Diagnostic Potential of Periostin, Galectin-3 and Tenascin C Serum Measurements in Inflammatory Bowel Disease: Pilot Study
Abstract
1. Introduction
2. Results
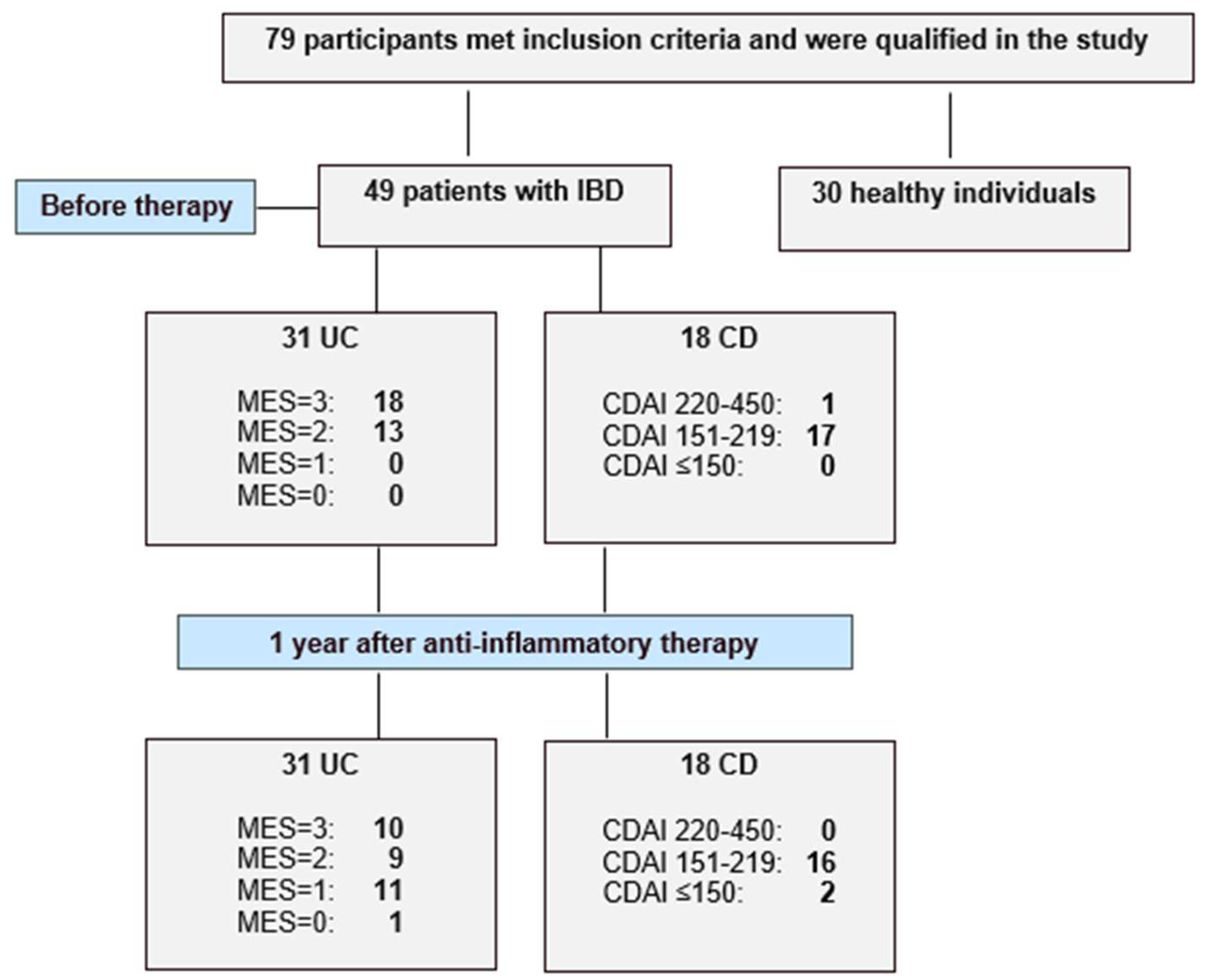
2.1. Characteristics of Patients
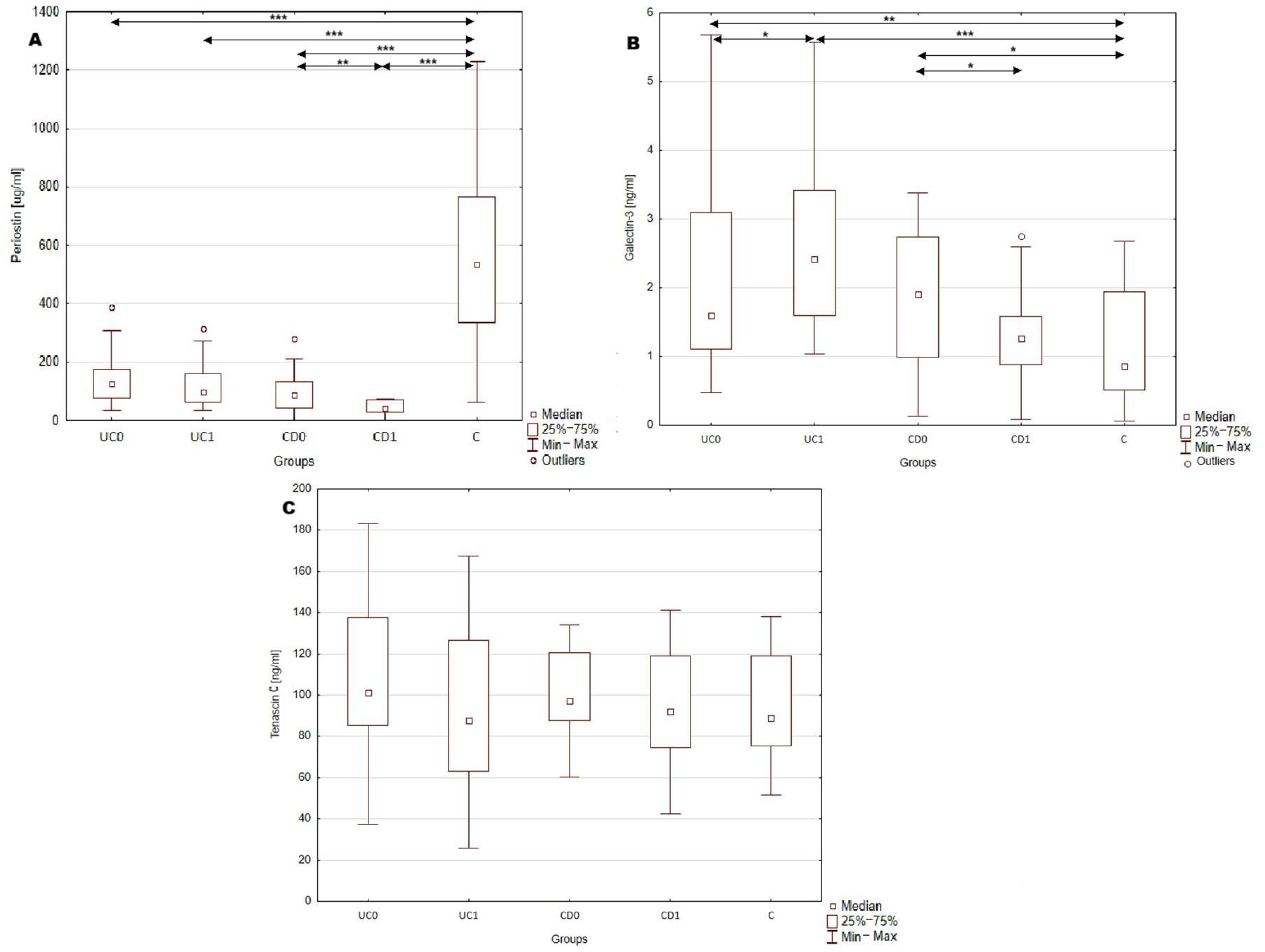
2.2. Serum Profile of Periostin, Galectin-3 and Tenascin C in Patients with Inflammatory Bowel Disease and Healthy Individuals
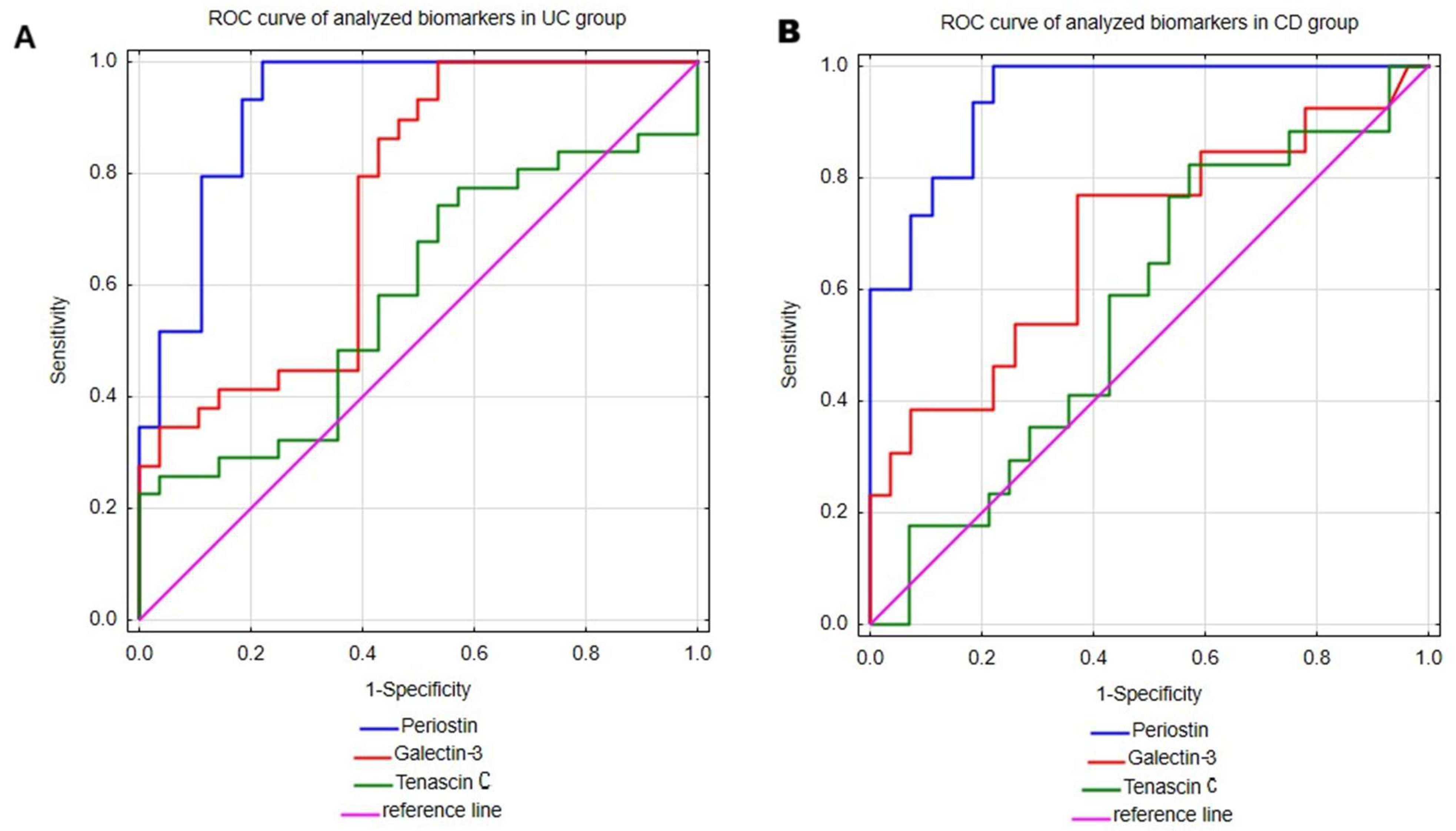
2.3. Evaluation of Serum Periostin, Galectin-3 and Tenascin C Profile as Diagnostic Biomarkers of Ulcerative Colitis and Crohn’s Disease
2.4. Utility of Serum Periostin, Galectin-3 and Tenascin C in Assessing Disease Activity in Inflammatory Bowel Disease
2.5. Effect of Implemented Treatment on the Serum Profile of Periostin, Galectin-3 and Tenascin C in Patients with IBD
3. Discussion
3.1. Serum Profile of Periostin, Galectin-3 and Tenascin C in Patients with Inflammatory Bowel Disease and Healthy Individuals
3.2. Utility of Serum Periostin, Galectin-3 and Tenascin C Profile in Evaluating Disease Activity and Response to Treatment in Patients with IBD
4. Materials and Methods
4.1. Study Population
4.2. Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef]
- Le Berre, C.; Ananthakrishnan, A.N.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative Colitis and Crohn’s Disease Have Similar Burden and Goals for Treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar] [CrossRef]
- Feakins, R.M. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology 2014, 64, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Norouzinia, M.; Chaleshi, V.; Alizadeh, A.H.M.; Zali, M.R. Biomarkers in inflammatory bowel diseases: Insight into diagnosis, prognosis and treatment. Gastroenterol. Hepatol. Bed Bench 2017, 10, 155–167. [Google Scholar] [PubMed]
- Mortensen, J.H.; Lindholm, M.; Langholm, L.L.; Kjeldsen, J.; Bay-Jensen, A.C.; Karsdal, M.A.; Manon-Jensen, T. The intestinal tissue homeostasis—The role of extracellular matrix remodeling in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 977–993. [Google Scholar] [CrossRef]
- Yang, L.; Guo, T.; Chen, Y.; Bian, K. The Multiple Roles of Periostin in Non-Neoplastic Disease. Cells 2022, 12, 50. [Google Scholar] [CrossRef]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy. Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Kii, I.; Ito, H. Periostin and its interacting proteins in the construction of extracellular architectures. Cell. Mol. Life Sci. 2017, 74, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, G.; Creber, K.; Hamilton, D.W. Wound healing and fibrosis: A contrasting role for periostin in skin and the oral mucosa. Am. J. Physiol. Cell Physiol. 2020, 318, C1065–C1077. [Google Scholar] [CrossRef]
- Koh, S.J.; Choi, Y.; Kim, B.G.; Lee, K.L.; Kim, D.W.; Kim, J.H.; Kim, J.W.; Kim, J.S. Matricellular Protein Periostin Mediates Intestinal Inflammation through the Activation of Nuclear Factor κB Signaling. PLoS ONE 2016, 11, e0149652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Liu, H. Tenascin-C: A Key Regulator in Angiogenesis during Wound Healing. Biomolecules 2022, 12, 1689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016, 7, 11703. [Google Scholar] [CrossRef]
- Giblin, S.P.; Midwood, K.S. Tenascin-C: Form versus function. Cell Adh. Migr. 2015, 9, 48–82. [Google Scholar] [CrossRef]
- McLeod, K.; Walker, J.T.; Hamilton, D.W. Galectin-3 regulation of wound healing and fibrotic processes: Insights for chronic skin wound therapeutics. J. Cell Commun. Signal. 2018, 12, 281–287. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Simovic Markovic, B.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V.; et al. Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J. Crohn’s Colitis 2016, 10, 593–606. [Google Scholar] [CrossRef]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef]
- Lippert, E.; Falk, W.; Bataille, F.; Kaehne, T.; Naumann, M.; Goeke, M.; Herfarth, H.; Schoelmerich, J.; Rogler, G. Soluble galectin-3 is a strong, colonic epithelial-cell-derived, lamina propria fibroblast-stimulating factor. Gut 2007, 56, 43–51. [Google Scholar] [CrossRef]
- Walker, J.T.; McLeod, K.; Kim, S.; Conway, S.J.; Hamilton, D.W. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res. 2016, 365, 453–465. [Google Scholar] [CrossRef]
- Coelho, T.; Sonnenberg-Riethmacher, E.; Gao, Y.; Mossotto, E.; Khojanazarov, A.; Griffin, A.; Mukanova, S.; Ashimkhanova, A.; Haggarty, R.; Borissenko, A.; et al. Expression profile of the matricellular protein periostin in paediatric inflammatory bowel disease. Sci. Rep. 2021, 11, 6194. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Kii, I.; Kashima, T.G.; Kikuchi, Y.; Ohazama, A.; Shimazaki, M.; Fukayama, M.; Kudo, A. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS ONE 2011, 6, e18410. [Google Scholar] [CrossRef] [PubMed]
- Takedomi, H.; Nunomura, S.; Nanri, Y.; Honda, Y.; Yokomizo, K.; Akutagawa, T.; Tsuruoka, N.; Sakata, Y.; Conway, S.; Kawaguchi, A.; et al. Serum Periostin is Able to Stratify Type 2-Dominant Ulcerative Colitis. Inflamm. Bowel Dis. 2025, 31, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.G.; Subramanian, S. Serum galectins as potential biomarkers of inflammatory bowel diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef]
- Frol’ová, L.; Smetana, K., Jr.; Borovská, D.; Kitanovicová, A.; Klimesová, K.; Janatková, I.; Malícková, K.; Lukás, M.; Drastich, P.; Benes, Z.; et al. Detection of galectin-3 in patients with inflammatory bowel diseases: New serum marker of active forms of IBD? Inflamm. Res. 2009, 58, 503–512. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Amin, H.Z.; Amin, L.Z.; Wijaya, I.P. Galectin-3: A novel biomarker for the prognosis of heart failure. Clujul Med. 2017, 90, 129–132. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Ning, L.; Li, S.; Gao, J.; Ding, L.; Wang, C.; Chen, W.; Shan, G.; Zhang, F.; Yu, J.; Xu, G. Tenascin-C Is Increased in Inflammatory Bowel Disease and Is Associated with response to Infliximab Therapy. BioMed Res. Int. 2019, 2019, 1475705. [Google Scholar] [CrossRef]
- Chen, S.S.; Sun, L.W.; Brickner, H.; Sun, P.Q. Downregulating galectin-3 inhibits proinflammatory cytokine production by human monocyte-derived dendritic cells via RNA interference. Cell. Immunol. 2015, 294, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar]
- Sakurai, T.; Saruta, M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion 2023, 104, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Kyle, B.D.; Agbor, T.A.; Sharif, S.; Chauhan, U.; Marshall, J.; Halder, S.L.S.; Ip, S.; Khan, W.I. Fecal Calprotectin, CRP and Leucocytes in IBD Patients: Comparison of Biomarkers With Biopsy Results. J. Can. Assoc. Gastroenterol. 2020, 4, 84–90. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Gut 2004, 53, 620–625. [Google Scholar] [CrossRef]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B. IBSEN Study Group. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Am. J. Gastroenterol. 2008, 103, 78–89. [Google Scholar]
- Qiu, X.N.; Hong, D.; Shi, Z.R.; Lu, S.Y.; Lai, Y.X.; Ren, Y.L.; Liu, X.T.; Guo, C.P.; Tan, G.Z.; Wang, L.C. TNF-α promotes CXCL-1/8 production in keratinocytes by downregulating galectin-3 through NF-κB and hsa-miR-27a-3p pathway to contribute psoriasis development. Immunopharmacol. Immunotoxicol. 2023, 45, 692–700. [Google Scholar] [CrossRef]
- Dabelic, S.; Novak, R.; Goreta, S.S.; Dumic, J. Galectin-3 expression in response to LPS, immunomodulatory drugs and exogenously added galectin-3 in monocyte-like THP-1 cells. In Vitr. Cell. Dev. Biol. Anim. 2012, 48, 518–527. [Google Scholar] [CrossRef]
- Takayama, G.; Arima, K.; Kanaji, T.; Toda, S.; Tanaka, H.; Shoji, S.; McKenzie, A.N.; Nagai, H.; Hotokebuchi, T.; Izuhara, K. Periostin: A novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J. Allergy Clin. Immunol. 2006, 118, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Luchak, A.; Solomon, L.A.; Kanagalingam, T.; Vijeyakumaran, M.; Rowe, B.H.; Cameron, L. Comparative efficacy of glucocorticoid receptor agonists on Th2 cell function and attenuation by progesterone. BMC Immunol. 2020, 21, 54. [Google Scholar] [CrossRef]
- Franchimont, D.; Louis, E.; Dewe, W.; Martens, H.; Vrindts-Gevaert, Y.; De Groote, D.; Belaiche, J.; Geenen, V. Effects of dexamethasone on the profile of cytokine secretion in human whole blood cell cultures. Regul. Pept. 1998, 73, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pittoni, V.; Bombardieri, M.; Spinelli, F.R.; Scrivo, R.; Alessandri, C.; Conti, F.; Spadaro, A.; Valesini, G. Anti-tumour necrosis factor (TNF) alpha treatment of rheumatoid arthritis (infliximab) selectively down regulates the production of interleukin (IL) 18 but not of IL12 and IL13. Ann. Rheum. Dis. 2002, 61, 723–725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magnusson, M.K.; Strid, H.; Isaksson, S.; Bajor, A.; Lasson, A.; Ung, K.A.; Öhman, L. Response to infliximab therapy in ulcerative colitis is associated with decreased monocyte activation, reduced CCL2 expression and downregulation of Tenascin C. J. Crohn’s Colitis 2015, 9, 56–65. [Google Scholar] [CrossRef]
| Parameter | Total (N = 49) | UC (N = 31) | CD (N = 18) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | UC0 | UC1 | p | CD0 | CD1 | p | ||
| Females, n (%) | 21 (42.8%) | 13 (41.9%) | - | 8 (44.4%) | - | ||||
| Males, n (%) | 28 (57.1%) | 18 (58.1%) | - | 10 (55.6%) | - | ||||
| Implemented treatment | Biological or conventional anti-inflammatory treatment | Biological, TNF-α inhibitors (adalimumab) | - | Conventional anti-inflammatory, steroids and immunomodulators (azathioprine or mercaptiopurine) | - | ||||
| Age [years] | 32.7 ± 12 | - | 33 ±13 | - | - | 32 ± 9.6 | - | ||
| BMI [kg/m2] | 23.2 ± 3.5 | 22.8 ± 3.3 | 24.3 ± 3.6 | 24.5 ± 4.2 | >0.05 | 20.6 ± 3.4 | 19.8 ± 2.8 | >0.05 | |
| Disease activity, n (%) | Severe | 35 (71.4%) | 26 (53.1%) | 18 (58.1%) | 10 (32.3%) | - | 17 (94.4%) | 16 (88.9%) | - |
| Moderate | 14 (28.6%) | 11 (22.4%) | 13 (41.9%) | 9 (29%) | 1 (5.6%) | 2 (11.1%) | |||
| Mild | 0 (0%) | 11 (22.4%) | 0 (0%) | 11 (35.5%) | 0 (0%) | 0 (0%) | |||
| Remission | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3.2%) | 0 (0%) | 0 (0%) | |||
| Disease activity in Mayo or CDAI scale | - | - | Mayo: 3 (2–3) | Mayo: 2 (1–3) | <0.005 | CDAI: 290.83 ± 35.53 | CDAI: 270.8 ± 44.3 | >0.05 | |
| CRP [mg/L] | 5.5 (2.3–19.9) | 5.2 (2.2–15.2) | 3.37 (1.26–15.20) | 2.41 (1.51–7.62) | >0.05 | 13.90 (3.20–31.40) | 15.2 (5.3–23.4) | >0.05 | |
| Serum calprotectin [ng/mL] | 2877.2 (1801.3–4754.5) | 2901.7 ± 1195.8 | 2782.9 (1674.2–4754.5) | 2708.3 ± 890.9 | >0.05 | 3537.52 ± 1893.78 | 2915.3 ± 1325.9 | >0.05 | |
| Parametr | UC | CD | Control | ||||
|---|---|---|---|---|---|---|---|
| UC0 | UC1 | p UC0 vs. C | CD0 | CD1 | p CD0 vs. C | ||
| Periostin [μg/mL] | 119.33 (77.83–167.85) | 99.45 (63.06–161.86) | <0.0001 | 98.03 ± 61.62 | 41.38 ± 25.19 | <0.0001 | 548.8 ± 307.46 |
| Galectin-3 [ng/mL] | 1.63 (1.14–3.09) | 2.42 (1.6–3.42) | <0.0005 | 1.92 ± 0.97 | 1.33 ± 0.77 | <0.05 | 0.95 (0.59–2.01) |
| Tenascin C [ng/mL] | 105.17 ± 36.69 | 93.48 ± 38.72 | >0.05 | 99.91 ± 22.69 | 93.88 ± 31.01 | >0.05 | 95.12 ± 26.18 |
| Parameter | Analyzed Groups | AUC (95% CI) | Youden Index | Cut-Off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Periostin | UC | 0.922 (0.85–0.995) | 0.78 | 308.36 μg/mL | 100 | 77.8 | 82.8 | 100 |
| CD | 0.943 (0.88–1) | 0.78 | 280.90 μg/mL | 100 | 77.8 | 71.4 | 100 | |
| Galectin-3 | UC | 0.745 (0.616–0.875) | 0.46 | 0.75 ng/mL | 100 | 46.4 | 65.9 | 100 |
| CD | 0.691 (0.506–0.876) | 0.40 | 1.22 ng/mL | 76.9 | 63.0 | 50.0 | 85.0 | |
| Tenascin C | UC | 0.582 (0.434–0.73) | 0.23 | 138.32 ng/mL | 22.6 | 100 | 100 | 53.8 |
| CD | 0.567 (0.395–0.739) | 0.25 | 85.28 ng/mL | 82.4 | 42.9 | 46.7 | 80.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górecka, A.; Jura-Półtorak, A.; Szeremeta, A.; Komosinska-Vassev, K. Diagnostic Potential of Periostin, Galectin-3 and Tenascin C Serum Measurements in Inflammatory Bowel Disease: Pilot Study. Int. J. Mol. Sci. 2025, 26, 10439. https://doi.org/10.3390/ijms262110439
Górecka A, Jura-Półtorak A, Szeremeta A, Komosinska-Vassev K. Diagnostic Potential of Periostin, Galectin-3 and Tenascin C Serum Measurements in Inflammatory Bowel Disease: Pilot Study. International Journal of Molecular Sciences. 2025; 26(21):10439. https://doi.org/10.3390/ijms262110439
Chicago/Turabian StyleGórecka, Aleksandra, Agnieszka Jura-Półtorak, Anna Szeremeta, and Katarzyna Komosinska-Vassev. 2025. "Diagnostic Potential of Periostin, Galectin-3 and Tenascin C Serum Measurements in Inflammatory Bowel Disease: Pilot Study" International Journal of Molecular Sciences 26, no. 21: 10439. https://doi.org/10.3390/ijms262110439
APA StyleGórecka, A., Jura-Półtorak, A., Szeremeta, A., & Komosinska-Vassev, K. (2025). Diagnostic Potential of Periostin, Galectin-3 and Tenascin C Serum Measurements in Inflammatory Bowel Disease: Pilot Study. International Journal of Molecular Sciences, 26(21), 10439. https://doi.org/10.3390/ijms262110439






