Crude Plant Extracts and Their Anti-Inflammatory Potential in Oral Inflammatory Cell Models: A Systematic Review of In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
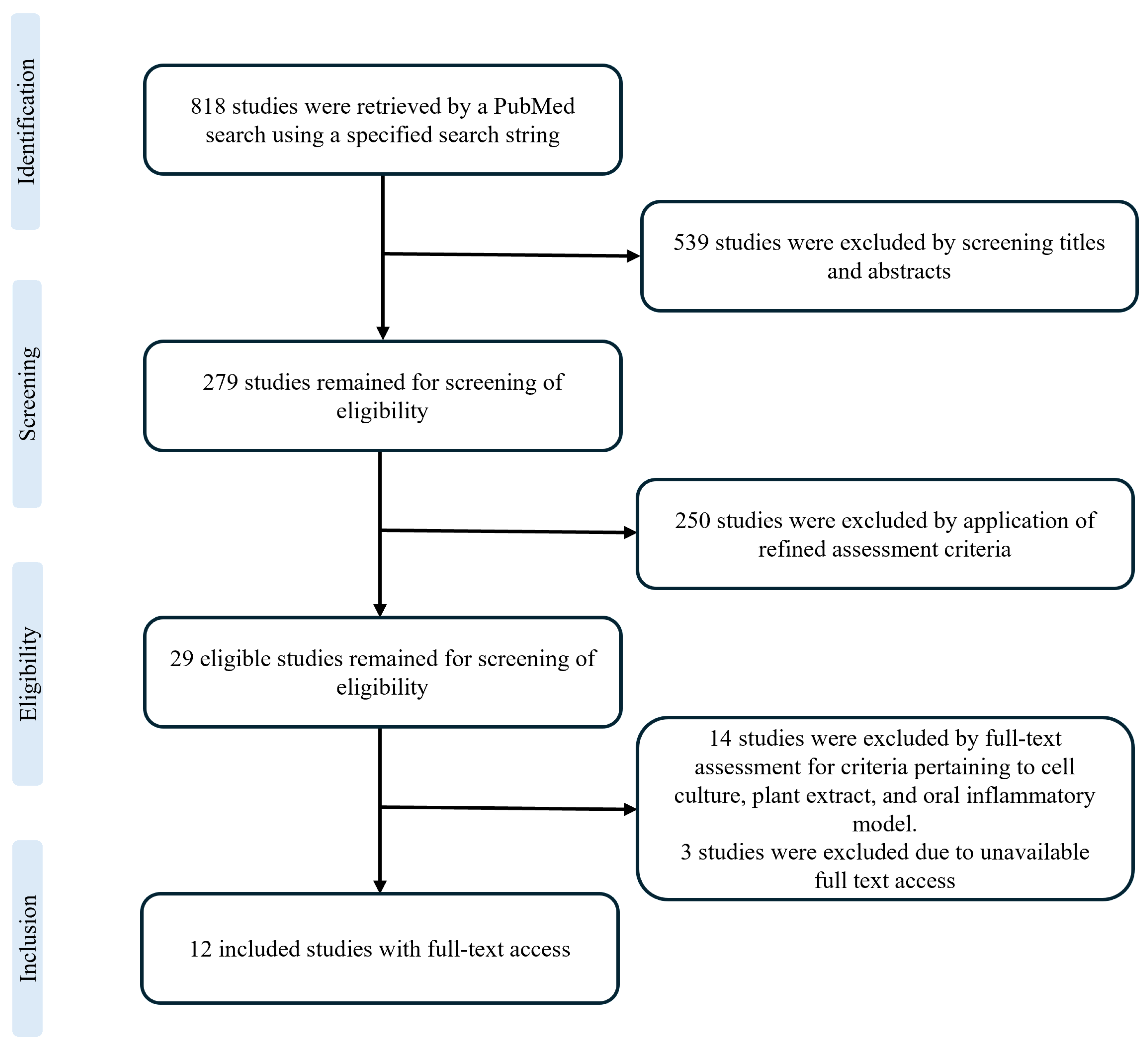
2.1. Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Collection Process
2.4. Quality Assessment
2.5. Synthesis Method
3. Results
3.1. Study Characteristics and Extract Preparation
3.2. Cell Models and Inflammatory Stimulation
3.3. Cell Viability and Cytotoxicity
3.4. Anti-Inflammatory Effects
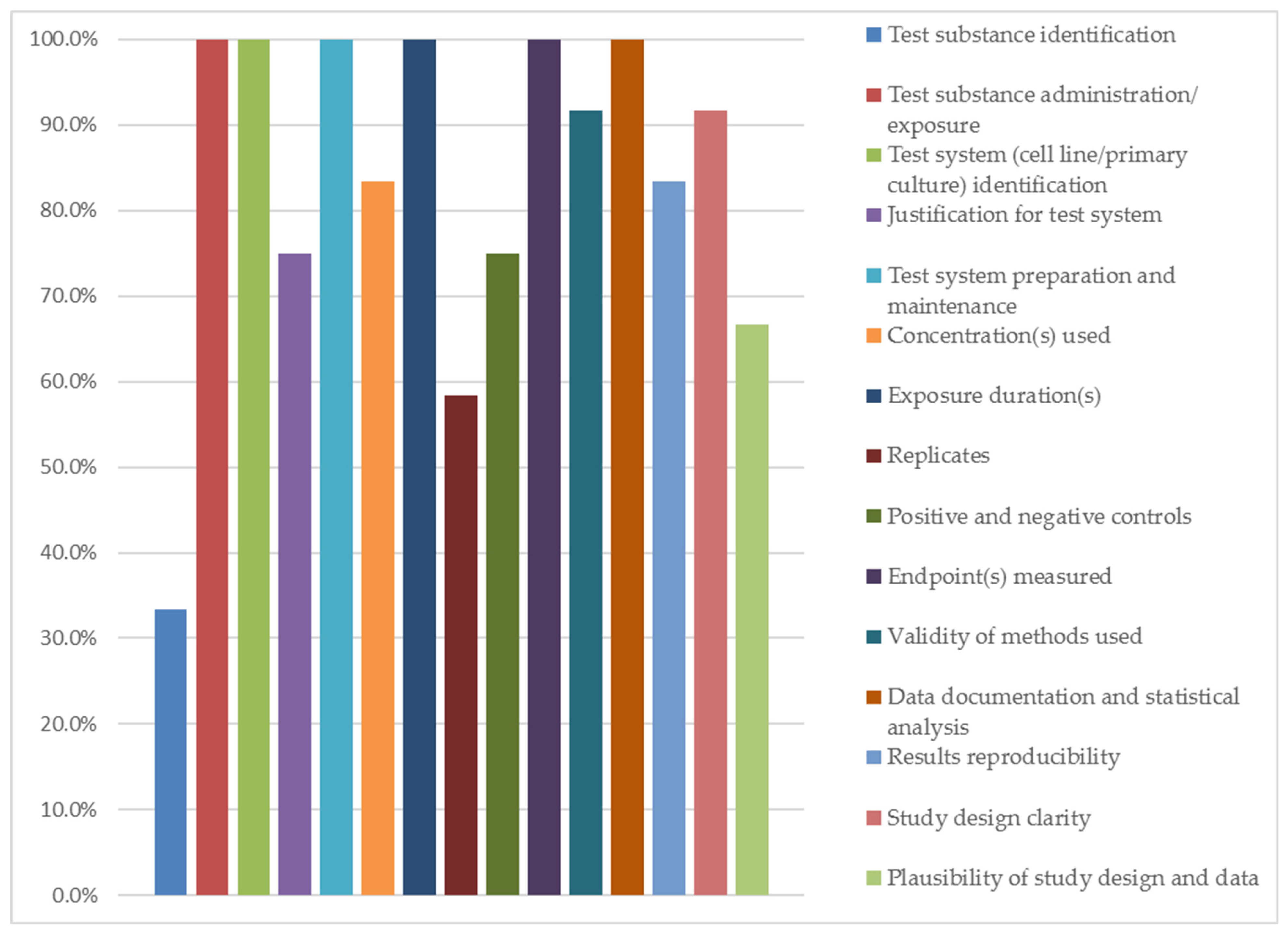
3.5. Quality Assessment Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MeSH | medical subject headings |
| NDR | no dose–response |
| PG-E2 | prostaglandin-E2 |
| COX2 | cyclooxygenase-2 |
| RAS | recurrent apthous stomatitis |
| PRISMA | preferred reporting items for systematic reviews and meta-analysis |
| PMA | phorbol 12-myristate 13-acetate |
| AgNP | silver nanoparticles |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| RT | room temperature |
| SC | stock concentration |
| WC | working concentration |
| D.H2O | distilled water |
| DMSO | dimethyl sulfoxide |
| LPS | lipopolysaccharide |
| ELISA | enzyme-linked immunosorbent assay |
| RT-PCR | real-time polymerase chain reaction |
| WST-1 | water-soluble tetrazolium salts |
| MTS | (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) |
References
- Menkin, V. The role of inflammation in immunity. Physiol. Rev. 1938, 18, 366–418. [Google Scholar] [CrossRef]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Garlet, G.P.; Fukada, S.Y.; Silva, J.S.d.; Cunha, F.d.Q. Chemokines in oral inflammatory diseases: Apical periodontitis and periodontal disease. J. Dent. Res. 2007, 86, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S.; Sabbah, W.; Donos, N.; Porter, S.; D’Aiuto, F. Common oral mucosal diseases, systemic inflammation, and cardiovascular diseases in a large cross-sectional US survey. Am. Heart J. 2011, 161, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp.(fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef]
- Napagoda, M.T.; Sundarapperuma, T.; Fonseka, D.; Amarasiri, S.; Gunaratna, P. An ethnobotanical study of the medicinal plants used as anti-inflammatory remedies in Gampaha District, Western Province, Sri Lanka. Scientifica 2018, 2018, 9395052. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Gbolade, A.A.; Ajaiyeoba, E.O. Ethnobotanical survey of plants used in treatment of inflammatory diseases in Ogun State of Nigeria. Eur. J. Sci. Res. 2010, 43, 183–191. [Google Scholar]
- Erdogrul, Ö.T. Antibacterial activities of some plant extracts used in folk medicine. Pharm. Biol. 2002, 40, 269–273. [Google Scholar] [CrossRef]
- Ariyawardana, A.; Cheng, K.K.F.; Kandwal, A.; Tilly, V.; Al-Azri, A.R.; Galiti, D.; Chiang, K.; Vaddi, A.; Ranna, V.; Nicolatou-Galitis, O.; et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3985–3995. [Google Scholar] [CrossRef]
- Sahebjamee, M.; Mansourian, A.; Hajimirzamohammad, M.; Zadeh, M.T.; Bekhradi, R.; Kazemian, A.; Manifar, S.; Ashnagar, S.; Doroudgar, K. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: A triple-blind, randomised, controlled clinical trial. Oral Health Prev. Dent. 2015, 13, 309–315. [Google Scholar]
- Mohandas, R.; Mohapatra, S. Comparative evaluation of the efficacy of herbal and benzydamine mouthwashes in preventing radiation-induced oral mucositis among head and neck cancer patients: A systematic review and network meta-analysis. Evid.-Based Dent. 2025, 26, 118. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Montiel, A. Benzydamine hydrochloride: An overview on a well-established drug with news in mechanisms of action. F1000Research 2024, 13, 350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. In Who Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- European Medicines Agency (EMA); Committee on Herbal Medicinal Products (HMPC). Guideline on Non-Clinical Documentation in Applications for Marketing Authorisation/Registration of Well-Established and Traditional Herbal Medicinal Products (EMA/HMPC/353983/2016). 2019. Available online: https://www.ema.europa.eu/en/non-clinical-documentation-applications-marketing-authorisation-registration-well-established-traditional-herbal-medicinal-products-scientific-guideline (accessed on 9 November 2025).
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Didilescu, A.C.; Chinthamani, S.; Scannapieco, F.A.; Sharma, A. NLRP3 inflammasome activity and periodontal disease pathogenesis—A bidirectional relationship. Oral Dis. 2024, 30, 4069–4077. [Google Scholar] [CrossRef]
- Aral, K.; Berdeli, E.; Cooper, P.R.; Milward, M.R.; Kapila, Y.; Karadede Ünal, B.; Aral, C.A.; Berdeli, A. Differential expression of inflammasome regulatory transcripts in periodontal disease. J. Periodontol. 2020, 91, 606–616. [Google Scholar] [CrossRef]
- Kim, K.O.; Lee, D.; Hiep, N.T.; Song, J.H.; Lee, H.-J.; Lee, D.; Kang, K.S. Protective Effect of Phenolic Compounds Isolated from Mugwort (Artemisia argyi) against Contrast-Induced Apoptosis in Kidney Epithelium Cell Line LLC-PK1. Molecules 2019, 24, 195. [Google Scholar] [CrossRef]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Zhao, L.; La, V.D.; Grenier, D. Antibacterial, antiadherence, antiprotease, and anti-inflammatory activities of various tea extracts: Potential benefits for periodontal diseases. J. Med. Food 2013, 16, 428–436. [Google Scholar] [CrossRef]
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-S.; Choi, Y.-G.; Jeong, M.-Y.; Lee, J.-H.; Lim, S. Moutan Cortex Radicis inhibits inflammatory changes of gene expression in lipopolysaccharide-stimulated gingival fibroblasts. J. Nat. Med. 2013, 67, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Lombardo Bedran, T.B.; Morin, M.-P.; Palomari Spolidorio, D.; Grenier, D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and β-defensin secretion in oral epithelial cells. PLoS ONE 2015, 10, e0143158. [Google Scholar] [CrossRef] [PubMed]
- Satthakarn, S.; Chung, W.; Promsong, A.; Nittayananta, W. H outtuynia cordata modulates oral innate immune mediators: Potential role of herbal plant on oral health. Oral Dis. 2015, 21, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Yun, I.-G.; Ahn, S.-H.; Yoon, W.-J.; Kim, C.S.; Lim, Y.K.; Kook, J.-K.; Jung, S.; Choi, C.-H.; Lee, T.-H. Litsea japonica leaf extract suppresses proinflammatory cytokine production in periodontal ligament fibroblasts Stimulated with oral pathogenic bacteria or interleukin-1β. Int. J. Mol. Sci. 2018, 19, 2494. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Inkielewicz-Stepniak, I.; Krauze-Baranowska, M. Anti-inflammatory and antioxidative effects of the buds from different species of Populus in human gingival fibroblast cells: Role of bioflavanones. Phytomedicine 2019, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Maquera Huacho, P.; Grenier, D. A cocoa (Theobroma cacao L.) extract impairs the growth, virulence properties, and inflammatory potential of Fusobacterium nucleatum and improves oral epithelial barrier function. PLoS ONE 2021, 16, e0252029. [Google Scholar] [CrossRef]
- Shiba, F.; Miyauchi, M.; Chea, C.; Furusho, H.; Iwasaki, S.; Shimizu, R.; Ohta, K.; Nishihara, T.; Takata, T. Anti-inflammatory effect of glycyrrhizin with Equisetum arvense extract. Odontology 2021, 109, 464–473. [Google Scholar] [CrossRef]
- Shin, S.W.; Hwang, Y.S. Anti-periodontitis effect of ethanol extracts of Alpinia katsumadai seeds. Nutrients 2021, 14, 136. [Google Scholar] [CrossRef]
- Wada, A.; Murakami, K.; Ishikawa, Y.; Amoh, T.; Hirao, K.; Hosokawa, Y.; Hinode, D.; Miyake, Y.; Yumoto, H. Anti-Inflammatory and Protective Effects of Juncus effusus L. Water Extract on Oral Keratinocytes. BioMed Res. Int. 2022, 2022, 9770899. [Google Scholar] [CrossRef]
- Al-Shibani, N.; Al-Kattan, R.; Alssum, L.; Allam, E. Effects of ginger (Zingiber officinale) on gingival fibroblasts: An in vitro study. Clin. Exp. Dent. Res. 2022, 8, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ding, Y.; Chen, G.; Sun, Y.; Zeng, X.; Ye, H. Components identification and nutritional value exploration of tea (Camellia sinensis L.) flower extract: Evidence for functional food. Food Res. Int. 2020, 132, 109100. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- He, J.; Dong, Y.; Liu, X.; Wan, Y.; Gu, T.; Zhou, X.; Liu, M. Comparison of chemical compositions, antioxidant, and anti-photoaging activities of Paeonia suffruticosa flowers at different flowering stages. Antioxidants 2019, 8, 345. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Lee, S.Y.; Min, B.S.; Kim, J.H.; Lee, J.; Kim, T.J.; Kim, C.S.; Kim, Y.H.; Lee, H.K. Flavonoids from the leaves of Litsea japonica and their anti-complement activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 273–276. [Google Scholar]
- Won, J.; Kim, J.-E.; Choi, D.H.; Han, M.-W.; Lee, D.-H.; Kang, S.C.; Song, Y.-J. Effects of compounds isolated from a Litsea japonica fruit extract on the TNF-α signaling pathway and cell viability. Mol. Cell. Toxicol. 2016, 12, 37–44. [Google Scholar] [CrossRef]
- Okińczyc, P.; Widelski, J.; Nowak, K.; Radwan, S.; Włodarczyk, M.; Kuś, P.M.; Susniak, K.; Korona-Głowniak, I. Phytochemical profiles and antimicrobial activity of selected Populus spp. bud extracts. Molecules 2024, 29, 437. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.; Figueroa, J.; Pérez-Álvarez, J.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Pallag, A.; Jurca, T.; Pasca, B.; Sirbu, V.; Honiges, A.; Costuleanu, M. Analysis of phenolic compounds composition by HPLC and assessment of antioxidant capacity in Equisetum arvense L. extracts. Rev. De Chim. 2016, 67, 1623–1627. [Google Scholar]
- Zhao, X.M.; You, H.L.; Yang, L.; Zhou, B.; Su, K.M.; Gajendran, B.; Shen, X.C.; Zhang, N.L. Flavonoids and Kavalactones Isolated from Seeds of Alpinia katsumadai Hayata and Their Cytotoxic Activities. Chem. Biodivers. 2025, 22, e202403429. [Google Scholar] [CrossRef]
- Park, N.-Y.; Kim, S.-G.; Park, H.-H.; Jeong, K.-T.; Lee, Y.J.; Lee, E. Anti-inflammatory effects of Juncus effusus extract (JEE) on LPS-stimulated RAW 264.7 cells and edema models. Pharm. Biol. 2016, 54, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.; Lu, F.; Peng, W.; Wu, C. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother. Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A.; Van Dyke, T.E. Oral inflammatory diseases and systemic inflammation: Role of the macrophage. Front. Immunol. 2012, 3, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wielento, A.; Lagosz-Cwik, K.B.; Potempa, J.; Grabiec, A.M. The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Scheres, N.; Laine, M.L.; Sipos, P.M.; Bosch-Tijhof, C.J.; Crielaard, W.; de Vries, T.J.; Everts, V. Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis. J. Periodontal Res. 2011, 46, 407–416. [Google Scholar] [CrossRef]
- Dai, M.; Dai, X.; Liang, Y.; Li, X.; Huang, H.; Zhao, W. Keratinocyte necroptosis promotes the progression of radiation-induced oral mucositis. BMC Oral Health 2025, 25, 941. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Almubarak, A.; Tanagala, K.K.K.; Papapanou, P.N.; Lalla, E.; Momen-Heravi, F. Disruption of Monocyte and Macrophage Homeostasis in Periodontitis. Front. Immunol. 2020, 11, 330. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, C.; Yang, J.; Li, B. Intersection between macrophages and periodontal pathogens in periodontitis. J. Leukoc. Biol. 2021, 110, 577–583. [Google Scholar] [CrossRef]
- Handschel, J.; Sunderkötter, C.; Prott, F.-J.; Meyer, U.; Kruse-Lösler, B.; Joos, U. Increase of RM3/1-positive macrophages in radiation-induced oral mucositis. J. Pathol. 2001, 193, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, G.; Galvan, O.; De Vries, A.; Ulmer, H.; Gunkel, A.; Lukas, P.; Thumfart, W. Local application of granulocyte-macrophage colony stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur. J. Cancer 2001, 37, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Kasurinen, S.; Happo, M.S.; Rönkkö, T.J.; Orasche, J.; Jokiniemi, J.; Kortelainen, M.; Tissari, J.; Zimmermann, R.; Hirvonen, M.R.; Jalava, P.I. Differences between co-cultures and monocultures in testing the toxicity of particulate matter derived from log wood and pellet combustion. PLoS ONE 2018, 13, e0192453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karri, V.; Lidén, C.; Fyhrquist, N.; Högberg, J.; Karlsson, H.L. Impact of mono-culture vs. Co-culture of keratinocytes and monocytes on cytokine responses induced by important skin sensitizers. J. Immunotoxicol. 2021, 18, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, O.; Dainty, S.J.; Kapas, S.; Taylor, J.J. A human oral keratinocyte cell line responds to human heat shock protein 60 through activation of ERK1/2 MAP kinases and up- regulation of IL-1beta. Clin. Exp. Immunol. 2005, 141, 307–314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hujiahemaiti, M.; Sun, X.; Zhou, J.; Lv, H.; Li, X.; Qi, M.; Chi, M.; Li, C.; Zhou, Y. Effects of quercetin on human oral keratinocytes during re-epithelialization: An in vitro study. Arch. Oral Biol. 2018, 95, 187–194. [Google Scholar] [CrossRef]
- Nakamura, M. Histological and immunological characteristics of the junctional epithelium. Jpn. Dent. Sci. Rev. 2018, 54, 59–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother. Pharmacol. 2009, 63, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Vaseenon, S.; Srisuwan, T.; Chattipakorn, N.; Chattipakorn, S.C. Lipopolysaccharides and hydrogen peroxide induce contrasting pathological conditions in dental pulpal cells. Int. Endod. J. 2023, 56, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Chayawatto, S.; Kornsuthisopon, C.; Linsuwanont, P. Effects of Lps and Hydrogen Peroxide on Cell Viability and Expression of Inflammatory Cytokines in Human Dental Pulp Cells: A Preliminary Study. Procedia Multidiscip. Res. 2025, 3, 44. [Google Scholar]
- Leng, S.X.; McElhaney, J.E.; Walston, J.D.; Xie, D.; Fedarko, N.S.; Kuchel, G.A. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 879–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Q.; Liu, B.; Yu, X.; Wei, X.; Xiao, Q. Integration of Immune Cell Signatures and Diagnostic Gene Markers in Pancreatitis: A Comprehensive Study on Therapeutic Targets and Predictive Diagnosis. Hum. Mutat. 2025, 2025, 7694723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- duPont, N.C.; Wang, K.; Wadhwa, P.D.; Culhane, J.F.; Nelson, E.L. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J. Reprod. Immunol. 2005, 66, 175–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ungaro, C.T.; Brown, S.D.; Wolfe, A.S. Comparative Analysis of Serum Cytokine ELISA and Multiplex Techniques. Biomed. J. Sci. Tech. Res. 2020, 32, 25325–25330. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.-S.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What is the best ethanol-water ratio for the extraction of antioxidants from rosemary? Impact of the solvent on yield, composition, and activity of the extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Effect of Organic Solvents and Water Extraction on the Phytochemical Profile and Antioxidant Activity of Clitoria ternatea Flowers. ACS Food Sci. Technol. 2021, 1, 1567–1577. [Google Scholar] [CrossRef]
- Aremu, A.O.; Pendota, S.C. Medicinal Plants for Mitigating Pain and Inflammatory-Related Conditions: An Appraisal of Ethnobotanical Uses and Patterns in South Africa. Front. Pharmacol. 2021, 12, 758583. [Google Scholar] [CrossRef] [PubMed]
- Namsa, N.D.; Tag, H.; Mandal, M.; Kalita, P.; Das, A.K. An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J. Ethnopharmacol. 2009, 125, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.P.; Li, M.; Budhathoki, C.; Yeh, C.H.; Ruble, K. Anti-inflammatory mouthwashes for the prevention of oral mucositis in cancer therapy: An integrative review and meta-analysis. Support. Care Cancer 2022, 30, 7205–7218. [Google Scholar] [CrossRef]
- Lima, I.C.G.d.S.; de Fátima Souto Maior, L.; Gueiros, L.A.M.; Leão, J.C.; Higino, J.S.; Carvalho, A.A.T. Clinical applicability of natural products for prevention and treatment of oral mucositis: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 4115–4124. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chen, Y.-W.; Kang, Y.-N.; Chen, J.-H.; Chen, C.; Lu, C.-Y.; Huang, T.-W.; Gautama, M.S.N. Efficacy of natural products in preventing oral mucositis resulting from cancer therapies: A network meta-analysis of randomized controlled trials. Crit. Rev. Oncol./Hematol. 2024, 199, 104373. [Google Scholar] [CrossRef]
- Balto, H.A.; Al-Manei, K.K.; Bin-Mohareb, T.M.; Shakoor, Z.A.; Al-Hadlaq, S.M. Cytotoxic effect of Salvadora persica extracts on human gingival fibroblast cells. Saudi Med. J. 2014, 35, 810–815. [Google Scholar]
- Verma, U.P.; Gupta, A.; Yadav, R.K.; Tiwari, R.; Sharma, R.; Balapure, A.K. Cytotoxicity of chlorhexidine and neem extract on cultured human gingival fibroblasts through fluorescence-activated cell sorting analysis: An in-vitro study. Eur. J. Dent. 2018, 12, 344–349. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Madorran, E.; Ambrož, M.; Knez, J.; Sobočan, M. An Overview of the Current State of Cell Viability Assessment Methods Using OECD Classification. Int. J. Mol. Sci. 2025, 26, 220. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, E.V.; Chesnokov, M.S.; Zhivotovsky, B.; Kopeina, G.S. Drug toxicity assessment: Cell proliferation versus cell death. Cell Death Discov. 2022, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systematic Toxicity Tests; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Chan, S.M.; Khoo, K.S.; Sit, N.W. Interactions between plant extracts and cell viability indicators during cytotoxicity testing: Implications for ethnopharmacological studies. Trop. J. Pharm. Res. 2015, 14, 1991–1998. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Corrigendum: Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2018, 9, 1178. [Google Scholar] [CrossRef]
- Stalinska, K.; Guzdek, A.; Rokicki, M.; Koj, A. Transcription factors as targets of the anti-inflammatory treatment. A cell culture study with extracts from some Mediterranean diet plants. J. Physiol. Pharmacol. Suppl. 2005, 56, 157–169. [Google Scholar]
- Lampronti, I.; Khan, M.; Bianchi, N.; Borgatti, M.; Gambari, R. Inhibitory effects of medicinal plant extracts on interactions between DNA and transcription factors involved in inflammation. Minerva Biotecnol. 2004, 16, 93. [Google Scholar]
- Lampronti, I.; Khan, M.; Bianchi, N.; Ather, A.; Borgatti, M.; Vizziello, L.; Fabbri, E.; Gambari, R. Bangladeshi medicinal plant extracts inhibiting molecular interactions between nuclear factors and target DNA sequences mimicking NF-kB binding sites. Med. Chem. 2005, 1, 327–333. [Google Scholar] [CrossRef]
- Eom, T.; Kim, I.-H.; Kim, H.-J.; Choi, Y.; Nam, T.-J. Calystegia soldanella Extract Exerts Anti-Oxidative and Anti-Inflammatory Effects via the Regulation of the NF-κB/Nrf-2 Pathways in Mouse Macrophages. Antioxidants 2021, 10, 1639. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Santos, A.R.d.O.d.; Carvalho, A.C.A.d.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.d.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and regulation of NF-kB in inflammatory bowel diseases: An overview of in vitro and in vivo effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D.; Parsanathan, R. l-Cysteine stimulates the effect of vitamin D on inhibition of oxidative stress, IL-8, and MCP-1 secretion in high glucose treated monocytes. J. Am. Coll. Nutr. 2021, 40, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, D.D. Correlation between oxidative stress markers and cytokines in different stages of breast cancer. Cytokine 2023, 161, 156082. [Google Scholar] [CrossRef]
- Choi, H.M.; Oh, D.H.; Bang, J.S.; Yang, H.I.; Yoo, M.C.; Kim, K.S. Differential effect of IL-1β and TNFα on the production of IL-6, IL-8 and PGE2 in fibroblast-like synoviocytes and THP-1 macrophages. Rheumatol. Int. 2010, 30, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Leimert, K.B.; Verstraeten, B.S.E.; Messer, A.; Nemati, R.; Blackadar, K.; Fang, X.; Robertson, S.A.; Chemtob, S.; Olson, D.M. Cooperative effects of sequential PGF2α and IL-1β on IL-6 and COX-2 expression in human myometrial cells. Biol. Reprod. 2019, 100, 1370–1385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Lankatillake, C.; Luo, S.; Flavel, M.; Lenon, G.B.; Gill, H.; Huynh, T.; Dias, D.A. Screening natural product extracts for potential enzyme inhibitors: Protocols, and the standardisation of the usage of blanks in α-amylase, α-glucosidase and lipase assays. Plant Methods 2021, 17, 3. [Google Scholar] [CrossRef]
- Mazumder, K.; Nabila, A.; Aktar, A.; Farahnaky, A. Bioactive Variability and In Vitro and In Vivo Antioxidant Activity of Unprocessed and Processed Flour of Nine Cultivars of Australian lupin Species: A Comprehensive Substantiation. Antioxidants 2020, 9, 282. [Google Scholar] [CrossRef]
| Author and Year | Plant Name | Extract Type | Extraction Temperature | Concentration | Medium/Vehicle | Purity | Cell Type | Inflammatory Stimulus | Stimulation Time/Order | Cell Viability Assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al., 2013 [23] | Camellia sinensis | aqueous | RT | SC: 2% WC:0.01%, 0.005%, 0.0025%, 0.0001% | D.H2O | 0.45 µm filter | Oral epithelial cell line | P. gingivalis extract | Extract for 2 h, then 24 h after stimulation | MTT |
| Ehrnhöfer-Ressler et al., 2013 [24] | Salvia officinalis | aqueous | 95 °C | SC: 1% WC: 1% | H2O | none | Gingival fibroblast cell line | PMA | Stimulation for 6 h, then plant extract for 6 h | WST-1 |
| Yun et al., 2013 [25] | Paeonia suffruticosa | ethanolic | RT | SC: 10% WC: 4% | DMSO | none | Primary gingival fibroblasts | LPS | Extract for 1 h, then LPS for 24 h | MTS |
| Lombardo Bedran et al., 2015 [26] | Camellia sinensis | aqueous | - | SC: 4% WC: 0.02% 0.01% | D.H2O | 0.2 µm filter | Oral epithelial cell line | LPS (A. actinomycetemcomitans) | Extract for 2 h, then stimulation for 24 h | MTT |
| Satthakarn et al., 2015 [27] | Houttuynia cordata | aqueous | - | SC: not mentioned WC: 0.02%, 0.01%, 0.005%, 0.0025% | H2O | none | Primary gingival epithelial cells | none | Extract for 18 h | CellTiter-Blue |
| Yun et al., 2018 [28] | Listea japonica | ethanolic | RT | WC: 0.01% | ethanol | none | Primary PDL fibroblast | LPS (P. gingivalis, T. forythea, T. denticola, F. nucleatum) | Dual (extract + LPS) for 0, 1, 3, 6, 12, 24 h Pre-treatment with the extract for 2 h, then LPS for 24 h | MTT |
| Pobłocka-Olech et al., 2019 [29] | Populus spp.: P. nigra, P. berolinensis, P. lasiocarpa | methanolic | 60 °C | SC: 5% WC: P.n + P.l: 0.0015% P.b: 0.00075% | methanol | none | Gingival fibroblast cell line | AgNP | Extract for 1 h, then stimulation for 18 h | MTT |
| Ben Lagha et al., 2021 [30] | Theobroma cacao | ethanolic | - | SC: 2% WC: 0.025% 0.0125% 0.0063% | DMSO | 0.22 µm filter | Oral epithelial cell lines | F. nucleatum | Extract for 30 min, then stimulation for 24 h | MTT |
| Shiba et al., 2021 [31] | Equisetum arvense | - | - | WC: 0.00004% | Commercially formulated: H2O + butylene glycol | none | Oral keratinocyte cell line, monocytic cell line | LPS (P. gingivalis, A. actinomycetemcomitans) | Co-stimulation for 6 h | None |
| Shin et al., 2021 [32] | Alpinia katsumadai | ethanolic | - | WC: 0.001% | ethanol | none | Gingival fibroblast and oral keratinocyte cell lines, murine macrophage cell line | LPS (dental plaque, P. gingivalis) | Co-stimulation for 24 h | MTT |
| Wada et al., 2022 [33] | Juncus effusus | aqueous | 60 °C | SC: 200% WC: 10% 2% | Ultra-pure H2O | 0.45 µm filter + centrigation 1200× g 15 min | Oral keratinocyte cell line | LPS (P. gingivalis) | Co-stimulation for 24 h, extract for 4 h, then stimulation for 24 h | None |
| Al-Shibani et al., 2022 [34] | Zingiber officinale | aqueous | RT | SC: 20% WC: 0.005% | D.H2O | 0.22 µm filter + centrifugation 3000× g 10 min | Primary gingival fibroblast | LPS (P. gingivalis) | Extract for 24 h, then LPS for 24 h, and vice versa | MTT |
| Author and Year | Assay | Outcome | Extract Highest and Non-Cytotoxic Concentration | Extract Lowest Effective Concentration |
|---|---|---|---|---|
| Zhao et al., 2013 [23] | ELISA | ↓ CCL5, IL-6, IL-8 | 100 µg/mL | 25 µg/mL |
| Ehrnhöfer-Ressler et al., 2013 [24] | Multiplex immunoassay | ↓ IL-6, IL-8 | NDR | NDR |
| Yun et al., 2013 [25] | RT-PCR | ↓ CXCL9, CXCL10, CXCL11 | NDR | NDR |
| Lombardo Bedran et al., 2015 [26] | ELISA | ↓ IL-8 | 200 µg/mL | 100 µg/mL |
| Satthakarn et al., 2015 [27] | RT-PCR ELISA | ↓ CXCL5 ↓ CCL2 | 200 µg/mL | 25 µg/mL |
| Yun et al., 2018 [28] | RT-PCR ELISA | ↓ IL-6, IL-8 ↓ IL-6, IL-8 | 100 µg/mL | 10 µg/mL |
| Pobłocka-Olech et al., 2019 [29] | RT-PCR ELISA | ↓ IL-6, IL-1ß ↓ IL-6, IL-1ß | NDR | NDR |
| Ben Lagha et al., 2021 [30] | ELISA | ↓ IL-6, IL-8 | 250 µg/mL | 125 µg/mL |
| Shiba et al., 2021 [31] | RT-PCR ELISA | ↓ TNF-α ↓ secretion of TNF-α | NDR | NDR |
| Shin et al., 2021 [32] | ELISA | ↓ PG-E2, COX-2 | NDR | NDR |
| Wada et al., 2022 [33] | ELISA | ↓ IL-8, CCL-20 | NDR | NDR |
| Al-Shibani et al., 2022 [34] | xMap Milliplex | ↓ IL-1ß, IL-8 | NDR | NDR |
| Author | Score | Comment |
|---|---|---|
| Zhao et al., 2013 [23] | 14 | reliable without restrictions |
| Ehrnhöfer-Ressler et al., 2013 [24] | 14 | reliable without restrictions |
| Yun et al., 2013 [25] | 11 | reliable with restrictions |
| Lombardo Bedran et al., 2015 [26] | 13 | reliable without restrictions |
| Satthakarn et al., 2015 [27] | 9 | reliable with restrictions |
| Yun et al., 2018 [28] | 13 | reliable without restrictions |
| Pobłocka-Olech et al., 2019 [29] | 14 | reliable without restrictions |
| Ben Lagha et al., 2021 [30] | 15 | reliable without restrictions |
| Shiba et al., 2021 [31] | 13 | reliable without restrictions |
| Shin et al., 2021 [32] | 11 | reliable with restrictions |
| Wada et al., 2022 [33] | 13 | reliable without restrictions |
| Al-Shibani et al., 2022 [34] | 11 | reliable with restrictions |
| Mean | 12.6 | |
| Median | 13 | |
| Standard Deviation | 1.7 | |
| Range | 6 | |
| Minimum | 9 | |
| Maximum | 15 | |
| Confidence Level (95.0%) | 1.099 | |
| Plant Species (Common Name) | Family | Major Phytochemical Groups | Principal Anti-Inflammatory Mechanisms |
|---|---|---|---|
| Camellia sinensis (Green tea) [36] | Theaceae | Polyphenols (catechins, EGCG, theaflavins) | Antioxidant; inhibits NF-κB, COX-2, and iNOS; activates Nrf2 |
| Salvia officinalis (Sage) [37] | Lamiaceae | Rosmarinic acid, carnosic acid, flavonoids, terpenes | NF-κB and MAPK suppression; COX-2 inhibition; antioxidant |
| Paeonia suffruticosa (Tree peony) [38] | Paeoniaceae | Paeonol, paeoniflorin, flavonoids | Inhibits MAPK and NF-κB; reduces IL-6, TNF-α; antioxidant |
| Houttuynia cordata (Chameleon plant) [39] | Saururaceae | Flavonoids, polysaccharides, and volatile oils | Immunomodulatory; suppresses IL-6, TNF-α, and NO; antiviral |
| Litsea japonica [40,41] | Lauraceae | Monoterpenes, flavonoids, lignans | Inhibits NO and TNF-α production; antioxidant |
| Populus spp. (poplar) [42] | Salicaceae | Phenolic glycosides (salicin, populin), flavonoids | COX inhibition; prostaglandin synthesis suppression |
| Theobroma cacao (Cocoa) [43] | Malvaceae | Polyphenols (procyanidins), theobromine, flavanols | Antioxidant; inhibits TNF-α, IL-6; enhances NO for vascular repair |
| Equisetum arvense (Horsetail) [44] | Equisetaceae | Silica, flavonoids, phenolic acids | Antioxidant; mild cytokine suppression; enhances collagen synthesis |
| Alpinia katsumadai (Katsumada galangal) [45] | Zingiberaceae | Diarylheptanoids, flavonoids, terpenes | Inhibits NO, COX-2, and IL-6; modulates MAPK |
| Juncus effusus (Soft rush) [46] | Juncaceae | Phenanthrenes, flavonoids, polysaccharides | Suppresses TNF-α, IL-6; antioxidant |
| Zingiber officinale (Ginger) [47] | Zingiberaceae | Gingerols, shogaols, zingerone | COX and LOX inhibition; NF-κB suppression; antioxidant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, I.; Gruber, R. Crude Plant Extracts and Their Anti-Inflammatory Potential in Oral Inflammatory Cell Models: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 11253. https://doi.org/10.3390/ijms262311253
Rasheed I, Gruber R. Crude Plant Extracts and Their Anti-Inflammatory Potential in Oral Inflammatory Cell Models: A Systematic Review of In Vitro Studies. International Journal of Molecular Sciences. 2025; 26(23):11253. https://doi.org/10.3390/ijms262311253
Chicago/Turabian StyleRasheed, Issam, and Reinhard Gruber. 2025. "Crude Plant Extracts and Their Anti-Inflammatory Potential in Oral Inflammatory Cell Models: A Systematic Review of In Vitro Studies" International Journal of Molecular Sciences 26, no. 23: 11253. https://doi.org/10.3390/ijms262311253
APA StyleRasheed, I., & Gruber, R. (2025). Crude Plant Extracts and Their Anti-Inflammatory Potential in Oral Inflammatory Cell Models: A Systematic Review of In Vitro Studies. International Journal of Molecular Sciences, 26(23), 11253. https://doi.org/10.3390/ijms262311253





