Early Supplementation with Branched-Chain Amino Acids Ameliorates Lipid Retention in Aortic Valves of ApoE-Knockout Mice
Abstract
1. Introduction
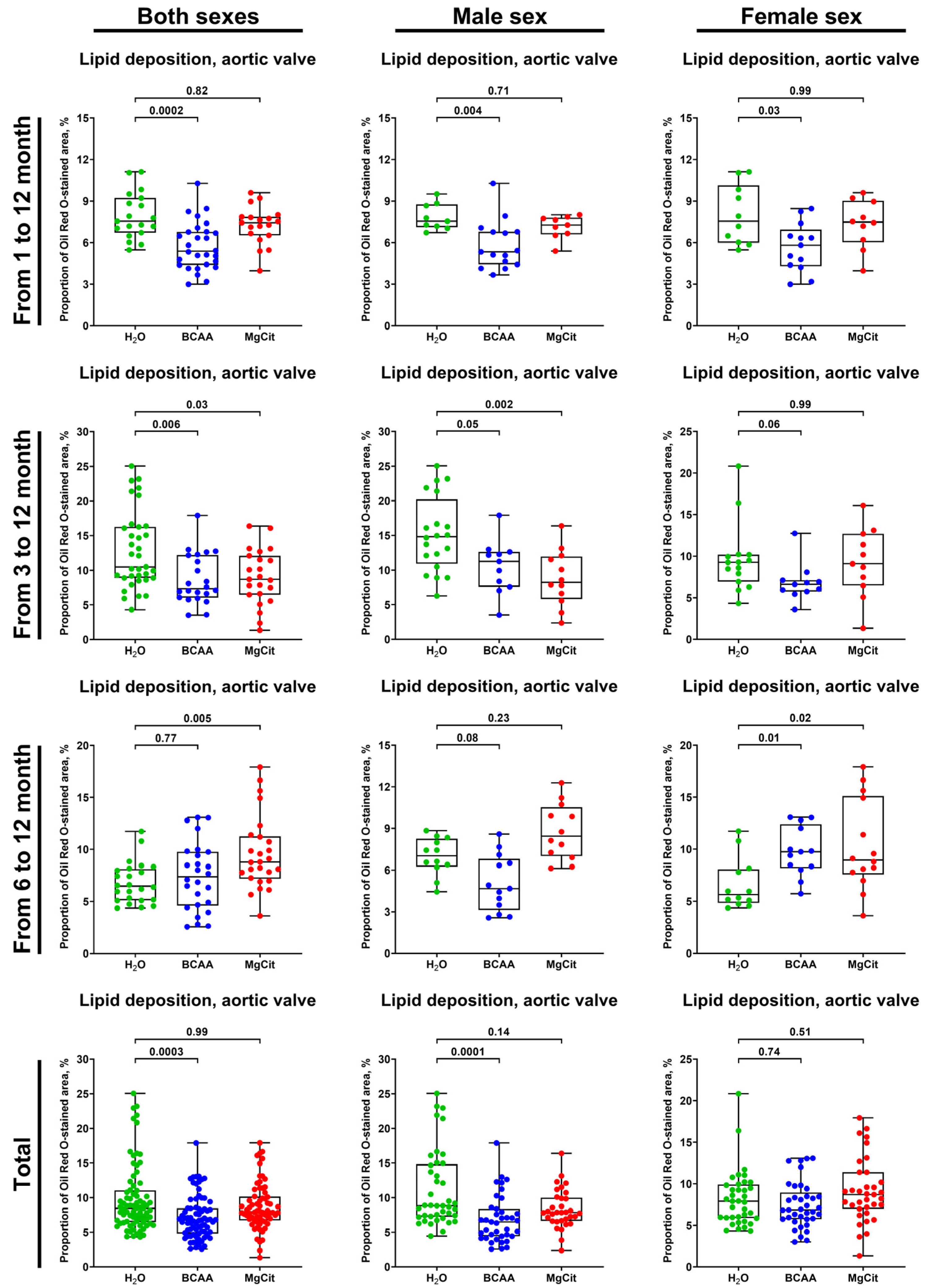
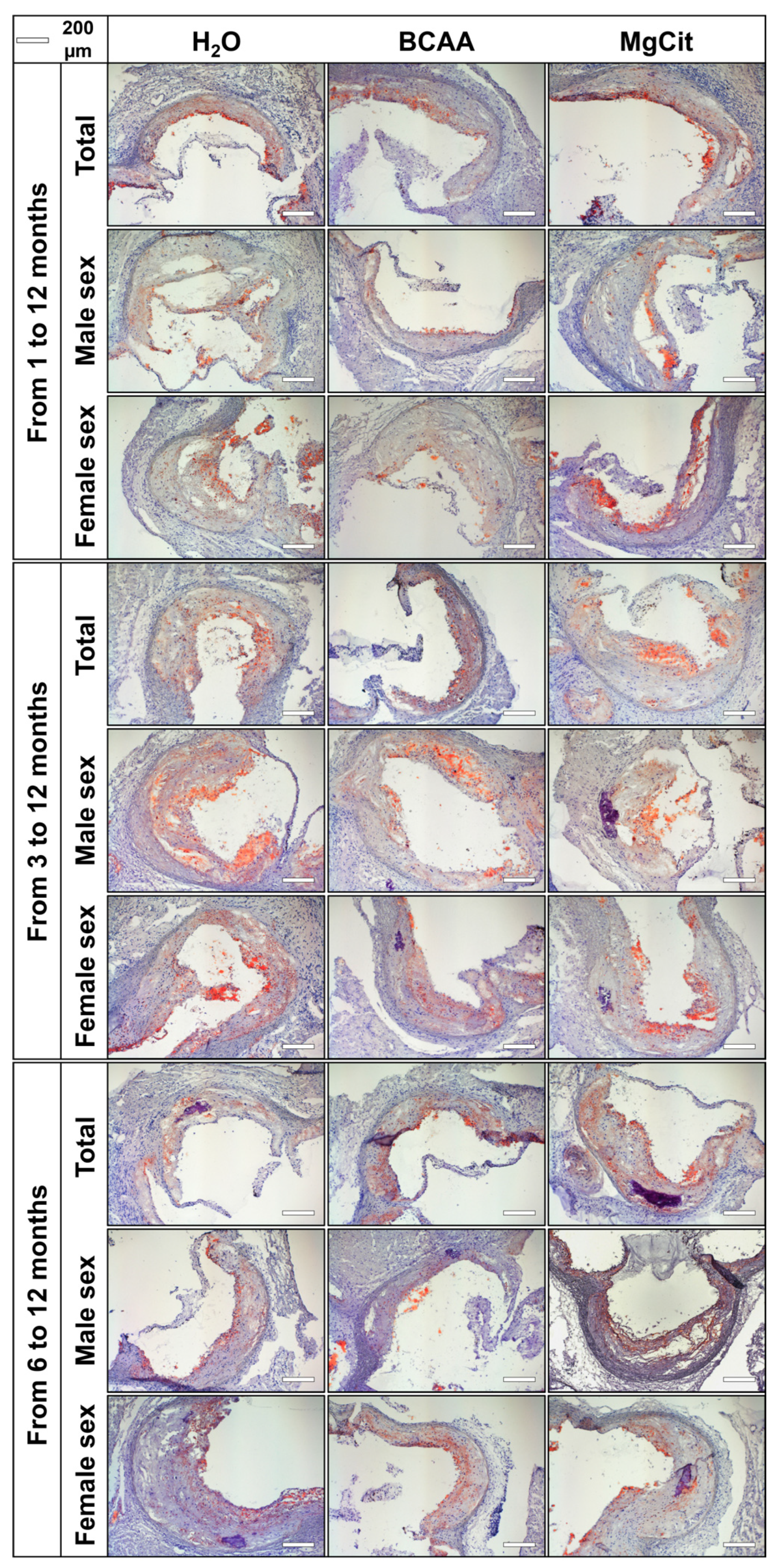
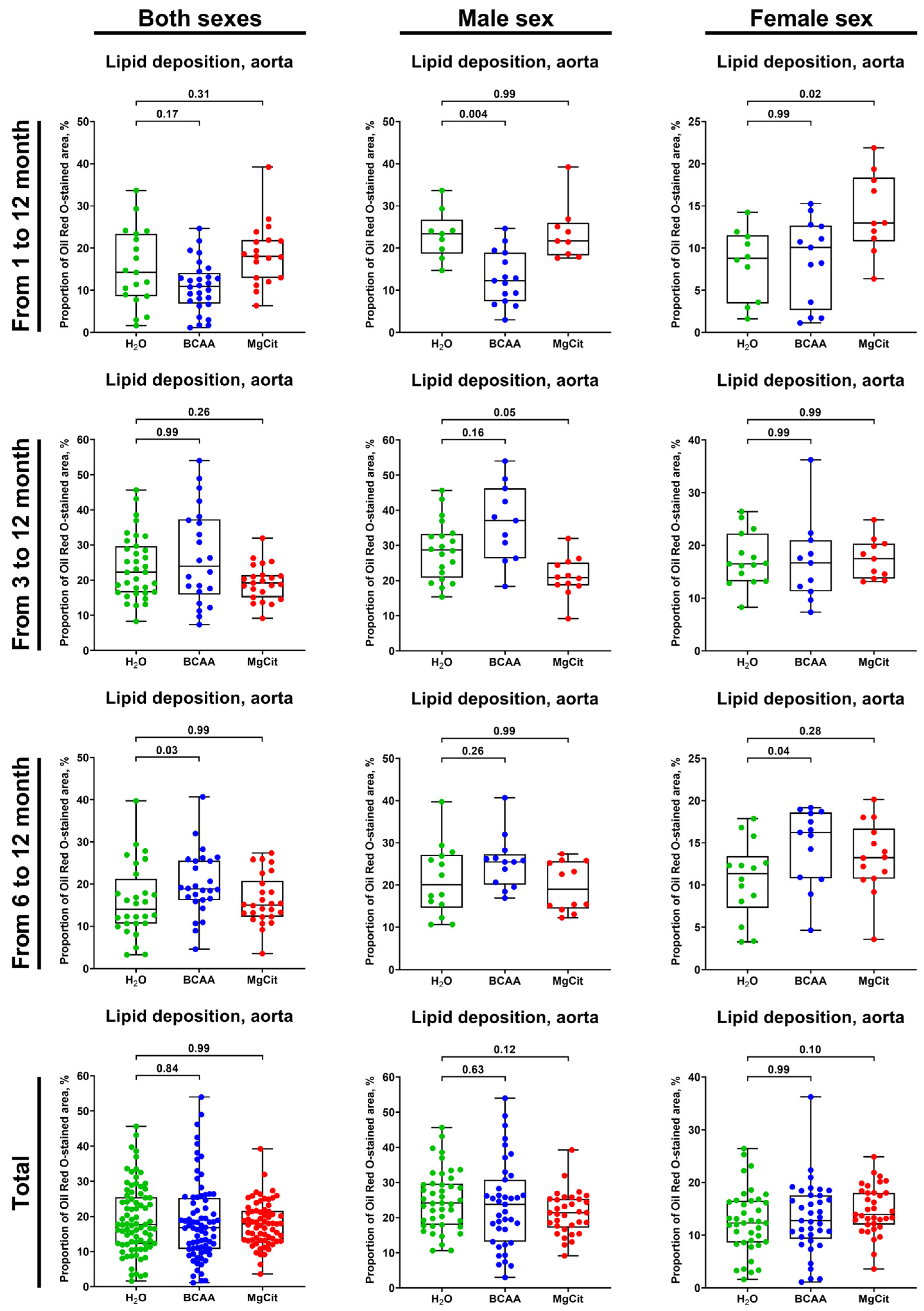
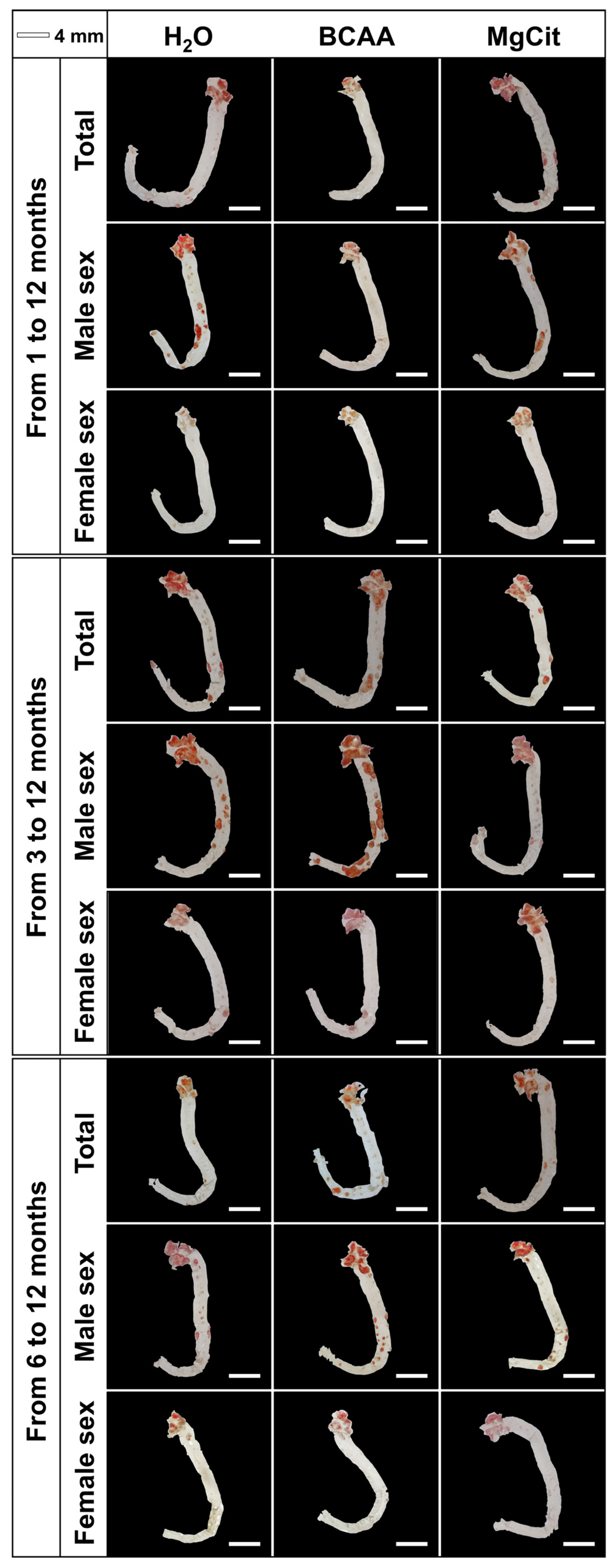
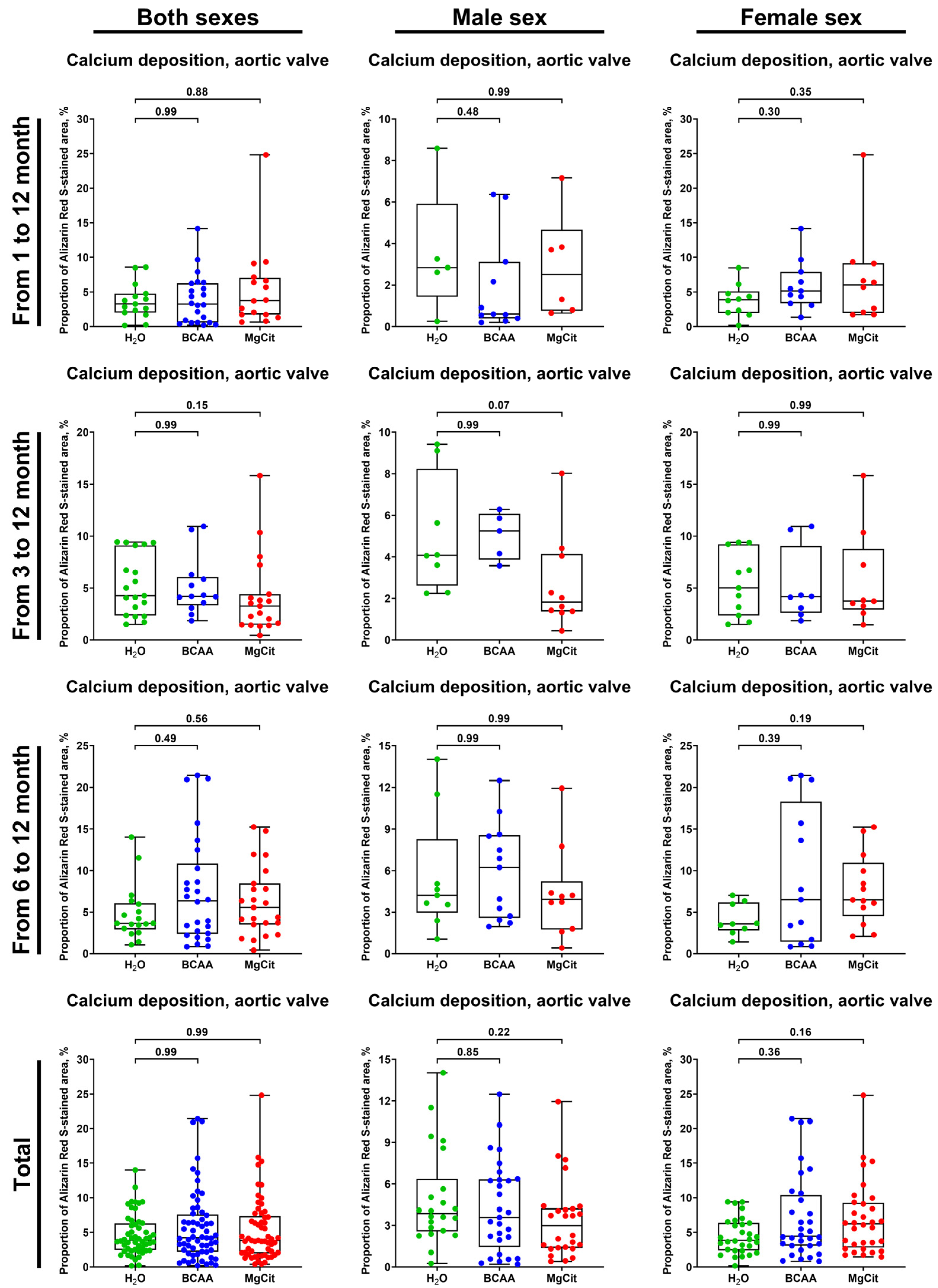
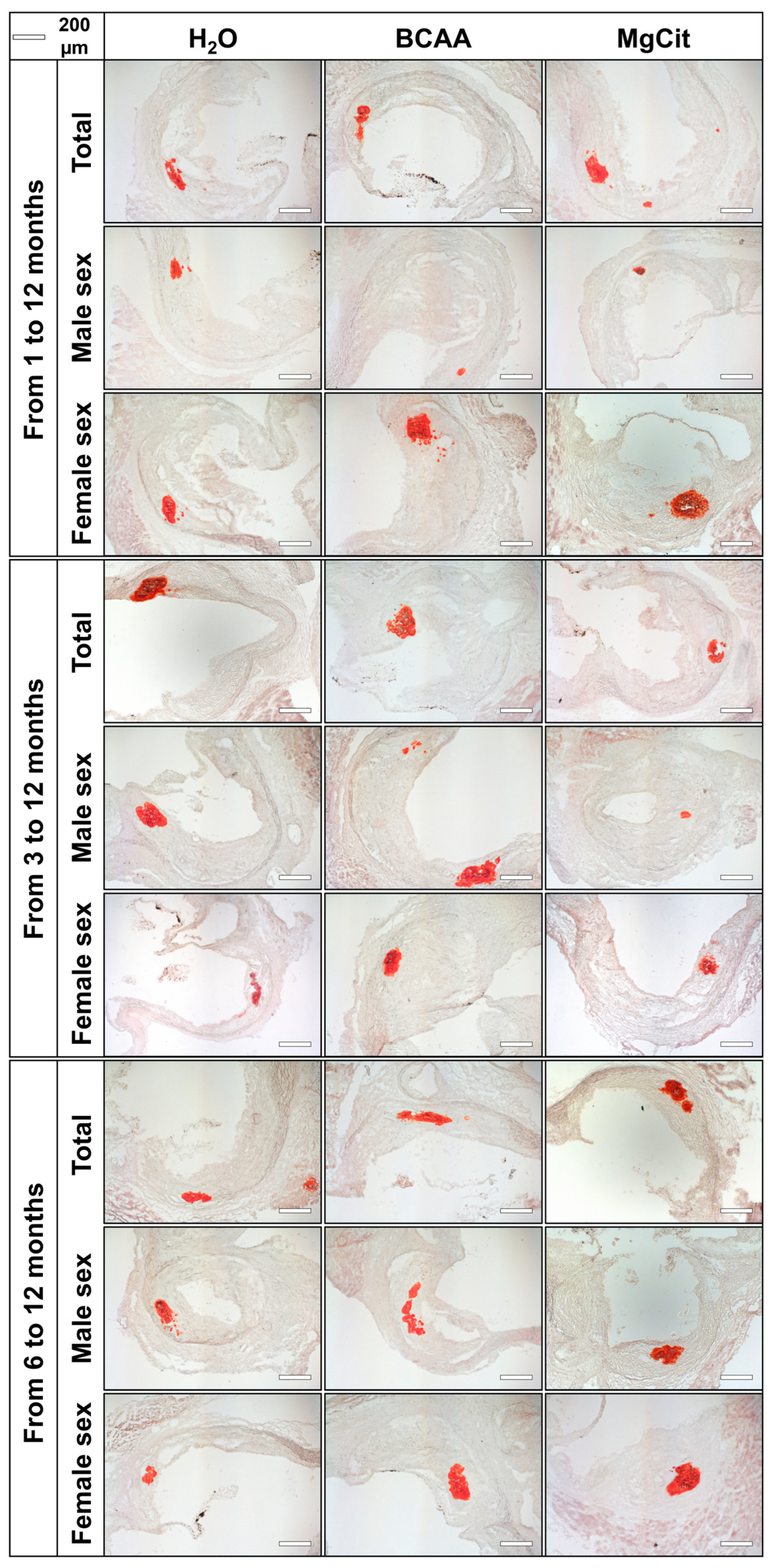
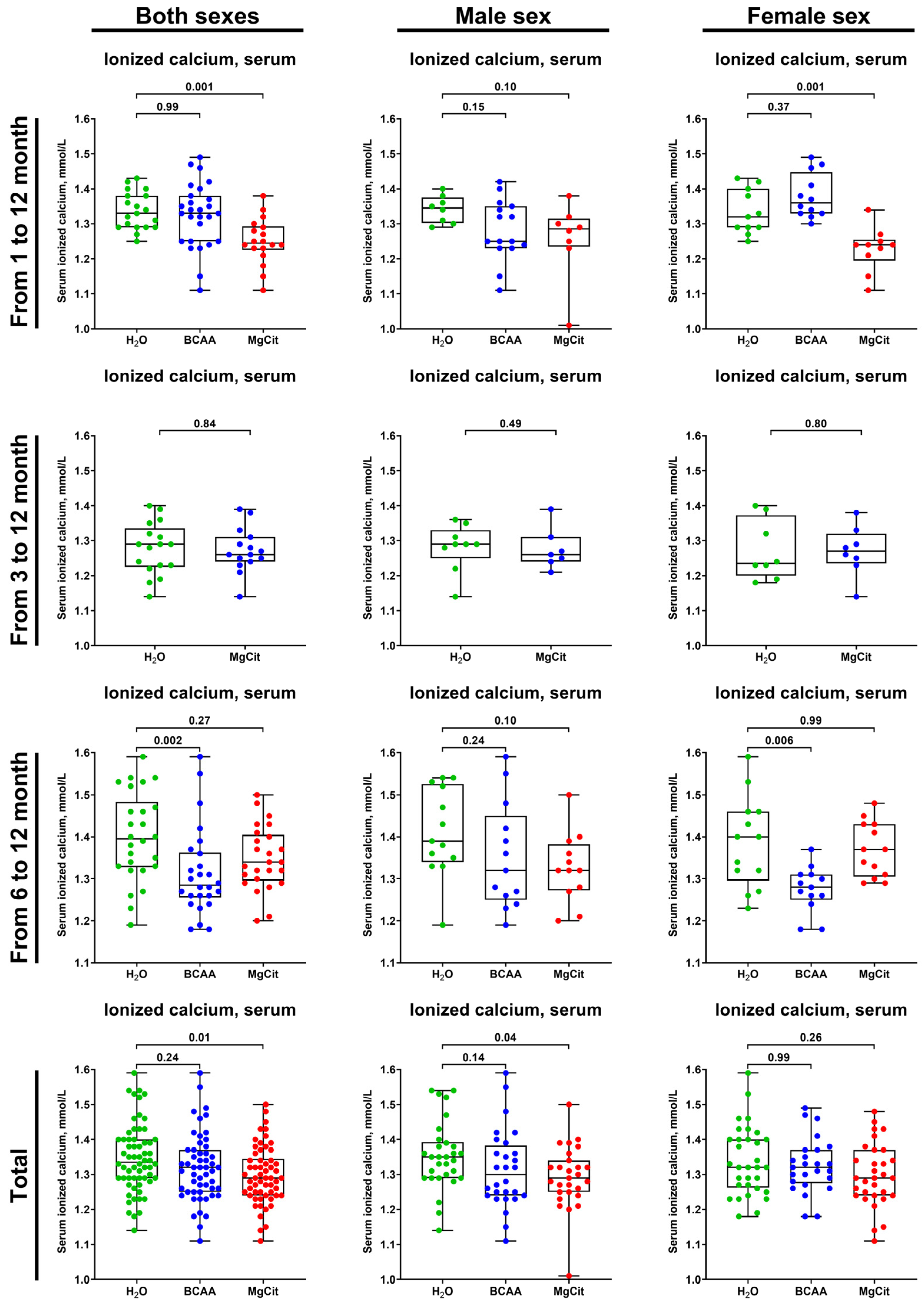
2. Results
3. Discussion
4. Materials and Methods
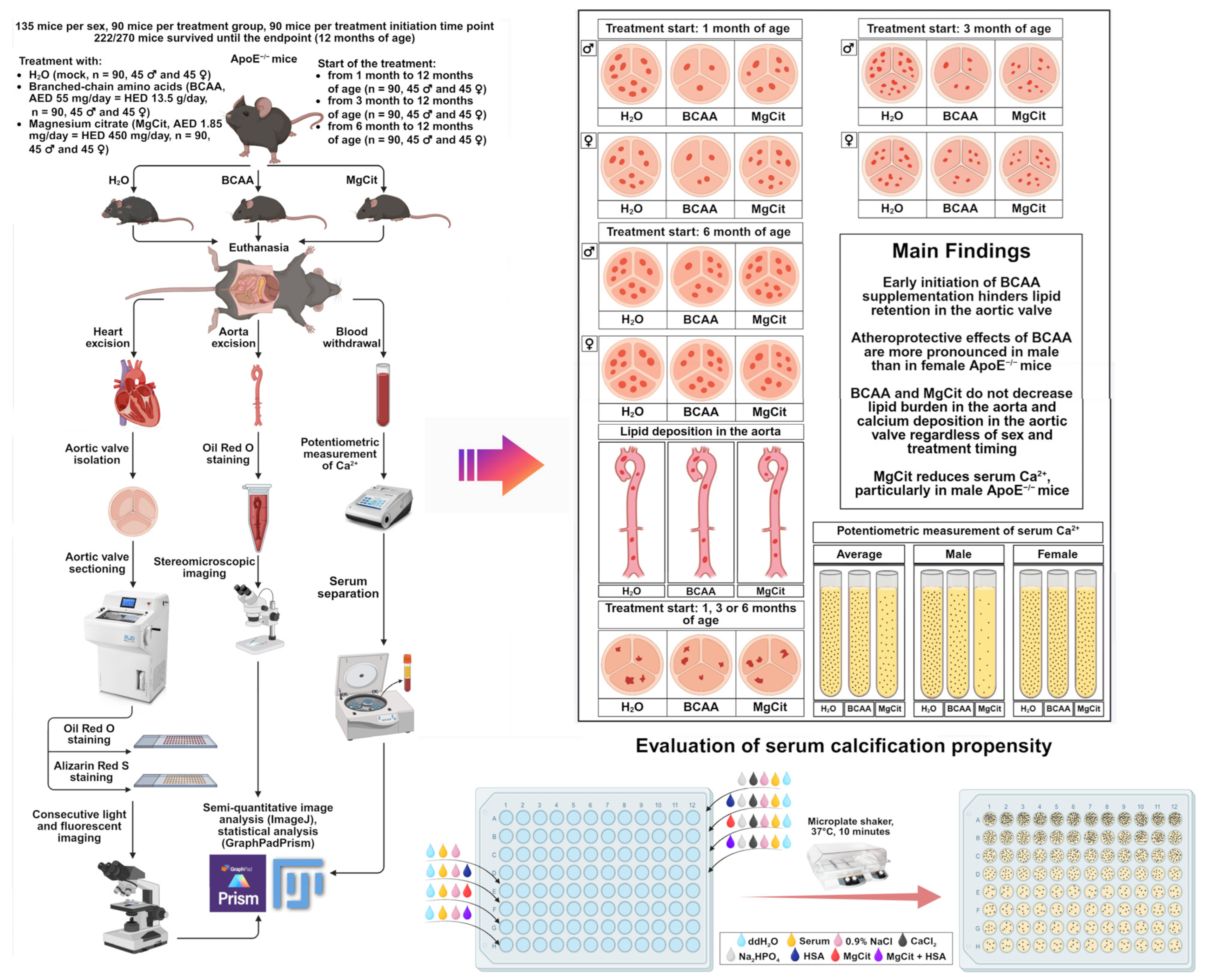
4.1. Animal Treatment Protocols
4.2. Histological Analysis
4.3. Measurement of Serum Ionized Calcium and Serum Calcification Propensity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCAA | Branched-chain amino acids |
| MgCit | Magnesium citrate |
| ApoE | Apolipoprotein E |
| AV | Aortic valve |
| HSA | Human serum albumin |
| STEMI | ST-segment elevation myocardial infarction |
| CAS | Chemical Abstracts Service |
References
- Nielsen, R.V.; Fuster, V.; Bundgaard, H.; Fuster, J.J.; Johri, A.M.; Kofoed, K.F.; Douglas, P.S.; Diederichsen, A.; Shapiro, M.D.; Nicholls, S.J.; et al. Personalized Intervention Based on Early Detection of Atherosclerosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 2112–2127. [Google Scholar] [CrossRef]
- Andersson, N.W.; Corn, G.; Dohlmann, T.L.; Melbye, M.; Wohlfahrt, J.; Lund, M. LDL-C Reduction with Lipid-Lowering Therapy for Primary Prevention of Major Vascular Events Among Older Individuals. J. Am. Coll. Cardiol. 2023, 82, 1381–1391. [Google Scholar] [CrossRef]
- Lee, S.J.; Joo, J.H.; Park, S.; Kim, C.; Choi, D.W.; Hong, S.J.; Ahn, C.M.; Kim, J.S.; Kim, B.K.; Ko, Y.G.; et al. Combination Lipid-Lowering Therapy in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2023, 82, 401–410. [Google Scholar] [CrossRef]
- McPherson, R.; Adreak, N.; Sharma, A. Medications for Lipid Control: Statins vs. Newer Drugs. Can. J. Cardiol. 2024, 40, S26–S34. [Google Scholar] [CrossRef]
- Ferri, N.; Ruscica, M.; Fazio, S.; Corsini, A. Low-Density Lipoprotein Cholesterol-Lowering Drugs: A Narrative Review. J. Clin. Med. 2024, 13, 943, Correction in J. Clin. Med. 2024, 13, 4582. [Google Scholar] [CrossRef]
- Tokgözoğlu, L.; Libby, P. The Dawn of a New Era of Targeted Lipid-Lowering Therapies. Eur. Heart J. 2022, 43, 3198–3208. [Google Scholar] [CrossRef]
- Nordestgaard, A.T.; Tybjærg-Hansen, A.; Mansbach, H.; Kersten, S.; Nordestgaard, B.G.; Rosenson, R.S. Target Populations for Novel Triglyceride-Lowering Therapies. J. Am. Coll. Cardiol. 2025, 85, 1876–1897. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Casula, M.; Cicero, A.F.G.; Corsini, A.; Borghi, C.; Catapano, A. Beyond Statins: New Pharmacological Targets to Decrease LDL-Cholesterol and Cardiovascular Events. Pharmacol. Ther. 2023, 250, 108507. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Cardiovascular Diseases and Risks 2023 Collaborators. Global, Regional, and National Burden of Cardiovascular Diseases and Risk Factors in 204 Countries and Territories, 1990–2023. J. Am. Coll. Cardiol. 2025; in press. [CrossRef]
- Moss, J.W.; Ramji, D.P. Nutraceutical Therapies for Atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Williams, J.O.; Ramji, D.P. Nutraceuticals as therapeutic agents for atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1562–1572. [Google Scholar] [CrossRef]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The role of nutraceuticals in statin intolerant patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef]
- Aquila, G.; Marracino, L.; Martino, V.; Calabria, D.; Campo, G.; Caliceti, C.; Rizzo, P. The use of nutraceuticals to counteract atherosclerosis: The role of the Notch pathway. Oxid. Med. Cell. Longev. 2019, 2019, 5470470. [Google Scholar] [CrossRef]
- Grant, J.K.; Dangl, M.; Ndumele, C.E.; Michos, E.D.; Martin, S.S. A historical, evidence-based, and narrative review on commonly used dietary supplements in lipid-lowering. J. Lipid Res. 2024, 65, 100493. [Google Scholar] [CrossRef]
- Riccardi, G.; Giosuè, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204. [Google Scholar] [CrossRef]
- Assadourian, J.N.; Peterson, E.D.; Gupta, A.; Navar, A.M. Use of dietary supplements among people with atherosclerotic cardiovascular disease in the United States: A population-based analysis from NHANES. J. Am. Heart Assoc. 2024, 13, e033748. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, R.; Mu, H.; Zhang, W.; Zeng, J.; Li, H.; Wang, S.; Zhao, X.; Chen, W.; Dong, J.; et al. Oral administration of branched-chain amino acids attenuates atherosclerosis by inhibiting the inflammatory response and regulating the gut microbiota in ApoE-deficient mice. Nutrients 2022, 14, 5065. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, E.; Jin, H.J.; Lee, Y.; Choi, S.J.; Lee, G.W.; Chang, P.S.; Paik, H.D. Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food Sci. Biotechnol. 2017, 26, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.R.E.V.; Makiyama, E.N.; Sampaio, G.R.; Soares-Freitas, R.A.M.; Bonvini, A.; Amaral, A.G.; Bordin, S.; Fock, R.A.; Rogero, M.M. Effects of branched-chain amino acids on the inflammatory response induced by LPS in Caco-2 cells. Metabolites 2024, 14, 76. [Google Scholar] [CrossRef]
- Ijichi, C.; Matsumura, T.; Tsuji, T.; Eto, Y. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem. Biophys. Res. Commun. 2003, 303, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Okabayashi, T.; Shima, Y.; Iiyama, T.; Takezaki, Y.; Munekage, M.; Namikawa, T.; Sugimoto, T.; Kobayashi, M.; Mimura, T.; et al. Branched-chain amino acid-enriched nutrients stimulate antioxidant DNA repair in a rat model of liver injury induced by carbon tetrachloride. Mol. Biol. Rep. 2012, 39, 10803–10810. [Google Scholar] [CrossRef]
- Konstantis, G.; Pourzitaki, C.; Chourdakis, M.; Kitsikidou, E.; Germanidis, G. Efficacy of branched chain amino acids supplementation in liver cirrhosis: A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1171–1190. [Google Scholar] [CrossRef]
- Sideris, G.A.; Tsaramanidis, S.; Vyllioti, A.T.; Njuguna, N. The role of branched-chain amino acid supplementation in combination with locoregional treatments for hepatocellular carcinoma: Systematic review and meta-analysis. Cancers 2023, 15, 926. [Google Scholar] [CrossRef]
- Hsu, Y.M.; Kuan, H.C.; Chen, Y.A.; Chiu, C.W.; Chen, P.C.; Tam, K.W. Effects of branched-chain amino acids supplementation on patients undergoing hepatic intervention: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2024, 131, 276–285. [Google Scholar] [CrossRef]
- Arques, S. Serum albumin and cardiovascular disease: State-of-the-art review. Ann. Cardiol. Angeiol. 2020, 69, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zoanni, B.; Brioschi, M.; Mallia, A.; Gianazza, E.; Eligini, S.; Carini, M.; Aldini, G.; Banfi, C. Novel insights about albumin in cardiovascular diseases: Focus on heart failure. Mass Spectrom. Rev. 2023, 42, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- van der Vusse, G.J. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.; Eckert, T.; Aretz, A.; Richtering, W.; van Dorp, W.; Schäfer, C.; Jahnen-Dechent, W. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J. Biol. Chem. 2008, 283, 14815–14825. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef]
- Watanabe, K.; Kinoshita, H.; Okamoto, T.; Sugiura, K.; Kawashima, S.; Kimura, T. Antioxidant properties of albumin and diseases related to obstetrics and gynecology. Antioxidants 2025, 14, 55. [Google Scholar] [CrossRef]
- Bar-Or, D.; Thomas, G.W.; Bar-Or, R.; Rael, L.T.; Scarborough, K.; Rao, N.; Shimonkevitz, R. Commercial human albumin preparations for clinical use are immunosuppressive in vitro. Crit. Care Med. 2006, 34, 1707–1712. [Google Scholar] [CrossRef]
- Rennie, C.; Donnelly, S.; McGrath, K. Albumin reduces hepatic steatosis and inflammation in high-fat-diet-fed mice. Int. J. Mol. Sci. 2025, 26, 7156. [Google Scholar] [CrossRef] [PubMed]
- Paar, M.; Rossmann, C.; Nusshold, C.; Wagner, T.; Schlagenhauf, A.; Leschnik, B.; Oettl, K.; Koestenberger, M.; Cvirn, G.; Hallström, S. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS ONE 2017, 12, e0182997. [Google Scholar] [CrossRef]
- Valeriani, E.; Pannunzio, A.; Palumbo, I.M.; Bartimoccia, S.; Cammisotto, V.; Castellani, V.; Porfidia, A.; Pignatelli, P.; Violi, F. Risk of venous thromboembolism and arterial events in patients with hypoalbuminemia: A comprehensive meta-analysis of more than 2 million patients. J. Thromb. Haemost. 2024, 22, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Seidu, S.; Kunutsor, S.K.; Khunti, K. Serum albumin, cardiometabolic and other adverse outcomes: Systematic review and meta-analyses of 48 published observational cohort studies involving 1,492,237 participants. Scand. Cardiovasc. J. 2020, 54, 280–293. [Google Scholar] [CrossRef]
- Ronit, A.; Kirkegaard-Klitbo, D.M.; Dohlmann, T.L.; Lundgren, J.; Sabin, C.A.; Phillips, A.N.; Nordestgaard, B.G.; Afzal, S. Plasma albumin and incident cardiovascular disease: Results from the CGPS and an updated meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Farcomeni, A.; Menichelli, D.; Pastori, D.; Violi, F. Serum albumin and risk of cardiovascular events in primary and secondary prevention: A systematic review of observational studies and Bayesian meta-regression analysis. Intern. Emerg. Med. 2020, 15, 135–143. [Google Scholar] [CrossRef]
- Thuemmler, R.J.; Pana, T.A.; Carter, B.; Mahmood, R.; Bettencourt-Silva, J.H.; Metcalf, A.K.; Mamas, M.A.; Potter, J.F.; Myint, P.K. Serum albumin and post-stroke outcomes: Analysis of UK regional registry data, systematic review, and meta-analysis. Nutrients 2024, 16, 1486. [Google Scholar] [CrossRef]
- Yao, Z.; Xu, Y.; Ma, W.; Sun, X.Y.; Jia, S.; Zheng, Y.; Liu, X.; Fan, Y.; Wang, C. Magnesium citrate protects against vascular calcification in an adenine-induced chronic renal failure rat model. J. Cardiovasc. Pharmacol. 2018, 72, 270–276. [Google Scholar] [CrossRef]
- Quiñones, H.; Hamdi, T.; Sakhaee, K.; Pasch, A.; Moe, O.W.; Pak, C.Y.C. Control of metabolic predisposition to cardiovascular complications of chronic kidney disease by effervescent calcium magnesium citrate: A feasibility study. J. Nephrol. 2019, 32, 93–100. [Google Scholar] [CrossRef]
- Zanforlini, B.M.; Ceolin, C.; Trevisan, C.; Alessi, A.; Seccia, D.M.; Noale, M.; Maggi, S.; Guarnieri, G.; Vianello, A.; Sergi, G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin. Exp. Res. 2022, 34, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Liu, J.; Miao, Y.; Wang, G.; Wang, X.; Liu, S.; Yang, L. The effects of magnesium and vitamin D/E co-supplementation on inflammation markers and lipid metabolism of obese/overweight population: A systematic review and meta-analysis. Front. Nutr. 2025, 12, 1563604. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.C.A.A.; Klein, M.R.S.T.; da Cunha, M.R.; de Souza Mattos, S.; de Paula Nogueira, L.; de Paula, T.; Corrêa, F.M.; Oigman, W.; Neves, M.F. Effects of oral magnesium supplementation on vascular function: A systematic review and meta-analysis of randomized controlled trials. High Blood Press. Cardiovasc. Prev. 2020, 27, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Darooghegi Mofrad, M.; Djafarian, K.; Mozaffari, H.; Shab-Bidar, S. Effect of magnesium supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2018, 273, 98–105. [Google Scholar] [CrossRef]
- Argeros, Z.; Xu, X.; Bhandari, B.; Harris, K.; Touyz, R.M.; Schutte, A.E. Magnesium supplementation and blood pressure: A systematic review and meta-analysis of randomized controlled trials. Hypertension, 2025; in press. [Google Scholar] [CrossRef]
- Amer, S.A.; Abo-Elnour, D.E.; Abbas, A.; Abdelrahman, A.S.; Hamdy, H.M.; Kenawy, S.; Sarhan, M.M.; Mohamed, O.H.; Elnaghy, M.Y.; Baker, M.; et al. Calcium, magnesium, and vitamin D supplementations as complementary therapy for hypertensive patients: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2025, 25, 89. [Google Scholar] [CrossRef]
- Behers, B.J.; Behers, B.M.; Stephenson-Moe, C.A.; Vargas, I.A.; Meng, Z.; Thompson, A.J.; Melchor, J.; Wojtas, C.N.; Rosario, M.A.; Baker, J.F.; et al. Magnesium and potassium supplementation for systolic blood pressure reduction in the general normotensive population: A systematic review and subgroup meta-analysis for optimal dosage and treatment length. Nutrients 2024, 16, 3617. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef]
- Asbaghi, O.; Moradi, S.; Nezamoleslami, S.; Moosavian, S.P.; Hojjati Kermani, M.A.; Lazaridi, A.V.; Miraghajani, M. The effects of magnesium supplementation on lipid profile among type 2 diabetes patients: A systematic review and meta-analysis of randomized controlled trials. Biol. Trace Elem. Res. 2021, 199, 861–873. [Google Scholar] [CrossRef]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef]
- Hariri, M.; Sohrabi, M.; Gholami, A. The effect of magnesium supplementation on serum concentration of lipid profile: An updated systematic review and dose-response meta-analysis on randomized controlled trials. Nutr. J. 2025, 24, 24. [Google Scholar] [CrossRef]
- Song, Y.; He, K.; Levitan, E.B.; Manson, J.E.; Liu, S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet. Med. 2006, 23, 1050–1056. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef]
- Liu, H.; Wang, R. Associations between the serum magnesium and all-cause or cardiovascular mortality in chronic kidney disease and end-stage renal disease patients: A meta-analysis. Medicine 2021, 100, e27486. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yang, C.C.; Hung, K.C.; Jiang, M.Y.; Huang, Y.T.; Hwang, J.C.; Hsieh, C.C.; Chuang, M.H.; Chen, J.Y. Association between hypomagnesemia and mortality among dialysis patients: A systematic review and meta-analysis. PeerJ 2022, 10, e14203. [Google Scholar] [CrossRef]
- Xiong, J.; He, T.; Wang, M.; Nie, L.; Zhang, Y.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: A systematic review and meta-analysis. J. Nephrol. 2019, 32, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Leenders, N.H.J.; Vermeulen, E.A.; van Ballegooijen, A.J.; Hoekstra, T.; de Vries, R.; Beulens, J.W.; Vervloet, M.G. The association between circulating magnesium and clinically relevant outcomes in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3133–3147. [Google Scholar] [CrossRef] [PubMed]
- Kobylecki, C.J.; Nordestgaard, B.G.; Afzal, S. Plasma ionized calcium and risk of cardiovascular disease: 106,774 individuals from the Copenhagen General Population Study. Clin. Chem. 2021, 67, 265–275. [Google Scholar] [CrossRef]
- Kobylecki, C.J.; Nordestgaard, B.G.; Afzal, S. Low plasma ionized calcium is associated with increased mortality: A population-based study of 106,768 individuals. J. Clin. Endocrinol. Metab. 2022, 107, e3039–e3047. [Google Scholar] [CrossRef]
- Mora, S.; Mann, G.; Adegoke, O.A.J. Sex differences in cachexia and branched-chain amino acid metabolism following chemotherapy in mice. Physiol. Rep. 2024, 12, e16003. [Google Scholar] [CrossRef]
- Liu, K.; Borreggine, R.; Gallart-Ayala, H.; Ivanisevic, J.; Marques-Vidal, P. Serum branched-chain amino acids are mainly associated with body mass index and waist circumference. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103880. [Google Scholar] [CrossRef]
- Muscella, A.; Felline, M.; Marsigliante, S. Sex-based effects of branched-chain amino acids on strength training performance and body composition. Sports 2024, 12, 275. [Google Scholar] [CrossRef]
- Wang, B.; Tan, X.; Yao, X.; Zhu, Z. Sex-specific differences in the association of magnesium intake with femoral neck bone mineral density among older adults. Endocr. Connect. 2025, 14, e250020. [Google Scholar] [CrossRef]
- Mazza, E.; Maurotti, S.; Ferro, Y.; Castagna, A.; Pujia, C.; Sciacqua, A.; Pujia, A.; Montalcini, T. Magnesium: Exploring gender differences in its health impact and dietary intake. Nutrients 2025, 17, 2226. [Google Scholar] [CrossRef]
- Obayashi, M.; Shimomura, Y.; Nakai, N.; Jeoung, N.H.; Nagasaki, M.; Murakami, T.; Sato, Y.; Harris, R.A. Estrogen controls branched-chain amino acid catabolism in female rats. J. Nutr. 2004, 134, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.; O’Connor, J.C.; Cortes, T.M.; Serra, M.C. Branched-chain amino acids combined with exercise improves physical function and quality of life in older adults: Results from a pilot randomized controlled trial. Dietetics 2025, 4, 32. [Google Scholar] [CrossRef]
- Sobhy, E.; Kamal, M.M.; Saad, Y.; Saleh, D.A.; Elgohary, R.; Hassan, M.S. Effect of branched-chain amino acid supplementation and exercise on quadriceps muscle quantity and quality in patients with cirrhosis as assessed by ultrasonography: A randomized controlled trial. Clin. Nutr. ESPEN 2024, 61, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Singh Tejavath, A.; Mathur, A.; Nathiya, D.; Singh, P.; Raj, P.; Suman, S.; Mundada, P.R.; Atif, S.; Rai, R.R.; Tomar, B.S. Impact of branched chain amino acid on muscle mass, muscle strength, physical performance, combined survival, and maintenance of liver function changes in laboratory and prognostic markers on sarcopenic patients with liver cirrhosis (BCAAS Study): A randomized clinical trial. Front. Nutr. 2021, 8, 715795. [Google Scholar] [CrossRef]
- Doma, K.; Singh, U.; Boullosa, D.; Connor, J.D. The effect of branched-chain amino acid on muscle damage markers and performance following strenuous exercise: A systematic review and meta-analysis. Appl. Physiol. Nutr. Metab. 2021, 46, 1303–1313. [Google Scholar] [CrossRef]
- Weber, M.G.; Dias, S.S.; de Angelis, T.R.; Fernandes, E.V.; Bernardes, A.G.; Milanez, V.F.; Jussiani, E.I.; de Paula Ramos, S. The use of BCAA to decrease delayed-onset muscle soreness after a single bout of exercise: A systematic review and meta-analysis. Amino Acids 2021, 53, 1663–1678. [Google Scholar] [CrossRef]
- Khemtong, C.; Kuo, C.H.; Chen, C.Y.; Jaime, S.J.; Condello, G. Does branched-chain amino acids (BCAAs) supplementation attenuate muscle damage markers and soreness after resistance exercise in trained males? A meta-analysis of randomized controlled trials. Nutrients 2021, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Ben Maaoui, K.; Jahrami, H.; AlMarzooqi, M.A.; Boukhris, O.; Messai, B.; Clark, C.C.T.; Glenn, J.M.; Ghazzaoui, H.A.; Bragazzi, N.L.; et al. Attenuating muscle damage biomarkers and muscle soreness after an exercise-induced muscle damage with branched-chain amino acid (BCAA) supplementation: A systematic review and meta-analysis with meta-regression. Sports Med. Open 2024, 10, 42. [Google Scholar] [CrossRef]
- Bai, G.H.; Tsai, M.C.; Tsai, H.W.; Chang, C.C.; Hou, W.H. Effects of branched-chain amino acid-rich supplementation on EWGSOP2 criteria for sarcopenia in older adults: A systematic review and meta-analysis. Eur. J. Nutr. 2022, 61, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Bucsa, C.; Farcas, A.; Leucuta, D.C.; Popa, S.L.; Dumitrascu, D.L. Effects of branched-chain amino acids on parameters evaluating sarcopenia in liver cirrhosis: Systematic review and meta-analysis. Front. Nutr. 2022, 9, 749969. [Google Scholar] [CrossRef]
- Siramolpiwat, S.; Limthanetkul, N.; Pornthisarn, B.; Vilaichone, R.K.; Chonprasertsuk, S.; Bhanthumkomol, P.; Nunanan, P.; Issariyakulkarn, N. Branched-chain amino acids supplementation improves liver frailty index in frail compensated cirrhotic patients: A randomized controlled trial. BMC Gastroenterol. 2023, 23, 154. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Yang, R.; Hu, C.; Huang, Z.; Zheng, C.; Liang, Q.; Gong, R.; Zhu, X.; Gong, H.; et al. Serum branched-chain amino acids are associated with leukocyte telomere length and frailty based on residents from Guangxi longevity county. Sci. Rep. 2020, 10, 10252. [Google Scholar] [CrossRef]
- van Dijk, A.M.; Bruins Slot, A.S.; Portincasa, P.; Siegerink, S.N.; Chargi, N.; Verstraete, C.J.R.; de Bruijne, J.; Vleggaar, F.P.; van Erpecum, K.J. Systematic review with meta-analysis: Branched-chain amino acid supplementation in liver disease. Eur. J. Clin. Investig. 2023, 53, e13909. [Google Scholar] [CrossRef]
- Noah, L.; Dye, L.; Bois De Fer, B.; Mazur, A.; Pickering, G.; Pouteau, E. Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post-hoc analysis of a randomised controlled trial. Stress Health 2021, 37, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Moabedi, M.; Aliakbari, M.; Erfanian, S.; Milajerdi, A. Magnesium supplementation beneficially affects depression in adults with depressive disorder: A systematic review and meta-analysis of randomized clinical trials. Front. Psychiatry 2023, 14, 1333261. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Mehboob, R.; Bokhari, S.S.; Ali, M.; Shabbir, A.; Mehboob, K.; Adnan, H.; Karami, M.M.; Shalabi, H.; Alshehri, B. Comparative efficacy of magnesium and potassium towards cholesterol and quality of life in patients with type 2 diabetes mellitus: A randomised single-blinded controlled clinical trial. Endocrinol. Diabetes Metab. 2024, 7, e511. [Google Scholar] [CrossRef]
- Cepeda, V.; Ródenas-Munar, M.; García, S.; Bouzas, C.; Tur, J.A. Unlocking the power of magnesium: A systematic review and meta-analysis regarding its role in oxidative stress and inflammation. Antioxidants 2025, 14, 740. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Zhao, T.; Zhang, D. Prognostic significance of nutrition-associated markers in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Arq. Bras. Cardiol. 2023, 120, e20220523. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: A systematic review and meta-analysis of prospective cohort studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Burgess, S.; Michaëlsson, K. Serum magnesium levels and risk of coronary artery disease: Mendelian randomisation study. BMC Med. 2018, 16, 68. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, M.; Yang, L.; Xu, H.; Song, W.; Qian, Y.; Zhao, M. Quantitative association between serum/dietary magnesium and cardiovascular disease/coronary heart disease risk: A dose-response meta-analysis of prospective cohort studies. J. Cardiovasc. Pharmacol. 2019, 74, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Alonso, A.; Folsom, A.R.; Michos, E.D.; Rebholz, C.M.; Misialek, J.R.; Chen, L.Y.; Dudley, S.; Lutsey, P.L. Serum magnesium and the incidence of coronary artery disease over a median 27 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) study and a meta-analysis. Am. J. Clin. Nutr. 2020, 111, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jin, F.; Hao, Y.; Li, H.; Tang, T.; Wang, H.; Yan, W.; Dai, K. Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e57720. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Arnold, A.P.; Reue, K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab. 2017, 25, 1216–1230. [Google Scholar] [CrossRef]
- Robinet, P.; Milewicz, D.M.; Cassis, L.A.; Leeper, N.J.; Lu, H.S.; Smith, J.D. Consideration of sex differences in design and reporting of experimental arterial pathology studies—Statement from ATVB Council. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 292–303. [Google Scholar] [CrossRef]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res. Cardiol. 2020, 115, 73. [Google Scholar] [CrossRef]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a biological variable in atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, J.I.; van Eenige, R.; Rensen, P.C.N.; Kooijman, S. Atherosclerosis: An overview of mouse models and a detailed methodology to quantify lesions in the aortic root. Vasc. Biol. 2024, 6, e230017. [Google Scholar] [CrossRef]
- Smit, V.; de Mol, J.; Kleijn, M.N.A.B.; Depuydt, M.A.C.; de Winther, M.P.J.; Bot, I.; Kuiper, J.; Foks, A.C. Sexual dimorphism in atherosclerotic plaques of aged Ldlr(−/−) mice. Immun. Ageing 2024, 21, 27. [Google Scholar] [CrossRef]
- Ravn, H.B.; Korsholm, T.L.; Falk, E. Oral magnesium supplementation induces favorable antiatherogenic changes in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 858–862. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, L.; Chen, Y.; Zhu, N.; Bai, Y.; Li, Q.; Zhao, S.; Fan, J.; Liu, E. Practical assessment of the quantification of atherosclerotic lesions in apoE−/− mice. Mol. Med. Rep. 2015, 12, 5298–5306. [Google Scholar] [CrossRef]
- Liu, F.Y.; Bai, P.; Jiang, Y.F.; Dong, N.G.; Li, G.; Chu, C. Role of interleukin 17A in aortic valve inflammation in apolipoprotein E-deficient mice. Curr. Med. Sci. 2020, 40, 729–738. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, N.; Kim, M.; Woo, S.H.; Han, I.; Park, J.; Kim, K.; Park, K.S.; Kim, K.; Shim, D.; et al. Single-cell transcriptomics reveal cellular diversity of aortic valve and the immunomodulation by PPARγ during hyperlipidemia. Nat. Commun. 2022, 13, 5461. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Hu, L.; Shan, J.; Zhang, H.; Hu, L.; Yuan, A.; Pu, J.; Xue, S. Mechanical injury accentuates lipid deposition in ApoE−/− mice and advance aortic valve stenosis: A novel modified aortic valve stenosis model. Front. Cardiovasc. Med. 2023, 10, 1119746. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, L.; Mo, P.; Chen, C.; Ji, Y.; Pang, L.; Chen, H.; Deng, Y.; Ou, W.; Liu, S.M. Apoe-knockout induces strong vascular oxidative stress and significant changes in the gene expression profile related to the pathways implicated in redox, inflammation, and endothelial function. Cell. Signal. 2023, 108, 110696. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Salagianni, M.; Varela, A.; Davos, C.H.; Galani, I.E.; Andreakos, E. Comprehensive analysis of 1-year-old female apolipoprotein E-deficient mice reveals advanced atherosclerosis with vulnerable plaque characteristics. Int. J. Mol. Sci. 2024, 25, 1355. [Google Scholar] [CrossRef]
- Xiang, A.; Guan, H.; Su, P.; Zhang, L.; Chen, X.; Yu, Q. IGFBP5 promotes atherosclerosis in APOE(−/−) mice through phenotypic transformation of VSMCs. Curr. Issues Mol. Biol. 2025, 47, 555. [Google Scholar] [CrossRef]
- Mencke, R.; Al Ali, L.; de Koning, M.L.Y.; Pasch, A.; Minnion, M.; Feelisch, M.; van Veldhuisen, D.J.; van der Horst, I.C.C.; Gansevoort, R.T.; Bakker, S.J.L.; et al. Serum calcification propensity is increased in myocardial infarction and hints at a pathophysiological role independent of classical cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1884–1894. [Google Scholar] [CrossRef]
- Kutikhin, A.G.; Shishkova, D.K.; Khryachkova, O.N.; Frolov, A.V.; Shabaev, A.R.; Zagorodnikov, N.I.; Markova, V.E.; Bogdanov, L.A.; Osyaev, N.Y.; Indukaeva, E.V.; et al. Formation of calcium phosphate bions in patients with carotid and coronary atherosclerosis. Russ. J. Cardiol. 2020, 25, 39–48. [Google Scholar] [CrossRef]
- Eelderink, C.; Te Velde-Keyzer, C.A.; Frenay, A.S.; Vermeulen, E.A.; Bachtler, M.; Aghagolzadeh, P.; van Dijk, P.R.; Gansevoort, R.T.; Vervloet, M.G.; Hillebrands, J.L.; et al. Serum calcification propensity and the risk of cardiovascular and all-cause mortality in the general population: The PREVEND study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Cai, X.; Mehta, R.C.; Scialla, J.J.; de Boer, I.H.; Hsu, C.Y.; Go, A.S.; Dobre, M.A.; Chen, J.; Rao, P.S.; et al. Serum calcification propensity and clinical events in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Dahle, D.O.; Åsberg, A.; Hartmann, A.; Holdaas, H.; Bachtler, M.; Jenssen, T.G.; Dionisi, M.; Pasch, A. Serum calcification propensity is a strong and independent determinant of cardiac and all-cause mortality in kidney transplant recipients. Am. J. Transplant. 2016, 16, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kurmann, R.; Buffle, E.; Pasch, A.; Seiler, C.; de Marchi, S.F. Predicting progression of aortic stenosis by measuring serum calcification propensity. Clin. Cardiol. 2022, 45, 1297–1302. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Scialla, J.J.; Dobre, M.A.; Chen, J.; Hsu, C.Y.; Leonard, M.B.; Go, A.S.; Rao, P.S.; Lash, J.P.; et al. Serum calcification propensity and coronary artery calcification among patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) study. Am. J. Kidney Dis. 2019, 73, 806–814. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Bolten, K.; Boschheidgen, M.; Schmidt, C.; Kolb, T.; Eckardt, K.U.; Pasch, A.; Schimmöller, L.; Rump, L.C.; Voelkl, J.; et al. Serum calcification propensity and calcification of the abdominal aorta in patients with primary aldosteronism. Front. Cardiovasc. Med. 2022, 9, 771096. [Google Scholar] [CrossRef]
- Nollet, L.; Van Gils, M.; Fischer, S.; Campens, L.; Karthik, S.; Pasch, A.; De Zaeytijd, J.; Leroy, B.P.; Devos, D.; De Backer, T.; et al. Serum calcification propensity T50 associates with disease severity in patients with pseudoxanthoma elasticum. J. Clin. Med. 2022, 11, 3727. [Google Scholar] [CrossRef]
- Koeppert, S.; Ghallab, A.; Peglow, S.; Winkler, C.F.; Graeber, S.; Büscher, A.; Hengstler, J.G.; Jahnen-Dechent, W. Live imaging of calciprotein particle clearance and receptor mediated uptake: Role of calciprotein monomers. Front. Cell Dev. Biol. 2021, 9, 633925. [Google Scholar] [CrossRef]
- Shishkova, D.; Markova, V.; Markova, Y.; Sinitsky, M.; Sinitskaya, A.; Matveeva, V.; Torgunakova, E.; Lazebnaya, A.; Stepanov, A.; Kutikhin, A. Physiological concentrations of calciprotein particles trigger activation and pro-inflammatory response in endothelial cells and monocytes. Biochemistry 2025, 90, 132–160. [Google Scholar] [CrossRef]
- Shishkova, D.K.; Markova, V.E.; Markova, Y.O.; Torgunakova, E.A.; Kondratiev, E.A.; Dyleva, Y.A.; Kutikhin, A.G. Patterns of calcium distribution by biochemical serum compartments in vitro modeling of mineral stress in endothelial dysfunction. Complex Issues Cardiovasc. Dis. 2024, 13, 60–71. [Google Scholar] [CrossRef]
- Banks, E.; Welsh, J.; Joshy, G.; Martin, M.; Paige, E.; Korda, R.J. Comparison of cardiovascular disease risk factors, assessment and management in men and women, including consideration of absolute risk: A nationally representative cross-sectional study. BMJ Open 2020, 10, e038761. [Google Scholar] [CrossRef] [PubMed]
- Bolijn, R.; Perini, W.; Tan, H.L.; Galenkamp, H.; Kunst, A.E.; van Valkengoed, I.G.M. Gender-related characteristics and disparities in estimated cardiovascular disease risk in a multi-ethnic general population: The HELIUS study. Int. J. Cardiol. 2021, 327, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cao, X.; Yu, K.; Pu, J.; Tang, Z.; Wei, N.; Wang, J.; Liu, F.; Li, S. Gender differences in all-cause and cardiovascular mortality among US adults: From NHANES 2005–2018. Front. Cardiovasc. Med. 2024, 11, 1283132. [Google Scholar] [CrossRef]
- Najman, J.M.; Kisely, S.; Scott, J.G.; Ushula, T.W.; Williams, G.M.; Clavarino, A.M.; McGee, T.R.; Mamun, A.A.; Wang, W.Y.S. Gender differences in cardiovascular disease risk: Adolescence to young adulthood. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, Z.; Jiang, C.; Wu, T.; Shi, W.; Liu, M.; Fan, Q.; Cui, G. Network Medicine-Based Repurposing of Mesalazine for Atherosclerosis Treatment. J. Cardiovasc. Transl. Res. 2025; online ahead of print. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkova, D.; Kanonykina, A.; Kondratiev, E.; Tyurina, A.; Morozova, A.; Poddubnyak, A.; Sinitskaya, A.; Sinitsky, M.; Markova, V.; Lazebnaya, A.; et al. Early Supplementation with Branched-Chain Amino Acids Ameliorates Lipid Retention in Aortic Valves of ApoE-Knockout Mice. Int. J. Mol. Sci. 2025, 26, 11259. https://doi.org/10.3390/ijms262311259
Shishkova D, Kanonykina A, Kondratiev E, Tyurina A, Morozova A, Poddubnyak A, Sinitskaya A, Sinitsky M, Markova V, Lazebnaya A, et al. Early Supplementation with Branched-Chain Amino Acids Ameliorates Lipid Retention in Aortic Valves of ApoE-Knockout Mice. International Journal of Molecular Sciences. 2025; 26(23):11259. https://doi.org/10.3390/ijms262311259
Chicago/Turabian StyleShishkova, Daria, Anastasia Kanonykina, Egor Kondratiev, Arina Tyurina, Alexandra Morozova, Alena Poddubnyak, Anna Sinitskaya, Maxim Sinitsky, Victoria Markova, Anastasia Lazebnaya, and et al. 2025. "Early Supplementation with Branched-Chain Amino Acids Ameliorates Lipid Retention in Aortic Valves of ApoE-Knockout Mice" International Journal of Molecular Sciences 26, no. 23: 11259. https://doi.org/10.3390/ijms262311259
APA StyleShishkova, D., Kanonykina, A., Kondratiev, E., Tyurina, A., Morozova, A., Poddubnyak, A., Sinitskaya, A., Sinitsky, M., Markova, V., Lazebnaya, A., Bogdanov, L., Stepanov, A., Agalaryan, S., & Kutikhin, A. (2025). Early Supplementation with Branched-Chain Amino Acids Ameliorates Lipid Retention in Aortic Valves of ApoE-Knockout Mice. International Journal of Molecular Sciences, 26(23), 11259. https://doi.org/10.3390/ijms262311259





