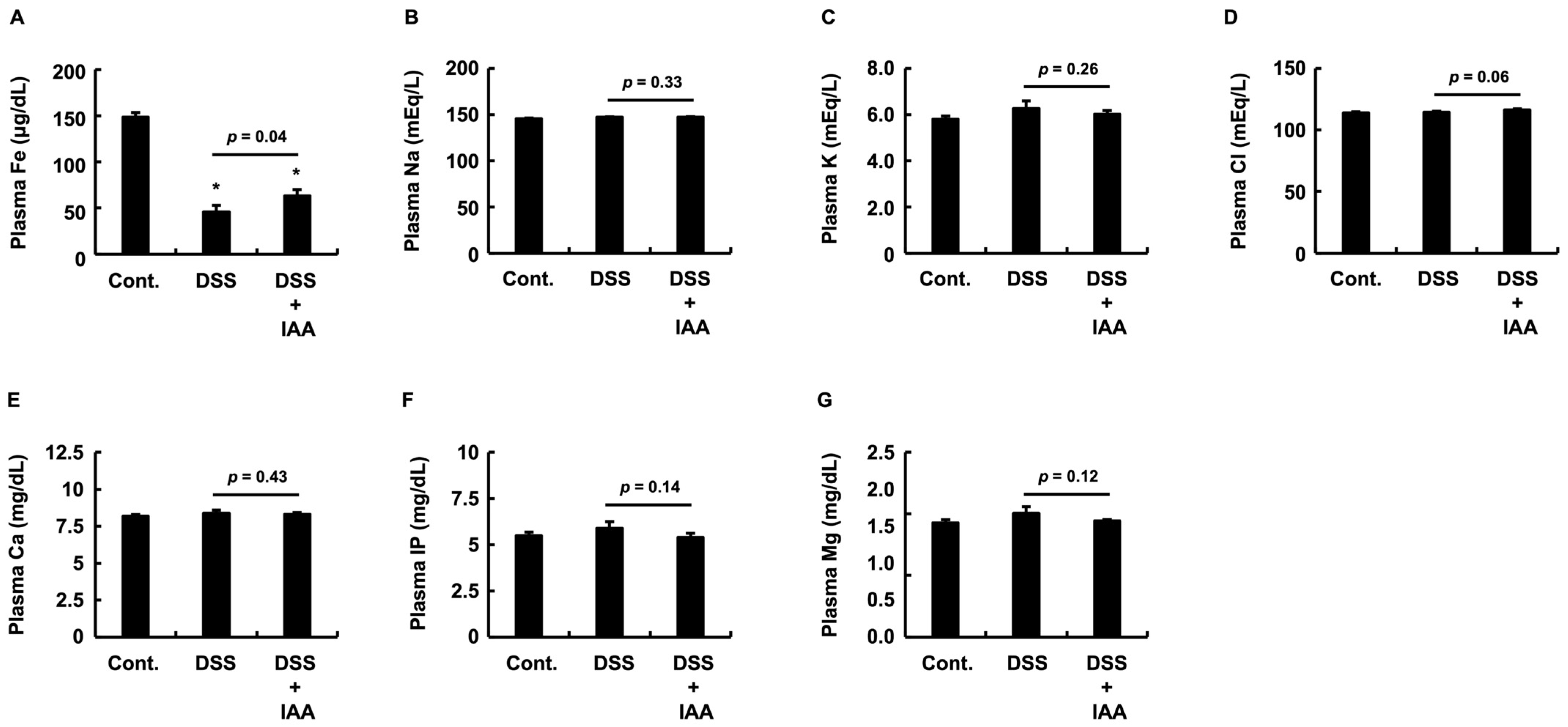
2.5. IAA Specifically Reverses Plasma Iron Depletion in DSS-Induced Colitis
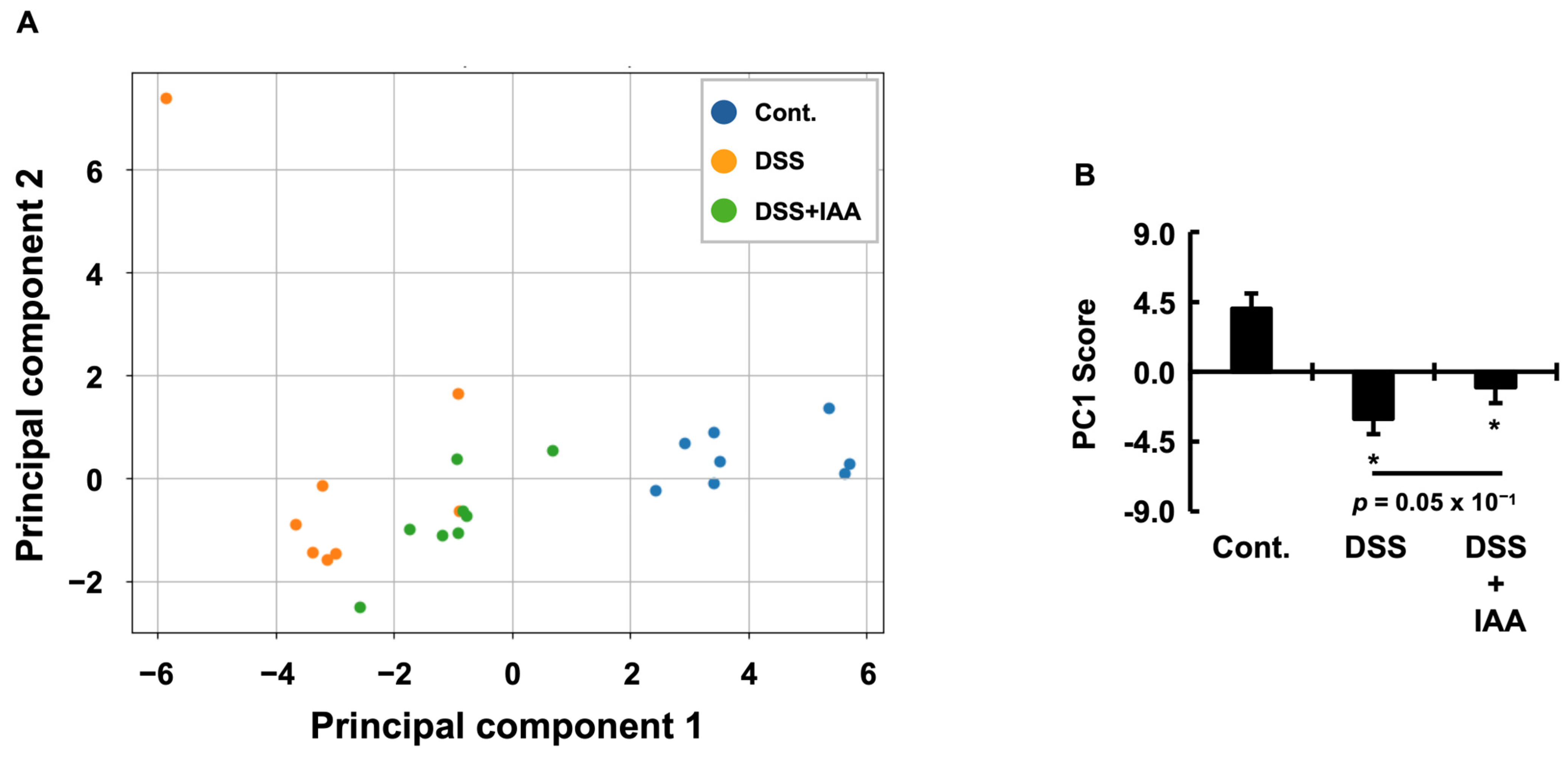
2.6. Integrative Assessment of Systemic Pathological State
2.7. IAA Remodels the Physiological Correlation Network Disrupted by DSS-Induced Colitis
3. Discussion
- Mechanism of Cachexia Amelioration: Investigate the “food intake-independent” effect by analyzing adipokine expression (e.g., ghrelin, leptin, adiponectin) and lipid metabolism genes in adipose tissue. Determine whether IAA acts directly on appetite centers via the brain–gut axis or indirectly via the resolution of inflammation.
- Scope of Renoprotective Effects: Conduct histopathological evaluations of renal tissue, including fibrosis assessment (e.g., Masson’s trichrome staining) and immunohistochemical analysis of injury markers.
- Effects on the Gut Microbiota: Comprehensive microbiome analyses, including 16S rRNA sequencing and metagenomics, will be performed to assess the influence of IAA on microbial composition and function.
4. Materials and Methods
4.1. Materials
4.2. Animal Experiments
- Pre-treatment phase (7 days): Mice were randomized into two groups. The IAA pre-treatment cohort (n = 8) received 5 mg/mL IAA dissolved in drinking water. The vehicle cohort (n = 16) received regular water intake. The mean body weights were matched between cohorts to ensure baseline equivalence.
- Disease induction phase (6 days): The vehicle cohort was further divided into two groups (n = 8 each): a healthy control group (receiving regular water) and a DSS group (receiving 2% w/v DSS in water). The IAA pre-treatment cohort was assigned to the DSS + IAA group (n = 8), which received a solution containing both 2% DSS and 5 mg/mL IAA. The mean body weights were matched across all three groups.
4.3. Measurement of Plasma Parameters
4.4. Statistical Analysis
- Principal Component Analysis (PCA) and Multivariate Score Tests: All parameters were standardized using z-score normalization prior to PCA. Principal component scores (PC1 and PC2) were calculated to capture the major biological variations. Inter-group differences in the multivariate space defined by PC1 and PC2 were assessed using multivariate analysis of variance (MANOVA), with Wilks’ Lambda and Pillai’s trace as test statistics. Pairwise group comparisons were further evaluated using Hotelling’s T2 test.
- Correlation Structure Analysis: Spearman’s rank correlation coefficients were calculated between the variables within each group. Fisher’s Z-transformation was applied to assess significant differences in correlation strength between groups.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Kirsner, J.B. The local and systemic complications of inflammatory bowel disease. JAMA 1979, 242, 1177–1183. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Faggiani, I.; Fanizza, J.; D’Amico, F.; Allocca, M.; Zilli, A.; Parigi, T.L.; Barchi, A.; Danese, S.; Furfaro, F. Extraintestinal Manifestations in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines 2024, 12, 1839. [Google Scholar] [CrossRef]
- Liu, L.F.; Fan, Y.W.; Lv, Y.; Liu, Z.X.; Dai, X.C. Strategies for assessing and preventing cardiovascular disease risk in inflammatory bowel disease patients: A meta-analysis and meta-regression and bibliometric review. PLoS ONE 2025, 20, e0327734. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Huangfu, G.; Yeaman, F.; Sukudom, S.; Lan, N.S.R.; Dwivedi, G.; Thin, L. Association between inflammatory bowel disease, current therapies, and cardiovascular events: A review and meta-analysis of data from 2.2 million individuals. J. Crohn’s Colitis 2025, 19, jjaf078. [Google Scholar] [CrossRef]
- Jaiswal, V.; Batra, N.; Dagar, M.; Butey, S.; Huang, H.; Chia, J.E.; Naz, S.; Endurance, E.O.; Raj, N.; Patel, S.; et al. Inflammatory bowel disease and associated cardiovascular disease outcomes: A systematic review. Medicine 2023, 102, e32775. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Non-alcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yanagi, K.; Yang, F.; Callaway, E.; Cheng, C.; Hensel, M.E.; Menon, R.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Oral supplementation of gut microbial metabolite indole-3-acetate alleviates diet-induced steatosis and inflammation in mice. eLife 2024, 12, RP87458. [Google Scholar] [CrossRef]
- Zhong, L.; Boopathi, S.; Purushothaman, B.; Tu, Q.; Zhang, Y. Gut microbiota-indole-3-acetic acid axis in cancer: Dual functions, mechanistic insights, and therapeutic potential. Microbiol. Res. 2025, 300, 128293. [Google Scholar] [CrossRef] [PubMed]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schonlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 615, 168, Erratum in Nature 2025, 641, E12–E13. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Yan, J.; Sun, J.; Wang, Y.; Huang, G.; Zhang, F.; Cao, H.; Li, D. Biotherapeutic potential of gut microbiota-derived indole-3-acetic acid. Crit. Rev. Microbiol. 2025, 1–21. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778, Erratum in Lancet 2020, 396, e56. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Zhang, Y.; Chung, H.; Fang, Q.W.; Xu, Y.R.; Zhang, Y.J.; Nakajo, K.; Wong, I.C.K.; Leung, W.K.; Qiu, H.; Li, X. Current and forecasted 10-year prevalence and incidence of inflammatory bowel disease in Hong Kong, Japan, and the United States. World J. Gastroenterol. 2025, 31, 105472. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic Dis. 2020, 11, 2040622319899297. [Google Scholar] [CrossRef]
- Caballero Mateos, A.M.; Cañadas de la Fuente, G.A.; Gros, B. Paradigm Shift in Inflammatory Bowel Disease Management: Precision Medicine, Artificial Intelligence, and Emerging Therapies. J. Clin. Med. 2025, 14, 1536. [Google Scholar] [CrossRef] [PubMed]
- Caron, B.; Habert, A.; Bonsack, O.; Camara, H.; Jeanbert, E.; Parigi, T.L.; Netter, P.; Danese, S.; Peyrin-Biroulet, L. Difficult-to-treat inflammatory bowel disease: Effectiveness and safety of 4th and 5th lines of treatment. United Eur. Gastroenterol. J. 2024, 12, 605–613. [Google Scholar] [CrossRef]
- Moss, A.C. Approach to Treatment Failure in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2022, 18, 360–363. [Google Scholar]
- State, M.; Negreanu, L. Defining the Failure of Medical Therapy for Inflammatory Bowel Disease in the Era of Advanced Therapies: A Systematic Review. Biomedicines 2023, 11, 544. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Tie, Y.; Huang, Y.; Chen, R.; Li, L.; Chen, M.; Zhang, S. Current insights on the roles of gut microbiota in inflammatory bowel disease-associated extra-intestinal manifestations: Pathophysiology and therapeutic targets. Gut Microbes 2023, 15, 2265028. [Google Scholar] [CrossRef]
- Shaheen, N.; Miao, J.; Xia, B.; Zhao, Y.; Zhao, J. Multifaceted Role of Microbiota-Derived Indole-3-Acetic Acid in Human Diseases and Its Potential Clinical Application. FASEB J. 2025, 39, e70574. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Deleu, S.; Puca, P.; Abreu, M.T.; Armuzzi, A.; Barbara, G.; Caprioli, F.; Chieng, S.; Costello, S.P.; Damiani, A.; et al. Guidance for Fecal Microbiota Transplantation Trials in Ulcerative Colitis: The Second ROME Consensus Conference. Inflamm. Bowel Dis. 2025, 31, 2408–2419. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, Y.; Wei, J.; Dong, Y.; Wang, J.; Dai, X.; Yan, J.; Chu, F.; Zhang, K.; Meng, F.; et al. Gut microbiota metabolite indole-3-acetic acid maintains intestinal epithelial homeostasis through mucin sulfation. Gut Microbes 2024, 16, 2377576. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248.e1. [Google Scholar] [CrossRef]
- Moutusy, S.I.; Ohsako, S. Gut Microbiome-Related Anti-Inflammatory Effects of Aryl Hydrocarbon Receptor Activation on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 3372. [Google Scholar] [CrossRef]
- Shiomi, Y.; Nishiumi, S.; Ooi, M.; Hatano, N.; Shinohara, M.; Yoshie, T.; Kondo, Y.; Furumatsu, K.; Shiomi, H.; Kutsumi, H.; et al. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm. Bowel Dis. 2011, 17, 2261–2274. [Google Scholar] [CrossRef]
- Ji, T.; Xu, C.; Sun, L.; Yu, M.; Peng, K.; Qiu, Y.; Xiao, W.; Yang, H. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig. Dis. Sci. 2015, 60, 1958–1966. [Google Scholar] [CrossRef]
- Vyhlídalová, B.; Krasulová, K.; Pečinková, P.; Marcalíková, A.; Vrzal, R.; Zemánková, L.; Vančo, J.; Trávníček, Z.; Vondráček, J.; Karasová, M.; et al. Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization. Int. J. Mol. Sci. 2020, 21, 2614. [Google Scholar] [CrossRef]
- Chowdhury, M.M.I.; Kurata, K.; Yuasa, K.; Koto, Y.; Nishimura, K.; Shimizu, H. Suppression of TNFα expression induced by indole-3-acetic acid is not mediated by AhR activation in Caco-2 cells. Biosci. Biotechnol. Biochem. 2021, 85, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.I.; Tomii, A.; Ishii, K.; Tahara, M.; Hitsuda, Y.; Koto, Y.; Kurata, K.; Yuasa, K.; Nishimura, K.; Shimizu, H. TLR4 may be a novel indole-3-acetic acid receptor that is implicated in the regulation of CYP1A1 and TNFα expression depending on the culture stage of Caco-2 cells. Biosci. Biotechnol. Biochem. 2021, 85, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Tomii, A.; Higa, M.; Naito, K.; Kurata, K.; Kobayashi, J.; Takei, C.; Yuasa, K.; Koto, Y.; Shimizu, H. Activation of the TLR4-JNK but not the TLR4-ERK pathway induced by indole-3-acetic acid exerts anti-proliferative effects on Caco-2 cells. Biosci. Biotechnol. Biochem. 2023, 87, 839–849. [Google Scholar] [CrossRef]
- Qu, X.; Song, Y.; Li, Q.; Xu, Q.; Li, Y.; Zhang, H.; Cheng, X.; Mackay, C.R.; Wang, Q.; Liu, W. Indole-3-acetic acid ameliorates dextran sulfate sodium-induced colitis via the ERK signaling pathway. Arch. Pharm. Res. 2024, 47, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, W.; Liang, Y.; Sun, L.; Yin, Y.; Zhang, W. Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells. Int. J. Mol. Sci. 2020, 21, 1579. [Google Scholar] [CrossRef]
- Fink, C.; Karagiannides, I.; Bakirtzi, K.; Pothoulakis, C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012, 18, 1550–1557. [Google Scholar] [CrossRef]
- Gonçalves, P.; Magro, F.; Martel, F. Metabolic inflammation in inflammatory bowel disease: Crosstalk between adipose tissue and bowel. Inflamm. Bowel Dis. 2015, 21, 453–467. [Google Scholar] [CrossRef]
- Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. [Google Scholar] [CrossRef]
- Eder, P.; Adler, M.; Dobrowolska, A.; Kamhieh-Milz, J.; Witowski, J. The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease. Cells 2019, 8, 628. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.; Kim, B.; Holzapfel, W.H.; Hyun, C.K. Disturbance of lipid metabolism in germ-free mice transplanted with gut microbiota of DSS-induced colitis mice. PLoS ONE 2023, 18, e0280850. [Google Scholar] [CrossRef]
- Aslam, T.; Mehmood, A. Prevalence and Risk Factors of Anemia in Inflammatory Bowel Diseases: A Case-Control Study. Cureus 2023, 15, e41990. [Google Scholar] [CrossRef]
- Eriksson, C.; Henriksson, I.; Brus, O.; Zhulina, Y.; Nyhlin, N.; Tysk, C.; Montgomery, S.; Halfvarson, J. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: A population-based cohort study. Aliment. Pharmacol. Ther. 2018, 48, 638–645. [Google Scholar] [CrossRef]
- Carrier, J.; Aghdassi, E.; Platt, I.; Cullen, J.; Allard, J.P. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment. Pharmacol. Ther. 2001, 15, 1989–1999. [Google Scholar] [CrossRef]
- Mahalhal, A.; Williams, J.M.; Johnson, S.; Ellaby, N.; Duckworth, C.A.; Burkitt, M.D.; Liu, X.; Hold, G.L.; Campbell, B.J.; Pritchard, D.M.; et al. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS ONE 2018, 13, e0202460. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, L.; Zeng, X.; Li, J.; Yin, Y.; Wang, Q.; Li, J.; Yang, H. Dietary High Dose of Iron Aggravates the Intestinal Injury but Promotes Intestinal Regeneration by Regulating Intestinal Stem Cells Activity in Adult Mice With Dextran Sodium Sulfate-Induced Colitis. Front. Vet. Sci. 2022, 9, 870303. [Google Scholar] [CrossRef] [PubMed]
- Loveikyte, R.; Bourgonje, A.R.; van Goor, H.; Dijkstra, G.; van der Meulen-de Jong, A.E. The effect of iron therapy on oxidative stress and intestinal microbiota in inflammatory bowel diseases: A review on the conundrum. Redox Biol. 2023, 68, 102950. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xiong, Q.; Kong, J.; Tian, C.; Miao, L.; Zhang, X.; Du, H. Intraperitoneal supplementation of iron alleviates dextran sodium sulfate-induced colitis by enhancing intestinal barrier function. Biomed. Pharmacother. 2021, 144, 112253. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.K.; Ellenbogen, S.; Trebicka, E.; Wang, L.; Mukhopadhyay, S.; Lacy-Hulbert, A.; Gallini, C.A.; Garrett, W.S.; Cherayil, B.J. Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS ONE 2012, 7, e38136. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684, Erratum in Nat. Genet. 2020, 52, 463. [Google Scholar] [CrossRef]
- Bessman, N.J.; Mathieu, J.R.R.; Renassia, C.; Zhou, L.; Fung, T.C.; Fernandez, K.C.; Austin, C.; Moeller, J.B.; Zumerle, S.; Louis, S.; et al. Dendritic cell-derived hepcidin sequesters iron from the microbiota to promote mucosal healing. Science 2020, 368, 186–189. [Google Scholar] [CrossRef]
- Han, X.; Xu, Z.; Chang, Y.; Li, H.; Hu, S.; Chang, S.; Liu, Y.; Yu, C.; Tang, T.; Li, Y. Concurrent chronic kidney disease in patients with inflammatory bowel disease, a systematic review and meta-analysis. Front. Med. 2024, 11, 1485087. [Google Scholar] [CrossRef] [PubMed]
- Zadora, W.; Innocenti, T.; Verstockt, B.; Meijers, B. Chronic Kidney Disease in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2024, 18, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ludvigsson, J.F.; Forss, A.; Faucon, A.L.; Faye, A.S.; Olén, O.; Sjölander, A.; Carrero, J.J. Risk of Kidney Failure in Patients With Inflammatory Bowel Disease Undergoing Colectomy: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2024, 22, 2291–2298.e17. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Zhang, J.; Su, H.; Ge, C. Causal effects of inflammatory bowel diseases on the risk of kidney stone disease: A two-sample bidirectional mendelian randomization. BMC Urol. 2023, 23, 162. [Google Scholar] [CrossRef]
- Caillard, P.; Bennis, Y.; Six, I.; Bodeau, S.; Kamel, S.; Choukroun, G.; Maizel, J.; Titeca-Beauport, D. The Role of Gut-Derived, Protein-Bound Uremic Toxins in the Cardiovascular Complications of Acute Kidney Injury. Toxins 2022, 14, 336. [Google Scholar] [CrossRef]
- Daneshamouz, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A. Protein-bound uremic toxins (PBUTs) in chronic kidney disease (CKD) patients: Production pathway, challenges and recent advances in renal PBUTs clearance. NanoImpact 2021, 21, 100299. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadvand, H.; Sotoodehnejadnematalahi, F.; Yaghmaei, P. The Protective Effects of Indole-Acetic Acid on the Renal Ischemia-Reperfusion Injury via Antioxidant and Antiapoptotic Properties in A Rat Model. Iran. J. Kidney Dis. 2022, 16, 125–134. [Google Scholar]
- Alhusaini, A.M.; Sarawi, W.; Mukhtar, N.; Aljubeiri, D.; Aljarboa, A.S.; Alduhailan, H.; Almutairi, F.; Mohammad, R.; Atteya, M.; Hasan, I. Role of Nrf2/HO-1 and cytoglobin signaling in the protective effect of indole-3-acetic acid and chenodeoxycholic acid against kidney injury induced by valproate. Heliyon 2024, 10, e41069. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Yan, X.; Wang, L.; Zhang, Y.; Qi, W.; Xi, J.; Hao, Z. Gut microbiota-derived indole-3-acetic acid ameliorates calcium oxalate renal stone formation via AHR/NF-κB axis. Urolithiasis 2025, 53, 134. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Sun, L.; Liu, X.; Zhang, W.; Hao, J. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut Microbes 2024, 16, 2329147. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Cho, K.A.; Kang, J.L.; Kim, K.H.; Woo, S.Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef]
- Wang, J.; Hao, Y.; Yang, Y.; Zhang, Y.; Xu, C.; Yang, R. Gut microbiota derived indole-3-acetic acid ameliorates precancerous inflammatory intestinal milieu to inhibit tumorigenesis through IL-35. J. Immunother. Cancer 2025, 13, e011155. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Qu, X.; Zhao, Y.; Zhang, X.; Cao, S.; Wang, X.; Song, Y.; Mackay, C.R.; Wang, Q. Indole-3-Acetic Acid Esterified with Waxy, Normal, and High-Amylose Maize Starches: Comparative Study on Colon-Targeted Delivery and Intestinal Health Impact. Nutrients 2024, 16, 3446. [Google Scholar] [CrossRef]
- Murthy, S.N.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef]

| Principal Component | Explained Variance Ratio (%) |
|---|---|
| PC1 | 49.4 |
| PC2 | 15.7 |
| PC1 + PC2 | 65.1 |
| Variable | PC1 Loading |
|---|---|
| Colon length | 0.29 |
| DAI | −0.29 |
| Body weight | 0.29 |
| ΔBody weight | 0.29 |
| Water intake (Day 6) | 0.29 |
| Plasma Fe concentration | 0.29 |
| Food intake (Day 6) | 0.29 |
| Fat weight | 0.28 |
| Fat/Body weight | 0.26 |
| Kidney weight | 0.24 |
| Food intake (DSS period) | 0.24 |
| Water intake (DSS period) | 0.14 |
| Plasma Na concentration | −0.12 |
| Kidney/Body weight | −0.12 |
| Plasma Mg concentration | −0.11 |
| Creatinine | −0.11 |
| BUN | −0.11 |
| Plasma K concentration | −0.10 |
| Plasma Cl concentration | −0.09 |
| Plasma IP concentration | −0.02 |
| Plasma Ca concentration | −0.02 × 10−1 |
| Variable | PC2 Loading |
|---|---|
| BUN | 0.45 |
| Plasma Mg concentration | 0.42 |
| Plasma IP concentration | 0.42 |
| Plasma Ca concentration | −0.38 |
| Kidney/Body weight | −0.33 |
| Food intake (DSS period) | 0.26 |
| Creatinine | 0.14 |
| Kidney weight | −0.14 |
| Water intake (DSS period) | 0.13 |
| Plasma Cl concentration | 0.12 |
| Fat/Body weight | −0.07 |
| Plasma Na concentration | −0.07 |
| Body weight | 0.06 |
| DAI | −0.05 |
| Plasma K concentration | −0.04 |
| Plasma Fe concentration | 0.04 |
| Food intake (Day 6) | 0.04 |
| Fat weight | −0.02 |
| ΔBody weight | −0.01 |
| Water intake (Day 6) | 0.01 |
| Colon length | −0.01 × 10−2 |
| Indicator | Value | p Value |
|---|---|---|
| Wilks’ lambda | 0.13 | 1.89 × 10−8 |
| Pillai’s trace | 0.9390 | 1.79 × 10−5 |
| Comparison Group | Hotelling T2 | F Value | p Value |
|---|---|---|---|
| Cont vs. DSS | 106.519 | 49.455 | 0.01 × 10−4 |
| Cont vs. IAA | 83.922 | 38.964 | 0.03 × 10−4 |
| DSS vs. IAA | 8.783 | 4.078 | 0.04 |
| Component | F Value | p Value |
|---|---|---|
| PC1 | 59.74715787 | 2.15 × 10−9 |
| PC2 | 1.019661616 | 0.37 |
| Group 1 | Group 2 | Variable 1 | Variable 2 | Spearman_r (Variable 1) | Spearman_r (Variable 2) | Z_Statistic | p Value |
|---|---|---|---|---|---|---|---|
| Cont | DSS | Body weight | Food intake (DSS period) | 0.83 | −0.25 | 2.30 | 0.02 |
| Cont | DSS | Body weight | K | 0.40 | −0.73 | 2.15 | 0.03 |
| Cont | DSS | Body weight | Ca | 0.87 | −0.03 | 2.19 | 0.02 |
| Cont | DSS | Body weight | IP | 0.97 | −0.16 | 3.57 | 0.03 × 10−2 |
| Cont | DSS | ΔBody weight | Water intake (Day 6) | 0.61 | 0.97 | −2.34 | 0.01 |
| Cont | DSS | ΔBody weight | Fat weight | 0.21 | 0.92 | −2.26 | 0.02 |
| Cont | DSS | ΔBody weight | Fat/Body weight | 0.14 | 0.90 | −2.14 | 0.03 |
| Cont | DSS | Food intake (DSS period) | Food intake (Day 6) | 0.92 | −0.03 | 2.66 | 0.07 × 10−1 |
| Cont | DSS | Food intake (DSS period) | Water intake (Day 6) | 0.92 | −0.25 | 3.01 | 0.02 × 10−1 |
| Cont | DSS | Food intake (DSS period) | Kidney weight | 0.90 | −0.27 | 2.81 | 0.04 × 10−1 |
| Cont | DSS | Food intake (DSS period) | Kidney/ Body weight | 0.85 | −0.35 | 2.62 | 0.08 × 10−1 |
| Cont | DSS | Food intake (DSS period) | Plasma Cl concentration | −0.49 | 0.74 | −2.35 | 0.01 |
| Cont | DSS | Food intake (Day 6) | Kidney/Body weight | 0.80 | −0.59 | 2.86 | 0.04 × 10−1 |
| Cont | DSS | Water intake (DSS period) | Water intake (Day 6) | 0.97 | 0.09 | 3.34 | 0.08 × 10−2 |
| Cont | DSS | Water intake (DSS period | Kidney weight | 0.88 | 0 | 2.18 | 0.02 |
| Cont | DSS | Water intake (DSS period) | Kidney/Body weight | 0.64 | −0.59 | 2.29 | 0.02 |
| Cont | DSS | Water intake (Day 6) | Colon length | 0.59 | 0.97 | −2.40 | 0.01 |
| Cont | DSS | Water intake (Day 6) | Fat weight | 0 | 0.90 | −2.36 | 0.01 |
| Cont | DSS | Water intake (Day 6) | Fat/Body weight | −0.23 | 0.88 | −2.56 | 0.01 |
| Cont | DSS | Fat/Body weigh | Plasma Fe concentration | −0.47 | 0.76 | −2.40 | 0.01 |
| Cont | DSS | Kidney weight | Plasma K concentration | 0.56 | −0.58 | 2.07 | 0.03 |
| Cont | DSS | Kidney weight | Plasma IP concentration | 0.80 | −0.16 | 2.01 | 0.04 |
| Cont | DSS | Cl | Plasma IP concentration | −0.77 | 0.32 | −2.18 | 0.02 |
| Cont | DSS | Ca | Plasma IP concentration | 0.79 | −0.27 | 2.16 | 0.03 |
| Cont | IAA | Body weight | Water intake (DSS period) | 0.92 | 0.35 | 2.01 | 0.04 |
| Cont | IAA | Body weight | Water intake (Day 6) | 0.90 | −0.13 | 2.57 | 0.09 × 10−1 |
| Cont | IAA | Body weight | Plasma Ca concentration | 0.87 | 0.02 | 2.09 | 0.03 |
| Cont | IAA | Body weight | Plasma IP concentration | 0.97 | 0.50 | 2.43 | 0.01 |
| Cont | IAA | ΔBody weight | Plasma Ca concentration | 0.79 | −0.63 | 2.89 | 0.03 × 10−1 |
| Cont | IAA | Food intake (DSS period) | Water intake (DSS period) | 0.90 | 0.04 | 2.29 | 0.02 |
| Cont | IAA | Food intake (DSS period) | Kidney weight | 0.90 | −0.17 | 2.65 | 0.07 × 10−1 |
| Cont | IAA | Food intake (DSS period) | Kidney/Body weight | 0.85 | −0.54 | 3.00 | 0.02 × 10−1 |
| Cont | IAA | Food intake (DSS period) | Plasma Ca concentration | 0.61 | −0.57 | 2.16 | 0.03 |
| Cont | IAA | Food intake (Day 6) | Water intake (DSS period) | 0.73 | −0.33 | 2.04 | 0.04 |
| Cont | IAA | Food intake (Day 6) | BUN | −0.64 | 0.45 | −1.97 | 0.04 |
| Cont | IAA | Food intake (Day 6) | Plasma Cl concentration | −0.23 | 0.78 | −2.05 | 0.03 |
| Cont | IAA | Water intake (DSS period) | Water intake (Day 6) | 0.97 | 0.03 | 3.43 | 0.05 × 10−2 |
| Cont | IAA | Water intake (Day 6) | Kidney weight | 0.90 | −0.06 | 2.46 | 0.01 |
| Cont | IAA | Water intake (Day 6) | Plasma Ca concentration | 0.75 | −0.41 | 2.25 | 0.02 |
| Cont | IAA | Colon length | Plasma Ca Concentration | 0.73 | −0.60 | 2.57 | 0.09 × 10−1 |
| Cont | IAA | Kidney/Body weight | BUN | −0.88 | 0.09 | −2.33 | 0.01 |
| Cont | IAA | BUN | Plasma K concentration | −0.44 | 0.71 | −2.15 | 0.03 |
| Cont | IAA | Creatinine | Plasma K concentration | 0.57 | −0.63 | 2.20 | 0.02 |
| Cont | IAA | Plasma Fe concentration | Plasma Ca concentration | 0.46 | −0.88 | 3.00 | 0.02 × 10−1 |
| Cont | IAA | Plasma Na concentration | Plasma Cl concentration | 0.91 | 0.21 | 2.07 | 0.03 |
| Cont | IAA | Plasma Ca concentration | Plasma IP concentration | 0.79 | −0.33 | 2.27 | 0.02 |
| DSS | IAA | DAI | Food intake (DSS period) | 0.21 | −0.77 | 1.99 | 0.04 |
| DSS | IAA | ΔBody weight | Water intake (Day 6) | 0.97 | 0.46 | 2.69 | 0.07 × 10−1 |
| DSS | IAA | Food intake (DSS period) | Creatinine | 0.66 | −0.71 | 2.69 | 0.07 × 10−1 |
| DSS | IAA | Food intake (DSS period) | Plasma Fe concentration | −0.49 | 0.85 | −2.87 | 0.04 × 10−1 |
| DSS | IAA | Water intake (Day 6) | Colon length | 0.97 | −0.06 | 3.59 | 0.03 × 10−2 |
| DSS | IAA | Water intake (Day 6) | Fat weight | 0.90 | 0.17 | 2.08 | 0.03 |
| DSS | IAA | Water intake (Day 6) | Fat/Body weight | 0.88 | 0.09 | 2.03 | 0.04 |
| DSS | IAA | Kidney weight | Plasma Fe concentration | 0.71 | −0.34 | 1.98 | 0.04 |
| DSS | IAA | Kidney weight | Plasma K concentration | −0.58 | 0.59 | −2.14 | 0.03 |
| DSS | IAA | Kidney/Body weight | Plasma Cl concentration | −0.59 | 0.52 | −1.99 | 0.04 |
| DSS | IAA | BUN | Creatinine | 0.49 | −0.76 | 2.46 | 0.01 |
| DSS | IAA | BUN | Plasma IP concentration | 0.66 | 0.56 | 2.27 | 0.02 |
| DSS | IAA | Creatinine | Plasma IP concentration | 0.87 | 0.08 | 2.02 | 0.04 |
| DSS | IAA | Creatinine | Plasma Mg concentration | 0.83 | −0.12 | 2.13 | 0.03 |
| DSS | IAA | Plasma Fe concentration | Plasma Ca concentration | −0.05 | −0.88 | 2.10 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomii, A.; Takei, C.; Yoshikiyo, K.; Shimizu, H. The Gut Microbial Metabolite Indole-3-Acetic Acid Reprograms Systemic Homeostasis and Ameliorates IBD-Associated Cachexia Independent of Food Intake. Int. J. Mol. Sci. 2025, 26, 11260. https://doi.org/10.3390/ijms262311260
Tomii A, Takei C, Yoshikiyo K, Shimizu H. The Gut Microbial Metabolite Indole-3-Acetic Acid Reprograms Systemic Homeostasis and Ameliorates IBD-Associated Cachexia Independent of Food Intake. International Journal of Molecular Sciences. 2025; 26(23):11260. https://doi.org/10.3390/ijms262311260
Chicago/Turabian StyleTomii, Ayame, Chihiro Takei, Keisuke Yoshikiyo, and Hidehisa Shimizu. 2025. "The Gut Microbial Metabolite Indole-3-Acetic Acid Reprograms Systemic Homeostasis and Ameliorates IBD-Associated Cachexia Independent of Food Intake" International Journal of Molecular Sciences 26, no. 23: 11260. https://doi.org/10.3390/ijms262311260
APA StyleTomii, A., Takei, C., Yoshikiyo, K., & Shimizu, H. (2025). The Gut Microbial Metabolite Indole-3-Acetic Acid Reprograms Systemic Homeostasis and Ameliorates IBD-Associated Cachexia Independent of Food Intake. International Journal of Molecular Sciences, 26(23), 11260. https://doi.org/10.3390/ijms262311260





